Abstract
One of the major frontiers in biomedical optics has been as an adjunct to gastrointestinal endoscopy. In particular, spectroscopy of elastic light scattering has the potential of addressing many of the vexing challenges confronting endoscopists. This review discusses the principles of spectroscopy and critically evaluates performance in clinically significant scenarios. One of the best established applications is optical biopsy (in situ histological determination), and a number of techniques such as elastic scattering spectroscopy have demonstrated the ability to discriminate between neoplastic and non-neoplastic polyps. For flat dysplasia detection in Barrett’s esophagus, some of the most promising spectroscopic technologies are angle-resolved low-coherence interferometry and endoscopic polarized scanning spectroscopy (the next generation light scattering spectroscopy). A new and exciting biological approach involves optical detection of field carcinogenesis. This can be exploited to reduce colonoscopic adenoma miss rate by assessing microcirculatory augmentation in the mucosa in the vicinity of the polyp using polarization-gatedspectroscopy. Furthermore, there are nano/micro-architectural correlates with diffuse field carcinogenesis throughout the colon. Indeed, technologies such as low coherence enhanced backscattering spectroscopy and partial wave spectroscopic microscopy have demonstrated that the detection of the nano-architectural alterations in the rectal mucosa can accurately sense advanced adenomas elsewhere in the colon. This may lend itself to a minimally intrusive risk stratification to identify patients who are most likely to harbor neoplasia and thus benefit from colonoscopy. Bridging these advances into the endoscopy suite requires pragmatic future development. Future studies need to focus on efficacy, cost, practicality (time required, etc), and particularly developing the paradigms that will impact upon clinical decision making.
Keywords: Spectroscopy, Endoscopy, Cancer Screening, Biophotonics, Field Carcinogenesis
Recently, a number of reports have heralded endoscopic triumphs in the cancer prevention arena. For instance, colonoscopic polypectomy resulted in an approximately 50% reduction in mortality from colorectal cancer (CRC).1 With regard to Barrett’s, radiofrequency ablation showed a remarkable approximately 90% decrease in esophageal adenocarcinoma development in patients harboring high-grade dysplasia.2 However, there is considerable work remaining as highlighted by the continued toll of CRC (second leading cause of cancer deaths) and the increasing incidence of esophageal adenocarcinoma. This provides the impetus for the development of adjunct endoscopic technologies.
For colonoscopy, one of the vexing obstacles is the marginal protection of colonoscopy against right-sided disease.3 In addition, the adenoma detection rate varies several fold even among well-trained endoscopists,4 and this appears to mirror CRC protection.5 Furthermore, modest patient compliance with colonoscopy has meant that much less accurate approaches (eg, a fecal immunohistochemical blood test) have an almost comparable rate of CRC diagnosis.6 Finally, because early stage CRC and advanced adenomas are infrequent in the screening population (~6%–7%), the vast majority of colonoscopies have no direct cancer prevention benefit that can be derived from polypectomy of biologically significant precursor lesions. This is also true for upper endoscopies for esophageal adenocarcinoma, given recent reports that Barrett’s progression to carcinoma is markedly lower than previous estimates.7
To address these hurdles, there have been a myriad of optical technologies developed. These span the gamut from improved visualization through modifications incorporated into the endoscope (eg, high-definition, narrow-band imaging and autofluorescence) to fiberoptic probes that can yield microscope-like images (including optical coherence tomography and confocal endomicroscopy) or visualize molecular probe or provide tissue microarchitectural quantification via various spectroscopic approaches.8 For this review, we focus primarily on spectroscopy because many nonspectroscopic techniques were covered in a recent Advances in Translational Science article.9
Principles of Spectroscopy
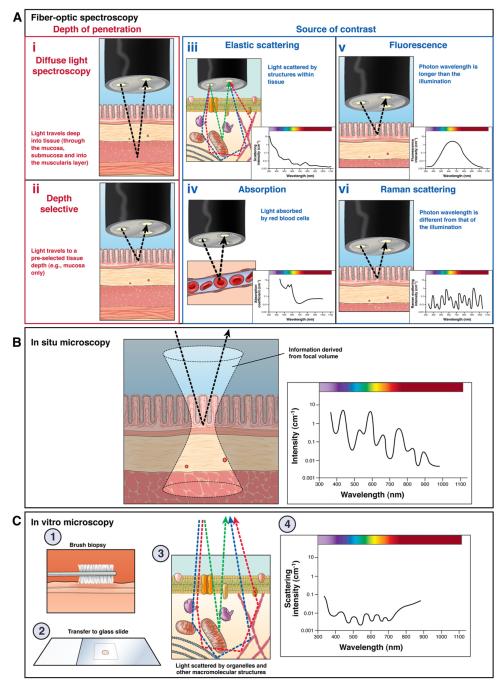
Spectroscopy characterizes objects based on how they interact with light. Light scattering can be dichotomized into elastic (no change in energy and hence wavelength with scattering; this is the dominant process of light transport in tissue) and nonelastic (scattering alters wavelength). Spectroscopic analysis typically harnesses light scattered in the backward direction (toward incident light) and can encompass a wide range of wavelengths. Elastic scattering is driven by spatial variations of tissue refractive index (determined by local macromolecular density) and thus provides fundamental insights into the size distribution of tissue structures (organelles, chromatin structure, collagen fibers, and so forth) and the spatial correlation of macromolecular density (Figure 1). Depending on the technique, length scales as large as a few micrometers (eg, cell nuclei) and as small as a few tens of nanometers (eg, macromolecular complexes) can be assessed. In addition, as light propagates through the tissue, absorption is inevitable and mainly is owing to hemoglobin. Thus, the absorption spectra can be used to measure tissue blood concentration, oxygen saturation, and sizes of blood vessels. With regard to inelastic light scattering the most commonly used is Raman spectroscopy, which measures vibrational and rotational aspects of molecules and thus provides insights into the molecular composition of the tissue, with its ability to discriminate proteins, nucleic acids, lipids, and so forth. Given that the majority of clinical trials to date have been related to elastic light scattering spectroscopy, we focus on these technologies.
Figure 1.
(A) Fiberoptic spectroscopy; (B) in situ microscopy; and (C) in vitro microscopy.
Spectroscopic techniques also can be classified based on a mode of signal acquisition (Figure 1), as follows: point measurement fiberoptic spectroscopy (the depth of measurements is determined by the source of contrast and probe design), in situ spectroscopic microscopy (eg, low-coherence interferometry, inverse scattering spectroscopic optical coherence tomography), and ex vivo spectroscopic microscopy (eg, partial wave spectroscopic nanocytology, quantitative phase microscopy).
Advantages of Spectroscopy
Spectroscopic output typically is quantitative and not an image, which is both a major strength and potential weakness. On the positive side, this does not require particular training in image analysis (ie, it does not require the gastroenterologist to be trained as a pathologist). In addition, tissue characteristics that otherwise are not possible to assess by means of endoscopic visualization can be assessed, such as chemical composition and nanoscale tissue structure. Conversely, the lack of image may lead to some reticence (more of a black box approach) and may not capture tissue/tumor heterogeneity adequately. Other positive attributes of spectroscopy include no requirements for contrast agents (in contradistinction to confocal or molecular imaging) and several-fold better resolution than conventional light microscopy (interrogating structures at submicrometer length scales).8 Furthermore, spectroscopy typically can yield rapid, almost real-time, assessment, which is mandatory for clinical practice.
Clinical Applications/Current State of the Art
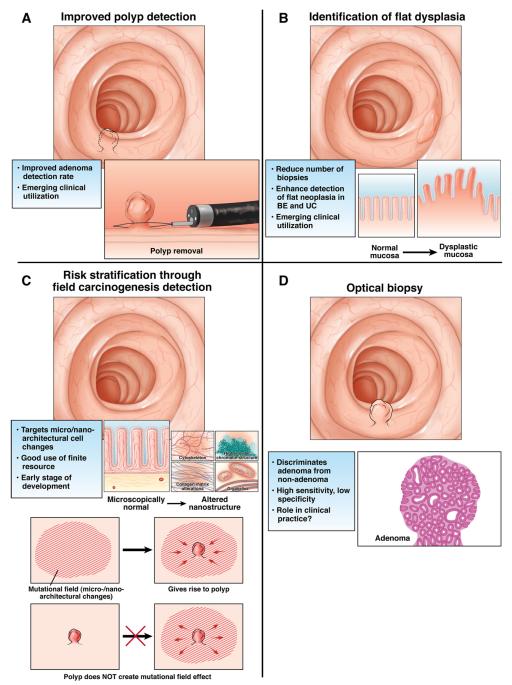
Instead of focusing on technology per se, it is more apropos to focus on clinical utility. As summarized in Figure 2, for biomedical optics in general, and spectroscopy in particular, these applications can be broadly subdivided into 4 major categories: (1) optical biopsy, (2) identification of flat dysplasia, (3) improved polyp detection, and (4) risk stratification through field carcinogenesis detection.
Figure 2.
(A) Improved polyp detection. (B) Identification of flat dysplasia. (C) Risk stratification through field carcinogenesis detection. (D) Optical biopsy.
Optical Biopsy
Clinical Need
Optical biopsy refers to in situ determination of the histology. This is attractive because it may allow avoidance of polypectomy (if the lesion is nonadenomatous), with its inherent risks and cost (pathology charges and so forth). Furthermore, occasionally polyp retrieval from the right colon necessitates complete colonoscope withdrawal and re-insertion, thereby increasing procedure time, patient discomfort, and risk of complications. Deciding which polyps can forego removal (diagnose and leave behind strategy) or pathologic analysis (resect and discard strategy) would increase the efficiency of endoscopic practice markedly.
Technology and Performance
There are a number of nonspectroscopic (confocal endomicroscopy, chromoendoscopy with pit-pattern analysis) as well as spectroscopic techniques designed for this indication. With regard to the latter, one example is elastic scattering spectroscopy (ESS). ESS has shown excellent discrimination between hyperplastic vs adenomatous tissue (84% sensitivity and 84% specificity).10 Whether this is sufficient for clinical practice is unclear (see Current Obstacles/Challenges for Implementation section later). ESS uses separated delivery and collection fibers to obtain a signal that arises largely from the first millimeter of tissue (mucosa and submucosa) and enables a quantitative analysis of tissue micromorphology and blood supply. Importantly, it already has been coupled with a polypectomy snare, fostering translation to clinical practice.
Identification of Polyps
Clinical Needs
Approaches to improve adenoma detection have either targeted endoscopic blind spots (behind folds, flexures) or visualized subtle lesions in the field of view (eg, flat and depressed lesions). Much of the efforts to date have been on improvements to the endoscope (high-definition, narrow-band imaging, autofluorescence, and so forth) or contrast agents (molecular imaging, chromoendoscopy), which have been reviewed recently.9
Technology and Performance
Because most spectroscopy typically is narrow-field (small amount of tissue interrogated), it is not necessarily conducive to this application. However, one approach has been polarization-gated spectroscopy for detection of the increase in blood supply (early increase in blood supply), found in the normal mucosa surrounding, or in the area of, a lesion. This can have a potential application as a “red flag” (identifying neoplasia-harboring colonic segments that warrant increased endoscopic scrutiny), with a sensitivity for advanced adenomas of 92% and specificity of 78%.11 Moreover, fine mapping may be possible because the magnitude of EIBS mirrors proximity to the lesion.
Flat Dysplasia Detection
Clinical Need
Flat dysplasia is the hallmark for carcinogenesis in Barrett’s and ulcerative colitis. The current state of the art is multiple random biopsy specimens looking for the proverbial needle in the haystack. However, obtaining a large number of random biopsy specimens is tedious, expensive, adds potential complications, and results in potential false negatives (because typically <5% of mucosa is sampled).
Technology and Performance
There are a plethora of techniques that have been developed with applications largely focused on Barrett’s esophagus. One of the most widely used techniques appears to be confocal endomicroscopy, although a recent multicenter study failed to validate this approach.12 Spectroscopic approaches for dysplasia identification in Barrett’s esophagus include the following.
Light-scattering spectroscopy
Light-scattering spectroscopy uses polarization to isolate scattering from the surface layer of the epithelium to provide information predominantly on nuclear size. In a small clinical trial of the first-generation system, light-scattering spectroscopy identified dysplasia with approximately 90% accuracy.13 The next generation system (endoscopic polarized scanning spectroscopy) had a sensitivity of 92% and a specificity of 96%.14
Angle-resolved low-coherence interferometry
This novel technique evaluates nuclear size with depth resolution, making it particularly powerful. Indeed, angle-resolved low-coherence interferometry nuclear measurement at a depth of 200–300 μm separated dysplastic from nondysplastic tissue with an area under the receiver operating characteristic curve (AUROC) of 0.91 (100% sensitivity and 84% specificity).15
Elastic scattering spectroscopy
Initial reports have indicated promising diagnostics for identifying high-risk mucosa with ESS, with a 92% sensitivity and 60% specificity.16
Risk Stratification Through Field Carcinogenesis Detection
Clinical Need
From a population perspective, the goal is to identify and remove all advanced adenomas to prevent future CRC. The challenges include the insufficient resources (funding, endoscopic capacity) and complication rate inherent in performing colonoscopy on the entire average-risk population (~100 million Americans older than age 50) to identify all neoplasia-harboring subjects. This is juxtaposed with the remarkably low yield of advanced adenomas in these screening/surveillance procedures, resulting in more than 90% of colonoscopies being unproductive from a cancer prevention perspective. Clearly, personalizing screening strategies is paramount. Because using demographic factors alone has been suboptimal (age, sex, diet, family history, and so forth yielded an AUROC of ~0.60 for CRC),17 attention has focused on other approaches, especially field carcinogenesis detection.
Field Carcinogenesis: Overview
Field carcinogenesis (also known as field effect, field defect) is the biological concept that the genetic/environmental milieu that results in a permissive environment for a focal adenoma/carcinoma to develop is diffuse and exists throughout the colon. The tumor location is determined by stochastic events (eg, truncation of adenomatous polyposis coli tumors suppressor gene or epigenetic silencing of human mutL homolog 1).18 This, in essence, is the rationale for surveillance colonoscopy (higher risk of recurrent adenomas throughout the colon). However, because adenomas are somewhat insensitive for colon carcinogenesis risk, research has focused on earlier events in the predysplastic (microscopically normal) mucosa. Indeed, various biomarkers including genomic, proteomic, cellular (apoptosis/proliferation), and epigenetic (methylation/microRNA) from the distal colon can correlate, albeit imperfectly, with the risk of neoplasia throughout the colon.19,20 However, these changes are heterogeneous but may share common microarchitectural aberrations.
Technology and Performance
Spectroscopic techniques have the power for submicrometer resolution and thus can detect/quantify the microarchitectural and nanoarchitectural consequences of these genetic/epigenetic changes.
Low-coherence enhanced backscattering spectroscopy
The advent of low-coherence enhanced backscattering spectroscopy (LEBS) represents a major advance for this application by providing quantitative insights into mucosal microarchitecture at length scales of 40 nm or more via a novel optical self-interference phenomenon. The proof-of-concept study for colonic risk stratification analyzed endoscopically normal rectal mucosal biopsy specimens from 273 patients undergoing screening/surveillance colonoscopy. Rectal LEBS was able to discriminate between patients with no neoplasia vs those with advanced adenomas with excellent performance (AUROC, 0.89).21 A recent in vivo fiberoptic LEBS probe study was performed in 574 subjects. Five LEBS readings (each requiring 250 ms) were recorded from the rectal mucosa before colonoscopy. In the blinded validation set, in vivo LEBS was able to discriminate between patients with no neoplasia vs those with advanced adenomas with 87% sensitivity and 78% specificity.22 The test was not confounded by patients’ demographic and risk factors or benign lesions and remained accurate regardless of adenoma location (distal vs proximal lesions).19
Other Techniques
Several other approaches have corroborated the ability to interrogate rectal mucosa to predict colonic neoplasia, such as rectal microvascular analysis with polarization-gated spectroscopy (AUROC, 0.88 for advanced adenomas in a study of 216 patients).23,24 Preliminary reports with ESS also showed strong diagnostics. Finally, nanocytology performed on rectal brushings via partial wave spectroscopic microscopy (sensitive to nanoscale cellular architecture) manifested an AUROC for advanced adenomas of 0.85.25 This prescreen paradigm may enable some proportion of procedures in neoplasia-free patients to be avoided, thereby allowing society to focus the finite endoscopic capacity for therapeutic (potentially cancer-preventing) procedures and include the currently unscreened population. These approaches may be translatable to ulcerative colitis dysplasia as indicated by a report using a related technique (spatial-domain, low-coherence, quantitative-phase microscopy) that yielded a sensitivity of 100% with a specificity of 75%26 in a small cohort (n = 28).
Current Obstacles/Challenges for Implementation
Optical Biopsy
One of the most fundamental issues for optical biopsy is developing the clinical paradigm.27 The “diagnose and leave behind” approach30 may be less attractive for small lesions because polypectomy is relatively safe/effective. Large lesions are more likely to be neoplastic and thus have a higher probability of needing removal (both adenomatous or even potentially serrated lesions), and thus optical biopsy may not change management.28 With the “resect and discard” strategy,30 small lesions are easy to recover (via suction), whereas in larger lesions one would need to be concerned about the presence of high-grade dysplasia, which might necessitate formal pathologic evaluation. On the other hand, there are several scenarios in which this information clearly would be very useful. For instance, in the anticoagulated patients, the presence of multiple pseudopolyps or those undergoing acute gastrointestinal bleed, an optical biopsy potentially would be of considerable value. Thus, a nuanced clinical vision is critical for implementation of these strategies.
Other concerns include the cost-benefit analysis with regard to both equipment (instrumentation/probes) and the associated time to set-up/take readings. Finally, there is the issue of risk management. The recently published American Society of Gastrointestinal Endoscopy taskforce consensus statement mandates that these technologies should have more than 90% performance with regards to both negative predictive value and concurrence with pathology for the strategies.29,30 Whether these guidelines will mitigate endoscopist liability for any future neoplasia in these patients needs to be determined.
Adenoma Detection
Spectroscopic false-positive readings may be time consuming and lead to considerable endoscopist frustration. In addition, with better endoscopes (higher definition), improvement in techniques such as right-sided retroflexion and more scrutiny of endoscopist performance (standardization of withdrawal time and adenoma detection rate), the added value of these techniques are less clear. The improvement in patient outcomes will need to be clarified because lesions detected only with the benefit of adjuvant techniques are probably small and unlikely to be clinically significant.
Flat Dysplasia Detection
Many of the issues center on the near field nature of spectroscopy (evaluating ~1 mm2 spot size). Thus, mapping the mucosa is difficult, although technical advances such as endoscopic polarized scanning spectroscopy appear to make this feasible.14 The cost of instrumentation/probes and extra endoscopic time is a major consideration for application of any of these technologies. The ability to biopsy the precise spot can be an issue, although the solution may be to couple the probe with biopsy forceps (such as with ESS). Finally, if more dysplasia is noted than with standard approaches, this may raise the question of an overdiagnosis bias.
Field Carcinogenesis Detection
The issues revolve around clinical implementation and rubrics. Will this be limited to the endoscopy suite or will it be able to transition to the primary care setting, which has its inherent challenges (disseminating instrumentation and possible need for disinfection facilities)? These studies will need to be performed in unprepared patients and thus it is unclear if the gastroenterologist (especially in the high-risk population) or primary care physician (mainly average risk patients). Although feasibility has been shown in pilot studies, this needs to be validated in larger-scale studies. Another issue that needs to be addressed is the impact of past neoplasia on the potential use for surveillance. From a population perspective, one needs to determine whether to target a high sensitivity and accept a lower specificity (very few false-negative results but an increased number of false-positive results requiring colonoscopy) or vice versa. Finally, although there are other potential applications in the gastrointestinal tract via extended field carcinogenesis (esophageal squamous mucosa → Barrett’s dysplasia or periampullary duodenal mucosa → pancreatic cancer),19 the diagnostic ability of these approaches needs to be determined in large-scale studies.
In conclusion, technological advances have engendered considerable enthusiasm among endoscopists. However, it is critical to focus on the particular clinical issues and ascertain how the technology will impact decision-making paradigms. The crux of the matter is patient outcomes and cost effectiveness. Furthermore, it is paramount to view these technologies in the context of the evolving landscape of clinical endoscopy (need for increased productivity and improved patient outcomes) and the macroeconomic health care environment (cost constraints and so forth). These issues are clearly surmountable and several technologies are undergoing the requisite large-scale validation necessary to bridge the bench-to-bedside chasm. These are exciting times to be an endoscopist at the front lines of this technological revolution in spectroscopy.
Acknowledgments
Funding Supported in part by grants from the National Institutes of Health (R01CA156186, R01CA128641, U01CA111257, R01CA165309, and R42 CA168055).
Abbreviations used in this paper
- AUROC
area under the receiver operating characteristic curve
- CRC
colorectal cancer
- ESS
elastic scattering spectroscopy
- LCI
low-coherence interferometry
- LEBS
low-coherence enhanced backscattering spectroscopy
Footnotes
Conflicts of interest The authors disclose the following: Drs Roy and Backman are co-founders/share holders of American BioOptics, LLC, and Nanocytomics, LLC.
References
- 1.Zauber AG, Winawer SJ, O’Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687–696. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaheen NJ, Sharma P, Overholt BF, et al. Radiofrequency ablation in Barrett’s esophagus with dysplasia. N Engl J Med. 2009;360:2277–2288. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 3.Brenner H, Chang-Claude J, Seiler CM, et al. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154:22–30. doi: 10.7326/0003-4819-154-1-201101040-00004. [DOI] [PubMed] [Google Scholar]
- 4.Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 5.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–1803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 6.Quintero E, Castells A, Bujanda L, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med. 2012;366:697–706. doi: 10.1056/NEJMoa1108895. [DOI] [PubMed] [Google Scholar]
- 7.Bhat S, Coleman HG, Yousef F, et al. Risk of malignant progression in Barrett’s esophagus patients: results from a large population-based study. J Natl Cancer Inst. 2011;103:1049–1057. doi: 10.1093/jnci/djr203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kiesslich R, Goetz M, Hoffman A, et al. New imaging techniques and opportunities in endoscopy. Nat Rev Gastroenterol Hepatol. 2011;8:547–553. doi: 10.1038/nrgastro.2011.152. [DOI] [PubMed] [Google Scholar]
- 9.Murthy S, Goetz M, Hoffman A, et al. Novel colonoscopic imaging. Clin Gastroenterol Hepatol. 2012;10:984–987. doi: 10.1016/j.cgh.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Dhar A, Johnson KS, Novelli MR, et al. Elastic scattering spectroscopy for the diagnosis of colonic lesions: initial results of a novel optical biopsy technique. Gastrointest Endosc. 2006;63:257–261. doi: 10.1016/j.gie.2005.07.026. [DOI] [PubMed] [Google Scholar]
- 11.Roy H, Liu Y, Kim Y, et al. Spectroscopic microvascular assessment from the endoscopically normal mucosa for colon adenoma identification. Gastroenterology. 2006;130:A102–A103. [Google Scholar]
- 12.Wallace MB, Crook JE, Saunders M, et al. Multicenter, randomized, controlled trial of confocal laser endomicroscopy assessment of residual metaplasia after mucosal ablation or resection of GI neoplasia in Barrett’s esophagus. Gastrointest Endosc. 2012;76:539–547.e1. doi: 10.1016/j.gie.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 13.Wallace MB, Perelman LT, Backman V, et al. Endoscopic detection of dysplasia in patients with Barrett’s esophagus using light-scattering spectroscopy. Gastroenterology. 2000;119:677–682. doi: 10.1053/gast.2000.16511. [DOI] [PubMed] [Google Scholar]
- 14.Qiu L, Pleskow DK, Chuttani R, et al. Multispectral scanning during endoscopy guides biopsy of dysplasia in Barrett’s esophagus. Nat Med. 2010;16:603–606. doi: 10.1038/nm.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wax A, Terry NG, Dellon ES, et al. Angle-resolved low coherence interferometry for detection of dysplasia in Barrett’s esophagus. Gastroenterology. 2011;141:443–447. doi: 10.1053/j.gastro.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lovat LB, Johnson K, Mackenzie GD, et al. Elastic scattering spectroscopy accurately detects high grade dysplasia and cancer in Barrett’s oesophagus. Gut. 2006;55:1078–1083. doi: 10.1136/gut.2005.081497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freedman AN, Slattery ML, Ballard-Barbash R, et al. Colorectal cancer risk prediction tool for white men and women without known susceptibility. J Clin Oncol. 2009;27:686–693. doi: 10.1200/JCO.2008.17.4797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giovannucci E, Ogino S. DNA methylation, field effects, and colorectal cancer. J Natl Cancer Inst. 2005;97:1317–1319. doi: 10.1093/jnci/dji305. [DOI] [PubMed] [Google Scholar]
- 19.Backman V, Roy HK. Light-scattering technologies for field carcinogenesis detection: a modality for endoscopic prescreening. Gastroenterology. 2011;140:35–41. doi: 10.1053/j.gastro.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chai H, Brown RE. Field effect in cancer-an update. Ann Clin Lab Sci. 2009;39:331–337. [PubMed] [Google Scholar]
- 21.Roy HK, Turzhitsky V, Kim Y, et al. Association between rectal optical signatures and colonic neoplasia: potential applications for screening. Cancer Res. 2009;69:4476–4483. doi: 10.1158/0008-5472.CAN-08-4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy HK, Mutyal NN, Du H, et al. In situ rectal spectral markers for colonic neoplasia risk stratification–a blinded validation study. Gastroenterology. 2012;142:S109. [Google Scholar]
- 23.Roy HK, Gomes A, Turzhitsky V, et al. Spectroscopic microvascular blood detection from the endoscopically normal colonic mucosa: biomarker for neoplasia risk. Gastroenterology. 2008;135:1069–1078. doi: 10.1053/j.gastro.2008.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomes AJ, Roy HK, Turzhitsky V, et al. Rectal mucosal microvascular blood supply increase is associated with colonic neoplasia. Clin Cancer Res. 2009;15:3110–3117. doi: 10.1158/1078-0432.CCR-08-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Damania D, Roy HK, Subramanian H, et al. Nanocytology of rectal colonocytes to assess risk of colon cancer based on field cancerization. Cancer Res. 2012;72:2720–2727. doi: 10.1158/0008-5472.CAN-11-3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bista RK, Brentnall TA, Bronner MP, et al. Using optical markers of nondysplastic rectal epithelial cells to identify patients with ulcerative colitis-associated neoplasia. Inflamm Bowel Dis. 2011;17:2427–2435. doi: 10.1002/ibd.21639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy HK, Goldberg MJ, Bajaj S, et al. Colonoscopy and optical biopsy: bridging technological advances to clinical practice. Gastroenterology. 2011;140:1863–1867. doi: 10.1053/j.gastro.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Rex DK, Kahi C, O’Brien M, et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc. 2011;73:419–422. doi: 10.1016/j.gie.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Coe SG, Wallace MB. Colonoscopy: new approaches to better outcomes. Curr Opin Gastroenterol. 2012;28:70–75. doi: 10.1097/MOG.0b013e32834ddab9. [DOI] [PubMed] [Google Scholar]