Abstract
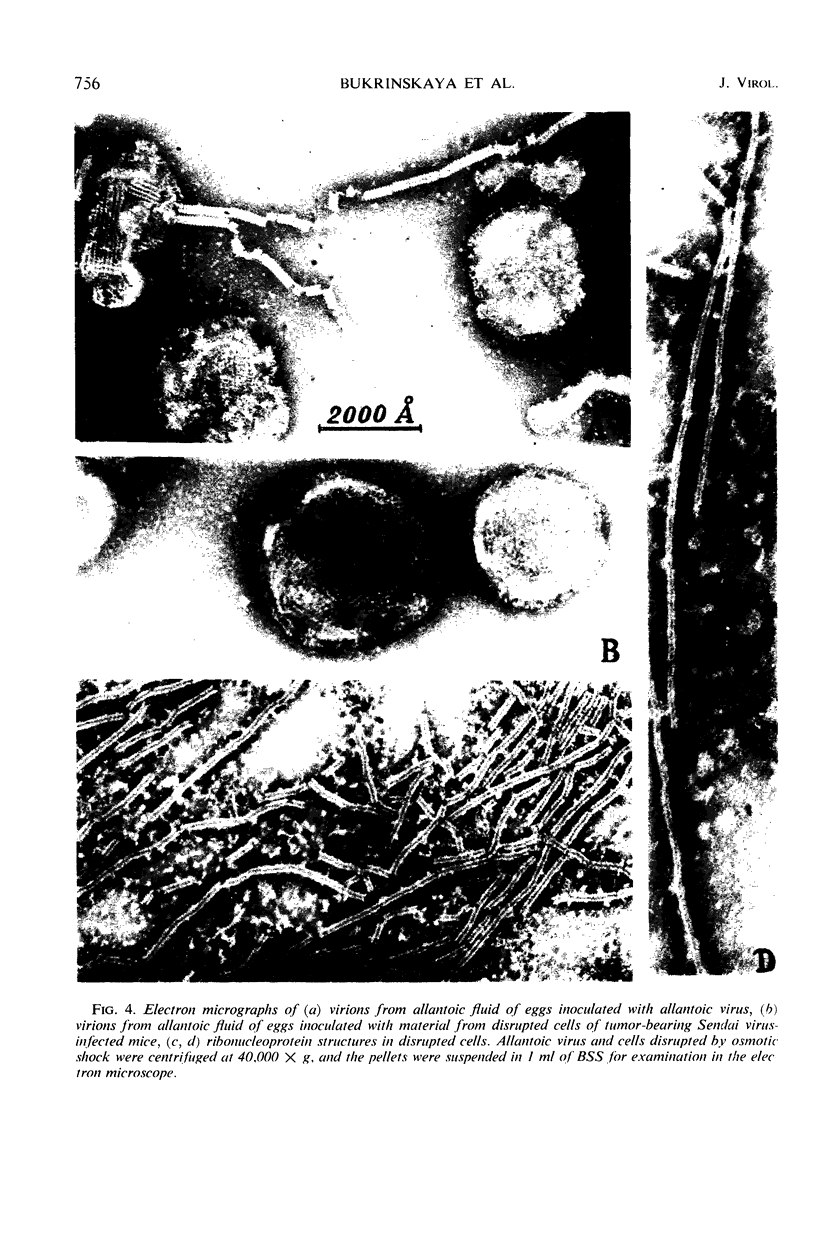
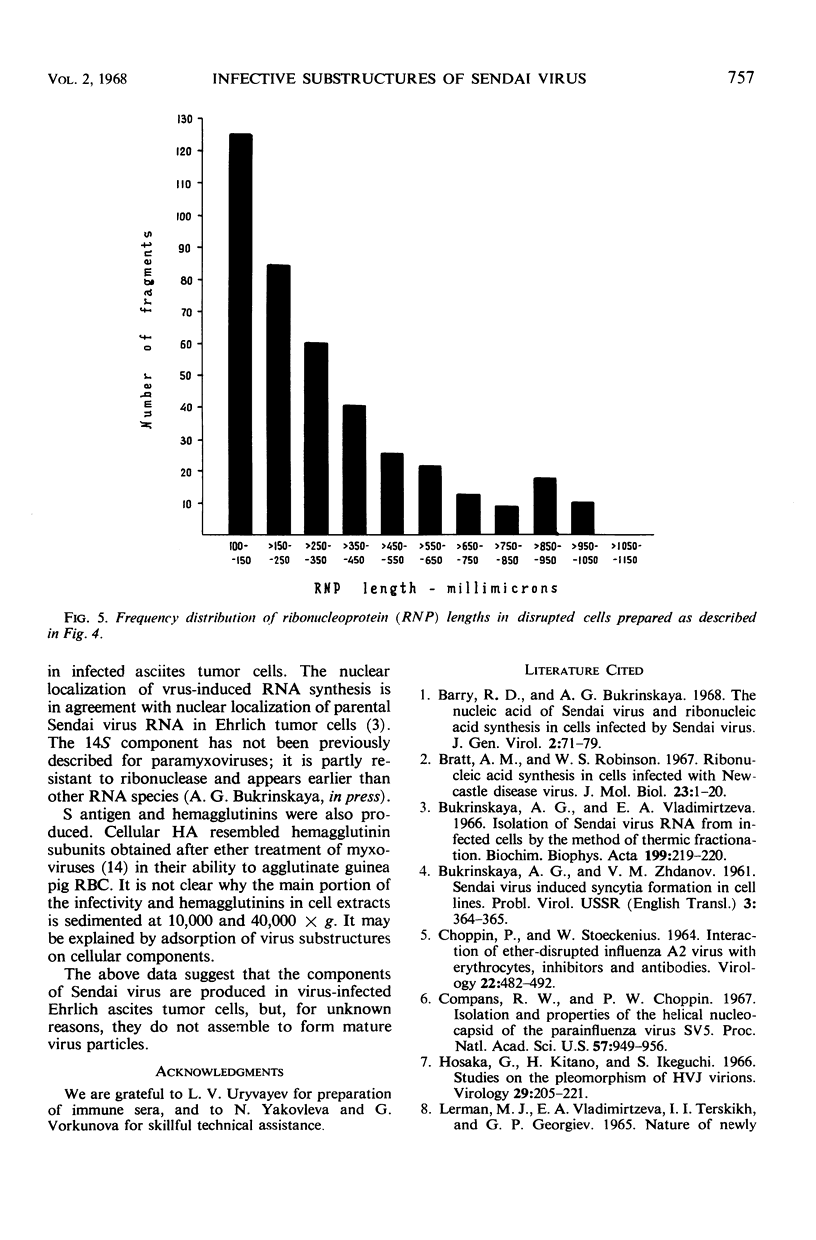
Increase of infectivity for embryonated eggs was observed in Ehrlich ascites tumor cells after intraperitoneal inoculation of Sendai virus into tumor-bearing mice. Virus-induced actinomycin-resistant ribonucleic acid consisting of 14S, 18S, 22S, 35S, and 48S was synthesized, and S antigen was produced in infected cells. The infectivity was suggested to be due to viral ribonucleoprotein for the following reasons: (i) the infectivity was unaffected by V antiserum but was abolished by whole hyperimmune serum, (ii) the infectivity was resistant to ribonuclease, (iii) virus particles were found neither in cells nor on red blood cell stroma treated with cellular extracts, (iv) structures similar to Sendai virus ribonucleoprotein with a maximal length of 10,500 A were observed in cellular extracts.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry R. D., Bukrinskaya A. G. The nucleic acid of Sendai virus and ribonucleic acid synthesis in cells infected by Sendai virus. J Gen Virol. 1968 Jan;2(1):71–79. doi: 10.1099/0022-1317-2-1-71. [DOI] [PubMed] [Google Scholar]
- Bukrinskaya A. G., Vladimirzeva E. A. Isolation of Sendai virus RNA from infected cells by the method of thermic fractionation. Biochim Biophys Acta. 1966 Apr 18;119(1):219–220. doi: 10.1016/0005-2787(66)90059-1. [DOI] [PubMed] [Google Scholar]
- CHOPPIN P. W., STOECKENIUS W. INTERACTIONS OF ETHER-DISRUPTED INFLUENZA A2 VIRUS WITH ERYTHROCYTES, INHIBITORS, AND ANTIBODIES. Virology. 1964 Apr;22:482–492. doi: 10.1016/0042-6822(64)90069-8. [DOI] [PubMed] [Google Scholar]
- Compans R. W., Choppin P. W. Isolation and properties of the helical nucleocapsid of the parainfluenza virus SV5. Proc Natl Acad Sci U S A. 1967 Apr;57(4):949–956. doi: 10.1073/pnas.57.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENLE W., LIEF F. S. Studies on the soluble antigen of influenza virus. I. The release of S antigen from elementary bodies by treatment with ether. Virology. 1956 Dec;2(6):753–771. doi: 10.1016/0042-6822(56)90056-3. [DOI] [PubMed] [Google Scholar]
- Hosaka Y., Kitano H., Ikeguchi S. Studies on the pleomorphism of HVJ virons. Virology. 1966 Jun;29(2):205–221. doi: 10.1016/0042-6822(66)90027-4. [DOI] [PubMed] [Google Scholar]
- OKADA Y. Analysis of giant polynuclear cell formation caused by HVJ virus from Ehrlich's ascites tumor cells. I. Microscopic observation of giant polynuclear cell formation. Exp Cell Res. 1962 Feb;26:98–107. doi: 10.1016/0014-4827(62)90205-7. [DOI] [PubMed] [Google Scholar]
- OKADA Y., TADOKORO J. Analysis of giant polynuclear cell formation caused by HVJ virus from Ehrlich's ascites tumor cells. II. Quantitative analysis of giant polynuclear cell formation. Exp Cell Res. 1962 Feb;26:108–118. doi: 10.1016/0014-4827(62)90206-9. [DOI] [PubMed] [Google Scholar]
- OKADA Y., TADOKORO J. THE DISTRIBUTION OF CELL FUSION CAPACITY AMONG SEVERAL CELL STRAINS OR CELLS CAUSED BY HVJ. Exp Cell Res. 1963 Dec;32:417–430. doi: 10.1016/0014-4827(63)90182-4. [DOI] [PubMed] [Google Scholar]