Abstract
Purpose
Individuals diagnosed with high survival cancers will often die of cardiovascular disease (CVD) rather than a recurrence of their cancer, yet CVD risk factors may be overlooked during survivorship care. We assess the prevalence of CVD risk factors among long-term cancer survivors and compare results to survey data from the general population in the same geographic region. We also characterize how often at-risk survivors discuss CVD-related health behaviors with their health care providers.
Methods
Survivors (n=1582) of breast, prostate, colorectal, and gynecologic cancers, 4–14 years after diagnosis, were recruited from two California cancer registries for a cross-sectional mail survey. We assessed CVD risk factors, including smoking, body mass index, physical inactivity, hypercholesterolemia, hypertension, and diabetes, as well as report of discussions with health care providers about diet, exercise, smoking, and lifestyle change assistance.
Results
With the exception of current smoking, CVD risk factors were more common among survivors than the general adult population. Of survivors, 62.0% were overweight or obese; 55.0% reported hypertension; 20.7% reported diabetes; 18.1% were inactive; and 5.1% were current smokers. Compared to white, non-Hispanic survivors, Hispanic (b= .37, p= .007) and African-American (b= .66, p<.0001), but not Asian survivors reported significantly more risk factors. One in three survivors with one or more risk factors for CVD did not report a health promotion discussion with their health care providers.
Conclusions
CVD risk factors are common among long-term survivors, but many at-risk survivors may not discuss lifestyle prevention with their health care team. Primary care and oncology should work together to deliver optimal survivorship care that addresses CVD risk factors, as well as prevalent disease.
Keywords: cancer survivorship, cardiovascular diseases, risk factors, health behaviors, epidemiology, cancer
Individuals diagnosed with cancers that typically have high survival rates (i.e., with 5-year relative survival exceeding 80%) will often die of cardiovascular disease (CVD) rather than progression or recurrence of their cancer [1–3]. Further, cancer survivors also have an elevated risk of non-cancer death compared to age-standardized mortality rates[3;4], with CVD being the most common cause. Several factors may converge to increase the risk of CVD among survivors[5]. First, survivors are typically older than the general population and may be more likely to develop CVD due to modifiable risk factors common to both cancer and CVD, (e.g., overweight/obesity, smoking and physical inactivity[6–8]). Second, the direct effects of cardiotoxic cancer therapies (radiation, chemotherapy) and the indirect effects of concomitant lifestyle changes (weight gain, reduced physical activity after treatment) further exacerbate CVD risk. Thus, CVD risk is an important clinical issue for the nearly 12 million cancer survivors in the US [9].
Limited data exist regarding the prevalence of CVD risk factors among survivors[10], and especially variation in risk factors by type of cancer. Many, but not all, recent studies of the general adult population have found elevated rates of CVD risk factors among racial/ethnic minorities, particularly African-Americans[11–13]. It remains unknown if patterns of CVD risk by race/ethnicity persist among survivors and how often survivors with CVD risk factors receive health promotion guidance from their providers. Prior survivor studies focusing on receipt of health promotion advice [14;15], have not focused on the population of survivors most at risk, those with behavioral risk factors.
To inform models of survivorship care that address prevention and management of CVD, we assessed the prevalence of CVD risk factors among long-term cancer survivors overall, and by race/ethnicity and cancer site, and compared the results to survey data from the general population in the same geographic region. We hypothesized that long-term survivors would have a higher prevalence of CVD risk factors than comparable adults. We also estimated the proportion of long-term cancer survivors who reported a health promotion discussion with a physician. Consistent with prior research[14;15], we expected that a substantial proportion of long-term cancer survivors would not report having health promotion discussions with a physician.
Methods
Cancer Survivor Sample [from the Follow Up Care Use in Survivors (FOCUS) study]
FOCUS study details have been described previously[16;17]. We identified survivors with a primary diagnosis of breast, prostate, colorectal, or gynecologic (endometrial or ovarian) cancers who, at the time of diagnosis (1992–1995 & 1997–2001), resided in the catchment area of the Los Angeles County Cancer Surveillance Program (LA CSP) and the Cancer Prevention Institute of California (CPIC) Surveillance, Epidemiology, and End Results (SEER) cancer registries. We selected a random sample of 6,391 survivors using strata defined by cancer site; time since diagnosis (4–9 and 11–14 years- centered around 5 & 10 years); age at diagnosis (21 to 64 and 65 to 79 years); gender; and race/ethnicity [non-Hispanic white, black or African-American, Asian Pacific Islander (API), and Hispanic]. After excluding survivors who were deceased, incapacitated, bedridden, unable to comprehend the questions, out of the country, did not read English, were currently receiving cancer treatment other than maintenance medications, whose physicians did not approve contact, or denied having cancer, there were 4,981 eligible survivors. We were unable to locate current contact information for 40.2% of eligible survivors, despite using extensive tracing methods. Of the 2,977 survivors we contacted, 1,667 completed the survey (55.6%). Among eligible located patients, the only factors significantly associated with lower response were older age, colorectal cancer diagnosis, and diagnosis 11–14 years ago. The final analytic sample consisted of 1,582 cancer survivors who reported they were cancer free.
Survey Procedure
The FOCUS study was approved by the Institutional Review Boards at the University of Southern California and the CPIC. Data collection by LA CSP and CPIC staff occurred between March 2005 and July 2006. Survivors were mailed a letter explaining the study and patient’s rights, along with the questionnaire. Interviewers conducted telephone follow-up to encourage survey completion if the survey was not returned after 3 weeks. Survivors received a $20 check (from the LA CSP) or a $25 money order (from CPIC) for completing the survey. Optical scan data entry and quality control processing occurred after scanning the paper questionnaires.
Smoking was defined as current (daily or “some days a month”, former (at least 100 lifetime cigarettes but not currently smoking), and never (less than 100 lifetime cigarettes). We classified survivors into three physical activity (PA) groups: meeting American College of Sports Medicine/ American Heart Association’s PA guidelines for adults[18] (≥ 150 minutes of moderate-intensity or 60 minutes of vigorous-intensity PA per week), some PA, but below guideline level, or no PA. Questions assessed the number of times per week (once, two to four, five to seven, eight to 10, or ≥ 10) and the minutes per episode (<10, 10–19, 20–29, 30–59, and ≥ 60) of moderate and vigorous PA. As in prior studies of cancer survivors[19], we set values equal to the minimum value in the range because adults tend to over-report PA[19].
We calculated body mass index (BMI, kg/m2) from self-reported height and weight and classified low/normal BMI as ≤ 25 kg/m2, overweight as 25–29.9 kg/m2, and obese as ≥ 30 kg/m2. We defined hypertension and diabetes as present if survivors reported an ever-diagnosis of hypertension or diabetes. We classified survivors as having a history of CVD if they reported that they had ever been diagnosed with irregular heartbeat, heart failure, cardiomyopathy, myocardial infarction, angina, pericarditis, or stiff or leaking heart valves.
Survivors were asked about discussions with a physician or other health professional during the past two years about 1) diet, 2) exercise, 3) smoking, and 4) assistance with making lifestyle changes. Self-reported sociodemographic variables included current age, race/ethnicity, and educational attainment (high school graduate or less, some college or technical degree, and college graduate). We used cancer registry data to determine cancer site and time since diagnosis, and to supplement sociodemographic data if necessary. We ascertained limited treatment data, defined as yes/no for surgery, chemotherapy, radiation, and maintenance hormonal therapy, by combining data from the cancer registries and questionnaire.
Comparison Sample
Data from the 2005 California Health Interview Survey (CHIS)[20], a an independently administered telephone survey of 43,020 adults in California, conducted by the UCLA Center for Health Policy Research in collaboration with the California Department of Public Health and the Department of Health Care Services, were used to generate prevalence estimates of CVD risk factors from the adult population living in the same geographic area as the cancer survivor sample[21]. We included CHIS data from Los Angeles county and 9 counties in the Greater Bay Area Cancer Registry (GBACR) catchment area and restricted the sample to white non-Hispanic, Hispanic, Black, and API adults ages 50–93 (an age range that was represented by 94% of the cancer survivor sample).
Although the surveys were conducted independently, the CVD risk factor questions were very similar, with the exception of physical activity which queried about activity in the last 7 days, rather than the last 4 weeks. We coded CHIS responses for smoking, BMI, hypertension, and diabetes using the same methodology we applied to the cancer survivor sample. For physical activity, CHIS participants were asked about walking for transportation and fun/exercise in addition to questions about moderate and vigorous physical activity in the last 7 days. In CHIS, PA responses were coded into a variable with three levels: regular PA (vigorous activity 20 minutes or more three or more days a week or moderate activity 30 minutes or more three or more days a week), some PA (walking at least 10 minutes for transportation or fun, vigorous activity in the past week, or at least 10 minutes of moderate activity in the past week), and sedentary/no PA (less than 10 min walking for transportation or fun or no PA in the past week). These levels are very similar to the levels in the FOCUS sample, especially for the binary comparison variable (any moderate or vigorous PA vs. none).
Data Analysis
We conducted weighted analyses using SUDAAN (Research Triangle Institute, version 10.0.1.) to account for the stratified sampling design. First, we examined the prevalence of CVD risk factors in FOCUS compared to CHIS. We used binary coding of the risk factor variables (Figure 1) for these comparisons. All CHIS CVD risk factor prevalence estimates were calculated using the AskCHIS analysis tool[22]. Risk factor prevalence in the FOCUS sample was compared to the CHIS sample using t-tests for independent samples.
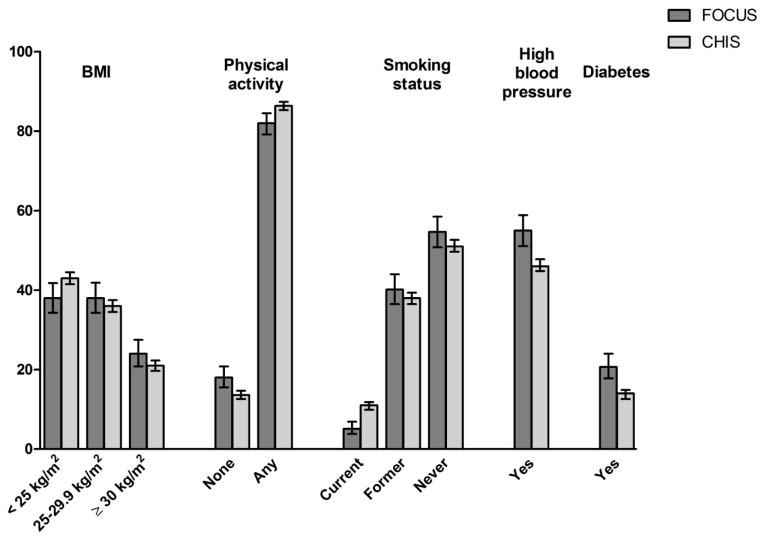
Figure 1.
Weighted prevalence (%) of cardiovascular risk factors in long-term cancer survivors (FOCUS) and a comparison sample (CHIS) Weighted prevalence (%) and 95% confidence interval are shown and reported in Online Resource 1. The FOCUS sample was comprised of 4–14 year cancer survivors of breast, prostate, colorectal, and gynecologic cancers recruited from SEER cancer registries in California. The California Health Interview Survey (CHIS) sample was comprised of adults aged 50 to 93 residing in the same California counties that were covered by the SEER cancer registries. With the exception of a history of former smoking, all paired comparisons are significantly different, p <.05.
Next for the survivor sample, we report the prevalence and 95% confidence intervals for each CVD risk factor stratified by race/ethnicity and cancer site. Missing data were omitted. Prevalence of missingness for CVD risk factors ranged from 3–7%. We also used bivariate logistic regression analyses to estimate the odds of having a given CVD risk factor by race/ethnicity, age and cancer site. The number of CVD risk factors was summed and race/ethnicity, age, and cancer site were examined as predictors of risk factor count using multivariable linear regression models. We report unadjusted odds ratios and 95% confidence intervals (OR, 95% CI) for these associations. Finally, we examined the prevalence of preventive health discussions with a provider for survivors with each risk factor. Race/ethnicity, age, and cancer site were examined as predictors of receiving provider health promotion discussions.
Results
Survivor Characteristics
Demographic and disease characteristics for the FOCUS sample reflect the sampling plan and are shown in Table 1. Half of the sample was female and non-Hispanic white, respectively. Two thirds were 65 years or older, and over half had been diagnosed with stage 0/1 cancer. Surgery was the most common cancer treatment (84%), followed by radiation (36.1%) and chemotherapy (30.6%).
Table 1.
Descriptive Characteristics for Long-term Adult Cancer Survivors (N= 1582)
| N (Weighted %) | |
|---|---|
| Cancer Site | |
| Breast | 394 (24.1) |
| Prostate | 393 (38.3) |
| Colorectal | 393 (21.4) |
| Gynecologic | 402 (16.1) |
| Sex | |
| Male | 592 (49.6) |
| Female | 990 (50.4) |
| Race/Ethnicity | |
| Non-Hispanic, white | 597 (50.4) |
| Hispanic, white | 221 (16.6) |
| Black or African-American | 378 (18.0) |
| Asian/Pacific Islander | 357 (13.6) |
| Educational Attainment | |
| High school or less | 419 (23.9) |
| Some college or technical school | 569 (36.2) |
| College graduate or more | 571 (38.2) |
| Age at Survey | |
| 29–64 | 511 (33.0) |
| 65–79 | 745 (46.8) |
| 80+ | 326 (20.2) |
| Time Since Diagnosis | |
| 4–9 years | 830 (52.6) |
| 10–15 years | 752 (47.4) |
| AJCC Stage (non-prostate) | |
| 0/I | 604 (51.8) |
| II | 319 (28.3) |
| III | 207 (15.3) |
| IV | 28 (1.5) |
| Unknown/Unstaged | 31 (3.0) |
| SEER Historic Stage (prostate) | |
| Local/Reg for Prostate | 376 (95.7) |
| Distant | 5 (0.9) |
| Unstaged | 12 (3.4) |
| Cancer Treatment (% Received)* | |
| Surgery | 1364 (84.0) |
| Chemotherapy | 595 (30.6) |
| Radiation | 535 (36.1) |
| Maintenance Hormonal Therapy | 336 (19.4) |
| Number of Cardiovascular Risk Factors† | |
| 0 | 265 (19.3) |
| 1 | 458 (29.6) |
| 2 | 494 (30.8) |
| 3 | 287 (15.8) |
| 4 | 75 (4.3) |
| 5 | 3 (0.2) |
Survivors often reported more than one treatment modality so the column percentages do not sum to 100%;
Cardiovascular risk factors included current cigarette smoking, no moderate or vigorous physical activity in the past month, overweight or obese body mass index, ever diagnosis with hypertension, and ever diagnosis with diabetes.
Comparison of Cardiovascular Risk Factors in Long-Term Cancer Survivors and the Comparison Sample
Figure 1 shows the prevalence of CVD risk factors in long-term cancer survivors (FOCUS) and the comparison population living in the same California counties during the same time period (CHIS). Compared to the comparison sample, a significantly higher percentage of cancer survivors were overweight or obese (t= 4.98, p=.006), physically inactive (t=4.62, p=.0003), and reported a history of hypertension (t=4.73, p<.0001), or diabetes (t=5.04, p<.0001). In contrast, a significantly lower percentage of cancer survivors were current smokers (t= 5.53, p=.0001); there was no difference in history of smoking (t= 1.27, p=.20).
Prevalence of Cardiovascular Risk Factors and CVD among Long-term Survivors
CVD risk factors were common in this sample of long-term cancer survivors. The most common risk factors included overweight (38%) or obese (24%) BMI, hypertension (55%), and diabetes (21%). Less common CVD risk factors included physical inactivity (18%) and current smoking (5%). A history of CVD was reported by 35.0% of cancer survivors. History of CVD did not vary by cancer site (X2= 0.60, p=0.61).
Prevalence of cardiovascular risk factors among survivors by cancer site, race/ethnicity, and age
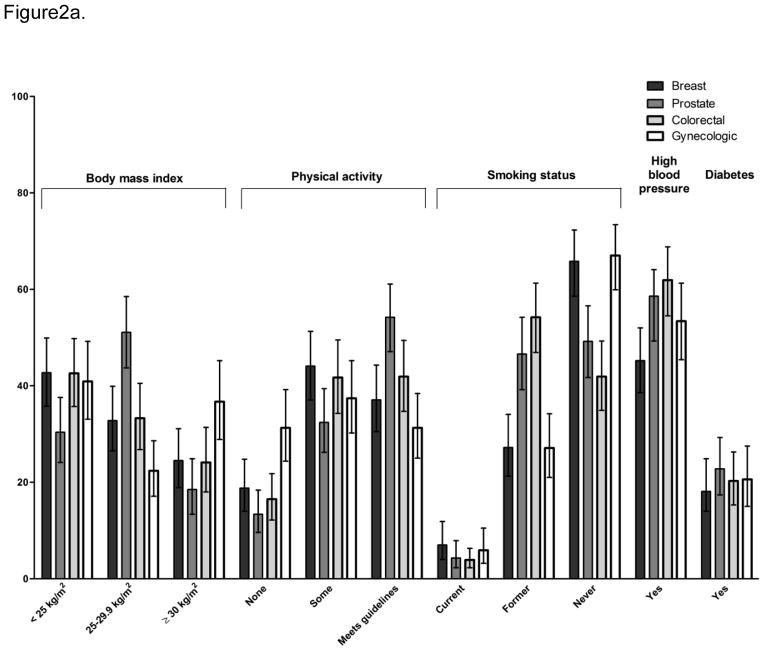
Figure 2a shows the prevalence of CVD risk factors by cancer site and values are reported in Online Resource 1. Compared to breast cancer survivors (57.3%), prostate cancer survivors (69.6%) were significantly more likely to be overweight or obese (OR=1.70, CI 1.10–2.62). Gynecologic cancer survivors (31.3%) were significantly more likely than breast cancer survivors (18.8%) to be physically inactive (1.98, 1.20–3.24), There were no significant differences in current smoking or diabetes by cancer site. Compared to breast cancer survivors (45.2%), prostate (56.8%) and colorectal (61.9%) cancer survivors were significantly more likely to report a history of hypertension (OR=1.60, CI 1.06–2.40 and OR=1.97, CI 1.31–2.97, respectively).
Figure 2.
Figure 2a. Weighted prevalence of cardiovascular risk factors among long-term survivors (FOCUS) by cancer site
Weighted prevalence and 95% confidence interval are shown and reported in Online Resource 1. Physical activity was classified as meeting American College of Sports Medicine/ American Heart Association’s physical activity guidelines for adults[18] (150 minutes of moderate-intensity or 60 minutes of vigorous-intensity physical activity per week), some moderate or vigorous physical activity, but below guideline level, or those reporting no moderate and no vigorous activity in the past 4 weeks. Self-report of ever diagnosis with hypertension or diabetes defined these risk factors.
2a-There were significant overall differences by cancer site for all of the cardiovascular risk factors except diabetes and high cholesterol (p<.05). Odds ratios for comparison of each site to breast cancer are reported in the text.
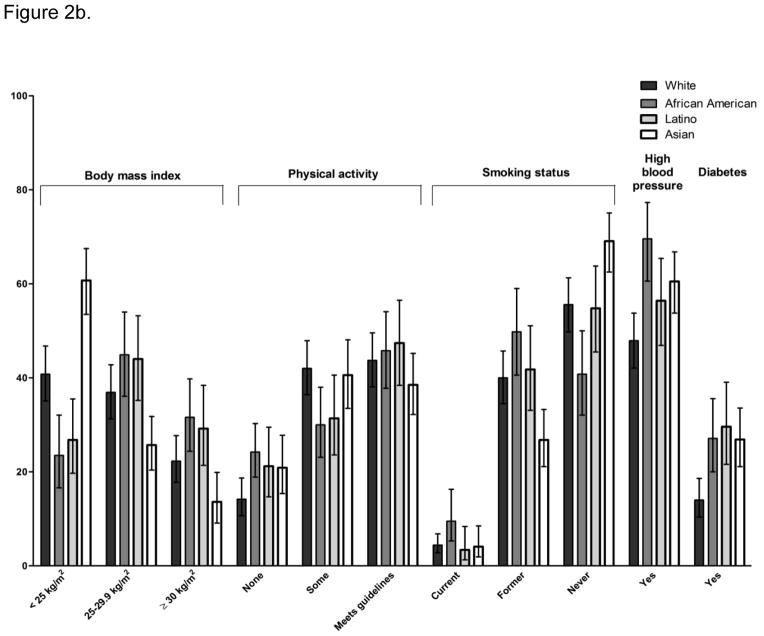
Figure 2b. Prevalence of cardiovascular risk factors among long-term cancer survivors (FOCUS) by race/ethnicity.
2b- There were significant overall differences by race/ethnicity for all of the cardiovascular risk factors except smoking (p<.05). Odds ratios for comparison of African American, Latino, and Asian survivors to white, non-Hispanic survivors are reported in the text.
There were significant differences by age/ethnicity for all CVD risk factors (see Figure 2b and Online Resource 1). Hispanic and African-American survivors were approximately twice as likely as non-Hispanic white survivors to be overweight or obese (ORHispanic= 1.88, CI 1.17–3.02 and ORAfrican-American= 2.25, CI 1.37–3.69). In contrast, API survivors were significantly less likely than non-Hispanic whites to be overweight or obese (OR=0.45, CI 0.30–0.66). African-American survivors were also significantly more likely than non-Hispanic white survivors to be physically inactive (OR=1.89, CI 1.21–2.95). There was a trend for Hispanic and API survivors to report no PA more often than white survivors, but this did not reach statistical significance. African-American survivors were more likely than non-Hispanic white survivors to be current smokers (OR=2.27, CI 1.05–4.94); significant differences were not observed for other racial and ethnic minority groups. API (OR=1.66, CI 1.16–2.39) and African-American (OR=2.49, CI 1.57–3.95), but not Hispanic (OR=1.40, CI 0.90–2.20), survivors were also more likely than non-Hispanic white to report a history of hypertension. All racial/ethnic minority groups were significantly more likely than non-Hispanic white survivors to report a history of diabetes (OR Hispanic= 2.59, CI 1.50–4.47, OR African-American= 2.29, CI 1.35–3.86, OR API= 2.26, CI 1.42–3.61).
There were no significant differences in physical inactivity, current smoking, or diabetes by age group. Compared to survivors under 65, 80+ survivors were less likely to be overweight or obese (OR=0.46, CI 0.30–0.71). Compared to the under-65 group, and older survivors were more likely to report a history of hypertension (OR 65–79 years= 2.38, CI 1.65–3.43 and OR 80+ years= 2.03, CI 1.30–3.18).
Predictors of Risk Factor Count
On average, cancer survivors reported a total of 1.6 CVD risk factors. In multivariable models, race/ethnicity and age, but not cancer site, were significant predictors of total number of CVD risk factors. Compared to white, non-Hispanic survivors, Hispanic (b= .37, p= .007) and African-American (b= .66, p<.0001), but not API (b= 0.18, p= .10) survivors reported significantly more risk factors. Survivors aged 65 to 79 years, but not the 80+ group, reported significantly more risk factors than younger survivors (b= 0.38, p=.0006).
Discussion about CVD Risk Reduction with Medical Providers
Most survivors reported dietary (61%), and exercise (68%) discussions with their doctors, as well as assistance with making lifestyle change (62%) within the last two years. Among the current smokers, 87% reported a discussion with their doctor about smoking. Reports of exercise and dietary discussions were significantly more common among those survivors with hypertension, diabetes, and those who were overweight or obese (Table 2). However, physically inactive survivors were not more likely than active survivors to report exercise discussions or lifestyle change assistance. Cancer survivors with a history of CVD were significantly more likely to report exercise discussion and lifestyle change assistance, but not dietary discussion (Table 2). Of the cancer survivors with one or more CVD risk factors, 33.6% did not report a discussion about diet, 29.2% did not report a discussion about PA, and 34.3% did not report assistance from a health care provider with lifestyle change. Cancer site, race/ethnicity, and age were not associated with health promotion discussions or health behavior change assistance in either bivariate or multivariable logistic regression models (data not shown).
Table 2.
Weighted Percent (and 95% confidence interval) of Long-Term Cancer Survivors who Report Receiving Health Promotion Discussions and Assistance from Health Care Providers
| Risk Factor | Lifestyle Change Assistance | Dietary Discussion | Exercise Discussion |
|---|---|---|---|
| Overweight or Obese | 65.2 (60.1, 70.0) | 67.3 (62.4, 71.9) | 72.7 (67.8, 77.0) |
| Not Overweight or Obese | 57.6 (51.4, 63.6) | 52.4 (46.1, 58.6) | 61.3 (55.0, 67.2) |
| Physically Inactive | 62.5 (54.4, 69.9) | 63.3 (55.0, 70.8) | 66.9 (58.7, 74.3) |
| Physically Active | 62.0 (57.5, 66.2) | 61.1 (56.7, 65.2) | 68.7 (64.4, 72.7) |
| Hypertension | 69.2 (64.2, 73.8) | 68.4 (63.4, 73.0) | 73.6 (68.8, 77.9) |
| No Hypertension | 53.1 (47.0, 59.0) | 52.8 (46.9, 58.7) | 61.8 (55.8, 67.4) |
| Diabetes | 77.5 (70.0, 83.6) | 81.3 (75.2, 86.2) | 82.7 (75.6, 88.0) |
| No Diabetes | 57.6 (53.2, 62.0) | 56.0 (51.5, 60.4) | 64.3 (59.9, 68.5) |
| History of CVD | 68.7 (62.4, 74.4) | 64.3 (57.9, 70.2) | 75.7 (69.8, 80.8) |
| No History of CVD | 58.0 (53.1, 62.8) | 59.7 (54.9, 64.4) | 63.9 (59.0, 68.5) |
Bold pairs are significantly different from each other, p<.05.; CVD= cardiovascular disease, including ever diagnosis with irregular heartbeat, heart failure, cardiomyopathy, myocardial infarction, angina, pericarditis, or stiff or leaking heart valves
Discussion
CVD risk factors and a history of CVD were common in this population of long-term survivors. All of the reported CVD risk factors are preventable or modifiable; however a substantial proportion of survivors (15–30%) at risk for CVD do not report health promotion discussions with their providers. The rates of health promotion discussion in our study were higher than those reported in a national sample of cancer survivors where less than half of survivors reported discussions about diet, exercise, or smoking[14]. Possible explanations for the differences include the cancer-specific nature of our questionnaire or the restriction of the current study sample to long-term survivors from California. Interestingly, we did not observe differences in provider health promotion discussion rates by age, race/ethnicity, or cancer site (a proxy for gender). Data from the National Ambulatory care survey suggests that ethnic minorities, women, and younger adults (<75 years) may be more likely to receive diet and physical activity counseling during ambulatory visits[23]. Our results suggest that it may be difficult to assess which survivors are not receiving health promotion discussion by risk factors or basic characteristics. Importantly, we relied on patients’ recollection of preventive health discussions. Provider perception or discussions documented in the medical record may not be consistent with patient report. Providers caring for cancer survivors may need additional education regarding how to screen and provide referrals or interventions for this patient population.
All CVD risk factors, with the exception of smoking, were more common among survivors compared to the comparison sample. In contrast, a prior study of cancer survivors and matched controls found elevated rates of current smoking and a lower prevalence of overweight/obese BMI among US survivors[10]. Our survivors were less likely to report current smoking (5%) compared to survivors in this study (35%), possibly because they were older and our sample did not include smoking-related cancers. Our study also included a much larger proportion of racial/ethnic minority survivors and was restricted to survivors diagnosed in California, one of the first states to implement comprehensive tobacco control strategies. Similar to what we observed in our cancer survivors, the CHIS adults appeared healthier on several CVD risk factors compared to national prevalence data (e.g. lower rates of current smoking and physical inactivity compared to National Health and Nutritional Examination Data[24] and a large cohort study[13]). Our comparison sample was not matched to our survivor sample, but was restricted to adults in the same California counties with roughly the same age range. Race/ethnicity distribution was similar in this comparison population, although the percent of African-Americans was slightly higher in our sample (18% vs. 9%). This approach is useful in order to provide a context for our results, which focused on long-term survivors, but does not account for differences in age, health status, or other potentially important CVD risk factor differences between survivors and the general adult population. In addition, although questions assessing risk factors in CHIS and FOCUS were similar, they were not identical, possibly leading to difference in measurement error between the two groups.
As is common in many surveys, we suspect there was over-reporting of physical activity and under-reporting of health compromising behaviors such as smoking or overweight/obese BMI by the survivors. We also did not have data regarding other important CVD risk factors including hypercholesterolemia, fasting glucose, diet quality, and inflammatory markers. Future studies should examine a larger set of CVD risk factors among long term cancer survivors. Further, we could not fully explore the role of gender because three of our four included cancer sites were restricted to one gender. In addition, because these data were cross-sectional we could not tell how CVD risk factors and discussions with medical providers changed over the cancer trajectory. Although our response rate among located survivors was similar to recent population-based studies of cancer survivors[25;26], nonresponse may have introduced error. Another significant limitation of this study was that treatment data were not ascertained from the medical records. Even though we asked survivors about treatments received and utilized data from the cancer registries, this approach was insufficient to determine if survivors received treatment regimens linked with cardiovascular complications, and thus could not be included in our analyses. Breast cancer survivors would have been most likely to receive treatment with cardiotoxic regimens (e.g., chest wall irradiation or treatment with anthracyclines), but we did not see an associated increase in risk factor prevalence or history of CVD in this group. Future studies need to examine risk factor prevalence and CVD prospectively in large samples with well-characterized treatment histories
This paper contributes to the growing literature on the importance of managing traditional risk factors for CVD in survivors of all ages[27]. Our data suggest health promotion discussions regarding risk factor reduction may not be taking place in the context of survivorship care. Risk factor counseling may be challenging in the context of brief oncology visits after cancer treatment and busy primary care encounters. Data from office visits indicate that <25% of adult patients presenting for routine exams received CVD prevention advice; internists had lower rates of counseling compared to cardiologists and family and general practitioners[28]. Data do suggest that survivors who see primary care providers for follow-up cancer care may be more likely to receive disease prevention advice[15].
Integrated models for cancer-related follow-up care emphasize the critical role of primary care, including internal medicine[29–31]. Administrative studies have suggested that survivors who see both oncology and primary care may be more likely to receive preventive health services[32;33], suggesting the crucial role of primary care in health promotion. With the increasing number of older cancer survivors and increased rates of long-term survival[34], the prevalence of CVD among cancer survivors is likely to rise. Primary care will play a crucial role in screening cancer patients for CVD risk factors and delivering appropriate care[35–37]. Ultimately evidence-based prevention programs and clinical practice guidelines are needed to prevent and treat CVD in this vulnerable patient population[7;35;36].
Supplementary Material
Implications for Cancer Survivors.
Cardiovascular disease may compromise cancer survivors’ long-term health and well-being, yet cardiovascular risk factors may be overlooked during survivorship care. We document that CVD risk factors are common among cancers survivors, yet nearly a third of survivors do not report health promotion discussions with their medical teams. Survivors should be aware of their cardiovascular risk factors and initiate discussions with their medical teams about health promotion topics, if appropriate.
Acknowledgments
Funding Sources:
This work was supported by the National Cancer Institute at the National Institutes of Health. Contracts No. N01-PC-35136 and HHSN 261201100189P. The ideas and opinions expressed herein are those of the authors and endorsement by the NCI, and or their contractors and subcontractors is not intended nor should be inferred.
Footnotes
Prior Presentations: A preliminary version of this data was presented at the Society of Behavioral Medicine Annual Meeting, April 2012
Conflict of Interest: None
References
- 1.Patnaik J, Byers T, DiGuiseppi C, Dabelea D, Denberg T. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Research. 2011;13:R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shikanov S, Kocherginsky M, Shalhav AL, Eggener SE. Cause-specific mortality following radical prostatectomy. Prostate Cancer and Prostatic Diseases. 2011;15:106–110. doi: 10.1038/pcan.2011.55. [DOI] [PubMed] [Google Scholar]
- 3.Fossa SD, Gilbert E, Dores GM, Chen J, McGlynn KA, Schonfeld S, et al. Noncancer Causes of Death in Survivors of Testicular Cancer. J Natl Cancer Inst. 2007;99:533–544. doi: 10.1093/jnci/djk111. [DOI] [PubMed] [Google Scholar]
- 4.Baade PD, Fritschi L, Eakin EG. Non-Cancer Mortality among People Diagnosed with Cancer (Australia) Cancer Causes & Control. 2006;17:287–297. doi: 10.1007/s10552-005-0530-0. [DOI] [PubMed] [Google Scholar]
- 5.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J Am Coll Cardiol. 2007;50:1435–1441. doi: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 6.Hawkes AL, Lynch BM, Owen N, Aitken JF. Lifestyle factors associated concurrently and prospectively with co-morbid cardiovascular disease in a population-based cohort of colorectal cancer survivors. Eur J Cancer. 2011;47:267–276. doi: 10.1016/j.ejca.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Wolin KY, Colditz GA. Implementing chronic disease prevention amongst cancer survivors. J Intern Med. 2011;269:85–87. doi: 10.1111/j.1365-2796.2010.02295.x. [DOI] [PubMed] [Google Scholar]
- 8.Haugnes HS, Wethal T, Aass N, Dahl O, Klepp Or, Langberg CW, et al. Cardiovascular Risk Factors and Morbidity in Long-Term Survivors of Testicular Cancer: A 20-Year Follow-Up Study. J Clin Oncol. 2010;28:4649–4657. doi: 10.1200/JCO.2010.29.9362. [DOI] [PubMed] [Google Scholar]
- 9.Howlander N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al., editors. SEER Cancer Statistics Review, 1975–2008. Bethesda, MD: National Cancer Institute; 2011. [Google Scholar]
- 10.Enright K, Krzyzanowska M. Control of cardiovascular risk factors among adult cancer survivors: a population-based survey. Cancer Causes Control. 2010;21:1867–1874. doi: 10.1007/s10552-010-9614-6. [DOI] [PubMed] [Google Scholar]
- 11.Bambs C, Kip KE, Dinga A, Mulukutla SR, Aiyer AN, Reis SE. Low prevalence of “ideal cardiovascular health” in a community-based population: the heart strategies concentrating on risk evaluation (Heart SCORE) study. Circulation. 2011;123:850–857. doi: 10.1161/CIRCULATIONAHA.110.980151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shay CM, Ning H, Allen NB, Carnethon MR, Chiuve SE, Greenlund KJ, et al. Status of Cardiovascular Health in US Adults: Prevalence Estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003–2008. Circulation. 2012;125:45–56. doi: 10.1161/CIRCULATIONAHA.111.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community Prevalence of Ideal Cardiovascular Health, by the American Heart Association Definition, and Relationship With Cardiovascular Disease Incidence. J Am Coll Cardiol. 2011;57:1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabatino SA, Coates RJ, Uhler RJ, Pollack LA, Alley LG, Zauderer LJ. Provider counseling about health behaviors among cancer survivors in the United States. J Clin Oncol. 2007;25:2100–2106. doi: 10.1200/JCO.2006.06.6340. [DOI] [PubMed] [Google Scholar]
- 15.Haggstrom DA, Arora NK, Helft P, Clayman ML, Oakley-Girvan I. Follow-up care delivery among colorectal cancer survivors most often seen by primary and subspecialty care physicians. J Gen Intern Med. 2009;24 (Suppl 2):S472–S479. doi: 10.1007/s11606-009-1017-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellizzi KM, Aziz NM, Rowland JH, Weaver K, Arora NK, Hamilton AS, et al. Double Jeopardy? Age, Race, and HRQOL in Older Adults with Cancer. Journal of Cancer Epidemiology. 2012;2012:1–9. doi: 10.1155/2012/478642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kent EE, Arora NK, Rowland JH, Bellizzi KM, Forsythe LP, Hamilton AS, et al. Health information needs and health-related quality of life in a diverse population of long-term cancer survivors. Patient Educ Couns. 2012 doi: 10.1016/j.pec.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haskell WL, Lee I-M, Pate RP, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A. Physical activity and public health: updated recommendation fro adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 19.Bellizzi KM, Rowland JH, Arora NK, Hamilton AS, Miller MF, Aziz NM. Physical activity and quality of life in adult survivors of non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27:960–966. doi: 10.1200/JCO.2008.17.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.California Health Interview Survey. CHIS 2005 Adult Public Use File. Los Angeles, CA: UCLA Center for Health Policy Research; 2011. [Google Scholar]
- 21.California Health Interview Survey. CHIS 2005 Metholdology Series: Report 4- Response Rates. Los Angeles, CA: UCLA Center for Health Policy Research; 2007. Available at: http://healthpolicy.ucla.edu/chis/design/Documents/CHIS2005_method4.pdf. [Google Scholar]
- 22.UCLA Center for Health Policy Research. California Health Interview Survey. Available at www.askchis.com.
- 23.Ma J, Urizar GG, Jr, Alehegn T, Stafford RS. Diet and physical activity counseling during ambulatory care visits in the United States. Prev Med. 2004;39:815–822. doi: 10.1016/j.ypmed.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Huffman MD, Capewell S, Ning H, Shay CM, Ford ES, Lloyd-Jones DM. Cardiovascular health behavior and health factor changes (1988–2008) and projections to 2020: results from the National Health and Nutrition Examination Surveys. Circulation. 2012;125:2595–2602. doi: 10.1161/CIRCULATIONAHA.111.070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baker F, Denniston M, Smith T, West MM. Adult cancer survivors: How are they faring? Cancer. 2005;104:2565–2576. doi: 10.1002/cncr.21488. [DOI] [PubMed] [Google Scholar]
- 26.Pakilit AT, Kahn BA, Petersen L, Abraham LS, Greendale GA, Ganz PA. Making effective use of tumor registries for cancer survivorship research. Cancer. 2001;92:1305–1314. doi: 10.1002/1097-0142(20010901)92:5<1305::aid-cncr1452>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 27.Landy DC, Miller TL, Lopez-Mitnik G, Lipsitz SR, Hinkle AS, Constine LS, et al. Aggregating traditional cardiovascular disease risk factors to assess the cardiometabolic health of childhood cancer survivors: an analysis from the Cardiac Risk Factors in Childhood Cancer Survivors Study. Am Heart J. 2012;163:295–301. doi: 10.1016/j.ahj.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Missed Opportunities in Preventive Counseling for Cardiovascular Disease–United States, 1995. JAMA: The Journal of the American Medical Association. 1998;279:741–742. [PubMed] [Google Scholar]
- 29.Hong S, Nekhlyudov L, Didwania A, Olopade O, Ganschow P. Cancer Survivorship Care: Exploring the Role of the General Internist. J Gen Intern Med. 2009;24:495–500. doi: 10.1007/s11606-009-1019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klabunde CN, Ambs A, Keating NL, He Y, Doucette WR, Tisnado D, et al. The Role of Primary Care Physicians in Cancer Care. J Gen Intern Med. 2009;24:1029–1036. doi: 10.1007/s11606-009-1058-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grunfeld E, Earle CC. The interface between primary and oncology specialty care: treatment through survivorship. J Natl Cancer Inst Monogr. 2010;2010:25–30. doi: 10.1093/jncimonographs/lgq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snyder C, Frick K, Peairs K, Kantsiper M, Herbert R, Blackford A, et al. Comparing Care for Breast Cancer Survivors to Non-Cancer Controls: A Five-Year Longitudinal Study. J Gen Intern Med. 2009;24:469–474. doi: 10.1007/s11606-009-0903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snyder C, Earle C, Herbert R, Neville B, Blackford A, Frick K. Trends in Follow-up and Preventive Care for Colorectal Cancer Survivors. J Gen Intern Med. 2008;23:254–259. doi: 10.1007/s11606-007-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parry C, Kent EE, Mariotto AB, Alfano CM, Rowland JH. Cancer Survivors: A Booming Population. Cancer Epidemiology Biomarkers & Prevention. 2011;20:1996–2005. doi: 10.1158/1055-9965.EPI-11-0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lenihan DJ, Cardinale D, Cipolla CM. The Compelling Need for a Cardiology and Oncology Partnership and the Birth of the International CardiOncology Society. Prog Cardiovasc Dis. 2010;53:88–93. doi: 10.1016/j.pcad.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Lindenfeld J, Kelly PA. Developing a Cardiology-Oncology Clinical Practice Guideline. Prog Cardiovasc Dis. 2010;53:173–179. doi: 10.1016/j.pcad.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Mann DL, Krone RJ. Cardiac Disease in Cancer Patients: An Overview. Prog Cardiovasc Dis. 2010;53:80–87. doi: 10.1016/j.pcad.2010.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.