Abstract
Objective
The purpose of this study was to quantify lumbar zygapophyseal (Z) joint space separation (gapping) in low back pain (LBP) subjects after spinal manipulative therapy (SMT) or side-posture positioning (SPP).
Methods
This was a controlled mechanisms trial with randomization and blinding. Acute LBP subjects (N=112, four n=28 MRI protocol groups) had 2 magnetic resonance imaging (MRI) appointments (initial enrollment [M1] and following 2 weeks of chiropractic treatment [M2]; receiving 2 MRI scans of the L4/L5 and L5/S1 Z joints at each MRI appointment. After the first MRI scan of each appointment, subjects were randomized (M1 appointment) or assigned (M2 appointment) into SPP (non-manipulation), SMT (manipulation), or control MRI protocol groups. After SPP or SMT, a second MRI was taken. The central anterior-posterior (A-P) joint space was measured. Difference between most painful side A-P measurements taken post- and pre-intervention was the Z joint “gapping difference.” Gapping differences were compared (ANOVA) among protocol groups. Secondary measures of pain visual analog scale (VAS), verbal numeric pain rating scale (VNPRS), and function Bournemouth questionnaire (BQ) were assessed.
Results
Gapping differences were significant at the first (adjusted, p=0.01; SPP=0.66 +0.48mm; SMT=0.23 +0.86; control=0.18 +0.71) and second (adjusted, p=0.0005; SPP=0.65 +0.92mm, SMT=0.89 +0.71; control=0.35 +0.32) MRI appointments. VNPRS differences were significant at first MRI appointment (p=0.04) with SMT showing the greatest improvement. VAS and BQ improved after two weeks of care in all groups (both p<0.0001).
Conclusions
SPP showed greatest gapping at baseline. After two weeks, SMT resulted in greatest gapping. SPP appeared to have additive therapeutic benefit to SMT.
Keywords: Manipulation, Spinal, Zygapophyseal Joint, Chiropractic, Low Back Pain, Lumbar vertebrae
Introduction
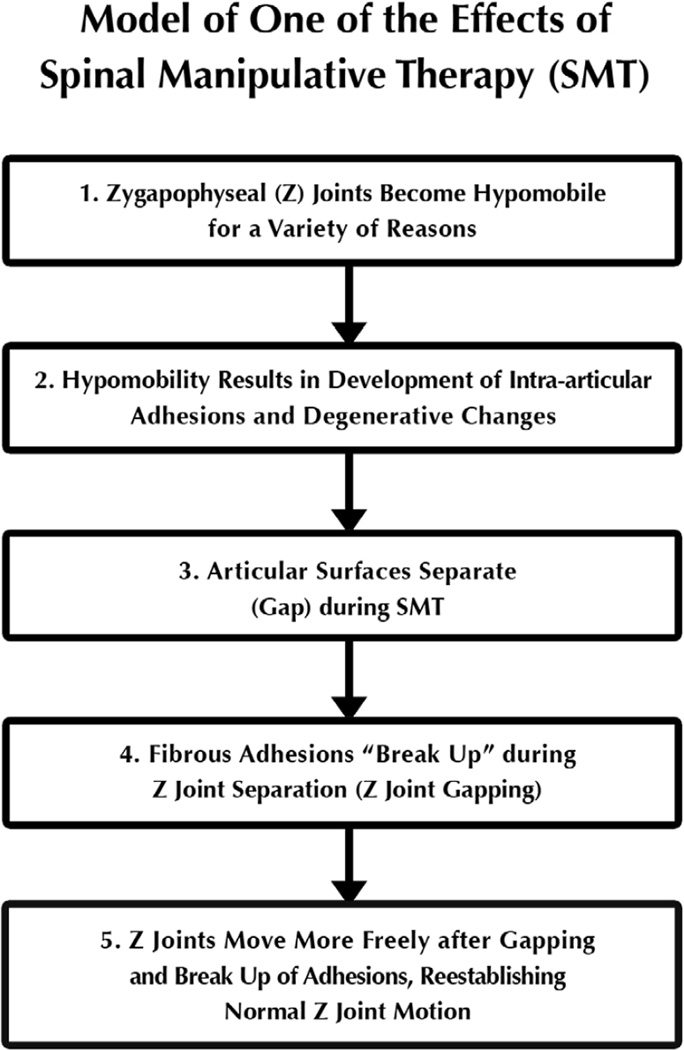
A fundamental hypothesis of a beneficial effect of chiropractic spinal manipulative therapy (SMT) is that adhesions developing in hypomobile zygapophyseal (Z) joints are broken during SMT by gapping of the Z joint articular surfaces1–3 (Figure 1).
Figure 1.
Theoretical model of one of the beneficial effects of spinal manipulative therapy (SMT, spinal adjusting). This project assessed Step 3 of the model, separation of the Z joint articular surfaces (therapeutic gapping).
Vertebral segmental hypomobility has been identified clinically, and low back pain patients with identified vertebral hypomobility have been found to respond more favorably to SMT than those without hypomobility.4,5 Putative reasons for Z joint hypomobility include: inactivity; injury; or repetitive, asymmetric motions (e.g., assembly line work). Such repetitive motions would tend to result in normal or increased movement of some of the Z joints while chronically loading others. The joints receiving the long-term loading would likely become relatively hypomobile.
Fibrous adhesions are thought to develop in hypomobile Z joints, further preventing normal joint motions.1–3 In fact, fibrous adhesions6 and degenerative changes7 have been quantified in hypomobile animal Z joints (Figure 1, Step 2). Gapping of the Z joints is thought to break-up intra-articular adhesions that have developed during hypomobility and aid in re-establishing normal range of motion to the Z joints (Figure 1, Steps 3–5).1,3,8 In the past, SMT was hypothesized to separate, or gap, the Z joint articular surfaces;3,8–14 and more recently, SMT and side-posture positioning (SPP) have been shown to gap the lumbar Z joints in healthy human volunteers, with SMT resulting in greater gapping than SPP alone.15–17 However, no previous studies assessed Z joint gapping in clinical (low back pain) patients.
The study reported here was designed to determine whether or not Z joints gap during lumbar side-posture SMT and SPP in acute low back pain (LBP) patients (Figure 1, Step 3). Z joint gapping was assessed from MRI scans taken at initial presentation and after 2 weeks of chiropractic care. Secondary outcomes assessing pain and functional impairment were also included.
Methods
Project Overview
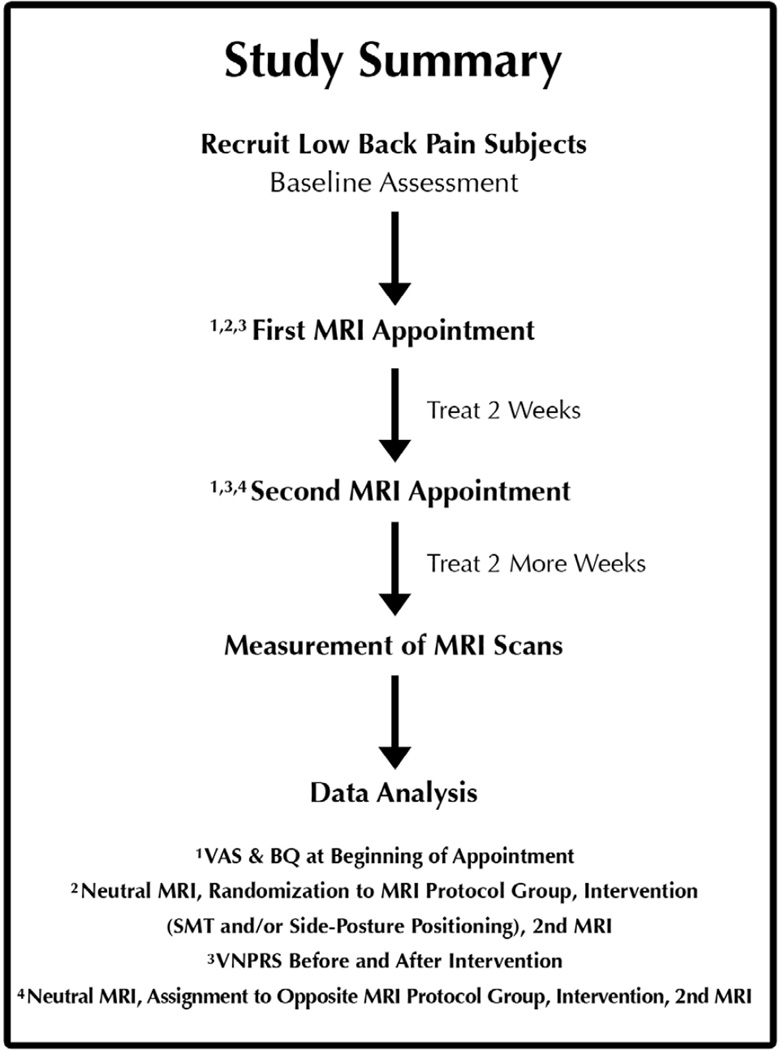
This controlled mechanisms trial with randomization and blinding used 4 MRI protocol groups (SPP, SMT, and 2 control groups) to assess a component of one of the proposed mechanisms of SMT. Figure 2 shows the general overview of the study. The study was not designed to assess the effectiveness of spinal manipulation as a treatment, other investigators are conducting such effectiveness studies;18–24 this study was designed to show gapping of the Z joints with SPP and SMT in LBP subjects. All subjects in this study received the same modalities of care during the treatment phase of the project.
Figure 2.
Flowchart showing the overview of the project. VAS = visual analog scale, BQ = Bournemouth Questionnaire of functional impairment,39 and VNPRS = verbal numeric pain rating scale.
This study was approved by the institutional review board of the National University of Health Sciences (IRB #H-0107) and was registered with the US NIH Clinical Trial Registry (NCT00284063).
Screening Examination
Table 1 shows the inclusion and exclusion criteria used at the screening examination. The acute low back pain subjects25 included in this study closely matched the patients described as “Category 1” (more specifically, Categories 1a and 1b) of the Quebec Task Force classification.26 Each subject’s most painful side (primary treatment side, PTS) was determined at the examination. The treating clinician (DG) asked the patient to describe her/his pain and to identify the most painful side. The subject’s reported most painful side became the PTS and did not change throughout the study. The PTS was the up-side during all SMT or SPP during the first (M1) and second (M2) MRI appointments.
Table 1.
Inclusion and exclusion criteria during the screening examination
| Inclusion | Exclusion | |
|---|---|---|
| Criteria |
|
|
MRI Scanning
Previously published methods were used for the MRI positioning and scanning.15,16 Each of the 112 subjects received 2 MRI scans (Hitachi MRP 5000, 0.2 Tesla MRI unit) on 2 separate occasions; the initial MRI appointment (M1) and after 2 weeks of chiropractic care (M2) (Figures 3 and 4).
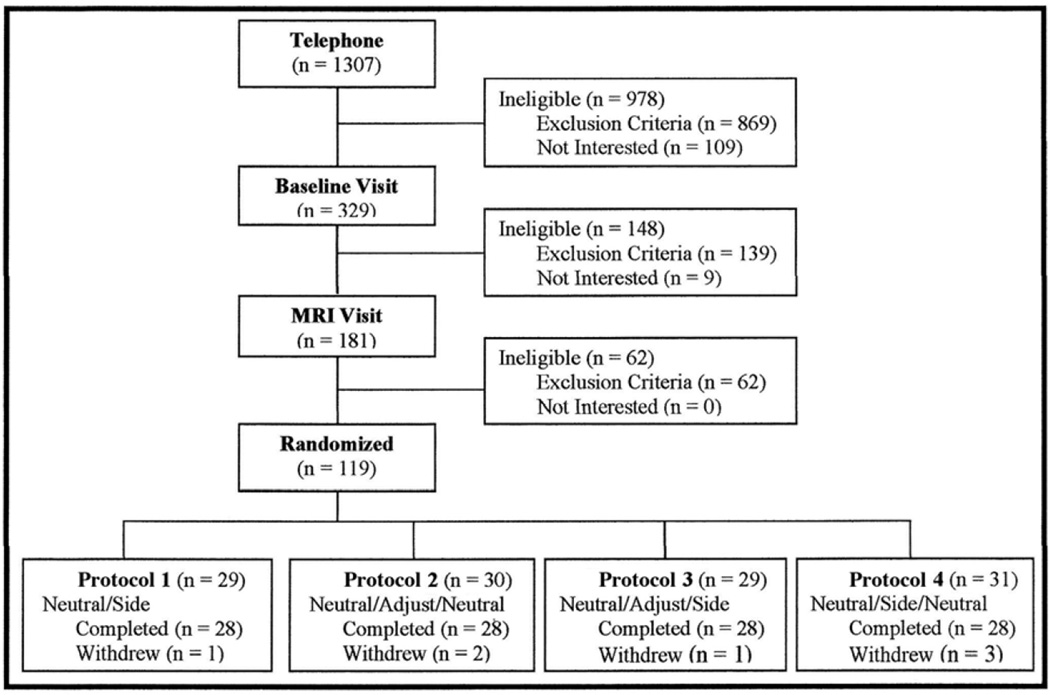
Figure 3.
Enrollment and exclusion of study subjects. Exclusions are listed along the right side of the flowchart. Withdrawals during the 2-week treatment period and second MRI scan are summarized in the last row of boxes (Protocols 1–4). The following abbreviations are used in the bottom row of boxes: neutral = supine position, side = side-posture positioning (SPP), SMT = spinal manipulative therapy. All SMT was performed with the most painful side (primary treatment side, PTS) as the up-side.
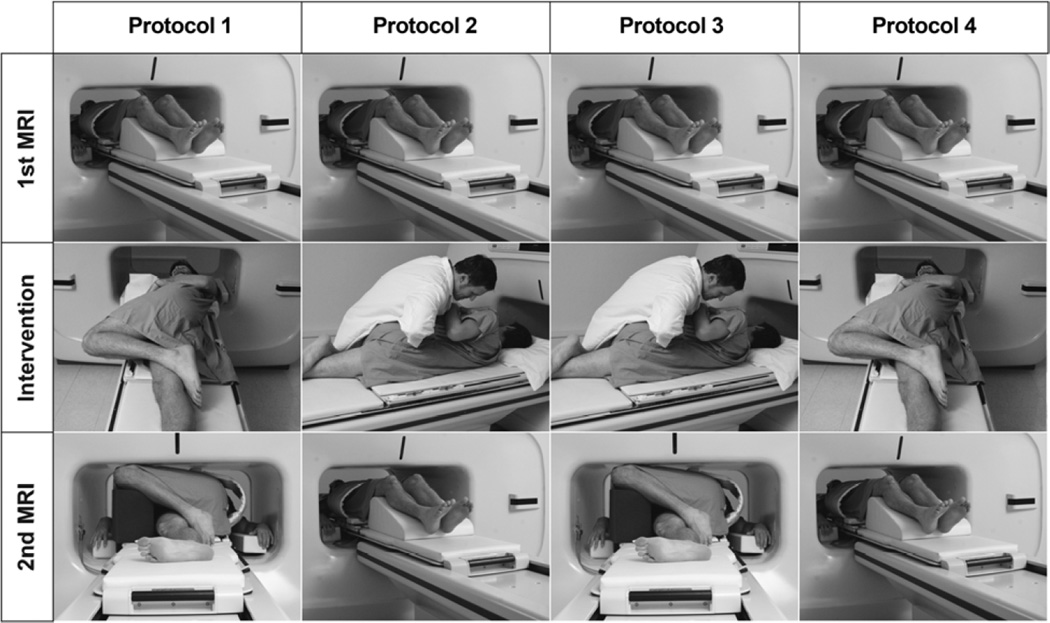
Figure 4.
Procedures used for each of the 4 study protocol groups. The protocols are described in the “MRI Scanning” subsection of the Material and Methods. Notice that all protocols began with an MRI scan in the neutral position (first row). Subjects were then randomized into one of 4 protocol groups (second and third rows) and were then scanned a second time in either the neutral or side-posture position (third row). Although subjects are shown receiving spinal manipulative therapy (SMT) or SPP with the left side as the up-side, the up-side was the subject’s most painful side (primary treatment side, PTS) at the examination appointment, which was frequently the right side (see Table 3).
Figure 4 shows the protocols used for the four protocol groups of the study. The most painful side, the primary treatment side (PTS), was always the up-side for side-posture positioning or SMT. The four protocol groups were as follows: Protocol 1 (SPP group): neutral positioning, followed by side-posture positioning (SPP), remaining in SPP for second MRI scan; Protocol 2 (SMT control): neutral positioning, followed by side-posture spinal manipulative therapy (SMT), followed by neutral positioning for second MRI; Protocol 3 (SMT group): neutral positioning, followed by side-posture SMT and remaining in side-posture for second MRI; and Protocol 4 (SPP control): neutral positioning, followed by brief SPP, followed by neutral positioning for second MRI.
MRI scans were taken with the subjects in the original neutral position and in the final position. The first scan of each MRI appointment was taken in the neutral (supine) position. This allowed for a baseline Z joint space (gapping) measurement to be obtained for each subject. The initial (neutral position) MRI was followed by an intervention (side-posture SMT or SPP), which was immediately followed by a second scan. The second MRI scan was taken either back in the supine (control Protocol Groups 2 and 4) or side-posture position (Protocol Groups 1 and 3). Imaging in the neutral position for the second MRI scans of Protocol Groups 2 and 4 served as controls for the SPP and SMT protocol groups imaged in the side-posture position for the second MRI scan (Protocol Groups 1 and 3). Previous studies showed that reloading the spine by placing the subject in the neutral position following the intervention resulted in no Z joint gapping difference between the first and second MRI scans.15,16 Consequently, Groups 2 and 4 were the control groups in the study (neutral position for the second MRI scan). The 2 MRI scans and interposed intervention were conducted over approximately 30 minutes (12:19 minutes per MRI scan; thus, side-posture positioning was held for this amount of time during the second MRI scan of Protocol Groups 1 and 3). All interventions were performed directly on the MRI gantry table after the first scan (Figures 3 and 4).
First MRI Appointment (M1)
The M1 appointment was conducted before any treatment began. Figures 3 and 4 summarize the design of the appointment.27,28 During the M1 appointment the subjects were randomized into one of 4 MRI protocol groups (see MRI Scanning, above). A technician not involved in patient contact used a random number generator to develop the randomization scheme. If a subject was eligible for study participation after the first scan of the M1 appointment, the scanning radiologist would leave the area to be blinded to SMT or no-SMT intervention, and the clinical research assistant pulled the next male or female randomization envelope from a safe. The research clinician then performed the SMT and/or positioned the subject according to randomized protocol. Once the clinician had completed the protocol with the subject remaining on his/her side, the scanning radiologist was called back and was told whether the subject should be positioned in the neutral or side-posture position for the second MRI scan.
Spinal Manipulation
The SMT; resisted mamillary push technique,29 used in the previous studies on healthy subjects,15,16 was also used in this trial (Figure 4, center row Protocols 2 and 3). One intent of the procedure is to open (gap) the up-side targeted joints, in this study the L4/L5 and L5/S1 Z joints.
Two Weeks of Treatment
Following the first MRI appointment, all subjects received chiropractic care for 2 weeks (1–3 visits per week as recommended by the treating clinician). The care included SMT and other modalities as deemed appropriate, including: hot moist packs, ultrasound, and/or interferential nerve stimulation. Only SMT was provided during the MRI appointments. Analgesic use during the previous week was recorded at the subject’s first appointment of each week. Study participants were asked to avoid any other form of care for his/her low back the two days prior to their MRI appointments. The subjects were also asked not to engage in heavy lifting (e.g. weight training) or prolonged walking or jogging during the same time period. These recommendations were made to avoid excessive loading of the Z joints for the two days prior to the MRI appointments. These were the only restrictions placed on subjects regarding outside care. Outside care was tracked at every visit (see Results for a description of the 3 subjects who sought outside care).
Second MRI Appointment (M2)
The M2 appointment occurred after 2 weeks of treatment (Figure 2). The M2 appointment was identical to the M1 appointment with the exception that each subject was assigned to the protocol group “opposite” the one to which she/he was randomized at the M1 appointment. That is, M1 Protocol 1 was assigned to M2 Protocol 2 and vice versa, and M1 Protocol 3 was assigned to M2 Protocol 4 and vice versa. This way during the study all subjects were in both an intervention and control group and all subjects were in an SPP and SMT group. Completion of the M2 appointment signified the completion of data collection for this study. Following the M2 appointment, subjects were provided as needed care for up to two additional weeks; however no information entered into their files during this final two weeks of care was used in the study.
Morphometry
The methods used to choose the MRI images to be measured and the procedures for making the measurements of the Z joint space from the images were described previously.30 A trained radiologist marked the Z joint images to be measured. During MR imaging, the scans were coded using random numbers so that all investigators, including the radiologist, were blinded to protocol group, MRI appointment (first or second), MRI scan (first or second for each appointment), and all subject identifying information.
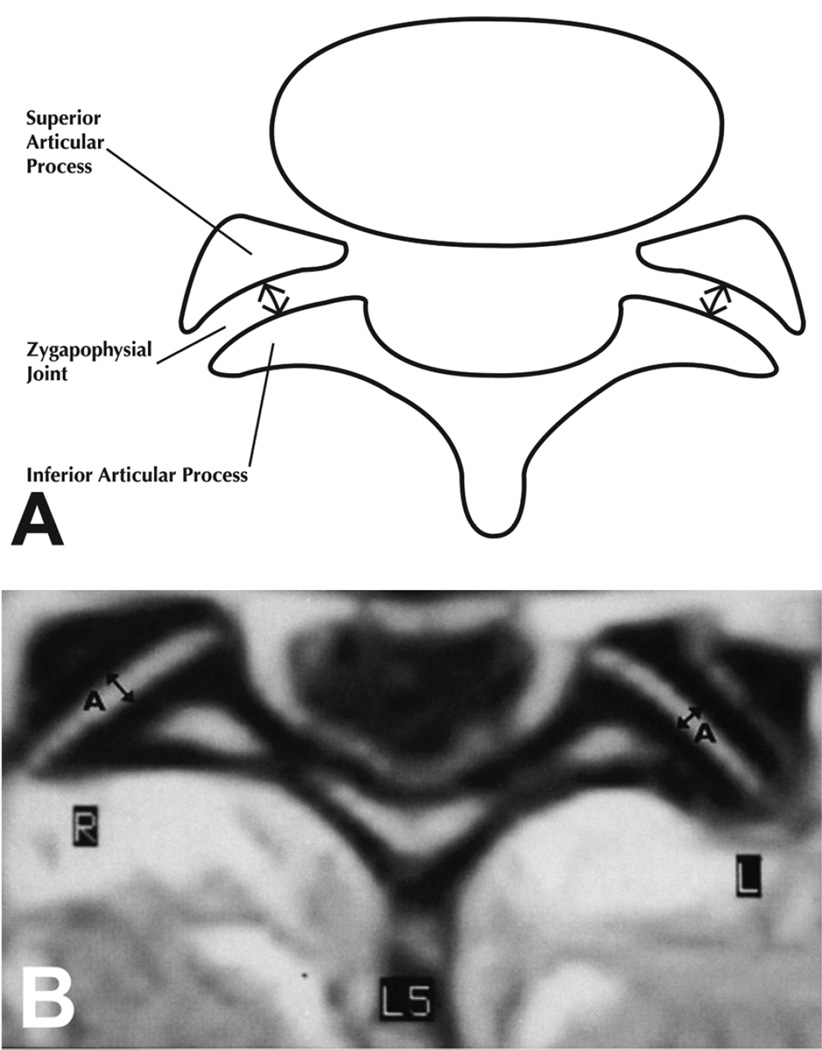
Using the procedures described and found to be reliable in a previous study,30 three trained observers measured the central anterior-posterior (A-P) Z joint space (Figure 5) on the coded scans. Measurements were made of the left and right L4/L5 and L5/S1 levels using a backlit GTCO Calcomp Drawing Board III digitizer (Source Graphics, Anaheim, CA) and were rounded to the nearest 0.1 mm. The observers were blinded to the other observers’ measurements.
Figure 5.
Illustration (A) and MRI scan (B) showing the central anterior to posterior (A-P) measurement of the Z joints that were made from the left and right L4/L5 and L5/S1 Z joints in this study.
Outcome Measures
Greatest Gapping Difference of the Primary Treatment Side (GGDPTS)
After all measurements were completed, the identification codes of the MRI scans were broken and the measurements from the first and second scans for each subject were paired. The value for each joint obtained from the first scan (pre-intervention) was subtracted from that of the second (post-intervention) scan to yield the “gapping difference” for each joint. A positive gapping difference indicated an increase in gapping following the SMT or SPP. SMT is not as specific as was previously supposed.31 Before the project began, the investigators determined that the L4/L5 or L5/S1 Z joint on the primary treatment side with the largest gap was the segment that received the primary force of the manipulation or the primary torque of the side-posture positioning. Therefore, the segment with the largest gap was used as the primary outcome for each subject for all of the protocol groups, including the controls (Protocol Groups 2 and 4); the greatest gapping difference on the primary treatment side (GGDPTS) was the primary outcome of the study and was calculated for all subjects in all 4 MRI protocol groups for both the M1 and M2 appointments.
Visual Analog Scale (VAS)
A 100 mm visual analog scale (VAS) was used to assess low back pain (anchors = “No Pain” and “Worst Pain Imaginable”).32–36
Verbal Numeric Pain Rating Scale (VNPRS)
A verbal numeric pain rating scale37,38 (VNPRS) was administered twice at each MRI appointment. After the initial, neutral-position MRI scan, a research assistant asked subjects to rate their current pain on a scale of 0–10 with 0 being no pain and 10 being the worst pain imaginable. The subject then received an intervention (SMT and/or SPP) while remaining on the MRI gantry table. The subject provided a second VNPRS after the intervention, which was immediately before the second MRI scan.
Bournemouth Questionnaire
Function was assessed by the reliable, seven-question Bournemouth Questionnaire (BQ) that scores impairment of function as a result of LBP on a scale of 0–70 (0 = no impairment, 70 = maximum impairment).39
Statistical Analysis
Sample Size
Sample size was determined by conducting a power analysis using the gapping differences and data variability of a previous study on healthy subjects and a pilot study on acute and chronic low back pain patients.16,40
Greatest Gapping Difference of the Primary Treatment Side (GGDPTS)
The GGDPTS values of each MRI appointment were analyzed to determine if differences existed between the 4 protocol groups (ANOVA with Tukey-Kramer post-hoc analysis). The Kruskal-Wallis test (ANOVA for nonparametric data) and Dunn’s post-hoc analysis were used when the data did not pass the normality test. Data were graphed and assessed for outliers. If outliers were found, secondary analyses were conducted with the outliers removed. GGDPTS analyses were conducted for both M1 and M2 data.
Greatest Gapping Differences of Males vs. Females
Previous authors have emphasized the importance of assessing the influence of gender on measurements of anatomic structures of the spine;41,42 consequently, GGDPTS of males and females for all protocol groups were assessed together using two-sided t-tests. If a difference was found, then sub-analyses were conducted for each of the four protocol groups. These analyses were conducted for both M1 and M2 data.
Pain, Function, and Gapping at the Second MRI Appointment (M2, after two weeks of treatment)
VAS and BQ data were assessed for all subjects before and after 2 weeks of treatment in order to give an indication of the overall change in pain and functional impairment, respectively. This was done by descriptive statistics and two-sided t-tests.
Difference in Pain (VNPRS) between the First and Second MRI Scans of Each MRI Appointment (M1 and M2)
Verbal numeric pain rating scale (VNPRS) differences before and after intervention were calculated for each MRI appointment (M1 and M2). VNPRS differences among the 4 MRI protocol groups were then compared using ANOVA. This was done separately for M1 and M2 data in order to determine changes in pain due to the intervention that took place at each MRI appointment.
Results
Figure 3 shows the flow of subjects through the study and includes numbers of subjects enrolled and numbers of subjects excluded or dropped out at each stage of the study. Table 2 shows the total number of exclusions for the study. Many subjects met multiple exclusion criteria; consequently, the number of exclusions in Table 2 is much higher than the number of subjects excluded as shown in Figure 3.
Table 2.
Reasons for subject exclusions
| Exclusion Reason | Telephone Screen |
Baseline Visit |
MRI Visit |
Total Excluded |
|---|---|---|---|---|
| Less than 21 years of age or greater than 69 years |
27 | 0 | 0 | 27 |
| Weight/BMI above allowable criteria | 312 | 36 | 0 | 348 |
| No low back pain or inability to reproduce pain at L4/L5 and/or L5/S1 |
50 | 16 | 0 | 66 |
| Chronic low back pain or recurrent episode of low back pain greater than 6 weeks |
1273 | 71 | 0 | 1344 |
| Scoliosis greater than 5 degrees | 7 | 3 | 3 | 13 |
| Presence of radiculopathy | 147 | 45 | 0 | 192 |
| Claustrophobia or other intolerance to MRI procedures |
25 | 0 | 3 | 28 |
| Pregnant or nursing | 3 | 0 | 0 | 3 |
| Transitional L4/L5 or L5/S1 segment | 0 | 0 | 17 | 17 |
| Severe arthritic change or osseous bridging |
0 | 0 | 14 | 14 |
| Disc protrusion/extrusion greater than 5mm |
0 | 0 | 26 | 26 |
| Spondylolysis or spondylolisthesis | 0 | 0 | 12 | 12 |
| Other significant pathology or contraindication to study participation |
100 | 16 | 9 | 125 |
| Not fluent or literate in English | 10 | 0 | 0 | 10 |
| Current or future health litigation | 32 | 3 | 0 | 35 |
| Total1 | 1986 | 190 | 84 | 2260 |
Totals are higher than Figure 3 due to frequent multiple exclusions per subject
Recruitment and enrollment lasted 22 months. Seven withdrawals occurred after the first MRI appointment. The reasons for the withdrawals included: not showing up for scheduled appointments or no longer interested in continuing (5), claustrophobia during the second M2 appointment (1), “not liking” the SMT (1). These withdrawals were evenly distributed among the MRI protocol groups (Figure 3). Because an important aspect of the study was to compare overall study outcomes at the M1 and M2 appointments (M2 after 2 weeks of care), MRI measurements were not taken from the M1 scans of withdrawn subjects. Enrollment was carefully tracked and intentionally slowed when only a few subjects were needed to reach the goal of 112 subjects. The study ended when the final subject completed the scheduled two weeks of treatment following the second MRI appointment.
First MRI Appointment (M1)
Subject Characteristics
Table 3 and the M1 (left) side of Table 4 show the subject characteristics for the four randomized protocol groups at M1. More males (n=75) were enrolled than females (n=37). With the possible exception of analgesic use (Table 4), subject characteristic differences among the protocol groups were not considered important to the outcome of the study. Subsequent analyses also found that analgesic use was not related to gapping (see Discussion).
Table 3.
Characteristics of subjects at first MRI appointment (M1)
| Protocol1 | Gender M=Male F=Female |
Primary Treatment Side (PTS) L=Left R=Right |
Age (years ±SD) |
Height (in ±SD) |
Weight (lbs ±SD) |
|---|---|---|---|---|---|
| 1 | M=21, F=7 | L=16 (57%), R=12 (43%) | 44.2 (12.7) | 69.0 (2.9) | 174.7 (26.2) |
| 2 | M=17, F=11 | L=16 (57%), R=12 (43%) | 42.7 (10.3) | 67.0 (4.2) | 160.8 (29.9) |
| 3 | M=20, F=8 | L=13 (46%), R=15 (54%) | 43.7 (12.7) | 68.4 (3.1) | 167.3 (28.2) |
| 4 | M=17, F=11 | L=14 (50%), R=14 (50%) | 47.6 (10.0) | 68.0 (3.9) | 172.5 (29.8) |
n=28 for each protocol group, total N=112
Table 4.
Pain (VAS), functional impairment (BQ) and analgesic use at first (M1) and second (M2) MRI appointments
| Protocol M11 |
VAS2 M1 | BQ3 M1 | Meds4 M1 | Protocol M21 |
VAS2 M2 | BQ3 M2 | Meds4 M2 |
|---|---|---|---|---|---|---|---|
| 1 | 30.3 (20.2) | 24.0 (13.0) | 15 (54%) | 1 (2)5 | 13.8 (15.0) | 14.4 (9.2) | 4 (14%) |
| 2 | 26.1 (15.7) | 22.9 (10.9) | 7 (25%) | 2 (1) | 17.1 (17.3) | 13.8 (12.2) | 7 (25%) |
| 3 | 32.3 (22.0) | 24.5 (11.7) | 9 (32%) | 3 (4) | 15.7 (14.3) | 12.4 (9.8) | 8 (29%) |
| 4 | 26.2 (13.6) | 23.9(11.1) | 8 (29%) | 4 (3) | 18.9 (20.7) | 13.2 (11.8) | 7 (25%) |
n=28 for each protocol group, total N=112
Visual analog scale scores (possible range 0–100) at the beginning of the MRI appointment [mean (SD)]
Bournemouth questionnaire of functional impairment scores (possible range 0–70) at the beginning of the MRI appointment [mean (SD)]
Subjects taking analgesic medications [subject count (percentage of subjects)]
Subjects were assigned to the protocol group opposite the protocol group to which they were randomized in the first MRI appointment (M1). The M1 protocol group is in parentheses.
Gapping Differences at M1
Analysis of the gapping differences for the four protocol groups at the first MRI appointment (n=28 for each protocol group, N=112) showed there was a significant difference (p=0.001, Kruskal-Wallis [KW]=16.3). Protocol 1 (SPP) had greater gapping than the other 3 protocol groups (mean gapping differences: Protocol 1=1.09 +1.22 mm; Protocol 2=0.24 +0.40; Protocol 3=0.19 +1.23; Protocol 4=0.18 +0.71).
Plotting the MRI gapping difference data revealed several outliers in Protocols 1 and 3. Consequently, an adjusted analysis was made that eliminated two values from Protocols 1 and 3 (the highest and lowest values of each protocol group). No values were eliminated from Protocols 2 and 4. The variability of Protocol 1 remained high, with 4 remaining Protocol 1 values markedly higher than the others. Therefore, secondary analyses (ANOVA) were performed with and without these four values in Protocol 1 (n=26 and n=22, respectively). Both analyses of the gapping differences for the four protocol groups at the first MRI appointment showed there was a significant difference (p=0.0005, KW=13.2; and p=0.009, KW=17.9 for N=108 and 104 subjects, respectively), with Protocol 1 (SPP) having greater gapping than the other 3 protocol groups (mean gapping differences for 104 subjects: Protocol 1=0.66 +0.48 mm; Protocol 2=0.24 +0.41; Protocol 3=0.23 +0.86; Protocol 4=0.18 +0.71).
Greatest Gapping Differences of Males vs. Females
No differences were found between males and females for GGPTS at either the M1 (p=0.81) or M2 (p=0.91) appointments.
Verbal Numeric Pain Rating Scale (VNPRS) at M1
Differences in VNPRS before and after the intervention were as follows: Protocol 1: 0.18 +1.19, Protocol 2: −0.04 +1.0, Protocol 3: 0.79 +1.4, Protocol 4: 0.18 +0.72. These VNPRS differences were significant (p=0.04) with Protocol 3 (SMT followed by SPP) showing the greatest difference (decreased pain after the intervention) and Protocol 2 (SMT followed by supine positioning) showing the least difference. The difference between these two protocol groups was significant (p<0.05). There were no significant differences between any other protocol groups.
Two Weeks of Treatment
Subjects received 2 weeks of care between the M1 and M2 appointments. Based on each subject’s clinical progress, the treating clinician determined the number of treatments and the modality (or modalities) of care given at each treatment. Excluding the MRI appointments, between 2 and 6 (mean=4.3) treatments per subject were given during the 2 weeks of care, totaling 483 treatments. Only 3 of these 483 did not include a lumbar side-posture SMT (1 treatment for 3 different subjects who were be assigned to 3 different protocol groups at the M2 appointment [Protocol Groups 2, 3, and 4)]. Table 5 provides detailed information of the other modalities used in the 2 weeks of treatments provided in the study. Only SMT was provided at the MRI appointments.
Table 5.
Treatment Modalities Used during Two Weeks of Care Between First (M1) and Second (M2) MRI Appointments1
| Protocol Group at M2 |
Total Treatments2 |
Cold Packs Only |
Hot Packs Only3 |
IFC Only | Ultrasound Only |
Soft Tissue Only |
Exercises Only |
Multiple Modalities |
Total Single and Multiple Modalities |
No Modalities |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 118 (4.2) | 0 (0.0) | 51 (43.2) | 3 (2.5) | 21 (17.8) | 5 (4.2) | 1 (0.8) | 31 (26.3) | 112 (94.9) | 6 (5.1) |
| 2 | 120 (4.3) | 0 (0.0) | 35 (29.2) | 6 (5.0) | 28 (23.3) | 5 (4.2) | 0 (0.0) | 31 (25.8) | 105 (87.5) | 15 (12.5) |
| 3 | 125 (4.5) | 0 (0.0) | 53 (42.4) | 4 (3.2) | 25 (20.0) | 9 (7.2) | 1 (0.8) | 28 (22.4) | 120 (96.0) | 5 (4.0) |
| 4 | 120 (4.3) | 0 (0.0) | 32 (26.7) | 5 (4.2) | 28 (23.3) | 8 (6.7) | 0 (0.0) | 36 (30.0) | 109 (90.8) | 11 (9.2) |
| Totals | 483 (4.3) | 0 (0.0) | 171 (35.4) | 18 (3.7) | 102 (21.1) | 27 (5.6) | 2 (0.4) | 126 (26.1) | 446 (92.3) | 37 (7.7) |
See text for discussion of numbers of spinal manipulative therapy (SMT) provided during the 2 weeks of care.
Values in parentheses are averages values for all other columns are percentages.
Values in parentheses in this column (and all other columns to the right) are percentages.
Three subjects sought outside care during the course of the study; 2 subjects (1 from second [M2] MRI Protocol Group 3 and 1 from M2 MRI Protocol Group 4) received a massage during the 2 weeks of care. A third subject (from M2 MRI Protocol Group 3) made an appointment and was seen by an orthopedic surgeon for a consultation. This third subject was then referred for an MRI (outside of the study), but received no additional treatment before the second MRI appointment and for at least the following 2 weeks (the duration of tracking in the study).
Second MRI Appointment (M2)
Changes in Subject Characteristics between First and Second MRI Appointments
The right (M2) side of Table 4 shows VAS, BQ, and analgesic medication use at M2. The only notable difference among protocol groups was that the overall percentage of individuals using analgesic medication was reduced from 35% (n=39) at M1 to 23% (n=26) at M2. Protocol 1 had fewer subjects using analgesics than the other M2 protocol groups (14% vs. 26%); however, this did not appear to influence the other variables.
Gapping Differences at M2
Data analysis for the second MRI appointment was initially performed on all 112 subjects. A significant difference (p=0.005, KW=12.9) existed, with Protocol 3 (SMT) having greater gapping than the other 3 protocol groups (mean gapping differences: Protocol 1=0.65 +0.92 mm; Protocol 2=0.18 +0.51; Protocol 3=0.76 +0.85; Protocol 4=0.44 +0.46).
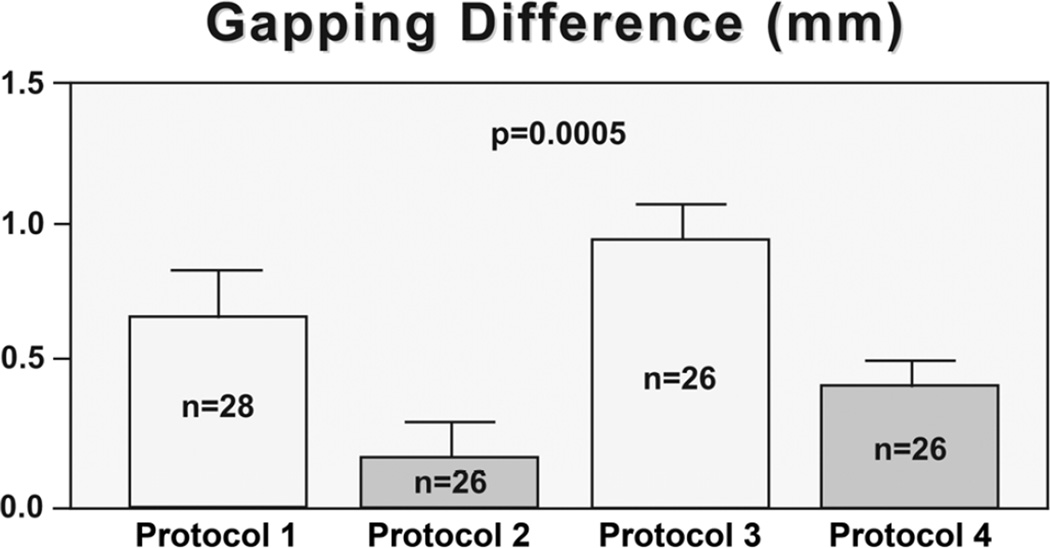
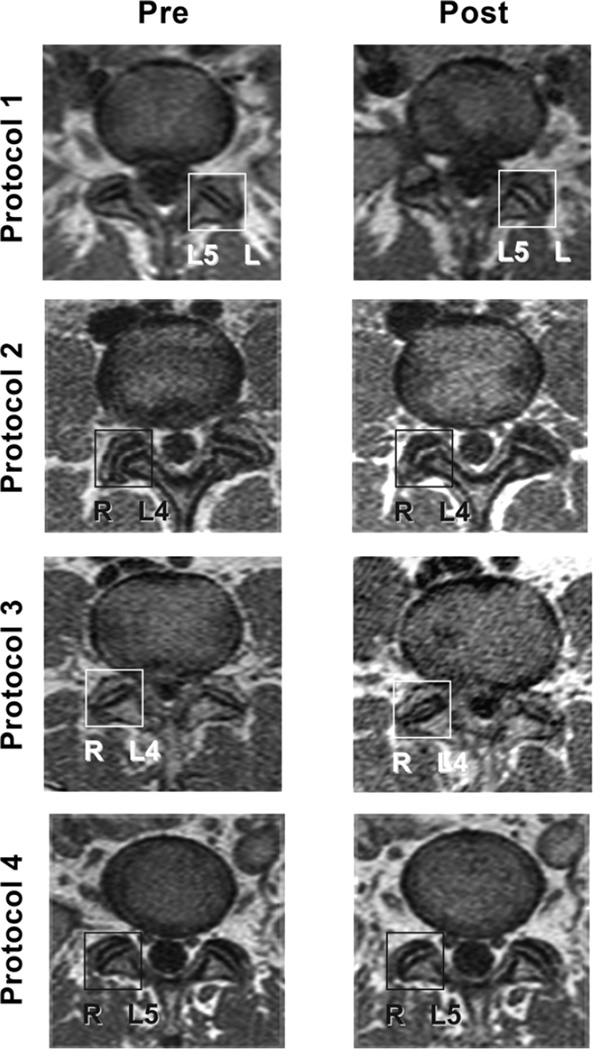
The values were plotted and several outliers were identified as follows: Protocol 1, no values; Protocol 2, 2 values (highest and lowest); Protocol 3, 2 values (lowest 2 values); Protocol 4, 2 values (highest 2 values). The remaining values were well-distributed following removal of the outliers. Consequently, the total number of subjects in the secondary analysis at the M2 appointment was 106. Differences remained significant (p=0.0005, F=6.5; Protocol 1=0.65 +0.92mm, Protocol 2=0.17 +0.38, Protocol 3=0.89 +0.71, Protocol 4=0.35 +0.32). In addition, Protocol 3 (SMT) was the only intervention that showed significantly more gapping than both the control protocols (Protocol 2, p<0.001, 95%CI −1.18 – −0.26; Protocol 4, p<0.05, 95%CI 0.08 – 1.0). Protocol 1 (SPP) showed a significant difference with Protocol 2 (p<0.05, 95%CI 0.02 – 0.93). Figure 6 shows the gapping differences for the 4 protocol groups and Figure 7 shows examples of pre-intervention and post-intervention scans for each protocol group.
Figure 6.
Gapping differences (in mm, Y-axis) between the A-P Z joint space measurements of the first and second MRI scans (value from the first scan was subtracted from the value of the second scan) at the second MRI appointment (M2). The greatest gapping differences of the primary treatment side (PTS) are presented here. Protocol 3 [spinal manipulative therapy (SMT) protocol groups] showed more gapping (therapeutic gapping) than the other protocol groups, followed by Protocol 1 [side-posture positioning (SPP) protocol group].
Figure 7.
Pre-intervention and post-intervention scans for each of the 4 study protocol groups. The box on each scan indicates the up-side Z joint during side-posture-positioning or side-posture SMT. This was also the most painful side of low back pain. L4 indicates the L4/L5 segmental level and L5 indicates the L5/S1 segmental level. Notice the low signal line within the center of the Protocol 3 “Post” R L4/L5 Z joint. This may be gas (most likely carbon dioxide) within the joint secondary to cavitation during SMT.
Changes in Pain (VAS) and function (BQ) at M2
Removal of outliers did not change the outcomes for M2 VAS, BQ or VNPRS. VAS changed from 28.8 +18.16 mm (range 1–82 mm) at the first MRI appointment to 16.4 +16.88 mm (range 0–81 mm) at the second MRI appointment, a difference of 12.4 mm (p<0.0001). Bournemouth Questionnaire (BQ) changed from 23.8 +11.56 (range 3–54) at the first MRI appointment to 13.4 + 10.69 (range 0–48) at the second MRI appointment, a difference of 10.4 (p<0.0001).
Verbal Numeric Pain Rating Scale (VNPRS) at M2
VNPRS pre-intervention values were low (little pain reported) at M2, and differences in VNPRS before and after the intervention at M2 were not significant (p=0.41)
No changes were made to the protocols and no adverse events, harms, or unintended effects were reported during the course of the study.
Discussion
Approximately twice as many males (n=75) were enrolled as females (n=37). This representation is different than is usually found in chiropractic practices, which have a slightly higher percentage of female patients.43–45 The incidence of low back pain in the general population is also slightly higher in females.46 The higher number of males in this study was a reflection of the numbers of males (n=798) and females (n=497) who responded to the recruiting advertisements (12 subjects declined before any data was recorded). Although recruitment for this study was based on methods used successfully in previous clinical trials,47,48 future studies will include more advertisements that target females and run on media and programming with a higher female demographic. Because no difference was found in gapping between male and female subjects at either the M1 or M2 appointments, the higher percentage of males most likely did not affect the outcome of the study.
First MRI Appointment
Subjects were allowed to take analgesic medications, both over-the-counter and previously prescribed prescription medication, and medication use between appointments was documented at every visit. Although a much higher percentage of M1 Protocol 1 subjects were taking analgesic medication, those Protocol 1 subjects taking analgesic medications had only 0.16 mm more gapping than those not taking analgesic medications; the GGDPTS for patients not taking and taking analgesic medications was 1.00 mm and 1.16 mm, respectively. Removal of Protocol Group 1 subjects taking analgesic medication did not alter the results.
First MRI appointment Protocol 1 (side-posture position [SPP] only) subjects showed more gapping than the other protocol groups, including Protocol 3 (side-posture SMT followed by SPP). Paraspinal muscles may have relaxed more during prolonged SPP in Protocol 1, whereas SMT may have resulted in transient increased muscle tightness in Protocol 3. This relationship reversed at the second MRI appointment (M2) with Protocol 3 showing more gapping than Protocol 1. The increased gapping of M2 Protocol 3 subjects may indicate that the paraspinal muscles were more relaxed following two weeks of treatment (including SMT) at the M2 appointment allowing more Z joint gapping with SMT.
Protocol 3 (SMT followed by SPP) was the only protocol group to show significant improvement in pain (VNPRS), whereas those subjects receiving side-posture SMT and then placed on their backs (Protocol 2) had almost no change in pain following the intervention. These results indicate that the lumbar side-posture position may have therapeutic benefit in acute LBP, increasing gapping in patients in acute pain (Protocol 1), and enhancing pain reduction following SMT (Protocol 3). This is consistent with the common recommendation by clinicians and the literature that lying on the side is of benefit for low back pain patients.49–54 The increased gapping that occurred with prolonged SPP (i.e., SPP of approximately 12 minutes) could conceivably promote the break-up of intra-articular Z joint adhesions.6 In addition, like SMT alone, SMT followed by SPP may also reduce pain by stimulating mechanoreceptors in the Z joint capsules55–57 and paraspinal muscles.58,59 Stimulation of such Z joint mechanoreceptors has been hypothesized as a mechanism of reducing pain via a gating mechanism in the spinal cord. This hypothesis is supported by animal studies showing that pressure on the Z joint (including the capsule) decreases activity of spinal cord dorsal horn neurons responding to nociceptive stimulation.60
Two Weeks of Treatment
The treatments in this study could be described as “structured pragmatic” in nature. Because the purpose of the study was to assess Z joint gapping at initial LBP presentation and after clinical improvement at 2 weeks, the purpose of the treatment was to provide usual chiropractic care that would result in the maximum improvement following 2 weeks of treatment. SMT was provided in almost all treatment appointments (only 3 of 483 treatments did not receive SMT). The study clinician was given the latitude to determine the number of treatments per week, within the parameters of 1–3 treatments, and choose from a “menu” of other modalities that could be used.
The goals of the treatments were successfully achieved. The results show that the subjects all received approximately the same number of treatments, SMT, and similar numbers and types of “other modalities.” In addition, after 2 weeks of care the subjects showed approximately the same improvement as measured by pain and functional impairment. Therefore, the study was able to measure Z joint gapping in a homogenous cohort at initial presentation and after similar improvement of LBP following 2 weeks of care. Recall, this was not a study assessing effectiveness of care (although all subjects significantly improved following 2 weeks of care), but was designed to assess Z joint gapping in acute LBP patients.
Second MRI Appointment
Consistent with previous studies,15,16 the Z joints resumed their normal spacing once they received the load of the supine position, explaining why Protocol 4 (brief SPP followed by neutral/supine position [SPP control group]) and Protocol 2 (SMT followed by neutral/supine position [SMT control group]) showed little gapping.
SMT followed by SPP (Protocol 3) resulted in the greatest amount of Z joint gapping in LBP subjects at the M2 appointment, followed by SPP (Protocol 1). These data indicate that SMT produced more gapping after subjects received two weeks of treatment. One would anticipate even greater differences between Protocol 1 (SPP) and Protocol 3 (SMT) mean gapping differences if the subjects had been assessed with a third MRI appointment after 4 weeks of care, because the subjects would then have been more similar in pain and function to the healthy subjects assessed in previous studies, where Protocol 3 showed 0.7 mm more gapping than Protocol 1.15,16 The increased gapping after two weeks of care in this study could have been due to reduction of intra-articular adhesions (and potentially other connective tissue adhesions, including adhesions within the fascia), and reduced muscle tension of the paraspinal muscles surrounding the Z joints. Future studies should assess changes in muscle activity, as measured by electromyography, at the M1 and M2 appointments.
The gapping changes were also accompanied with an overall reduction of pain and improved functional impairment. The Protocol 3 (SMT followed by SPP) findings also indicate that keeping a person in the side-posture position for several minutes following SMT may have therapeutic benefit. Future work in animals and humans should further assess the unique effects of SPP alone and SMT followed by SPP.
This study provides additional evidence that normal Z joints (i.e., Z joints within normal anatomic limits) gap with SMT and SPP, which is different from conclusions of previous authors61 who believed that Z joints that were within normal anatomic limits do not gap. These previous authors strongly indicated that chiropractors were misinforming their patients when describing Z joint gapping as a mechanism of SMT. Recall that subjects with anomalous Z joints were excluded from this study. The study conducted by the other investigators placed the cadaveric spines in a more extended posture, which significantly reduces Z joint rotation, and consequently Z joint gapping. The standard SMT of this study is administered with the Z joints in a flexed position, which allows for rotation62–64 and gapping. The results of this and previous studies15,16 indicate that typical Z joints do gap with SMT and SPP. The evidence that Z joints do gap can lead to a different approach to patient care than an assumption that they do not gap. When combined with other studies showing that adhesions develop in hypomobile Z joints6 and LBP patients with clinical hypomobility respond favorably to SMT,4,5 the results of this study further buttress the theory provided in Figure 1. This theory begins with the a priori assumption that Z joints become hypomobile for a variety of reasons (e.g., sedentary lifestyle, injury, repetitive asymmetrical tasks at work); that hypomobile Z joints develop adhesions, which further reduces motion; SMT gaps the Z joint surfaces, thus breaking up Z joint adhesions and reestablishing spinal motion.
Limitations
This study was conducted on the lumbar spine. Additional research assessing the cervical and thoracic regions are needed to determine the effects of positioning and gapping of the Z joints in other regions of the vertebral column.
As discussed previously, future studies should target female subjects for recruitment in order to obtain a more equal distribution of male and female subjects.
The ideal design would have been four MRI appointments; one before commencement of treatment (M1 in this study), one after one week of treatment, one after two weeks of treatment (M2 in this study), and one after four weeks of treatment. This would not only have allowed for assessment of gapping at additional time point in the LBP continuum, but would also have allowed each subject to be in each of the 4 protocol groups. However, four MRI appointments could not be justified from cost and patient burden standpoints. In addition, future research assessing gapping differences in subjects with chronic LBP should be performed. Future work could also include a medication only control treatment protocol group.
CONCLUSION
In this study of acute LBP subjects, SPP subjects (Protocol 1) showed the greatest Z joint gapping at the baseline MRI appointment. After two weeks of standard chiropractic treatment, SMT followed by SPP (Protocol 3) resulted in the greatest amount of Z joint gapping, followed by SPP alone (Protocol 1); these results are consistent with those of previous studies on healthy subjects.15,16 The side-posture position appeared to have additive therapeutic benefit to SMT, with acute LBP subjects receiving SMT and remaining in side-posture experiencing the greatest reduction of pain, independent of Z joint gapping, at the first appointment and the greatest amount of Z joint gapping after 2 weeks of care.
Zygapophyseal (Z) joint gapping is hypothesized to be related to a therapeutic benefit of spinal manipulative therapy (SMT) (“therapeutic gapping”)
Previous studies of healthy subjects found that Z joints receiving SMT gapped more than those receiving side-posture positioning alone
In this study of acute low back pain subjects, side-posture positioning showed the greatest Z joint gapping at the baseline MRI appointment
After two weeks of standard chiropractic treatment, SMT followed by side-posture positioning resulted in the greatest amount of Z joint gapping, followed by side-posture positioning alone
The side-posture position appeared to have additive benefit to SMT regarding pain reduction and Z joint gapping
Acknowledgements
We gratefully acknowledge the technical support of Joshua Healy, DC and the observers who made the morphometric measurements: Frank Balester, MSOM, LAc; Tyra Horner, DC; and Derek Simpson, DC, ND.
Funding Source: This project was made possible by Grant Number R01-AT000123 from the National Center for Complementary and Alternative Medicine (NCCAM). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No Conflict of Interest: No conflict of interest was reported by any of the authors.
Contributor Information
Gregory D. Cramer, Department of Research, National University of Health Sciences.
Jerrilyn Cambron, Department of Research, National University of Health Sciences.
Joe A Cantu, Charlottesville, Virginia.
Jennifer M. Dexheimer, Department of Research, National University of Health Sciences.
Judith D Pocius, Department of Research, National University of Health Sciences.
Douglas Gregerson, Department of Research, National University of Health Sciences.
Michael Fergus, Department of Diagnostic Imaging, National University of Health Sciences.
Ray McKinnis, Winfield, IL.
Thomas J Grieve, Department of Research, National University of Health Sciences.
References
- 1.Janse J. In: Principles and practice of chiropractic: an anthology. Hildebrandt RW, editor. Wheaton: Kjellberg & Sons; 1976. p. 326. [Google Scholar]
- 2.Triano JJ. In: Interaction of spinal biomechanics and physiology. Principles and practice of chiropractic. 2nd ed. Haldeman S, editor. East Norwalk, Conn: Appleton & Lange; 1992. 1992. pp. 225–257. [Google Scholar]
- 3.Evans DW. Mechanisms and effects of spinal high-velocity, low-amplitude thrust manipulation: previous theories. J Manipulative Physiol Ther. 2002;25(4):251–262. doi: 10.1067/mmt.2002.123166. [DOI] [PubMed] [Google Scholar]
- 4.Flynn T, Fritz J, Whitman J, Wainner R, Magel J, Rendeiro D, et al. A clinical prediction rule for classifying patients with low back pain who demonstrate short-term improvement with spinal manipulation. Spine (Phila Pa 1976) 2002;27(24):2835–43. doi: 10.1097/00007632-200212150-00021. [DOI] [PubMed] [Google Scholar]
- 5.Fritz JM, Whitman JM, Childs JD. Lumbar spine segmental mobility assessment: an examination of validity for determining intervention strategies in patients with low back pain. Arch Phys Med Rehabil. 2005;86(9):1745–1752. doi: 10.1016/j.apmr.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 6.Cramer GD, Henderson CN, Little JW, Daley C, Grieve TJ. Zygapophyseal joint adhesions after induced hypomobility. J Manipulative Physiol Ther. 2010;33(7):508–518. doi: 10.1016/j.jmpt.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Cramer GD, Fournier JT, Henderson CN, Wolcott CC. Degenerative changes following spinal fixation in a small animal model. J Manipulative Physiol Ther. 2004;27(3):141–154. doi: 10.1016/j.jmpt.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 8.Sandoz R. Some physical mechanisms and effects of spinal adjustments. Ann Swiss Chirop Assoc. 1976;6:91–141. [Google Scholar]
- 9.Cassidy J, Kirkaldy-Willis W. Manipulation. In: Kirkaldy-Willis W, Burton V, editors. Managing low back pain. 3rd ed. New York: Churchill Livingstone; 1992. pp. 283–296. [Google Scholar]
- 10.Engel R, Bogduk N. The menisci of the lumbar zygapophysial joints. J Anat. 1982;135(Pt 4):795–809. [PMC free article] [PubMed] [Google Scholar]
- 11.Giles LG, Taylor JR. Human zygapophyseal joint capsule and synovial fold innervation. Br J Rheumatol. 1987;26(2):93–98. doi: 10.1093/rheumatology/26.2.93. [DOI] [PubMed] [Google Scholar]
- 12.Kirkaldy-Willis W. The pathology and pathogenesis of low back pain. In: Kirkaldy-Willis WH, Burton V, editors. Managing low back pain. 3rd ed. New York: Churchill Livingstone; 1992. pp. 283–296. [Google Scholar]
- 13.Kos J, Wolf J. Les menisques intervertebraux et le role possible dans les blocages vertebraux (translation) J Orthop Sports Phys Ther. 1972;1:8–9. [Google Scholar]
- 14.Sandoz R. Some reflex phenomena associated with spinal derangements and adjustments. Ann Swiss Chirop Assoc. 1981;7:45–65. [Google Scholar]
- 15.Cramer GD, Tuck NR, Jr, Knudsen JT, Fonda SD, Schliesser JS, Fournier JT, et al. Effects of side-posture positioning and side-posture adjusting on the lumbar zygapophysial joints as evaluated by magnetic resonance imaging: a before and after study with randomization. J Manipulative Physiol Ther. 2000;23(6):380–394. doi: 10.1067/mmt.2000.108145. [DOI] [PubMed] [Google Scholar]
- 16.Cramer GD, Gregerson DM, Knudsen JT, Hubbard BB, Ustas LM, Cantu JA. The effects of side-posture positioning and spinal adjusting on the lumbar Z joints: a randomized controlled trial with sixty-four subjects. Spine (Phila Pa 1976) 2002;27(22):2459–2466. doi: 10.1097/00007632-200211150-00008. [DOI] [PubMed] [Google Scholar]
- 17.Cramer GD, Ross K, Pocius J, Cantu JA, Laptook E, Fergus M, et al. Evaluating the relationship among cavitation, zygapophyseal joint gapping, and spinal manipulation: an exploratory case series. J Manipulative Physiol Ther. 2011;34(1):2–14. doi: 10.1016/j.jmpt.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bronfort G, Evans RL, Maiers M, Anderson AV. Spinal manipulation, epidural injections, and self-care for sciatica: a pilot study for a randomized clinical trial. J Manipulative Physiol Ther. 2004;27(8):503–508. doi: 10.1016/j.jmpt.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Bronfort G, Goldsmith CH, Nelson CF, Boline PD, Anderson AV. Trunk exercise combined with spinal manipulative or NSAID therapy for chronic low back pain: a randomized, observer-blinded clinical trial. J Manipulative Physiol Ther. 1996;19(9):570–582. [PubMed] [Google Scholar]
- 20.Bronfort G, Haas M, Evans R, Kawchuk G, Dagenais S. Evidence-informed management of chronic low back pain with spinal manipulation and mobilization. Spine J. 2008;8(1):213–225. doi: 10.1016/j.spinee.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 21.Bronfort G, Haas M, Evans R, Leiniger B, Triano J. Effectiveness of manual therapies: the UK evidence report. Chiropr Osteopat. 2010;18(1):3. doi: 10.1186/1746-1340-18-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bronfort G, Haas M, Evans RL, Bouter LM. Efficacy of spinal manipulation and mobilization for low back pain and neck pain: a systematic review and best evidence synthesis. Spine J. 2004;4(3):335–356. doi: 10.1016/j.spinee.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Haas M, Goldberg B, Aickin M, Ganger B, Attwood M. A practice-based study of patients with acute and chronic low back pain attending primary care and chiropractic physicians: two-week to 48-month follow-up. J Manipulative Physiol Ther. 2004;27(3):160–169. doi: 10.1016/j.jmpt.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 24.Haas M, Groupp E, Kraemer DF. Dose-response for chiropractic care of chronic low back pain. Spine J. 2004;4(5):574–583. doi: 10.1016/j.spinee.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Frymoyer J. Back pain and sciatica. N Engl J Med. 1988;318:291–300. doi: 10.1056/NEJM198802043180506. [DOI] [PubMed] [Google Scholar]
- 26.Spitzer WOLF, Dupuis M, et al. Quebec Task Force on Spinal Disorders. Scientific approach to the assessment and management of activity-related spinal disorders: a monograph for clinicians. Spine (Phila Pa 1976) 1987;12(7 Suppl):S1–S59. [PubMed] [Google Scholar]
- 27.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152(11):726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson D, Bergmann T. Chiropractic technique. 3 ed. New York: Churchill Livingstone; 2002. p. 810. [Google Scholar]
- 30.Cramer GD, Cantu JA, Pocius JD, Cambron JA, McKinnis RA. Reliability of zygapophysial joint space measurements made from magnetic resonance imaging scans of acute low back pain subjects: comparison of 2 statistical methods. J Manipulative Physiol Ther. 2010;33(3):220–225. doi: 10.1016/j.jmpt.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross JK, Bereznick DE, McGill SM. Determining cavitation location during lumbar and thoracic spinal manipulation: is spinal manipulation accurate and specific? Spine. 2004;29(13):1452–1457. doi: 10.1097/01.brs.0000129024.95630.57. [DOI] [PubMed] [Google Scholar]
- 32.Dixon JS, Bird HA. Reproducibility along a 10 cm vertical visual analogue scale. Ann Rheum Dis. 1981;40(1):87–89. doi: 10.1136/ard.40.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carlsson AM. Assessment of Chronic Pain. I. Aspects of the reliability and validity of the visual analog scale. Pain. 1983;16:87–101. doi: 10.1016/0304-3959(83)90088-X. [DOI] [PubMed] [Google Scholar]
- 34.Machin D, Lewith GT, Wylson S. Pain Measurement in randomized clinical trials: A comparison of two pain scales. The Clinical Journal of Pain. 1988;4:161–168. [Google Scholar]
- 35.Love A, Leboeuf C, Crisp TC. Chiropractic chronic low back pain sufferers and self-report assessment methods. Part I. A reliability study of the Visual Analogue Scale, the Pain Drawing, and the McGill Pain Questionnaire. J Manipulative Physiol Ther. 1989;12:21–25. [PubMed] [Google Scholar]
- 36.Giles LG, Muller R. Chronic spinal pain syndromes: a clinical pilot trial comparing acupuncture, a nonsteroidal anti-inflammatory drug, and spinal manipulation. J Manipulative Physiol Ther. 1999;22(6):376–381. doi: 10.1016/s0161-4754(99)70082-5. [DOI] [PubMed] [Google Scholar]
- 37.Von Korff M, Jensen MP, Karoly P. Assessing global pain severity by self-report in clinical and health services research. Spine (Phila Pa 1976) 2000;25(24):3140–3151. doi: 10.1097/00007632-200012150-00009. [DOI] [PubMed] [Google Scholar]
- 38.Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1–2):9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 39.Bolton JE, Breen AC. The Bournemouth Questionnaire: a short-form comprehensive outcome measure. I. Psychometric properties in back pain patients. J Manipulative Physiol Ther. 1999;22(8):503–510. doi: 10.1016/s0161-4754(99)70001-1. [DOI] [PubMed] [Google Scholar]
- 40.Cramer GD, Wolcott CC, Cantu J, Fergus M, Cambron JA, Gregerson DM, et al. The effects of side-posture adjusting on the lumbar zygapophysial joints of low back pain patients as evaluated by magnetic resonance imaging: a preliminary study. Journal of Chiropractic Education. 2004;18(1):4. [Google Scholar]
- 41.Fujiwara A, Lim TH, An HS, Tanaka N, Jeon CH, Andersson GB, et al. The effect of disc degeneration and facet joint osteoarthritis on the segmental flexibility of the lumbar spine. Spine (Phila Pa 1976) 2000;25(23):3036–3044. doi: 10.1097/00007632-200012010-00011. [DOI] [PubMed] [Google Scholar]
- 42.Nachemson AL, Schultz AB, Berkson MH. Mechanical properties of human lumbar spine motion segments. Influence of age, sex, disc level, and degeneration. Spine (Phila Pa 1976) 1979;4(1):1–8. doi: 10.1097/00007632-197901000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Coulter ID, Hurwitz EL, Adams AH, Genovese BJ, Hays R, Shekelle PG. Patients using chiropractors in North America: who are they, and why are they in chiropractic care? Spine (Phila Pa 1976) 2002;27(3):291–6. doi: 10.1097/00007632-200202010-00018. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 44.Mootz RD, Cherkin DC, Odegard CE, Eisenberg DM, Barassi JP, Deyo RA. Characteristics of chiropractic practitioners, patients, and encounters in Massachusetts and Arizona. J Manipulative Physiol Ther. 2005;28(9):645–653. doi: 10.1016/j.jmpt.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 45.Ndetan HT, Bae S, Evans MW, Jr, Rupert RL, Singh KP. Characterization of health status and modifiable risk behavior among United States adults using chiropractic care as compared with general medical care. J Manipulative Physiol Ther. 2009;32(6):414–422. doi: 10.1016/j.jmpt.2009.06.012. [DOI] [PubMed] [Google Scholar]
- 46.Waterman BR, Belmont PJ, Jr, Schoenfeld AJ. Low back pain in the United States: incidence and risk factors for presentation in the emergency setting. Spine J. 2012;12(1):63–70. doi: 10.1016/j.spinee.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 47.Gudavalli MR, Cambron JA, McGregor M, Jedlicka J, Keenum M, Ghanayem AJ, et al. A randomized clinical trial and subgroup analysis to compare flexion-distraction with active exercise for chronic low back pain. Eur Spine J. 2006;15(7):1070–1082. doi: 10.1007/s00586-005-0021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cambron JA, Dexheimer JM, Chang M, Cramer GD. Recruitment methods and costs for a randomized, placebo-controlled trial of chiropractic care for lumbar spinal stenosis: a single-site pilot study. J Manipulative Physiol Ther. 2010;33(1):56–61. doi: 10.1016/j.jmpt.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 49.Gracovetsky SA. The resting spine. A conceptual approach to the avoidance of spinal reinjury during rest. Phys Ther. 1987;67(4):549–553. doi: 10.1093/ptj/67.4.549. [DOI] [PubMed] [Google Scholar]
- 50.Finneson BE. Low Back Pain. Second Ed. Hagerstown: Lippincott Williams & Wilkins; 1981. p. 597. [Google Scholar]
- 51.Normand MC, Descarreaux M, Poulin C, Richer N, Mailhot D, Black P, et al. Biomechanical effects of a lumbar support in a mattress. J Can Chiropr Assoc. 2005;49(2):96–101. [PMC free article] [PubMed] [Google Scholar]
- 52.Burkhart L. Proper Sleep Ergonomics. Journal of the American Chiropractic Association. 2011;48(4):1–2. [Google Scholar]
- 53.NASS. Back Pain Basics. Burr Ridge, IL: North American Spine Society; 2007. Available from: http://www.knowyourback.org/documents/back_pain_basics_web.pdf. [Google Scholar]
- 54.DeVocht JW, Wilder DG, Bandstra ER, Spratt KF. Biomechanical evaluation of four different mattresses. Appl Ergon. 2006;37(3):297–304. doi: 10.1016/j.apergo.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Ianuzzi A, Khalsa PS. Comparison of human lumbar facet joint capsule strains during simulated high-velocity, low-amplitude spinal manipulation versus physiological motions. Spine J. 2005;5(3):277–290. doi: 10.1016/j.spinee.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ianuzzi A, Pickar JG, Khalsa PS. Relationships between joint motion and facet joint capsule strain during cat and human lumbar spinal motions. J Manipulative Physiol Ther. 2011;34(7):420–431. doi: 10.1016/j.jmpt.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McLain RF, Pickar JG. Mechanoreceptor endings in human thoracic and lumbar facet joints. Spine (Phila Pa 1976) 1998;23(2):168–173. doi: 10.1097/00007632-199801150-00004. [DOI] [PubMed] [Google Scholar]
- 58.Pickar JG, Wheeler JD. Response of muscle proprioceptors to spinal manipulative-like loads in the anesthetized cat. J Manipulative and Physiol Ther. 2001;24(1):2–11. doi: 10.1067/mmt.2001.112017. [DOI] [PubMed] [Google Scholar]
- 59.Sung PS, Kang YM, Pickar JG. Effect of spinal manipulation duration on low threshold mechanoreceptors in lumbar paraspinal muscles. Spine. 2004;30(1):115–122. doi: 10.1097/01.brs.0000147800.88242.48. [DOI] [PubMed] [Google Scholar]
- 60.Gillette RG, Kramis RC, Roberts WJ. Suppression of activity in spinal nocireceptive ‘low back’ neurons by paravertebral somatic stimuli in the cat. Neurosci Lett. 1998;241(1):45–48. doi: 10.1016/s0304-3940(97)00977-4. [DOI] [PubMed] [Google Scholar]
- 61.McFadden KD, Taylor JR. Axial rotation in the lumbar spine and gaping of the zygapophyseal joints. Spine. 1990;15:295–299. doi: 10.1097/00007632-199004000-00009. [DOI] [PubMed] [Google Scholar]
- 62.White AA, Panjabi MM. Clinical biomechanics of the spine. 2nd ed. Philadelphia: JB Lippincott; 1990. [Google Scholar]
- 63.Williams PL, Banniser LH, Berry MM, Collins P, Dussek JE, Dyson M, et al. Gray’s anatomy. Edinburgh: Churchill Livingstone; 1995. [Google Scholar]
- 64.Kapandji IA. The physiology of the joints. Annotated diagrams of the mechanics of the human joints. 6th ed. Edinburgh: Churchill Livingstone; 2008. [Google Scholar]