Abstract
Impact of dose reduction of atypical antipsychotics on cognitive function has not been investigated in stable patients with schizophrenia. In this open-label, 28-week, randomized controlled trial, stable patients with schizophrenia treated with risperidone or olanzapine were randomly assigned to the reduction group (dose reduced by 50% in 4 weeks and then maintained) or maintenance group (dose kept constant). Assessments at baseline and week 28 included the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS), Positive and Negative Syndrome Scale (PANSS), and Drug-Induced Extrapyramidal Symptoms Scale (DIEPSS). Sixty-one patients were enrolled; 2 of 31 (6.5%) and 5 of 30 (16.7%) patients prematurely withdrew from the study in the reduction and maintenance groups, respectively. While no significant differences in change in the PANSS total score were observed between the 2 groups, the reduction group showed significantly greater improvements in the RBANS and DIEPSS total scores compared with the maintenance group (mean ± SD, +7.0±7.1 vs −0.1±8.0, P < .001; −0.9±1.7 vs +0.1±1.2, P = .010, respectively). This 6-month pilot study suggests that risperidone or olanzapine dose reduction of 50% can improve cognitive function for stable patients with schizophrenia. Due to the open-label design, small sample size, and short study duration, however, there is a need to confirm the finding through double-blind, larger scale trials with longer follow-up periods. Moreover, potential risks of relapse following antipsychotic dose reduction should be thoroughly investigated in longer term studies.
Key words: antipsychotics, cognitive function, dose reduction, olanzapine, risperidone, schizophrenia
Introduction
While the effects of antipsychotic drugs on cognitive function remain controversial in patients with schizophrenia,1 excessive dopaminergic blockade has been related to cognitive impairment2 as well as extrapyramidal symptoms.3 In fact, recent studies have reported that higher dose of antipsychotics impairs cognitive function in patients with schizophrenia,4 , 5 even treated with atypical antipsychotics.6 However, to date there has been no clinical trial that has evaluated the effects of atypical antipsychotic dose reduction on cognitive function in patients with schizophrenia. To address this gap in the literature, we conducted an open-label, 28-week, randomized, controlled, pilot study investigating the impact of risperidone and olanzapine dose reduction on cognitive function in stable patients with schizophrenia.
Methods
Study Design and Setting
This pilot study represented a multicenter, open-label, parallel-group, 28-week, randomized, controlled trial. It was conducted at 6 psychiatric hospitals and clinics in Tokyo from April 2009 to August 2011. The trial protocol was approved by the institutional review board at each participating site. After full description of the study, all participants provided written informed consent prior to entering the study. This trial was registered at UMIN Clinical Trials Registry (UMIN-CTR) on March 2009 (UMIN000001834).
Patients
Patients were included if they were ≥18 years, diagnosed with schizophrenia according to DSM-IV,7 receiving a stable dose of either risperidone >2mg/day or olanzapine >5mg/day as antipsychotic monotherapy for at least 3 months, and in remission with respect to positive symptoms, as defined by a score of ≤3 (mild) on all of the following 4 Positive and Negative Syndrome Scale (PANSS)8 Positive subscale items: delusion (P1), conceptual disorganization (P2), hallucinatory behavior (P3), and suspiciousness (P6). Patients on antipsychotic polypharmacy were excluded, although concomitant use of ≤50mg/day of chlorpromazine or levomepromazine was allowed because these medications are often used as hypnotics in Japan, and it was considered that such low doses would not be associated with antipsychotic effects. Concomitant medications other than antipsychotics were allowed. Patients were also excluded if they suffered from any significant medical or neurological illnesses, or were pregnant or lactating.
Procedures
Patients who met inclusion criteria were randomly assigned to either the reduction or maintenance groups by central randomization stratified by their antipsychotics type (ie, risperidone or olanzapine). In the reduction group, risperidone or olanzapine were reduced by 25% at baseline and week 4, followed by the treatment with half the baseline dose over the next 24 weeks. For safety reasons, the dose was not reduced beyond the lower limit of the dose range recommended for maintenance treatment of schizophrenia, ie, 2mg/day for risperidone and 5mg/day for olanzapine.9 In the maintenance group, patients received the same regimen for the entire 28 weeks. For each treatment arm, concomitant medications, including anticholinergic drugs, were kept constant throughout the study period. Due to the nature of open-label study design, both patients and raters were not blind to patients’ group assignment.
Outcome Measures
The following assessments were performed at baseline and 28 weeks: the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)10 , 11 for cognitive function; the PANSS, the Clinical Global Impression–Severity scale (CGI-S),12 and the Calgary Depression Scale for Schizophrenia (CDSS)13 for clinical psychopathology; and, the Drug-Induced Extrapyramidal Symptoms Scale (DIEPSS)14 for extrapyramidal symptoms.
In this study, relapse was defined as a score of ≥4 (moderate) on at least one of the 4 PANSS Positive subscale items identified: delusion (P1), conceptual disorganization (P2), hallucinatory behavior (P3), and suspiciousness (P6).
Statistical Analyses
Statistical analyses were performed on an intention-to-treat basis. The primary outcome was change in RBANS Total scale scores. Baseline demographic and clinical characteristics were compared between the 2 groups by the Fisher’s exact test for categorical variables and by the Student’s t test for continuous variables. The between-group differences in changes at last visit from baseline in all the efficacy endpoints were tested by the Student’s t test using a last observation carried forward (LOCF) method as a primary analysis. A 2-tailed P value of <.05 was considered statistically significant for all tests. All statistical analyses were conducted using the IBM SPSS Statistics version 19 (IBM Corporation, Armonk, NY).
Results
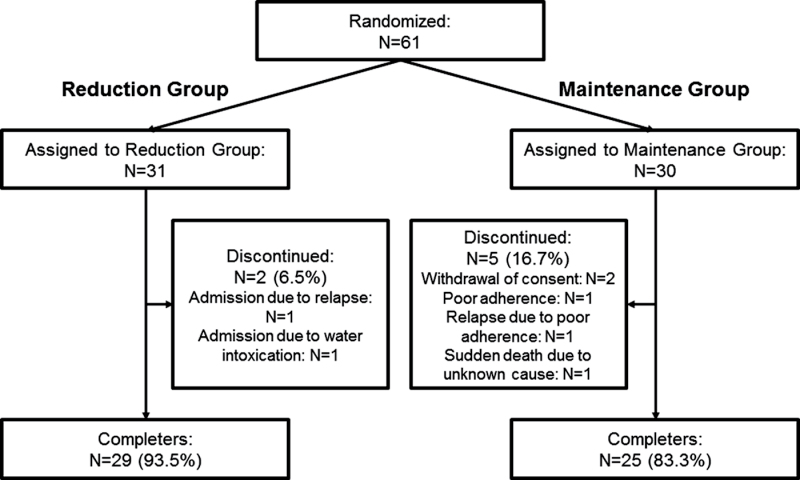
A total of 61 patients were enrolled and randomly assigned to the reduction group (n = 31) or the maintenance group (n = 30). A total of 54 patients completed all study procedures; 2 and 5 patients prematurely withdrew from the study in the reduction group (6.5%) and the maintenance group (16.7%), respectively; this did not represent a statistically significant difference (Figure 1).
Fig. 1.
Flow of patient disposition.
Patient Characteristics
Baseline demographic and clinical characteristics of the patients are shown in Tables 1 and 2. There were no statistical differences in any baseline values between the 2 groups (Tables 1 and 2). In the reduction group, 10 patients (32.3%) received an antipsychotic dose reduction of less than 50%; each received a dose reduction of one-third, in keeping with the position that dose reduction was not performed beyond the lower limit of the recommended dose range (ie, risperidone 2mg/day or olanzapine 5mg/day), as described in “Methods” section.
Table 1.
Demographic and Clinical Characteristics of Patients in Reduction and Maintenance Groupsa
| Reduction Group (n = 31) | Maintenance Group (n = 30) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Male | 18 | 58.1 | 19 | 63.3 |
| Outpatient | 28 | 90.3 | 28 | 93.3 |
| Patient treated with risperidone | 12 | 38.7 | 11 | 36.7 |
| Patient treated with olanzapine | 19 | 61.3 | 19 | 63.3 |
| Mean | SD | Mean | SD | |
| Age (years) | 40.9 | 12.2 | 38.4 | 14.3 |
| Duration of education (years) | 13.6 | 2.4 | 13.1 | 2.2 |
| Duration of illness (years) | 15.5 | 11.3 | 12.9 | 13.0 |
| Number of admissions | 1.5 | 1.5 | 1.4 | 1.3 |
| Dose of risperidone [range] (mg/day) | 3.7 [3–6] | 1.0 | 4.5 [3–12] | 2.8 |
| Dose of risperidone after reduction [range] (mg/day) | 2.1 [2–3] | 0.3 | N/A | N/A |
| Dose of olanzapine [range] (mg/day) | 13.8 [7.5–20] | 5.2 | 14.1 [10–20] | 4.3 |
| Dose of olanzapine after reduction [range] (mg/day) | 7.1 [5–10] | 2.4 | N/A | N/A |
| n | % | n | % | |
| Concomitant medications | ||||
| Chlorpromazine or levomepromazine (≤50mg/day) | 3 | 9.7 | 2 | 6.7 |
| Anticholinergic drugs | 15 | 48.4 | 8 | 26.7 |
| Benzodiazepines | 17 | 54.8 | 17 | 56.7 |
| Antidepressants | 5 | 16.1 | 3 | 10.0 |
| Mood stabilizers | 2 | 6.5 | 6 | 20.0 |
| Antihistamine drugs | 4 | 12.9 | 1 | 3.3 |
aNo significant differences in all values between the 2 groups.
Table 2.
Cognitive Function, Clinical Psychopathology, and Extrapyramidal Symptoms in Reduction and Maintenance Groupsa
| Reduction Group (n = 31) | Maintenance Group (n = 30) | Difference in Change Between Groupsb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Change | Baseline | Change | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | t | P | |
| Cognitive function (RBANS) | ||||||||||
| Total scale | 83.0 | 14.1 | 7.0 | 7.1 | 78.2 | 13.2 | −0.1 | 8.0 | 3.67 | <.001* |
| Immediate memory | 71.5 | 16.7 | 11.0 | 12.2 | 70.0 | 16.0 | 5.3 | 9.3 | 2.04 | .046* |
| Visuospatial/constructional | 105 | 15.7 | 1.9 | 13.7 | 97.3 | 18.2 | −2.4 | 12.5 | 1.28 | .205 |
| Language | 91.9 | 15.5 | 3.7 | 10.3 | 90.4 | 11.5 | −4.1 | 9.6 | 3.04 | .004* |
| Attention | 84.5 | 17.3 | 6.0 | 12.1 | 81.6 | 12.4 | 1.2 | 11.1 | 1.61 | .112 |
| Delayed memory | 82.2 | 16.0 | 5.1 | 13.4 | 76.1 | 20.1 | −0.4 | 16.4 | 1.44 | .156 |
| Clinical psychopathology | ||||||||||
| PANSS | ||||||||||
| Total | 56.4 | 15.1 | −6.7 | 10.3 | 56.3 | 11.7 | −5.7 | 6.9 | −0.43 | .666 |
| Positive | 10.7 | 3.3 | −1.0 | 2.0 | 10.4 | 3.3 | −1.4 | 2.8 | 0.59 | .559 |
| Negative | 16.8 | 6.2 | −3.0 | 3.7 | 17.3 | 6.3 | −1.3 | 2.8 | −2.01 | .049* |
| General psychopathology | 28.9 | 7.3 | −2.7 | 6.2 | 28.7 | 5.6 | −3.1 | 4.6 | 0.25 | .801 |
| CGI-S | 2.8 | 1.1 | −0.2 | 0.7 | 2.8 | 0.9 | 0.0 | 0.6 | −1.34 | .186 |
| CDSS total | 3.1 | 2.7 | −0.9 | 2.7 | 2.8 | 2.0 | −0.5 | 1.8 | −0.69 | .492 |
| Extrapyramidal symptoms (DIEPSS) | ||||||||||
| Total | 3.1 | 3.3 | −0.9 | 1.7 | 2.9 | 2.6 | 0.1 | 1.2 | −2.64 | .010* |
Note: RBANS; Repeatable Battery for the Assessment of Neuropsychological Status, PANSS; Positive and Negative Syndrome Scale, CGI-S; Clinical Global Impression–Severity scale, CDSS; Calgary Depression Scale for Schizophrenia, DIEPSS; Drug-Induced Extrapyramidal Symptoms Scale.
aLast observation carried forward (LOCF) method data.
bNo significant differences in all baseline values between the 2 groups by Student’s t test.
*P < .05.
Cognitive Function
A significantly greater improvement in the RBANS Total scale score was observed in the reduction vs maintenance group (Table 2), and effect size (Cohen’s d) for the endpoint was large, .95 (P < .001). When patients who took anticholinergic drugs were excluded, a significant difference was also found between the reduction group (n = 16, mean ± SD = +8.1±7.7) and the maintenance group (n = 22, mean ± SD = −1.6±7.8) (P < .001). No significant difference was found between the patients undergoing a one-half and a one-third reduction. Exploratory analyses for the 5 cognitive domains revealed significant improvements in the Immediate Memory and Language index scores in the reduction group compared with the maintenance group (Table 2), although improvement in the Immediate Memory index score failed to remain significant after Bonferroni correction (ie, P value multiplied by 5).
Clinical Psychopathology and Extrapyramidal Symptoms
Only 1 patient in each group met criteria for relapse. No significant differences between the 2 groups were found in overall changes from baseline to last visit on the PANSS Total, Positive or General Psychopathology subscale scores, as well as the CGI-S and CDSS (Table 2). On the other hand, a significant reduction in the PANSS Negative subscale score was observed in the reduction group compared with the maintenance group (Table 2), although it failed to remain significant after Bonferroni correction (ie, P value multiplied by 3). As expected, the DIEPSS total score decreased significantly in the reduction group compared with the maintenance group (Table 2).
Discussion
To the best of our knowledge, this is the first study to investigate the impact of atypical antipsychotic dose reduction on cognitive function in stabilized patients with schizophrenia. Our preliminary findings are 2-fold: (1) cognitive function can be improved by reducing the doses of risperidone and olanzapine and (2) dose reduction of risperidone and olanzapine did not increase the risk of worsening in clinical psychopathology over a 6-month interval in patients who currently do not demonstrate significant positive symptoms.
Most notably, a simple dose reduction strategy resulted in significant improvements in cognitive function; moreover, the effect size of general cognitive improvement between groups was large, ie, .95. This is striking when one considers that clinical trials for cognitive enhancers in schizophrenia have demonstrated only modest efficacy or failed to show a separation from placebo,15 and highlights the potential of dose reduction as a strategy that may be effective through minimizing antipsychotic-induced cognitive impairment. Other studies have reported that dose is inversely correlated with general cognitive function4 , 6 as well as specific cognitive domains such as processing speed5 and verbal learning in patients receiving risperidone or olanzapine.6 Our results are consistent with these findings; both verbal memory and processing speed were improved by reducing antipsychotic dose. This said, the point has been made that a single generalized cognitive score should be used because specified domains do not exist independently.16 From this perspective, the improvement in the RBANS Total scale score in the reduction group also endorses the benefits of this strategy in enhancing cognition.
From a mechanistic point of view, cognitive impro vement following antipsychotic dose reduction may be a product of decreased dopamine D2 receptor blockade. This notion is in line with our recent cross-sectional analysis, using the data from the CATIE study, which demonstrated impaired general cognitive function in patients with antipsychotic-related high estimated D2 receptor occupancy.2 Another possible mechanism is that of anticholinergic activity, which is also associated with cognitive impairment, especially in the domains of attention and memory.17 Regardless of mechanism, though, findings underscore the need for using the lowest possible antipsychotic dose, typical or atypical, to minimize or prevent such cognitive side effects.
The risk of worsening in clinical psychopathology was not increased after dose reduction, at least over an interval of 6 months. This favorable result may be due to the inclusion of clinically stable patients without significant positive symptoms (ie, scores ≤3 on P1, P2, P3, and P6). In addition, our study did not allow for a dose decrease beyond the lower limit of currently recommended doses (ie, risperidone 2mg/day or olanzapine 5mg/day). Indeed, our recent meta-analysis revealed that the efficacy of moderately low and standard doses are comparable in preventing relapse in schizophrenia, while less than half the standard dose may increase the risk of relapse.18 Thus, low-dose strategies are effective in maintenance treatment although there still may remain a lower threshold beyond which risk of relapse rises.
There are limitations related to the present study that warrant comment. First, the open-label design could have influenced results; more specifically, the cognitive improvement in the reduction group may have been attributable to patients’ and raters’ expectation bias. Second, the follow-up period of 6 months may be too short to assess long-term outcomes, especially relapse rates, in schizophrenia. In fact, while one previous 6-month study reported no increase in relapse after approximately a 25% dose reduction of olanzapine,19 a 1-year study did demonstrate an increase in relapse after a 50% dose reduction of risperidone.20 In addition, previous longer term randomized controlled trials of typical antipsychotic dose reduction have shown that the relapse rates keep gradually increasing also beyond 6 months although the doses were reduced to one-fifth or less in those studies.21 – 23 Third, the sample size was small, which did not allow us to separately analyze the data for risperidone and olanzapine. Finally, medication adherence was assessed based on clinical interview at each visit without any objective measures such as pill count or electronic monitoring. Going forward, double-blind, larger scale, and longer term trials are needed to replicate our findings.
In conclusion, a 50% dose reduction in risperidone or olanzapine improved cognitive function without increasing risk of worsening in clinical psychopathology in stabilized patients with schizophrenia over 6 months in this pilot study. This highlights the fact that risperidone and olanzapine can induce cognitive impairment in a dose-dependent fashion, although these preliminary findings should be confirmed in further studies.
Funding
Inokashira Hospital and Research Group for Schizophrenia.
Acknowledgments
The authors thank Dr Y. Yamashima for permission of the use of the Japanese version of the RBANS and Drs Y. Imasaka, Y. Kimura, S. Nakajima, J. Hirano, A. Nagata, Y. Fujita, M. Nishimoto, K. Funaki, H. Ataka, and T. Suzuki for their continuous keen support. Conflicts of interest: Dr H.T. has received fellowship grants from the Japanese Society of Clinical Neuropsychopharmacology and Astellas Foundation for Research on Metabolic Disorders; speaker’s honoraria from Dainippon Sumitomo Pharma, Eli Lilly, GlaxoSmithKlein, Janssen Pharmaceutical, Meiji Seika Pharma, and Otsuka Pharmaceutical; and manuscript fees from Dainippon Sumitomo Pharma within the past 5 years. Dr T.S. has received fellowship grants from the Japanese Society of Clinical Neuropsychopharmacology, Government of Canada Post-Doctoral Research Fellowships, Kanae Foundation, and Mochida Memorial Foundation; speaker’s honoraria from Eli Lilly, Shionogi, and Yoshitomiyakuhin; and manuscript fees from Dainippon Sumitomo Pharma and Kyowa Hakko Kirin within the past 5 years. Dr G.R. has received research support from Novartis, Medicure, and Neurocrine Bioscience; consultant fees from Roche; and speaker’s fees from Novartis. He holds no commercial investments in any pharmaceutical company within the past 5 years. Dr R.R.B. has received grants from Eli Lilly through the Indiana CTSI as well as from Merck and Company through Regenstrief Institute within the past 5 years. Dr T.A. has no competing interests to disclose. Dr A.G.-G. has received grant support from National Institute of Health, National Institute of Mental Health, Canadian Institutes of Health Research, Ontario Mental Health Foundation, National Council for Science and Technology, and Janssen and has served as consultant and/or speaker for Abbott Laboratories, Gedeon Richter Plc, and Eli Lilly within the past 5 years. Dr K.W. has served on the advisory board of Dainippon Sumitomo Pharma, Eli Lilly, and Otsuka Pharmaceutical; received grants from Dainippon Sumitomo Pharma, GlaxoSmithKline, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Otsuka Pharmaceutical, and Pfizer Japan; and speaker’s honoraria or manuscript fees from Astellas Pharma, Dainippon Sumitomo Pharma, Eli Lilly, GlaxoSmithKline, Janssen Pharmaceutical, Meiji Seika Pharma, Mitsubishi Tanabe Pharma, Mochida Pharmaceutical, Otsuka Pharmaceutical, Pfizer Japan, Shionogi, and Yoshitomiyakuhin within the past 5 years. Dr M.M. has received grants or consultant fees from Astellas Pharma, GlaxoSmithKline, Eisai, and Meiji Seika Pharma and speaker’s honoraria from Astellas Pharma, Dainippon Sumitomo Pharma, Eli Lilly, GlaxoSmithKline, Janssen Pharmaceutical, Meiji Seika Pharma, Otsuka Pharmaceutical, Pfizer Japan, and Yoshitomiyakuhin within the past 5 years. Dr H.U. has received grants from Astellas Pharma, Dainippon Sumitomo Pharma, Eisai, Eli Lilly, GlaxoSmithKline, Janssen Pharmaceutical, Meiji Seika Pharma, Mochida Pharmaceutical, Otsuka Pharmaceutical, Pfizer Japan, Shionogi, and Yoshitomiyakuhin and speaker’s honoraria from Dainippon Sumitomo Pharma, Eli Lilly, GlaxoSmithKline, Janssen Pharmaceutical, Novartis Pharma, Otsuka Pharmaceutical, Shionogi, and Yoshitomiyakuhin within the past 5 years.
References
- 1. Keefe RS, Harvey PD. Cognitive impairment in schizophrenia. Handb Exp Pharmacol. 2012;11–37 [DOI] [PubMed] [Google Scholar]
- 2. Sakurai H, Bies RR, Stroup ST, et al. Dopamine D2 receptor occupancy and cognition in schizophrenia: analysis of the CATIE data. Schizophr Bull. 2013;39:564–574 doi:10.1093/schbul/sbr189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Uchida H, Takeuchi H, Graff-Guerrero A, Suzuki T, Watanabe K, Mamo DC. Dopamine D2 receptor occupancy and clinical effects: a systematic review and pooled analysis. J Clin Psychopharmacol. 2011;31:497–502 [DOI] [PubMed] [Google Scholar]
- 4. Elie D, Poirier M, Chianetta J, Durand M, Grégoire C, Grignon S. Cognitive effects of antipsychotic dosage and polypharmacy: a study with the BACS in patients with schizophrenia and schizoaffective disorder. J Psychopharmacol. 2010;24:1037–1044 [DOI] [PubMed] [Google Scholar]
- 5. Knowles EE, David AS, Reichenberg A. Processing speed deficits in schizophrenia: reexamining the evidence. Am J Psychiatry. 2010;167:828–835 [DOI] [PubMed] [Google Scholar]
- 6. Hori H, Yoshimura R, Katsuki A, et al. The cognitive profile of aripiprazole differs from that of other atypical antipsychotics in schizophrenia patients. J Psychiatr Res. 2012;46:757–761 [DOI] [PubMed] [Google Scholar]
- 7. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 4th ed Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 8. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276 [DOI] [PubMed] [Google Scholar]
- 9. Falkai P, Wobrock T, Lieberman J, Glenthoj B, Gattaz WF, Möller HJ. WFSBP Task Force on Treatment Guidelines for Schizophrenia World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: long-term treatment of schizophrenia. World J Biol Psychiatry. 2006;7:5–40 [DOI] [PubMed] [Google Scholar]
- 10. Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status. San Antonio, TX: Psychological Corp; 1998. [Google Scholar]
- 11. Yamashima T, Yoshida M, Kumahashi K, et al. [The Japanese version of RBANS (Repeatable Battery for the Assessment of Neuropsychological Status)]. No To Shinkei. 2002;54:463–471 [PubMed] [Google Scholar]
- 12. Guy W. ECDEU Assessment Manual for Psychopharmacology. Washington, DC: US Department of Health, Education, and Welfare; 1976 [Google Scholar]
- 13. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3:247–251 [DOI] [PubMed] [Google Scholar]
- 14. Inada T. DIEPSS: A Second-Generation Rating Scale for Antipsychotic-Induced Extrapyramidal Symptoms: Drug-Induced Extrapyramidal Symptoms Scale. Tokyo, Japan: Seiwa Shoten; 2009. [Google Scholar]
- 15. Millan MJ, Agid Y, Brüne M, et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11:141–168 [DOI] [PubMed] [Google Scholar]
- 16. Dickinson D, Gold JM. Less unique variance than meets the eye: overlap among traditional neuropsychological dimensions in schizophrenia. Schizophr Bull. 2008;34:423–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Minzenberg MJ, Poole JH, Benton C, Vinogradov S. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiatry. 2004;161:116–124 [DOI] [PubMed] [Google Scholar]
- 18. Uchida H, Suzuki T, Takeuchi H, Arenovich T, Mamo DC. Low dose vs standard dose of antipsychotics for relapse prevention in schizophrenia: meta-analysis. Schizophr Bull. 2011;37:788–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rouillon F, Chartier F, Gasquet I. Strategies of treatment with olanzapine in schizophrenic patients during stable phase: results of a pilot study. Eur Neuropsychopharmacol. 2008;18:646–652 [DOI] [PubMed] [Google Scholar]
- 20. Wang CY, Xiang YT, Cai ZJ, et al. ; Risperidone Maintenance Treatment in Schizophrenia (RMTS) investigators Risperidone maintenance treatment in schizophrenia: a randomized, controlled trial. Am J Psychiatry. 2010;167:676–685 [DOI] [PubMed] [Google Scholar]
- 21. Kane JM, Rifkin A, Woerner M, et al. Low-dose neuroleptic treatment of outpatient schizophrenics. I. Preliminary results for relapse rates. Arch Gen Psychiatry. 1983;40:893–896 [DOI] [PubMed] [Google Scholar]
- 22. Hogarty GE, McEvoy JP, Munetz M, et al. Dose of fluphenazine, familial expressed emotion, and outcome in schizophrenia. Results of a two-year controlled study. Arch Gen Psychiatry. 1988;45:797–805 [DOI] [PubMed] [Google Scholar]
- 23. Schooler NR, Keith SJ, Severe JB, et al. Relapse and rehospitalization during maintenance treatment of schizophrenia. The effects of dose reduction and family treatment. Arch Gen Psychiatry. 1997;54:453–463 [DOI] [PubMed] [Google Scholar]