Abstract
Objectives
The goal of this study was to examine the prospective association between oxidation-specific biomarkers, primarily oxidized phospholipids (OxPL) on apolipoprotein B-100-containing lipoproteins (OxPL/apoB) and lipoprotein (a) [Lp(a)], and risk of peripheral artery disease (PAD). As secondary analyses, we examined indirect measures of oxidized lipoproteins, including autoantibodies to malondialdehyde-modified low density lipoprotein (MDA-LDL) and apolipoprotein B-100 immune complexes (ApoB-IC).
Background
Biomarkers to predict the development of PAD are lacking. OxPL circulate in plasma, are transported by Lp(a), and deposit in the vascular wall and induce local inflammation.
Methods
The study population included two parallel nested case-control studies of 143 men within the Health Professionals Follow-up Study (1994-2008) and 144 women within the Nurses’ Health Study (1990-2010) with incident confirmed cases of clinically significant PAD, matched 1:3 to controls.
Results
Levels of OxPL/apoB were positively associated with risk of PAD in men and women: pooled relative risk (RR) 1.37, 95% CI, 1.19-1.58 for each 1-standard deviation increase after adjusting age, smoking, fasting status, month of blood draw, lipids, body mass index, and other cardiovascular disease risk factors. Lp(a) was similarly associated with risk of PAD (pooled adjusted RR, 1.36; 95% CI, 1.18-1.57 for each 1-standard deviation increase). Autoantibodies to MDA-LDL and ApoB-IC were not consistently associated with risk of PAD.
Conclusions
OxPL/apoB were positively associated with risk of PAD in men and women. The major lipoprotein carrier of OxPL, Lp(a), was also associated with risk of PAD, reinforcing the key role of OxPL in the pathophysiology of atherosclerosis mediated by Lp(a).
Keywords: peripheral artery disease, oxidized phospholipids, lipoprotein (a), biomarker, autoantibodies, immune complex
INTRODUCTION
Approximately 10 million U.S. adults have peripheral artery disease (PAD), including 23% of those age 70 years or older (1,2). PAD is associated with substantial morbidity, cost(3), functional decline (4) and may require limb amputation in extreme cases. Despite its high prevalence and associated morbidity, risk factors for PAD are less well studied than those for coronary and cerebrovascular disease.
Evidence from cellular and animal experiments suggests that oxidative stress plays a key, modifiable role in the etiology of atherosclerosis (5,6). However, there is a lack of appropriate epidemiological markers to measure oxidation: many biomarkers lack the necessary combination of reliability, accuracy, cost-effectiveness, and ease of measurement and few have been examined specifically with respect to PAD. Oxidized phospholipids (OxPL), a marker of lipid oxidation transported by lipoprotein (a) [Lp(a)] in plasma (7,8), may provide insight about the role of oxidative stress in atherosclerosis. Pro-inflammatory OxPL on Lp(a) and other apoB-100-containing lipoproteins upregulate pro-inflammatory genes and pro-inflammatory responses of several arterial wall cells, and initiate a localized inflammatory cascade (9,10).
These oxidation-specific epitopes, such as OxPL and malondialdehyde (MDA) epitopes, represent danger-associated molecular patterns (DAMPs) that are detrimental to the host, are present on apoptotic cells, oxidized LDL and lipids, and often share molecular identity/mimicry with epitopes on pathogens (11). In response to such DAMPs, macrophage scavenger receptors, immune effector proteins such as autoantibodies to malondialdehyde-modified low density lipoprotein (MDA-LDL), complement factor H (12) and C-reactive protein have been selected and expanded to bind and neutralize their pro-inflammatory effects.
OxPL on apolipoprotein B-100-containing lipoproteins (OxPL/apoB) have been associated with carotid and femoral atherosclerosis measured by ultrasound (13), myocardial infarction (MI), stroke, revascularization, and total mortality in selected, largely clinical populations (7). IgM autoantibodies to MDA-LDL have also been inversely associated with ultrasound-detected carotid and femoral atherosclerosis (14,15) and in another small cross-sectional study, autoantibody titers against oxidized LDL were increased in patients with early onset PAD (16). However, no adequately powered prospective cohort studies have examined the association between oxidation-specific biomarkers and risk of incident clinically manifested PAD in healthy populations. The goal of this study was to examine the prospective association between oxidation-specific biomarkers, primarily oxidized phospholipids (OxPL) on apolipoprotein B-100-containing lipoproteins (OxPL/apoB) and lipoprotein (a) [Lp(a)], and risk of peripheral artery disease (PAD). As secondary analyses, we examined indirect measures of oxidized lipoproteins, including autoantibodies to malondialdehyde-modified low density lipoprotein(MDA-LDL) and apolipoprotein B-100 immune complexes (ApoB-IC).
METHODS
Study Population
The Health Professionals Follow-up Study (HPFS) is a prospective cohort study of 51,529 male dentists, optometrists, pharmacists, podiatrists, osteopathic physicians, and veterinarians aged 40 to 75 years that began in 1986. The Nurses’ Health Study (NHS) is a prospective cohort study of 121,700 female nurses aged 30 to 55 years that began in 1976. From individuals in these two studies, 18,224 men provided blood specimens in 1994 and 32,826 women provided a blood sample in 1989. We excluded individuals who had a history of cardiovascular disease (CVD), including MI, surgical/percutaneous revascularization of the coronary, carotid, or peripheral beds, confirmed PAD, stroke, and transient ischemic attack.
The case-control analytic datasets include 143 incident cases and 429 controls in the HPFS and 144 incident cases and 432 controls in the NHS. Cases were matched 1:3 to controls on age, race (NHS only) month of blood draw (within 3 months), fasting status, and smoking history (never/former/current). We selected controls at random, conditional on the matching factors, from participants free of PAD at the time the case occurred (risk set sampling). Since older age categories had fewer participants, we relaxed the age match range year-by-year if necessary to a maximum of within 3 years. The Harvard School of Public Health Human Subjects Committee approved both studies.
Assessment of OxPL, Lp(a), autoantibodies and immune complexes
Blood samples were shipped overnight with a cold pack to the central laboratory, centrifuged on arrival, aliquotted, and stored in liquid nitrogen at −130°C to −196°C. HPFS specimens were anticoagulated with EDTA and NHS with heparin, and 95% of HPFS bloods and 97% of NHS bloods were received within 24 hours. A validated plasma assay was used to measure OxPL/apoB, using the murine monoclonal antibody E06 that recognizes the phosphocholine (PC) group on oxidized but not on native phospholipids (reviewed in detail in Taleb et al (7) and references therein). E06 similarly recognizes the PC covalently bound to BSA, as in PC-BSA. A 1:50 dilution of plasma in PBS is added to microtiter wells coated with monoclonal antibody MB47, which binds a saturating amount of apoB-100 to each well. Finally, biotinylated E06 is used to determine OxPL/apoB in relative light units (RLU). Within-person 5-year reproducibility of frozen samples is high (r=0.78) (13) and pilot-tests showed that OxPL/apoB levels are stable over 24 hours on ice (ICC=0.96).
To facilitate comparison of absolute OxPL/apoB levels across studies, we also describe the reporting of OxPL/apoB levels as nanomolar (nM) OxPL rather than RLU OxPL using a novel standard curve of phosphocholine (PC) equivalents. The standard curve is generated by plating known concentrations of PC-modified bovine serum albumin (PC-BSA), which has approximately 16 moles of PC per mole of BSA (Biotech technologies, Novato, CA), and is recognized by E06. Biotin-E06 is then added to detect the number of moles of PC in the linear range on the plate and is measured in RLU. This standard curve is then used to convert the RLU derived from wells containing individual human samples to OxPL equivalents. This new method is reported as nanomoles of PC found on OxPL per liter plasma (i.e nanoMolar or nM) for each sample. Since each mole of OxPL has one PC headgroup recognized by E06, this can be reported as nM OxPL.
Lp(a) levels were determined using a chemiluminescent ELISA with MB47-coated wells, a 1:400 plasma dilution, and biotinylated monoclonal antibody LPA4, as described previously (17). Chemiluminescent ELISA was used to measure IgG and IgM autoantibodies to MDA-LDL and apoB-immune complexes (ApoB-IC), as previously described (18). Measured CVs in duplicate samples were 8% for OxPL/apoB, 13% for Lp(a), 11% for IgG AA, 9% for IgM AA, 10% for IgG ApoB-IC, and 10% for IgM ApoB-IC in the HPFS. CVs were 21% for OxPL/apoB, 13% for Lp(a), 9% for IgG AA, 7% for IgM AA, 16% for IgG ApoB-IC, and 25% for IgM ApoB-IC in the NHS.
Assessment of PAD
Participants reported the occurrence of professionally diagnosed medical conditions, including claudication and revascularization for arterial disease of the leg, during the previous 2 years on biennial mailed questionnaires. We collected medical records from treating physicians and hospitals for those who reported either condition. Professionals blinded to biomarker status reviewed and confirmed PAD diagnoses using medical records.
We defined PAD as arterial disease below the aortic bifurcation (i.e., excluding abdominal aortic aneurysm and renal artery stenosis) as described previously (19). Confirmed PAD required at least one of the following (in order of severity/certainty): (1) report of amputation, bypass, or other revascularization procedure (ex: angioplasty) for occlusive artery disease, (2) angiogram showing at least 50% stenosis of at least one artery with congruent symptoms in the ipsilateral limb, or (3) ankle-brachial index < 0.9, or (4) physician’s diagnosis confirmed by medical record review.
Assessment of Covariates
Questionnaires provided information about medical history and lifestyle habits, including detailed information about smoking, medication use, weight, parental history of MI, alcohol, physical activity, diet, and postmenopausal hormone use. Participants reported the average amount of time they spent per week on various activities like walking, jogging, running, bicycling, and tennis. This information was used to calculate weekly energy expenditure in metabolic equivalent task-hours. We calculated body mass index (BMI) by dividing weight (kg) by squared height (m2). Both physical activity and BMI measures are highly valid (20-22).
A laboratory certified by the National Heart, Lung and Blood Institute/Centers for Disease Control and Prevention Lipid standardization Program analyzed all other biochemical markers by means of commercially available analytic systems. The lab measured high-density lipoprotein cholesterol (HDL-C) and triglycerides enzymatically and low-density lipoprotein cholesterol (LDL-C) by a homogenous direct method from Roche Diagnostics (Indianapolis, IN). An immunoturbidimetric assay on the Roche P Modular system from Roche Diagnostics (Indianapolis, IN) quantified the concentration of high-sensitivity C-reactive protein (hsCRP), using reagents and calibrators from DiaSorin (Stillwater, MN). The Roche P Modular system uses turbidimetric immunoinhibition and a hemolyzed whole blood or packed red cells to determine hemoglobin A1c (HbA1c) (Roche Diagnostics, Indianapolis, IN).
Statistical Analysis
We used generalized linear mixed models and Cochran-Mantel-Haenszel tests to compare continuous variables and categorical variables by case/control status, respectively, accounting for clustering by matching status. We examined the shape of the associations of OxPL/apoB, Lp(a), autoantibodies and ApoB-IC with PAD risk using cubic splines, adjusting for all covariates. We used conditional logistic regression, conditioning on matching factors, to estimate odds ratios for PAD according to level of OxPL/apoB and present estimates adjusted for matching factors and additionally for other PAD risk factors. Because we employed risk set sampling, we report these odds ratios as unbiased estimates of the incidence rate ratio (RR).
We included covariates in our models as linear variables if appropriate or as categorical variables if discrete or their association with PAD was non-linear. We checked for multicollinearity among covariates using variance inflation factors. Because the test for heterogeneity was not statistically significant, we used meta-analysis with fixed effects to pool our RR estimates for OxPL/apoB and Lp(a). We tested effect modification by including interaction terms between OxPL/apoB and the potential effect modifier (continuous variable) in our models and pooled the interaction terms for men and women using a meta-analysis with fixed effects. We analyzed levels of autoantibodies and ApoB-IC in similar fashion. All analyses used SAS statistical software version 9.2 (Cary, North Carolina).
RESULTS
Among men, medical reports of amputation, bypass, or other revascularization procedure confirmed 87 PAD cases (61%), angiogram or Doppler ultrasound 15 cases (10%), ABI <0.9 23 cases (16%), and physician diagnosis 18 cases (13%). Among women, surgery or procedure confirmed 74 cases (51%), angiogram confirmed 24 cases (17%), ABI confirmed 39 cases (27%), and physician diagnosis 7 cases (5%). Compared to controls, PAD cases had higher levels of traditional CVD risk factors (Table 1). Although cases and controls were matched on current smoking status, cases still had a significantly higher average number of pack-years compared to controls.
Table 1.
Baseline characteristics of cases and matched controls.
| WOMEN | MEN | |||||
|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |||
| n = 144 | n = 432 | p-value* | n = 143 | n = 429 | p-value* | |
| Age (y) | 59.9 (5.2) | 60.0 (5.2) | Matched | 65.4 (8.1) | 65.3 (8.1) | Matched |
| Oxidation-related factors | ||||||
| OxPL/apoB (nM) | 8.46 (3.9,19.3) | 5.00 (2.9,10.6) | <0.001 | 15.24 (12.4,23.6) | 14.15 (12.3,19.4) | 0.02 |
| OxPL/apoB (RLU) | 4882 (2237,11135) | 2887 (1649,6132) | <0.001 | 8800 (7162,13601) | 8173 (7126,11184) | 0.02 |
| Lipoprotein (a) (mg/dL) | 35.1 (8.7,72.3) | 13.1 (4.9,43.1) | <0.001 | 7.6 (2.8,34.3) | 4.5 (1.6,17.7) | 0.007 |
| IgG AA to MDA-LDL (RLU) | 8776 (6394,12669) | 8541 (5572,12758) | 0.34 | 2313 (1411,4949) | 2733 (1741,4350) | 0.36 |
| IgM AA to MDA-LDL (RLU) | 20457 (14001,26529) | 18866 (13903,25145) | 0.22 | 9684 (5836,14986) | 9213 (6363,13823) | 0.16 |
| IgG ApoB-IC (RLU) | 1106 (862,1439) | 1076 (791,1494) | 0.94 | 1620 (1265,2243) | 1748 (1376,2374) | 0.22 |
| IgM ApoB-IC (RLU) | 1490 (897,2574) | 1478 (960,2393) | 0.17 | 885 (562,1600) | 921 (601,1425) | 0.29 |
| Lipids | ||||||
| Triglycerides (mg/dL) | 110 (85,161) | 106 (73,145) | 0.34 | 143 (105,195) | 115 (80,165) | 0.001 |
| HDL-C (mg/dL) | 60.5 (19.9) | 62.1 (17.1) | 0.42 | 41.7 (11) | 48.5 (14) | <0.001 |
| LDL-C (mg/dL) | 148 (44) | 142 (38) | 0.16 | 139 (35) | 131 (33) | 0.02 |
| High-sensitivity CRP (mg/L) | 2.56 (1.25,4.70) | 1.62 (0.73,3.33) | 0.07 | 2.24 (1.2,3.5) | 1.18 (0.5,2.3) | 0.005 |
| HbA1c (%) | 5.46 (5.28,5.72) | 5.33 (5.16,5.55) | <0.001 | 5.56 (5.3,6.0) | 5.41 (5.2,5.6) | <0.001 |
| Smoking status | ||||||
| Never | 30 (21%) | 90 (21%) | Matched | 23 (18%) | 82 (20%) | Matched |
| Past | 56 (39%) | 170 (39%) | Matched | 78 (60%) | 242 (59%) | Matched |
| Current | 58 (40%) | 172 (40%) | Matched | 32 (22%) | 90 (21%) | Matched |
| 1-14 cigarettes/d | 18 (13%) | 86 (20%) | 15 (11%) | 44 (11%) | ||
| 15-34 cigarettes/d | 31 (22%) | 80 (19%) | 11 (8%) | 33 (8%) | ||
| 35+ cigarettes/d | 9 (6%) | 5 (1%) | 4 (3%) | 9 (2%) | ||
| Pack-years (y) | 32.3 (25.6) | 22.0 (21.3) | <0.001 | 28.7 (24) | 22.5 (22) | <0.001 |
| Physical activity (MET hr/wk) | 12.8 (4.7,24.9) | 13.6 (5.0,28.1) | 0.69 | 22.7 (8.0,43.8) | 27.4 (10.3,52.8) | 0.003 |
| History of hypertension | 68 (47%) | 137 (32%) | <0.001 | 70 (49%) | 130 (30%) | <0.001 |
| History of diabetes | 19 (13%) | 12 (3%) | <0.001 | 28 (20%) | 16 (4%) | <0.001 |
| History of hypercholesterolemia | 84 (58%) | 200 (46%) | 0.01 | 82 (57%) | 187 (44%) | 0.005 |
| Alcohol | ||||||
| Never drinker | 49 (34%) | 130 (30%) | 35 (24%) | 84 (20%) | ||
| Former drinker | 44 (31%) | 137 (32%) | 24 (17%) | 80 (19%) | ||
| < 1 drink/day | 29 (20%) | 109 (25%) | 0.43 | 39 (27%) | 123 (29%) | 0.81 |
| 1-1.9 drinks/day | 12 (8%) | 20 (5%) | 23 (16%) | 65 (15%) | ||
| 2+ drinks/day | 9 (6%) | 31 (7%) | 22 (15%) | 76 (18%) | ||
| Parental history of MI < age 60 y | 31 (22%) | 61 (14%) | 0.03 | 22 (15%) | 44 (10%) | 0.09 |
| BMI category | ||||||
| < 25 | 84 (58%) | 259 (60%) | 62 (43%) | 204 (48%) | ||
| 25-29.9 | 38 (26%) | 132 (31%) | 0.13 | 67 (47%) | 196 (46%) | 0.42 |
| 30+ | 22 (15%) | 41 (9%) | 14 (10%) | 29 (7%) | ||
| Aspirin use | 29 (20%) | 89 (21%) | 0.91 | 80 (56%) | 184 (43%) | 0.01 |
| Postmenopausal | 131 (95%) | 390 (95%) | 0.72 | |||
| Ever used postmenopausal hormones† | 96 (72%) | 257 (63%) | 0.04 | |||
| Currently using postmenopausal hormones** |
57 (43%) | 183 (45%) | 0.84 | |||
Generalized linear mixed models for continuous variables and Cochran-Mantel-Haenszel test for categorical variables (to account for matching/correlation between controls), matching criteria were age, month of blood draw, fasting status, and smoking status.
Abbreviations: OxPL/apoB, oxidized phospholipids on apolipoprotein B-100-containing lipoproteins; AA to MDA-LDL, autoantibodies to malondialdehydemodified LDL-C; ApoB-IC, apolipoprotein B-100 immune complexes; RLU, relative light units; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; CRP, C-reactive protein; HbA1c, hemoglobin A1c; MET-hr, metabolic equivalent task-hours; MI, myocardial infarction.
Note: data are expressed as mean (SD), median (interquartile range), or n (%).
Among postmenopausal women
OxPL/apoB and Lp(a)
Women had considerably lower levels of OxPL/apoB compared to men, while the distribution of Lp(a) overlapped substantially across sexes with somewhat greater levels among women. OxPL/apoB was not strongly correlated with standard CVD risk factors (Supplemental Table).
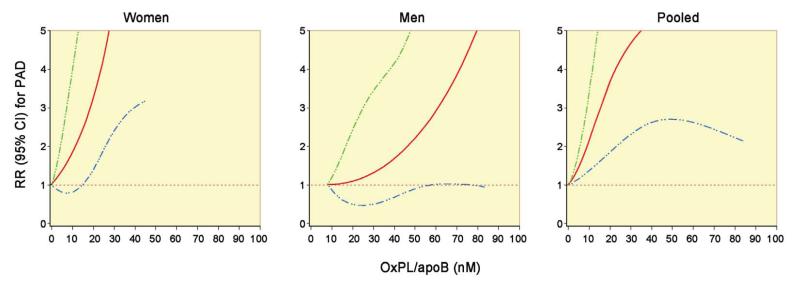
The association between OxPL/apoB and Lp(a) and risk of PAD appeared linear (Figures 1-3), and likelihood ratio tests for non-linearity were not statistically significant (p > 0.05) comparing a model with the linear term to a model with the linear and cubic spline terms. Therefore we discuss our results using units of one standard deviation (SD) of nM OxPL/apoB. Since all previous work used OxPL/apoB measured in RLU, we have additionally presented our results using RLU. There was a 51% (95% CI, 24-85%) increase in risk of PAD for each 1-SD increase in OxPL/apoB in women and a 23% (95% CI, 0-52%) increase in men in full multivariable models (Table 2). These estimates were essentially the same for matching-adjusted models (53% in women and 24% in men). Similarly, there was a 52% (95% CI, 24-87%) increase in risk of PAD for each 1-SD increase in Lp(a) in women and a 24% (95% CI, 0-53%) increase in men in full multivariable models (Table 3).
Figure 1. Adjusted relationship between plasma levels of OxPL/apoB (nM) and relative risk of PAD.
Data derive from a cubic spline conditional logistic regression model with age, race (women only), smoking status, fasting status and date of blood sampling as matching variables. The model is adjusted for triglycerides, HDL-C, LDL-C, hsCRP, HbA1c, history of diabetes, history of hypertension, pack-years of smoking, parental history of MI before age 60, aspirin use, BMI, physical activity, postmenopausal hormone use (women only), and gender (pooled only). The 95% CI is indicated by the dashed lines.
Figure 3. Adjusted relationship between plasma levels of OxPL/apoB (RLU) and relative risk of PAD.
Legend as per Figure 1.
Table 2.
Relative risks and 95% confidence intervals for peripheral artery disease according to level of OxPL/apoB.
|
WOMEN
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Per 1 SD increase (7.17) |
Tertile | Per 1 SD increase (4142) |
Tertile | ||||||
| 1 | 2 | 3 | 1 | 2 | 3 | ||||
| Range (nM) | 0.00-3.72 | 3.73-9.55 | 9.56-46.09 | Range (RLU) | 0-2150 | 2151-5517 | 5518-26622 | ||
| No. Cases | 144 | 33 | 43 | 68 | No. Cases | 144 | 33 | 43 | 68 |
| Model 1 | 1.53 (1.30-1.79) | 1.0 (ref) | 1.44 (0.86-2.41) | 2.73 (1.67-4.48) | Model 1 | 1.53 (1.30-1.79) | 1.0 (ref) | 1.44 (0.86-2.41) | 2.73 (1.67-4.48) |
| Model 2 | 1.50 (1.26-1.79) | 1.0 (ref) | 1.58 (0.91-2.75) | 2.92 (1.71-5.00) | Model 2 | 1.50 (1.26-1.79) | 1.0 (ref) | 1.58 (0.91-2.75) | 2.92 (1.71-5.00) |
| Model 3 | 1.51 (1.24-1.85) | 1.0 (ref) | 1.38 (0.75-2.54) | 2.55 (1.41-4.64) | Model 3 | 1.51 (1.24-1.85) | 1.0 (ref) | 1.38 (0.75-2.54) | 2.55 (1.41-4.64) |
|
| |||||||||
| MEN | |||||||||
|
| |||||||||
| Per 1 SD increase (10.38) |
Tertile | Per 1 SD increase (5999) |
Tertile | ||||||
| 1 | 2 | 3 | 1 | 2 | 3 | ||||
| Range (nM) | 7.85-12.93 | 12.94-16.85 | 16.86-82.75 | Range (RLU) | 4535-7518 | 7519-9730 | 9731-47792 | ||
| No. Cases | 143 | 41 | 47 | 55 | No. Cases | 143 | 42 | 46 | 55 |
| Model 1 | 1.24 (1.05-1.46) | 1.0 (ref) | 1.15 (0.70-1.87) | 1.50 (0.92-2.46) | Model 1 | 1.24 (1.05-1.46) | 1.0 (ref) | 1.09 (0.67-1.78) | 1.46 (0.90-2.38) |
| Model 2 | 1.31 (1.09-1.58) | 1.0 (ref) | 1.23 (0.72-2.08) | 1.78 (1.03-3.09) | Model 2 | 1.31 (1.09-1.58) | 1.0 (ref) | 1.15 (0.68-1.96) | 1.72 (0.99-2.97) |
| Model 3 | 1.23 (1.00-1.52) | 1.0 (ref) | 1.03 (0.57-1.86) | 1.50 (0.81-2.78) | Model 3 | 1.23 (1.00-1.52) | 1.0 (ref) | 0.99 (0.55-1.79) | 1.46 (0.79-2.70) |
|
| |||||||||
| POOLED | |||||||||
|
| |||||||||
| Model 3 | 1.37 (1.19-1.58) | 1.0 (ref) | 1.18 (0.77-1.82) | 1.97 (1.28-3.03) | Model 3 | 1.37 (1.18-1.58) | 1.0 (ref) | 1.17 (0.76-1.80) | 1.95 (1.27-3.00) |
Model 1: adjusted for matching factors (age, race (women only), month of blood draw, fasting status, and smoking).
Model 2: adjusted for matching factors, triglycerides, HDL-C, LDL-C, hsCRP, and HbA1c.
Model 3: adjusted for matching factors, triglycerides, HDL-C, LDL-C, hsCRP, HbA1c, parental history of MI before age 60, pack-years of smoking, physical activity, hypertension, diabetes, hypercholesterolemia, BMI, aspirin use and postmenopausal hormone use (women only).
Table 3.
Relative risks and 95% confidence intervals for peripheral artery disease according to level of Lp(a).
|
WOMEN
| ||||
|---|---|---|---|---|
| Per 1 SD increase (28.8) |
Tertile | |||
| 1 | 2 | 3 | ||
| Range (mg/dL) | 0.1-8.4 | 8.5-35.0 | 35.1-144.6 | |
| No. Cases | 144 | 35 | 37 | 72 |
| Model 1 | 1.57 (1.33-1.86) | 1.0 (ref) | 1.04 (0.62-1.75) | 2.64 (1.64-4.26) |
| Model 2 | 1.54 (1.28-1.85) | 1.0 (ref) | 1.11 (0.64-1.94) | 2.76 (1.64-4.67) |
| Model 3 | 1.52 (1.24-1.87) | 1.0 (ref) | 1.37 (0.72-2.59) | 2.91 (1.58-5.36) |
|
| ||||
| MEN | ||||
|
| ||||
| Per 1 SD increase (22.8) |
Tertile | |||
| 1 | 2 | 3 | ||
| Range (mg/dL) | 0.1-2.4 | 2.5-12.0 | 12.1-129.1 | |
| No. Cases | 143 | 31 | 52 | 60 |
| Model 1 | 1.29 (1.09-1.52) | 1.0 (ref) | 1.88 (1.13-3.13) | 2.51 (1.49-4.21) |
| Model 2 | 1.27 (1.05-1.53) | 1.0 (ref) | 1.27 (0.73-2.22) | 1.91 (1.08-3.39) |
| Model 3 | 1.24 (1.00-1.53) | 1.0 (ref) | 1.20 (0.64-2.25) | 1.59 (0.84-3.00) |
|
| ||||
| POOLED | ||||
|
| ||||
| Model 3 | 1.36 (1.18-1.57) | 1.0 (ref) | 1.28 (0.82-2.00) | 2.18 (1.40-3.39) |
Model 1: adjusted for matching factors (age, race (women only), month of blood draw, fasting status, and smoking).
Model 2: adjusted for matching factors, triglycerides, HDL-C, LDL-C, hsCRP, and HbA1c.
Model 3: adjusted for matching factors, triglycerides, HDL-C, LDL-C, hsCRP, HbA1c, parental history of MI before age 60, pack-years of smoking, physical activity, hypertension, diabetes, hypercholesterolemia, BMI, aspirin use and postmenopausal hormone use (women only).
Because we did not observe statistically significant heterogeneity between NHS and HPFS, we pooled our results for OxPL/apoB and Lp(a) in women and men (Tables 2 and 3). In pooled analyses, a 1-SD increase in OxPL/apoB was associated with a 37% (95% CI, 19-58%) increased risk of PAD, adjusting for all covariates. A 1-SD increase in Lp(a) was associated with almost the same magnitude of increased risk of PAD. Using tertiles to categorize OxPL/apoB and Lp(a), men and women in the highest compared to lowest tertile of both biomarkers had approximately double the risk of PAD. In additional analyses, we found no interactions of OxPL/apoB with age, years of follow-up, LDL-C, autoantibodies to MDA-LDL, or ApoB-IC.
IgG and IgM Autoantibodies and Immune Complexes
We did not find consistent associations of autoantibodies to MDA-LDL with risk of PAD. The relative risks fluctuated across tertiles and the confidence intervals were consistent with a broad range of risk estimates (Table 4), although it was of interest that there was a significant inverse relationship of IgG to MDA-LDL in men but not women. IgG and IgM ApoB-IC were not statistically significantly associated with risk of PAD in men or in women, either as continuous variables or in tertiles (Table 5).
Table 4.
Relative risks and 95% confidence intervals for peripheral artery disease according to level of AA to MDA-LDL.
| IgG AA | IgM AA | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| WOMEN | |||||||||
|
| |||||||||
| Per 1 SD increase |
Tertile | Per 1 SD increase |
Tertile | ||||||
| log(IgG AA) | 1 | 2 | 3 | 1 | 2 | 3 | |||
| Range (RLU) | 1,465-6,580 | 6,581-11,017 | 11,018-57,009 | Range (RLU) | 2,013-15,741 | 15,742-22,494 | 22,495-77,968 | ||
| No. Cases | 144 | 40 | 56 | 48 | No. Cases | 144 | 50 | 34 | 60 |
| Model 1 | 1.16 (0.96-1.40) | 1.0 (ref) | 1.53 (0.95-2.45) | 1.26 (0.78-2.02) | Model 1 | 1.13 (0.95-1.36) | 1.0 (ref) | 0.59 (0.35-0.98) | 1.34 (0.84-2.13) |
| Model 2 | 1.16 (0.95-1.42) | 1.0 (ref) | 1.48 (0.89-2.46) | 1.32 (0.80-2.17) | Model 2 | 1.10 (0.91-1.34) | 1.0 (ref) | 0.57 (0.34-0.97) | 1.32 (0.81-2.17) |
| Model 3 | 1.10 (0.88-1.37) | 1.0 (ref) | 1.42 (0.79-2.53) | 1.13 (0.65-1.96) | Model 3 | 1.00 (0.80-1.26) | 1.0 (ref) | 0.47 (0.26-0.85) | 1.05 (0.60-1.85) |
|
| |||||||||
| MEN | |||||||||
|
| |||||||||
| Per 1 SD increase |
Tertile | Per 1 SD increase |
Tertile | ||||||
| log(IgG AA) | 1 | 2 | 3 | 1 | 2 | 3 | |||
| Range (RLU) | 485-1,940 | 1,941-3,690 | 3,691-64,956 | Range (RLU) | 732-7,203 | 7,204-11,833 | 11,834-94,623 | ||
| No. Cases | 143 | 57 | 40 | 46 | No. Cases | 143 | 45 | 44 | 54 |
| Model 1 | 0.94 (0.79-1.12) | 1.0 (ref) | 0.57 (0.35-0.93) | 0.73 (0.46-1.15) | Model 1 | 1.13 (0.96-1.34) | 1.0 (ref) | 0.91 (0.56-1.49) | 1.25 (0.80-1.97) |
| Model 2 | 0.87 (0.71-1.06) | 1.0 (ref) | 0.52 (0.30-0.89) | 0.54 (0.32-0.92) | Model 2 | 1.18 (0.97-1.43) | 1.0 (ref) | 0.83 (0.48-1.43) | 1.17 (0.70-1.96) |
| Model 3 | 0.84 (0.67-1.06) | 1.0 (ref) | 0.58 (0.31-1.08) | 0.52 (0.29-0.95) | Model 3 | 1.13 (0.93-1.39) | 1.0 (ref) | 1.02 (0.55-1.87) | 1.26 (0.71-2.23) |
Model 1: adjusted for matching factors (age, race (women only), month of blood draw, fasting status, and smoking status).
Model 2: adjusted for matching factors, triglycerides, HDL-C, LDL-C, hsCRP, and HbA1c.
Model 3: adjusted for matching factors, triglycerides, HDL-C, LDL-C, hsCRP, HbA1c, parental history of MI before age 60, pack-years of smoking, physical activity, hypertension, diabetes, hypercholesterolemia, BMI, aspirin use and postmenopausal hormone use (women only).
Table 5.
Relative risks and 95% confidence intervals for peripheral artery disease according to level of immune complexes (IC).
| IgG ApoB-IC | IgM ApoB-IC | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
WOMEN
| |||||||||
| Per 1 SD increase |
Tertile | Per 1 SD increase |
Tertile | ||||||
| 1 | 2 | 3 | 1 | 2 | 3 | ||||
| Range (RLU) |
272-906 | 907-1,328 | 1,329-12,372 | Range (RLU) |
23-1,142 | 1,143-2,050 | 2,051-21,500 | ||
| No. Cases | 144 | 41 | 58 | 45 | No. Cases | 144 | 49 | 52 | 53 |
| Model 1 | 1.00 (0.82-1.21) | 1.0 (ref) | 1.53 (0.96-2.44) | 1.15 (0.71-1.88) | Model 1 | 1.13 (0.95-1.33) | 1.0 (ref) | 0.78 (0.48-1.26) | 1.13 (0.71-1.80) |
| Model 2 | 0.97 (0.78-1.20) | 1.0 (ref) | 1.55 (0.94-2.55) | 1.08 (0.64-1.83) | Model 2 | 1.15 (0.96-1.38) | 1.0 (ref) | 0.82 (0.49-1.38) | 1.21 (0.74-1.98) |
| Model 3 | 0.96 (0.73-1.24) | 1.0 (ref) | 1.67 (0.96-2.93) | 0.94 (0.51-1.74) | Model 3 | 1.14 (0.92-1.41) | 1.0 (ref) | 0.78 (0.45-1.37) | 1.08 (0.61-1.91) |
|
| |||||||||
|
MEN
| |||||||||
| Per 1 SD increase |
Tertile | Per 1 SD increase |
Tertile | ||||||
| 1 | 2 | 3 | 1 | 2 | 3 | ||||
| Range (RLU) |
459-1,460 | 1,461-2,061 | 2,062-28,725 | Range (RLU) |
96-685 | 686-1,235 | 1,236-59,411 | ||
| No. Cases | 143 | 54 | 43 | 46 | No. Cases | 143 | 49 | 47 | 47 |
| Model 1 | 0.88 (0.68-1.13) | 1.0 (ref) | 0.68 (0.43-1.08) | 0.77 (0.48-1.24) | Model 1 | 1.08 (0.97-1.21) | 1.0 (ref) | 0.90 (0.57-1.43) | 0.94 (0.60-1.48) |
| Model 2 | 0.89 (0.68-1.15) | 1.0 (ref) | 0.65 (0.38-1.09) | 0.84 (0.49-1.44) | Model 2 | 1.10 (0.93-1.31) | 1.0 (ref) | 0.90 (0.54-1.52) | 0.97 (0.59-1.59) |
| Model 3 | 0.86 (0.63-1.17) | 1.0 (ref) | 0.65 (0.36-1.19) | 0.98 (0.53-1.82) | Model 3 | 1.13 (0.91-1.42) | 1.0 (ref) | 1.11 (0.63-1.97) | 0.97 (0.55-1.73) |
Model 1: adjusted for matching factors (age, race (women only), month of blood draw, fasting status, and smoking status).
Model 2: adjusted for matching factors, triglycerides, HDL-C, LDL-C, hsCRP, and HbA1c.
Model 3: adjusted for matching factors, triglycerides, HDL-C, LDL-C, hsCRP, HbA1c, parental history of MI before age 60, pack-years of smoking, physical activity, hypertension, diabetes, hypercholesterolemia, BMI, aspirin use and postmenopausal hormone use (women only).
OxPL/apoB and Lp(a) in Context
In women, the C-statistic value for area under the receiver operating characteristic curve increased from 0.725 to 0.756 and 0.761 with the addition of OxPL/apoB and Lp(a), respectively, to a list of traditional risk factors (Table 6). These risk factors include age, race (women only), month of blood draw, fasting status, smoking status, parental history of MI before age 60, pack-years of smoking, physical activity, hypertension, diabetes, hypercholesterolemia, BMI, aspirin use and postmenopausal hormone use (women only). In men, the improvement was smaller: 0.728 to 0.731 and 0.735 with the addition of OxPL/apoB and Lp(a), respectively. Comparing OxPL/apoB and Lp(a) to other standard biomarkers, the magnitude of association between a 1-standard deviation increase in OxPL/apoB and Lp(a) and risk of PAD was similar to that for LDL-C and HDL-C in men (Figure 4).
Table 6.
C-statistic values for area under the receiver operating characteristic curves.
| Women | Men | |
|---|---|---|
|
|
||
| Traditional risk factors | 0.725 | 0.728 |
| Traditional risk factors + OxPL/apoB | 0.756 | 0.731 |
| Traditional risk factors + Lp(a) | 0.761 | 0.735 |
| Traditional risk factors + triglycerides | 0.727 | 0.741 |
| Traditional risk factors + HDL-C | 0.726 | 0.761 |
| Traditional risk factors + LDL-C | 0.727 | 0.734 |
| Traditional risk factors, OxPL/apoB, Lp(a) | 0.759 | 0.736 |
| Traditional risk factors, OxPL/apoB, Lp(a), triglycerides | 0.762 | 0.749 |
| Traditional risk factors, OxPL/apoB, Lp(a), triglycerides, HDL-C | 0.763 | 0.769 |
| Traditional risk factors, OxPL/apoB, Lp(a), triglycerides, HDL-C, LDL-C | 0.763 | 0.780 |
Traditional risk factors: matching factors (age, race (women only), month of blood draw, fasting status, and smoking status), parental history of MI before age 60, pack-years of smoking, physical activity, hypertension, diabetes, hypercholesterolemia, BMI, aspirin use and postmenopausal hormone use (women only)
Figure 4. Adjusted relationship between oxidation –specific and standard biomarkers and relative risk of PAD.
Adjusted for age, race (women only), fasting status, smoking, triglycerides, HDL-C, LDL-C, hsCRP, HbA1c, parental history of MI before age 60, pack-years of smoking, physical activity, hypertension, diabetes, hypercholesterolemia, BMI, aspirin use and postmenopausal hormone use (women only). RR and 95% CI.
DISCUSSION
This study demonstrates that OxPL/apoB levels are positively associated with risk of PAD in men and women, with no appreciable attenuation after adjustment for conventional risk factors. Risk of developing PAD was approximately doubled among those in the highest compared with the lowest tertile. Lp(a), the main lipoprotein carrier of OxPL, was similarly associated with risk of PAD. In contrast, we found no consistent relationship of indirect measures of oxidized lipoproteins such as autoantibodies to MDA-LDL and apoB-immune complexes with risk of PAD.
The role of OxPL and the role of the innate and adaptive immune system in mediating many pro-atherogenic processes is well-established in experimental models (10,11,23). However, clinical evidence is limited due to the absence of adequate diagnostic and therapeutic tools to measure atherosclerosis. In particular, biomarkers to predict PAD have been lacking (24). OxPL/apoB is a validated biomarker that reflects anatomical coronary, carotid and femoral artery disease, atherosclerosis, and predicts myocardial infarction and stroke (7).
In this study, we demonstrate the novel observation that elevated levels of OxPL/apoB are associated with PAD in both men and women in nested case-control studies within two, well-validated epidemiological cohorts. The odds ratios were robust, averaging ~2.0 per 1 SD increase, which is in line with prior data on CVD endpoints (25,26). Interestingly, in women, the curve was shifted to the left compared to men, suggesting a stronger association with lower OxPL/apoB values. Furthermore, the OxPL/apoB relationship to PAD was different than the Lp(a) relationship in women, with a similar left shift. This may reflect differences in OxPL content among Lp(a) particles, with higher OxPL in Lp(a) associated with small isoforms (27). This hypothesis-generating observation needs to be replicated, but it does suggest OxPL/apoB may be particularly useful in women. Our finding that an easily-measured marker of lipid oxidation is independently associated with risk of PAD also provides evidence that oxidative stress may indeed be an important contributor to atherosclerosis and provides a potentially useful tool to test this hypothesis in future studies.
We likewise found a positive association between Lp(a) and risk of PAD, an area where previous studies have been discordant. Some (28-35) but not all (29,36-38) cross-sectional studies tend to show positive associations between Lp(a) and PAD or PAD progression and one longitudinal study found a positive association (35). In contrast to PAD, data on the role of Lp(a) in the development of CVD is fairly consistent for a modest, independent association (39,40).
A recent pooled analysis found that the RRs of CHD and ischemic stroke per 1 SD increase in Lp(a) were 1.13 (95% CI 1.09-1.18) and 1.10 (95% CI 0.97-1.04), respectively, after adjusting for lipids and other conventional risk factors (40). We observed a pooled RR of PAD per 1-SD that was more than twice as strong as the previous estimate for CHD (estimated ln[RR] of 0.12 versus 0.31), suggesting that Lp(a) may be a stronger risk factor for PAD than for other forms of CVD, however more studies are needed to confirm this observation definitively.
Despite a recent focus on OxPL and Lp(a) as cardiovascular disease risk factors, the etiologic link between Lp(a) and atherosclerosis remains unclear. Lp(a) mediates atherogenesis through mechanisms linked to its LDL and apo(a) components and its associated pro-inflammatory OxPL (41). A link was recently established between OxPL and Lp(a) in mediating macrophage apoptosis, a common mechanism for plaque progression and destabilization (42).
Given that atherosclerosis represents a dynamic process of lipid deposition and reverse transport, clearance of oxidized particles would be anticipated to prevent atherosclerotic progression. In this study, we measured autoantibodies to MDA-LDL, which would be anticipated to serve as a sink for circulating oxidized LDL particles. Some, but not all (43), previous studies found an inverse association between levels of IgM autoantibodies to MDA-LDL and cardiovascular disease, including coronary artery disease (44), hypertension (45), MI (46-48), and carotid and femoral atherosclerosis (14,15). It is interesting that we found an inverse relationship between IgG to MDA-LDL and risk of PAD in men but not in women. With this exception, we found no other consistent associations of either autoantibodies or IC to LDL with risk of PAD.
Strengths of our study include adjudicated cases of clinically meaningful PAD in both sexes, the high quality measurement of both biochemical and behavioral confounders, and the use of risk set sampling to select controls which minimizes bias due to controls not accurately reflecting the source population from which the cases came. In addition, this is the first study to our knowledge that prospectively examines the association between novel cardiovascular risk factors OxPL/apoB, autoantibodies and IC and risk of PAD.
Our study is not without limitation. There is a possibility of unmeasured or residual confounding, and because the NHS and HPFS contain predominantly white participants, we cannot necessarily generalize to minority populations, some of whom are at increased risk for PAD. However, we have no evidence that oxidative stress is less important in these populations, and indeed recent evidence suggests that Lp(a) is strongly associated with CHD and ischemic stroke in both blacks and whites (49).
A potential limitation of studies of clinically significant PAD like this one is the possibility that some controls have undiagnosed PAD. Although this is a potential weakness of all studies of CVD events (e.g., individuals free of MI may nonetheless have asymptomatic coronary atherosclerosis), our results may be a conservative estimate of the association between OxPL/apoB and risk of PAD if controls have unrecognized PAD. However, all study participants are health professionals who report almost universal access to healthcare, which would tend to minimize undiagnosed cases. Regardless, our endpoint reflects clinically significant and meaningful cases of greatest concern to both patients and physicians. Finally, our results alone cannot definitively separate OxPL and Lp(a) as individual determinants of PAD, given their inherent biological interrelationship.
In conclusion, OxPL/apoB are positively associated with risk of PAD and are a unique oxidation-specific biomarker that it is not associated with other traditional CVD risk factors besides Lp(a), the major lipoprotein carrier of OxPL. Future research should continue to explore the mechanisms that link oxidation to risk of PAD and test whether modifiable risk factors, potentially including novel therapies that reduce levels of OxPL, may prevent the development of atherosclerotic diseases such as PAD.
Supplementary Material
Figure 2. Adjusted relationship between plasma levels of Lp(a) and relative risk of PAD.
Legend as per Figure 1.
Acknowledgements
We would like to acknowledge the Channing Division of Network Medicine, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School. We thank the participants of the Nurses’ Health and Health Professionals Follow-up Studies for their ongoing dedication.
This study was supported by grants R01 HL091874, HL35464, HL086559, HL 088093, P01 CA55075, P01 CA87969, and R01 CA49449 from the National Institutes of Health, and the Foundation Leducq.
Abbreviations
- PAD
peripheral artery disease
- OxPL
oxidized phospholipids
- Lp(a)
lipoprotein (a)
- MDA
malondialdehyde
- DAMP
danger-associated molecular pattern
- ApoB
apolipoprotein B-100-containing lipoproteins
- MI
myocardial infarction
- HPFS
Health Professionals Follow-up Study
- NHS
Nurses’ Health Study
- CVD
cardiovascular disease
- PC
phosphocholine
- RLU
relative light units
- BSA
bovine serum albumin
- ApoB-IC
apoB-immune complexes
- BMI
body mass index
- HDL-C
high-density lipoprotein cholesterol
- LDL-C
low-density lipoprotein cholesterol
- hsCRP
high-sensitivity C-reactive protein
- HbA1c
hemoglobin A1c
- RR
incidence rate ratio/relative risk
- SD
standard deviation
Footnotes
Disclosures: Drs. Tsimikas and Witztum are named as co-inventors of patents for the commercial use of oxidation-specific antibodies that are held by the University of California, San Diego, are co-founders of Atherotope, Inc and are consultants to Quest, Isis Pharmaceutical Inc. and Regulus Therapeutics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Criqui MH. Peripheral arterial disease--epidemiological aspects. Vasc Med. 2001;6:3–7. doi: 10.1177/1358836X0100600i102. [DOI] [PubMed] [Google Scholar]
- 2.Ostchega Y, Paulose-Ram R, Dillon CF, Gu Q, Hughes JP. Prevalence of peripheral arterial disease and risk factors in persons aged 60 and older: data from the National Health and Nutrition Examination Survey 1999-2004. J Am Geriatr Soc. 2007;55:583–9. doi: 10.1111/j.1532-5415.2007.01123.x. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 4.McDermott MM, Liu K, Greenland P, et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–61. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 5.Berliner JA, Navab M, Fogelman AM, et al. Atherosclerosis: basic mechanisms. Oxidation, inflammation, and genetics. Circulation. 1995;91:2488–96. doi: 10.1161/01.cir.91.9.2488. [DOI] [PubMed] [Google Scholar]
- 6.Navab M, Imes SS, Hama SY, et al. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991;88:2039–46. doi: 10.1172/JCI115532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taleb A, Witztum JL, Tsimikas S. Oxidized phospholipids on apoB-100-containing lipoproteins: a biomarker predicting cardiovascular disease and cardiovascular events. Biomarkers Med. 2011;5:673–94. doi: 10.2217/bmm.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergmark C, Dewan A, Orsoni A, et al. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res. 2008;49:2230–9. doi: 10.1194/jlr.M800174-JLR200. [DOI] [PubMed] [Google Scholar]
- 9.Berliner JA, Leitinger N, Tsimikas S. The role of oxidized phospholipids in atherosclerosis. J Lipid Res. 2009;50(Suppl):S207–12. doi: 10.1194/jlr.R800074-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stockl J. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal. 2010;12:1009–59. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller YI, Choi SH, Wiesner P, et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–48. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weismann D, Hartvigsen K, Lauer N, et al. Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature. 2011;478:76–81. doi: 10.1038/nature10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsimikas S, Kiechl S, Willeit J, et al. Oxidized phospholipids predict the presence and progression of carotid and femoral atherosclerosis and symptomatic cardiovascular disease: five-year prospective results from the Bruneck study. J Am Coll Cardiol. 2006;47:2219–28. doi: 10.1016/j.jacc.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Hulthe J, Bokemark L, Fagerberg B. Antibodies to oxidized LDL in relation to intimamedia thickness in carotid and femoral arteries in 58-year-old subjectively clinically healthy men. Arterioscler Thromb Vasc Biol. 2001;21:101–7. doi: 10.1161/01.atv.21.1.101. [DOI] [PubMed] [Google Scholar]
- 15.Karvonen J, Paivansalo M, Kesaniemi YA, Horkko S. Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation. 2003;108:2107–12. doi: 10.1161/01.CIR.0000092891.55157.A7. [DOI] [PubMed] [Google Scholar]
- 16.Bergmark C, Wu R, de Faire U, Lefvert AK, Swedenborg J. Patients with early-onset peripheral vascular disease have increased levels of autoantibodies against oxidized LDL. Arterioscler Thromb Vasc Biol. 1995;15:441–5. doi: 10.1161/01.atv.15.4.441. [DOI] [PubMed] [Google Scholar]
- 17.Tsimikas S, Lau HK, Han KR, et al. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation. 2004;109:3164–70. doi: 10.1161/01.CIR.0000130844.01174.55. [DOI] [PubMed] [Google Scholar]
- 18.Tsimikas S, Witztum JL, Miller ER, et al. High-dose atorvastatin reduces total plasma levels of oxidized phospholipids and immune complexes present on apolipoprotein B-100 in patients with acute coronary syndromes in the MIRACL trial. Circulation. 2004;110:1406–12. doi: 10.1161/01.CIR.0000141728.23033.B5. [DOI] [PubMed] [Google Scholar]
- 19.Joosten MM, Pai JK, Bertoia ML, et al. Associations between conventional cardiovascular risk factors and risk of peripheral artery disease in men. JAMA. 2012;308:1660–7. doi: 10.1001/jama.2012.13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chasan-Taber S, Rimm EB, Stampfer MJ, et al. Reproducibility and validity of a self-administered physical activity questionnaire for male health professionals. Epidemiology. 1996;7:81–6. doi: 10.1097/00001648-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–73. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–9. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 23.Lahoute C, Herbin O, Mallat Z, Tedgui A. Adaptive immunity in atherosclerosis: mechanisms and future therapeutic targets. Nat Rev Cardiol. 2011;8:348–58. doi: 10.1038/nrcardio.2011.62. [DOI] [PubMed] [Google Scholar]
- 24.Cooke JP, Wilson AM. Biomarkers of peripheral arterial disease. J Am Coll Cardiol. 2010;55:2017–23. doi: 10.1016/j.jacc.2009.08.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsimikas S, Mallat Z, Talmud PJ, et al. Oxidation-specific biomarkers, lipoprotein(a), and risk of fatal and nonfatal coronary events. J Am Coll Cardiol. 2010;56:946–55. doi: 10.1016/j.jacc.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 26.Kiechl S, Willeit J, Mayr M, et al. Oxidized phospholipids, lipoprotein(a), lipoprotein-associated phospholipase A2 activity, and 10-year cardiovascular outcomes: prospective results from the Bruneck study. Arterioscler Thromb Vasc Biol. 2007;27:1788–95. doi: 10.1161/ATVBAHA.107.145805. [DOI] [PubMed] [Google Scholar]
- 27.Tsimikas S, Clopton P, Brilakis ES, et al. Relationship of oxidized phospholipids on apolipoprotein B-100 particles to race/ethnicity, apolipoprotein(a) isoform size, and cardiovascular risk factors: results from the Dallas Heart Study. Circulation. 2009;119:1711–9. doi: 10.1161/CIRCULATIONAHA.108.836940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volpato S, Vigna GB, McDermott MM, et al. Lipoprotein(a), inflammation, and peripheral arterial disease in a community-based sample of older men and women (the InCHIANTI study) Am J Cardiol. 2010;105:1825–30. doi: 10.1016/j.amjcard.2010.01.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pradhan AD, Shrivastava S, Cook NR, Rifai N, Creager MA, Ridker PM. Symptomatic peripheral arterial disease in women: nontraditional biomarkers of elevated risk. Circulation. 2008;117:823–31. doi: 10.1161/CIRCULATIONAHA.107.719369. [DOI] [PubMed] [Google Scholar]
- 30.Jones GT, van Rij AM, Cole J, et al. Plasma lipoprotein(a) indicates risk for 4 distinct forms of vascular disease. Clin Chem. 2007;53:679–85. doi: 10.1373/clinchem.2006.079947. [DOI] [PubMed] [Google Scholar]
- 31.Dieplinger B, Lingenhel A, Baumgartner N, et al. Increased serum lipoprotein(a) concentrations and low molecular weight phenotypes of apolipoprotein(a) are associated with symptomatic peripheral arterial disease. Clin Chem. 2007;53:1298–305. doi: 10.1373/clinchem.2007.088013. [DOI] [PubMed] [Google Scholar]
- 32.Widmann MD, Sumpio BE. Lipoprotein (a): a risk factor for peripheral vascular disease. Ann Vasc Surg. 1993;7:446–51. doi: 10.1007/BF02002128. [DOI] [PubMed] [Google Scholar]
- 33.Tseng CH. Lipoprotein(a) is an independent risk factor for peripheral arterial disease in Chinese type 2 diabetic patients in Taiwan. Diabetes Care. 2004;27:517–21. doi: 10.2337/diacare.27.2.517. [DOI] [PubMed] [Google Scholar]
- 34.Tyrrell J, Cooke T, Reilly M, et al. Lipoprotein [Lp(a)] and peripheral vascular disease. J Intern Med. 1992;232:349–52. doi: 10.1111/j.1365-2796.1992.tb00596.x. [DOI] [PubMed] [Google Scholar]
- 35.Gurdasani D, Sjouke B, Tsimikas S, et al. Lipoprotein(a) and Risk of Coronary, Cerebrovascular, and Peripheral Artery Disease: The EPIC-Norfolk Prospective Population Study. Arterioscler Thromb Vasc Biol. 2012;32:3058–65. doi: 10.1161/ATVBAHA.112.255521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ix JH, Allison MA, Denenberg JO, Cushman M, Criqui MH. Novel cardiovascular risk factors do not completely explain the higher prevalence of peripheral arterial disease among African Americans. The San Diego Population Study. J Am Coll Cardiol. 2008;51:2347–54. doi: 10.1016/j.jacc.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 37.Ridker PM, Stampfer MJ, Rifai N. Novel risk factors for systemic atherosclerosis: a comparison of C-reactive protein, fibrinogen, homocysteine, lipoprotein(a), and standard cholesterol screening as predictors of peripheral arterial disease. JAMA. 2001;285:2481–5. doi: 10.1001/jama.285.19.2481. [DOI] [PubMed] [Google Scholar]
- 38.Aboyans V, Criqui MH, Denenberg JO, Knoke JD, Ridker PM, Fronek A. Risk factors for progression of peripheral arterial disease in large and small vessels. Circulation. 2006;113:2623–9. doi: 10.1161/CIRCULATIONAHA.105.608679. [DOI] [PubMed] [Google Scholar]
- 39.Dube JB, Boffa MB, Hegele RA, Koschinsky ML. Lipoprotein(a): more interesting than ever after 50 years. Curr Opin Lipidol. 2012;23:133–40. doi: 10.1097/MOL.0b013e32835111d8. [DOI] [PubMed] [Google Scholar]
- 40.Erqou S, Kaptoge S, Perry PL, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–23. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spence JD, Koschinsky M. Mechanisms of lipoprotein(a) pathogenicity: prothrombotic, proatherosclerotic, or both? Arterioscler Thromb Vasc Biol. 2012;32:1550–1. doi: 10.1161/ATVBAHA.112.251306. [DOI] [PubMed] [Google Scholar]
- 42.Seimon TA, Nadolski MJ, Liao X, et al. Atherogenic lipids and lipoproteins trigger CD36-TLR2-dependent apoptosis in macrophages undergoing endoplasmic reticulum stress. Cell Metab. 2010;12:467–82. doi: 10.1016/j.cmet.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mayr M, Kiechl S, Tsimikas S, et al. Oxidized low-density lipoprotein autoantibodies, chronic infections, and carotid atherosclerosis in a population-based study. J Am Coll Cardiol. 2006;47:2436–43. doi: 10.1016/j.jacc.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 44.Tsimikas S, Bergmark C, Beyer RW, et al. Temporal increases in plasma markers of oxidized low-density lipoprotein strongly reflect the presence of acute coronary syndromes. J Am Coll Cardiol. 2003;41:360–70. doi: 10.1016/s0735-1097(02)02769-9. [DOI] [PubMed] [Google Scholar]
- 45.Wu R, de Faire U, Lemne C, Witztum JL, Frostegard J. Autoantibodies to OxLDL are decreased in individuals with borderline hypertension. Hypertension. 1999;33:53–9. doi: 10.1161/01.hyp.33.1.53. [DOI] [PubMed] [Google Scholar]
- 46.Puurunen M, Manttari M, Manninen V, et al. Antibody against oxidized low-density lipoprotein predicting myocardial infarction. Arch Intern Med. 1994;154:2605–9. [PubMed] [Google Scholar]
- 47.Erkkila AT, Narvanen O, Lehto S, Uusitupa MI, Yla-Herttuala S. Autoantibodies against oxidized low-density lipoprotein and cardiolipin in patients with coronary heart disease. Arterioscler Thromb Vasc Biol. 2000;20:204–9. doi: 10.1161/01.atv.20.1.204. [DOI] [PubMed] [Google Scholar]
- 48.Mustafa A, Nityanand S, Berglund L, Lithell H, Lefvert AK. Circulating immune complexes in 50-year-old men as a strong and independent risk factor for myocardial infarction. Circulation. 2000;102:2576–81. doi: 10.1161/01.cir.102.21.2576. [DOI] [PubMed] [Google Scholar]
- 49.Virani SS, Brautbar A, Davis BC, et al. Associations between lipoprotein(a) levels and cardiovascular outcomes in black and white subjects: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2012;125:241–9. doi: 10.1161/CIRCULATIONAHA.111.045120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.