Abstract
Low birth weight (LBW) and hypoxia are among the environmental factors most reliably associated with schizophrenia; however, the nature of this relationship is unclear and both gene-environment interaction and gene-environment covariation models have been proposed as explanations. High-risk (HR) designs that explore whether obstetric complications differentially predict outcomes in offspring at low risk (LR) vs HR for schizophrenia, while accounting for differences in rates of maternal risk factors, may shed light on this question. This study used prospectively obtained data to examine relationships between LBW and hypoxia on school outcome at age 15–16 years in a Finnish sample of 1070 offspring at LR for schizophrenia and 373 offspring at HR for schizophrenia, based on parental psychiatric history. Controlling for offspring sex, maternal smoking, social support, parity, age, and number of prenatal care visits, HR offspring performed worse than LR offspring across academic, nonacademic, and physical education domains. LBW predicted poorer academic and physical education performance in HR offspring, but not in LR offspring, and this association was similar for offspring of fathers vs mothers with schizophrenia. Hypoxia predicted poorer physical education score across risk groups. Rates of LBW and hypoxia were similar for LR and HR offspring and for offspring of fathers vs mothers with schizophrenia. Results support the hypothesis that genetic susceptibility to schizophrenia confers augmented vulnerability of the developing brain to the effects of obstetric complications, possibly via epigenetic mechanisms.
Key words: low birth weight, hypoxia, high risk, academic, physical education
Introduction
Schizophrenia is a neurodevelopmental disorder that stems from altered brain developmental processes beginning as early as the first or second trimester in utero. 1 Although frankly psychotic symptoms do not generally emerge until late adolescence, subtler behavioral problems are often evident much earlier. Individuals who later manifest the syndrome often show premorbid deficits in motor, social, intellectual, and academic functioning, 2–5 and some offspring of parents with schizophrenia show a constellation of similar deficits that is predictive of later psychosis. 6–8 Progress in understanding the pathways through which various etiologic factors disrupt brain development in schizophrenia has been slow. However, studying risk factors for schizophrenia and how they relate to its developmental precursors may shed light on these pathways.
Schizophrenia is determined multifactorially by genetic and environmental factors, 9 and at least some cases are thought to result from the interaction of environmental factors with genetic risk. 10 Pregnancy and delivery complications are among the environmental factors most frequently associated with schizophrenia. Meta-analyses report pooled odds ratios for exposure to obstetric complications on schizophrenia likelihood to be around 2.0, indicating that individuals with schizophrenia are about twice as likely to have experienced obstetric complications compared with healthy controls. 11 , 12 However, most individuals exposed to obstetric complications do not develop schizophrenia. 13 Thus, obstetric complications appear insufficient to cause schizophrenia, raising the question of whether genetic risk for schizophrenia results in a developing brain that is more vulnerable to insult via obstetric complications.
If obstetric complications and genetic risk for schizophrenia interact to affect development, obstetric complications should differentially predict neurobehavioral outcomes in individuals at high risk (HR) for the illness (ie, having 1 or 2 parents with schizophrenia) vs those at low risk (LR; ie, having parents with no psychiatric illnesses). Early studies using this design reported that obstetric complications predicted motor impairments in HR but not in LR individuals at age 10 7 and interacted with genetic risk to predict ventricular enlargement, such that the relationship between birth complications and ventricular enlargement was greater in individuals with 2 vs 1 affected parent and greater in individuals with one vs no affected parents. 14 , 15 Although these studies provided intriguing evidence that individuals at genetic risk for schizophrenia may be more sensitive to the effects of obstetric complications, they were limited by small samples of individuals exposed to obstetric complications and did not control for variables such as maternal risk behaviors, social factors, and prenatal care that may differentiate HR and LR offspring and potentially contribute to poorer outcomes in offspring of parents with schizophrenia. 16 Clarke et al. 17 also reported recently that the difference in risk for later development of schizophrenia between siblings exposed vs not exposed to prenatal infection was 5 times larger in HR compared with LR offspring. In contrast to these studies, Goldstein et al. 18 found no interaction of genetic risk with hypoxia-ischemia on intelligence at age 7 after controlling for maternal age, ethnicity, socioeconomic status, and sex. Further, rates of obstetric complications are often higher in the obstetric histories of offspring of mothers with schizophrenia, 16 , 18 – 20 raising the possibility that obstetric complications themselves could be associated with susceptibility genes for schizophrenia, as in a gene-environment covariation model. Obstetric complications could therefore be markers for schizophrenia risk rather than causal factors in its etiologic pathway. 21 In light of these findings, additional studies that examine whether genetic risk modulates the relationship between obstetric complications and outcome are needed in larger samples, after accounting for group differences in risk factors and obstetric complications.
Low birth weight (LBW) 22–25 and hypoxia-related complications 26 , 27 have been found more frequently in the obstetric histories of schizophrenia patients than control subjects and are among the specific obstetric complications most reliably associated with schizophrenia. 12 , 25 , 28 , 29 This study therefore used prospectively obtained data to examine how LBW and hypoxia related to school outcome at 15–16 years in one of the largest studies to date of offspring at HR vs LR for schizophrenia. Potential group differences in rates of LBW and hypoxia were assessed to examine differential predictions of the gene-environment interaction vs covariation models, and maternal risk factors were assessed to explore if these contributed to relationships among genetic risk, obstetric complications, and outcome. We hypothesized that HR offspring would show poorer school performance than LR offspring and that HR offspring who experienced obstetric complications would show the poorest performance overall. The gene-environment interaction model would predict that differential relationships between obstetric complications and school outcome would exist independent of group differences in rates of LBW or hypoxia; conversely, the gene-environment covariation model would predict that the HR offspring would experience higher rates of LBW and/or hypoxia and that differential relationships between obstetric complications and outcome for HR vs LR offspring would not exist after accounting for this.
Methods
Ascertainment of Study Cases
The study was a registry-based, records-coding study with no direct contact with subjects. Study protocol was approved by the Institutional Review Boards of the Finnish National Public Health Institute and the University of California, Los Angeles. Study cohort consisted of individuals born in Finland between January 2, 1987 and December 31, 1993 identified as LR or HR for schizophrenia based on parental psychiatric history. HR subjects were ascertained by searching the Hospital Discharge Register for all men and women with schizophrenia diagnoses and crossing their social security numbers with the Finnish Perinatal Register to identify children born to these individuals between 1987 and 1993. The Hospital Discharge Register records diagnoses for all hospital admissions in Finland and was searched from 1969 to 2006. Finnish citizens have access to free psychiatric care and approximately 90% of Finnish individuals with schizophrenia have been hospitalized at least once. 30 , 31 For admissions before 1987, schizophrenia was identified as an International Classification of Diseases, Eighth Revision (ICD-8) diagnosis under 295; for admissions between 1987 and 1993, schizophrenia was identified as an ICD-9, Finnish version, diagnosis under 295, and for admissions between 1994 and 2006, schizophrenia was identified as an ICD-10 diagnosis under F21, F22, or F25. As the Hospital Discharge Register was searched for diagnoses of schizophrenia beyond the years of the births of HR offspring included in the study, some mothers and fathers with schizophrenia may have experienced their first hospitalization for psychosis and/or psychotic episode after the birth of offspring included in the study. LR subjects were ascertained at a ratio of approximately 3 LR:1 HR subject from children born at the same hospitals during these years to mothers and fathers with no psychiatric diagnoses in the Hospital Discharge Register. LR subjects were selected to be as close in gender, date of birth, and birth hospital to the identified HR offspring as possible. Offspring were included in the study if they were born in the Helsinki area (Helsinki-Espoo-Kauniainen-Vantaa) and had data available from schools in the Helsinki area. This resulted in the identification of 373 HR offspring (171 females) and 1070 LR offspring (552 females) from 107 schools in the Helsinki area. Ten LR offspring (1% of LR subjects) and 136 HR offspring (36% of HR subjects) shared a parent with another subject in the study. Of these subjects, 139 shared both mother and father with their included sibling(s); the remaining 7 subjects shared only a mother with a sibling in the study. No subjects shared only a father. Of the HR offspring, 173 had a father with schizophrenia, 197 had a mother with schizophrenia, and 3 had both a mother and father with schizophrenia. The limited number of HR subjects with both a father and mother with schizophrenia prohibited exploration of these subjects as a separate group. These subjects were grouped with offspring of mothers with schizophrenia for any analyses comparing offspring of fathers vs mothers with schizophrenia, as risk factors would be expected to be most severe in mothers with schizophrenia. 16
Pregnancy and Delivery Records
In the Finnish health care system, expectant mothers access complete examinations throughout pregnancy at Well Mother-Baby Clinics during which midwives and obstetricians fill out standard forms noting prenatal maternal and fetal health. At delivery, prior to 1990, Finnish hospitals used medical record sheets to document delivery and postnatal health information for the mother and infant. From January 1, 1990, onward, delivery and postnatal health information were recorded in a computerized perinatal registry that included many of the variables recorded in the paper records and some unique variables. A standardized coding form was used to transcribe prenatal and perinatal fetal and maternal health information for this study.
Low Birth Weight
Subjects were identified as LBW if they were less than 2500g at birth, as defined by the World Health Organization.
Hypoxia
Prior to 1990, hypoxia was based on ICD-9 diagnoses under intrauterine hypoxia and birth asphyxia (768) in the medical record sheets, and it included diagnoses of mild-to-moderate birth asphyxia (768.6), severe birth asphyxia (768.5), fetal distress first noted during labor and delivery in liveborn infant (768.3), fetal distress unspecified as to time of onset in liveborn infant (768.4), and fetal distress affecting management of mother (656.3). From 1990, onward, information regarding hypoxia was available in the computerized registry based on a specific item for hypoxia coded yes/no by the attending physician. Additionally, beginning September 28, 1990, information on arterial cord pH at delivery was available. Subjects with arterial cord pH values below 7.2 were also identified as having a history of hypoxia. 32
Maternal Risk Factors
Number of Well Mother-Baby Clinic visits, maternal age, parity, smoking, and social support were also examined. Maternal smoking was coded according to the presence or absence of smoking during pregnancy. Social support was coded according to the presence or absence of a partner that the mother was either living with or married to during pregnancy.
School Records
In Finland, the compulsory education program for children is a 9-year comprehensive curriculum that begins the year children have their seventh birthday. Mandatory curriculum consists of Finnish, Swedish, one foreign language, history, religion, mathematics, biology, geography, physics, chemistry, music, art, home economics, handiwork, and physical education. Grading is standardized across schools using national guidelines and ranges from 4 (fail) to 10 (excellent). Grades are assigned by relevant subject teachers and are intended to be nationally comparable. Numeric grades in this study were for mandatory subjects in the certificates of graduation when pupils were 15–16 years of age.
Statistical Analyses
Statistical analyses were conducted using Predictive Analytics Software (PASW) and Statistical Analysis System (SAS). A principal component analysis was conducted to reduce the 15 school subjects to a smaller number of underlying dimensions. The criterion for significant loading by individual school subject was set at 0.5. The criterion for significant communality value was set at 0.5. On the first iteration, physical education did not meet the communality value criterion, so it was removed, and the principal component analysis was re-run. The final solution yielded 2 factors with eigenvalues greater than 1.0: an academic and a nonacademic factor. School subjects that loaded onto each factor are listed in table 1. Grades for subject areas that loaded on each factor were summed and divided by the number of subject areas per factor to create average academic and nonacademic scores. Physical education was analyzed separately.
Table 1.
Principal Components Analysis of School Subject Grades (With Varimax Rotation)a
| School Subjects | Factor 1 (Academic) | Factor 2 (Nonacademic) |
|---|---|---|
| Finnish | .728 | .456 |
| Swedish | .781 | .369 |
| Foreign language | .762 | .162 |
| Math | .829 | .253 |
| Biology | .802 | .370 |
| Geography | .804 | .368 |
| Physics | .842 | .270 |
| Chemistry | .809 | .360 |
| History | .825 | .259 |
| Religion | .738 | .419 |
| Music | .255 | .676 |
| Art | .169 | .771 |
| Housekeeping | .387 | .685 |
| Handiwork | .275 | .657 |
aBold face type indicates variables with loadings greater than 0.5.
Potential differences in offspring gestational age and birth weight, number of prenatal care visits, maternal age, and parity for LR vs HR offspring, and offspring of fathers vs mothers with schizophrenia were examined using independent samples t tests. Differences in offspring sex, maternal smoking, and social support were examined using chi-square frequency tests. Rates of LBW and hypoxia between LR and HR offspring, and offspring of fathers vs mothers with schizophrenia, were compared using chi-square frequency tests.
We assessed the dependence of each of the school variables (ie, academic, nonacademic, and physical education score) on genetic risk and LBW, or genetic risk and hypoxia, in separate sets of parallel analyses using multilevel modeling. Multilevel modeling with school and family entered as random variables, and family nested within school, was chosen over MANOVA to adjust for correlations between observations from siblings and to more accurately model the hierarchical nature of the data where subjects were nested within families, and families were nested within schools. Genetic risk, birth weight group, and their interaction, or genetic risk, hypoxia group, and their interaction, were entered as fixed effects in their respective models. In cases where the interaction term was not significant, it was removed and the model was re-run. To control for the effects of potential confounders, this procedure was repeated with offspring sex, maternal smoking, social support, parity, age, and number of prenatal care visits included in the models. Offspring sex, maternal smoking status, and social support status were entered as fixed effects; parity, maternal age, and number of prenatal care visits were entered as continuous effects.
Following results of the initial analyses, additional multilevel models were conducted within HR subjects only to explore if dependence of academic and physical education scores on LBW interacted with parent source of risk (ie, whether the father or mother had schizophrenia). Parent source of risk, birth weight group, and their interaction were entered as fixed effects. The covariates were similarly included in the models. An α level of 0.05 was used to determine significance for all tests.
Results
Demographic and Obstetric Characteristics
Table 2 shows the demographic characteristics and rates of LBW and hypoxia for LR offspring and for HR offspring stratified by parent source of risk. Between LR and HR offspring, there were significant differences in maternal age, smoking, and social support, and a near significant difference in sex distribution. Between offspring of fathers vs mothers with schizophrenia, there was a significant difference in maternal smoking. There were no significant differences in rates of LBW or hypoxia between LR vs HR offspring and/or offspring of fathers vs mothers with schizophrenia.
Table 2.
Demographic and Obstetric Characteristics for Low-Risk (LR) Offspring and High-Risk (HR) Offspring Stratified by Parent Source of Schizophrenia (SZ)a
| LR Offspring | HR Offspring— Father With SZ | HR Offspring—Mother With SZ | LR vs HR Offspring | HR Offspring—Father vs Mother With SZ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) or % | n | Mean (SD) or % | n | Mean (SD) or % | n | t or χ2 | P | t or χ2 | P | |
| Child characteristics | ||||||||||
| Male | 48.40 | 1070 | 57.8 | 173 | 51.0 | 200 | 3.65 | .06 | 1.73 | .19 |
| Gestational age | 39.77 (1.83) | 1061 | 39.87 (2.10) | 171 | 39.64 (1.90) | 199 | 0.21 | .84 | 1.13 | .26 |
| Weight at birth (g) | 3511.82 (561.69) | 1063 | 3484.01 (613.83) | 171 | 3440.93 (576.55) | 199 | 1.48 | .14 | 0.70 | .49 |
| Mother characteristics | ||||||||||
| Age at birthb | 29.53 (4.93) | 1070 | 28.43 (5.36) | 173 | 28.74 (5.75) | 200 | 3.07 | .002 | 0.53 | .60 |
| Parity | 0.77 (.94) | 1059 | 0.63 (.77) | 172 | 0.80 (.99) | 198 | 1.05 | .30 | 1.83 | .07 |
| Well-Mother Baby Clinic visits | 15.42 (4.14) | 1051 | 15.44 (4.22) | 172 | 15.22 (4.42) | 196 | 0.39 | .70 | 0.47 | .64 |
| Maternal smoking during pregnancyb ,c | 19.8 | 1034 | 22.0 | 168 | 43.1 | 197 | 27.85 | <.001 | 18.18 | <.001 |
| Social support during pregnancyb | 90.6 | 1070 | 87.3 | 173 | 81.0 | 200 | 12.33 | <.001 | 2.71 | .10 |
| Rates of obstetric complications | ||||||||||
| Low birth weight (LBW) | 4.0 | 1070 | 5.2 | 173 | 3.5 | 200 | 0.52 | .82 | 0.66 | .42 |
| Hypoxia | 8.1 | 1070 | 11.6 | 173 | 10.0 | 200 | 2.317 | .13 | 0.24 | .63 |
aBold face type indicates significant test statistic.
bSignificant difference between LR and HR offspring.
cSignificant difference between offspring of father SZ vs mother SZ.
Dependence of School Outcomes on Genetic Risk and LBW
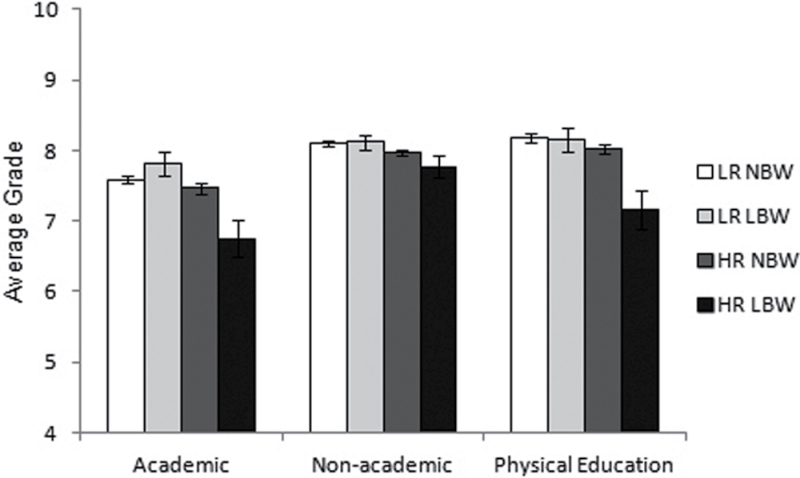
Results of the multilevel models testing the dependence of academic, nonacademic, and physical education score on genetic risk and LBW are shown before and after controlling for the covariates in table 3 and are depicted in figure 1. In the model for academic score, there was a significant effect of genetic risk, with HR subjects performing worse than LR subjects, and a significant interaction of genetic risk with birth weight group. Follow-up tests revealed that HR subjects with LBW performed significantly worse than HR subjects without LBW, (β = −0.68, se = 0.28, P = .016); conversely, there was no significant effect of LBW in LR subjects (β = 0.27, se = 0.17, P = .11). In the model for nonacademic score, the interaction between genetic risk and birth weight group was not significant (F(1,47) = 1.95, P = .17), so the model was rerun with the interaction term removed. The simplified model showed a significant effect of genetic risk, with HR subjects performing worse than LR subjects, and no effect of LBW. In the model for physical education score, there were significant main effects of genetic risk and birth weight group and a significant interaction of genetic risk with birth weight group. HR subjects performed worse than LR subjects, and LBW subjects performed worse than subjects without LBW. However, tests of simple effects within each genetic group revealed that LBW predicted poorer physical education scores within HR offspring, (β = −0.82, se = 0.28, P = .004), but not within LR offspring, (β = −0.04, se = 0.17, P = .83). Multilevel models with offspring sex, maternal smoking, social support, parity, age, and number of prenatal care visits included as covariates revealed the same pattern of results. Thus, the interactions between genetic risk and birth weight group remained significant: HR subjects with LBW performed significantly worse than HR subjects without LBW for academic (β = −0.73, se = 0.26, P = .006) and physical education score (β = −0.85, se = 0.28, P = .002). Conversely, there was no difference between LR subjects with and without LBW for academic (β = 0.23, se = 0.16, P = .16) or physical education score (β = −0.02, se = 0.17, P = .91). The interaction of genetic risk and birth weight group was not significant for nonacademic score (F(1,47) = 1.95, P = .17).
Table 3.
Dependence of School Outcome Scores on Genetic Risk and Low Birth Weight (LBW) Statusa
| Academic | Nonacademicb | Physical Education | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F | df | P | F | df | P | F | df | P | |
| Without potential confounders | |||||||||
| Genetic risk | 18.07 | 1,47 | <.001 | 23.21 | 1,47 | <.001 | 15.36 | 1,47 | <.001 |
| LBW | 1.51 | 1,47 | .23 | 0.03 | 1,47 | .87 | 6.69 | 1,47 | .01 |
| Genetic risk × LBW | 8.26 | 1,47 | .006 | — | — | — | 5.54 | 1,47 | .02 |
| With potential confoundersc | |||||||||
| Genetic risk | 15.49 | 1,37 | <.001 | 12.92 | 1,37 | <.001 | 12.15 | 1,37 | .001 |
| LBW | 2.58 | 1,37 | .12 | 0.12 | 1,37 | .73 | 6.85 | 1,37 | .01 |
| Genetic risk × LBW | 9.59 | 1,37 | .004 | — | — | — | 6.33 | 1,37 | .02 |
aBold face type indicates significant test statistic.
bTest statistics presented from the simplified model after removing the nonsignificant genetic risk x LBW interaction term.
cControlling for main effects of offspring sex, maternal smoking status, social support status, parity, age, and number of Well Mother-Baby Clinic visits.
Fig. 1.
Mean ± SE average school outcome scores for offspring at low risk (LR) or high risk (HR) for schizophrenia with normal birth weight (NBW) or low birth weight (LBW).
Dependence of School Outcomes on Genetic Risk and Hypoxia
The models testing the dependence of the school variables on genetic risk and hypoxia showed no significant interactions between genetic risk and hypoxia group for academic (F(1,47) = 0.33, P =.57), nonacademic (F(1,47) = 0.03, P = .85), or physical education score (F(1,47) = 0.11, P = .75), so the models were rerun with the interaction term removed. The simplified models showed a significant effect of genetic risk across school domains and a significant effect of hypoxia for physical education score only; subjects who experienced hypoxia performed significantly worse than subjects who did not experience hypoxia (β = −0.21, se = 0.10, P = .046). After controlling for potential confounding variables, the same pattern was evident, with no significant interactions between genetic risk and hypoxia for any school variable (Ps > .05); the simplified model for physical education similarly showed a main effect of hypoxia (β = −0.22, se = 0.10, P = .038). Results before and after controlling for the covariates are shown in table 4.
Table 4.
Dependence of School Outcome Scores on Genetic Risk and Hypoxia Statusa
| Academicb | Nonacademicb | Physical Educationb | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F | df | P | F | df | P | F | df | P | |
| Without potential confounders | |||||||||
| Genetic risk | 15.40 | 1,47 | <.001 | 22.98 | 1,47 | <.001 | 18.08 | 1,47 | <.001 |
| Hypoxia | 0.59 | 1,47 | .44 | 0.53 | 1,47 | .47 | 4.22 | 1,47 | .046 |
| Genetic risk × hypoxia | — | — | — | — | — | — | — | — | — |
| With potential confoundersc | |||||||||
| Genetic risk | 6.94 | 1,37 | .01 | 12.85 | 1,37 | .001 | 7.85 | 1,37 | .008 |
| Hypoxia | 1.24 | 1,37 | .27 | 0.07 | 1,37 | .79 | 4.64 | 1,37 | .038 |
| Genetic risk × hypoxia | — | — | — | — | — | — | — | — | — |
aBold face type indicates significant test statistic.
bTest statistics presented from the simplified model after removing the nonsignificant genetic risk x hypoxia interaction term.
cControlling for main effects of offspring sex, maternal smoking status, social support status, parity, age, and number of Well Mother-Baby Clinic visits.
LBW Effect Within HR Offspring by Parent Schizophrenia Group
Models comparing offspring of fathers vs mothers with schizophrenia showed no modulation of the LBW effect on academic or physical education score by parent schizophrenia group. Thus, across HR offspring, there was a significant effect of LBW on academic and physical education score. The main effect for parent schizophrenia group and interaction of parent schizophrenia group with LBW were not significant. (See online supplementary material for table S1 and figure S1.)
Discussion
This study examined relationships among genetic risk for schizophrenia, obstetric complications, and adolescent school outcome using prospectively obtained data in one of the largest samples comparing developmental outcomes of LR vs HR offspring. Controlling for offspring sex, maternal smoking, social support, parity, age, and number of prenatal care visits, HR offspring performed worse than LR offspring across school domains. LBW predicted poorer academic and physical education scores in HR offspring, but not in LR offspring, and these effects were present regardless of whether offspring had a father or mother with schizophrenia. There was no interaction of hypoxia with genetic risk for any school domain; however, hypoxia predicted a subtle decrease in physical education score across groups. Similar rates of LBW and hypoxia in LR and HR offspring suggest that the relationships between LBW and school outcome in HR offspring are not accounted for by gene-environment covariation.
That HR offspring showed worse performance across school domains is consistent with findings that offspring of parents with schizophrenia show deficits across numerous domains including attention, memory, and motor skill 6–8 , 18 and that individuals who later develop schizophrenia show premorbid deficits across cognitive, motor, and social functioning. 2–5 , 33 Individuals who later develop schizophrenia also show poorer performance on tests of academic achievement 4 , 34 , 35 and poorer school outcomes compared with peers 3 , 36 , 37 ; however, patterns of poor academic outcome in preschizophrenia children using school data have been less consistent than those using standardized measures, possibly due to different characteristics of education systems at different historical times. 33
Outcomes of LBW infants are heterogeneous and range from severe neonatal brain injury and neurologic dysfunction to normal functioning. LBW babies are at increased risk for subnormal growth, adverse health conditions, and neurodevelopmental problems; however, the majority of LBW infants have normal outcomes. 38 , 39 Thus, studies report a downward shift in average IQ score for LBW infants relative to normal birth weight infants, but this shift is small and the majority of LBW infants score in the normal IQ range. 39 , 40 Similarly, LBW infants show excess motor problems in childhood and adolescence compared with normal birth weight infants; however, most LBW infants do not show motor problems. 40–42
In light of these heterogeneous outcomes, it is notable that LBW predicted poorer academic and physical education scores in HR offspring, but not in LR offspring, despite similar rates of LBW across groups. The development of the central nervous system requires coordinated activity between an individual’s genetic program and embryonic environment and is dependent on sufficient glucose and oxygen supply via the uteroplacental unit. LBW provides little information about the specific factors that disrupt development in utero. Nevertheless, it is widely associated with conditions of altered uteroplacental flow such as maternal malnutrition, smoking, and preeclampsia, 43–45 as well as increased incidence of neonatal complications that involve deprivation of oxygen and other substrates, including hypoxia, ischemia, and respiratory distress syndrome. 46–48 That LBW predicted poorer school outcomes in HR offspring, but not in LR offspring, is consistent with a gene-environment interaction model and may reflect altered epigenetic modulation of brain development following early deprivation of key substrates such as glucose and oxygen. Thus, obstetric complications have been suggested to alter gene expression relevant to neurodevelopmental outcomes, 43 , 49 and insufficient availability of such substrates has been shown to initiate signaling cascades that alter gene expression for vascular and metabolic function. 43 , 50 , 51 Interestingly, Schmidt-Kastner et al. 52 reported that over half the genes identified as candidate schizophrenia genes are regulated by hypoxia-ischemia and/or are expressed in the cerebrovasculature. In line with this, Nicodemus et al. 53 reported that schizophrenia patients who experienced serious obstetric complications showed distorted transmission of allele variations in several hypoxia-regulated candidate genes for schizophrenia, and Joo et al. 54 reported that allelic variations in the hypoxia-regulating gene AKT-1 was associated with severity of obstetric complications in female schizophrenia patients. Structural brain abnormalities such as decreased hippocampal and gray matter volume and increased ventricular volumes that are reliably found in patients with schizophrenia 55 are augmented in patients who have histories of obstetric complications. 56–58 That similar augmentations of abnormality in these structures have been found in the unaffected siblings, 56 , 59 twins, 57 and offspring of schizophrenia patients with histories of obstetric complications, 14 , 15 , 60 , 61 in the absence of parallel relationships for control subjects, 14 , 15 , 56 , 58 suggest that genes carried by individuals at risk for schizophrenia may lead to less adaptive epigenetic programming in response to glucose or oxygen deprivation during early prenatal or neonatal life.
Given the theoretical emphasis placed on hypoxia in the relationship between obstetric complications and schizophrenia, it is surprising that hypoxia was not associated with academic scores in HR subjects and only predicted subtle decreases in physical education score across risk groups. Individuals with a history of perinatal hypoxia often show impairments in motor, cognitive, and school performance; however, some show differential deficits in cognitive vs motor functioning, and many show no deficits at all. 62–64 That hypoxia was associated with only subtle impairment in physical education performance and not with academic performance in this study is consistent with this variable picture of outcome and may also reflect the greater difficulty of identifying hypoxia. In contrast to birth weight, which is accurately and routinely assessed, hypoxia is inferred from a variety of obstetric indicators such as Apgar scores, acidosis, abnormal electroencephalography (EEG), and neurological anomalies in early postnatal life. Thus, as reflected by the wide variation in definitions of hypoxia used across studies (eg, 18 , 56 , 62–64 ), accurately and reliably assessing hypoxia poses a significant challenge for physicians and researchers alike. Further, our understanding of how severity, timing, and duration of hypoxia affects outcome is lacking. In this study, we used a narrow definition that required a formal diagnosis of intrauterine hypoxia or birth asphyxia or an arterial pH value below 7.2, in part because differences in the record system for births before and after 1990 restricted which maternal and fetal health variables at delivery were available across the cohort. A different definition that incorporated broader markers of hypoxia may have yielded different results. However, having a larger sample to examine relationships among schizophrenia risk, obstetric complications, and outcome seemed of greater benefit than exploring additional variables that have been less robustly linked to schizophrenia in a smaller sample.
Some other potential limitations to this study should be noted. First, although we controlled for numerous potentially confounding variables and subject ascertainment dictated that all subjects were from an urban area, it is possible that other risk factors that were not directly assessed, such as low socioeconomic status, could have modulated the differential relationship between LBW and outcome in HR vs LR offspring. Evidence that social risk amplifies negative effects of LBW on neurodevelopmental outcomes, however, is equivocal. 39 , 65–68 In addition, because Finnish children attend schools within their district, our inclusion of school as a random factor in all models would likely also control for some variance in social factors that vary by district. Further, Finnish state policies limit income disparity and its effects, 69 thereby minimizing potential contributions of social factors to differential relationships between LBW and outcome in HR and LR offspring. Second, we were only able to assess adolescent outcome using school data. Standardized measures of intellectual and motor functioning are more precise measures of neurodevelopmental outcome. However, the advantage of using school data was the opportunity to assess how long-term outcomes related to prospectively collected obstetric data in a large cohort of HR offspring. Finally, genetic risk was inferred based on parental schizophrenia status, rather than modeled directly using DNA markers. Although genome-wide screening methods are now available, these approaches require extremely large sample sizes to achieve adequate power to detect genetic associations of small effect, and even larger samples to screen for interactions of genotypes by environmental risk exposures. The patterns of results detected here are consistent with gene-environment interaction, though parental schizophrenia status could also reflect nongenetic influences or gene by environment interactive influences. We modeled and ruled out a number of environmental influences (eg, maternal smoking, social support), but our ability to do so was limited to variables available through the Finnish register system. Additionally, it was not possible to explore or model potential gene by environment interactive influences inherit within schizophrenia parental status using this study design.
In summary, HR offspring performed worse across school domains than LR offspring. LBW predicted poorer academic and physical education outcomes only in HR offspring. That LBW predicted academic and physical education scores, but not nonacademic scores in HR offspring suggests that these effects are not likely due to global disengagement of these individuals from school and may indicate greater effects of LBW on brain development related to cognitive and motor function. Comparable effects of LBW in offspring of fathers vs mothers with schizophrenia in the absence of group differences in rates of obstetric complications provides novel support for the hypothesis that susceptibility genes for schizophrenia confer augmented vulnerability of the developing brain to the detrimental effects of obstetric complications. That hypoxia did not predict academic scores and predicted only subtle decrements in physical education score likely reflects the greater challenge in reliably identifying hypoxia. Studies that continue to follow these individuals until adulthood would be beneficial to examine continuities in relationships among genetic risk, obstetric complications, school outcomes, and adult psychopathology.
Funding
Grant from the March of Dimes, gifts to the UCLA Foundation by Garen and Shari Staglin, and National Science Foundation Graduate Research Fellowship to JKF.
Supplementary Material
Supplementary materials are available at http://schizo phreniabulletin.oxfordjournals.org.
Acknowledgments
Dr Suvisaari has served as a consultant to Janssen-Cilag and has received lecture fees from AstraZeneca. The authors have no other conflicts of interest to report.
References
- 1. Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669 [DOI] [PubMed] [Google Scholar]
- 2. Done DJ, Crow TJ, Johnstone EC, Sacker A. Childhood antecedents of schizophrenia and affective illness: social adjustment at ages 7 and 11. BMJ. 1994;309:699–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. MacCabe JH, Lambe MP, Cnattingius S, et al. Scholastic achievement at age 16 and risk of schizophrenia and other psychoses: a national cohort study. Psychol Med. 2008;38:1133–1140 [DOI] [PubMed] [Google Scholar]
- 4. Jones P, Rodgers B, Murray R, Marmot M. Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet. 1994;344:1398–1402 [DOI] [PubMed] [Google Scholar]
- 5. Walker EF, Savoie T, Davis D. Neuromotor precursors of schizophrenia. Schizophr Bull. 1994;20:441–451 [DOI] [PubMed] [Google Scholar]
- 6. Erlenmeyer-Kimling L, Rock D, Roberts SA, et al. Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychoses: the New York High-Risk Project. Am J Psychiatry. 2000;157:1416–1422 [DOI] [PubMed] [Google Scholar]
- 7. Fish B, Marcus J, Hans SL, Auerbach JG, Perdue S. Infants at risk for schizophrenia: sequelae of a genetic neurointegrative defect. A review and replication analysis of pandysmaturation in the Jerusalem Infant Development Study. Arch Gen Psychiatry. 1992;49:221–235 [DOI] [PubMed] [Google Scholar]
- 8. Niemi LT, Suvisaari JM, Tuulio-Henriksson A, Lönnqvist JK. Childhood developmental abnormalities in schizophrenia: evidence from high-risk studies. Schizophr Res. 2003;60:239–258 [DOI] [PubMed] [Google Scholar]
- 9. Gottesman II. Schizophrenia Genesis. New York:Freeman;1991 [Google Scholar]
- 10. Tsuang MT, Faraone SV. The case for heterogeneity in the etiology of schizophrenia. Schizophr Res. 1995;17:161–175 [DOI] [PubMed] [Google Scholar]
- 11. Geddes JR, Lawrie SM. Obstetric complications and schizophrenia: a meta-analysis. Br J Psychiatry. 1995;167:786–793 [DOI] [PubMed] [Google Scholar]
- 12. Cannon M, Jones PB, Murray RM. Obstetric complications and schizophrenia: historical and meta-analytic review. Am J Psychiatry. 2002;159:1080–1092 [DOI] [PubMed] [Google Scholar]
- 13. Buka SL, Tsuang MT, Lipsitt LP. Pregnancy/delivery complications and psychiatric diagnosis. A prospective study. Arch Gen Psychiatry. 1993;50:151–156 [DOI] [PubMed] [Google Scholar]
- 14. Cannon TD, Mednick SA, Parnas J. Genetic and perinatal determinants of structural brain deficits in schizophrenia. Arch Gen Psychiatry. 1989;46:883–889 [DOI] [PubMed] [Google Scholar]
- 15. Cannon TD, Mednick SA, Parnas J, Schulsinger F, Praestholm J, Vestergaard A. Developmental brain abnormalities in the offspring of schizophrenic mothers. I. Contributions of genetic and perinatal factors. Arch Gen Psychiatry. 1993;50:551–564 [DOI] [PubMed] [Google Scholar]
- 16. Jablensky AV, Morgan V, Zubrick SR, Bower C, Yellachich LA. Pregnancy, delivery, and neonatal complications in a population cohort of women with schizophrenia and major affective disorders. Am J Psychiatry. 2005;162:79–91 [DOI] [PubMed] [Google Scholar]
- 17. Clarke MC, Tanskanen A, Huttunen M, Whittaker JC, Cannon M. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am J Psychiatry. 2009;166:1025–1030 [DOI] [PubMed] [Google Scholar]
- 18. Goldstein JM, Seidman LJ, Buka SL, et al. Impact of genetic vulnerability and hypoxia on overall intelligence by age 7 in offspring at high risk for schizophrenia compared with affective psychoses. Schizophr Bull. 2000;26:323–334 [DOI] [PubMed] [Google Scholar]
- 19. Ellman LM, Huttunen M, Lönnqvist J, Cannon TD. The effects of genetic liability for schizophrenia and maternal smoking during pregnancy on obstetric complications. Schizophr Res. 2007;93:229–236 [DOI] [PubMed] [Google Scholar]
- 20. Nilsson E, Lichtenstein P, Cnattingius S, Murray RM, Hultman CM. Women with schizophrenia: pregnancy outcome and infant death among their offspring. Schizophr Res. 2002;58:221–229 [DOI] [PubMed] [Google Scholar]
- 21. Mittal VA, Ellman LM, Cannon TD. Gene-environment interaction and covariation in schizophrenia: the role of obstetric complications. Schizophr Bull. 2008;34:1083–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abel KM, Wicks S, Susser ES, et al. Birth weight, schizophrenia, and adult mental disorder: is risk confined to the smallest babies? Arch Gen Psychiatry. 2010;67:923–930 [DOI] [PubMed] [Google Scholar]
- 23. Dalman C, Allebeck P, Cullberg J, Grunewald C, Köster M. Obstetric complications and the risk of schizophrenia: a longitudinal study of a national birth cohort. Arch Gen Psychiatry. 1999;56:234–240 [DOI] [PubMed] [Google Scholar]
- 24. Jones PB, Rantakallio P, Hartikainen AL, Isohanni M, Sipila P. Schizophrenia as a long-term outcome of pregnancy, delivery, and perinatal complications: a 28-year follow-up of the 1966 north Finland general population birth cohort. Am J Psychiatry. 1998;155:355–364 [DOI] [PubMed] [Google Scholar]
- 25. Kunugi H, Nanko S, Murray RM. Obstetric complications and schizophrenia: prenatal underdevelopment and subsequent neurodevelopmental impairment. Br J Psychiatry Suppl. 2001;40:s25–s29 [DOI] [PubMed] [Google Scholar]
- 26. Dalman C, Thomas HV, David AS, Gentz J, Lewis G, Allebeck P. Signs of asphyxia at birth and risk of schizophrenia. Br J Psychiatry. 2001;179:403–408 [DOI] [PubMed] [Google Scholar]
- 27. Zornberg GL, Buka SL, Tsuang MT. Hypoxic-ischemia-related fetal/neonatal complications and risk of schizophrenia and other nonaffective psychoses: a 19-year longitudinal study. Am J Psychiatry. 2000;157:196–202 [DOI] [PubMed] [Google Scholar]
- 28. Cannon TD. On the nature and mechanisms of obstetric influences in schizophrenia: a review and synthesis of epidemiologic studies. Int Rev Psychiatr. 1997;9:387–397 [Google Scholar]
- 29. Clarke MC, Harley M, Cannon M. The role of obstetric events in schizophrenia. Schizophr Bull. 2006;32:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suvisaari J, Perala J, Saarni SI, Juvonen H, Tuulio-Henriksson A, Lonnqvist J. The epidemiology and descriptive and predictive validity of DSM-IV delusional disorder and subtypes of schizophrenia. Clin Schizophr Relat Psychoses. 2009;2:289–297 [Google Scholar]
- 31. Perälä J, Suvisaari J, Saarni SI, et al. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry. 2007;64:19–28 [DOI] [PubMed] [Google Scholar]
- 32. Anslow P. Birth asphyxia. Eur J Radiol. 1998;26:148–153 [DOI] [PubMed] [Google Scholar]
- 33. Dickson H, Laurens KR, Cullen AE, Hodgins S. Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psychol Med. 2011;1–13 [DOI] [PubMed] [Google Scholar]
- 34. Bilder RM, Reiter G, Bates J, et al. Cognitive development in schizophrenia: follow-back from the first episode. J Clin Exp Neuropsychol. 2006;28:270–282 [DOI] [PubMed] [Google Scholar]
- 35. Fuller R, Nopoulos P, Arndt S, O’Leary D, Ho BC, Andreasen NC. Longitudinal assessment of premorbid cognitive functioning in patients with schizophrenia through examination of standardized scholastic test performance. Am J Psychiatry. 2002;159:1183–1189 [DOI] [PubMed] [Google Scholar]
- 36. Cannon M, Jones P, Huttunen MO, et al. School performance in Finnish children and later development of schizophrenia: a population-based longitudinal study. Arch Gen Psychiatry. 1999;56:457–463 [DOI] [PubMed] [Google Scholar]
- 37. Isohanni I, Järvelin MR, Nieminen P, et al. School performance as a predictor of psychiatric hospitalization in adult life. A 28-year follow-up in the Northern Finland 1966 Birth Cohort. Psychol Med. 1998;28:967–974 [DOI] [PubMed] [Google Scholar]
- 38. Tanskanen P, Valkama M, Haapea M, et al. Is prematurity associated with adult cognitive outcome and brain structure? Pediatr Neurol. 2011;44:12–20 [DOI] [PubMed] [Google Scholar]
- 39. Hack M, Klein NK, Taylor HG. Long-term developmental outcomes of low birth weight infants. Future Child. 1995;5:176–196 [PubMed] [Google Scholar]
- 40. Whitaker AH, Feldman JF, Lorenz JM, et al. Motor and cognitive outcomes in nondisabled low-birth-weight adolescents: early determinants. Arch Pediatr Adolesc Med. 2006;160:1040–1046 [DOI] [PubMed] [Google Scholar]
- 41. Evensen KA, Vik T, Helbostad J, Indredavik MS, Kulseng S, Brubakk AM. Motor skills in adolescents with low birth weight. Arch Dis Child Fetal Neonatal Ed. 2004;89:F451–F455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Weindrich D, Jennen-Steinmetz C, Laucht M, Schmidt MH. Late sequelae of low birthweight: mediators of poor school performance at 11 years. Dev Med Child Neurol. 2003;45:463–469 [DOI] [PubMed] [Google Scholar]
- 43. Myatt L. Placental adaptive responses and fetal programming. J Physiol (Lond). 2006;572:25–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Valero De Bernabé J, Soriano T, Albaladejo R, et al. Risk factors for low birth weight: a review. Eur J Obstet Gynecol Reprod Biol. 2004;116:3–15 [DOI] [PubMed] [Google Scholar]
- 45. Goldenberg RI, Culhane JR, Iams JD, Romero R. Epide miology and causes of preterm birth. Lancet. 2008;371:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Janvier A, Khairy M, Kokkotis A, Cormier C, Messmer D, Barrington KJ. Apnea is associated with neurodevelopmental impairment in very low birth weight infants. J Perinatol. 2004;24:763–768 [DOI] [PubMed] [Google Scholar]
- 47. McIntire DD, Bloom SL, Casey BM, Leveno KJ. Birth weight in relation to morbidity and mortality among newborn infants. N Engl J Med. 1999;340:1234–1238 [DOI] [PubMed] [Google Scholar]
- 48. Resnik R. Intrauterine growth restriction. Obstet Gynecol. 2002;99:490–496 [DOI] [PubMed] [Google Scholar]
- 49. Bale TL, Baram TZ, Brown AS, et al. Early life programming and neurodevelopmental disorders. Biol Psychiatry. 2010;68:314–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kietzmann T, Knabe W, Schmidt-Kastner R. Hypoxia and hypoxia-inducible factor modulated gene expression in brain: involvement in neuroprotection and cell death. Eur Arch Psychiatry Clin Neurosci. 2001;251:170–178 [DOI] [PubMed] [Google Scholar]
- 51. Ross MG, Beall MH. Adult sequelae of intrauterine growth restriction. Semin Perinatol. 2008;32:213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schmidt-Kastner R, van Os J, W M Steinbusch H, Schmitz C. Gene regulation by hypoxia and the neurodevelopmental origin of schizophrenia. Schizophr Res. 2006;84:253–271 [DOI] [PubMed] [Google Scholar]
- 53. Nicodemus KK, Marenco S, Batten AJ, et al. Serious obstetric complications interact with hypoxia-regulated/vascular-expression genes to influence schizophrenia risk. Mol Psychiatry. 2008;13:873–877 [DOI] [PubMed] [Google Scholar]
- 54. Joo EJ, Lee KY, Jeong SH, et al. AKT1 Gene Polymorphisms and Obstetric Complications in the Patients with Schizophrenia. Psychiatry Investig. 2009;6:102–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cannon TD, van Erp TG, Rosso IM, et al. Fetal hypoxia and structural brain abnormalities in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 2002;59:35–41 [DOI] [PubMed] [Google Scholar]
- 57. McNeil TF, Cantor-Graae E, Weinberger DR. Relationship of obstetric complications and differences in size of brain structures in monozygotic twin pairs discordant for schizophrenia. Am J Psychiatry. 2000;157:203–212 [DOI] [PubMed] [Google Scholar]
- 58. Van Erp TG, Saleh PA, Rosso IM, et al. Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. Am J Psychiatry. 2002;159:1514–1520 [DOI] [PubMed] [Google Scholar]
- 59. Ebner F, Tepest R, Dani I, et al. The hippocampus in families with schizophrenia in relation to obstetric complications. Schizophr Res. 2008;104:71–78 [DOI] [PubMed] [Google Scholar]
- 60. Schulsinger F, Parnas J, Petersen ET, et al. Cerebral ventricular size in the offspring of schizophrenic mothers. A preliminary study. Arch Gen Psychiatry. 1984;41:602–606 [DOI] [PubMed] [Google Scholar]
- 61. Silverton L, Finello KM, Mednick SA, Schulsinger F. Low birth weight and ventricular enlargement in a high-risk sample. J Abnorm Psychol. 1985;94:405–409 [DOI] [PubMed] [Google Scholar]
- 62. Mañeru C, Junqué C, Botet F, Tallada M, Guardia J. Neuropsychological long-term sequelae of perinatal asphyxia. Brain Inj. 2001;15:1029–1039 [DOI] [PubMed] [Google Scholar]
- 63. Barnett AL, Guzzetta A, Mercuri E, et al. Can the Griffiths scales predict neuromotor and perceptual-motor impairment in term infants with neonatal encephalopathy? Arch Dis Child. 2004;89:637–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gonzalez FF, Miller SP. Does perinatal asphyxia impair cognitive function without cerebral palsy? Arch Dis Child Fetal Neonatal Ed. 2006;91:F454–F459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Currie J, Hyson R. Is the impact of health shocks cushioned by socioeconomic status? The case of low birthweight. Am Econ Rev. 1999;89:245–250 [Google Scholar]
- 66. Breslau N. Psychiatric sequelae of low birth weight. Epidemiol Rev. 1995;17:96–106 [DOI] [PubMed] [Google Scholar]
- 67. Pyhälä R, Lahti J, Heinonen K, et al. Neurocognitive abilities in young adults with very low birth weight. Neurology. 2011;77:2052–2060 [DOI] [PubMed] [Google Scholar]
- 68. Hack M, Breslau N, Aram D, Weissman B, Klein N, Borawski-Clark E. The effect of very low birth weight and social risk on neurocognitive abilities at school age. Develop Behav Pediatr. 1992;13:412–420 [PubMed] [Google Scholar]
- 69. Forster M, d’Ercole MM. Income Distribution and Poverty in OECD Countries in the Second Half of the 1990’s 2005. Retrieved August 12, 2012, from http://www.oecd.org/tax/34483698.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.