Abstract
Conduct disorder (CD) prior to age 15 is a precursor of schizophrenia in a minority of cases and is associated with violent behavior through adulthood, after taking account of substance misuse. The present study used structural magnetic imaging to examine gray matter (GM) volumes among 27 men with schizophrenia preceded by CD (SZ+CD), 23 men with schizophrenia but without CD (SZ–CD), 27 men with CD only (CD), and 25 healthy (H) men. The groups with schizophrenia were similar in terms of age of onset and duration of illness, levels of psychotic symptoms, and medication. The 2 groups with CD were similar as to number of CD symptoms, lifelong aggressive behavior, and number of criminal convictions. Men with SZ+CD, relative to those with SZ–CD, displayed (1) increased GM volumes in the hypothalamus, the left putamen, the right cuneus/precuneus, and the right inferior parietal cortex after controlling for age, alcohol, and drug misuse and (2) decreased GM volumes in the inferior frontal region. Men with SZ+CD (relative to the SZ–CD group) and CD (relative to the H group) displayed increased GM volumes of the hypothalamus and the inferior and superior parietal lobes, which were not associated with substance misuse. Aggressive behavior, both prior to age 15 and lifetime tendency, was positively correlated with the GM volume of the hypothalamus. Thus, among males, SZ+CD represents a distinct subtype of schizophrenia. Although differences in behavior emerge in childhood and remain stable through adulthood, further research is needed to determine whether the differences in GM volumes result from abnormal neural development distinct from that of other males developing schizophrenia.
Key words: conduct problems, antisocial behavior, violence, structural brain alterations
Many years ago, Lee Robins found that conduct disorder (CD) was a precursor of schizophrenia 1 and later confirmed this finding. 2 , 3 Subsequent evidence concurs. For example, a prospective investigation that followed a birth cohort to age 26 determined that 40% of the cohort members who developed schizophreniform disorders had displayed CD prior to age 15. 4 The CD modules of the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental (DSM) disorders (fourth edition, DSM-IV), SCID in short, were designed to diagnose CD prior to age 15 among adults. 5 Several studies have used this interview protocol, some supplementing self-reports with information from family members, school, social service, and justice files, to diagnose CD among adults with schizophrenia. Among men and women with schizophrenia in general psychiatric services, the prevalence of CD prior to age 15 ranged from 20% to 45%, 6 , 7 with higher rates in samples recruited from forensic hospitals and correctional facilities. 6 CD is a precursor of schizophrenia, and it is more common among people with schizophrenia than in the general population. 6
Among people with schizophrenia, CD prior to age 15 continues to be associated with antisocial and violent behavior through adult life after taking account of past and current substance misuse. 6–12 Among people with schizophrenia, 10 , 13–17 as in the general population, 18–20 those who present CD in childhood commit a disproportionate number of violent crimes. While some studies show that positive symptoms are associated with aggressive behavior even after taking account of CD, 21 those with CD are not distinguished from other patients with schizophrenia by profiles of positive and negative symptoms. 22
Prospective investigations show that adults with schizophrenia and prior CD (SZ+CD) displayed aggressive behavior, psychotic-like experiences as children, 23 and poor academic achievement. 24 Retrospective studies report that adults with SZ+CD, as compared to those with SZ–CD, obtained lower-than-average marks in elementary school, failed to graduate from secondary school, abused substances in adolescence, and experienced physical abuse. 6 , 24–27 Criminality and substance misuse are elevated among fathers and brothers of men with SZ+CD, whereas rates of mental illness are similar to that found among patients with SZ–CD. 6 , 27 , 28
Among the non–mentally ill men, a small group present CD from an early age, persistent antisocial and aggressive behavior through adulthood, and abnormalities in brain structure relative to healthy men. 29–37 Results of structural magnetic resonance imaging studies (sMRI) measuring gray matter (GM) volumes are inconsistent regarding the type (larger or smaller) and regions of abnormality. 32 , 35–37 Among men with SZ+CD, however, there no studies. 38 , 39 A few studies of brain structure have been conducted among men with schizophrenia who display different ages of onset and patterns of aggressive behavior, including persistent aggressive behavior and poor response to antipsychotic medication, no previous aggressive behavior and 1 violent offence, no aggressive behavior prior to onset followed by persistent aggression, and finally the largest group comprising those who show conduct problems from childhood that persist across their life span. The extant literature is limited and difficult to aggregate, but it does suggest differences specific to each pattern of violent behavior. 38–43
Two studies examined male offenders with schizophrenia: one compared those with and without comorbid antisocial personality disorder (ASPD), which requires, by definition, CD prior to age 15; 44 and another compared those with and without high psychopathy scores. 45 Both included small samples and reported fewer neuropsychological deficits among the antisocial participants in tests tapping the dorsolateral prefrontal and the orbital frontal cortex (OFC) functions. 38 A recent meta-analysis reported that among persons with schizophrenia, those defined very broadly as antisocial, as compared to the nonantisocial, were characterized by lower intelligence quotients (IQs) and memory dysfunction, whereas compared to non–mentally ill antisocial participants, they exhibited deficits in IQ, attention, executive function, and memory. 46
Among patients with schizophrenia, scores on the Life History of Aggression (LHA) measure were associated with increased diffusivity in the inferior frontal white matter and lower functional connectivity between the amygdala and the ventral prefrontal cortex. 39 Diffusivity has been associated with increased cerebrospinal fluid (CSF). 47 Functional MRI (fMRI) studies of violent offenders with schizophrenia observed decreased frontal basal activation during a Go/NoGo task, increased activity in the motor, premotor, and anterior cingulate regions among those with ASPD, 48 and attenuated amygdala activation to fearful faces among those with high psychopathy scores. 49
Thus, among men with schizophrenia, at least one in five people presents CD prior to age 15 and persistent antisocial and aggressive behavior. Identifying distinctive subtypes of schizophrenia may facilitate etiological research 50 and inform the development of effective treatments for both the illness and the antisocial and aggressive behavior. 51 Both schizophrenia 52 and CD 53 , 54 are neurodevelopmental disorders. In both disorders, from conception onwards, combinations of genes, in addition and in interaction with environmental events, are thought to modify brain structure and function. Thus, we reasoned that when schizophrenia develops in parallel with CD, neurodevelopment would be distinct from both that associated with schizophrenia and that associated with CD. We hypothesized that in adulthood, men with SZ+CD would show cognitive and structural brain abnormalities relative to healthy men, and both similarities and differences relative to men with SZ–CD and those with CD and no mental illness.
Almost all persons with childhood onset of CD and persistence of antisocial and aggressive behavior in adulthood also display childhood onset and persistent pattern of substance misuse. 55–57 This is true among those with and without schizophrenia. 22 , 26–28 Although substance misuse is an integral part of a heritable pattern of lifelong antisocial behavior, 58 disentangling the cognitive and structural abnormalities consequent to substance use from those associated with persistent antisocial and aggressive behavior is necessary to understand the mechanisms underlying these behaviors. However, neither statistical controls nor studying groups of antisocial persons without substance misuse provide an ideal solution to this problem. 59 Further, prospective studies indicate that heavy cannabis use in adolescence may play a causal role in schizophrenia 60 , 61 by altering brain development, 62 , 63 and 1 study has shown that among persons experiencing a first episode of psychosis, CD increased the likelihood of cannabis use before age 14. 64 In addition, histories of substance misuse that can be obtained from middle-aged adults are imprecise measures of different phenomena—past and current use by type, combinations, and doses of substances. This led us to obtain careful histories of use of substances and to statistically control for group differences in use.
Four groups of men, with SZ+CD, SZ–CD, CD, and no schizophrenia or history of CD (H), were compared on sociodemographic, clinical, and forensic characteristics, and their GM brain volumes were assessed using sMRI.
Method
Participants
The initial sample included men living in Germany, of whom 71 were offenders and 52 nonoffenders. Offenders with no history of CD were excluded. Participants with schizophrenia were recruited from general and forensic psychiatric services: 27 with CD prior to age 15 (SZ+CD), and 23 with no history of CD (SZ–CD). Participants with no Axis I or II diagnoses other than past substance use disorders were recruited through general and forensic psychiatric services, from prisons, and through advertisements: 27 with CD prior to age 15 and 25 healthy (H) men.
As presented in table 1, the 4 groups were similar with reference to their mean age and years of education. No participant had a history of medical/ neurological illness or head injury resulting in loss of consciousness for more than 30min. All participants were right-handed, with IQ scores of 80 or higher on the multiple choice vocabulary test (MWT-B). 65 Self-reports indicated that none of the participants had consumed any substance in the year prior to study entry. Urine tests were available for 22 of the 27 SZ+CD, 12 of the 23 SZ–CD, 23 of the 27 CD, and 6 of the 25 H men. Results indicated that 2 CD participants were clean for the 6 months prior to testing and all others for 12 months. All of the participants with schizophrenia, except 1, were on antipsychotic medications: SZ+CD: 2 were on typical agents, 18 on atypical agents, 7 on both typical and atypical agents, and none were receiving other medications; SZ–CD: 11 were on atypicals, 11 on both typicals and atypicals; and 1 was also receiving benzodiazepines.
Table 1.
Comparisons of the Sociodemographic, Forensic, and Clinical Characteristics of Men With Schizophrenia and Conduct Disorder, Men With Schizophrenia and No Conduct Disorder, Men With Conduct Disorder, and Healthy Men
| SZ+CD (n = 27) | Healthy (n = 25) | SZ–CD (n = 23) | CD (n = 27) | Group Comparisons (ANOVA or Chi-Square) Bonferroni Adjusted (P < .003) | ||
|---|---|---|---|---|---|---|
| Statistics | Post Hoc Tukey Tests (Bonferroni) | |||||
| Demographic characteristics | ||||||
| Mean age (years) | 36.2±7.7 | 33.0±10.0 | 35.7±8.7 | 36.0±7.9 | F 3,98 = 0.7, P = .532 | NA |
| Mean years of education | 9.5±1.2 | 9.9±1.6 | 10.0±1.9 | 9.6±1.2 | F 3,98 = 1.4, P = .226 | NA |
| Clinical characteristics | ||||||
| Mean age at schizophrenia onset | 24.9±6.9 | NA | 23.4±6.6 | NA | F 1,48 = 0.6, P = .432 | NA |
| Mean duration of illness | 11.3±5.9 | NA | 12.6±7.4 | NA | F 1,48 = 0.5, P = .497 | NA |
| Mean score positive symptoms | 13.6±4.8 | NA | 14.4±4.0 | NA | F 1,48 = 0.4, P = .531 | NA |
| Mean score negative symptoms | 17.5±6.7 | NA | 17.2±6.4 | NA | F 1,48 = 0.0, P = .872 | NA |
| Mean general psychopathology | 31.9±8.3 | NA | 31.3±6.9 | NA | F 1,48 = 0.1, P = .777 | NA |
| Mean PANSS total score | 63.7±17.2 | NA | 63.0±14.1 | NA | F 1,48 = 0.0, P = .862 | NA |
| Mean chlorpromazine units equivalents (mg/day) | 577±296 | NA | 616±418 | NA | F 1,48 = 0.0, P = .975 | NA |
| Premorbid IQ | 101±15 | 109±13 | 102±13 | 105±14 | F 3,98 = 1.7, P = .175 | NA |
| Antisocial behavior | ||||||
| Mean number of CD symptoms | 7.1±3.3 | 1.0±0.8 | 1.2±0.8 | 6.3±2.5 | F 3,98 = 54.5, P < .001 | SZ+CD, CD > H, SZ–CD |
| Antisocial personality disorder (N) | N = 13 | N = 0 | N = 0 | N = 16 | χ23,102 = 34.7, P < .001 | NA |
| Mean score life history of aggression (0–55) | 24.6±11.2 | 11.2±4.3 | 9.5±3.4 | 23.0±8.1 | F 3,98 = 27.0, P < .001 | SZ+CD, CD > H, SZ–CD |
| Mean number of criminal convictions | 4.4±5.6 | 0.0±0.0 | 0.0±0.0 | 5.6±3.4 | F 3,98=16.4, P < .001 | SZ+CD, CD > H, SZ–CD |
| Past substance misuse | ||||||
| Alcohol use disorders | N = 16 | N = 4 | N = 4 | N = 15 | χ23,102 = 17.9, P < .001 | NA |
| Polysubstance dependence | N = 12 | N = 3 | N = 1 | N = 8 | χ23,102 = 13.7, P = .003 | NA |
| Nicotine dependence (DSM-IV: 305.10) | N = 19 | N = 14 | N = 14 | N = 16 | χ23,102 = 1.3, P = .734 | NA |
| Cannabis abuse (DSM-IV: 305.20) | N = 13 | N = 6 | N = 7 | N = 11 | χ23,102 = 3.9, P = .278 | NA |
| Hallucinogen abuse (DSM-IV: 305.30) | N = 5 | N = 2 | N = 2 | N = 3 | χ23,102 = 1.8, P = .626 | NA |
| Sedative abuse (DSM-IV: 305.40) | N = 2 | N = 0 | N = 1 | N = 2 | χ23,102 = 2.0, P = .566 | NA |
| Opiate abuse (DSM-IV: 305.50) | N = 6 | N = 1 | N = 3 | N = 5 | χ23,102 = 3.9, P = .277 | NA |
| Cocaine abuse (DSM-IV: 305.60) | N = 8 | N = 3 | N = 3 | N = 7 | χ23,102 = 3.8, P = .290 | NA |
| Stimulant abuse (DSM-IV: 305.70) | N = 8 | N = 1 | N = 4 | N = 4 | χ23,102 = 6.2, P = .101 | NA |
| Mean score: MAST | 8.6±6.3 | 3.5±5.6 | 4.4±5.4 | 6.3±5.8 | F 3,98 = 3.9, P = .012 | SZ+CD > H, SZ–CD |
| Mean score: DAST | 10.6±6.1 | 2.9±5.1 | 4.5±4.7 | 8.4±7.3 | F 3,98 = 9.1, P < .001 | SZ+CD, CD > H, SZ–CD |
Note: SZ+CD, schizophrenia and conduct disorder prior to age 15; SZ–CD, schizophrenia and no conduct disorder; CD, conduct disorder prior to age 15 and no schizophrenia; NA, Not applicable; PANSS, Positive and Negative Symptom Scale; MAST, Michigan Alcohol Screening Test; and DAST, Drug Abuse Screening Test; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; IQ, intelligence quotient.
The study was approved by the Local Ethics Committee and was carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). After a detailed description of the study, written informed consent was obtained from each participant.
Measures
Clinical Assessment.
The SCID I and II 5 were administered by an experienced psychiatrist trained to use these instruments. Symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS) 66 by 2 clinicians trained to use this instrument. The intraclass correlation for positive symptoms was .771 and for negative symptoms .823.
Aggressive Behavior.
A semistructured interview, the LHA, 67 assessed history of temper tantrums, verbal assaults, property assaults, physical fights, and assaults.
Substance Misuse.
The Michigan Alcohol Screening Test (MAST) 68 and the Drug Abuse Screening Test (DAST-20) 69 were completed by participants to provide scores for lifetime use of alcohol and illicit drugs.
Criminal Convictions.
Information on offending was extracted from official criminal records. Violent crimes were defined as parts 13, 16–18, and 20 of the German penal code; all other crimes were defined as nonviolent. In Germany, plea bargaining is rare.
MRI Data Acquisition
Brain images were acquired on a 1.5-T MRI system (Siemens Sonata, Erlangen, Germany) using a 3D T1-weighted sequence with the following parameters: repetition time = 1900ms; echo time = 3.93ms; inversion time = 800 ms; flip angle = 15°; 160 contiguous 1-mm sagittal slices; field of view = 240×240mm; 2 matrix size = 240×240; voxel size = 1.0×0.9×1.0mm.
Voxel-Based Morphometry
Data were processed and examined using the SPM8 software 70 and the voxel-based morphometry VBM8 toolbox 71 with default parameters. Images were bias corrected, tissue classified, and registered using linear (12-parameter affine) and nonlinear transformations (warping) within a unified model. 72 Subsequently, analyses were performed on GM segments, which were multiplied by the nonlinear components derived from the normalization matrix in order to preserve the actual GM values locally (modulated GM volumes). Importantly, the segments were not multiplied by the linear components of the registration in order to account for individual differences in brain orientation, alignment, and size globally. Finally, the modulated volumes were smoothed with a Gaussian kernel of 8mm full-width-at-half maximum.
Statistical Analyses
Chi-square tests, one-way analyses of variance, and post hoc Tukey tests were used to compare groups with reference to their sociodemographic, clinical, and forensic characteristics using SPSS version 19.0 software.
Voxel-wise GM differences between SZ+CD men and SZ–CD, CD, or H men and between H and the groups SZ–CD and CD were examined using independent sample t tests, controlling initially for age, then for age and MAST scores, and finally for age, MAST, and DAST scores. In order to avoid possible edge effects between different tissue types, we excluded all voxels with GM values less than 0.1 (absolute threshold masking). For all comparisons, a value of P < .05, false discovery rate (FDR) corrected for multiple comparison, was applied.
Results
Comparisons of Participants With SZ+CD, SZ–CD, CD, and Neither Schizophrenia Nor CD
As presented in table 1, the 2 groups of participants with schizophrenia were similar in terms of age of onset and duration of illness, scores for positive and negative psychotic symptoms, and dose of medication measured in chlorpromazine equivalent units. The 2 groups with CD were similar in the mean number of CD symptoms prior to age 15, scores for lifelong aggression, numbers of criminal convictions, and proportions with a diagnosis of ASPD. Although both the SZ+CD and the CD groups had higher DAST scores than both groups without CD, those with SZ+CD, but not those with CD, obtained significantly higher scores for lifetime alcohol use than either the H or SZ–CD participants. Diagnoses of past polysubstance dependence and alcohol use disorders were obtained by similar proportions of both groups with CD, significantly more than those among the participants without CD. Two of the participants with only CD and none of the other participants met the criteria for a drug use disorder in the previous year.
Global Brain Volume
As presented in table 2, the 4 groups did not differ with respect to total brain volumes. After adjusting for total brain volume, the groups were similar with reference to white matter volume and differed in their GM and CSF volumes. Post hoc Tukey tests revealed that participants with SZ–CD showed smaller GM volumes than the CD men and that both schizophrenia groups exhibited increased CSF volumes compared to both groups without schizophrenia.
Table 2.
Comparisons of Global Volume Measures (cm3) of the 4 Groups of Men With Schizophrenia and Conduct Disorder, Men With Schizophrenia and No Conduct Disorder, Men With Conduct Disorder, and Healthy Men
| Global Volume Measures | SZ+CD (n = 27) | Healthy (n = 25) | SZ–CD (n = 23) | CD (n = 27) | Group Comparisons (ANOVA) Bonferroni Adjusted (P < .003) | |
|---|---|---|---|---|---|---|
| Statistics | Post Hoc Tukey tests (Bonferroni) | |||||
| Mean total brain volume ± SD | 1436±111 | 1491±141 | 1502±103 | 1460±111 | F 3,98 = 1.7, P = .172 | NA |
| Mean total GM volume ± SDa | 598±30 | 614±44 | 594±41 | 621±29 | F 3,98 = 3.3, P = .023 | CD > SZ–CD |
| Mean total WM volume ± SDa | 628±25 | 635±38 | 629±32 | 630±24 | F 3,98 = 0.2, P = .882 | NA |
| Mean total CSF volume ± SDa | 243±20 | 220±28 | 246±32 | 217±26 | F 3,98 = 8.0, P < .001 | SZ+CD, SZ–CD > H, CD |
Note: Following abbreviations are explained in the footnote to table 1: SZ+CD, SZ–CD, CD, NA; GM, gray matter; WM, white matter; and CSF, cerebrospinal fluid.
aVolumes adjusted by brain size (ie, scaled by each individual’s total brain volume).
Whole-Brain Analyses
Table 3 presents the significant results of comparisons of GM volumes of the SZ+CD men and the healthy and SZ–CD men, initially without covariates, then covarying for age, covarying for age and MAST scores, and covarying for age, MAST, and DAST scores.
Table 3.
Comparisons of Regional GM Volumes of Men With Schizophrenia and Conduct Disorder as Compared to Healthy Men, Men With Schizophrenia and No Conduct Disorder, and Men With Conduct Disorder and No Schizophrenia
| Group Contrast (Brain Region) | BA | Side | Significant Group Differences With a Height Threshold of P < .05 FDR Corrected for Multiple Comparisons | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Covariates | |||||||||||||
| MNI Coordinates | None | Age | Age and MAST Scores | Age, MAST, and DAST Scores | |||||||||
| x | y | z | Cluster Size | z | Cluster Size | z | Cluster Size | z | Cluster Size | z | |||
| SZ+CD>H | |||||||||||||
| Hypothalamus | — | 7 | –3 | –8 | R | 280 | 4.34 | 444 | 4.99 | 441 | 5.06 | 605 | 5.67* |
| — | –8 | –1 | –5 | L | 178 | 4.12 | 313 | 4.25 | 254 | 4.17 | 355 | 4.13 | |
| Superior parietal | 7 | –15 | –44 | 65 | L | — | — | — | — | 103 | 4.18 | 121 | 4.18 |
| Cerebellum | — | 15 | –50 | –46 | R | 452 | 4.44 | 758 | 4.63 | 342 | 4.80 | 615 | 4.20 |
| SZ+CD < H | |||||||||||||
| Superior temporal/inferior frontal | 11,38,47 | –30 | 22 | –21 | L | 998 | 4.65 | 1067 | 4.50 | 660 | 4.05 | — | — |
| Middle/superior temporal | 21,22 | 68 | –42 | –1 | R | 910 | 5.04 | 960 | 4.83 | 183 | 4.01 | 179 | 4.14 |
| Inferior/middle temporal | 20 | 52 | 5 | –43 | R | — | — | — | — | 123 | 4.31 | — | — |
| Inferior temporal | 20 | 65 | –12 | –31 | R | — | — | — | — | 551 | 4.26 | — | — |
| Temporoparietal junction | 43 | 51 | –17 | 14 | R | — | — | — | — | 386 | 4.08 | 155 | 4.02 |
| Middle frontal | 11 | –41 | 51 | –16 | L | 121 | 4.31 | 119 | 4.02 | — | — | — | — |
| Superior frontal | 11 | 7 | 67 | –12 | R | 132 | 4.36 | 199 | 4.31 | 114 | 4.10 | — | — |
| 9,10 | 23 | 57 | 33 | R | 148 | 4.34 | 150 | 4.05 | — | — | — | — | |
| 9 | –30 | 47 | 37 | L | 255 | 5.25 | 255 | 4.98 | 180 | 4.45 | — | — | |
| 8 | 12 | 43 | 51 | R | 107 | 4.34 | 129 | 4.11 | 108 | 4.00 | — | — | |
| Inferior frontal | 47 | –37 | 19 | –10 | L | 107 | 4.12 | — | — | 292 | 4.06 | — | — |
| SZ+CD > SZ–CD | |||||||||||||
| Hypothalamus | — | –6 | –1 | –11 | L | 1037 | 5.15 | 985 | 5.05 | 444 | 4.45 | 430 | 4.34 |
| — | 6 | 0 | –10 | R | 606 | 5.33 | 622 | 5.46* | |||||
| Putamen | — | –25 | –5 | –8 | L | — | — | — | — | — | — | 159 | 4.15 |
| Cuneus/precuneus | 7,31 | –13 | –77 | 30 | L | 116 | 4.29 | 118 | 4.30 | 143 | 4.31 | 200 | 4.35 |
| — | –34 | –37 | 46 | L | 110 | 4.12 | — | — | — | — | — | — | |
| Inferior parietal | 40 | 43 | –50 | 48 | R | 61 | 3.92 | 155 | 4.25 | 206 | 4.20 | 128 | 3.79 |
| SZ+CD < SZ–CD | |||||||||||||
| Inferior frontal | 11,47 | –25 | 17 | –21 | L | 510 | 4.21 | 397 | 4.11 | — | — | — | — |
| SZ+CD > CD | |||||||||||||
| No region | — | — | — | — | — | — | — | — | — | — | — | — | — |
| SZ+CD < CD | |||||||||||||
| Superior temporal | 38 | 50 | 20 | –23 | R | 436 | 4.51 | 468 | 4.59 | 333 | 4.43 | 296 | 4.35 |
| Inferior temporal | 20 | 64 | –54 | –19 | R | 161 | 3.95 | 574 | 4.11 | 458 | 4.24 | 289 | 4.63 |
| Middle temporal | 21 | 69 | –41 | –15 | R | 178 | 4.12 | 188 | 4.15 | ||||
| Superior temporal/insula | 13,38 | –42 | 7 | –8 | L | 156 | 3.87 | 1264 | 4.48 | 1466 | 4.42 | 1196 | 4.34 |
| 13,22,38 | 48 | 8 | –1 | R | 247 | 4.30 | 449 | 4.26 | 912 | 4.54 | 756 | 4.49 | |
| Superior temporal | 21,22 | –57 | 10 | 1 | L | — | — | 148 | 4.19 | 278 | 4.28 | 225 | 4.20 |
| Middle/superior temporal | 21,22 | 65 | –56 | 8 | R | 463 | 4.84 | 568 | 4.91 | 322 | 4.69 | 248 | 4.26 |
| Temporoparietal junction | 19,39 | –53 | –72 | 18 | L | 484 | 4.71 | 582 | 4.99 | 442 | 4.82 | 402 | 4.83 |
| — | –59 | –59 | 12 | L | — | — | 237 | 4.16 | — | — | — | — | |
| 39,40 | 56 | –66 | 37 | R | 279 | 4.29 | 686 | 4.47 | 261 | 4.21 | 211 | 4.14 | |
| Middle/superior frontal | 10 | –31 | 64 | –4 | L | 110 | 4.18 | 314 | 4.75 | 315 | 4.80 | 289 | 4.77 |
| 10 | 45 | 55 | 3 | R | 140 | 4.16 | 173 | 4.24 | — | — | — | — | |
| 10,46 | –35 | 60 | 8 | L | 337 | 4.19 | 509 | 4.43 | 339 | 4.25 | 273 | 4.18 | |
| 9 | –28 | 48 | 37 | L | 163 | 4.44 | 314 | 4.64 | 332 | 4.68 | 315 | 4.64 | |
| Inferior/middle frontal | 9 | 60 | 10 | 32 | R | 120 | 4.30 | 144 | 4.40 | 117 | 4.26 | 114 | 4.24 |
| Inferior frontal | 11 | 23 | 36 | –22 | R | — | — | — | — | — | — | 111 | 4.05 |
| Postcentral | 2 | 50 | –31 | 57 | R | — | — | 109 | 4.23 | 136 | 4.29 | 121 | 4.24 |
| Thalamus (pulvinar) | 7 | –22 | 17 | R | 136 | 3.99 | 466 | 4.21 | 766 | 4.65 | 705 | 4.58 | |
| Uncus/parahippocampal | 28,34 | 14 | 5 | –23 | R | — | — | 483 | 4.57 | 308 | 4.35 | 278 | 4.28 |
| 28,34 | –18 | 2 | –19 | L | — | — | 437 | 4.19 | 349 | 4.18 | 303 | 4.11 | |
Note: BA, Brodmann area. Following abbreviations are explained in the footnote to table 1: SZ+CD, SZ–CD, MAST, and DAST; FDR, false discovery rate, MNI, Montreal Neurological Institute.
*Peak voxel significant at P < .05; corrected for multiple comparisons after Family Wise Error (FWE).
SZ+CD vs Healthy.
As compared to the healthy men, participants with SZ+CD exhibited increased GM volume in the hypothalamus, the right superior parietal cortex (BA7), and parts of the cerebellum. As compared to the healthy men, participants with SZ+CD exhibited reduced GM volumes of the inferior, medial, and superior temporal lobes (BA20, 21, 22, and 38), the temporoparietal junction (BA43), the bilateral inferior frontal operculum (BA47), and the OFC (BA11).
SZ+CD vs SZ–CD.
The SZ+CD participants, as compared to those with SZ–CD, exhibited greater GM volume in the right hypothalamus extending into the mammillary bodies, the putamen, the left cuneus/precuneus (BA7, 31), and the inferior parietal cortex (BA7). The SZ+CD participants exhibited no regions in which GM volumes were reduced in comparison to the SZ–CD participants.
SZ+CD vs CD.
Men with SZ+CD exhibited no regions in which GM volumes were increased as compared to the CD participants. The SZ+CD participants exhibited volume decreases relative to CD in the inferior, medial, and superior temporal lobes (BA20, 21, 22, and 38), the temporoparietal junction (BA19, 39, 40), the parahippocampal gyrus, the dorsolateral prefrontal cortex (BA9, 46), the frontopolar regions (BA10), and the thalamus.
SZ–CD vs Healthy.
As presented in table 4, the SZ–CD participants exhibited volume decreases relative to healthy men in the inferior, medial, and superior temporal lobes (BA20, 21, and 38), the parahippocampal gyrus (BA28), orbitofrontal and superior frontal cortices (BA9, 47), the insula (BA13) a,nd the thalamus—in particular, the mediodorsal and pulvinar nuclei.
Table 4.
Comparisons of Regional GM Volumes of Men With Schizophrenia and No Conduct Disorder and Healthy Men and Comparisons of Men With Conduct Disorder and Healthy Men
| Group Contrast (Brain Region) | BA | Side | Significant Group Differences With a Height Threshold of P < .05 FDR Corrected for Multiple Comparisons | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MNI Coordinates | None | Age | Age and MAST Scores | Age, MAST, and DAST Scores | |||||||||
| x | y | z | Cluster Size | z | Cluster Size | z | Cluster Size | z | Cluster Size | z | |||
| SZ–CD > H | |||||||||||||
| No region | — | — | — | — | — | — | — | — | — | — | — | — | — |
| SZ–CD < H | |||||||||||||
| Inferior temporal | 20 | –55 | –1 | –40 | L | 158 | 4.24 | 164 | 4.54 | 150 | 4.47 | 119 | 4.29 |
| Inferior/middle temporal | 20 | 55 | 2 | –40 | R | 225 | 3.89 | 218 | 4.09 | 183 | 4.04 | 172 | 4.05 |
| Parahippocampal/hippocampus | 28 | –25 | –16 | –20 | L | 884 | 4.95 | 611 | 4.76 | 741 | 4.83 | 602 | 4.69 |
| Superior temporal | 38 | –44 | 2 | –13 | L | 302 | 3.88 | 199 | 3.78 | 168 | 3.77 | — | — |
| Middle temporal | 21 | 62 | –6 | –11 | R | 175 | 3.93 | 134 | 3.97 | 118 | 3.97 | — | — |
| Inferior frontal | 47 | 35 | 22 | –13 | R | 147 | 3.83 | — | — | — | — | — | — |
| 47 | –38 | 20 | –7 | L | 144 | 3.79 | — | — | — | — | — | — | |
| Thalamus (mediodorsal, pulvinar) | — | 4 | –24 | 1 | R/L | 1588 | 4.16 | 1016 | 4.13 | 874 | 4.11 | 764 | 4.12 |
| Insula | 13 | 40 | 11 | 2 | R | 117 | 3.64 | — | — | — | — | — | — |
| Superior frontal | 9 | –31 | 48 | 37 | L | 266 | 4.94 | 233 | 5.05 | 217 | 5.02 | 180 | 4.86 |
| Precuneus/superior parietal | 7 | 5 | –73 | 50 | R | 341 | 4.10 | 222 | 4.05 | 211 | 4.06 | 166 | 3.96 |
| Superior parietal | 7 | 32 | –71 | 50 | R | 280 | 4.50 | 230 | 4.49 | 207 | 4.41 | 209 | 4.41 |
| CD > H | |||||||||||||
| Uncus/superior temporal (extending | 28,38 | –23 | 13 | –41 | L | 257 | 4.54 | 266 | 4.44 | 282 | 4.19 | 243 | 4.13 |
| into amygdala and hypothalamus | 28 | –25 | 4 | –23 | L | — | — | — | — | 475 | 4.39 | 454 | 4.28 |
| at the right hemisphere) | 28,34,38 | 13 | 5 | –26 | R | 248 | 4.10 | 412 | 4.35 | 1187 | 4.56 | 1739 | 4.68 |
| Caudate head | — | 17 | 19 | 9 | R | — | — | — | — | 523 | 4.09 | 632 | 3.95 |
| Superior temporal | 38 | 55 | 17 | –20 | R | 174 | 4.14 | 225 | 4.21 | 321 | 4.32 | 533 | 4.97 |
| Middle temporal | 39 | –56 | –73 | 19 | L | — | — | — | — | 169 | 4.28 | 471 | 4.48 |
| Temporoparietal junction | 39,40 | 58 | –62 | 33 | R | — | — | 112 | 4.12 | 392 | 4.34 | 982 | 4.93 |
| Inferior parietal | 40 | 67 | –33 | 26 | R | — | — | — | — | — | — | 722 | 4.24 |
| Inferior parietal/postcentral | 2,40 | 51 | –31 | 50 | R | — | — | — | — | — | — | 201 | 4.26 |
| Inferior parietal | 40 | –58 | –41 | 46 | L | — | — | — | — | 115 | 3.93 | 164 | 4.03 |
| Postcentral | 2 | –58 | –28 | 47 | L | — | — | — | — | — | — | 129 | 3.99 |
| Inferior/superior parietal/postcentral | 5,7 | –27 | –51 | 61 | L | — | — | — | — | 117 | 3.93 | 305 | 4.23 |
| Posterior cingulate | 31 | 3 | –25 | 42 | R | — | — | — | — | — | — | 218 | 3.94 |
| Precentral | 6 | 39 | –14 | 48 | R | — | — | — | — | — | — | 118 | 3.82 |
| CD < H | |||||||||||||
| Inferior frontal | 11 | –22 | 49 | –13 | L | 163 | 4.01 | 222 | 3.94 | — | — | — | — |
| 47 | 32 | 28 | –3 | R | 161 | 4.12 | 108 | 3.90 | — | — | — | — | |
Note: Following abbreviations are explained in the footnote to table 1: SZ+CD, SZ–CD, MAST, DAST, and MNI.
CD vs Healthy.
The CD participants exhibited volume increases, relative to healthy men, in a large cluster including the uncus and the superior temporal cortex (extending into the right amygdala and the right hypothalamus), the temporoparietal junction (BA39, 40), the inferior and superior parietal regions (BA2, 5, 7, 40), the posterior cingulate (BA31), and the pre- and postcentral gyri (BA2, 6).
Post Hoc Analyses
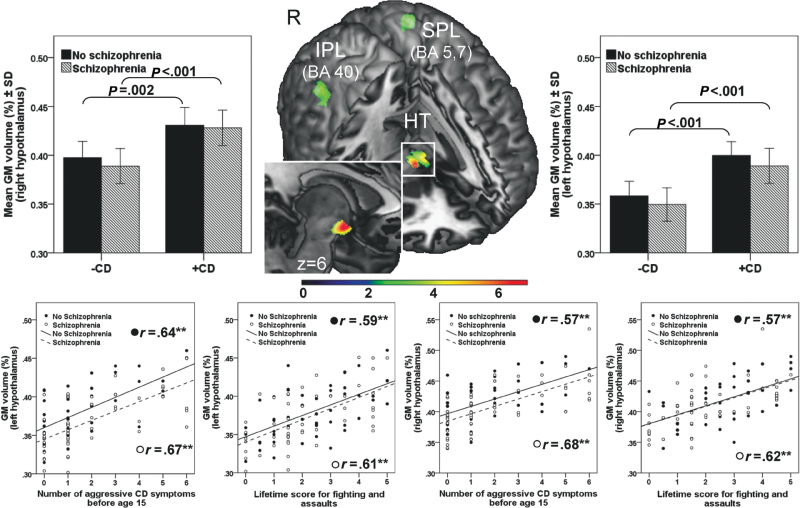
Conjunction Analysis. In order to identify GM abnormalities that were similar in men with SZ+CD and CD, a voxel-wise conjunction analysis, 73 corrected for multiple comparisons (FDR) with a value of P < 0.05, was conducted. The brain map provided in figure 1 illustrates all voxels where GM volumes of SZ+CD were greater than SZ–CD volumes and volumes of CD were greater than H volumes. The set of voxels that met these criteria were not associated with age, or the MAST or DAST scores and were located in the hypothalamus (Montreal Neurological Institute [MNI] coordinates: 6, 0, –10; z = 6.06; k = 699; and MNI: –5,–2,–1; z = 4.59; k = 175), the right inferior parietal cortex (BA40; MNI coordinates: 41, –48, 48; z = 4.02; k = 358), and the left superior parietal cortex (BA5,7; MNI coordinates: –29, –48, 61; z = 4.14; k = 431).
Fig. 1.
Gray matter volumes associated with conduct disorder and aggressive behavior. At the center of the top panel in the cut out view brain map, the foci of gray matter (GM) volumes that significantly distinguished the SZ+CD from the SZ–CD men and the CD from the H men, after controlling for age, MAST, and DAST scores, are presented. These sets of voxels are located in the hypothalamus (HT), the right inferior parietal cortex (IPL), and the left superior parietal cortex (SPL). Coordinates of clusters, as well as their sizes and peak z scores are provided in the text. In the top panel, to the left and right of the brain map, the bar charts illustrate the associations between GM volumes of the right and left hypothalamus, respectively, comparing (1) SZ+CD and SZ–CD and (2) CD and H. The bottom panel presents the correlations among GM volumes of the left and right hypothalamus, the number of aggressive CD symptoms prior to age 15, and the mean score for lifetime fighting and assaults.
Correlation Analysis.
A diagnosis of CD may be made in the absence of aggressive behavior. Given the finding of enlarged hypothalamic volumes among both SZ+CD and CD men, and animal studies indicating that the hypothalamus plays a prominent role in aggression, 74 we undertook post hoc analyses to determine whether hypothalamic volumes were specifically associated with aggressive behavior. Aggressive behavior was assessed by 2 items from the LHA (fighting and assaults), and the total number of aggressive CD symptoms (bullies, threatens, or intimidates others; initiates physical fights; used weapon; physical cruelty to people; physical cruelty to animals; stolen while confronting a victim). GM volumes of brain clusters that varied as a function of CD in the conjunction analysis were extracted by volume-of-interest analysis (first eigenvariate) and associations with measures of aggression were separately estimated for men with and without schizophrenia using Spearman’s rho rank correlation coefficient. As presented in figure 1 (bottom half), the GM volumes of the hypothalamus were significantly correlated with the scores for fighting and assault and the number of aggressive CD symptoms.
Discussion
SZ+CD men were characterized by a lifelong pattern of antisocial and aggressive behavior, substance misuse, and criminality. As compared to H, despite similar IQ scores, they displayed widespread abnormalities of GM volumes. Compared to SZ–CD men and men with CD, they showed both similarities and differences.
Relative to men with SZ–CD, those with SZ+CD displayed increased GM volumes in the hypothalamus, the left putamen, the right cuneus/precuneus, and the right inferior parietal cortex, after controlling for age and MAST/DAST scores; they also showed 1 region of decreased GM volumes. Yet, these 2 groups of men with schizophrenia were similar in terms of their levels of positive and negative symptoms at the time of testing, age of onset and duration of illness, and dose and class of antipsychotic medications.
Among those with CD, relative to H, increased GM volumes in a widespread network of temporoparietal and subcortical regions, including the inferior and superior parietal regions, the precuneus, the pre- and postcentral gyri, the posterior cingulate as well as temporal and dorsolateral prefrontal cortices, the left amygdala, the right caudate, and the right hypothalamus, were detected after controlling for age and MAST/DAST scores. Similar abnormalities have been previously identified among adult males displaying lifelong patterns of antisocial behavior. 34 , 37 To our knowledge, this is the first study to report abnormal GM volumes of the hypothalamus in antisocial males.
The SZ+CD men were similar to the men with CD in terms of an early onset and persistence of antisocial and aggressive behavior, substance misuse, criminality, and increased GM volumes of the hypothalamus, the right inferior parietal cortex, and the left superior parietal cortex. Spatial impairments are evident by age 3 among males whose antisocial and criminal behavior persists into adulthood, 75 structural abnormalities of the parietal lobes have been reported in boys with CD, 32 , 35 and functional abnormalities in male offenders with high psychopathy scores. 34 , 76 The right hemisphere is dominant in the first months and years of life, and atypical development has been hypothesized to impair the child’s recognition of mother’s facial expressions and consequent mother–child interactions. 77
Among both the SZ+CD and the CD men, GM volumes of the hypothalamus were positively associated with aggressive behavior prior to age 15 and with lifetime scores for fighting and assaults. Few studies of humans have reported hypothalamic abnormalities associated with aggression. Lower metabolism in the right hypothalamus was observed in violent men with high LHA scores 78 and children with gelastic seizures and hypothalamic hamartomas present elevated rates of CD. 79 In rodents and cats, stimulation of the hypothalamus leads to aggressive behavior. 74 , 80 The amygdala–hypothalamus–periaqueductal gray system within the midbrain is thought to mediate reactive aggression in response to a real or perceived threat. 81–83 This system is organized hierarchically such that aggression evoked by stimulation of the periaqueductal gray is not dependent on the functional integrity of the amygdala. 81–83 Moreover, fMRI studies indicate that in contrast to healthy individuals, people with schizophrenia, 84 even those with high psychopathy scores or ASPD, 49 show little activation in the amygdala in response to threatening faces, reduced connectivity from the amygdala to the precuneus and parietal regions, 85 and reduced activation in the OFC and basal regions during a Go/NoGo task. 48 Elevated scores for lifelong aggression, assessed as in the present study with the LHA, have been positively correlated with CSF arginine vasopressin 86 and negatively correlated with brain serotonin levels, 87 5-hydroxytryptamine transporter platelet-binding sites, 88 reduced CSF oxytocin, 89 and, as noted, with reduced metabolism in the right hypothalamus. 77 Thus, among men with SZ+CD and those with CD, aggressive behavior may be associated with dysfunction at the bottom of this neural circuit. 90 , 91 Although much evidence has accumulated to show that altered serotonergic functioning is associated with reactive aggressive behavior, impulsive antisocial temperament is associated with excess neurochemical and functional engagement of the mesolimbic dopamine system in response to reward. 92 Research in rodents suggests that mesolimbic dopamine is critical for the expression of aggression 93–95 and that genetic manipulations that reduce striatal dopamine clearance increase aggressive behavior. 95 Increased striatal dopamine synthesis capacity predates the onset of schizophrenia. 96 Thus, abnormalities of dopamine dysfunction in the striatum are associated with both psychosis and impulsive antisocial behavior, and future research is needed to determine whether they are linked to structural abnormalities.
The SZ+CD participants, but not the CD participants, displayed larger cerebellar volumes than the healthy men. Similar increases have been reported in boys with CD 35 and male offenders with ASPD. 97 Because antipsychotic medications are associated with reduced cerebellar volumes, 98 the increased volumes observed among the SZ+CD men may have been even greater prior to treatment. The cerebellum develops late and is particularly vulnerable to environmental insults. 99 , 100
The participants with SZ+CD and CD also exhibited reductions in GM volumes in specific regions, with both groups showing reductions in the inferior frontal regions, including the OFC, relative to the healthy men. 32 , 34 , 97 , 101 , 102 Abnormalities of GM volumes, both increases and decreases, have been reported among children and adolescents with CD 32 , 33 , 35 , 36 , 103–105 and among adults with ASPD, 106 , 107 high psychopathy scores, 34 , 97 , 102 , 108–110 and histories of criminal offense. 59 The results however, are inconsistent. This may be due to the heterogeneity of samples with respect to anxiety disorders that characterize approximately half of male offenders with ASPD, 111 a large proportion of children with CD, 53 and proportions who meet criteria for the syndrome of psychopathy. 34 , 111
Although this was a cross-sectional study of adult men, the results may be best understood in a developmental context. Aggressive behavior is as stable across the life span as IQ. 112–114 Aggressive behavior prior to age 15 was associated with GM abnormalities among the men with SZ+CD. Some of these alterations may have occurred very early in life, leading to further abnormalities in higher structures, while others resulted from subsequent brain insults. Early alterations to the hypothalamus would affect the development of pituitary–adrenal functioning, as well as connections with the limbic system and related cortical regions. Maltreatment in childhood is associated with high scores on the LHA in adulthood, 115 with CD, 116 and with schizophrenia. 117 The effect of maltreatment and other forms of pre- and postnatal stress on the brain vary by genetic vulnerability, timing and duration of the trauma, and the presence of protective factors such as secure attachment to parents. 118 , 119 Consequently, although abnormality in the hippocampi are adult markers of maltreatment in childhood, 120 the absence of such abnormalities among the men with SZ+CD may simply reflect the moderating effects of all, or some, of these factors.
Limitations and Strengths of the Present Study
Among people with schizophrenia, GM volumes of various structures are modified by antipsychotic medications, illness duration and severity, 98 cognitive treatment, 121 exercise, 122 and stress. 123 In the present study, no information was available on the latter 3 factors. Although the use of alcohol and most, but not all, illicit drugs is associated with reductions of GM volumes, 124 the SZ+CD men exhibited increased GM volume in regions associated with aggressive behavior. There was no evidence that the group differences were associated with substance misuse, consistent with a longitudinal study of patients with schizophrenia, which reported that substance misuse was associated with increased CSF and decreased cerebellar volume, but not with GM cortical volumes, after controlling for follow-up duration, illness severity, and lifetime antipsychotic treatment. 98 The larger putamen among the SZ+CD men, as compared to SZ–CD men, could be due to antipsychotic medications, as could many of the reductions in GM volume observed in participants with schizophrenia as compared to healthy men. 98 If the SZ+CD and SZ–CD groups are genetically distinct, all of these environmental factors may affect their brains differently. Another limitation of the present study was the retrospective measure of CD. However, CD was assessed using a protocol developed for use with adults to describe behaviors in childhood. Strengths of the study include the relatively large sample. The SZ+CD SZ–CD participants were closely matched with reference to the age of onset and duration of illness, and dose and type of medication. The SZ+CD participants were also matched to the CD participants on the number of CD symptoms, proportions who presented ASPD, and scores for lifelong aggressive behavior. The MRI images were of high resolution, optimized for discerning morphological anomalies, and were analyzed using a fully automated, whole-brain technique.
Conclusions
Men with schizophrenia and a prior history of CD presented alterations in GM volumes distinct from other men with schizophrenia and similar to those observed among non–mentally ill men with CD. Men with SZ+CD present a challenge to clinical services. Identifying the specific neurobiological mechanisms that underlie this type of schizophrenia would allow for the development of treatments that specifically target these mechanisms.
Funding
Landschaftsverband Rheinland, Germany to Dr Schiffer.
Supplementary Material
Acknowledgments
Christina Pawliczek and Alexander Wormit assisted with data collection and analyses. The Authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Robins LN. Deviant Children Grown Up. Baltimore, MD: Williams & Williams; 1966 [Google Scholar]
- 2. Robins LN. Childhood conduct problems, adult psychopathology, and crime. In: Hodgins S, ed. Mental Disorder and Crime. Newbury Park, CA: Sage Publications Inc; 1993:173–207 [Google Scholar]
- 3. Robins LN, Tipp J, Przybeck T. Antisocial personality. In: Robins LN, Regier D, eds. Psychiatric Disorders in America: The Epidemiologic Catchment Area Study. New York, NY: Macmillan/Free Press; 1991:258–290 [Google Scholar]
- 4. Kim-Cohen J, Caspi A, Moffitt TE, Harrington H, Milne BJ, Poulton R. Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Arch Gen Psychiatry. 2003;60:709–717 [DOI] [PubMed] [Google Scholar]
- 5. First MB, Gibbon M, Spitzer RL, Williams JBW. User’s Guide for the Structured Clinical Interview for DSM-IV Axis 1 Disorders. Washington, DC: American Psychiatric Press, Inc; 1996 [Google Scholar]
- 6. Hodgins S. Violent behaviour among people with schizophrenia: a framework for investigations of causes, and effective treatment, and prevention. Philos Trans R Soc Lond, B, Biol Sci. 2008;363:2505–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hodgins S, Cree A, Alderton J, Mak T. From conduct disorder to severe mental illness: associations with aggressive behaviour, crime and victimization. Psychol Med. 2008;38:975–987 [DOI] [PubMed] [Google Scholar]
- 8. Jones RM, Van den Bree M, Ferriter M, Taylor PJ. Childhood risk factors for offending before first psychiatric admission for people with schizophrenia: a case-control study of high security hospital admissions. Behav Sci Law. 2010;28:351–365 [DOI] [PubMed] [Google Scholar]
- 9. Rice ME, Harris GT. Psychopathy, schizophrenia, alcohol abuse, and violent recidivism. Int J Law Psychiatry. 1995;18:333–342 [DOI] [PubMed] [Google Scholar]
- 10. Fulwiler C, Ruthazer R. Premorbid risk factors for violence in adult mental illness. Compr Psychiatry. 1999;40:96–100 [DOI] [PubMed] [Google Scholar]
- 11. Tengström A, Hodgins S, Grann M, Långström N, Kullgren G. Schizophrenia and criminal offending: the role of psychopathy and substance use disorders. Crim Justice Behav. 2004;31:367–391 [Google Scholar]
- 12. Swanson JW, Swartz MS, Van Dorn RA, et al. A national study of violent behavior in persons with schizophrenia. Arch Gen Psychiatry. 2006;63:490–499 [DOI] [PubMed] [Google Scholar]
- 13. Crocker AG, Mueser KT, Drake RE, et al. Antisocial personality, psychopathy, and violence in persons with dual disorders: a longitudinal analysis. Crim Justice Behav. 2005;32:452–476 [Google Scholar]
- 14. Mueser KT, Crocker AG, Frisman LB, Drake RE, Covell NH, Essock SM. Conduct disorder and antisocial personality disorder in persons with severe psychiatric and substance use disorders. Schizophr Bull. 2006;32:626–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hodgins S. Criminal and antisocial behaviours and schizophrenia: a neglected topic. In: Gattaz WF, Häfner H, eds. Search for the Causes of Schizophrenia. Vol 5 Darmstadt, Germany: Steinkopff Verlag; 2004:315–341 [Google Scholar]
- 16. Hodgins S, Côté G. Major mental disorder and antisocial personality disorder: a criminal combination. Bull Am Acad Psychiatry Law. 1993;21:155–160 [PubMed] [Google Scholar]
- 17. Schug RA, Raine A, Wilcox RR. Psychophysiological and behavioural characteristics of individuals comorbid for antisocial personality disorder and schizophrenia-spectrum personality disorder. Br J Psychiatry. 2007;191:408–414 [DOI] [PubMed] [Google Scholar]
- 18. Farrington DP, West DJ. Criminal, penal and life histories of chronic offenders: risk and protective factors and early identification. Crim Behav Ment Health. 1993;3:492–523 [Google Scholar]
- 19. Kratzer L, Hodgins S. A typology of offenders: a test of Moffitt’s theory among males and females from childhood to age 30. Crim Behav Ment Health. 1999;9:57–73 [Google Scholar]
- 20. Moffitt TE, Caspi A, Harrington H, Milne BJ. Males on the life-course-persistent and adolescence-limited antisocial pathways: follow-up at age 26 years. Dev Psychopathol. 2002;14:179–207 [DOI] [PubMed] [Google Scholar]
- 21. Hodgins S, Hiscoke UL, Freese R. The antecedents of aggressive behavior among men with schizophrenia: a prospective investigation of patients in community treatment. Behav Sci Law. 2003;21:523–546 [DOI] [PubMed] [Google Scholar]
- 22. Moran P, Hodgins S. The correlates of comorbid antisocial personality disorder in schizophrenia. Schizophr Bull. 2004;30:791–802 [DOI] [PubMed] [Google Scholar]
- 23. Arseneault L, Cannon M, Murray R, Poulton R, Caspi A, Moffitt TE. Childhood origins of violent behaviour in adults with schizophreniform disorder. Br J Psychiatry. 2003;183:520–525 [DOI] [PubMed] [Google Scholar]
- 24. Hodgins S. ed. The etiology and development of offending among persons with major mental disorders: conceptual and methodological issues and some preliminary findings. In: Violence Among the Mentally Ill: Effective Treatments and Management Strategies. Dordrecht, the Netherlands: Kluwer Academic Publishers; 2000:89–116 [Google Scholar]
- 25. Schanda H, Földes P, Topitz A, Fliedl R, Knecht G. Premorbid adjustment of schizophrenic criminal offenders. Acta Psychiatr Scand. 1992;86:121–126 [DOI] [PubMed] [Google Scholar]
- 26. Fulwiler C, Grossman H, Forbes C, Ruthazer R. Early-onset substance abuse and community violence by outpatients with chronic mental illness. Psychiatr Serv. 1997;48:1181–1185 [DOI] [PubMed] [Google Scholar]
- 27. Tengström A, Hodgins S, Kullgren G. Men with schizophrenia who behave violently: the usefulness of an early- versus late-start offender typology. Schizophr Bull. 2001;27:205–218 [DOI] [PubMed] [Google Scholar]
- 28. Mueser KT, Rosenberg SD, Drake RE, et al. Conduct disorder, antisocial personality disorder and substance use disorders in schizophrenia and major affective disorders. J Stud Alcohol. 1999;60:278–284 [DOI] [PubMed] [Google Scholar]
- 29. Blair RJ. Neuroimaging of psychopathy and antisocial behavior: a targeted review. Curr Psychiatry Rep. 2010;12:76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Arch Gen Psychiatry. 2000;57:119–27; discussion 128 [DOI] [PubMed] [Google Scholar]
- 31. Yang Y, Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Volume reduction in prefrontal gray matter in unsuccessful criminal psychopaths. Biol Psychiatry. 2005;57:1103–1108 [DOI] [PubMed] [Google Scholar]
- 32. Hyatt CJ, Haney-Caron E, Stevens MC. Cortical thickness and folding deficits in conduct-disordered adolescents. Biol Psychiatry. December 29, 2011;. 10.1016/j.biopsych.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sterzer P, Stadler C. Neuroimaging of aggressive and violent behaviour in children and adolescents. Front Behav Neurosci. 2009;3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koenigs M, Baskin-Sommers A, Zeier J, Newman JP. Investigating the neural correlates of psychopathy: a critical review. Mol Psychiatry. 2011;16:792–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Brito SA, Mechelli A, Wilke M, et al. Size matters: increased grey matter in boys with conduct problems and callous-unemotional traits. Brain. 2009;132:843–852 [DOI] [PubMed] [Google Scholar]
- 36. Fairchild G, Passamonti L, Hurford G, et al. Brain structure abnormalities in early-onset and adolescent-onset conduct disorder. Am J Psychiatry. 2011;168:624–633 [DOI] [PubMed] [Google Scholar]
- 37. Nordstrom BR, Gao Y, Glenn AL, et al. Neurocriminology. Adv Genet. 2011;75:255–283 [DOI] [PubMed] [Google Scholar]
- 38. Naudts K, Hodgins S. Neurobiological correlates of violent behavior among persons with schizophrenia. Schizophr Bull. 2006;32:562–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hoptman MJ, Antonius D. Neuroimaging correlates of aggression in schizophrenia: an update. Curr Opin Psychiatry. 2011;24:100–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Barkataki I, Kumari V, Das M, Taylor P, Sharma T. Volumetric structural brain abnormalities in men with schizophrenia or antisocial personality disorder. Behav Brain Res. 2006;169:239–247 [DOI] [PubMed] [Google Scholar]
- 41. Narayan VM, Narr KL, Kumari V, et al. Regional cortical thinning in subjects with violent antisocial personality disorder or schizophrenia. Am J Psychiatry. 2007;164:1418–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schiffer B, Müller BW, Scherbaum N, et al. Impulsivity-related brain volume deficits in schizophrenia-addiction comorbidity. Brain. 2010;133:3093–3103 [DOI] [PubMed] [Google Scholar]
- 43. Yang Y, Raine A, Han CB, Schug RA, Toga AW, Narr KL. Reduced hippocampal and parahippocampal volumes in murderers with schizophrenia. Psychiatry Res. 2010;182:9–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lapierre D, Braun CM, Hodgins S. Ventral frontal deficits in psychopathy: neuropsychological test findings. Neuropsychologia. 1995;33:139–151 [DOI] [PubMed] [Google Scholar]
- 45. Rasmussen K, Levander S, Sletvold H. Aggressive and non-aggressive schizophrenics: symptom profile and neuropsychological differences. Psychol Crime Law. 1995;2:119–129 [Google Scholar]
- 46. Schug RA, Raine A. Comparative meta-analyses of neuropsychological functioning in antisocial schizophrenic persons. Clin Psychol Rev. 2009;29:230–242 [DOI] [PubMed] [Google Scholar]
- 47. Narr KL, Hageman N, Woods RP, et al. Mean diffusivity: a biomarker for CSF-related disease and genetic liability effects in schizophrenia. Psychiatry Res. 2009;171:20–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Joyal CC, Putkonen A, Mancini-Marïe A, et al. Violent persons with schizophrenia and comorbid disorders: a functional magnetic resonance imaging study. Schizophr Res. 2007;91:97–102 [DOI] [PubMed] [Google Scholar]
- 49. Dolan MC, Fullam RS. Psychopathy and functional magnetic resonance imaging blood oxygenation level-dependent responses to emotional faces in violent patients with schizophrenia. Biol Psychiatry. 2009;66:570–577 [DOI] [PubMed] [Google Scholar]
- 50. Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophr Res. 2008;102:1–18 [DOI] [PubMed] [Google Scholar]
- 51. Volavka J, Citrome L. Heterogeneity of violence in schizophrenia and implications for long-term treatment. Int J Clin Pract. 2008;62:1237–1245 [DOI] [PubMed] [Google Scholar]
- 52. Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669 [DOI] [PubMed] [Google Scholar]
- 53. Stadler C, Poustka F, Sterzer P. The heterogeneity of disruptive behavior disorders - implications for neurobiological research and treatment. Front Psychiatry. 2010;1:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Blair RJ, Peschardt KS, Budhani S, Mitchell DG, Pine DS. The development of psychopathy. J Child Psychol Psychiatry. 2006;47:262–276 [DOI] [PubMed] [Google Scholar]
- 55. Robins LN, McEvoy L. Conduct problems as predictors of substance abuse. In: Robins LN, Rutter M, eds. Straight and Devious Pathways From Childhood to Adulthood. Cambridge, United Kingdom: Cambridge University Press; 1990:182–204 [Google Scholar]
- 56. Kessler RC, Nelson CB, McGonagle KA, Edlund MJ, Frank RG, Leaf PJ. The epidemiology of co-occurring addictive and mental disorders: implications for prevention and service utilization. Am J Orthopsychiatry. 1996;66:17–31 [DOI] [PubMed] [Google Scholar]
- 57. Compton WM, Conway KP, Stinson FS, Colliver JD, Grant BF. Prevalence, correlates, and comorbidity of DSM-IV antisocial personality syndromes and alcohol and specific drug use disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry. 2005;66:677–685 [DOI] [PubMed] [Google Scholar]
- 58. Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: modeling the externalizing spectrum. J Abnorm Psychol. 2002;111:411–424 [PubMed] [Google Scholar]
- 59. Schiffer B, Müller BW, Scherbaum N, et al. Disentangling structural brain alterations associated with violent behavior from those associated with substance use disorders. Arch Gen Psychiatry. 2011;68:1039–1049 [DOI] [PubMed] [Google Scholar]
- 60. Arseneault L, Cannon M, Witton J, Murray RM. Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry. 2004;184:110–117 [DOI] [PubMed] [Google Scholar]
- 61. McGrath J, Welham J, Scott J, et al. Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch Gen Psychiatry. 2010;67:440–447 [DOI] [PubMed] [Google Scholar]
- 62. Shapiro GK, Buckley-Hunter L. What every adolescent needs to know: cannabis can cause psychosis. J Psychosom Res. 2010;69:533–539 [DOI] [PubMed] [Google Scholar]
- 63. Malone DT, Hill MN, Rubino T. Adolescent cannabis use and psychosis: epidemiology and neurodevelopmental models. Br J Pharmacol. 2010;160:511–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Malcolm CP, Picchioni MM, DiForti M, et al. Pre-morbid Conduct Disorder symptoms are associated with cannabis use among individuals with a first episode of psychosis. Schizophr Res. 2011;126:81–86 [DOI] [PubMed] [Google Scholar]
- 65. Lehrl S, Triebig G, Fischer B. Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol Scand. 1995;91:335–345 [DOI] [PubMed] [Google Scholar]
- 66. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276 [DOI] [PubMed] [Google Scholar]
- 67. Coccaro EF, Berman ME, Kavoussi RJ. Assessment of life history of aggression: development and psychometric characteristics. Psychiatry Res. 1997;73:147–157 [DOI] [PubMed] [Google Scholar]
- 68. Selzer ML. The Michigan alcoholism screening test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–1658 [DOI] [PubMed] [Google Scholar]
- 69. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363–371 [DOI] [PubMed] [Google Scholar]
- 70. Wellcome Department of Imaging Neuroscience SPM8http://www.fil.ion.ucl.ac.uk/spm Accessed January 17, 2012.
- 71. Structural Brain Mapping Group. VBM8 Toolbox. http://dbm.neuro.uni-jena.de/vbm/ Accessed January 17, 2012. [Google Scholar]
- 72. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851 [DOI] [PubMed] [Google Scholar]
- 73. Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660 [DOI] [PubMed] [Google Scholar]
- 74. Lin D, Boyle MP, Dollar P, et al. Functional identification of an aggression locus in the mouse hypothalamus. Nature. 2011;470:221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Raine A, Moffitt TE, Caspi A, Loeber R, Stouthamer-Loeber M, Lynam D. Neurocognitive impairments in boys on the life-course persistent antisocial path. J Abnorm Psychol. 2005;114:38–49 [DOI] [PubMed] [Google Scholar]
- 76. Birbaumer N, Veit R, Lotze M, et al. Deficient fear conditioning in psychopathy: a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2005;62:799–805 [DOI] [PubMed] [Google Scholar]
- 77. Raine A, Yaralian PS, Reynolds C, Venables PH, Mednick SA. Spatial but not verbal cognitive deficits at age 3 years in persistently antisocial individuals. Dev Psychopathol. 2002;14:25–44 [DOI] [PubMed] [Google Scholar]
- 78. George DT, Rawlings RR, Williams WA, et al. A select group of perpetrators of domestic violence: evidence of decreased metabolism in the right hypothalamus and reduced relationships between cortical/subcortical brain structures in position emission tomography. Psychiatry Res. 2004;130:11–25 [DOI] [PubMed] [Google Scholar]
- 79. Weissenberger AA, Dell ML, Liow K, et al. Aggression and psychiatric comorbidity in children with hypothalamic hamartomas and their unaffected siblings. J Am Acad Child Adolesc Psychiatry. 2001;40:696–703 [DOI] [PubMed] [Google Scholar]
- 80. Herman JP, Flak J, Jankord R. Chronic stress plasticity in the hypothalamic paraventricular nucleus. Prog Brain Res. 2008;170:353–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Panksepp J. Affective Neuroscience: The Foundations of Human and Animal Emotions. New York, NY: Oxford University Press; 1998 [Google Scholar]
- 82. Gregg TR, Siegel A. Brain structures and neurotransmitters regulating aggression in cats: implications for human aggression. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:91–140 [DOI] [PubMed] [Google Scholar]
- 83. Blair RJR. Considering anger from a cognitive neuroscience perspective. WIREs Cogn Sci. 2012;3:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gur RE, Loughead J, Kohler CG, et al. Limbic activation associated with misidentification of fearful faces and flat affect in schizophrenia. Arch Gen Psychiatry. 2007;64:1356–1366 [DOI] [PubMed] [Google Scholar]
- 85. Mukherjee P, Whalley HC, McKirdy JW, et al. Lower effective connectivity between amygdala and parietal regions in response to fearful faces in schizophrenia. Schizophr Res. 2012;134:118–124 [DOI] [PubMed] [Google Scholar]
- 86. Coccaro EF, Kavoussi RJ, Hauger RL, Cooper TB, Ferris CF. Cerebrospinal fluid vasopressin levels: correlates with aggression and serotonin function in personality-disordered subjects. Arch Gen Psychiatry. 1998;55:708–714 [DOI] [PubMed] [Google Scholar]
- 87. Coccaro EF, Sripada CS, Yanowitch RN, Phan KL. Corticolimbic function in impulsive aggressive behavior. Biol Psychiatry. 2011;69:1153–1159 [DOI] [PubMed] [Google Scholar]
- 88. Coccaro EF, Lee R, Kavoussi RJ. Inverse relationship between numbers of 5-HT transporter binding sites and life history of aggression and intermittent explosive disorder. J Psychiatr Res. 2010;44:137–142 [DOI] [PubMed] [Google Scholar]
- 89. Lee R, Ferris C, Van de Kar LD, Coccaro EF. Cerebrospinal fluid oxytocin, life history of aggression, and personality disorder. Psychoneuroendocrinology. 2009;34:1567–1573 [DOI] [PubMed] [Google Scholar]
- 90. Blair RJ. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain Cogn. 2004;55:198–208 [DOI] [PubMed] [Google Scholar]
- 91. Lotze M, Veit R, Anders S, Birbaumer N. Evidence for a different role of the ventral and dorsal medial prefrontal cortex for social reactive aggression: an interactive fMRI study. Neuroimage. 2007;34:470–478 [DOI] [PubMed] [Google Scholar]
- 92. Buckholtz JW, Treadway MT, Cowan RL, et al. Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci. 2010;13:419–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Lewis MH, Gariépy JL, Gendreau P, Nichols DE, Mailman RB. Social reactivity and D1 dopamine receptors: studies in mice selectively bred for high and low levels of aggression. Neuropsychopharmacology. 1994;10:115–122 [DOI] [PubMed] [Google Scholar]
- 94. Couppis MH, Kennedy CH. The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacology (Berl). 2008;197:449–456 [DOI] [PubMed] [Google Scholar]
- 95. Rodriguiz RM, Chu R, Caron MG, Wetsel WC. Aberrant responses in social interaction of dopamine transporter knockout mice. Behav Brain Res. 2004;148:185–198 [DOI] [PubMed] [Google Scholar]
- 96. Howes OD, Bose SK, Turkheimer F, et al. Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am J Psychiatry. 2011;11:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tiihonen J, Rossi R, Laakso MP, et al. Brain anatomy of persistent violent offenders: more rather than less. Psychiatry Res. 2008;163:201–212 [DOI] [PubMed] [Google Scholar]
- 98. Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68:128–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. Neuroimage. 2010;49:63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. ten Donkelaar HJ, Lammens M, Wesseling P, Thijssen HO, Renier WO. Development and developmental disorders of the human cerebellum. J Neurol. 2003;250:1025–1036 [DOI] [PubMed] [Google Scholar]
- 101. de Oliveira-Souza R, Hare RD, Bramati IE, et al. Psychopathy as a disorder of the moral brain: fronto-temporo-limbic grey matter reductions demonstrated by voxel-based morphometry. Neuroimage. 2008;40:1202–1213 [DOI] [PubMed] [Google Scholar]
- 102. Boccardi M, Frisoni GB, Hare RD, et al. Cortex and amygdala morphology in psychopathy. Psychiatry Res. 2011;193:85–92 [DOI] [PubMed] [Google Scholar]
- 103. Huebner T, Vloet TD, Marx I, et al. Morphometric brain abnormalities in boys with conduct disorder. J Am Acad Child Adolesc Psychiatry. 2008;47:540–547 [DOI] [PubMed] [Google Scholar]
- 104. Kruesi MJ, Casanova MF, Mannheim G, Johnson-Bilder A. Reduced temporal lobe volume in early onset conduct disorder. Psychiatry Res. 2004;132:1–11 [DOI] [PubMed] [Google Scholar]
- 105. Fahim C, He Y, Yoon U, Chen J, Evans A, Pérusse D. Neuroanatomy of childhood disruptive behavior disorders. Aggress Behav. 2011;37:326–337 [DOI] [PubMed] [Google Scholar]
- 106. Yang Y, Raine A, Narr KL, Colletti P, Toga AW. Localization of deformations within the amygdala in individuals with psychopathy. Arch Gen Psychiatry. 2009;66:986–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ermer E, Cope LM, Nyalakanti PK, Calhoun VD, Kiehl KA. Aberrant paralimbic gray matter in criminal psychopathy. J Abnorm Psychol. December 12, 2011;. 10.1037/a0026371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Glenn AL, Raine A, Yaralian PS, Yang Y. Increased volume of the striatum in psychopathic individuals. Biol Psychiatry. 2010;67:52–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Glenn AL, Yang Y, Raine A, Colletti P. No volumetric differences in the anterior cingulate of psychopathic individuals. Psychiatry Res. 2010;183:140–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Yang Y, Raine A, Colletti P, Toga AW, Narr KL. Abnormal temporal and prefrontal cortical gray matter thinning in psychopaths. Mol Psychiatry. 2009;14:561–2, 555 [DOI] [PubMed] [Google Scholar]
- 111. Hodgins S, de Brito S, Simonoff E, Vloet T, Viding E. Getting the phenotypes right: an essential ingredient for understanding aetiological mechanisms underlying persistent violence and developing effective treatments. Front Behav Neurosci. 2009;3:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Olweus D. Stability of aggressive reaction patterns in males: a review. Psychol Bull. 1979;86:852–875 [PubMed] [Google Scholar]
- 113. Coccaro EF, Lawrence T, Trestman R, Gabriel S, Klar HM, Siever LJ. Growth hormone responses to intravenous clonidine challenge correlate with behavioral irritability in psychiatric patients and healthy volunteers. Psychiatry Res. 1991;39:129–139 [DOI] [PubMed] [Google Scholar]
- 114. Broidy LM, Nagin DS, Tremblay RE, et al. Developmental trajectories of childhood disruptive behaviors and adolescent delinquency: a six-site, cross-national study. Dev Psychol. 2003;39:222–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Chen P, Coccaro EF, Lee R, Jacobson KC. Moderating effects of childhood maltreatment on associations between social information processing and adult aggression. Psychol Med. 2011;19:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Hofvander B, Ståhlberg O, Nydén A, et al. Life History of Aggression scores are predicted by childhood hyperactivity, conduct disorder, adult substance abuse, and low cooperativeness in adult psychiatric patients. Psychiatry Res. 2011;185:280–285 [DOI] [PubMed] [Google Scholar]
- 117. Heins M, Simons C, Lataster T, et al. Childhood trauma and psychosis: a case-control and case-sibling comparison across different levels of genetic liability, psychopathology, and type of trauma. Am J Psychiatry. 2011;168:1286–1294 [DOI] [PubMed] [Google Scholar]
- 118. Sánchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev Psychopathol. 2001;13:419–449 [DOI] [PubMed] [Google Scholar]
- 119. Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–445 [DOI] [PubMed] [Google Scholar]
- 120. McCrory E, De Brito SA, Viding E. Research review: the neurobiology and genetics of maltreatment and adversity. J Child Psychol Psychiatry. 2010;51:1079–1095 [DOI] [PubMed] [Google Scholar]
- 121. Kumari V, Fannon D, Peters ER, et al. Neural changes following cognitive behaviour therapy for psychosis: a longitudinal study. Brain. 2011;134:2396–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Pajonk FG, Wobrock T, Gruber O, et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67:133–143 [DOI] [PubMed] [Google Scholar]
- 123. Mondelli V, Dazzan P, Hepgul N, et al. Abnormal cortisol levels during the day and cortisol awakening response in first-episode psychosis: the role of stress and of antipsychotic treatment. Schizophr Res. 2010;116:234–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Lingford-Hughes AR, Davies SJ, McIver S, Williams TM, Daglish MR, Nutt DJ. Addiction. Br Med Bull. 2003;65:209–222 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.