Abstract
Poor adherence to medication leads to symptom exacerbation and interferes with the recovery process for patients with schizophrenia. Following baseline assessment, 142 patients in medication maintenance at a community mental health center were randomized to one of 3 treatments for 9 months: (1) PharmCAT, supports including pill containers, signs, alarms, checklists and the organization of belongings established in weekly home visits from a PharmCAT therapist; (2) Med-eMonitor (MM), an electronic medication monitor that prompts use of medication, cues the taking of medication, warns patients when they are taking the wrong medication or taking it at the wrong time, record complaints, and, through modem hookup, alerts treatment staff of failures to take medication as prescribed; (3) Treatment as Usual (TAU). All patients received the Med-eMonitor device to record medication adherence. The device was programmed for intervention only in the MM group. Data on symptoms, global functioning, and contact with emergency services and police were obtained every 3 months. Repeated measures analyses of variance for mixed models indicated that adherence to medication was significantly better in both active conditions than in TAU (both p<0.0001). Adherence in active treatments ranged from 90–92% compared to 73% in TAU based on electronic monitoring. In-person and electronic interventions significantly improved adherence to medication, but that did not translate to improved clinical outcomes. Implications for treatment and health care costs are discussed.
Key words: medication adherence, medication compliance, cognitive adaptation, training, environmental supports, electronic adherence intervention, smart, pill containers
Introduction
Adherence to medication treatments is poor across a wide range of physical and psychiatric disorders.1,2 In over 35 double-blind trials that have included more than 3500 patients, the role of antipsychotic medications in preventing relapse and rehospitalization in schizophrenia has been firmly established.3–6 Approximately 50% of patients with schizophrenia do not take medications as prescribed.1,2,7,8
Adherence problems complicate the treatment for prescribers as well. Psychiatrists may have difficulty distinguishing between poor response to medication and poor adherence. Providers may unnecessarily or prematurely discontinue medications, increase doses, and add concomitant medications.9 After a number of failed trials on different medications, providers may begin to consider (nonadherent) individuals to be resistant to treatment.9
While adherence problems are often multidetermined, prominent among reasons for poor adherence are forgetfulness and failing to establish routines that promote adherence.1,8–11 Cognitive Adaptation Training (CAT; a psychosocial treatment using environmental supports such as signs, labels, pill containers, and checklists to cue and sequence adaptive behavior in the community, including taking medication) and its counterpart PharmCAT (which only uses supports targeting adherence) have been found to improve adherence to oral medication and to reduce rates of relapse in comparison to standard treatment for individuals with schizophrenia.10 While PharmCAT has been shown to be effective in improving both adherence and community tenure, the intervention requires that customized supports for cueing adherence to medication and attendance at medication appointments be established in the home based upon assessments of cognition, behavior, and the environment. Supports are maintained or altered on weekly home visits. While we have successfully implemented CAT techniques in community mental health centers in the United States and Canada, technology has advanced to the point that much of what is done in PharmCAT using home visits may be accomplished via smart pill containers with case managers following up by telephone when needed. Treatments that rely to a greater extent on technology vs home visits may make the use of environmental supports more affordable and more widely available.
We compare 3 groups (1) treatment as usual (TAU), (2) PharmCAT—application of environmental supports maintained on weekly home visits by a case worker, and (3) a smart pill container known as the Med-eMonitor™ (MM) capable of cueing the taking of medication, warning patients when they are taking the wrong medication, recording side effect complaints, and (through modem hookup to a secure Web site) promptly alerting treatment staff of failures to take medication as prescribed. MM staff follow-up with the patient by telephone when adherence problems are identified.
Both MM and PharmCAT prompt the taking of medication and are designed to bypass controlled processes (Do I really feel like taking this now?) in favor of automatic processes (ie, responding to an alarm) and habit formation. Both reinforce the taking of medication either with electronic messages or with social reinforcement, address fluctuating motivation by connecting adherence to recovery goals, and address side effects by forming a plan to either minimize their impact or get them resolved (eg, ask the doctor if a sedating medication can be taken at night instead of morning). Treatments differ in that PharmCAT utilizes an array of supports and is delivered during weekly home visits, while in MM, the monitor is the only support and once it is placed in the home, all contact is via telephone on an as-needed basis. The goal of the study is to determine whether these interventions improve adherence relative to TAU in patients with schizophrenia.
To our knowledge, this is the first randomized trial of home-delivered supports and electronic adherence intervention in serious mental illness. Our primary hypothesis is that both PharmCAT and MM will improve adherence as measured by electronic monitoring in comparison to TAU. In addition, we hypothesized that the treatment groups would have improved scores on a global measure of functional outcome in comparison to TAU. Secondary outcome measures included adherence assessed during unannounced pill counts conducted in the home, positive symptoms, and contact with emergency services.
Methods
Study Design
Participants had a diagnosis of schizophrenia or schizoaffective disorder and were being seen at a community mental health center for medication follow-up. They received a baseline assessment of adherence lasting for 1 month in which adherence was monitored via the MM and pill count and then were randomized into 1 of 3 treatment conditions for 9 months. Randomization was stratified by gender and age using a computer-generated algorithm created by the statistician who had no patient contact. Assessments of symptomatology and functioning were conducted at baseline and every 3 months.
Participants
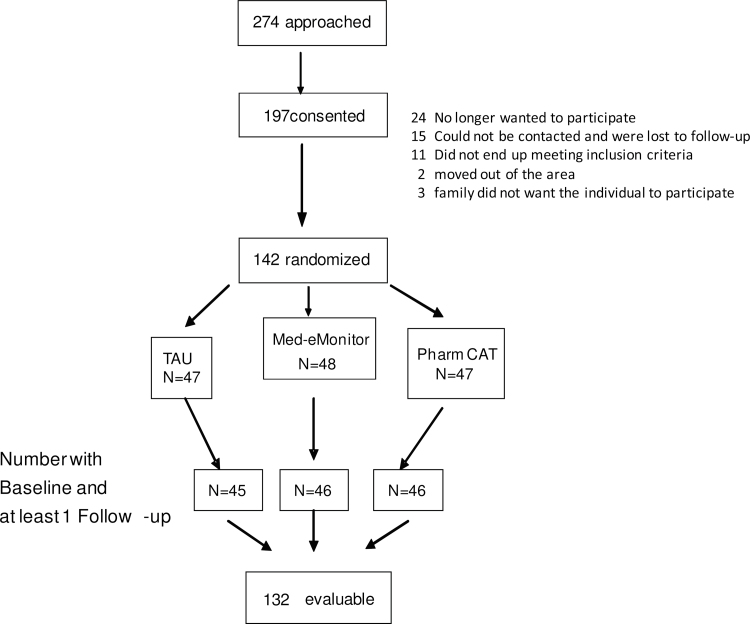
Participants were recruited from public mental health clinics in 2 counties in Texas, identified through chart reviews by research staff credentialed at participating sites in accordance with Health Insurance Portability and Accountability Act requirements. All participants signed a written consent form approved by an Institutional Review Board. Procedures were consistent with internationally recognized standards for ethical conduct of human research. The study was registered with ClinicalTrials.gov (identifier #NCT00406718). The Consort Diagram appears in figure 1. A total of 197 participants were consented, and 142 made it through the 1-month adherence assessment and were randomized.
Fig. 1.
Consort diagram.
Diagnoses were confirmed utilizing the Structured Clinical Interview for Diagnosis.12 Participants were between the ages of 18 and 60, were receiving ongoing treatment with an oral antipsychotic, had primary responsibility for taking their own medications, had missed at least one dose of medication in the preceding week, had a stable residence, and were able to understand and complete assessments. Individuals were excluded if they were on a depot antipsychotic medication; had a hospitalization in the past 3 months (to decrease early dropout due to readmission); had a documented history of significant head trauma, seizure disorder, or mental retardation; had a history of substance abuse or dependence in the past month; or had a history of violence in the past 6 months (as a safety measure for staff making home visits).
Of the 142 participants with baseline assessments, 75 were male and 67 were female. Of them, 55 were Hispanic, 49 were non-Hispanic White, 35 were African American, and 3 were from other or mixed ethnic groups. Mean age was 42.52 (SD = 10.27); 24% (n = 42) patients were on aripiprazole, 20% (n = 35) were on olanzapine, 17% (n = 30) were on quetiapine, 15% were on geodone (n = 26), 14% (n = 25) were on risperidone, and the remaining 9% were on other first- or second-generation antipsychotics. Almost 80% (n = 114) were on concomitant medications for side effects, mood, anxiety, or sleep. Symptoms of psychosis as rated from the Brief Psychiatric Rating Scale (BPRS) Psychosis Factor were in the mild range (M = 2.73 SD = 2.34). There were no significant differences in demographics for participants not making it to randomization vs those randomized to treatment (all P values >.20).
Treatment Groups
PharmCAT—PharmCAT (a subset of techniques from the CAT program) is manual driven and uses environmental supports such as signs, alarms, calendars, checklists, and notebooks to record questions for their prescriber, organization of belongings, and pill containers to improve medication adherence.10,13–15 Prior to treatment, patients were administered a cognitive test battery, a behavioral measure examining problems in goal-directed activity that identifies whether behavior is more dominated by apathy or disinhibition, an environmental assessment to identify problems with organization and behavioral triggers that interfere with the regular taking of medication and appointment adherence and a functional assessment. Interventions in PharmCAT are individualized based upon level of impairment in executive functioning (fair or poor) and whether behavior is characterized to a greater extent by apathy or disinhibition. Only interventions that specifically targeted adherence were used. Additional issues such as transportation were addressed only if they related to taking medication or making it to clinic appointments (eg, picking up prescriptions). Patients in PharmCAT were seen once weekly in their home for 30min.10,13–18 PharmCAT therapists were individuals with bachelor’s or master’s degrees in psychology or related fields trained using a combination of didactic and in vivo strategies. An independent specialist made fidelity checks using the PharmCAT fidelity scale for 20% of tape recordings of home visits. Scores are expressed in percentiles. Mean fidelity scores for 7 therapists was 0.96 (SD = 0.02) with a range from 0.94 to 0.99.
Med-eMonitor.
MM treatment consists of an MM therapist programming prescribing information into the device, setting up the device in the home to fit into the patient’s routine (eg, set alarm to take medication about 30min after waking, place in a location where he/she is likely to hear the alarm, etc.), assisting the patient in accurately filling the device, training the patient how to use the device and providing ongoing trouble shooting. Every 3 days the MM therapist was required to check the secure Web site to determine whether medication was being taken as prescribed and intervene by telephone if the patient was missing doses. All phone contacts to intervene were scripted to address either practical issues (eg, ways to discuss problems with the doctor, what to do if medication was left somewhere) or issues of motivation for adherence and focused on the connection between adherence and recovery (eg, discussing how stable medication can help one stay in school). All therapists were trained in technical aspects of monitor use. Supervision was conducted regularly to ensure adherence to the model.
Treatment as Usual.
TAU was case management and psychiatry appointments at a community mental health center.
Assessments
Diagnosis.
The Structured Clinical Interview for the Diagnostic and Statistical Manual for Mental Disorders was utilized to make DSM-IV diagnoses.
Medication Adherence.
Two objective measures of medication adherence were obtained. Our primary measure was percent adherence derived from the electronic monitor. Adherence was calculated by comparing the number of doses the patient was supposed to take with the number of times the container was opened within a specified dosing window and the patient indicated that a pill was ingested. This method goes one step further than an electronic cap, which records only openings of the bottle but does not eliminate openings for reasons other than taking medication (eg, filling the container). All individuals in all groups received a MM to assess adherence and contact with emergency services, but only in the MM group was the monitor set to prompt adherence.
In addition to adherence from the electronic monitor, unannounced pill counts were conducted in participants’ homes monthly. The pill counting procedure is described in detail in a recent publication and involves a setup in the home in which all bottles of medication are counted, old medication is bagged and stapled, and reminders not-to-throw-out medication bottles are strategically placed.10 We aggregated adherence data across each 3 month period to increase reliability of measurement. The correlation between pill count adherence and adherence from the electronic monitor during the 1-month baseline assessment was 0.61 P < .0001.
Symptoms, Use of Inpatient or Emergency Services, and Functional Assessment
Symptom and functional assessments were administered by Bachelor’s, Master’s or Doctoral level psychologists required to reach a criterion of 0.80 intraclass correlation coefficient on a series of interviews. We conducted checks on rater competency throughout the study to prevent rater/scorer drift.19 All raters were blind to treatment condition.
Symptomatology.
Symptoms over the past week were assessed using the expanded version of the BPRS.19,20 The psychosis factor score, the mean of items assessing hallucinations, unusual thought content, suspiciousness and conceptual disorganization, was a measure of positive symptoms.21,22 Higher scores indicate greater symptomatology.
Contact With Hospital and Emergency Services.
Use of hospital and emergency services for psychiatric reasons was collected because these services drive health care costs. Each week, an alarm on the monitor sounded and the participant was asked whether they used (yes or no) any of these services for their mental health problems.
Functional Outcome.
Global functioning over the past 3 months was assessed on a scale from 1–100, using the Social and Occupational Functioning Scale (SOFAS).13 Higher scores indicate better adaptive function. The SOFAS score was based upon a lengthy semistructured role-functioning interview.
Data Analysis
We examined group differences in medication adherence (MM adherence data and pill counts), symptomatology (BPRS positive symptom score), and functioning (SOFAS score) over time (3, 6, and 9 months) by treatment group (PharmCAT, MM, and TAU) using mixed effects regression with repeated measures, covarying for baseline scores.23 Group differences were tested using the overall F test and pairwise t tests at each visit. Time to contact with hospital or emergency psychiatric services was examined by calculating the Kaplan-Meier survival curve and testing group differences with the log-rank test. Standardized effect sizes were calculated utilizing the square root of the estimated residual variance for the outcome variable adjusting for baseline. In addition, for significant effects, we report number needed to treat (NNT) using Kraemer’s method for estimating NNT from Cohen’s d.24,25
Power was estimated at 0.80 to detect a moderate effect size (d = 0.43) for pairwise contrasts for 3 groups of N = 45 (with autoregressive structure) and r = .50 (with a baseline covariate) and 3 postbaseline measures, all normally distributed with equal variances and constant effect size over time.
Results
There were no statistically significant group differences with respect to demographic or clinical data (table 1). Baseline adherence as assessed from the electronic monitor differed by treatment group (P < .01, PharmCAT > TAU > MM), but there were no significant baseline differences for adherence based on pill counts (P < .11). (Because monitoring adherence is in itself an intervention, baseline represents adherence under monitored conditions rather than a true prestudy baseline. Baseline values were covaried.)
Table 1.
Baseline Characteristics by Treatment Group
| Treatment As Usual (n = 47)a | Med-eMonitor (n = 48)a | PharmCAT (n = 47)a | |
|---|---|---|---|
| % Male | 52.82 | 55.32 | 52.08 |
| % Hispanic | 27.66 | 40.43 | 47.92 |
| % Non-Hispanic White | 34.04 | 42.55 | 27.08 |
| % African American | 34.04 | 14.89 | 14.89 |
| Age | 42 (SD = 9.27) | 43 (SD = 10.15) | 43 (SD = 11.04) |
| Education | 12.4 (SD = 2.3) | 12.3 (SD=2.4) | 12.3 (SD= 2.2) |
| BPRS psychosis factor | 2.7 (SD = 2.34) | 2.6 (SD = 1.47) | 2.5(SD = 1.34) |
| Social and Occupational Functioning Scale | 45.6 (SD = 8.07) | 45.5 (SD = 8.90) | 45.9 (SD = 8.19) |
| Baseline medication | |||
| % Risperidone | 41.4 | 21.9 | 38.2 |
| % Olanzapine | 34.5 | 46.9 | 41.2 |
| % Othera | 24.1 | 31.2 | 20.6 |
Note: No significant group differences (all P values with one exception >.20). BPRS, Brief Psychiatric Rating Scale.
aSymptoms and functioning—3 months, n = 137: 6 months, n = 124; and 9 months, n = 120. Adherence from the electronic monitor and pill count, respectively—baseline, n = 138 and 135; 3 months n = 136 and 127; 6 months, n = 123 and 117; 9 months, n = 117 and 109.
We examined differential dropout (table 1) by treatment group using a proportional hazards regression model. Individuals in MM were more likely to drop out of the study  = 6.13 P < .046). Fourteen participants (29.8%) dropped from MM, four from PharmCAT (8.3%), and eight from TAU (17.2%).
= 6.13 P < .046). Fourteen participants (29.8%) dropped from MM, four from PharmCAT (8.3%), and eight from TAU (17.2%).
Medication Adherence
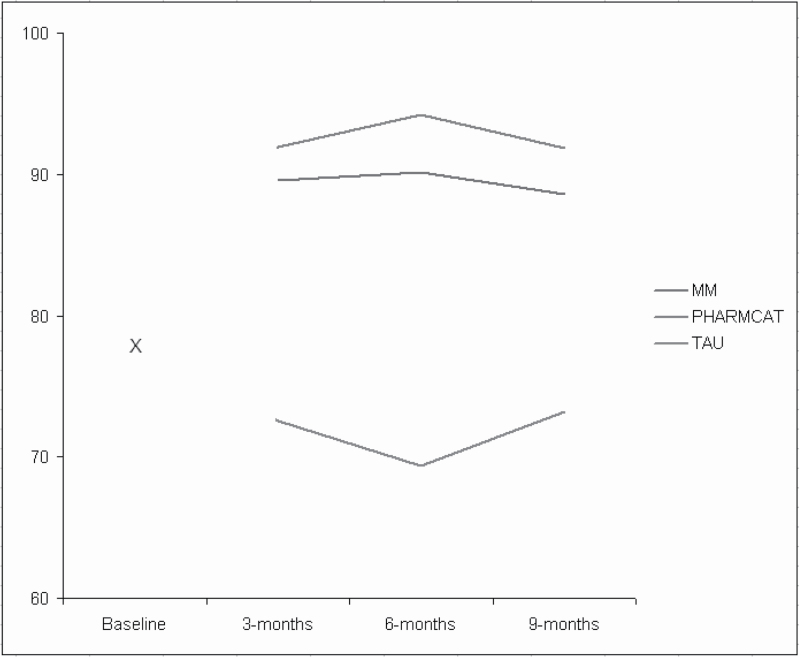
For MM-based adherence, the mixed-effects regression model yielded a significant treatment group effect (F = 47.29, df = 2, 365, P < .0001) and nonsignificant effects for time (F = 0.06, df = 2, 365, P > .94) and time by group (F = 0.44, df = 4,365, P > .77). Across time, adherence averaged 91% for MM, 90% for PharmCat, and 72% for the TAU group.
Both PharmCAT and MM were significantly better than TAU at all time points through treatment and follow-up (all P values < .0001). Differences between PharmCAT and MM were not significant (all P values > .43). Figure 2 depicts estimated means derived from the regression model at specified time points by treatment group and the common baseline used in analysis.
Fig. 2.
Adherence over time by group as measured by electronic monitoring. Main effect of group F(2,265) = 47.29; P < .0001; Main effect of visit F(2,365) = 0.06 P > .94; Group by visit F(4,365) = 0.44; P > .77. P values for PharmCAT and Med-eMonitor vs standard treatment were <.0001 at all time points. P values for PharmCAT vs MM were >.43 at all time points.
Averaged across the time points, the effect sizes for PharmCAT and MM compared with TAU were 1.03 and 0.98, respectively, values that correspond to NNT of 1.9.25 According to Cohen’s conventions,26 these are large (≥0.8) treatment effects.
We also examined pill counts conducted on monthly home visits. The mixed-effects regression model yielded a significant main effect of group (F = 7.83, df = 2, 116, P < .0001). Effects of time (F = <1, df = 2, 212, P = .53) and the group by time interaction (F = 2.34, df = 4, 414, P = .06) were not significant at P < .05. The trend for treatment by time was due to the 6-month period in which the MM and TAU groups did not differ. The PharmCAT group had higher adherence by pill count (91%) than either MM (86%, t = 2.05, df = 116, P = .04) or TAU (80%, t = 3.95, df = 115, P = .0001). The difference between MM and TAU approached significance (t = 1.82, df = 119, P = 0.072).
Symptoms and Functioning
For SOFAS scores and BPRS, results of mixed-effects regression models yielded no significant main effects or interactions (all P values > .09).
Use of Emergency Services
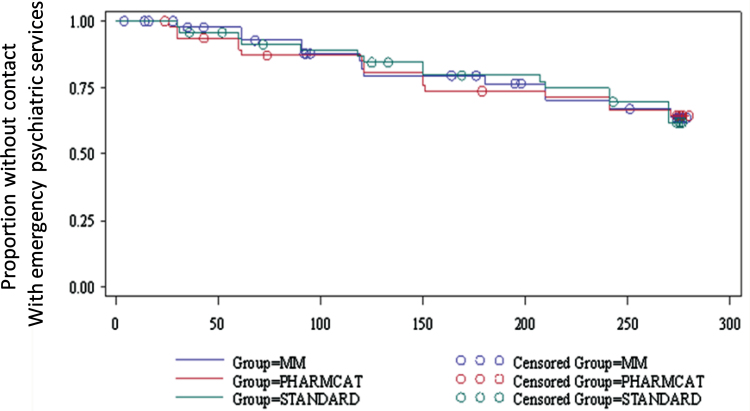
Based upon self-report elicited weekly by the MM device, 13 out of 47 patients (27.7%) in the MM group, 16 out of 48 (33.3%) in PharmCAT, and 16 out of 47 (34%) in TAU had contact with hospital or emergency psychiatric services. There were no differences between groups (χ2 = 0.53, df = 2, P = .77).
Time to use of these services did not differ significantly by treatment group  =3.29; P > .19). The survival curves for each treatment group over time are presented in figure 3.
=3.29; P > .19). The survival curves for each treatment group over time are presented in figure 3.
Fig. 3.
Proportion of patients who had contact with emergency services as obtained by weekly queries from a smart pill container. There were no differences in numbers of patients using psychiatric emergency services by treatment group (2× value = 0.5327, P value = .7662).
Additional Analyses
Separate logistic regression analyses were conducted to examine baseline variables predicting dropout, including treatment group, each covariate in turn, and the group by covariate interaction. Age was significantly negative correlated with dropout such that younger people dropped out at a higher rate (age effect: χ2 = 12.4, df = 1, P = .0004), but this did not differ by treatment group (χ2 = 3.2, df = 2, P = .20). No other baseline variables predicted dropout. Exit interviews were conducted on a subset of the sample (n = 21 in PharmCAT and n = 16 in MM). Respondents rated overall satisfaction with treatment on a scale from 1 to 7 (not at all to extremely satisfied). Scores were higher for those in PharmCAT (M = 6.80±0.54) than those in MM (M = 6.10±1.61), but the difference was not significant (t = 1.67, df = 17.6, P = 0.11). However, only 1 of the 21 respondents (5%) in PharmCAT vs 6 of the 16 (38%) in MM listed a complaint about the treatment when prompted for things they disliked (χ2 = 6.3, df = 1, P = .012, Fisher’s Exact P = .029). The primary complaint was the beeping of the device.
Costs of Treatment
Average costs of treatment per patient, per month in PharmCAT and MM were $180 and $130, respectively, after eliminating procedures and visits for research purposes only. Costs included mileage for home visits, price of the monitor with web support, PharmCAT supplies and staff time for checking the Web site, making telephone calls, and making home visits for PharmCAT or to fill the monitor for MM. The majority of the cost in MM was for the monitor and web support.
Discussion
PharmCAT and the MM improved adherence significantly over TAU. Average rates of adherence based upon electronic monitoring were about 90% for the active treatments compared to about 73% for TAU. For pill counts, individuals in PharmCAT had the best adherence followed by MM and then TAU. Both active treatments received high satisfaction ratings, but dropout and complaints were both higher in MM. The findings indicate that home visits and the use of electronic prompting are both effective ways to increase adherence with prescribed medication among individuals with schizophrenia. Unfortunately, these improvements in adherence did not translate into improvements in functional or symptom outcomes in comparison to TAU. There was no difference in time to emergency contact among groups. This is the first study to compare these adherence interventions.
These findings are consistent with our previous study,10 in which we reported that both PharmCAT and the full CAT program improved adherence to oral medications but not symptoms relative to TAU. In that study, we found some improvement in the PharmCAT group in global functional outcome, but the effect size was small and participants in the full CAT program made much more robust gains in functioning. We also found that patients in both PharmCAT and CAT had a longer time to relapse than those in TAU, in contrast to this trial that did not reveal differences in time to contact with psychiatric emergency services.
A majority of participants in our earlier trial were recruited as inpatients and were therefore more likely to experience rehospitalization or emergency contact. Moreover, the patients in TAU in our previous trial were less medication compliant than those in the current trial, with mean adherence levels at least 10% lower. Higher levels of adherence in TAU would be expected to attenuate group differences in outcomes associated with improved adherence in active treatments. Thus, our current and past results suggest that adherence interventions may have greater impact on individuals with lower levels of adherence and those who have been recently discharged from hospital. Studies examining novel adherence interventions may be more successful at improving global outcomes if focused on this group.
The finding that significant improvement in adherence to oral medication does not significantly improve levels of symptom severity is puzzling. One possibility is that the measurement window is too brief. Ratings on the BPRS capture only 1 week of the 3-month time interval between ratings, and scores on the day of rating may not be representative of symptom levels over time. Assessing symptoms daily or weekly using a mobile device may more accurately capture this. The topic of using mobile devices for more sensitive assessment of symptoms and functioning in schizophrenia was discussed extensively in a recent special issue of this journal.27
Another, more sobering possibility is that that prescribers do not know how much medication is needed to control symptoms for particular individuals, such that taking 90% of the prescribed dose provides as much benefit as taking 70%. In the current treatment environment, providers may spend as little as 15min with a patient every few months. Determining how well symptoms are controlled between visits is difficult given that there is no way to accurately measure this. Psychiatry has nothing akin to Hemoglobin A1C for diabetes.
If medications are prescribed in amounts that exceed doses necessary to control symptoms, 100% adherence is not needed to maintain symptom stability, and unnecessary health care costs may be incurred. But when the true level of medication adherence is not known, it is extremely difficult to identify an appropriate dose. Increasing the utilization of long-acting antipsychotics that allow prescribers to separate problems with efficacy from problems with adherence or otherwise monitoring adherence may be helpful in identifying appropriate doses to control symptomatology.
Adherence data obtained from unannounced, in-home pill counts suggested more robust treatment effects for PharmCAT than for MM. It may be that some individuals in MM opened the pill drawer (recording the opening of the specific drawer, date, and time) and responded affirmatively to the query asking whether they took their pill, but did not really take a pill. This same problem can occur with electronic monitoring caps (often considered a “gold standard” of adherence measurement), where the bottle is opened and the medication is not taken. The measure of adherence neither from the monitor nor from the in-home pill counting procedure is able to identify the amount of medication that is actually ingested by the participant.
The feasibility of these treatments needs to be considered. For MM, a case worker needs to be available to help the client setup the monitor in the home, monitor adherence via the secure Web site, and call the patient when adherence is outside a predetermined threshold. For PharmCAT, a caseworker must make home visits weekly, at least initially. Therefore, PharmCAT is more expensive and is only applicable in funding environments that reimburse for home visits by a case manager.
Two days of hospitalization per 9 months would pay for the cost of either MM or PharmCAT treatment. However, there are a number of ways in which the cost of MM could be substantially reduced. If the technology were mass produced, the cost would drop. Monitors can be reused with new trays bringing the cost per person down. Also, added to the cost of the monitor were monthly home visits to fill the tray for at least half the participants who were unable to fill the trays themselves. This cost would disappear if trays could be filled by a relative or at a pharmacy. With some of these changes, the cost of MM treatment could be as low as $50 monthly per person, if employed on a large scale in community mental health. This approach is less intensive and less costly than home visits to monitor and improve adherence and could be cost effective. Formal cost effectiveness studies should be undertaken.
These results must be examined in the context of the study’s limitations. All assessments of medication adherence involve error. There were a significant number of individuals approached for the trial who were not interested in participating, and others who dropped during the 1-month baseline period. The relatively high baseline level of adherence could have minimized outcome differences between treatments. There were baseline adherence differences based on electronic monitoring, so it could be argued that higher levels of adherence were maintained rather than adherence being improved in PharmCAT. However, significant improvements in adherence for PharmCAT were found based upon pill count data as well, with no significant differences at baseline on this measure. The fact that medication adherence improved while psychotic symptoms did not suggests that our notions about adequate adherence and optimal dosing may have been formed in the ambiguous environment of partial adherence.
A higher dropout in the MM condition relative to other groups could not be explained by demographic or clinical characteristics. Our exit interview suggested that the beeping of the device was annoying to some, but vast majority of our participants made it through treatment, and the average satisfaction rating was very high. It may be that our sample was less comfortable with technology than the general population, who are more likely than our participants to own cell phones and computers. As familiarity with technology increases, satisfaction with devices such as the MM may improve. Customized alarms and other novel features may add to the appeal of such devices. Assessing level of comfort with technology may be useful as a way of identifying those for whom technological treatments would be most appropriate.
The study demonstrates that adherence to oral medication can be maintained at high levels in this population with either an in-home treatment based on behavioral techniques or an electronic device designed to cue adherence coupled with telephone intervention if problems are identified. The value of adherence in terms of personal and social costs has been demonstrated.
Funding
National Institutes of Health (5R01MH074047-05).
Acknowledgments
We wish to thank the participants and staff from the Center for Health Care Services (Executive Director: Leon Evans) and Austin-Travis County Integrated Care Services (Executive Director: David Evans) for their ongoing support of our research program. The Authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Sackett DL, Haynes RB. Compliance with therapeutic regimens. Baltimore, MD: Johns Hopkins University Press; 1976 [Google Scholar]
- 2. Velligan DI, Weiden PJ, Sajatovic M, et al. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;7(Suppl 4):1–46; quiz 47–48. [PubMed] [Google Scholar]
- 3. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330 [DOI] [PubMed] [Google Scholar]
- 4. Tandon R. Antipsychotics in the treatment of schizophrenia: an overview. J Clin Psychiatry. 2011;72(Suppl 1):4–8 [DOI] [PubMed] [Google Scholar]
- 5. Palmer BW, Dawes SE, Heaton RK. What do we know about neuropsychological aspects of schizophrenia? Neuropsychol Rev. 2009;19:365–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hirsch SR, Barnes TRE. The clinical treatment of schizophrenia with antipsychotic medications. In: Hirsch SR, Weinberger DR, eds. Schizophrenia. Oxford: Blackwell Science; 1995 [Google Scholar]
- 7. Dolder CR, Lacro JP, Dunn LB, Jeste DV. Antipsychotic medication adherence: is there a difference between typical and atypical agents? Am J Psychiatry. 2002;159:103–108 [DOI] [PubMed] [Google Scholar]
- 8. Goff DC, Hill M, Freudenreich O. Strategies for improving treatment adherence in schizophrenia and schizoaffective disorder. J Clin Psychiatry. 2010;71(Suppl 2):20–26 [DOI] [PubMed] [Google Scholar]
- 9. Velligan DI, Dicocco M, Castillo D, et al. Obstacles in assessing adherence to oral antipsychotic medications. Schizophr Res. 2003;15(Suppl 1):330 [Google Scholar]
- 10. Velligan DI, Diamond PM, Mintz J, et al. The use of individually tailored environmental supports to improve medication adherence and outcomes in schizophrenia. Schizophr Bull. 2008;34:483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weiden PJ. Understanding and addressing adherence issues in schizophrenia: from theory to practice. J Clin Psychiatry. 2007;68(Suppl 14):14–19 [PubMed] [Google Scholar]
- 12. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 13. Velligan DI, Bow-Thomas CC, Huntzinger C, et al. Randomized controlled trial of the use of compensatory strategies to enhance adaptive functioning in outpatients with schizophrenia. Am J Psychiatry. 2000;157:1317–1323 [DOI] [PubMed] [Google Scholar]
- 14. Velligan DI, Prihoda TJ, Ritch JL, Maples N, Bow-Thomas CC, Dassori A. A randomized single-blind pilot study of compensatory strategies in schizophrenia outpatients. Schizophr Bull. 2002;28:283–292 [DOI] [PubMed] [Google Scholar]
- 15. Velligan DI, Gonzalez JM. Rehabilitation and recovery in schizophrenia. Psychiatr Clin North Am. 2007;30:535–548 [DOI] [PubMed] [Google Scholar]
- 16. Velligan DI, Bow-Thomas CC. Two case studies of cognitive adaptation training for outpatients with schizophrenia. Psychiatr Serv. 2000;51:25–29 [DOI] [PubMed] [Google Scholar]
- 17. Velligan DI, Mahurin RK, True JE, Lefton RS, Flores CV. Preliminary evaluation of cognitive adaptation training to compensate for cognitive deficits in schizophrenia. Psychiatr Serv. 1996;47:415–417 [DOI] [PubMed] [Google Scholar]
- 18. Velligan DI, Diamond PM, Maples NJ, et al. Comparing the efficacy of interventions that use environmental supports to improve outcomes in patients with schizophrenia. Schizophr Res. 2008;102:312–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance with the Brief Psychiatric Rating Scale: “The drift busters”. Int J Methods Psychiatr Res. 1993;3:221–244 [Google Scholar]
- 20. Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A. Manual for the Expanded Brief Psychiatric Rating Scale. Int J Methods Psychiatr Res. 1993;3:227–244 [Google Scholar]
- 21. Ventura J, Nuechterlein KH, Subotnik KL, Gutkind D, Gilbert EA. Symptom dimensions in recent-onset schizophrenia and mania: a principal components analysis of the 24-item Brief Psychiatric Rating Scale. Psychiatry Res. 2000;97:129–135 [DOI] [PubMed] [Google Scholar]
- 22. Velligan D, Prihoda T, Dennehy E, et al. Brief psychiatric rating scale expanded version: How do new items affect factor structure? Psychiatry Res. 2005;135:217–228 [DOI] [PubMed] [Google Scholar]
- 23. SAS Institute Inc SAS/STAT® 9.2 User’s Guide. Cary, NC: SAS Institute Inc.; 2008 [Google Scholar]
- 24. Kraemer HC, Blasey CM. Centring in regression analyses: a strategy to prevent errors in statistical inference. Int J Methods Psychiatr Res. 2004;13:141–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59:990–996 [DOI] [PubMed] [Google Scholar]
- 26. Cohen TJ. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988 [Google Scholar]
- 27. Ben-Zeev D, McHugo GJ, Xie H, Dobbins K, Young MA. Comparing retrospective reports to real-time/real-place mobile assessments in individuals with schizophrenia and a nonclinical comparison group. Schizophr Bull. 2012;38:396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]