Abstract
Background: In patients with schizophrenia, the misattribution of self-generated events to an external source is associated with the presence of psychotic symptoms. The aim of this study was to investigate how this misattribution is influenced by dysfunction of attentional processing, which is also impaired in schizophrenia. Methods: Participants underwent functional Magnetic Resonance Imaging (fMRI) while listening to prerecorded speech. Their expectancies were manipulated using visual cues that were either congruent (valid) or incongruent (invalid) with the speech. The source (self/other) and the acoustic quality (undistorted/distorted) of the speech were also manipulated. Twenty patients with first-episode psychosis (FEP) and 20 matched healthy controls (HC) were tested. Results: When listening to self-generated speech preceded by an invalid (other speech) cue, relative to HC, FEP patients showed a trend to misidentify their own speech as that of another person. Analysis of fMRI data showed that FEP patients had reduced activation in the right middle temporal gyrus (MTG) and left precuneus (Pc) relative to HC. Within the FEP group, the level of activation in the right MTG was negatively correlated with the severity of their positive psychotic symptoms. Conclusions: Impaired attentional modulation in schizophrenia may contribute to the tendency for FEP patients to misattribute the source of self-generated material, and this may be mediated by the right MTG and Pc, regions that are involved in both self-referential processing and the integration of sensory information.
Key words: psychosis, schizophrenia, speech, self-recognition, attention, top-down, bottom-up
Introduction
Phenomenologically oriented research has proposed that disturbance of the sense of self is a psychopathological trait marker of psychotic vulnerability, particularly for schizophrenia-spectrum disorders.1 Such a disturbance in self-referential processing may be particularly relevant to the experience of positive symptoms such as auditory verbal hallucinations (AVH) and other passivity phenomena in schizophrenia.1,2 A recent meta-analysis reports impaired self-recognition performance across a range of tasks in patients with schizophrenia and, in particular, the misattribution of self-generated speech to an external source in patients with AVH and/or delusions.3,4
The evaluation of speech involves a network including the inferior frontal gyrus, anterior cingulate cortex (ACC), and voice-selective regions in the superior temporal gyrus along the upper bank of the superior temporal sulcus (STS) and the bilateral middle temporal gyrus (MTG).5 These regions are consistently implicated in the pathophysiology of psychosis.6–9 In patients with AVH and delusions, the misidentification of self-generated speech is associated with reduced activation in ACC, as well as functional abnormalities in the left temporal cortex.10,11 The involvement of the ACC is consistent with evidence that cortical midline structures (CMS) are fundamental to self-referent processes12 and that CMS function is altered in patients with schizophrenia.1,13
Although self-recognition deficits may contribute to the misattribution of self-generated stimuli to an external origin, biased or impaired attentional processes may also be involved.14–16 For example, neurocognitive models propose an altered interaction between bottom-up and top-down attentional processes in schizophrenia17 that may influence conscious perception leading to AVH.14 Specifically, bottom–up or exogenous attentional control is stimulus driven (ie, attention is spontaneously oriented toward an incoming stimulus). In contrast, top-down or endogenous attentional control is intentional and cognitively driven (ie, directed by knowledge, expectation, and current goals).18 Importantly, top-down and bottom-up processes represent overlapping organizational principles and interact to optimize attentional performance.19 Behavioral studies report that when bottom-up and top-down processes conflict, patients with schizophrenia exhibit significantly worse performance than healthy controls (HC). This has been shown during invalidly cued trials in visual attention tasks17 and during imagery/perception comparison tasks in visual and auditory modality.14
To examine the effect of attentional modulation in the context of a source attribution task, we adapted a paradigm previously used by our group3,20 to modulate participants’ expectancies regarding the source of prerecorded speech.15 In addition to the manipulation of speech source (ie, self-/other speech) and ambiguity (undistorted/distorted speech), we also manipulated participants’ top-down (endogenous) expectancies by using visual cues that were either congruent (valid) or incongruent (invalid) with the source of subsequently perceived speech. In our previous study, we found that relative to HC, chronic schizophrenia patients were more likely to misidentify their own speech as originating from another person when it was distorted. Moreover, patients made significantly more errors across all invalid cue conditions, suggesting a greater influence of top-down expectancy than in HC and/or a failure to properly integrate bottom-up information.15 In HC, differential networks have been implicated in both top-down and bottom-up attentional processings in both visual21–23 and auditory24 modalities. In particular, the inferior parietal cortex (IPC) is crucial for the integration of bottom-up and top-down attentional demands.23,25 Neuroimaging studies also suggest a role for the posterior CMS in conscious awareness and integration of self-referential stimuli26 and temporal lobe (TL) regions for source attribution/monitoring.7,10,11
The aim of this study was to examine the neural correlates of attentional modulation during a source attribution task in patients with first-episode psychosis (FEP) and in HC. We chose to study FEP patients to avoid confounds associated with prolonged medication and illness chronicity associated with established schizophrenia. We adapted the task previously used by Ilankovic and colleagues for use in a functional Magnetic Resonance Imaging (fMRI) study.15 Based on previous findings, we predicted that during a cued source attribution task, FEP patients would make more source attribution errors than controls on trials preceded by an invalid cue. We also predicted that this impairment in task performance would be associated with altered activation in the IPC, CMS, and TL, regions involved in the integration of attentional demands, self-referential processes, and source attributions, respectively. Finally, we predicted that in FEP patients, altered activation in these regions would be associated with the severity of their positive psychotic symptoms.
Methods
Participants
Patient Group. Twenty patients who met DSM-IV27 criteria for psychosis were recruited through the South London and Maudsley National Health Service Trust. Clinical teams were systematically contacted with a request to identify patients with FEP who had prominent positive symptoms (hallucinations and/or delusions). This information was corroborated by careful review of the patients’ clinical records. Potentially eligible patients were then approached by the investigators and assessed on the day of testing using Positive And Negative Syndrome Scales (PANSS)28 and the Psychotic Symptom Rating Scale (PSYRATS).29 (Symptom scores are reported in table 1). Patients were medicated with regular, stable doses of atypical antipsychotic medication (Aripriprazole, Seroquel, Olanzapine, Risperidon, and Quetapine) except for 3 patients who had ceased medication. Exclusion criteria for the patient group was the presence of an Axis II DSM-IV diagnosis or another Axis I diagnosis, a neurological disorder, or history of or current alcohol or substance dependence. Participants with a premorbid intelligence quotient (IQ) < 80 (determined by the Wide Range Achievement Test; WRAT)30 were excluded from the study. Because HC had a significantly higher premorbid IQ than the FEP group (table 1), IQ scores were included as a covariate in all subsequent analyses. All participants gave written informed consent for a protocol (09/H0807/47) approved by the the Joint South London and Maudsley and the Institute of Psychiatry NHS Research Ethics Committee.
Table 1.
Demographic Psychopathological and Behavioral Data
| First-Episode Patients (N = 20) M(SD) | Controls (N = 20) M(SD) | Statistics, t (P) | |
|---|---|---|---|
| Age (y) | 25.8 (6.3) | 26.2 (6.1) | .17 (0.86) |
| Premorbid IQ | 100.9 (10.6) | 110.1 (7.4) | 3.17 (0.003)* |
| Years of education | 15.3 (3.1) | 13.4 (3.2) | 1.88 (0.067) |
| Gender ratio, M/F | 14/6 | 14/6 | |
| Duration of illness | 1–18 months | ||
| PANSS: positive symptomsa | 14.0 (5.5) | — | |
| PANSS: negative symptomsa | 14.2 (6.0) | — | |
| PANSS: general psychopathology | 29.2 (8.66) | — | |
| Total medicationb | 47243.24 (37873.96) | — | |
| Mean medication/dayc | 252.62 (216.57) | — | |
| PSYRATS auditory hallucinationsa | 9.50 (14.0) | — | |
| PSYRATS delusionsa | 8.40 (8.2) |
Note: M/F, male/female; PSYRATS, Psychotic Symptom Rating Scale; PANSS = Positive And Negative Syndrome Scale.
aSymptom profile recorded at time of scan.
bTotal Medication refers to the average absolute amount of medication taken by that group in standardized mg units of Chlorpromazine ± 1SD.
cMean Medication/day is the average medication dosage taken by each subject during their period of treatment in standardized mg units of Chlorpromazine ± 1SD.
Healthy Controls. Twenty healthy, right-handed English- speaking volunteers (14 males, 18–35 years of age) with normal hearing and corrected-to-normal vision (in 9 participants) who were matched to FEP for age and gender participated in the study. None of the subjects had a history of medical disorder or drug or alcohol misuse, or was receiving medication.
fMRI Task
Participants listened to recordings of their own or another person’s speech, preceded by a picture of either their own face or another person’s face. One hundred and sixty adjectives applicable to people were used (eg, “perfect,” “tall”). All the words were monosyllabic or disyllabic and were selected from lists used in a previous study.3,15 These 160 words consisted of 70 positive, 70 negative, and 20 neutral words. The sets of words presented in each condition were balanced for the number of syllables (ie, equal amounts of 1- and 2-syllable words), word frequency, and valence (equal amounts of positive, negative, and neutral words).
A grayscale portrait picture (2304 × 3072 pixel size) was taken of each participant using an Olympus digital camera (D-425: 4.0 megapixel). Pictures were matched for size, color intensity, brightness, and contrast by using Adobe Photoshop Software (http://www.adobe.com/de/products/photoshop/). The picture of a male and a female researcher who were unknown to the participants was used as the “other” face picture. Only the face was presented to the participants (without hair and neck), in order to avoid additional elements that could interfere with the face processing. The task used a factorial design with 2 levels of validity (valid, invalid), source of speech (self, other), and distortion (0, −4 semitones) yielding a 2 × 2 × 2 design. Self-speech preceded by a picture of the subject’s own face (self-picture cue) and other speech preceded by another person’s face (other picture cue), respectively, constituted valid trials. Self-speech preceded by another person’s face and other speech preceded by the subject’s own face represented invalid trials. The task consisted of 70% valid and 30% invalid cues when the ratio of valid to invalid cues is high; attention to the cued stimuli is purposefully allocated through top-down mechanisms. There were 28 words in each of the valid conditions and 12 words in each of the invalid conditions.
Procedure
Approximately 1–2 hours before scanning, all participants were presented with a list of 160 words on a piece of paper and asked to read them aloud in a clear voice at a rate of approximately 1 word/s. Participants read all 160 words even though half would subsequently be replaced and presented to them in another person’s voice; this was to ensure that participants could not make judgments based on source memory during the task. They were not asked to remember the words. Their speech was recorded by a computer (Cool Edit 2000 for Windows XP). A male and a female researcher who were unknown to the participants recorded the words for the non-self- (other) condition (80 words in total). The researchers used a neutral English pronunciation. The experimenter edited the recordings so that 80 of the words were replaced by a recording of the same word spoken in another person’s voice, and 80 words (50%) of all the words (both self- and other speech) were distorted using −4 semitone pitch shift. The subsets of words that were replaced and pitch shifted, respectively, were predesignated (allocated so that the subsets were matched for word length, frequency, and valence). The level of pitch distortion was based on findings from previous studies.3,15 The same subsets of words were used for all participants.
Once participants had been placed in the scanner, a standardized instruction script was read to them. Subjects were instructed to attend to each face-voice combination but to make a decision regarding the source of the speech, not the face. Each trial started with a face picture cue that was presented for 200ms, followed by a variable SOA (300 or 800ms) between the face picture and the voice to avoid the habituation effects. Auditory stimuli were presented via fMRI suitable headphones (Confon HP_S 01), and visual stimuli were presented via Magnetic Resonance (MR) compatible goggles (Nordic neurolab’s VisualSystem). The volume of the auditory stimuli was checked to ensure it was sufficiently loud for participants to hear the speech without difficulty. All participants reported that speech stimuli were clearly audible. The participants were able to register a response of either “self,” “unsure,” or “other” via a joystick. The option to register an unsure response was included to avoid the need for participants to make a forced choice between a “self-” or “other” response when they were unsure. During rest periods participants viewed a black screen. Response accuracy and reaction times were recorded online. Participant’s occasional failures to press a button were recorded as none responses.
MRI Data Acquisition
Imaging was performed with a 3.0-T whole-body MRI scanner (GE Signa Excite) at the Centre for Neuroimaging Sciences, Institute of Psychiatry King’s College London. A standard head coil (8 channels) was used for a radiofrequency transmission and reception. A compressed T2-weighted whole-brain echo planer pulse sequence was acquired with 744 images (axial mode = 2D; scan timing: an effective time of repetition (TR) of 2 s; composed of 1.2 s acquisition period; and silent period = 0.8, time to echo (TE) = 30ms; flip angle = 70°; matrix = 64 × 64; slice thickness = 4mm; interslice gap = 0.4mm). The compressed sequence provided a simple and robust means of monitoring task performance in the absence of the acoustic scanner noise. Of 744 images, 160 were experimental and the remainder were null events (a black screen). Each whole-brain volume consisted of 24 axial slices acquired parallel to the anterior-posterior intercommissural line. Stimuli were presented in random order in an event-related design, with a variable intertrial interval ranging from 6–8 s in order to provide optimal hemodynamic refractoriness and avoid habituation effects. Each response time was locked to the beginning of the word presentation.
The mean proportions of total error and unsure trials were calculated for each subject. Total errors were all misattribution errors (misidentification of the source of the speech) plus unsure responses. ANCOVA was used to test for task, group, and interaction effects while covarying for premorbid IQ. The associations between performance and PANSS/ PSYRATS scores were examined using Pearson’s correlation. The data were analyzed using Statistical Package for Social Science SPSS 15. All statistical tests were two tailed, and reported at a significance level P < .05. To estimate the effect size we used the eta square measure (η2).
Image Analysis
Image processing and statistical analyses were conducted using SPM8 (Wellcome Department of Cognitive Neurology, University College London, UK) running in MATLAB 7.4 (The Mathworks Inc, Natick, MA, USA). Movement correction of MRI scans was performed after the first 3 volumes were removed to allow for steady-state magnetization. The remaining 741 images were realigned to the first scan as a reference and resliced with sinc interpolation. There were no differences in the interscan movement parameters between groups (P > .05 for all parameters). Scans were spatially normalized to a standard Montreal Neurological Institute (MNI)-305 template using nonlinear-basis functions. Functional data were spatially smoothed with an 8mm full width at half maximum isotropic Gaussian kernel to compensate for residual variability in functional anatomy after spatial normalization.
A standard first-level random-effects statistical analysis of regional responses was performed to identify regional activations in each subject independently. To remove low-frequency drifts, the data were high-pass filtered using a set of discrete cosine basis functions with a cutoff period of 128 s. Activations at the onset of all trials, from the epoch of the face picture cue, were modeled using an event-related analysis. A first-level model with 8 regressors was specified (valid self-speech, valid self-distorted speech, valid other speech, valid other distorted, invalid self-speech, invalid self-distorted speech, invalid other speech and invalid other-distorted speech). All speech trials conditions were modeled independently by convolving onset times with a canonical hemodynamic response function. Trials were modeled against a low-level baseline consisting of null trials. Response estimations from the first-level analysis were entered into a series of second-level general linear models. First, paired t tests were used to examine the main task effects of validity (valid vs invalid), source (self vs other), and distortion (distorted vs nondistorted) regardless of group. To examine the interaction between group and task, first-level contrast images specifying the source × distortion interaction term, in both valid and invalid experimental conditions separately, were entered into a 2 × 2 (group × validity) flexible factorial ANCOVA, with IQ as a covariate of no interest. The same analysis was rerun using first-level contrasts based on correct trials only and is reported in the online supplementary material section. Subsequent interaction effects were interrogated by plotting subject-specific activations extracted from voxels showing maximum effects (contrast estimates, mean Beta values). All main and interaction effects are reported at a whole-brain voxelwise-corrected level (Family Wise Error, FWE, P < .05). We used a whole-brain voxelwise approach, rather than a region-of-interest approach, to detect potential within- and between-subject differences across the brain and not just in hypothesized region. A whole-brain voxelwise multiple comparisons correction is (a) stringent and (b) appropriate when activation across the whole brain is being tested.31 Associations with symptom scores were examined using Pearson correlations with Bonferroni correction.
Results
Behavioral Results
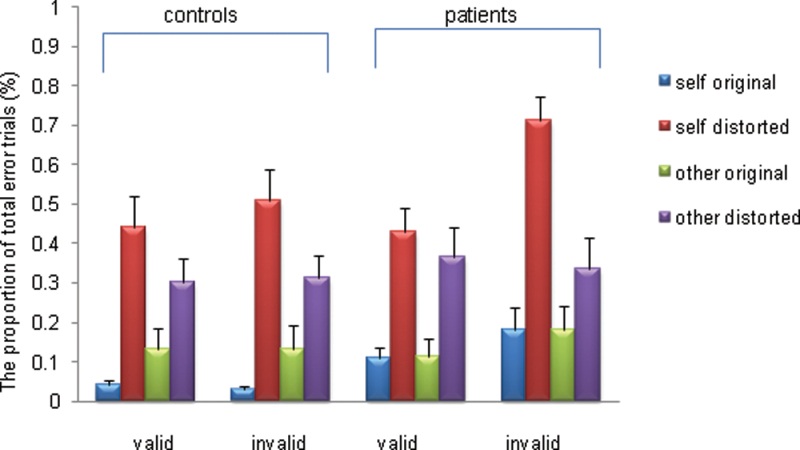
Total Errors. Across all participants, there were significant main effects for validity (F = 4.03, df = 38, P = .05, ηp 2 = 0.095; invalid > valid), source (F = 4.22, df = 38, P = .04, ηp 2 = 0.097; self-speech > other speech), and distortion (F = 112.6, df = 38, P = .00, ηp 2 = 0.747; distorted speech > undistorted speech). The interaction between group and validity was nonsignificant (F = 2.13, df = 38, P = .15). However, there was a trend for an interaction between validity, source, and group (F = 3.67, df = 38, P = .063). Post hoc tests showed that the patients made significantly more total errors than HC when listening to their own voice preceded by an invalid cue (ie, non-self-cue) (z = −2.35, P = .035, figure 1). Neither the interaction between source, distortion, and group (F = 0.094, df = 38, P = .761) nor the interaction between validity, distortion, and group was significant (F = 0.034, df = 38, P = .855). The mean number of omission errors was 0.10 trials out of 160 in HC and 0.39 trials out of 160 in FEP. We found no condition-specific group differences in failure to press.
Fig. 1.
Proportion of the total error trials for the healthy controls and patients through the task.
Unsure Responses. The main effect of distortion (F = 22.950, df = 38, P = .000, ηp 2 = 0.603) and interaction between the source of speech and distortion (F = 1.948, df = 38, P = .045, ηp 2 = 0.113) were significant. All participants made more unsure responses when listening to their own distorted voice. The main effects of validity and source were nonsignificant. The interaction between validity and the group (F = 2. 624, df = 38, P = .114) was also nonsignificant.
Functional Magnetic Resonance Imaging
Across all conditions in all subjects, the task was associated with activation in the left postcentral, supramarginal, middle frontal, posterior cingulate, middle temporal gyri, the right insula, and occipital cortex and the supplementary motor area bilaterally.
Main Effect for Source (Self vs Other Trials)
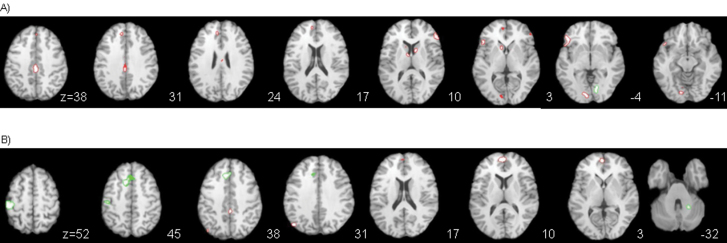
Relative to other-speech trials, listening to self-generated speech was associated with activation in the inferior frontal gyri; the left lingual, anterior, and posterior cingulate; the left thalamus; and the caudate nucleus bilaterally, and reduced deactivation in the left medial frontal gyrus (figure 2A; table 2, for the activation graphs see online supplementary materials). Conversely, listening to other speech, relative to self-speech, was associated with activation in the right lingual gyrus (figure 2A, table 2).
Fig. 2.
Statistical parametric maps of the main effect contrast for A) source of speech; red = self > alien speech and green = alien > self speech; B) distortion; red = original > distorted speech and green = distorted > original speech. The left side of the brain is showed on the left side of the images. The level of axial sections is indicated in Z coordinates in mm.
Table 2.
Coordinates of Foci of Activation for the Main Effects and Interactions
| Cerebral Region | Side | Coordinates | Cluster Size | z | BA | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Main effect of source | |||||||
| Self > alien | |||||||
| Inferior frontal gyrus | L | −45 | 23 | −2 | 173 | 5.75 | 47 |
| R | 49 | 37 | 3 | 74 | 5.08 | 46 | |
| R | 43 | 18 | −12 | 5 | 4.70 | 47 | |
| R | 33 | 16 | −19 | 4 | 4.77 | 47 | |
| Lingual gyrus | L | −7 | −85 | 2 | 123 | 5.51 | 17 |
| Cingulate gyrus (middle) | L | 0 | −27 | 36 | 153 | 5.56 | 31 |
| L | −3 | −8 | 31 | 9 | 4.81 | 24 | |
| Medial frontal gyrus | L | −5 | 48 | 3 | 41 | 5.31 | 10 |
| L | −7 | 49 | 14 | 9 | 4.75 | 10 | |
| L | 1 | 38 | 33 | 4 | 4.67 | 9 | |
| Anterior cingulate gyrus | R | −7 | 38 | 23 | 36 | 5.07 | 32 |
| Thalamus (anterior nucleus) | L | −5 | −3 | 9 | 13 | 4.78 | — |
| Caudate (head) | L | −7 | 11 | 4 | 41 | 5.13 | — |
| Caudate (body) | R | 9 | 6 | 8 | 33 | 4.91 | — |
| Alien > self | |||||||
| Lingual gyrus | R | 11 | −71 | −1 | 72 | 5.28 | 18 |
| Main effect of distortion | |||||||
| Distorted > undistorted | |||||||
| Postcentral gyrus | L | −47 | −26 | 52 | 227 | 5.78 | 2 |
| Anterior cingulate gyrus | L | −7 | 24 | 31 | 235 | 5.69 | 32 |
| Cerebellum (culmen) | R | 21 | −53 | −22 | 29 | 4.96 | — |
| Undistorted > distorted | |||||||
| Medial frontal gyrus | L | −1 | 50 | 6 | 153 | 5.53 | 10 |
| Angular gyrus | L | −45 | −70 | 29 | 62 | 5.19 | 39 |
| Posterior cingulate gyrus | R | 3 | −33 | 34 | 32 | 4.83 | 31 |
| Posterior cingulate gyrus | R | 3 | −51 | 9 | 1 | 4.60 | 29 |
| Main effect validity | |||||||
| No supra threshold effect | |||||||
| Interactions | |||||||
| Validity × source × group | |||||||
| Middle temporal gyrus | R | 51 | −48 | 0 | 2 | 4.69 | 22 |
| Precuneus | L | −1 | −68 | 38 | 1 | 4.59 | 7 |
| Post hoc (self-speech trials in HC) | |||||||
| (in controls and self-speech trials) | |||||||
| Precuneus | L | 1 | −54 | 32 | 49 | 5.01 | 31 |
| Middle temporal gyrus | R | 61 | −14 | −11 | 29 | 4.93 | 21 |
| Insula | L | −47 | −39 | 24 | 18 | 4.73 | 13 |
| Self-speech trials in FEP | |||||||
| No supra threshold effect | |||||||
Note: BA, Brodmann area. Coordinates refer to the stereotactic space as defined in the atlas of Talairach and Tournoux (1988).
Main Effect for Distortion (Distorted vs Undistorted Speech Trials)
Relative to undistorted speech, distorted speech trials were associated with greater activation in the left postcentral gyrus, left ACC, and right cerebellum (figure 2B; table 2). Conversely, listening to undistorted speech trials was associated with relatively greater activation in the left medial frontal gyrus and the right posterior cingulate gyrus, and reduced deactivation in the left angular gyrus (figure 2B; table 2, for the activation graphs see online supplementary materials).
Main Effect for Validity (Valid vs Iinvalid Trials)
The main effect for validity did not survive correction for multiple comparisons (FWE < .05). At an uncorrected threshold (P < .001), relative to valid trials, invalid trials were associated with activation in the left ACC, the medial frontal gyrus bilaterally, and in the left inferior orbitofrontal gyrus. Activation in the left fusiform gyrus was seen during valid relative to invalid trials.
Interaction Effects
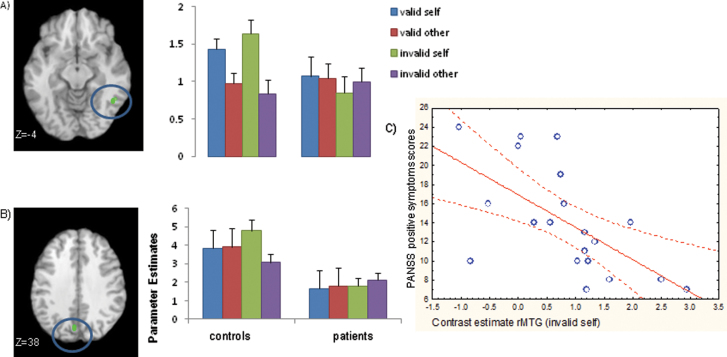
There was a significant interaction between group, validity, and source in the right MTG and in the left precuneus (Pc). In both these regions, HC showed greater activation for self-speech relative to other speech during invalidly cued (but not validly cued) trials. FEP patients however, demonstrated relatively unaltered activation during both source and validity manipulations in these regions (figures 3A and 3B). A post hoc analysis shows that in HC, relative to valid self-trials, invalid self-trials were associated with greater activation in the right Pc, MTG, and left insula (table 2). In FEP, there were no areas more active during invalid relative to valid trials. The interactions between group and validity and between group, validity, source, and distortion were nonsignificant (FWE < .05).
Fig. 3.
Brain activation map for the interaction between the source of speech, validity and group A) in the right middle temporal gyrus (rMTG) B) in the left precuneus C) scatter plot showing negative correlation between activation in the rMTG during invalid self trials and PANSS positive symptoms subscale.
Symptom Correlations
In FEP patients, there was a significant negative correlation between activation in the right MTG (as identified in the group × validity × source interaction) and ratings on both the PANSS positive symptoms subscale (r = −.620, P < .004) and the PSYRATS delusion items (r = −.451, P < .046). However, after correcting for multiple comparisons (Bonferroni correction), only the relationship between the right MTG and PANSS positive symptom scale remained significant (figure 3C). There were no significant correlations between right MTG or left Pc activation and PSYRATS hallucination scores (r = −.138, P = .561).
Because most of the patients who took part in the study were receiving antipsychotic medication, we examined if the level of medication exposure could explain the failure to activate right MTG/left Pc. No significant correlations were found for either the total current dose of medication (right MTG: r = −.039, P = .881, n = 17/left Pc: r = .127, P = .626, n = 17), total duration of medication treatment (right MTG: r = .234, P = .365, n = 17/left Pc: r = .290, P = .259, n = 17) or the mean daily dose over the period they had been taking antipsychotic medication (right MTG: r = −.162, P = .536, n = 17/r = −.097, P = .710, n = 17).
Discussion
Our aim was to investigate the neural correlates of attentional modulation during a source attribution task in FEP patients. Specifically, we tested if (a) the FEP patients would make more source attribution errors than controls on trials preceded by an invalid cue and (b) activation in IPC, CMS, and TL regions was altered in FEP patients relative to HC when top-down attention (ie, expectancy) was manipulated while participants judged the source of prerecorded speech.
Unlike our previous behavioral study in patients with established schizophrenia,15 FEP patients did not make significantly more misattribution errors when speech stimuli were invalidly cued. This study may have been underpowered to detect the behavioral effects seen previously. Although not reaching significance, FEP patients did demonstrate a trend to misattribute their own speech to an external source when it was preceded by an invalid (non-self) cue. This may be due to a general tendency in FEP patients to make external source attributions2 (ie, exacerbated when bottom-up and top-down influences conflict).15,17 Further, as FEP patients made more misattribution errors for invalidly cued self-speech trials, rather than for all invalidly cued trials, it seems unlikely that patients were unable to learn the cuing contingency.
Across all conditions, the experimental task was associated with activation in a network of parietal, temporal, and frontal regions, including the supramarginal gyrus, middle temporal, middle frontal, and posterior cingulate gyrus. The engagement of the frontal and parietal cortex is consistent with their role in top-down attentional modulation.21,23,25 The left middle frontal gyrus is important for the top-down updating of cue-related information,32 while the MTG is commonly activated when subjects are attending to endogenous (top-down) cues, and when reorienting auditory attention.24 Furthermore, the MTG and adjacent STS are selectively activated by voice stimuli,5 and activation in these regions is reported in previous source and speech monitoring fMRI studies.11,33 The left supramarginal gyrus is involved in the integration of top-down and bottom-up attention,23 and the insula,34 lateral temporal cortex35 and inferior frontal gyrus36 are involved in audio-visual integration.
In all participants, relative to other speech, listening to self-speech was associated with activation in prefrontal regions (bilateral inferior frontal gyrus) and CMS (cingulate, lingual gyri), as well as the left thalamus and bilateral caudate. Deactivation was seen in the left medial frontal gyrus. Activation in the inferior frontal gyrus20 and thalamus37 during source attribution tasks has been reported previously, suggesting these regions may be involved in successful source monitoring.37 Activation seen in the cingulate gyrus and during self-speech trials is consistent with previous findings that CMS are involved in self-processing.26 Activation in bilateral inferior frontal gyrus during self-speech trials has also been reported20 in HC.
There was a significant interaction effect between group, validity, and speech source in the right MTG and left Pc. In HC, the right MTG showed greater activation during self-speech relative to other-speech trials particularly when preceded by an invalid cue. In FEP patients, however, activation in the right MTG did not appear to differentiate between self/other or valid/invalid speech trials. TL regions, have previously been shown to facilitate source attribution in HC7,11 and have also been implicated in crossmodal/audiovisual integration.34 The right MTG in particular has been shown to play an important role in audio-visual matching38 and may be important for facilitating the integration of self-referential sensory information. Furthermore, in FEP patients, there was a negative association between right MTG activation and positive symptoms. An association between MTG dysfunction and psychosis has been reported previously and particularly between positive symptoms such as persecution and disorganization.37
We did not confirm our hypothesis that impaired task performance in FEP patients would be associated with altered IPC activation. It is possible that integration of sensory information in the IPC is specific to the integration of spatial23 and/or visual39 stimuli integration. However, in HC, activation in the left Pc (a CMS) was greater during invalid self-speech trials than during other and validly cued speech trials. The precuneus, as the postero-medial portion of the parietal lobe, has also been linked to top-down attention23 and is involved in the integration of self-referential information,12 self-processing operations, and the experience of agency.26,40 In FEP patients, attenuated activation in right MTG and left Pc during invalidly cued self-speech trials may be associated with impaired integration of top-down and bottom-up information particularly when that information is self-referential.9 It is possible that dysfunction in this network results in an impaired ability to integrate or fully take into account bottom-up information and partly accounts for the information gathering bias (ie, jumping to conclusions) often seen in patients with schizophrenia and positive symptoms.41
The study has some limitations. First, the results could not dissociate neural networks mediating top-down and bottom-up control as in tasks examining visuospatial selective attention.23 However, this was not the aim of our study as we sought to identify regions involved in top-down and bottom-up integrations in the context of source judgments about externally presented speech. Second, because the majority of our patients were receiving antipsychotic medication, their symptoms were likely to have been at least partially attenuated. These effects need to be considered when interpreting group differences in activation between medicated patients and controls.
Another limitation is the group difference in IQ, which we addressed using an ANCOVA model. A supplementary analysis (reported in the online supplementary materials) confirmed that excluding the IQ covariate had no appreciable effect on the results. All significant clusters remained so, and the position of cluster peaks were not appreciably altered such that all cluster labels remained unchanged.
To conclude, impaired attentional modulation in FEP patients may lead to erroneous source attributions. In FEP patients, relative to HC, task performance was associated with reduced activation in the right MTG and left Pc, both regions involved in self-referential processing and integration of sensory information. Relatively reduced activation in the right MTG during the task was also associated with positive symptoms although a specific association with AVH was not established. Future work is needed to establish the attentional hierarchy in this network.
Supplementary Material
Supplementary material is available at http://schizophre niabulletin.oxfordjournals.org.
Supplementary Material
Acknowledgments
We are grateful to the Barbara-Wengeler-Stiftung Scholarship awarded to Lana Kambeitz-Ilankovic. We would like to thank LEO (Lambeth Early Onset), STEP (Southwark Team for Early Intervention in Psychosis), COAST (Croydon Early Intervention Team) and Maudsley Hospital services for their assistance in the recruitment of the patients. The authors declare no conflict of interest.
References
- 1. Nelson B, Fornito A, Harrison BJ, et al. A disturbed sense of self in the psychosis prodrome: linking phenomenology and neurobiology. Neurosci Biobehav Rev. 2009;33:807–817 [DOI] [PubMed] [Google Scholar]
- 2. Waters F, Woodward T, Allen P, Aleman A, Sommer I. Self-recognition deficits in schizophrenia patients with auditory hallucinations: a meta-analysis of the literature. Schizophr Bull. 2012;38:741–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Allen PP, Johns LC, Fu CH, Broome MR, Vythelingum GN, McGuire PK. Misattribution of external speech in patients with hallucinations and delusions. Schizophr Res. 2004;69:277–287 [DOI] [PubMed] [Google Scholar]
- 4. Johns LC, Gregg L, Allen P, McGuire PK. Impaired verbal self-monitoring in psychosis: effects of state, trait and diagnosis. Psychol Med. 2006;36:465–474 [DOI] [PubMed] [Google Scholar]
- 5. Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000;403:309–312 [DOI] [PubMed] [Google Scholar]
- 6. Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2011;168:73–81 [DOI] [PubMed] [Google Scholar]
- 7. McGuire PK, Silbersweig DA, Frith CD. Functional neuroanatomy of verbal self-monitoring. Brain. 1996;119 (Pt 3):907–917 [DOI] [PubMed] [Google Scholar]
- 8. Shergill SS, Brammer MJ, Williams SC, Murray RM, McGuire PK. Mapping auditory hallucinations in schizophrenia using functional magnetic resonance imaging. Arch Gen Psychiatry. 2000;57:1033–1038 [DOI] [PubMed] [Google Scholar]
- 9. Allen P, Larøi F, McGuire PK, Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev. 2008;32:175–191 [DOI] [PubMed] [Google Scholar]
- 10. Fu CH, Brammer MJ, Yágüez L, et al. Increased superior temporal activation associated with external misattributions of self-generated speech in schizophrenia. Schizophr Res. 2008;100:361–363 [DOI] [PubMed] [Google Scholar]
- 11. Allen P, Aleman A, McGuire PK. Inner speech models of auditory verbal hallucinations: evidence from behavioural and neuroimaging studies. Int Rev Psychiatry. 2007;19:407–415 [DOI] [PubMed] [Google Scholar]
- 12. Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci (Regul Ed). 2004;8:102–107 [DOI] [PubMed] [Google Scholar]
- 13. van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev. 2010;34:935–946 [DOI] [PubMed] [Google Scholar]
- 14. Aleman A, Böcker KB, Hijman R, de Haan EH, Kahn RS. Cognitive basis of hallucinations in schizophrenia: role of top-down information processing. Schizophr Res. 2003;64:175–185 [DOI] [PubMed] [Google Scholar]
- 15. Ilankovic LM, Allen PP, Engel R, et al. Attentional modulation of external speech attribution in patients with hallucinations and delusions. Neuropsychologia. 2011;49:805–812 [DOI] [PubMed] [Google Scholar]
- 16. Garety PA, Kuipers E, Fowler D, Freeman D, Bebbington PE. A cognitive model of the positive symptoms of psychosis. Psychol Med. 2001;31:189–195 [DOI] [PubMed] [Google Scholar]
- 17. Maruff P, Danckert J, Pantelis C, Currie J. Saccadic and attentional abnormalities in patients with schizophrenia. Psychol Med. 1998;28:1091–1100 [DOI] [PubMed] [Google Scholar]
- 18. Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annu Rev Neurosci. 1995;18:193–222 [DOI] [PubMed] [Google Scholar]
- 19. Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Brain Res Rev. 2001;35:146–160 [DOI] [PubMed] [Google Scholar]
- 20. Allen PP, Amaro E, Fu CH, et al. Neural correlates of the misattribution of self-generated speech. Hum Brain Mapp. 2005;26:44–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of top-down attentional control. Nat Neurosci. 2000;3:284–291 [DOI] [PubMed] [Google Scholar]
- 22. Giesbrecht B, Woldorff MG, Song AW, Mangun GR. Neural mechanisms of top-down control during spatial and feature attention. Neuroimage. 2003;19:496–512 [DOI] [PubMed] [Google Scholar]
- 23. Hahn B, Ross TJ, Stein EA. Neuroanatomical dissociation between bottom-up and top-down processes of visuospatial selective attention. Neuroimage. 2006;32:842–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mayer AR, Harrington D, Adair JC, Lee R. The neural networks underlying endogenous auditory covert orienting and reorienting. Neuroimage. 2006;30:938–949 [DOI] [PubMed] [Google Scholar]
- 25. Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215 [DOI] [PubMed] [Google Scholar]
- 26. Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain–a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457 [DOI] [PubMed] [Google Scholar]
- 27. Anon Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 28. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276 [DOI] [PubMed] [Google Scholar]
- 29. Haddock G, McCarron J, Tarrier N, Faragher EB. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS). Psychol Med. 1999;29:879–889 [DOI] [PubMed] [Google Scholar]
- 30. Jastak SR, Wilkinson GS. WRAT-R: Wide Range Achievement Test-Revised Administration Manual. Wilmington, DE: Jastak Associates, Inc; 1984 [Google Scholar]
- 31. Friston KJ. Testing for anatomically specified regional effects. Hum Brain Mapp. 1997;5:133–136 [DOI] [PubMed] [Google Scholar]
- 32. Pessoa L, Rossi A, Japee S, Desimone R, Ungerleider LG. Attentional control during the transient updating of cue information. Brain Res. 2009;1247:149–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang L, Metzak PD, Woodward TS. Aberrant connectivity during self-other source monitoring in schizophrenia. Schizophr Res. 2011;125:136–142 [DOI] [PubMed] [Google Scholar]
- 34. Bushara KO, Hanakawa T, Immisch I, Toma K, Kansaku K, Hallett M. Neural correlates of cross-modal binding. Nat Neurosci. 2003;6:190–195 [DOI] [PubMed] [Google Scholar]
- 35. Park JY, Gu BM, Kang DH, et al. Integration of cross-modal emotional information in the human brain: an fMRI study. Cortex. 2010;46:161–169 [DOI] [PubMed] [Google Scholar]
- 36. Calvert GA, Campbell R, Brammer MJ. Evidence from functional magnetic resonance imaging of crossmodal binding in the human heteromodal cortex. Curr Biol. 2000;10:649–657 [DOI] [PubMed] [Google Scholar]
- 37. Kumari V, Fannon D, Ffytche DH, et al. Functional MRI of verbal self-monitoring in schizophrenia: performance and illness-specific effects. Schizophr Bull. 2010;36:740–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saito DN, Yoshimura K, Kochiyama T, Okada T, Honda M, Sadato N. Cross-modal binding and activated attentional networks during audio-visual speech integration: a functional MRI study. Cereb Cortex. 2005;15:1750–1760 [DOI] [PubMed] [Google Scholar]
- 39. Wojciulik E, Kanwisher N. The generality of parietal involvement in visual attention. Neuron. 1999;23:747–764 [DOI] [PubMed] [Google Scholar]
- 40. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583 [DOI] [PubMed] [Google Scholar]
- 41. Moritz S, Vitzthum F, Randjbar S, Veckenstedt R, Woodward TS. Detecting and defusing cognitive traps: metacognitive intervention in schizophrenia. Curr Opin Psychiatry. 2010;23:561–569 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.