Abstract
Background: STOP/MAP6 null (KO) mice recapitulate behavioral abnormalities related to positive and negative symptoms and cognitive deficits of schizophrenia. Here, we investigated whether decreased expression of STOP/MAP6 proteins in heterozygous mice (only one allele expressed) would result in abnormal behavior related to those displayed by STOP null mice. Methods: Using a comprehensive test battery, we investigated the behavioral phenotype of STOP heterozygous (Het) mice compared with STOP KO and wild type (WT) mice on animals raised either in standard conditions (controls) or submitted to maternal deprivation. Results: Control Het mice displayed prominent deficits in social interaction and learning, resembling KO mice. In contrast, they exhibited short-lasting locomotor hyperreactivity to acute mild stress and no impaired locomotor response to amphetamine, much like WT mice. Additionally, perinatal stress deteriorated Het mouse phenotype by exacerbating alterations related to positive symptoms such as their locomotor reactivity to acute mild stress and psychostimulant challenge. Conclusion: Results show that the dosage of susceptibility genes modulates their putative phenotypic contribution and that STOP expression has a high penetrance on cognitive abilities. Hence, STOP Het mice might be useful to investigate cognitive defects related to those observed in mental diseases and ultimately might be a valuable experimental model to evaluate preventive treatments.
Key words: schizophrenia, animal model, microtubule, neurodevelopment, behavioral phenotype, gene x environment interaction
Introduction
Schizophrenia is thought to be based on subtle neuro developmental disturbances altering nervous system plas ticity through deficits in neuronal connectivity linked to synapse alterations in their structure and/or functioning.1,2 In this respect, many genes encoding proteins essential for morphological transformation of neurons, including, DISC1, dysbindin-1, and STOP (also known as MAP6) are considered as susceptibility genes of schizophrenia and proposed as potential biomarkers for early detection.1–4 The heterogeneity of the disease is underlain by a polygenic contribution cooperating with environmental factors.5,6 Beyond genetic heterogeneity, factors such as epigenetic or regulatory changes could be involved.5,6 Increasing evidence indicates that genetic variations at regulatory regions underlie differences in gene expression and could be a major contributor to phenotypic diversity in humans.5,6 Thus, determining how genetic variation within complex gene regulatory networks results in phenotypic alterations may serve to understand human evolution and disease. Given the complexity of this question in humans, as a first step, animals genetically controlled would be invaluable tools to evaluate how varying dosages of a susceptibility gene will affect its contribution to a putative phenotype.6
Many animal models have been successfully developed to study the pathophysiology of schizophrenia (see http://www.schizophreniaforum.org). Of particular relevance are the genetic models mimicking the clinical course of the disease. Total deletion of STOP/MAP6 gene in mice leads, in adulthood, to behavioral alterations related to positive7,8 and negative symptoms9–11 and to cognitive impairments seen in schizophrenia.10,12,13 The strong behavioral phenotype of STOP null (KO) mice may be the consequence of the many biological dysfunctions found in these mice and known to be implicated in schizophrenia, such as nervous system plasticity impairments,9,11,14 and alterations in many neurotransmission systems.7,8,14–17 Behavioral and biological alterations exhibited by STOP KO mice are sensitive to drugs.7,9,11,18 The progression of behavioral defects over time, subtle in the prepubertal period to major in adulthood, suggests a neurodevelopmental progression in biological dysfunctions.8
STOP KO mice exhibit a severe full-blown phenotype, and we can hypothesize that a partial deletion of STOP/MAP6 would give rise to a blunted phenotype. Indeed, modulations of the level of expression of susceptibility genes of schizophrenia have been shown to influence the behavioral phenotype in experimental animal models. Eg, mice heterozygous and KO for dysbindin-1 share common behavioral alterations but with more or less severity.19
A similar report has been made for mice Het or KO for catechol-O-methyltransferase.20 Also, both down regulation and overexpression of neuregulin-1 gene in mice result in alterations of cognitive abilities.21,22
Here, we aimed at characterizing the behavioral phenotype of STOP heterozygous (Het) mice—expressing only one allele of STOP/MAP6 by comparison to that of STOP KO and wild type (WT) mice. While STOP/MAP6 proteins are totally absent in STOP KO mice, STOP Het mice may display a partial dosage of STOP/MAP6 proteins, as suggested by a reduced abundance of the encoding mRNA.23 Further, we asked whether environmental factors such as stress might aggravate the specific phenotype of STOP Het mice.
Methods
Animals in Two Experimental Conditions
Knock out (KO/STOP−/−),9 heterozygous (Het/STOP+/−) mice and their wild type (WT/STOP+/+) littermates were C57BL6/129SvPas F1 (50%/50%) animals (for details, see online supplementary material). Mice were housed in a temperature (22°C) controlled environment under a 12h/12h light/dark cycle (light from 6:00 am to 6:00 pm), with ad libitum access to food and water. After mating, dams were housed individually and daily checked for delivery. On the day of parturition [postnatal day (PND)] 0, each litter (6–12 pups) was assigned to 1 of the 2 experimental conditions: maternal deprivation (MD) or not (controls). MD was carried out daily for 6h (10:00–16:00) from PND 2 to 14 by removing each litter from their home cage, while the dams were left undisturbed. Each litter was placed in a temperature-(31±3°C) and humidity-controlled incubator in a room separated from their mother.
At weaning, for each experimental condition, mice were sorted by sex and genotyped by polymerase chain reaction (for details, see online supplementary material). Males were housed eight per cage (L42 × l26 × h15cm) with an effort to balance genotype ratio, and they were raised in standard facility conditions.
The behavioral tests of control and maternally deprived mice were conducted independently in adult male mice (11–16 weeks old; for details, see online supplementary material).
Ethics Statement
In accordance with the policy of the French legislation, experiments were done in compliance with the European Community Council Directive of November 24, 1986 (86/609/EEC; for a detailed description of ethics statement, see online supplementary material).
STOP Protein Assay
STOP protein levels were detected by western blot analysis: total brains or micro-dissected regions from WT, KO, and Het STOP mice were homogenized in radioimmunoprecipitation assay Buffer (50 Mm Tris pH 8, 150mM NaCl, 1% NP40, 0.5% sodium desoxycholate and 0.1% sodium dodecyl sulfate) and centrifuged at 21 000g for 20min at 4°C. Supernatants (5 µg) were resolved on SDS-PAGE and revealed using primary [MAP6, 23C antibody24 and Neurone Specific Enolase (Millipore)] and secondary antibodies coupled to horseradish peroxidase.
Locomotor Activity
Horizontal activity was continuously video recorded in an open field, cumulated over intervals, as appropriate, computerized, and expressed as the distance traveled (Videotrack, Viewpoint, Lyon, France). The locomotor activity of each mouse was assessed in response to mild stress and psychostimulant in a single recording session (for details, see online supplementary material). Tests were applied in the following order: exposure to a novel environment, saline injection [10ml/kg, intraperitoneal (i.p.)] and D-amphetamine (Sigma, St Louis, MO) injection (1.5 or 3mg/kg, 10ml/kg, i.p.).
The locomotor activity in response to a novel environment was also measured in the Y-maze test (see below).
Y-maze Test
Spontaneous alternation behavior and exploratory activity were video recorded in a Y-shaped maze during an 8-min session of free roaming (for details, see online supplementary material). The movement and location of the mice were monitored online and computerized (Videotrack, View Point, Lyon, France). An alternation was defined as entries into all 3 arms on consecutive choices. The alternation score (%) for each mouse was automatically calculated as the ratio of the actual number of alternations to the possible number (defined as the total number of arm entries minus 2), as shown by the following equation: % alternation = [(Number of alternations)/(Total arm entries-2)] × 100. Additionally, the total distance traveled was considered as an index of locomotor reactivity to novel environment.
Conspecific Recognition Test
Home-cage social interaction was assessed as the duration of sniffing investigation displayed by a resident mouse in response to presentation of an anesthetized intruder mouse (for a detailed description of the paradigm, see online supplementary material). In this test, WT, Het, and KO mice, used as residents, were individually housed for 1 week before testing to permit establishment of a home-cage territory. Het mice, used as intruders, were unfamiliar with mice used as residents. Intruder mice were deeply anesthetized by an i.p. injection of urethane (1.8g/kg, 10ml/kg) at least 10min prior to the experiment. In each trial, an intruder was placed prone at the centre of each resident home cage (L20 × l10 × h13cm), and the exploration activity of the resident was immediately video recorded. Four trials lasting 3min were repeated with a 12-min intertrial period. The first trial (T1) was used to assess home-cage social interaction. In the first 3 trials (T1 to T3), the same anesthetized intruder (mouse 1) was used to assess habituation. In the fourth trial (T4), a novel anesthetized intruder (mouse 2), representing a novel challenge, was used to assess discrimination. The exploration activity of the resident, measured as the time the resident spent sniffing the intruder animal, was manually counted from video recordings. Sniffing was defined as olfactory exploration and close contact (<1cm) between the resident nose or vibrissae and the body of the intruder.
Acoustic Startle and Prepulse Inhibition Test
Sensorimotor gating capacities were analyzed as the inhibition of the acoustic startle response by a preliminary nonstartling acoustic stimulus, termed prepulse inhibition (PPI) using a Panlab PPI equipment (Spain; for a detailed description of the PPI equipment, see online supplementary material). Mice were exposed throughout the test to a 60 dB background noise with white sounds. Following a 10-min acclimation, mice were given first an acoustic pulse (110 dB, 40ms) and then 5 types of trials with different acoustic stimuli: trials with 1 “pulse alone” (110 dB, 40ms), trials with “no stimulus”, and 3 different “prepulse + pulse” trials, in which a 68, 76, or 84 dB stimulus was presented for 20ms followed 50ms later by a pulse (110 dB, 40ms). Each trial type was presented 10 times in an irregular counter-balanced order with a variable intertrial interval of 10–20 s (15 s average). The startle response was recorded for 100ms, beginning with each trial onset. Percent prepulse inhibition (%PPI) at each prepulse level was calculated as follows: [1 − (startle response to “prepulse + pulse”)/(startle response to “pulse alone”)] × 100.
Statistical Analysis
All data were analyzed with Prism4 (GraphPad Software, San Diego, CA). When indicated, comparisons between genotypes were performed as nonparametric one-way ANOVAs (Kruskal-Wallis tests), with genotype as intersubject factor followed by Dunn’s post hoc tests when appropriate.
In social habituation condition of conspecific recognition studies, results (T1–T3) within each genotype were analyzed by nonparametric one-way repeated measures ANOVAs followed by Dunn’s post hoc tests when appropriate. To study social discrimination, observations at T3 and T4 were compared within each genotype by paired nonparametric Mann-Whitney’s t tests.
%PPI data were checked for normality (Shapiro-Wilk test), followed by a two-way ANOVAs, with genotype as between-subject independent variable and prepulse intensities as within-subject variable, followed by Bonferroni’s post hoc comparisons when appropriate.
Results
The present study was performed in F1 mice generated from crossings of STOP heterozygous mice of C57BL6 and STOP heterozygous mice of 129SvPas genetic backgrounds.
STOP/MAP6 Protein Level
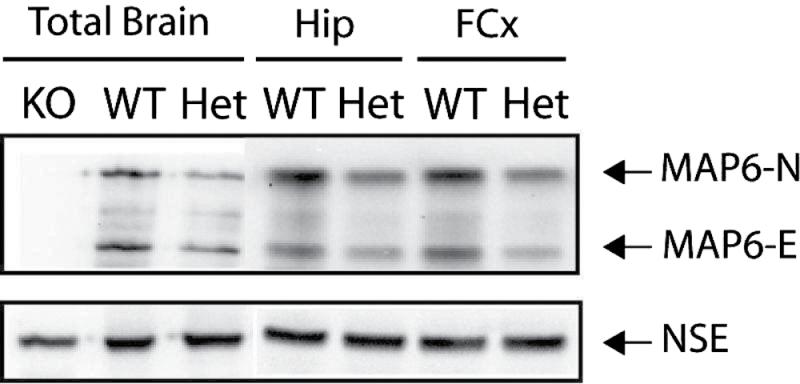
Total brains or specific brain areas were used to prepare protein extracts. The amounts of MAP6 isoforms (MAP6–N and MAP6-E) in extracts from adult WT or STOP Het mice were evaluated in several independent experiments. As illustrated in figure 1, the expression of both isoforms of MAP6 was lower in STOP Het brain extracts than in WT extracts. The decrease is visible in total-brain extracts, as well as in the hippocampal and prefrontal extracts. This clearly indicated that the gene dosage of STOP/MAP6 resulted in a halved expression of the corresponding proteins.
Fig. 1.
STOP/MAP6 protein level in control mice. Both MAP6-N and MAP6-E protein levels were lower in heterozygous (Het) STOP mice brain tissue than in wild type (WT). Representative western blot of the amounts of STOP/MAP6 neuronal isoforms (MAP6-N and MAP6-E) present in brain extracts from adult WT, Het, or STOP null (KO) mice. Neuronal Specific Enolase was used as loading control. (Hip, hippocampus; FCx, frontal cortex).
Behavioral Phenotype of STOP Het Mice Raised in Control Conditions
The behavioral phenotype of control STOP Het mice was analyzed using a test battery (for a detailed test order, see online supplementary figure 1S). In each test, the response of STOP Het mice was compared with that of corresponding WT and KO mice.
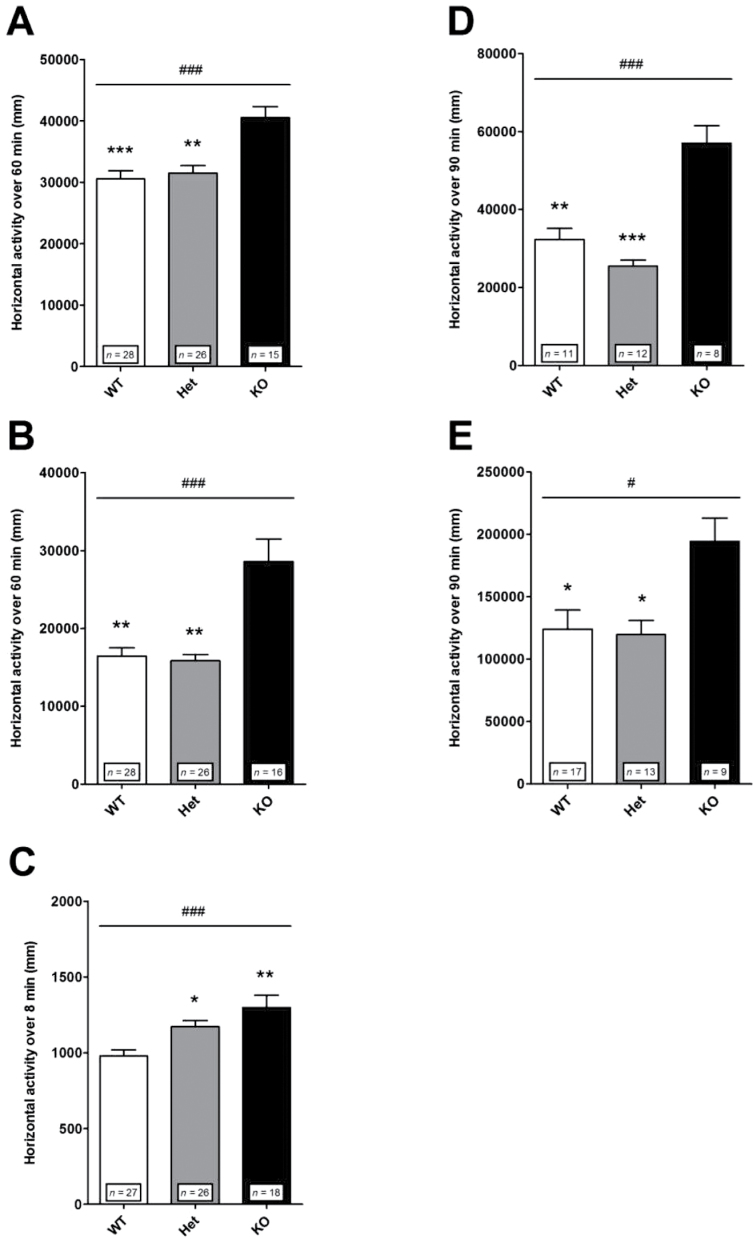
Locomotor Reactivity to Acute Mild Stress. Mice were tested in different paradigms of acute mild stress. Locomotor activity was assessed in an open field in a novel environment condition (figure 2A) and after saline injection (figure 2B), and expressed as the total distance traveled cumulated over 60min. The responses exhibited by Het mice were not different from those of WT mice, while KO mice exhibited enhanced locomotor reactivity to both exposure to novelty (figure 2A) and saline injection (figure 2C). These results suggest that the mild stress-dependent reactivity of Het mice is close to that of WT mice. An exception was the locomomotor activity in response to a novel environment when assessed in a Y-maze test (figure 2C). In this test, the locomotor activity was measured as the total distance traveled for a shorter duration (8min) in a more confined environment than in the open field condition. Het and KO mice were found hyperreactive relative to WT mice. These results show that Het mice can exhibit short-lasting locomotor hyperactivity in response to acute mild stress as already reported.25
Fig. 2.
Locomotor reactivity to acute mild stress and to amphetamine in control mice. Following acute mild stress and amphetamine, KO mice exhibited a larger locomotor response than WT and Het mice. (A) Exposure to a novel environment in an open field. Nonparametric one-way ANOVA showed a significant effect of genotype [F(2,66) = 16.77; ### P < .001]. Post hoc analysis revealed significant differences between KO and WT (*** P < .001) or Het (** P < .01) mice. (B) Exposure to a saline injection in an open field. Nonparametric one-way ANOVA showed a significant effect of genotype [F(2,67) = 15.45; ### P < .001]. Post hoc analysis revealed significant differences between KO and WT (** P < .01) or Het (** P < .01) mice. (C) Exposure to a novel environment in a Y-maze. Nonparametric one-way ANOVA showed a significant effect of genotype [F(2,68) = 14.18; ### P < .001]. Post hoc analysis revealed significant differences between WT and KO (** P < .01) or Het (* P < .05) mice. (D) Amphetamine 1.5mg/kg. Nonparametric one-way ANOVA showed a significant effect of genotype [F(2,28) = 18.90; ### P < .001]. Post hoc analysis revealed significant differences between KO and WT (** P < .001) or Het (*** P < .001) mice. (E) Amphetamine 3mg/kg. Nonparametric one-way ANOVA showed a significant effect of genotype [F(2,36) = 8.43; # P < .05]. Post hoc analysis revealed significant differences between KO and WT (* P < 0.05) or Het (* P < .05) mice. Horizontal locomotor activity is presented as the distance traveled just after exposure to a novel environment, after a saline injection or after amphetamine injection and cumulated over (A, B) 60min, (C) 8min, or (D, E) 90min. Values represent mean ± standard error of mean for the indicated numbers of animals.
Locomotor Reactivity to Psychostimulant. In this test, the locomotor effect of the indirect dopamine agonist amphetamine was evaluated. Mice were injected with amphetamine 1.5 (figure 2D) or 3mg/kg (figure 2E). At each dose used, the locomotor reaction of Het mice did not differ from that of WT mice, while KO mice were hyperreactive. Note that in each genotype the response to amphetamine increased with increasing doses (figure 2).
Spatial Working Memory. Spatial working memory was assessed by testing spontaneous alternation in a Y-maze (see online supplementary figure 2S). No genotype exhibited a significantly different percent of spontaneous alternation, suggesting that the working memory of mice is not modified by STOP gene deletion, as tested in our paradigm (for a specific discussion, see online supplementary material).
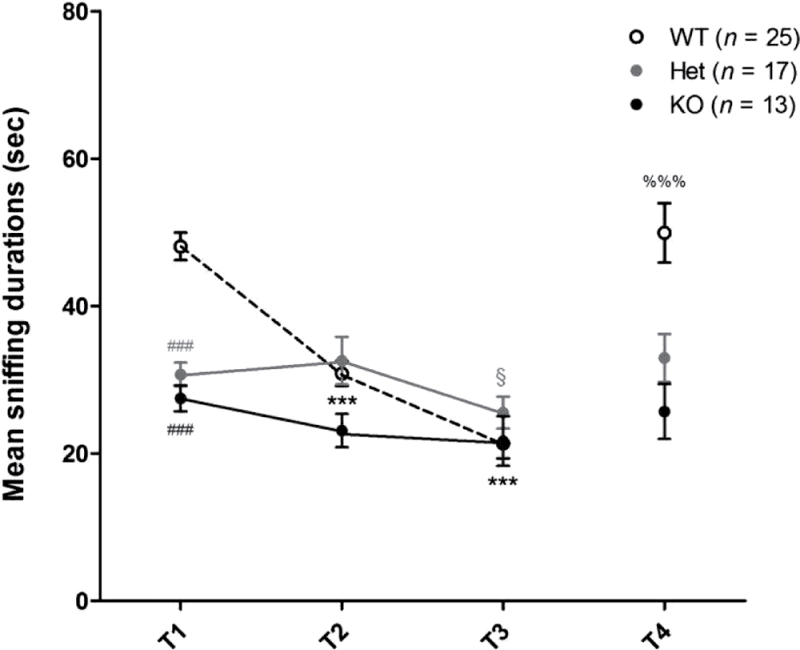
Conspecific Recognition. Social interaction and recognition have been defined as the sniffing investigation displayed in response to 4 repeated presentations of anesthetized intruder mice. The same mouse was used as intruder in the first 3 trials (T1–T3) and a new one in the fourth trial (T4). Considering the level of social interaction assessed by sniffing duration during the first presentation (T1), Het and KO mice exhibited deep deficits compared with WT mice (figure 3).
Fig. 3.
Social interaction and recognition in control mice. Social interaction and recognition, including habituation and discrimination, were impaired in Het and KO mice compared with WT mice. (T1) Social interaction. Nonparametric one-way ANOVA showed a significant effect of genotype [F(2,52) = 35.72; P < .001]. Post hoc analysis revealed significant differences between WT mice and Het (### P < .001) or KO mice (### P < 0.001). (T1–T3) Social recognition-habituation. When each genotype was considered, nonparametric one-way ANOVA of repeated measures revealed that the duration of sniffing was significantly different from T1 to T3 in WT [F(2,24) = 38.48; P < .001] and Het mice [F(2,16) = 7.18; P < .05] only. Post hoc analysis revealed significant differences between T1 and T2 (*** P < .001) or T3 (*** P < .001) for WT mice and between T2 and T3 for Het mice (§ P > .05). (T4) Social recognition-discrimination. Nonparametric paired t tests revealed a significantly longer sniffing duration in T4 than in T3 for WT mice only (%%% P < .001). The exploration activity is presented as the duration of sniffing cumulated for each 3-min trial (T1–T4). Values represent mean ± standard error of mean for the indicated numbers of animals.
Social recognition was studied by analyzing the profile of sniffing durations displayed by each genotype over the 4 trials (figure 3). Across trials T1–T3, WT mice showed a highly significant decline in the time spent investigating the first intruder followed by a highly significant recovery after the introduction of a second one (T4 compared with T3). These characteristic responses reflect the social memory abilities of WT mice in terms of habituation and discrimination. In contrast, Het mice and KO mice did not exhibit different durations of sniffing over the first 3 trials (T1–T3). Note that in Het mice, a slightly significant decrease was observed between T2 and T3 only (figure 3). Moreover, no significant recovery was observed at T4 with either genotype. Altogether, these results suggest that Het mice exhibit clear deficits in social cognitive abilities, including social withdrawal and impairment in social habituation and discrimination, much like KO mice.
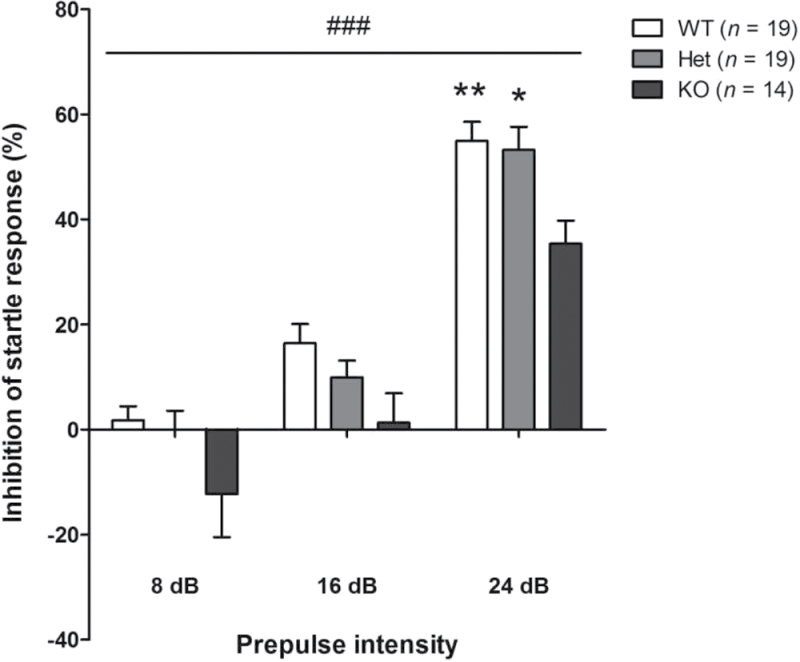
Prepulse Inhibition. Sensorimotor gating capacities were analyzed by assessing PPI of acoustic startle response (PPI). Compared with WT mice, KO mice globally showed impaired PPI; the impairment was significant using 24 dB prepulses (figure 4). PPI in Het mice were mostly not significantly different from those exhibited by WT or KO mice, suggesting that Het mice displayed an intermediate level of response. However, using 24 dB prepulses, PPI in Het mice became slightly different from PPI in KO mice (figure 4). In summary, Het mice could be slightly impaired in sensorimotor gating capacities, while KO mice exhibit forthright deficits.
Fig. 4.
Prepulse inhibition of a startle response in control mice. KO mice were impaired in the prepulse inhibition of an acoustic startle response. Two-way ANOVA showed a significant effect of genotype [F(2,49) = 5.28; ### P < .001] and prepulse intensity [F(2,49) = 56.12; P < .001]; post hoc analysis revealed significant differences at 24 dB between KO and WT (** P < .01) or Het mice (* P < .05). Values represent mean ± standard error of mean for the indicated numbers of animals.
Behavioral Phenotype of STOP Het Mice Submitted to Maternal Deprivation
Mice from 3 genotypes, namely WT, Het, and KO mice, were submitted to maternal separation for 6h from P2 to P14 and then raised in standard conditions. Maternally deprived animals were tested in adulthood by using some of the paradigms used with control mice as described above.
Locomotor Reactivity to Acute Mild Stress and Psychostimulant. Maternally deprived mice were tested for locomotor reaction in a novel environment condition both in an open field and in a Y-maze test, and after injection of amphetamine 3mg/kg. In the 3 paradigms, as in control animals, deprived KO mice were hyperreactive as compared with corresponding WT mice (figure 5A, 5B, 5C). Concerning deprived Het mice, as in control mice, their locomotor reactivity was not significantly different from that of deprived WT mice in response both to novelty in an open field (compare figures 5A and 2A) and to amphetamine (compare figures 5C and 2E), but in contrast to control mice, the locomotor reactivity of deprived Het mice was no longer significantly different from that of deprived KO mice either. In the Y-maze, as in control mice, deprived Het mice were not different from KO mice and were significantly hyperactive relative to corresponding WT mice (compare figures 5B and 2C).
Fig. 5.
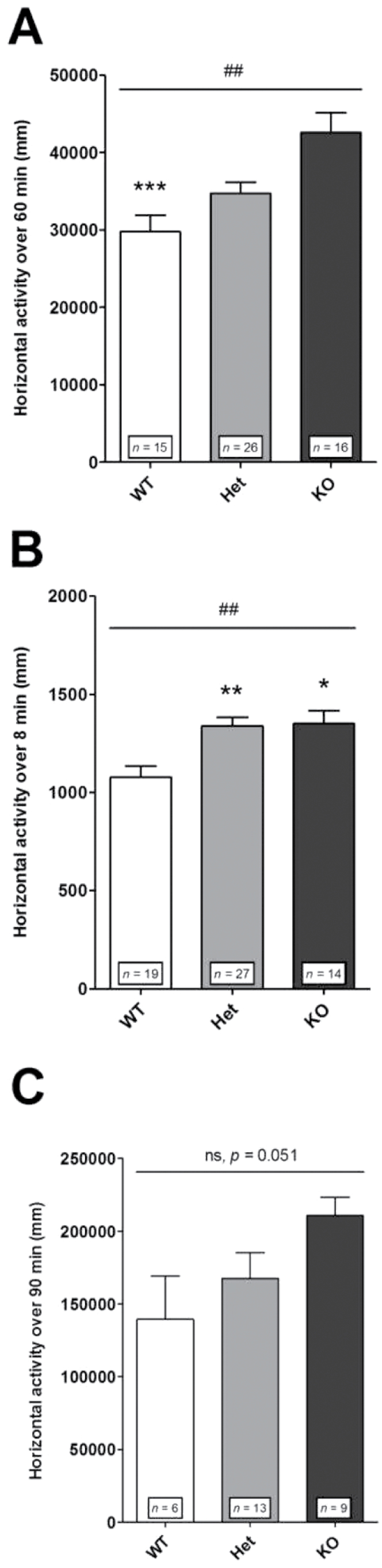
Locomotor reactivity in maternally deprived mice. In maternal deprivation condition, following mild stress and amphetamine challenge, the locomotor response of Het mice tended to be closer to the response exhibited by KO mice. (A) Exposure to a novel environment in an open field. Nonparametric one-way ANOVA showed a significant effect of genotype [(F(2,54) = 13.18; ## P < .05]. Post hoc analysis revealed significant differences between KO and WT (*** P < .05) and no longer between Het and KO mice. (B) Exposure to a novel environment in a Y-maze. Nonparametric one-way ANOVA showed a significant effect of genotype [(F(2,57) = 11.98; ## P < .01]. Post hoc analysis revealed significant differences between WT and Het (** P < .01), or KO mice (* P < .01). (C) Amphetamine injection 3mg/kg. Nonparametric one-way ANOVA showed no significant effect of genotype [(F(2,25) = 5.95; P = .051]. Horizontal locomotor activity was measured as in figure 2. Values represent mean ± standard error of mean for the indicated numbers of animals.
When working memory, conspecific recognition abilities, and PPI were assessed in deprived animals, the responses exhibited by the 3 genotypes showed the same pattern of difference as in control animals (data not shown).
Altogether, these results suggest that, between control and maternal deprivation conditions, the locomotor reactivity phenotype of deprived Het mice is slightly shifted from the phenotype of corresponding WT mice toward that of KO mice.
Discussion
First, this study shows that total STOP/MAP6 gene deletion in C57BL6/129SvPas background mice induces clear behavioral alterations, including locomotor hyperreactivity to acute mild stress and psychostimulant, social withdrawal, social learning, and sensori gating impairment as previously reported in STOP KO mice of BALBc/129SvPas background. The supersensitive responses to mild stress and to psychostimulant are features that also occur in patients with schizophrenia and are thought to be a signature of hyperdopaminergic transmission linked to positive symptoms.26 Thus, the alterations found in STOP KO mice correlate for the positive and negative symptoms and the cognitive impairments classically observed in schizophrenia, giving the model face validity, independently from the genetic background.
Second, the present results demonstrate that mice heterozygous for STOP/MAP6 gene displayed a gene-dosage effect on STOP/MAP6 protein levels associated with a specific behavioral phenotype. Compared with WT mice, Het mice displayed an altered phenotype. Compared with KO mice, the behavioral phenotype of Het mice was blunted, but the blunting was not homogeneous in number and intensity along the behavioral components. Het mice displayed prominent social interaction and learning deficits, thus resembling KO mice, while Het mice exhibited only short-lasting locomotor hyperreactivity to acute mild stress and no impaired locomotor response to amphetamine, in contrast to KO mice. Further, Het mice have been shown impaired in object recognition/discrimination and spatial memory.25 Altogether, these results indicate a strong link between STOP/MAP6 protein and cognition.
Microtubules and some effectors that modulate their dynamics or stability, including STOP/MAP6,27–29 dysbindin-1,30 and DISC11 proteins are essential for neuronal morphogenesis and connectivity during development, as well as for synaptic plasticity in adulthood. Variations of microtubule dynamics and stability are related to integrated brain functions, including memory, attention, and executive functioning such as social behavior and involving the prefrontal cortex among other structures,31,32 and their alterations are involved in the physiopathology of neurodegenerative and psychiatric diseases.1,2 Consistently, variations in dysbindin-1 or DISC1 gene have been associated with schizophrenia and/or bipolar disorder.1,2,4 Likewise, genetic modifications of dysbindin-1 or DISC1 in mice induce a set of disorders evocative of schizophrenia, with a specific influence on cognition.33 STOP proteins24 are increasingly involved in the physiopathology of psychiatric disorders.34 The gene encoding STOP/MAP6 proteins is localized in the human 11q14 chromosomal region containing many susceptibility genes for schizophrenia.35 Polymorphisms in gene STOP/MAP6 have been associated with schizophrenia,36 and STOP/MAP6 protein expression is altered in the prefrontal cortex of patients with schizophrenia.4 The subtle to overt cognitive deficits found in STOP Het, reminiscent of cognitive impairment of schizophrenia, are consistent with human and animal data described above and argue for a high penetrance of STOP/MAP6 mutation on cognition in association with schizophrenia.
However, the behavioral phenotype exhibited by STOP Het mice is not totally reminiscent of overt schizophrenia: the only slight and short-lasting locomotor hyperreactivity to acute mild stress and the absence of hyperreactivity to amphetamine in STOP Het mice suggest only mild alterations related to dopaminergic transmission and thus to positive symptoms.26 The Het mouse phenotype could be then related to the syndromes described in individuals with an increased risk of developing schizophrenia, including the ultra-high-risk subjects. Interestingly, some individuals at ultra-high risk of psychosis have genetic risk combined with functional deterioration, often linked to cognitive deficits affecting mainly social cognition.37 Moreover, ultra-high-risk individuals can experience mild psychotic symptoms, which are attenuated, brief, limited, or intermittent and associated with slight dopaminergic impairment,38–40 whereas clear positive symptoms are absent.37
Hence, STOP Het mice recapitulate behavioral abnormalities that resemble several well-recognized features of human individuals at ultra-high risk of transition to schizophrenia, including cognitive and social impairment and mild psychotic symptoms.
To propose STOP Het mice as an animal model to study the transition to psychosis and to develop preventive treatment, we attempted to alter Het mouse phenotype according to the current etiological model of schizophrenia, known as the stress-vulnerability model.6 This model assumes that genetic factors operate by making individuals vulnerable to environmental risks during sensitive periods, including perinatal and pubertal periods. Although it is very difficult to provide data substantiating this model,6 it is interesting to transfer experiences of social stress in humans to a potential animal model. Exposure of rodents to early maternal separation negatively affects brain development and adult behavior. Here, we show Het mice early stressed by a maternal separation exhibit in adulthood enhanced locomotor reactivity to acute mild stress and psychostimulant challenge, as revealed by a shift of their locomotor reactivity phenotype toward that of KO mice. Interestingly, because the behavioral responses exhibited by control, and maternally deprived WT mice can be qualitatively considered similar, the exacerbated locomotor reactivity seen in adult deprived Het mice suggests that Het mice are more sensitive to perinatal stress. Altogether, our results suggest that perinatal environmental stress can worsen STOP Het mouse behavioral phenotype by exacerbating behavioral alterations related to positive symptoms. Even if most studies indicate that prolonged maternal separation is associated with long-term dopaminergic sensitization, the precise mechanisms involved are still not understood. There is evidence that postnatal hypothalamic-pituitary-adrenal (HPA) axis activation can influence the functioning of dopaminergic system in adulthood.41 However, the effects of maternal separation on the different parameters of HPA axis are not consistent.42 The change in dopaminergic system, seen in Het mice, might also be linked to abnormal development of hypothalamic circuitries combined with an abnormal neuroendocrine transmission, impaired neurogenesis and neurotrophic signaling in the hippocampus, as well as epigenetic regulation.41
Concerning the cognitive phenotype, there are several possible reasons for the lack of aggravation in maternally deprived mice. First, considering cognition- and emotion-related behavior, maternal deprivation in mice is strain-specific and C57Bl/6 strain is considered as a stress-resilient strain.43 Second, because Het mice exhibit prominent social cognitive deficits resembling to KO, they might have reached the maximal level of response. Finally, maternal deprivation as performed in the present study may not be optimal. Indeed, the behavioral effect induced by maternal deprivation largely depends on the inbred strain of mice, the behavioral component tested, and the stress parameters.43 A combination of different environmental disturbances applied early and late associated with other genic variations5,6 could be more suitable to aggravate the phenotype of Het mice.
Conclusion
The present study shows that STOP KO mice exhibit behavioral alterations independently from their genetic background, thereby highlighting the robustness of the phenotype induced by STOP/MAP6 gene deletion and reinforcing the face validity of this model for schizophrenia.
In control conditions and despite the same environmental cues, we provide evidence that Het mice exhibit a blunted phenotype compared with KO mice, corroborating that the dosages of susceptibility genes modulate their putative phenotypic contribution. The clear behavioral deficits in Het mice argue for a high penetrance of STOP/MAP6 mutation on cognition. According to human studies proposing social cognitive deficits in ultra-high-risk populations as robust premorbid predictive markers of a subsequent psychotic disorder,37 we propose STOP Het mice as a translational animal model for the genetic ultra-high-risk states of schizophrenia. The validity of the model is supported by the exacerbation, through maternal deprivation, of the behavioral alterations related to positive symptoms. Such a model will help to elucidate transition factors from ultra-high-risk states to full-blown psychosis and to decipher the mechanisms underlying this transition. Moreover, because social cognitive deficits in ultra-high-risk populations have been proposed as targets for early intervention,37 we propose that STOP Het mice would be valuable tools to study preventive strategies.
Funding
JV held a doctoral fellowship from la Région Rhône- Alpes, France (06.010768.02; 07.019481.01; 08.019466.01).
Supplementary Material
Supplementary material is available at http://schizo phreniabulletin.oxfordjournals.org.
Acknowledgments
The authors thank Annie Schweitzer for her assistance.The Authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Brandon NJ, Sawa A. Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat Rev Neurosci. 2011;12:707–722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14:285–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martins-De-Souza D, Dias-Neto E, Schmitt A, et al. Proteome analysis of schizophrenia brain tissue. World J Biol Psychiatry. 2010;11:110–120 [DOI] [PubMed] [Google Scholar]
- 4. Choi KH, Zepp ME, Higgs BW, Weickert CS, Webster MJ. Expression profiles of schizophrenia susceptibility genes during human prefrontal cortical development. J Psychiatry Neurosci. 2009;34:450–458 [PMC free article] [PubMed] [Google Scholar]
- 5. Burmeister M, McInnis MG, Zöllner S. Psychiatric genetics: progress amid controversy. Nat Rev Genet. 2008;9:527–540 [DOI] [PubMed] [Google Scholar]
- 6. van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212 [DOI] [PubMed] [Google Scholar]
- 7. Brun P, Bégou M, Andrieux A, et al. Dopaminergic transmission in STOP null mice. J Neurochem. 2005;94:63–73 [DOI] [PubMed] [Google Scholar]
- 8. Bégou M, Brun P, Bertrand JB, et al. Post-pubertal emergence of alterations in locomotor activity in STOP null mice. Synapse. 2007;61:689–697 [DOI] [PubMed] [Google Scholar]
- 9. Andrieux A, Salin PA, Vernet M, et al. The suppression of brain cold-stable microtubules in mice induces synaptic defects associated with neuroleptic-sensitive behavioral disorders. Genes Dev. 2002;16:2350–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bégou M, Volle J, Bertrand JB, et al. The stop null mice model for schizophrenia displays [corrected] cognitive and social deficits partly alleviated by neuroleptics. Neuroscience. 2008;157:29–39 [DOI] [PubMed] [Google Scholar]
- 11. Delotterie D, Ruiz G, Brocard J, et al. Chronic administration of atypical antipsychotics improves behavioral and synaptic defects of STOP null mice. Psychopharmacology. 2010;208:131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Powell KJ, Hori SE, Leslie R, et al. Cognitive impairments in the STOP null mouse model of schizophrenia. Behav Neurosci. 2007;121:826–835 [DOI] [PubMed] [Google Scholar]
- 13. Fradley RL, O’Meara GF, Newman RJ, Andrieux A, Job D, Reynolds DS. STOP knockout and NMDA NR1 hypomorphic mice exhibit deficits in sensorimotor gating. Behav Brain Res. 2005;163:257–264 [DOI] [PubMed] [Google Scholar]
- 14. Fournet V, Jany M, Fabre V, et al. The deletion of the microtubule-associated STOP protein affects the serotonergic mouse brain network. J Neurochem. 2010;115:1579–1594 [DOI] [PubMed] [Google Scholar]
- 15. Bouvrais-Veret C, Weiss S, Hanoun N, et al. Microtubule- associated STOP protein deletion triggers restricted changes in dopaminergic neurotransmission. J Neurochem. 2008;104:745–756 [DOI] [PubMed] [Google Scholar]
- 16. Brenner E, Sonnewald U, Schweitzer A, Andrieux A, Nehlig A. Hypoglutamatergic activity in the STOP knockout mouse: a potential model for chronic untreated schizophrenia. Journal of neuroscience research. 2007;85:3487–3493. [DOI] [PubMed] [Google Scholar]
- 17. Bouvrais-Veret C, Weiss S, Andrieux A, et al. Sustained increase of alpha7 nicotinic receptors and choline-induced improvement of learning deficit in STOP knock-out mice. Neuropharmacology. 2007;52:1691–1700 [DOI] [PubMed] [Google Scholar]
- 18. Andrieux A, Salin P, Schweitzer A, et al. Microtubule stabilizer ameliorates synaptic function and behavior in a mouse model for schizophrenia. Biol Psychiatry. 2006;60:1224–1230 [DOI] [PubMed] [Google Scholar]
- 19. Bhardwaj SK, Baharnoori M, Sharif-Askari B, Kamath A, Williams S, Srivastava LK. Behavioral characterization of dysbindin-1 deficient sandy mice. Behav Brain Res. 2009;197:435–441 [DOI] [PubMed] [Google Scholar]
- 20. Babovic D, O’Tuathaigh CM, O’Connor AM, et al. Phenotypic characterization of cognition and social behavior in mice with heterozygous versus homozygous deletion of catechol-O-methyltransferase. Neuroscience. 2008;155:1021–1029 [DOI] [PubMed] [Google Scholar]
- 21. O’Tuathaigh CM, Babovic D, O’Sullivan GJ, et al. Phenotypic characterization of spatial cognition and social behavior in mice with ‘knockout’ of the schizophrenia risk gene neuregulin 1. Neuroscience. 2007;147:18–27 [DOI] [PubMed] [Google Scholar]
- 22. Kato T, Kasai A, Mizuno M, et al. Phenotypic characterization of transgenic mice overexpressing neuregulin-1. PLoS ONE. 2010;5:e14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eastwood SL, Lyon L, George L, Andrieux A, Job D, Harrison PJ. Altered expression of synaptic protein mRNAs in STOP (MAP6) mutant mice. J Psychopharmacol. 2007;21:635–644 [DOI] [PubMed] [Google Scholar]
- 24. Galiano MR, Bosc C, Schweitzer A, Andrieux A, Job D, Hallak ME. Astrocytes and oligodendrocytes express different STOP protein isoforms. J Neurosci Res. 2004;78:329–337 [DOI] [PubMed] [Google Scholar]
- 25. Merenlender-Wagner A, Pikman R, Giladi E, Andrieux A, Gozes I. NAP (davunetide) enhances cognitive behavior in the STOP heterozygous mouse–a microtubule-deficient model of schizophrenia. Peptides. 2010;31:1368–1373 [DOI] [PubMed] [Google Scholar]
- 26. Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacology. 2000;23:223–239 [DOI] [PubMed] [Google Scholar]
- 27. Benardais K, Kasem B, Couegnas A, et al. Loss of STOP protein impairs peripheral olfactory neurogenesis. PLoS ONE. 2010;5:e12753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bosc C, Cronk JD, Pirollet F, et al. Cloning, expression, and properties of the microtubule-stabilizing protein STOP. Proc Natl Acad Sci USA. 1996;93:2125–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Guillaud L, Bosc C, Fourest-Lieuvin A, et al. STOP proteins are responsible for the high degree of microtubule stabilization observed in neuronal cells. J Cell Biol. 1998;142:167–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Talbot K, Cho DS, Ong WY, et al. Dysbindin-1 is a synaptic and microtubular protein that binds brain snapin. Hum Mol Genet. 2006;15:3041–3054 [DOI] [PubMed] [Google Scholar]
- 31. Burdick KE, Lencz T, Funke B, et al. Genetic variation in DTNBP1 influences general cognitive ability. Hum Mol Genet. 2006;15:1563–1568 [DOI] [PubMed] [Google Scholar]
- 32. Fallgatter AJ, Herrmann MJ, Hohoff C, et al. DTNBP1 (dysbindin) gene variants modulate prefrontal brain function in healthy individuals. Neuropsychopharmacology. 2006;31:2002–2010 [DOI] [PubMed] [Google Scholar]
- 33. Papaleo F, Lipska BK, Weinberger DR. Mouse models of genetic effects on cognition: relevance to schizophrenia. Neuropharmacology. 2012;62:1204–1220 [DOI] [PubMed] [Google Scholar]
- 34. Schloss P, Lau T. STOP - in the Name of Mood: microtubule-associated proteins in mood and cognition. J Neurochem. 2012;121:1–3 [DOI] [PubMed] [Google Scholar]
- 35. Lewis CM, Levinson DF, Wise LH, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet. 2003;73:34–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shimizu H, Iwayama Y, Yamada K, et al. Genetic and expression analyses of the STOP (MAP6) gene in schizophrenia. Schizophr Res. 2006;84:244–252 [DOI] [PubMed] [Google Scholar]
- 37. Thompson AD, Bartholomeusz C, Yung AR. Social cognition deficits and the ‘ultra high risk’ for psychosis population: a review of literature. Early Interv Psychiatry. 2011;5:192–202 [DOI] [PubMed] [Google Scholar]
- 38. Brunelin J, d’Amato T, van Os J, Cochet A, Suaud-Chagny MF, Saoud M. Effects of acute metabolic stress on the dopaminergic and pituitary-adrenal axis activity in patients with schizophrenia, their unaffected siblings and controls. Schizophr Res. 2008;100:206–211 [DOI] [PubMed] [Google Scholar]
- 39. Brunelin J, d’Amato T, Van Os J, Costes N, Suaud Chagny MF, Saoud M. Increased left striatal dopamine transmission in unaffected siblings of schizophrenia patients in response to acute metabolic stress. Psychiatry Res. 2010;181:130–135 [DOI] [PubMed] [Google Scholar]
- 40. Hirvonen J, Hietala J. Dysfunctional brain networks and genetic risk for schizophrenia: specific neurotransmitter systems. CNS Neurosci Ther. 2011;17:89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Walker E, Mittal V, Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu Rev Clin Psychol. 2008;4:189–216 [DOI] [PubMed] [Google Scholar]
- 42. Pryce CR, Feldon J. Long-term neurobehavioural impact of the postnatal environment in rats: manipulations, effects and mediating mechanisms. Neurosci Biobehav Rev. 2003;27:57–71 [DOI] [PubMed] [Google Scholar]
- 43. Millstein RA, Holmes A. Effects of repeated maternal separation on anxiety- and depression-related phenotypes in different mouse strains. Neurosci Biobehav Rev. 2007;31:3–17 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.