Abstract
Objective
The only treatment for celiac disease is lifelong adherence to a gluten-free diet, yet adherence is limited and factors influencing adherence are poorly understood. The purpose of this study was to determine factors influencing gluten-free diet adherence in adults with celiac disease.
Methods
A questionnaire was developed and administered to 154 adults with celiac disease who then underwent a standardized gluten-free diet evaluation by an experienced nutritionist. Multivariate analysis was conducted to determine factors associated with adherence level.
Results
Thirteen factors hypothesized to contribute to gluten-free diet adherence were found to be significantly associated with improved adherence including: understanding of the gluten-free diet, membership of a celiac disease advocacy group, and perceived ability to maintain adherence despite travel or changes in mood or stress (P < 0.001).
Conclusions
This study identified specific factors correlated with gluten-free diet adherence. These results provide a foundation for the design of educational interventions to improve adherence.
Keywords: Celiac disease, Gluten free diet, Compliance, Adherence
Introduction
There is rapidly rising clinical awareness of celiac disease (CD), which has resulted in increasing rates of diagnosis. These changes reflect recent advances in our understanding of the epidemiology and broad spectrum of clinical presentations of CD [1]. Accurate serological assays to identify untreated CD have resulted in a reevaluation of the population prevalence of CD in the United States and Europe. Multiple studies report the prevalence of CD in populations of European decent to be between 1:67 and 1:250 [2–5]. Furthermore, a growing body of literature supports the notation that CD is a common disease in diverse populations across the globe, especially the Near and Far East, and North Africa [6].
There are important ramifications for an individual who receives a diagnosis of CD. CD is a systemic, immunological disorder in which the sentinel lesion is an enteropathy triggered by polypeptides derived primarily from the prolamine proteins found in wheat, rye, and barley. Ingestion of the offending proteins leads to inflammation and intestinal mucosal damage, which may result in a spectrum of gastrointestinal symptoms, nutritional abnormalities, and systemic complications ranging from anemia and osteoporosis to secondary autoimmunity and malignancy.
The only accepted treatment for CD is lifelong adherence to a gluten-free diet (GFD). Maintenance of a GFD entails the avoidance of the ingestion of any products containing even small amounts of wheat, rye, and barley. Complete gluten withdrawal in patients diagnosed with classic symptoms has been shown to lead to normalization of standardized mortality rate [7, 8], as well as improvement in the majority of related problems including osteoporosis and osteopenia [9], anemia [10], risk of malignancy [7, 11], gastrointestinal symptoms [10], and in several studies, psychological well-being and quality of life [12–15].
Despite the proven benefits of the GFD, it can be exceedingly difficult to completely avoid gluten-containing foods, and adherence to a GFD is estimated to be only 45–80% [16, 17]. Comprehensive understanding of the factors associated with optimal GFD adherence is needed to develop strategies and resources to assist individuals with CD maintain a GFD. The primary aim of this study was to identify factors prospectively that are independently correlated with GFD adherence.
Methods
An expert panel was assembled to identify factors perceived to be important in living with CD and influential in GFD adherence. The panel included gastroenterologists, nutritionists, psychologists, and adults diagnosed with CD. Over a series of meetings, a set of domains relevant to life with CD were elucidated. These domains included the psychosocial burden of disease (e.g., concern about future problems, difficulty socializing), symptoms, social and health support, self-efficacy (e.g., an individual's perception of their ability to accomplish necessary actions), perceived adherence, and general health. A bank of items was developed from these domains that were hypothesized to be representative of the areas in question. These items were assessed for clarity and comprehensiveness by two successive focus groups of 8–12 adults with biopsy confirmed CD before incorporation into the final questionnaire, the global celiac assessment scale (GCAS), consisting of 142 items.
Adults (≥18 years old) diagnosed with biopsy-confirmed CD for longer than three months were then enlisted to participate through recruitment posters that were mailed to New England support groups and advertisements placed in regional CD newsletters and publications frequented by individuals with CD. In addition, eligible patients with CD being treated at the Celiac Center at Beth Israel Deaconess Medical Center (BIDMC) were invited to participate.
All participants enrolled in the study by reviewing and signing the approved informed consent form followed by completion of the GCAS and had blood drawn for IgA antitissue transglutaminase (tTG) antibody titer. Prior to completion of the study visit, the questionnaire was reviewed for completeness by a study investigator. Finally, a highly skilled nutritionist with over five years of experience working with over 450 patients with CD evaluated participants' GFD adherence. The nutritional evaluation was done in a standardized fashion using analysis of three-day food records (or 24-h food recall when a three-day food record was unavailable), a food ingredient quiz, and a clinical interview. Global GFD adherence was recorded on a six-point Likert scale ranging from ‘Excellent adherence: Consuming gluten less than three times per year’ to ‘Not currently following a GFD’ (see Appendix 1). This expert dietician evaluation was used as the gold standard for GFD adherence. Analysis of tTG titers was done by enzymelinked immunosorbent assay (ELISA) with recombinant human antigen (INOVA Quanta Lite human-tTG IgA, San Diego, USA; sensitivity 97%, specificity 99%) [18].
Data were entered into a secure database (Access, Microsoft Office, Microsoft Corp. Redmond, WA) and reviewed for errors prior to analysis. Statistical analyses were completed using SPSS release 13.0 (SPSS Inc., Chicago, IL, 2004) for Windows. Because the sample was skewed toward better adherence [mean expert nutritionist evaluation score = 1.92 (1.11), skewness = 1.49, kurtosis = 2.20], values were transformed using a natural log, after which the distribution reached acceptable levels (skewness = 0.50, kurtosis = −0.79). Correlations were performed using Fisher's exact test, Spearman's correlation, and Pearson's product moment correlation. The study was approved by the Beth Israel Deaconess Medical Center (BIDMC) committee on clinical investigations and treatment of participants was in accordance with the ethical principles of the BIDMC CCI.
Results
One hundred and fifty-four adults participated in this study, of which 111 (72%) participated for research only and 43 (28%) participated during a clinical visit. There was no difference in mean compliance level between the two groups (P = 0.165). There was also no relationship between participation in a celiac disease patient group and study participation for research only or in conjunction with a clinical visit. In this population 53.5% of patients seen on a clinical visit belonged to a celiac support group compared to 46.5% seen for research only (P = 0.464). Mean length of time on a gluten-free diet was 58 months (range 3– 576 months), the mean age at diagnosis was 44.8 (range 1– 89) years, and the mean age at participation was 50.3 (range 22–91) years. Of these, time on gluten-free diet was skewed toward more recent diet adoption, while age at diagnosis and participation were equally distributed.
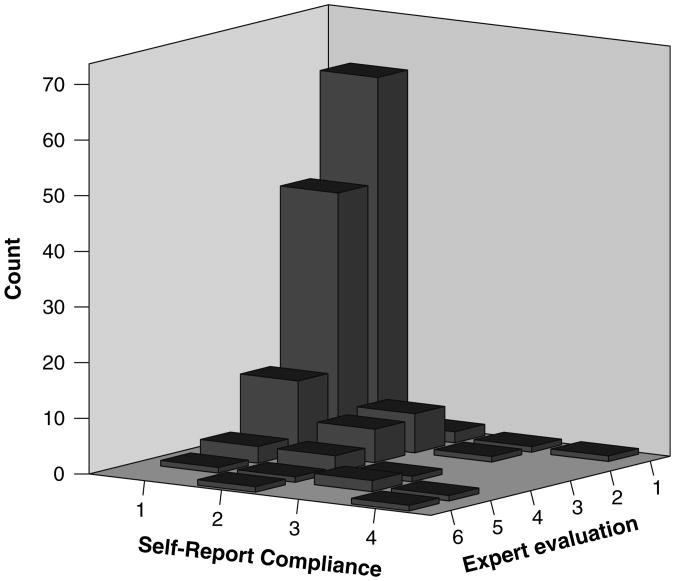
With the exception of a lower prevalence of comorbid psychiatric conditions the demographic characteristics of the study population were not significantly different from those of the overall CD population treated at BIDMC (see Table 1). In general, the study population was found to adhere well to a GFD, with 44.2% and 34.4% being rated as ‘Excellent’ or ‘Good’, respectively, by the expert nutritionist. However, individuals tended to overestimate their adherence, as 70.1% reported that they were strictly adherent to the GFD and no participants indicated that they were not following the diet at all (see Fig. 1). Similarly, tTG was normal in the group that the nutritionist rated as being highly adherent (mean = 19.8 IU/ml, 95%CI: 10.4– 29.2), but was elevated in the group that self-reported strict adherence (mean = 38.8 IU/ml, 95%CI: 22.1–55.5).
Table 1. Characteristics of the study population.
| Study group (n = 154) | CD population seen at BIDMC (n = 601) | P | |
|---|---|---|---|
| Mean age of diagnosis in years (range) | 44.8 (1–89) | 43.7 (1–90) | 0.45 |
| % Female | 76.6 | 71.7 | 0.23 |
| Race | |||
| White | 152 | 598 | 0.58 |
| Other | 2 | 3 | |
| Percentage comorbid psychiatric conditiona | 16.9 | 25.3 | 0.03 |
| Percentage otherautoimmune disorderb | 30.5 | 27.1 | 0.42 |
Predominantly depression and anxiety
Predominantly thyroid disease > type 1 diabetes mellitus > Raynaud's phenomenon > inflammatory bowel disease > sarcoidosis > psoriasis
Fig. 1.
Distribution of reported and assessed gluten-free diet adherence expert evaluation scoring: 1—excellent, participant eats gluten fewer than three times per year (<3 per year); 2—good, participant eats gluten one time per month; 3—fair, participant eats gluten 2–3 times per month; 4—poor, participant eats gluten 1–2 times per week; 5—very poor, participant eats gluten more than two times per week (>2 per week); 6—participant does not follow the gluten-free diet. Self-reporting scoring: 1—highly compliant with the gluten-free diet; 2—moderately compliant with the gluten free diet, 3—moderately noncompliant with the gluten-free diet, 4—highly noncompliant with the gluten-free diet, 5—I am not following a gluten-free diet at this time, 6—participant does not follow a gluten-free diet
Married participants were more adherent than non-married participants (P = 0.05) while other demographic factors including gender, age at participation, age at diagnosis of CD, length of time on a GFD, educational achievement, and employment status were not found to be correlated with adherence. Twenty-seven participants (17.5%) were either asymptomatic or had only atypical symptoms prior to diagnosis with CD (see Table 2), and adherence was not different between this group and the group diagnosed with classic CD symptoms (P = 0.450). The presence of other autoimmune disorders (30.5% of participants) or the presence of psychological disturbances (16.9% of participants) did not influence adherence P = 0.98 and P = 0.20, respectively (see Tables 3 and 4).
Table 2. Categorization of reported symptoms preceding the diagnosis of celiac disease.
| Classic symptomsa | Asymptomatic/atypical symptoms |
|---|---|
| Diarrhea | No symptoms |
| Abdominal pain | Anemia |
| Weight loss | Laboratory abnormalities including iron deficiency, liver function test abnormalities |
| Dermatitis herpetiformis | Osteopenia or osteoporosis |
| Fatigue or lethargy | Constipation |
| Nausea or vomiting | Gastroesophageal reflux disease or heartburn |
| Bloating or excess intestinal gas | Myalgia |
| Arthralgia | |
| Alopecia | |
| Recurrent headache | |
| Asthma | |
| Infertility |
Only one needed to qualify for classic presentation
Table 3. Association of binary demographic factors with GFD adherence.
| Factor | Percentage with excellent to good adherence | P |
|---|---|---|
| Presence of other food intolerances | 87.9 | 0.028 |
| Absence of other food intolerances | 72.9 | |
| Female gender | 79.7 | 0.551 |
| Male gender | 75.0 | |
| Comorbid autoimmune disorders | 78.7 | 0.976 |
| No comorbid autoimmune disorders | 78.5 | |
| Comorbid psychological disorders | 69.2 | 0.203 |
| No comorbid psychological disorders | 80.5 | |
| Classic CD symptoms | 79.2 | 0.450 |
| No classic CD symptoms | 75.9 | |
| Employed | 81.5 | 0.177 |
| Not employed | 71.7 | |
| Married | 83.5 | 0.052 |
| Not married | 70.2 |
Table 4. Association of ordinal/continuous demographic factors with GFD adherence.
| Factor | Mean value for participants with excellent to good adherence | Mean value for participants with fair to poor adherence | P |
|---|---|---|---|
| Educational achievementa | 6.6 | 6.3 | 0.488 |
| Age | 50.11 | 51.24 | 0.967 |
| Age at CD diagnosis | 44.82 | 44.70 | 0.721 |
| Months on GFD | 56.21 | 66.30 | 0.829 |
Reported on a scale of 1 to 10 where 1 equals completion of 8th grade or less and 10 is a doctorate
However, the presence of additional food intolerances, reported in 37.7% of participants, was correlated with improved adherence (P = 0.028; Tables 3 and 4).
Significant correlations between GCAS items and adherence are listed in Table 5. Of the participants, 75.3% did not feel that cost made it difficult to adhere to the GFD but 51.3% reported that cost was an important issue in living with CD. Furthermore, self-reporting that cost made adherence difficult was correlated with poor GFD adherence (P = 0.011), but the perception that cost was an important issue in living with celiac disease was not. Also, 56.5% reported difficulty finding GF foods when eating outside the home, 24% reported difficulty finding GF foods when shopping, and 75.3% reported that the quality of GF foods is a significant concern. However, these factors were not significantly correlated with GFD adherence as determined by multivariate analysis (P = 0.171, P = 0.182, and P = 0.731, respectively). The ability to follow a gluten-free diet while traveling, dining out, and at social events were all correlated with better GFD adherence while the avoidance of these activities in order to follow the diet was not. Similarly, although a large number of participants reported concern regarding the accessibility and quality of gluten-free foods, these factors were not associated with level of adherence.
Table 5. Factors associated with better GFD adherence.
| Factor | P (univariate) | P (multivariate)a |
|---|---|---|
| Cost makes GFD adherence difficult | 0.011 | 0.011 |
| Concern with purposeful gluten exposureb | <0.001 | 0.001 |
| Concern with accidental gluten exposureb | 0.001 | <0.001 |
| Reported understanding of GFD | 0.001 | 0.001 |
| Better score in GFD quiz | 0.001 | 0.002 |
| Ability to follow GFD when travelingb | 0.01 | 0.012 |
| Ability to follow GFD when dining outb | <0.001 | <0.001 |
| Ability to follow GFD during social eventsb | 0.004 | 0.007 |
| Membership of CD advocacy groupb | 0.004 | 0.008 |
| Comfort following GFD at work | 0.002 | 0.003 |
| Belief that avoiding gluten is important for health | 0.027 | 0.027 |
| Mood does not affect GFD adherence | 0.003 | 0.006 |
| Level of stress does not affect GFD adherenceb | 0.006 | 0.008 |
Controlling for age, age of diagnosis, time on GFD, gender, education, and marital status
Independently associated with tTG.
Of the participants, 75.3% and 79.2% believed that accidental and purposeful exposure to gluten has important health ramifications, respectively, and these responses were correlated with better GFD adherence (P = 0.001 and P < 0.001, respectively). The majority of participants reported excellent (43.5%) or good (46.1%) understanding of the GFD, and diet adherence was positively associated with reported understanding (P = 0.001). Similarly, results of a standardized GFD knowledge quiz (see Appendix 2) revealed a positive correlation between test score and adherence; adherers correctly answered a mean of 16.4 of 28 questions (58.6%) compared to 14.2 (50.7%) in non-adherers (P = 0.001).
Regarding travel, 74.7% reported that they were able to follow a GFD when traveling, whereas 24.0% reported avoiding travel in order to maintain a GFD. Of the participants, 85.7% reported that they were able to follow a GFD when eating outside the home; however 44.2% reported avoiding eating outside the home to ensure that food was GF. The percentage reporting they were able to follow a GFD while at social events such as parties and dates was 81.2%, while 21.4% reported avoiding social events to maintain a GFD. Only 45.5% felt that they were able to follow a GFD in religious practice and 37% reported avoiding certain religious practices in order to maintain a GFD. Of the participants, 58.9% belonged to a CD advocacy group and, of these, 86.5% felt that this involvement was beneficial. The percentage of participants who reported feeling comfortable following a GFD around friends and that they had sufficient family support in maintaining the GFD was 89.6%, whereas 74.7% reported that they felt comfortable following a GFD at work.
Participants reported receiving adequate information and support from their healthcare providers as follows: dietician 63.0%, gastroenterologist 57.1%, primary care physician 35.7%, and pharmacist 22.7%. Sources that were cited as being helpful in learning about the GFD were: the Internet 85.1%, dietician 64.9%, gastroenterologist 50.6%, friends with CD 48.7%, friends without CD 44.8%, other media (magazines, television, radio) 43.5%, and primary care physician 24.7%. None of these factors was correlated with GFD adherence.
Of the participants, 40.9% reported that keeping a GFD increased their level of stress, but only 18.8% reported that their stress level affected their ability to follow a GFD. Following a GFD was reported to have negative effects on social life by 33.8% of participants, while 13.6% felt that it positively affected their social life. Mood was reported to affect their ability to follow a GFD by 20.8% of participants, with 39.0% reporting a positive and 24% a negative effect on mood due to following the GFD. Increased anxiety due to having to keep a GFD was reported by 31.8%, while 27.3% reported decreased anxiety. Being diagnosed with CD was reported has having positively affected their life by 61.7%. Anger and sadness were reported by 31.2% and 40.2%, respectively, at the diagnosis of CD, with 59.8% reporting relief and 16.9% happiness. Participants who reported that they were able to follow the GFD despite changes in mood and stress had improved adherence (P = 0.006 and P = 0.008, respectively), while none of the other factors listed above was significantly correlated with adherence.
All but one participant (99.4%) reported avoiding gluten because doing so is perceived as being important for health. Worrying over the long-term consequences of CD was reported by 96.1%, while 83.8% reported avoiding gluten in order to avoid symptoms. Avoidance of gluten due to guilt and concern for their family was reported by 38.6% and 48.7%, respectively. Of these factors, only the avoidance of gluten ‘because it is important for health’ was correlated with adherence.
Discussion
In this study we demonstrated that GFD adherence is associated with a number of factors, including the presence of other food intolerances, concern over cost, concern with gluten exposure, subjective and objective perceptions of the GFD, ability to follow a GFD outside the home, and the ability to follow the GFD irrespective of mood and stress. Many individuals were not content with the services provided by their health-care team to help them manage CD. Unlike previous studies [19], females were not found to adhere better than males and demographic factors in general had surprisingly little association with GFD adherence.
Complying with a GFD can be extremely challenging for individuals with CD. Previous studies have shown that adolescents and those diagnosed with CD by serologic screening are less likely to adhere strictly to a GFD when compared to younger children and adults diagnosed because of classical symptoms [20–23]. Rates of adherence in adolescent populations vary from 56% to 83% [21, 24], while individuals diagnosed with CD at a very young age are reported to have the highest rates of adherence [20]. Furthermore, in a Swedish study, only 36% of adults who were diagnosed with CD at four years of age or older were found to be compliant with the GFD, as compared to 80% of those adults diagnosed prior to their fourth birthday [16]. In our population, only two participants were diagnosed before the age of four, which precluded our ability to examine this association.
Rates of strict adherence to a GFD in adults have been found to vary between 17% and 45% [16, 17, 25, 26]. Fewer than 50% of adults with CD studied in France and Belgium strictly adhered to a GFD for more than one year after being diagnosed [27]. Similarly, Ciacci et al., in a long-term follow-up study of adults with CD, found that 24% were nonadherent and had severe intestinal damage, while 33% were mildly nonadherent and had milder intestinal damage [17]. Our study population was similar in that fewer than 50% of participants had excellent GFD adherence according to the nutritionist assessment.
This low global level of adherence to a GFD in individuals with CD is troubling given the known morbidity and mortality associated with long-term untreated symptomatic CD [7, 11, 28, 29] and the lack of any other effective treatment. There are a variety of reasons why it is difficult to follow a GFD, including the fact that wheat-based food products are a major staple in the North American and European diet, and increasingly complex lifestyles have contributed to a greater reliance on packaged convenience foods and meals eaten away from home. Additionally, there are cost and access issues and psychological barriers to GFD adherence. Indeed, 85% of adults and 90% of children surveyed by the Canadian Celiac Association reported that finding gluten-free foods was a major barrier to complying with a GFD, and 83% indicated that finding high-quality gluten-free foods was a major obstacle [30].
Despite this clear evidence of poor adherence and anecdotal information regarding barriers to adherence, there is a striking lack of published data regarding the factors that actually influence GFD adherence in CD. The few studies that address this issue have measured adherence through self-report and/or questionnaires [16, 26, 31]. However, the questionnaires have not been validated, and self-reporting has been shown to be inaccurate [32]. Conversely, trained nutritionist evaluation shows a high correlation with intestinal biopsy abnormalities [17]. Reliable information on the factors that influence GFD adherence is crucial to enable the design of interventions aimed at improving GFD adherence and thereby the health outcomes of individuals with CD.
In addition to indicating patient qualities and attitudes that present challenges to adherence, our study data also highlight striking deficiencies both in the quality of information and in the level of support that patients receive from their health-care providers. This was true across a number of disciplines, with two-thirds of subjects rating their nutritionists positively compared to half for gastroenterologists and one-third or less for primary care physicians. Although limited to one geographic region, the finding that friends with or without celiac disease are better sources of information and support about celiac disease than primary care physicians, and nearly as good as gastroenterologists, not only reveals a deficit on the part of the medical community but highlights the great importance of social supports and patient advocacy groups in living with celiac disease. Clearly, if health-care professionals expect individuals with CD to adhere strictly to the GFD despite the difficult lifestyle changes involved then the level of education, encouragement, and assistance they provide to support GFD adherence should improve substantially.
Our study adds to the available data regarding the significant factors that play a role in gluten-free diet adherence. The large number of participants allows for multivariate analysis and is likely a representative segment of the average clinically identified American celiac population. The standardized evaluation, including a three-day food record by a highly trained nutritionist, allows for as accurate as possible an evaluation of long-term gluten intake and the GCAS was carefully developed to cover a very broad range of factors.
However, a few limitations of this study are notable. First, adherence was not confirmed with concurrent histological evaluation. However it is not clear if biopsy provides a better assessment of long-term adherence than nutritionist evaluation. In fact, prior studies showed only a modest correlation of histology with clinical presentation or assessed dietary adherence [17, 33]. Although for brevity, in the nutritionist evaluation found in Appendix 1, only frequency is noted, the evaluation was designed to be standardized, dynamic, and comprehensive. For example, whereas ‘Fair’ on the Appendix is defined as ‘Participant eats gluten 2–3 times per month’, the full description, as used clinically at our center for ‘fair’ is: ‘Does not ask questions in restaurants or when dining out—guesses or takes chances. Checks some but not all meds, supplements, body care products. Shows some confusion over label reading. Review of diet shows some obvious gluten exposure. Consumes gluten on occasion—intentionally or unintentionally per diet/lifestyle recall. May rely on partner/family member for some caregiving regarding: diet. Has not eliminated cross contamination potential in kitchen. May have mental or behavioral issues that make following the diet more difficult’. Certainly in our society even the strictest of patients will have occasional issues with contamination, and predisposition to react to a given level of gluten varies from patient to patient. There are clearly some people who are aware that they are being less vigilant than necessary with the diet whereas others truly believe that they are following the diet but are making regular errors due to poor understanding or other reasons. This likely accounts for much of the discrepancy between self-reporting and nutritionist assessment.
Also, although the study size was large in comparison to many similar studies, the data were collected from participants almost exclusively in Massachusetts and surrounding states and in a specialized teaching hospital celiac disease clinic, which may limit generalizability.
There are also a few potential sources of bias in our study. As was clear from the distribution of adherence levels, voluntary participation in a study such as this likely selects for more adherent individuals, and findings may not apply to a more poorly adherent group. Another bias may be gender distribution, although the fact that the ratio of males to females who participated in our study is nearly identical to that in our overall celiac population (76% versus 72% female) is reassuring. Similarly, age at diagnosis and age at participation did not have significant skew or kurtosis and so are less likely to bias results. Time on the gluten-free diet, however, was skewed toward recently beginning the diet, which may have bias results and obscured an association between time on the diet and adherence.
It was also unexpected that compliance was similar between classic celiac symptoms and those diagnosed by screening due to screening or atypical signs or symptoms. It could be argued that anemia is a classic manifestation of celiac disease and increasing numbers of individuals are currently tested based on this finding alone. However, similar to liver function test abnormalities, asymptomatic anemia has not traditionally prompted evaluation for celiac disease. For this reason we chose to count anemia alone in the atypical/asymptomatic category however if fatigue or GI symptoms were also present this would qualify for the classic symptoms category. Additionally, only six individuals would be affected if this change were made and there would be no overall change in significant results. Further, only 27 cases (17.5%) had nonclassic symptoms at diagnosis but adherence between the these two groups was almost identical, with a mean expert evaluation score of 1.96 versus 1.91 in classical symptom patients (P = 0.835). There were only 12 (7.8%) truly asymptomatic patients. These patients did have a believable trend toward worse adherence based on the mean expert evaluation score of 2.42, versus 1.88 for all symptomatic patients. However due to the small number, this was not statistically significant (P = 0.171).
The results of this study point to a number of areas, both obvious and obscure, that may be productive targets for interventions aimed at improving dietary adherence in individuals with CD. For instance, areas highly associated with adherence such as understanding of the GFD (measured by both reported understanding and dietary knowledge score) suggest that educational programs aimed at teaching individuals with CD more about CD and the GFD may be helpful. Similarly, the high correlation of adherence with reported ability to follow the GFD outside the home implies that providing training aimed at enhancing an individual's ability to follow a GFD at social events and when traveling, perhaps through the use of informational cards, lists of CD friendly establishments, and assertiveness training, may be beneficial. Similarly, the associations between adherence and perceived ability to follow a GFD despite changes in mood and stress level suggest that psychological and/or personality factors may be significant in determining which patients are able to comply better with dietary recommendations. Our future studies will seek to confirm the validity of these associations and develop evidence-based interventions to facilitate GFD adherence in the growing number of individuals diagnosed with CD.
Acknowledgments
This study was supported by charitable donations to the Celiac Center at BIDMC, the Celiac Sprue Association, NIH T32 training grant DK07760, and the Harvard-Thorndike General Clinical Research Center M01 RR01032.
Abbreviations
- BIDMC
Beth Israel Deaconess Medical Center
- CD
Celiac disease
- GCAS
Global celiac assessment scale
- GFD
Gluten-free diet
- tTG
Tissue transglutaminase
Appendices.
Appendix 1.
Expert dietitian evaluation of gluten-free diet adherence.
| (1) In general how compliant do you believe the participant is with the gluten-free diet (include accidental and/or intentional ingestion of gluten in analysis)? | |
| (1) Excellent; Participant eats gluten fewer than 3 times per year (<3 per year) | |
| (2) Good; Participant eats gluten 1 time per month | |
| (3) Fair; Participant eats gluten 2–3 times per month | |
| (4) Poor; Participant eats gluten 1–2 times per week | |
| (5) Very Poor; Participant eats gluten more than 2 times per week (>2 per week) | |
| (6) Participant does not follow the gluten-free diet | |
| (2) Food label quiz (circle ingredients that participant incorrectly classifies) | |
| (1) Dehydrated potatoes | (2) Oat gum |
| (3) Sugar | (4) Corn oil |
| (5) Partially hydrogenated corn oil | (6) Sea salt |
| (7) Soy lecithin | (8) Wheat flour |
| (9) Leavening (sodium bicarbonate) | |
| (10) Natural flavors | |
| (11) Sucrose | |
| (12) Molasses | (13) Spices |
| (14) Wheat starch | (15) Tomato paste |
| (16) Dextrose | |
| (17) Malt extract | (18) Maltodextrin |
| (19) Extracts of paprika | (20) Citric acid |
| (21) Beef fat | (22) Soy flour |
| (23) Corn syrup solids | (24) Barley malt flour |
| (25) Lactic acid | (26) Egg yolk |
| (27) Casein | (28) Peanut oil |
| (This ingredient list is a modified ingredient list for potato chips from a major manufacturer) | |
Appendix 2.
Gluten-free diet knowledge quiz.
| The single word “starch” on a US food label indicates that the food: | |||
| (1) Contains gluten | (2) May contain gluten | (3) Does not contain gluten | |
| In the United States distilled vinegar is gluten-free: | |||
| (1) True | (2) False | ||
| ‘Wheat-free’ is the same as gluten-free: | |||
| (1) True | (2) False | ||
| Which of the following ingredients clearly DOES NOT contain gluten: | |||
| (1) Dextrin | (2) Maltodextrin | (3) Seasonings | (4) Malt flavoring |
| Which of the following grains/flours are gluten-free in the US? | |||
| Buckwheat | Yes | No | |
| Spelt | Yes | No | |
| Teff | Yes | No | |
| Amaranth | Yes | No | |
| Kamut | Yes | No | |
| Rice pilaf | Yes | No | |
| Wild rice | Yes | No | |
| Chickpea flour | Yes | No | |
| Triticale | Yes | No | |
| Quinoa | Yes | No | |
| Natural and artificial flavorings may contain gluten: | |||
| (1) True | (2) False | ||
| Individuals with celiac disease are recommended to follow the gluten-free diet in order to avoid the following possible complications: | |||
| (1) Osteoporosis (bone loss) | Yes | No | |
| (2) Iron-deficiency anemia | Yes | No | |
| (3) Heart attack | Yes | No | |
| (4) Thyroid disorders | Yes | No | |
| (5) Cancer | Yes | No | |
| (6) Urinary tract infections | Yes | No | |
| (7) Weight loss | Yes | No | |
| (8) Diarrhea | Yes | No | |
| (9) Constipation | Yes | No | |
| (10) Renal (kidney failure) | Yes | No | |
| (11) Fertility issues | Yes | No | |
| (12) Bloating and/or gas | Yes | No | |
Contributor Information
Daniel A. Leffler, Email: dleffler@caregroup.harvard.edu, Department of Gastroenterology, The Celiac Center, Dana 501, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215, USA.
Jessica Edwards-George, Department of Gastroenterology, The Celiac Center, Dana 501, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215, USA; Department of Psychology, Northeastern University, Boston, MA, USA.
Melinda Dennis, Department of Gastroenterology, The Celiac Center, Dana 501, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215, USA.
Detlef Schuppan, Department of Gastroenterology, The Celiac Center, Dana 501, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215, USA.
Francis Cook, Department of Epidemiology, Harvard School of Public Health, Boston, MA, USA.
Debra L. Franko, Department of Psychology, Northeastern University, Boston, MA, USA
Jessica Blom-Hoffman, Department of Psychology, Northeastern University, Boston, MA, USA.
Ciaran P. Kelly, Department of Gastroenterology, The Celiac Center, Dana 501, Beth Israel Deaconess Medical Center, 330 Brookline Ave, Boston, MA 02215, USA
References
- 1.Dieterich W, Esslinger B, Schuppan D. Pathomechanisms in celiac disease. Int Arch Allergy Immunol. 2003;132:98–108. doi: 10.1159/000073710. [DOI] [PubMed] [Google Scholar]
- 2.Fasano A, Berti I, Gerarduzzi T, Not T, Colletti RB, Drago S, Elitsur Y, Green PH, Guandalini S, Hill ID, Pietzak M, Ventura A, Thorpe M, Kryszak D, Fornaroli F, Wasserman SS, Murray JA, Horvath K. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 3.Not T, Horvath K, Hill ID, Partanen J, Hammed A, Magazzu G, Fasano A. Celiac disease risk in the USA: high prevalence of antiendomysium antibodies in healthy blood donors. Scand J Gastroenterol. 1998;33:494–498. doi: 10.1080/00365529850172052. [DOI] [PubMed] [Google Scholar]
- 4.Catassi C, Fabiani E, Ratsch IM, Coppa GV, Giorgi PL, Pierdomenico R, Alessandrini S, Iwanejko G, Domenici R, Mei E, Miano A, Marani M, Bottaro G, Spina M, Dotti M, Montanelli A, Barbato M, Viola F, Lazzari R, Vallini M, Guariso G, Plebani M, Cataldo F, Traverso G, Ventura A, et al. The coeliac iceberg in Italy. A multicentre antigliadin antibodies screening for coeliac disease in school-age subjects. Acta Paediatr Suppl. 1996;412:29–35. doi: 10.1111/j.1651-2227.1996.tb14244.x. [DOI] [PubMed] [Google Scholar]
- 5.Maki M, Mustalahti K, Kokkonen J, Kulmala P, Haapalahti M, Karttunen T, Ilonen J, Laurila K, Dahlbom I, Hansson T, Hopfl P, Knip M. Prevalence of Celiac disease among children in Finland. N Engl J Med. 2003;348:2517–2524. doi: 10.1056/NEJMoa021687. [DOI] [PubMed] [Google Scholar]
- 6.Accomando S, Cataldo F. The global village of celiac disease. Dig Liver Dis. 2004;36:492–498. doi: 10.1016/j.dld.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 7.West J, Logan RF, Smith CJ, Hubbard RB, Card TR. Malignancy and mortality in people with coeliac disease: population based cohort study. BMJ. 2004;329:716–719. doi: 10.1136/bmj.38169.486701.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corrao G, Corazza GR, Bagnardi V, Brusco G, Ciacci C, Cottone M, Sategna Guidetti C, Usai P, Cesari P, Pelli MA, Loperfido S, Volta U, Calabro A, Certo M. Mortality in patients with coeliac disease and their relatives: a cohort study. Lancet. 2001;358:356–361. doi: 10.1016/s0140-6736(01)05554-4. [DOI] [PubMed] [Google Scholar]
- 9.Tau C, Mautalen C, De Rosa S, Roca A, Valenzuela X. Bone mineral density in children with celiac disease. Effect of a Gluten-free diet. Eur J Clin Nutr. 2006;60:358–363. doi: 10.1038/sj.ejcn.1602323. [DOI] [PubMed] [Google Scholar]
- 10.Dewar DH, Ciclitira PJ. Clinical features and diagnosis of celiac disease. Gastroenterology. 2005;128:S19–S24. doi: 10.1053/j.gastro.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Green PH, Fleischauer AT, Bhagat G, Goyal R, Jabri B, Neugut AI. Risk of malignancy in patients with celiac disease. Am J Med. 2003;115:191–195. doi: 10.1016/s0002-9343(03)00302-4. [DOI] [PubMed] [Google Scholar]
- 12.Zarkadas M, Cranney A, Case S, Molloy M, Switzer C, Graham ID, Butzner JD, Rashid M, Warren RE, Burrows V. The impact of a gluten-free diet on adults with coeliac disease: results of a national survey. J Hum Nutr Diet. 2006;19:41–49. doi: 10.1111/j.1365-277X.2006.00659.x. [DOI] [PubMed] [Google Scholar]
- 13.Mustalahti K, Lohiniemi S, Collin P, Vuolteenaho N, Laippala P, Maki M. Gluten-free diet and quality of life in patients with screen-detected celiac disease. Eff Clin Pract. 2002;5:105–113. [PubMed] [Google Scholar]
- 14.Pynnonen PA, Isometsa ET, Verkasalo MA, Kahkonen SA, Sipila I, Savilahti E, Aalberg VA. Gluten-free diet may alleviate depressive and behavioural symptoms in adolescents with coeliac disease: a prospective follow-up case-series study. BMC Psychiatry. 2005;5:14. doi: 10.1186/1471-244X-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Addolorato G, Capristo E, Ghittoni G, Valeri C, Masciana R, Ancona C, Gasbarrini G. Anxiety but not depression decreases in coeliac patients after one-year gluten-free diet: a longitudinal study. Scand J Gastroenterol. 2001;36:502–506. doi: 10.1080/00365520119754. [DOI] [PubMed] [Google Scholar]
- 16.Hogberg L, Grodzinsky E, Stenhammar L. Better dietary compliance in patients with coeliac disease diagnosed in early childhood. Scand J Gastroenterol. 2003;38:751–754. doi: 10.1080/00365520310003318. [DOI] [PubMed] [Google Scholar]
- 17.Ciacci C, Cirillo M, Cavallaro R, Mazzacca G. Long-term follow-up of celiac adults on gluten-free diet: prevalence and correlates of intestinal damage. Digestion. 2002;66:178–185. doi: 10.1159/000066757. [DOI] [PubMed] [Google Scholar]
- 18.Van Meensel B, Hiele M, Hoffman I, Vermeire S, Rutgeerts P, Geboes K, Bossuyt X. Diagnostic accuracy of ten second-generation (human) tissue transglutaminase antibody assays in celiac disease. Clin Chem. 2004;50:2125–2135. doi: 10.1373/clinchem.2004.035832. [DOI] [PubMed] [Google Scholar]
- 19.Ciacci C, D'Agate C, De Rosa A, Franzese C, Errichiello S, Gasperi V, Pardi A, Quagliata D, Visentini S, Greco L. Self-rated quality of life in celiac disease. Dig Dis Sci. 2003;48:2216–2220. doi: 10.1023/b:ddas.0000004530.11738.a2. [DOI] [PubMed] [Google Scholar]
- 20.Pietzak MM. Follow-up of patients with celiac disease: achieving compliance with treatment. Gastroenterology. 2005;128:S135–S141. doi: 10.1053/j.gastro.2005.02.025. [DOI] [PubMed] [Google Scholar]
- 21.Mayer M, Greco L, Troncone R, Auricchio S, Marsh MN. Compliance of adolescents with coeliac disease with a gluten free diet. Gut. 1991;32:881–885. doi: 10.1136/gut.32.8.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fabiani E, Catassi C, Villari A, Gismondi P, Pierdomenico R, Ratsch IM, Coppa GV, Giorgi PL. Dietary compliance in screening-detected coeliac disease adolescents. Acta Paediatr Suppl. 1996;412:65–67. doi: 10.1111/j.1651-2227.1996.tb14256.x. [DOI] [PubMed] [Google Scholar]
- 23.Fabiani E, Taccari LM, Ratsch IM, Di Giuseppe S, Coppa GV, Catassi C. Compliance with gluten-free diet in adolescents with screening-detected celiac disease: a 5-year follow-up study. J Pediatr. 2000;136:841–843. [PubMed] [Google Scholar]
- 24.Kumar PJ, Walker-Smith J, Milla P, Harris G, Colyer J, Halliday R. The teenage coeliac: follow up study of 102 patients. Arch Dis Child. 1988;63:916–920. doi: 10.1136/adc.63.8.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bardella MT, Molteni N, Prampolini L, Giunta AM, Baldassarri AR, Morganti D, Bianchi PA. Need for follow up in coeliac disease. Arch Dis Child. 1994;70:211–213. doi: 10.1136/adc.70.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rashid M, Cranney A, Zarkadas M, Graham ID, Switzer C, Case S, Molloy M, Warren RE, Burrows V, Butzner JD. Celiac disease: evaluation of the diagnosis and dietary compliance in Canadian children. Pediatrics. 2005;116:e754–e759. doi: 10.1542/peds.2005-0904. [DOI] [PubMed] [Google Scholar]
- 27.Vahedi K, Mascart F, Mary JY, Laberenne JE, Bouhnik Y, Morin MC, Ocmant A, Velly C, Colombel JF, Matuchansky C. Reliability of antitransglutaminase antibodies as predictors of gluten-free diet compliance in adult celiac disease. Am J Gastroenterol. 2003;98:1079–1087. doi: 10.1111/j.1572-0241.2003.07284.x. [DOI] [PubMed] [Google Scholar]
- 28.Peters U, Askling J, Gridley G, Ekbom A, Linet M. Causes of death in patients with celiac disease in a population-based Swedish cohort. Arch Intern Med. 2003;163:1566–1572. doi: 10.1001/archinte.163.13.1566. [DOI] [PubMed] [Google Scholar]
- 29.Sheiner E, Peleg R, Levy A. Pregnancy outcome of patients with known celiac disease. Eur J Obstet Gynecol Reprod Biol. 2006;129(1):41–45. doi: 10.1016/j.ejogrb.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 30.Cranney A, Zarkadas M, Graham ID, Switzer C. The Canadian celiac health survey—the Ottawa chapter pilot. BMC Gastroenterol. 2003;3:8. doi: 10.1186/1471-230X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green PHR, Stavropoulos SN, Panagi SG, Goldstein SL, McMahon DJ, Absan H, Neugut AI. Characteristics of adult celiac disease in the USA: results of a national survey. Am J Gastroenterol. 2001;96:126–131. doi: 10.1111/j.1572-0241.2001.03462.x. [DOI] [PubMed] [Google Scholar]
- 32.Fera T, Cascio B, Angelini G, Martini S, Guidetti CS. Affective disorders and quality of life in adult coeliac disease patients on a gluten-free diet. Eur J Gastroenterol Hepatol. 2003;15:1287–1292. doi: 10.1097/00042737-200312000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Lee SK, Lo W, Memeo L, Rotterdam H, Green PH. Duodenal histology in patients with celiac disease after treatment with a gluten-free diet. Gastrointest Endosc. 2003;57:187–191. doi: 10.1067/mge.2003.54. [DOI] [PubMed] [Google Scholar]