Abstract
Obesity and obesity-related complications are epidemic issues currently plaguing much of the developed world with increasing associated morbidity, mortality, and economic burden. In this brief review, we discuss emerging evidence and remaining questions regarding the possible role for mitochondrial sirtuin 3 as a therapeutic target for the treatment of obesity-related metabolic diseases.
Introduction
Acetylation is a reversible form of posttranslational modification that has recently emerged as an important mechanism for controlling the activity of a broad array of metabolic pathways [1,2]. The sirtuins are a family of NAD+-dependent deacetylases (and perhaps more appropriately, de-acylases) that modify protein activity and gene expression in response to changes in nutrient status. Thus, this family of proteins provides an important link between cellular energy availability and control of metabolism. Many of the sirtuin targets identified to date are enzymes and genes that regulate metabolic processes important for cancer, obesity, type 2 diabetes, and longevity, making them highly attractive targets for the pharmaceutical treatment of many diseases. Herein we specifically discuss the possible role for mitochondrial sirtuin 3 (Sirt3) as a therapeutic target for the treatment of obesity-related metabolic diseases.
Sirtuin3 is a key regulator of mitochondrial metabolism
Since their initial recognition as silencing factors in yeast, sirtuins have received a tremendous amount of attention as regulators of both histone and non-histone protein (de)acetylation, initially due to their perceived role in caloric restriction-induced longevity [3]. Subsequently, a growing list of genes, metabolic pathways, and physiologic processes shown to be regulated by various sirtuins has been paralleled by growing interest in their expression, regulation, and functions. For a recent, comprehensive review of this topic, we direct readers to the excellent work of Houtkooper et al. [4]. We instead will focus exclusively on mitochondrial-localized sirtuins, and in particular the mounting interest in Sirt3.
Despite a conserved deacetylase domain among all of the seven mammalian sirtuins (Sirt1-7), some of the sirtuins display little-to-no known deacetylase activity in vivo. This is true for two of the three mitochondrial-localized sirtuins, including Sirt4 and Sirt5, which have recently been shown to have ADP-ribosylation, and demalonylation and desuccinylation activities, respectively [5-7]. Still, relatively little is known about the physiological significance of the activity of these two mitochondrial sirtuins on mitochondrial function, which is an emerging area of interest. In contrast, we and others have helped to characterize the functional role(s) of Sirt3, the primary mitochondrial-localized deacetylase, under conditions of nutrient excess [8-10]. Development of interest in Sirt3 in the pathophysiology of obesity was fueled by findings that Sirt3 protein content is downregulated with obesity, and that Sirt3 deficient mice (Sirt3-/-) display augmented mitochondrial protein hyperacetylation and accelerated development of the metabolic syndrome when placed on a high fat diet [9,10]. Furthermore, mass spectrometry-based proteomics has led to the estimation that at least 20% of the mitochondrial proteome is acetylated [11], with essentially every major pathway of intermediary metabolism being represented, suggesting that the deacetylase activity of Sirt3 may help to regulate a broad array of metabolic processes. Whether altered mitochondrial function is a cause or consequence of each of these disease conditions is not well established, but the diverse factors known to provoke these conditions, including oxidative stress, endoplasmic reticulum stress, hypoxia, high fatty acid availability, and altered inflammatory and immune responses, are often related to changes in mitochondrial function. Thus, understanding the regulation of mitochondrial metabolism and function appears paramount for deconstructing the pathophysiology of a growing number of disease conditions. Below we explore mechanisms of Sirt3 activity that could make it an attractive therapeutic drug target, discuss what is known about the regulation of Sirt3, and throughout highlight key questions presently unanswered that will be important to evaluate prior to the use of Sirt3 as a therapeutic drug target.
Sirt3 facilitates fatty acid oxidation and electron transport system activity
Insufficient oxidation of fatty acids has long been implicated as a cause of ectopic lipid accumulation and the development of insulin resistance [12,13]. However, it is important to recognize that excess weight gain is due to a chronic mismatch between energy intake and whole body energy expenditure resulting in positive energy balance, rather than insufficient oxidation of fatty acids per se. Nevertheless, targeting mitochondrial fatty acid oxidation as a means to enhance total energy expenditure, and thereby affect total energy balance, remains a possible mechanism to treat obesity and obesity-related metabolic diseases. Sirt3 deacetylates a number of enzymes in the pathways essential for fatty acid oxidation, and more importantly, several of these Sirt3-dependent modifications have been shown to regulate enzyme activity. Hirschey et al. [14], first demonstrated that fatty acid oxidation in mitochondrial extracts from liver, heart, and brown adipose tissue of Sirt3-/- mice is significantly reduced when compared with wild type mice. In the same report the activity of long-chain acyl-CoA dehydrogenase (LCAD), a key enzyme of the fatty acid β-oxidation pathway, was enhanced by Sirt3-mediated deacetylation in response to changes in cellular nutrient status, thereby providing an enzymatic mechanism for Sirt3-mediated regulation of fatty acid oxidation [14]. The upstream activator of AMP-activated protein kinase (AMPK), liver kinase B1 (LKB1) was also recently shown to be a target for Sirt3-mediated deacetylation, providing a mechanism for a “global” increase in fatty acid oxidation and increased mitochondrial resynthesis of ATP [15].
Perhaps even more important, multiple independent investigations have reported Sirt3-mediated (positive) regulation of multiple enzymes within the TCA cycle [16-18], as well as multiple protein subunits of complexes comprising the electron transport system (ETS; e.g., complex I [19], complex II [20,21], complex III [22]), providing support for the role of Sirt3 as a key regulator of total mitochondrial activity. Indeed, Sirt3-/- mice have lowered fasting ATP and NAD+ concentrations, lowered complex I-dependent O2 consumption in isolated mitochondria, impaired fasting cold tolerance, and, not surprisingly, display accelerated weight gain compared with wild type animals when placed on high fat diet (independent of genotype-specific hyperphagia or spontaneous activity) [9,19]. These findings clearly indicate impaired energy expenditure in Sirt3-/- mice, at least partly explain their accelerated development of the metabolic syndrome (via accelerated weight gain), and strongly support Sirt3 as a key regulator of mitochondrial energy metabolism. Thus, stimulating Sirt3 activity may provide a mechanism of increasing energy expenditure, making it an obvious target of interest for the treatment of obesity and obesity-related metabolic diseases.
Sirt3-mediated deacetylation enhances mitochondrial antioxidant defense
Sirt3-mediated deacetylation is also a key regulator of mitochondrial antioxidant defense enzymes, and thus oxidative stress. Like Sirt1, Sirt3 is reported to at least partially mediate the beneficial effects of caloric restriction, including protection against age-related hearing loss [17], and it is likely that these effects can largely be attributed to control of cellular reactive oxygen species (ROS). Indeed, the attenuation in cellular oxidative stress that occurs with caloric restriction is lost in the absence of Sirt3 [17,23], suggesting that Sirt3 may be a critical regulator of cellular redox balance. Most cellular ROS production occurs in mitochondria, as superoxide (O2-) is a natural byproduct of ETS metabolism. And because increased oxidative damage is a known pathological component of cancer, obesity, type 2 diabetes, cardiovascular disease, and many other aging-related disorders, Sirt3 may be an attractive drug target for treatment of numerous disease conditions via improved redox balance.
Substantial recent evidence indicates that mitochondrial superoxide dismutase (MnSOD/SOD2), the enzyme responsible for catalyzing the dismutation of O2- to the less reactive H2O2, is regulated by Sirt3-mediated deacetylation. Multiple conserved lysine residues of MnSOD are known to be targets of Sirt3 deacetylation, and Sirt3-mediated deacetylation of these residues increases the activity of MnSOD [23-25]. We also recently observed a strong inverse correlation between loss of Sirt3 activity and MnSOD hyperacetylation in skeletal muscle from obese pregnant women compared with lean pregnant women, providing the first functional human data suggesting that impaired Sirt3 activity may trigger broad-ranging metabolic consequences via diminished antioxidant protection (KE Boyle et al., unpublished). Like MnSOD, Sirt3 has been shown to deacetylate and thereby enhance the activity of isocitrate dehydrogenase 2 (IDH2), an enzyme that catalyzes the generation of NADPH from NADP+ [17]. Because NADPH is required for regeneration of the glutathione ROS detoxification pathway, Sirt3-mediated enhancement of NADPH synthesis (via regulation of IDH2) is critical for normal function of the glutathione system and complete detoxification of mitochondrial ROS. Thus, Sirt3 activity aids clearance of mitochondrial ROS at multiple steps of the detoxification pathway (as shown in Figure 1). Whether Sirt3 activity also helps to lower mitochondrial ROS production (for a given level of O2 consumption) via its known deacetylation of various subunits of the ETS complexes is currently unknown, but is an important area of interest of future investigation.
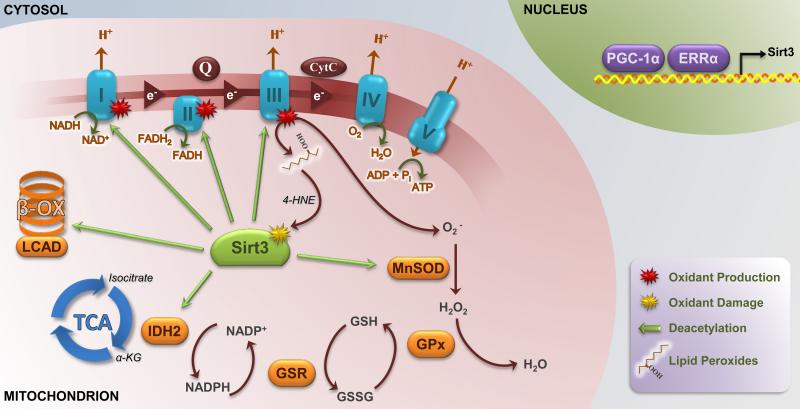
Figure 1.
Overview of Sirt3 deacetylation targets, and regulation of Sirt3 expression and activity. Sirt3 is a mitochondrial matrix-localized deacetylase that targets various subunits of complexes within the electron transport system, and appears to be a critical regulator of cellular ATP. Sirt3 also deacetylates enzymes within the β-oxidation pathway, including LCAD, and within the TCA cycle, including IDH2. Sirt3 is also a key regulator of antioxidant defense pathways. Enhanced IDH2 activity yields critical regeneration of NADPH for the glutathione detoxification pathway, and deacetylation of MnSOD on multiple conserved sites has been shown to enhance the activity of this mitochondrial-localized dismutase. Sirt3 expression is regulated by PGC-1α and ERRα, while its activity can be inhibited by 4-HNE-mediated carbonylation. Sirt3, sirtuin 3; LCAD, long-chain acyl-CoA dehydrogenase; TCA, tricarboxylic acid cycle; IDH2, isocitrate dehydrogenase 2; MnSOD, manganese superoxide dismutase; PGC-1α, PPARγ coactivator-1α; ERRα, estrogen-related receptor α; 4-HNE, 4-hydroxynonenol; β-OX, β-oxidation; α-KG, α-ketoglutarate; GSR, glutathione reductase; GPx, glutathione peroxidase; Q, co-enzyme Q; CytC; cytochrome C.
Regulation of Sirt3 activity is not well understood
Despite growing interest in Sirt3, the regulation of its activity is still poorly understood. Sirt3 is highly expressed in mitochondria-rich tissues, including liver, kidney, heart, brown adipose, and skeletal muscle, and is sensitive to changes in cellular energy status (e.g., fasting/re-feeding, caloric restriction, exercise) [23,26-28]. During fasting (i.e., low energy status), Sirt3 mRNA and protein are generally increased, and this corresponds with decreased mitochondrial protein acetylation of Sirt3 target proteins [14]. In contrast, chronic high fat feeding (i.e., diet-induced obesity and excess energy availability) is associated with decreased cellular NAD+ levels, decreased Sirt3 protein content and/or activity, and gross mitochondrial protein hyperacetylation [9,10]. Sirt3 expression is regulated by the activity of the transcriptional co-activator PPARγ coactivator-1α (PGC-1α), and estrogen-related receptor α (ERRα) [29,30]. Importantly, attenuated PGC-1α content with chronic high fat feeding is likely responsible for lowered Sirt3 protein content in obesity [9]. However, because Sirt3 requires NAD+ for its deacetylase activity, the obesity-related decline in cellular NAD+ concentration demonstrated by our laboratory [10] and others [31] may also play a critical role in the loss of Sirt3 activity in obesity. This attenuation in cellular NAD+ with obesity may be due in part to downregulation of nicotinamide phosphoribosyltransferase (Nampt), the rate-limiting enzyme in the NAD+ salvage pathway [32].
Surprisingly, little is known about regulation of Sirt3 activity independent of Sirt3 protein content and cellular NAD+ concentration, particularly acute and/or reversible posttranslational modification. Fritz et al. [33] recently demonstrated that Sirt3 activity could be directly inhibited via 4-hydroxynonenol (4-HNE)-mediated carbonylation at Cys280, a critical zinc-binding residue. Because 4-HNE is a product of lipid peroxidation, a common consequence of obesity, it is possible that carbonylation of Sirt3 may explain evidence of impaired Sirt3 activity without a reduction in Sirt3 protein during chronic high fat feeding [10]. To our knowledge, no other posttranslational regulation of Sirt3 activity has been characterized.
Similarly, pharmaceutical and/or nutriceutical activation of Sirt3 is obviously an area of great interest, but to date we are unaware of any known Sirt3-specific agonist or antagonist. Intriguingly, resveratrol is reportedly not a direct activator of Sirt3 (and perhaps even a weak inhibitor) [34]; however, the resveratrol-like compound trans-(-)-ε-Viniferin was recently found to have profoundly protective effects in a model of Huntington disease that were Sirt3-dependent [15]. Still, the effect of trans-(-)-ε-Viniferin in this model was not due to direct activation of Sirt3, but rather due to an increase in Sirt3 protein, likely via robust induction of PGC-1α [15]. Conversely, an analogue of nicotinamide, a known inhibitor of sirtuins, has been developed with relative specificity for Sirt3 inhibition over Sirt1 and Sirt2, but it is likely that off-target effects of this compound will limit its use to non-clinical applications [35]. A better understanding of how Sirt3 may be acutely and reversibly activated is essential for the evolution of Sirt3 as a potential therapeutic target for the treatment of metabolic diseases.
Is Sirt3 a viable pharmaceutical target for the treatment of obesity-related metabolic disease?
If there were an available Sirt3 agonist, could it be useful in treating or preventing obesity-related metabolic disease including insulin resistance and type 2 diabetes? There has been ample demonstration that restoration of Sirt3 in Sirt3-depleted animals and tissue cultures is beneficial for mitochondrial metabolism and redox balance [8,19]; however, there is currently little-to-no direct evidence that induction of Sirt3 protein or activity is beneficial for the prevention or treatment of obesity-related metabolic disease. Specifically, there is currently no experimental evidence that tissue-specific Sirt3 overexpression (in wild type animals) is protective against the deleterious effects of chronic high fat feeding and obesity. These and other related experiments will be critical for understanding the possible therapeutic value of a Sirt3 agonist.
Considering the possibility that Sirt3 itself may not be a viable target for the treatment of obesity-related metabolic diseases, it is noteworthy that Sirt3 activity has been shown to mediate fasting acetaminophen-induced liver injury [36]. And in addition to the many known actions of Sirt3 not discussed here (for review, see [37]), there are undoubtedly numerous functional targets of this enzyme that currently remain unstudied, raising the possibility that agonizing Sirt3 could certainly have negative consequences. Along these lines, it is also conceivable that the obesity-related attenuation in Sirt3 protein/activity is an adaptive mechanism, perhaps in part to protect against excessive fatty acid-induced ROS production. For example, Anderson et al. [38] demonstrated that excessive availability of fatty acids and the subsequent increase in mitochondrial fatty acid oxidation following high fat feeding increases mitochondrial H2O2 emission potential, and that this is a critical component of fatty acid-induced insulin resistance. Accordingly, if targeting Sirt3 activity facilitates additional mitochondrial fatty acid oxidation, it is possible that this could add to cellular oxidative burden (via increased H2O2 emission) and thereby compound obesity-related metabolic dysfunction. Conversely, it also remains possible that Sirt3-mediated induction of antioxidant defense pathways (as described above), and perhaps also via improved ETS complex activity that limits the increase in superoxide production, may help to attenuate this less desirable consequence of increased mitochondrial fatty acid oxidation. This is just one of many possible scenarios that will need to be closely evaluated during future investigations into the possible use of a Sirt3 agonist in therapeutic treatment of metabolic disease.
Concluding remarks
We have presented recent evidence supporting the role of Sirt3 in the control of overall metabolic function of the cell, including fatty acid oxidation, ETS function, and antioxidant defense pathways (summarized in Figure 1). Because obesity is a condition characterized by excess availability of energy, attenuated Sirt3-mediated support of mitochondrial metabolism and antioxidant defense pathways presents a situation whereby the complications of obesity linked with the development of insulin resistance and many other metabolic diseases may be undesirably worsened (Figure 2). In this regard, it is not terribly surprising that Sirt3-/- mice develop accelerated obesity, insulin resistance, and other characteristics of the metabolic syndrome when placed on a calorically dense, high fat diet. Together, these findings strongly suggest that an obesity-related downregulation of Sirt3 activity may help to link obesity with the development of numerous metabolic disease conditions, and that restoration of Sirt3 activity may help to attenuate the progression of this deleterious cycle. Nevertheless, many questions vital to the complete understanding of the potential benefits and consequences of a Sirt3-targeted approach for the treatment of obesity-related metabolic diseases remain unanswered. The coming years will undoubtedly bring substantial advances in this area of research, and in turn, yield novel therapeutic strategies for the prevention and/or treatment of obesity and the many related metabolic disease conditions. We remain hopeful that Sirt3 may be a promising therapeutic drug target for a wide range of diseases.
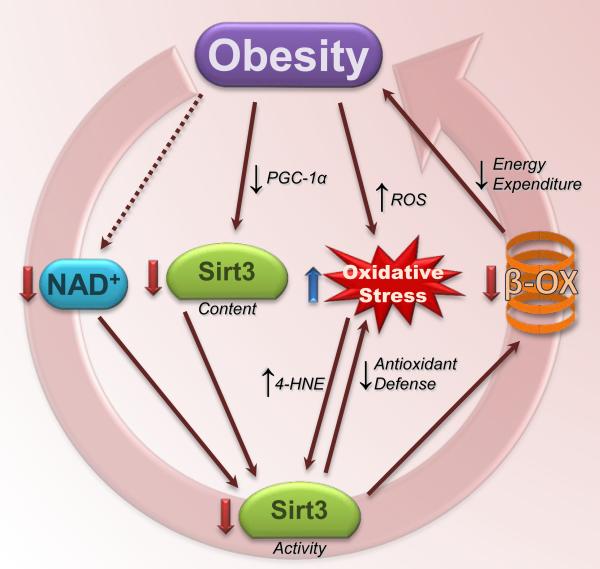
Figure 2.
A proposed deleterious cycle of obesity and downregulation of Sirt3 activity. Obesity is associated with decreased cellular NAD+ concentration and decreased Sirt3 protein content, both of which likely contributed to attenuated Sirt3 activity. In turn, the loss of Sirt3-mediated antioxidant defense may contribute to increased oxidative stress, and impaired fatty acid oxidation and lowered energy expenditure may increase the likelihood of positive energy balance and continued/additional weight gain. Not pictured here (for simplicity) are the many other deleterious effects associated with obesity that may then by worsened as a result of this proposed cycle. Sirt3, sirtuin 3; 4-HNE, 4-hydroxynonenol; PGC-1α, PPARγ coactivator-1α; β-OX, β-oxidation.
Highlights.
- Reversible lysine acetylation has emerged as a key regulator of mitochondrial fatty acid oxidation and antioxidant defense
- Sirtuin 3 is the primary mitochondrial deacetylase, but is downregulated in obesity, resulting in mitochondrial protein hyperacetylation
- Animals lacking sirtuin 3 display accelerated weight gain and development of the metabolic syndrome on a high fat diet
- Whether restoration of sirtuin 3 activity in obesity holds metabolic benefit remains unknown
Acknowledgments
The authors would like to acknowledge the excellent work of many others in this area, and apologize for any contributions that could not be included in this brief review due to space limitations. The authors would also like to thank Rachel Janssen for helpful editorial assistance in the preparation of this manuscript. This work was supported in part by grants NIH DK 5R01DK062155 and Colorado Nutrition and Obesity Research (NORC) NIH P30 DK048520. SAN is supported by NIH-F32 DK 095509. KEB is supported by NIHK12 HD 057022-06.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors have no conflicts of interest to report.
References (annotations included below highlighted references)
- 1.Wang Q, et al. Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science. 2010;327(5968):1004–1007. doi: 10.1126/science.1179687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao S, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327(5968):1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen D, Guarente L. SIR2: a potential target for calorie restriction mimetics. Trends Mol Med. 2007;13(2):64–71. doi: 10.1016/j.molmed.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Houtkooper RH, et al. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13(4):225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haigis MC, et al. SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell. 2006;126(5):941–954. doi: 10.1016/j.cell.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 6.Du J, et al. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334(6057):806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng C, et al. The first identification of lysine malonylation substrates and its regulatory enzyme. Mol Cell Proteomics. 2011;10(12):M111, 012658. doi: 10.1074/mcp.M111.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Bao J, et al. SIRT3 is regulated by nutrient excess and modulates hepatic susceptibility to lipotoxicity. Free Radic Biol Med. 2010;49(7):1230–1237. doi: 10.1016/j.freeradbiomed.2010.07.009. [Early characterization of the decrease in hepatic Sirt3 with chronic high fat feeding.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Hirschey MD, et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol Cell. 2011;44(2):177–190. doi: 10.1016/j.molcel.2011.07.019. [Excellent characterization of the deleterious effects of Sirt3 deficiency in mice chronically fed a high fat diet.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Kendrick AA, et al. Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation. Biochem J. 2011;433(3):505–514. doi: 10.1042/BJ20100791. [Identification of hyperacetylation patterns in liver proteins of chronically high fat-fed mice.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SC, et al. Substrate and functional diversity of lysine acetylation revealed by a proteomics survey. Mol Cell. 2006;23(4):607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 12.Kelley DE, et al. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277(6 Pt 1):E1130–1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 13.Kelley DE, et al. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51(10):2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 14••.Hirschey MD, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464(7285):121–125. doi: 10.1038/nature08778. [Identification of LCAD as a target of Sirt3-mediated deacetylation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu J, et al. trans-(-)-epsilon-Viniferin increases mitochondrial sirtuin 3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of Huntington Disease. J Biol Chem. 2012;287(29):24460–24472. doi: 10.1074/jbc.M112.382226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lombard DB, et al. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27(24):8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17••.Someya S, et al. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143(5):802–812. doi: 10.1016/j.cell.2010.10.002. [Identification of IDH2 as a target of Sirt3-mediated deacetylation, and Sirt3 as a key regulator of the reduction in oxidative damage with caloric restriction.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlicker C, et al. Substrates and regulation mechanisms for the human mitochondrial sirtuins Sirt3 and Sirt5. J Mol Biol. 2008;382(3):790–801. doi: 10.1016/j.jmb.2008.07.048. [DOI] [PubMed] [Google Scholar]
- 19•.Ahn BH, et al. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci U S A. 2008;105(38):14447–14452. doi: 10.1073/pnas.0803790105. [Seminal characterization of Sirt3 as a critical regulator of energy homeostasis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cimen H, et al. Regulation of succinate dehydrogenase activity by SIRT3 in mammalian mitochondria. Biochemistry. 2010;49(2):304–311. doi: 10.1021/bi901627u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finley LW, et al. Succinate dehydrogenase is a direct target of sirtuin 3 deacetylase activity. PLoS One. 2011;6(8):e23295. doi: 10.1371/journal.pone.0023295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22•.Jing E, et al. Sirtuin-3 (Sirt3) regulates skeletal muscle metabolism and insulin signaling via altered mitochondrial oxidation and reactive oxygen species production. Proc Natl Acad Sci U S A. 2011;108(35):14608–14613. doi: 10.1073/pnas.1111308108. [Characterization of Sirt3 as a key regulator of skeletal muscle mitochondrial metabolism.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23••.Qiu X, et al. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12(6):662–667. doi: 10.1016/j.cmet.2010.11.015. [Identification of MnSOD as a Sirt3 target during caloric restriction.] [DOI] [PubMed] [Google Scholar]
- 24••.Tao R, et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40(6):893–904. doi: 10.1016/j.molcel.2010.12.013. [Primary identification of a conserved Sirt3-regulated acetylation site on MnSOD.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, et al. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011;12(6):534–541. doi: 10.1038/embor.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palacios OM, et al. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging (Albany NY) 2009;1(9):771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurd BJ, et al. In mammalian muscle, SIRT3 is present in mitochondria and not in the nucleus; and SIRT3 is upregulated by chronic muscle contraction in an adenosine monophosphate-activated protein kinase-independent manner. Metabolism. 2012;61(5):733–741. doi: 10.1016/j.metabol.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Hokari F, et al. Muscle contractile activity regulates Sirt3 protein expression in rat skeletal muscles. J Appl Physiol. 2010;109(2):332–340. doi: 10.1152/japplphysiol.00335.2009. [DOI] [PubMed] [Google Scholar]
- 29.Giralt A, et al. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha controls transcription of the Sirt3 gene, an essential component of the thermogenic brown adipocyte phenotype. J Biol Chem. 2011;286(19):16958–16966. doi: 10.1074/jbc.M110.202390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30•.Kong X, et al. Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One. 2010;5(7):e11707. doi: 10.1371/journal.pone.0011707. [Identification of Sirt3 as a key gene target of PGC-1α.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim HJ, et al. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J Proteome Res. 2011;10(2):722–731. doi: 10.1021/pr100892r. [DOI] [PubMed] [Google Scholar]
- 32.Dahl TB, et al. Intracellular nicotinamide phosphoribosyltransferase protects against hepatocyte apoptosis and is down-regulated in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2010;95(6):3039–3047. doi: 10.1210/jc.2009-2148. [DOI] [PubMed] [Google Scholar]
- 33•.Fritz KS, et al. 4-Hydroxynonenal inhibits SIRT3 via thiol-specific modification. Chem Res Toxicol. 2011;24(5):651–662. doi: 10.1021/tx100355a. [Initial report of Sirt3 carbonylation, and impaired Sirt3 activity in the presence of 4-HNE.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gertz M, et al. A molecular mechanism for direct sirtuin activation by resveratrol. PLoS One. 2012;7(11):e49761. doi: 10.1371/journal.pone.0049761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galli U, et al. Identification of a sirtuin 3 inhibitor that displays selectivity over sirtuin 1 and 2. Eur J Med Chem. 2012;55:58–66. doi: 10.1016/j.ejmech.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 36•.Lu Z, et al. SIRT3-dependent deacetylation exacerbates acetaminophen hepatotoxicity. EMBO Rep. 2011;12(8):840–846. doi: 10.1038/embor.2011.121. [Acetaminophen-induced liver injury shown to be mediated in part by Sirt3 deacetylase activity.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giralt A, Villarroya F. SIRT3, a pivotal actor in mitochondrial functions: metabolism, cell death and aging. Biochem J. 2012;444(1):1–10. doi: 10.1042/BJ20120030. [DOI] [PubMed] [Google Scholar]
- 38.Anderson EJ, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119(3):573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]