Abstract
Trichodysplasia spinulosa (TS) is a folliculocentric and clinically papular dermatological disorder occurring in the setting of immunosuppression typically in association with solid organ transplantation or hematolymphoid malignancies. We report the occurrence of TS in a 7-year-old girl with Down syndrome and pre-B-acute lymphoblastic leukemia (pre-B-ALL) who was completing chemotherapy at onset. The patient’s affected follicles were dilated by an expansion of a dystrophic follicular inner root sheath cell population displaying enlarged trichohyaline cytoplasmic granules and progressing centrally to keratotic and parakeratotic debris, and superficially demonstrating some diminutive hair shaft-like material within the keratotic spicules. Electron microscopic studies of a follicular lesion demonstrated extracellular viral particles suggestive of a polyomavirus within the central follicular keratotic debris. DNA Polymerase chain reaction (PCR) and gene sequencing studies, performed on the tissue of the microscopic slide and paraffin block, for the recently identified trichodysplasia spinulosa-associated polyomavirus (TSPyV), were resulted as positive for TSPyV. PCR for the Merkel cell polyomavirus (MCPyV) was negative. To date this case is unique in representing the first case of TS confirmed by electron microscopy in which a related viral pathogen has been molecularly identified. An additional 19 reported cases classifiable as TS are tabulated and reviewed.
Introduction
Trichodysplasia spinulosa (TS) is a folliculocentric process which presents as keratotic spiny papules typically distributed over the ears and structures of the central face and less commonly involves the extremities, trunk, and scalp. Alopecia, usually most severely affecting the eyebrows and eyelashes, often accompanies the disorder. The dermatological condition was initially identified in both adult and pediatric patients who had undergone allograft solid organ transplants and were then placed on immunosuppressants to protect against allograft rejection.1,2 Soon thereafter the condition was recognized in adult and pediatric patients immunocompromised secondary to malignant hematolymphoproliferative disorders and the associated treatment with antineoplastic chemotherapeutic agents.3,4 Although the disorder was first described as trichodysplasia spinulosa in 19991, a number of similar entities—trichodysplasia of immunosuppression5,6,7, viral-associated trichodysplasia2,3,7, virus-associated trichodysplasia spinulosa4,8, follicular dystrophy of immunosuppression9, cyclosporine-induced folliculodystrophy10, pilomatrix dysplasia11 and spiny hyperkeratosis (hair-like hyperkeratosis)12–have been described and are likely related. Recent characterization of a new human polyomavirus associated with TS occurring in immunocompromised patients has been reported, and this newly studied agent has been designated trichodysplasia spinulosa-associated polyomavirus (TSPyV or TSV).13
Materials and methods
Tissue samples (4 μm tissue rolls or slide sections) for polymerase chain reaction (PCR) were deparaffinized with xylene (Sigma) and were then subjected to 12 h proteinase-K digestion at 55°C. DNA was extracted with a spin column according to the manufacturer’s protocol (Qiagen DNAeasy Blood and Tissue Kit). The resulting DNA was eluted with water and assessed for concentration and protein contamination. Approximately 40ng of genomic DNA was used for each PCR reaction (Fisher Hi Fidelity, Takara Ex Taq).14 Primers amplifying a 152bp segment of the β2-microglobulin gene were used as positive controls for the quality of the DNA. Three previously published primer sets targeting conserved sequences in the MCPyV genome designated as LT315, PN116, and MCVPS117 were used as previously described. In addition, nested primer sets for the LT3 and PN1 products were designed to increase the sensitivity of the MCPyV assay. The MCPyV study thermocycler conditions were as follows: denaturation 5min at 95°C, 30 cycles of denaturation at 94°C for 30s, annealing at 52°C for 30s, and polymerization at 72°C for 30s, and a final polymerization at 72°C for 10 min. Additionally, three published primer pairs targeting the recently described TSPyV genome designated as NCCR, VP1, and LT were used to detect TSPyV.13 Annealing for the TSPyV primers was at 62°C for 30s, otherwise cycling conditions were identical to those of the MCPyV protocol. For sequencing, PCR products were purified over a spin column (Qiagen) and sent for automated sequencing using both forward and reverse primers. Properties of the relevant MCPyV and TSPyV primers are summarized in Table 1.
Table 1.
Summarization of the relevant MCPyV and TSPyV associated PCR primer properties
| Primer Name | Forward | Reverse | Amplicon size (bp) |
Reference |
|---|---|---|---|---|
| β-globin | ACACAACTgTgTTCACTAgC | CAACTTCATCCACgTTCACC | 110 | Duncavage19 |
| MCVPS1 | TCAgCgTCCCAggCTTCAgA | TggTggTCTCCTCTCTgCTACTg | 109 | Duncavage19 |
| LT3 | TTgTCTCgCCAgCATTgTAg | ATATAggggCCTCgTCAACC | 308 | Feng17 |
| LT3 nested | gATTCTAAgTgCgCTTgTAT | CTCCACCATTCTTTgAATTT | 370 | This study |
| PN4 | gCAAgCTTTTggAgATTgCT | TCCAAAgggTgTTCAATTCC | 137 | Garneski18 |
| PN4 nested | TCTgCATATAgACAAgATgg | TgTggATATTTTgCTggAAT | 195 | This study |
| TS_NCCR | TCATACTgCCACAAACACAggAAg | AgAACACAgAgCgggAggATg | 156 | van der Meijden13 |
| TS_VP1 | AgTCTAAggACAACTATgTTACAg | ATTACAggTTAggTCCTCATTCAAC | 142 | van der Meijden13 |
| TS_LT | TgTgTTTggAAACCAgAATCATTTg | TgCTACCTTgCTATTAAATgTggAg | 119 | van der Meijden13 |
Case report and results
A 7 year-old Hispanic female with Down syndrome developed a pruritic rash 2 months prior to finishing chemotherapy for pre-B ALL. The pruritus was relieved by topical corticosteroids but the rash persisted. After completing chemotherapy, the rash improved but never resolved. Approximately 6 months after completing chemotherapy the rash worsened and the patient was subsequently diagnosed with recurrence of pre-B ALL by bone marrow biopsy. At that time dermatology was consulted to biopsy “atypical eczema.” On examination, there were multiple fine erythematous papules on the central face, ears, and extremities (Fig. 1). There were a few similar papules on the trunk. Most of the papules had a central, fine, white spiny projection. A diagnosis of phrynoderma was considered but retinol and retinyl palmitate levels were normal.
Figure 1.
Patient with multiple fine erythematous folliculocentric papules with central, fine, white, keratotic spiny projection over the central face, ears, and extremities.
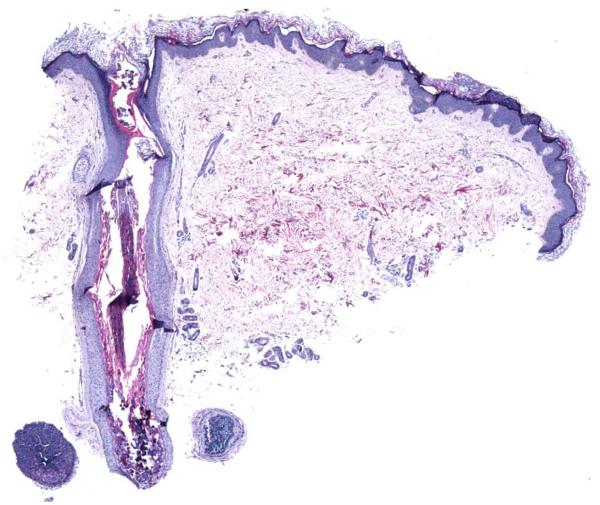
A skin biopsy was obtained from the left forearm for routine microscopy. Histopathologically, distorted and dilated anagen hair follicles were filled with an expansion of enlarged, disorganized inner root sheath cells with cytoplasmic distensions by both enlarged trichohyaline granules and gray-blue cytoplasmic material (Figs. 2, 3). Well defined dermal hair papillae were lacking and parakeratotic material progressing superficially to compacted keratin occupied the lower follicular segment regions where keratinizing hair shafts would normally occur. However, identifiable hair cuticle formation and well developed hair cortex type keratinization were not present at the follicular sites of typical occurrence. Curiously, some thin dystrophic refractile hair shaft-like material was present in the follicular infundibular region and within the spiny hyperkeratotic spicule that projected from the biopsied hair follicle (Fig. 4). This aforementioned histologic picture supported a diagnosis of trichodysplasia spinulosa, and overall the findings were similar to those previously described by Haycox et al and Sperling et al.1,2 A second skin biopsy was obtained from the right thigh for electron microscopy (EM). EM demonstrated the presence of viral particle arrays distributed in the deep follicular lower segment within the central extracellular hair follicle region containing the aggregated parakeratotic and keratotic debris, but curiously virus particles were not identified in the affected hair follicle inner root sheath cells themselves (Fig. 5). The ultrastructural findings further supported the occurrence of viral-associated trichodysplasia spinulosa related to immunosuppression. The family elected to continue with topical steroids for relief of pruritus but wanted to avoid any systemic therapies while the child was undergoing chemotherapy. The child expired 1 month after re-starting chemotherapy due to sepsis.
Figure 2.
Dilated anagen follicle with central keratotic and parakeratotic debris (hematoxylin and eosin, X40).
Figure 3.
Disorganized inner root sheath cells with cytoplasmic distensions by both enlarged trichohyaline granules and gray-blue cytoplasmic material and central accumulation of parakeratotic debris (hematoxylin and eosin, X600).
Figure 4.
Superficial follicular projecting white spiny keratotic spicule with central refractile thin fragmented hair shaft-like material (hematoxylin and eosin, X600).
Figure 5.
Electronmicrograph of viral particle arrays admixed in central extracellular follicular keratotic material (X20,000, direct). Inset: viral particle arrays with icosahedral virus pattern and 42 nm average diameter (X100,000, direct).
Given the striking ultrastructural viral particle arrays found in this and other cases of TS, infection by a polyomavirus is likely related to the pathogenesis of this disorder. MCPyV has been found to be important in the pathogenesis of the eponymous cutaneous carcinoma as well as associated with subsets of common cutaneous warts, squamous cell carcinomas in situ, cutaneous lymphomas, inflammatory skin conditions and possibly the general population.13,15,16,17,18,19 The van der Meijden and Feltkamp group recently molecularly studied the case of a pediatric patient with TS who had previously undergone heart transplantation.13 In this study they detected the presence of a new human polyomavirus associated with TS (TSPyV ).13 As such, for the current case under report, we sought to confirm these aforementioned findings and additionally study for the presence of MCPyV. TS current case extracted DNA was prepared using both the remainder of the paraffin tissue block and tissue scraped from the microscopic slides containing the abnormal hair follicles. Using 3 sets of primers specific for MCPyV, including completion of a highly sensitive nested primer set protocol, we were unable to detect MCPyV viral sequences from either the microscopic slides of affected hair follicles or the remaining paraffin tissue block (Fig. 6a and data not shown).14 However, in all cases, we were able to amplify the β2-microglobulin gene as a positive control for the quality of the DNA preparation. Moreover, MCPyV sequences were amplified from a MCPyV-positive Merkel cell carcinoma when run in parallel (Fig. 6a, lane 4).14 This assay has been able to detect MCPyV DNA in 20 out of 30 cases of Merkel cell carcinoma (R.C. Wang, unpublished data). Furthermore, this level of MCPyV associated sensitivity is comparable to what has been reported in the literature.16,17 Importantly, when we used the primers described in the newly discovered and sequenced trichodysplasia spinulosa-associated polyomavirus, TSPyV DNA was amplified from both the hair follicles with lesion and the remaining paraffin tissue block using all three published primer pairs (Fig. 6b).13 The patient samples, but not the dH20 negative control, generated products of the expected size (Table 1). Furthermore, when the PCR products were purified and sent for sequencing, each of the products was found to be identical to the sequence reported by the van der Meijden and Feltkamp group (data not shown).13
Figure 6.
(a) PCR using the primer pairs listed (right) was performed using the input DNA (top) as described in the methods. MCC, Merkel cell carcinoma; dH2O, negative control lane. (b) PCR of TDS (trichodysplasia spinulosa) patient sample using 3 independent primer pairs (right) unique to TSPyV. dH2O, negative control lane.
Discussion
Trichodysplasia spinulosa was first coined and described in 1999 by Haycox and colleagues.1 They reported the development of friable follicular spinous processes, erythematous indurated papules, and progressive alopecia in a 44 year-old male transplant patient on immunosuppressive regimen.1 On biopsy, light microscopy revealed changes within the follicular epithelium.1 There was follicular expansion and dilatation with keratotic plugging of the infundibula with enlarged trichohyaline granules within the dystrophic inner root sheath cell population.1 Normal hair shafts were absent, and the inner root sheath epithelium was also characterized by acantholysis and apoptotic cells.1
In 2004, Sperling and colleagues described the first child with ultrastructurally demonstrated viral-associated trichodysplasia: a 13 year-old female who had undergone kidney transplant 9-months previously.2 Sperling et al extensively described the histopathology of the affected hair follicles and surrounding epidermal components in their TS report.2 Furthermore, the current case and the majority of the reported TS cases to date have documented histopathological characteristics similar or identical to those of the aforementioned Haycox et al and Sperling et al descriptions.1,2 Of note several investigators have reported some additional findings not demonstrated in the Haycox et al and Sperling et al case studies.1,2 Namely, Izakovic et al and Lee et al studied the hair-like spikes emanating from the affected follicles and noted the spikes to be composed of compacted hyperkeratotic and inner root sheath material with and without central small, poorly formed hair shafts.12,20 We of the case under report, as well as other investigators, note the presence of the hyperkeratotic projecting spicules associated with the affected follicles.3,6,10,12,13,20,21,22,23 Additionally, we and several other authors also detected small dystrophic hair shaft-like material centrally within some of the keratotic spicules.10,12,13,20
In reporting their 2 cases of TS, Wyatt and colleagues described the first case of TS in a non-transplant patient: a 19 year-old male with pre-B cell acute lymphocytic leukemia.4 Wyatt and colleagues also note three prior reports of abnormal follicular maturation in solid organ transplant recipients with similar clinical and histology findings to those of trichodysplasia patients.4,10,11,12 The authors of these prior reports suspected cyclosporine as the direct cause of this follicular pathology. Sperling further suggests that similar cases labeled with various diagnostic terms likely represent the same entity.7
Importantly, in 2006 Osswald et al reported the first case of viral-associated trichodysplasia related to lymphoma occurring in a 68 year old man with a history of treated low grade follicular B-cell lymphoma who experienced lymphoma relapse about 15 months after onset of the trichodysplasia lesions.3 The child in the current case under report demonstrated a worsening of the trichodysplasia skin lesions corresponding to pre-B -ALL relapse. Hence, as Osswald et al originally asserted clinical appearance or worsening of the trichodysplasia lesions may be a forerunner of relapse in those patients with medical histories of hematolymphoid malignancies.3
Medical treatment of clinical TS lesions has proven difficult relative to a uniform successful treatment for all cases.2,3,5,6,13,22,23 TS lesions tend to persist in patients with ongoing immunosuppression.23 Nonetheless, several investigators have reported little to marked improvement of the clinical TS lesions with topical cidofovir 1-3% cream.2,3,13,23 Oral valganciclovir has also been reported to be of limited to dramatic benefit in several patients with the majority of patients experiencing at least modest improvement of the clinical TS lesions.5,22,23
Table 2 describes the clinical, histopathological, electron microscopic and molecular findings for all 20 published cases identifiable as TS or likely TS. Tabulation of these reported cases allows for the detection of some trends and disease patterns. For 19 informative cases, 12 of the patients were male and 7 were female. The age range of TS in the reported cases is 5 to 70 years. Patients appear to be nearly equally divided among the pediatric and adult age groups. Thirteen patients received allograft solid organ transplantation with anti-rejection medicines and the remaining 7 patients experienced hematolymphoid malignancies and chemotherapy. Of the aforementioned 13 transplant patients, a single pediatric patient underwent cardiac transplantation and 1 year later began treatment for Epstein-Barr virus related large B-cell lymphoma at the time of TS onset.13 The majority of TS cases occurring in association with a treated hematolymphoid malignancy onset within a few months before or following chemotherapy completion.3,4,20,21 Only a fraction of patients received cyclosporine, thus TS occurs both within and outside the setting of cyclosporine administration. The most common medication constituting the anti-rejection protocols was mycophenolate, a potent B-cell and T-cell cytostatic/anti-proliferative agent, with the majority of transplant patients receiving this and other anti-rejection medications.
Table 2.
Comparison of clinical, molecular, ultrastructural and histomorphological features amongst reported TS cases@
| Reference (Year) |
Age - Years/ Gender% |
Medical history, Immune status cause |
Tricho- dysplasia spinulosa (TS) onset |
TS duration |
Immuno- suppressant medications, other concurrent medicines, treatment |
Clinical distribution of papular follicular lesions |
Clinical distribution of alopecia |
Anti-viral and other medications post diagnosis |
Molecular and viral culture study results |
Electron Microscopy (EM)/ virus distribution |
Hair follicle pathology# |
Hair shaft features In affected follicles |
Inner root sheath cell cytoplasmic features# |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Current case (2010) |
7/F | Down syndrome, Pre-B-Acute Lymphoblast ic Leukemia |
3 years post start and 2 months prior completion of chemo- therapy |
12 months |
Completed chemotherapy: ARA-C, etoposide, ifosfamise, intrathecal methotrexate, hydrocortisone |
Central face, ears, extremities, trunk |
None, but sparse eyebrows and eyelashes |
None | Positive for trichodysplasia spinulosa- associated polyomavirus (TSPyV) by PCR and DNA sequencing methods, negative for MCPyV by PCR (all studies on paraffin tissue block and microscopic slides with histopathology) |
Virus particles in follicle lower segment in extracellular central keratotic debris - not inner root sheath follicular epithelium, icosahedral 39-45 nm |
Similar to Haycox/Sperli ng |
Single and multiple dysmorphic and diminutive hair shaft- like structures in follicular infundibulum and spiny keratotic projections |
Enlarged cytoplasmic contents with either trichohyalin e granules or gray-blue material |
| Elaba, Hughey, et al24,& (2010) |
62/M | Bi-lateral lung transplant (due to idiopathic pulmonary fibrosis) |
6 years post transplant |
> 8 years, sporadical ly with resolution on valacyclo- vir |
Tacrolimus, mycophenolate mofetil, prednisone, |
Central face | Generalized alopecia |
Valacyclovir for shingles (TS lesions temporarily resolve) |
None& | Intracellular viral particles 28 nm from EM on paraffin tissue block |
Similar to Haycox/Sperli ng |
Absent | Similar to Haycox/ Sperling |
| de Luzuriaga, Petronic- Rosic, et al27,& (2010) |
Not Reporte d in abstract& |
Kidney transplant on dialysis |
Not Reported in abstract& |
< 2 years | Not Reported in abstract& |
Nose, lower extremities |
Not Reported in abstract& |
Not Reported in abstract& |
Not Reported in abstract& |
Not Reported in abstract& |
Similar to Haycox/Sperli ng |
Not Reported in abstract& |
Similar to Haycox/ Sperling |
| Benoit, Bacelieri, et al23 (2010) |
5/M | Heart transplant (due to Kabuki and hypoplastic left heart syndromes) |
1 year post transplant |
> 7 months |
Tacrolimus, mycophenolate mofetil, prednisone, intravenous immunoglobulin, rituximab, cyclophosphamid e, plasma- pheresis |
Central face, nose, chin (with leonine faces), ears, trunk, proximal thighs |
Not reported | Ammonium lactate cream, 0.1% triamcinolone cream, then tretinoin cream 0.025%, urea cream 40, then BID cidofovir ointment 3% (all failure) , then significant improvement but not total resolution following systemic valganciclovir |
None | EM not performed |
Similar to Haycox/Sperli ng |
Absent | Similar to Haycox/ Sperling |
| Schwieger- Briel, Balma- Mena, et al22 (2010) |
5/F | Heart transplant (due to dilated cardiomyopa thy) |
9 months post transplant |
>15 months |
Tacrolimus, mycophenolate mofetil (short trial of azathioprine, sirolimus replaced tacrolimus) |
Face, chin, with pustules over extensor surfaces of upper extremities |
Eyebrows | Trimethoprim then erythromycin, topical vitamin A acid, systemic isotretinoin, topical acyclovir (limited response), oral valganciclovir (modest improvement) |
None | No virus particles found on several EM attempts |
Similar to Haycox/Sperling |
Absent | Similar to Haycox/ Sperling |
| van der Meijden, Janssens, et al13,&& (2010) |
15/M | Heart transplant (due to dilated cardiomyo- pathy), stroke, EBV associated large B cell lymphoma |
1 year post transplant |
>18 months |
Tacrolimus, mycophenolate mofetil, methylpredniso- lone, amplodipine, pravastatine, levetiracetam, (lymphoma- rituximab |
Central face, eyebrows, nose, ears, malar- region, forehead, then legs |
Eyebrows, partially- eyelashes |
Topical cidofovir cream 1% BID with considerable improvement over 3 months; viral load reduced to 104 copies per cell by quantitative PCR |
Positive for new human polyomavirus from plucked facial spines by rolling-circle genome amplification/ quantitative TSPyV- specific PCR with 105 copies per cell |
Attempted but failed due to poor sample quality |
Distended and enlarged follicles with hyperplastic hair bulbs and some hair papillae diminished in size |
Some follicles with poorly formed hairs |
Not reported |
| Holzer and Hughey5 (2009) |
37/F | Heart transplant |
8 months post transplant |
5 months | Cyclosporine/ sirolimus, mycophenolic acid, prednisone, valganciclovir then acyclovir |
Face then trunk, extremities |
Face, trunk, upper extremities, partial madarosis |
Imiquimod (no improvement), tazarotene (not tolerated), oral valganciclovir, topical acyclovir (improved)/ valganciclovir 900 mg ea day maintenance |
PCR for all known human papillomavirus types resulted negative |
No virus Identified on EM |
Similar to Haycox/Sperling |
Absent | Similar to Haycox/ Sperling |
| Lee, Frederiksen,et al20 (2008) |
70/M | Chronic Lymphocytic Leukemia (CLL), in remission |
2 months post cessation chemo- therapy, 4 years post CLL diagnosis |
>13 months |
No immuno- suppressive medications during outbreak: completed chemotherapy: 6 courses – fludarabine, cyclophosph- amide, rituximab |
Face, nose, malar area, forehead, eyelids, eyebrows, ears, arms, trunk, thighs, legs, arms |
Eyebrows (mild) |
Minocycline, topical keratolytic cream with 10% urea, 5% lactic acid (no improvement) |
None | EM not performed |
Similar to Haycox/Sperling with some hair papillae formation but diminished in size, and prominent hyperkeratotic projecting spicules of thin hair shafts encased by inner root sheath keratin |
Some poorly formed hairs with surrounding parakera-tosis, non- disengaged from internal root sheath keratinization |
Similar to Haycox/ Sperling, also noted myrrmecial- like large trichohyaline granules |
| Osswald, Kulick, et al3 (2007) |
68/M | Non- Hodgkin lymphoma, low grade follicular type |
12 years post original lymphoma diagnosis and about 15 months prior to 3rd lymphoma relapse |
About 15 months |
Post TS diagnosis: fludarabine, rituximab, IV- acyclovir for lymphoma relapse and generalized herpes zoster |
Nose, glabella, eyebrows, chin, ears; then shoulders, posterior arms, upper abdomen, legs |
Eyebrows, eyelashes beard/chin area, then shoulders, posterior arms, upper abdomen, legs |
Cidofovir 1% in Vanicream™ (marked improvement) |
None | Numerous intranuclear virus particles in affected inner root sheath epithelia, 40-45 nm diameter, icosahedral, polyomavirus |
Similar to Haycox/Sperling , with some follicular inner root sheath spinous keratotic excrescences lacking hair shafts |
Absent | Similar to Haycox/ Sperling |
| Sadler, Halbert, et al21 (2007) |
Patient1: 8/M |
T-cell acute lymphocytic leukemia (T- ALL), then hypo- gamma- globulinemia (IgG1, IgG, absent CD19 B- cells) |
2 years post diagnosis of T-ALL, during maintenance chemo- therapy |
2 years, with rapid regressio n 6 months post chemo- therapy |
Children’s Cancer Group protocol 1961 chemotherapy with ongoing maintenance: vincristine, 6- mercaptopurine, methotrexate |
Face, trunk, limbs |
Eyebrows (mild) |
Topical salicyclic acid 4%, ammonium lactate 17.5%, tretinoin 0.05%, oral acitretin (eruption persisted), IV-IgG |
PCR for JC, BK polyoma- viruses resulted negative |
Virus particles in stratum corneum, 30 nm, papovavirus |
Similar to Haycox/Sperling , with absent hair papillae and some projecting follicular keratin spicules |
Absent | Similar to Haycox/ Sperling |
| Sadler, Halbert, et al21 (2007) |
Patient2: 6/M |
T-ALL | 2 years post diagnosis of T-ALL, 4 months post completion of chemo- therapy |
9 months | New York II protocol chemotherapy, prophylactic cranial irradiation, trimethoprin- sulpha- methoxazole |
Face, trunk, limbs |
None | No anti-viral medication, continued oral trimethoprin- sulpha- methoxazole |
None | No viral particles identified by EM of paraffin- embedded material |
Similar to Haycox/Sperling , with absent hair papillae and some projecting follicular keratin spicules |
Absent | Similar to Haycox/ Sperling |
| Khoury, Kibbi, et al8,& (2007) |
6/M | Acute lymphoblasti c leukemia |
Oral abstract& |
Oral abstract& |
Methotrexate, 6- mercaptopurine |
Oral abstract& |
Oral abstract& |
Oral abstract& |
Oral abstract& |
Oral abstract& |
Similar to Haycox/Sperling |
Oral abstract& |
Similar to Haycox/ Sperling |
| Campbell, Ney, et al6 (2006) |
48/F | Kidney Transplant secondary to lupus nephropathy |
9 years post renal failure and renal transplant |
>6 months |
tacrolimus, mycophenolate mofetil |
Nose, malar eminences, upper cutaneous lip, arms, thighs |
None | 0.25% tretinoin cream (no improvement) then 0.5% tazarotene gel (significant improvement) |
PCR and immuno- histochemical studies failed to identify virus |
No virus particles demonstrated by EM study |
Similar to Haycox/Sperling with some abortive hair shaft keratin formation |
Absent | Similar to Haycox/ Sperling |
| Wyatt, Sachs, et al4 (2005) |
Patient1: 19/M |
Pre-B-cell acute lymphocytic leukemia (pre-B-ALL) |
2.5 years post diagnosis of B-ALL, 3 months post completion of chemo- therapy |
Not reported |
Cyclophos- phamide, vincristine, high dose prednisone, intrathecal methotrexate (NY II protocol) |
Face, posterior arms, thighs |
Eyebrows (partial) |
Various topical therapies including corticosteroids (no improvement) |
BK viral cultures negative |
Rare virus in nuclei of follicular inner root sheath epithelial cells, 46 nm polyoma- virus |
Similar to Haycox/Sperling |
Absent at 1st biopsy, some mature hair shafts noted at 2nd biopsy 1 month post 1st biopsy |
Similar to Haycox/ Sperling |
| Wyatt, Sachs, et al4 (2005) |
Patient2: 8/M |
Henoch- Schonlein purpura, Kidney transplant |
8 months post kidney transplant |
Not reported |
Prednisone, tacrolimus, mycophenolate mofetil |
Face with concentra- tion over nose, ears |
None | Lac-hydrin 12% lotion, cidofovir 3% cream |
None | Numerous viral particles in nuclei and cytoplasm of follicular inner root sheath cells, 46 nm polyoma- Virus |
Similar to Haycox/Sperling |
Absent | Similar to Haycox/ Sperling |
| Sperling, Tomaszewski et al2 (2004) |
13/F | Kidney transplant |
9 months post transplant |
>9 months |
Prednisone, tacrolimus, mycophenolate mofetil |
Mid facial: nose, malar region, glabella, chin |
Eyebrows, eyelashes, (scalp spared) |
Topical corticosteroids(n o improvement) then imiquimod creme (minimal improvement) then cidofovir 3% cream (steady improvement) |
None | Virus in nuclei of follicular inner root sheath epithelial cells, 43 nm icosahedral papovavirus or polyoma virus |
No hair papillae, with anagen follicular distention by inner root sheath epithelial cells with enlarged cytoplasmic trichohyaline granules and central keratinaceous and cellular apoptotic debris, thin outer basal germinative cells. |
Absent | Enlarged trichohyaline granules |
| Heaphy, Shamma, et al10,## (2004) |
34/F | Systemic lupus erythema- tosus, kidney transplant (rejected), hemo- dialysis, gingival hyperplasia |
11 years post SLE onset, 3 years post renal transplant, post more than 2 years continuous immuno- suppression |
>12 months |
Cyclosporine, mycophenolate mofetil, prednisone, tacrolimus, amlodipine, famotidine, chlorpheniramine, pseudoephedrine , bumetanide, doxazosin, clonidine, sertraline |
Face, nose, eyebrows, chin, ears, trunk, extremities (scalp spared) |
Eyebrows, eyelashes (scalp spared) |
Desoximeta- sone, topical antibiotics, minocycline, tacrolimus, tretinoin 0.05% cream (all unsuccessful) |
None | EM not performed |
Clinically: central facial follicular papules with some dysmorphic hairs and keratinous hairlike spicules; histologically: enlarged aberrant anagen follicles, some bifid with dilated follicular lumina filled with masses of parkeratotic cells and eosinophilic debris |
Dysmorphic hairs present in some clinically affected follicles |
Enlarged and disordered follicular bulbs with eosinophilic perinuclear cytoplasmic globules or cytoplasmic vacuoles in enlarged supra- matrical cells |
| Chastain, Millikan, et al11,@@ (2000) |
13/F | Cystic fibrosis with bronchiolitis obliterans bilateral lung transplant, gingival hypertrophy, hyperten- sion, osteopenia, normocytic anemia |
3 years post bilateral lung transplant/ immuno- suppression |
>6 months |
Cyclosporine, mycophenolate mofetil, prednisone, methotrexate, trimethoprim- sulpha- methoxazole, nifedipine, pancreatic enzymes, triamcinolone inhaler, salmeterol inhaler |
Ears, nose and surrounding facial areas, proximal extremities |
Eyebrows, eyelashes |
Benzoyl peroxide (no improvement) switch from cyclosporine to tacrolimus (mild improvement) |
Attempts to detect human papillomavirus (HPV) by PCR unsuccessful |
EM not performed |
Dilated anagen follicles with thickened inner root sheath cell layer and containing disorganized hyperkeratotic and parakeratotic luminal debris rather than well developed hair shafts, Ki-67 immunostain revealed high proliferative rate in matrical cells |
Vellus-like hairs on face, ears noted clinically, hair shafts absent in histologically examined affected follicles |
Many inner root sheath cells with pale, vacuolated cytoplasm |
| Haycox, Kim, et al1 (1999) |
44/M | Type I diabetes mellitus, combined renal/ pancreas transplant |
Post 3 years combined renal/ pancreas transplant |
>7 months | Tacrolimus, azathioprine, prednisone |
Nose, ears, forehead, some coalescence of papules into plaques with accom-panied progression to infiltrated look of leonine facies |
Eyebrows then entire body with sparse sparing of terminal scalp and pubic region, small white friable follicular spinous processes in alopecia areas projecting from follicular orifices |
None | Immunohisto- chemistry for papillomavirus and BK virus large-T antigen each negative; PCR for HPV subtypes 6/11, 16, 18, 31/33/35/39, 40/42/53/54, 51/52/55/58, 45/56 all negative; Fungal, bacterial, mycobacterial and special histochemical microbial stains each negative; No report of JC virus status – JC virus studies were underway but not resulted at publication |
Intracellular 38 nm icosahedral papovavirus - smaller size favors polyomavirus |
Pathologic changes limited to follicular epithelium: no hair papillae, with anagen follicular distention by inner root sheath epithelial proliferation with infundibular plugging by keratinaceous and cellular apoptotic debris, thin outer basal germinative cells. |
No normal hair shafts present |
Enlarged irregular tricho-hyaline granules; Immuno- histochemis- try for Ki-67 showed marked proliferative activity of follicular epithelial cells, Antibody AE-15 showed dramatic expansion of expression in inner root sheath cells |
| Izakovic, Buchner, et al12,%% (1995) |
31/M | Kidney transplant secondary to membrano- proliferative glomerulo- nephritis |
Papules presented post 7 months start of cyclosporine; Spicules presented post 9 months start of cyclosporine |
Cleared post 3 months of lowering cyclo- sporine from 400 mg BID to 175 mg BID |
Prednisone with taper followed by, cyclosporine A |
Centrofacial, eyelids and nares heavily affected; global distribution on trunk and limbs but lower density, followed 2 months latter by follicular hyper- keratotic spicules |
None described |
Follicular spiny hyper- keratoses treated with metronidazole and tretinoin topicals |
None | Scanning EM demonstrated hair-like structures in some of the spiny hyper-keratoses; Transmission EM for virus not performed |
Marked hyperkeratosis of hair follicles with hair-like spikes alone or in connection with hairs, hyperplastic follicles with cystic widening and lumina containing amorphous eosinophilic material, “sebaceous gland hyperplasia” |
Hair structures occasionally present in diseased follicles |
Not described |
Chart data and format adapted, modified and expanded from Osswald, et al3.
M, male; F, female.
Histological findings reported similar to those documented by Haycox, et al1 and Sperling, et al2 unless otherwise listed.
Current case under report.
Cases presented in oral or poster abstract form and denoted data not provided in the written abstract.
Case represents the seminal identification and molecular description of the trichodysplasia spinulosa-associated polyomavirus (TSPyV).
Case reported as: “Cyclosporine-induced folliculodystrophy”
Case reported as: “Pilomatrix dysplasia”
Case reported as: “Hair-like hyperkeratosis, spiny hyperkeratosis”
EM study searching for virus particles has been undertaken in 12 informative cases of the 20 cases tabulated.1,2,3,4,5,6,21,22,24 Of these 12 cases viral particles have been ultrastructurally identified in 8 cases with virion diameters of 28-46 nm reported.1,2,3,4,21,24 Overall, the virion diameter measurements, icosahedral shape and other ultrastructural features are most consistent with a polyomavirus as human papilloma virus (HPV) family particles typically measure larger at a diameter of 50-55 nm.25 In the current case under report arrays of viral particles (with diameters of 39-45 nm) were demonstrated in the central intra-follicular lower segment compacted keratotic extracellular debris but not within the dystrophic expanded inner root sheath cell population. Sadler et al. also reported extracellular virus particles of polyomavirus dimensions in the stratum corneum in one of their 2 reported cases.21 However, the majority of investigators have reported ultrastructural evidence of viral particles within the cytoplasm and/or nuclei of the dystrophic inner root sheath cell population.1,2,3,4 Hence, as would be expected, the highest yield for EM virus detection is in examination of the affected hair follicle cellular and related extracellular components.
Molecular, immunohistochemical and/or virus culture studies were attempted on 8 informative cases.1,4,5,6,11,13,21 Various examinations for HPV, the highly seroprevalent BK and JC polyomaviruses, fungal, bacterial and mycobacterial agents have all been resulted as failed or negative.1,4,5,6,11,21,26 However, recent work has been completed by van der Meijden et al. in which an unique as to yet uncharacterized human polyomavirus was detected and molecularly described in TS lesions obtained from a single pediatric patient in the absence of EM viral particle verification within lesion components.13 This newly isolated human polyomavirus (TSPyV) has demonstrated a close phylogenetic relationship with the Bornean orangutan polyomavirus and a more distant relationship to the MCPyV.13 van der Meijden et al. further demonstrated the presence of very low copy numbers of TSPyV in 4% of 69 long term renal transplant patients lacking visible signs and symptoms of TS, thereby suggestive that TSPyV may participate in low level subclinical or latent infections in the immunocompromised and/or general population.13 As such, following the EM detection of polyomavirus-like particles in the material of the current case, we too molecularly demonstrated the presence of TSPyV in affected TS tissues duplicating and confirming the van der Meijden13 PCR and sequencing results in concert with demonstrating an absence of MCPyV by PCR methodology.
In summary, trichodysplasia spinulosa is a recently recognized condition seen in immunocompromised patients. We, as those investigators before us, suspect that this condition is under-diagnosed and is fairly common due to the high number of patients receiving immunosuppressive therapy or with medical conditions resulting in a substantial immunosuppressed state. Herein we describe a pediatric TS case with clinical, histopathological, EM and molecular identification of the recently identified trichodysplasia spinulosa-associated polyomavirus in the context of negative molecular studies for the Merkel cell polyomavirus. As such, this report serves to further assert TSPyV as the likely unique causative agent of viral-associated trichodysplasia of immunosuppression. However, additional studies will need to be conducted before it can be definitively concluded that TSPyV is indeed the sole causative agent of TS and that other viral agents are not involved in the pathogenesis of this interesting disorder.
Note: The Merkel cell polyomavirus molecular data of this case was presented in part at the 70th Society for Investigative Dermatology meeting 2010, Atlanta, Georgia, in poster written abstract form.14
Acknowledgements
Special thanks to: Sandra C. Osswald, MD for promoting and facilitating the collaboration between the authors; Beth Levine for providing reagents required for the PCR experiments; Loderick Matthews for his invaluable technical assistance in helping to perform PCR studies; and Torsten Ehrig, MD for German to English translation services.
References
- 1.Haycox CL, Kim S, Fleckman P, et al. Trichodysplasia spinulosa – a newly decscribed folliculocentric viral infection in an immunocompromised host. J Invest Dermatol. 1999;4:268. doi: 10.1038/sj.jidsp.5640227. [DOI] [PubMed] [Google Scholar]
- 2.Sperling LC, Tomaszewski M, Thomas D. Viral-associated trichodysplasia in patients who are immunocompromiused. J Am Acad Dermatol. 2004;50:318. doi: 10.1016/s0190-9622(03)01490-7. [DOI] [PubMed] [Google Scholar]
- 3.Osswald SS, Kulick KB, Tomaszewski MM, Sperling LC. Viral-associated trichodysplasia in a patient with lymphoma: a case report and review. J Cutan Pathol. 2007;34:721. doi: 10.1111/j.1600-0560.2006.00693.x. [DOI] [PubMed] [Google Scholar]
- 4.Wyatt AJ, Sachs DL, Shia J, Delgado R, Busam KJ. Virus-associated trichodysplasia spinulosa. Am J Surg Pathol. 2005;29:241. doi: 10.1097/01.pas.0000149691.83086.dc. [DOI] [PubMed] [Google Scholar]
- 5.Holzer AM, Hughey LC. Trichodysplasia of immunosuppression treated with oral valganciclovir. J Am Acad Dermatol. 2009;60:169. doi: 10.1016/j.jaad.2008.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell RM, Ney A, Gohh R, Robinson-Bostom L. Spiny hyperkeratotic projections on the face and extremities of a kidney transplant recipient. Arch Dermatol. 2006;142:1643. doi: 10.1001/archderm.142.12.1643-d. [DOI] [PubMed] [Google Scholar]
- 7.Sperling LC. Reply to follicular dystrophy of immunosuppresion. J Am Acad Dermatol. 2004;50:540. doi: 10.1016/j.jaad.2004.07.059. [DOI] [PubMed] [Google Scholar]
- 8.Khoury F, Kibbi A, Ghosn S. Virus-associated trichodysplasia spinulosa in a child with acute lymphoblastic leukemia. J Cutan Pathol. 2007;34:77. Oral Abstract. [Google Scholar]
- 9.Daneshpazhooh M, Asgari M. Follicular dystrophy of immunosuppression. J Am Acad Dermatol. 2004;50:540. doi: 10.1016/j.jaad.2004.07.059. [DOI] [PubMed] [Google Scholar]
- 10.Heaphy MR, Shamma HN, Hickmann M, White MJ. Cyclosporine-induced folliculodystrophy. J Am Acad Dermatol. 2004;50:310. doi: 10.1016/s0190-9622(03)00774-6. [DOI] [PubMed] [Google Scholar]
- 11.Chastain MA, Millikan LE. Pilomatrix dysplasia in an immunosuppressed patient. J Am Acad Dermatol. 2000;43:118. doi: 10.1067/mjd.2000.100967. [DOI] [PubMed] [Google Scholar]
- 12.Izakovic J, Buchner SA, Duggelin M, Guggenheim R, Itin PH. Hair-like hyperkeratosis in patients with kidney transplants: a new cyclosporine side-effect. Hautarzt. 1995;46:841. doi: 10.1007/s001050050350. [DOI] [PubMed] [Google Scholar]
- 13.van der Meijden E, Janssens RW, Lauber C, Bouwes Bavinck JN, Gorbalenya AE, Feltkamp MC. Discovery of a new human polyomavirus associated with trichodysplasia spinulosa in an immunocompromised patient. PLoS Pathog. 2010 Jul 29;6(7):e1001024. doi: 10.1371/journal.ppat.1001024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang RC, Matthews MR, Matthews L, et al. Merkel cell polyomavirus is present in Merkel cell cancer (MCC) but absent in gastrointestinal neuroendocrine carcinomas and trichodysplasia spinulosa. Journal of Investigative Dermatology. 2010;130(supplement 1S) abstract 141. [Google Scholar]
- 15.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garneski KM, Warcola AH, Feng Q, Kiviat NB, Leonard JH, Nghiem P. Merkel cell polyomavirus is more frequently present in North American than Australian Merkel cell carcinoma tumors. J Invest Dermatol. 2009;129:246. doi: 10.1038/jid.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duncavage EJ, Zehnbauer BA, Pfeifer JD. Prevalence of Merkel cell polyomavirus in Merkel cell carcinoma. Mod Pathol. 2009;22:516. doi: 10.1038/modpathol.2009.3. [DOI] [PubMed] [Google Scholar]
- 18.Mertz KD, Pfaltz M, Junt T, et al. Merkel cell polyomavirus is present in common warts and carcinoma in situ of the skin. Human Pathology. 2010;41:1369. doi: 10.1016/j.humpath.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 19.Andres C, Puchta U, Sander CA, Ruzicka T, Flaig MJ. Prevalence of merkel cell polyomavirus DNA in cutaneous lymphomas, pseudolymphomas, and inflammatory skin diseases. Am J Dermatopathol. 2010;32:593. doi: 10.1097/DAD.0b013e3181ce8beb. [DOI] [PubMed] [Google Scholar]
- 20.Lee JS, Frederiksen P, Kossard S. Progressive trichodysplasia spinulosa in a patient with chronic lymphocytic leukaemia in remission. Australs J Dermatol. 2008;49:57. doi: 10.1111/j.1440-0960.2007.00422.x. [DOI] [PubMed] [Google Scholar]
- 21.Sadler GM, Halbert AR, Smith N, Rogers M. Trichodysplasia spinulosa associated with chemotherapy for acute lymphocytic leukaemia. Australs J Dermatol. 2007;48:110. doi: 10.1111/j.1440-0960.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- 22.Schwieger-Briel A, Balma-Mena A, Ngan B, Dipchand A, Pope E. Trichodysplasia spinulosa – a rare complication in immunosuppressed patients. Pediatric Dermatology. 2010;27:509. doi: 10.1111/j.1525-1470.2010.01278.x. [DOI] [PubMed] [Google Scholar]
- 23.Benoit T, Bacelieri R, Morrell DS, Metcalf J. Viral-associated trichodysplasia of immunosuppression: report of a pediatric patient with response to oral valganciclovir. Arch Dermatol. 2010;146:871. doi: 10.1001/archdermatol.2010.175. [DOI] [PubMed] [Google Scholar]
- 24.Elaba Z, Hughey SL, Andea A. Trichodysplasia spinulosa in a lung transplant patient. The American Society of Dermatopathology 47th Annual Meeting; Atlanta, GA. 2010. Poster abstract 239. [Google Scholar]
- 25.Howatson AF, Nagai M, Zu Rhien GM. Polyoma-like virions in human demyelinating brain disease. The Canadian Medical Association J. 1965;93:379. [PMC free article] [PubMed] [Google Scholar]
- 26.Stolt A, Sasnauskas K, Koskela P, Lehtinen M, Dillner J. Seroepidemiology of the human polyomaviruses. J Gen Virol. 2003;319:1096. doi: 10.1099/vir.0.18842-0. [DOI] [PubMed] [Google Scholar]
- 27.de Luzuriaga AR, Petronic-Rosic V, Finch T, Wasserman J. Trichodysplasia of immunosuppression. The American Society of Dermatopathology 47th Annual Meeting; Atlanta, GA. 2010. Poster abstract 238. [Google Scholar]