Abstract
Our study indicated the relationship between tumor length and clinicopathologic characteristics as well as long-term survival in esophageal cancer. A total of 116 patients who underwent curative surgery for thoracic esophageal cancer with standard lymphadenectomy in 2 fields between 2000 and 2010 were included in the study. The medical records of these patients were retrospectively reviewed. The patients with tumor length ≥3 cm had a highly significant difference in the involvement of adventitia and lymph node stations. The patients with tumor length ≤3 cm had significantly lower rates of involvement of the adventitia and lymph node stations. Tumor length could have a significant impact on both the overall survival and disease-free survival of patients with resected esophageal carcinomas and may provide additional prognostic value to the current tumor, node, and metastasis staging system before patients receive any cancer-specific treatment.
Keywords: Tumor length, Esophageal cancer, Prognostic factor
Esophageal cancer is one of the most mortal malignancies worldwide. Most patients with esophageal cancer die within 1 year of diagnosis; overall, 5-year survival chances are only 8% to 20%.1 Esophageal carcinomas are diagnosed in the advanced stages. Improvements in the early detection of esophageal cancer, used in the preoperative term of accurate staging modalities, selection of appropriate treatment for patients, and optimal selection of surgical candidates, may improve the overall survival rates.
In the newly published seventh edition of the American Joint Cancer Committee (AJCC) tumor, node, metastasis (TNM) staging system, histologic grading and tumor location, as well as depth of esophageal wall invasion, are regarded as the prognostic factors for esophageal cancer. The influence of tumor length has not been comprehensively evaluated on new TNM classification that changed the T and N factors.
Previously and recently, published results indicated that tumor length was an independent predictor of survival in esophageal cancer.2–5 The present study aims to investigate the impact of tumor length as an independent prognostic factor for the survival of patients with esophageal cancer.
Patients and Methods
All patients who underwent esophagectomy with a curative purpose for invasive esophageal squamous cell carcinoma (ESCC) and adenocarcinoma of the esophagus between 2000 and 2010 were included. The medical records of these patients were reviewed retrospectively.
Patients who had received neoadjuvant chemoradiotherapy after a noncurative resection and patients who died in the hospital in the early postoperative term were excluded. All patients were evaluated by means of barium meal, esophagogastroscopy, and computed tomography (CT) of the chest and abdomen for preoperative staging. Some patients received positron emission tomography–CT (PET-CT). Pulmonary and cardiac function studies and other blood tests were also done in order to assess surgical tolerance.
Surgical procedures
Patients were combined with mediastinal and intraperitoneal lymph node (LN) dissection with intrathoracic esophagus tumors (n = 116), and they underwent esophagectomy via a right-sided transthoracic esophagectomy, midline laparotomy.
Tumor grading and histopathologic assessment
Histopathologic examination included the evaluation of tumor length, histologic grade, and tumor depth invasion (T factor), with a number of positive and dissected LN stations. Tumors were classified according to the seventh editions of the Union for International Cancer Committee–AJCC staging systems. Tumor recurrence was classified as locoregional, distant, or both locoregional and distant. Locoregional recurrence was described as the recurrence at an anastomotic site or within the area of previous resection. Distant recurrence was described to be hematogenous metastasis to solid organs or recurrence in another body cavity.
Follow-up
Patients were observed every 3 months for the first 2 years after surgery, then decreasing to 6-month intervals for 5 years or until death. Survival time was considered as the number of days from the patient's date of surgery to the date of death and recurrence.
Statistical analysis
Survival probability was calculated by the Kaplan-Meier method. The prognostic impact of clinicopathologic characteristics and the difference between survival curves were assessed by the log-rank test. Statistical analysis was performed with SPSS 20 for Windows (SPSS Inc, Chicago, Illinois). Univariate analysis was performed by the Kaplan-Meier test. Multivariate analysis of survival was performed using then Cox regression model to identify prognostic factors. The level of significance was established as P < 0.05.
Results
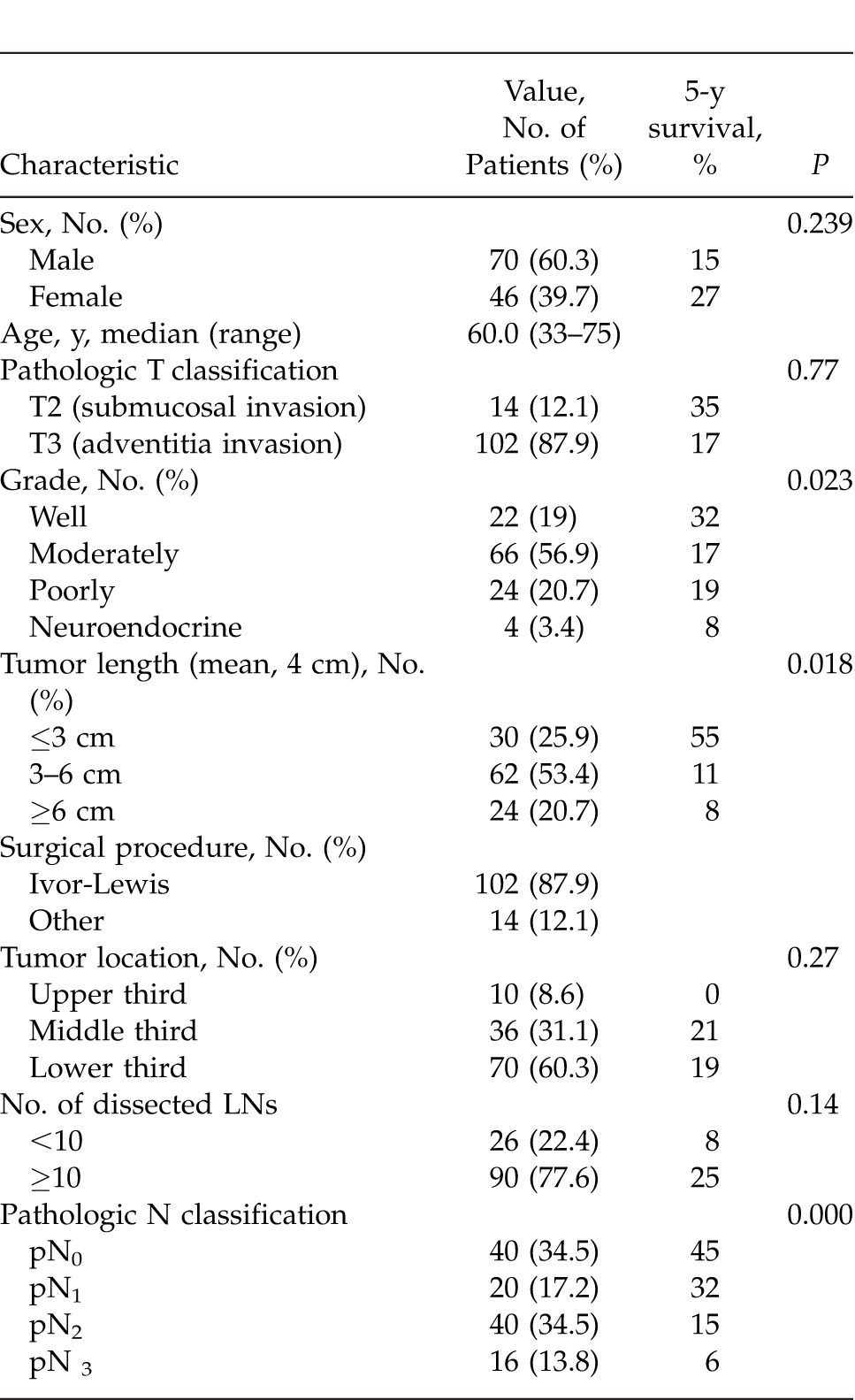
A total of 116 esophageal carcinoma patients receiving primary R0 (tumor-free surgical margin) surgical resection between 2000 and 2010 were considered. There were 116 patients (70 men and 46 women), with a median age of 60 years (range, 36–81 years). Characteristics and overall survival rates of patients are summarized in Table 1.
Table 1 .
Patient demographic data and result of univariate survival analysis
There were 102 patients who had undergone Ivor-Lewis esophageal resection. The pathologic study results showed that adenocarcinoma was found in 48 patients (41.4%), and ESCC was found in 68 patients (58.6%).
The distribution stages were according to the seventh edition of the AJCC TNM classification: stage IIA (n = 31; 26.7%), stage IIB (n = 10; 8.6%), stage IIIA (n = 21; 18.1%), stage IIIB (n = 36; 31%), and stage IIIC (n = 18; 15.5%). Median follow-up time was 39.7 months.
At follow-up, 24 patients were alive (including 2 patients with recurrent cancer), 83 patients had died from the original cancer, and 9 patients died from different causes without any evidence of tumor recurrence. The pattern of recurrence included locoregional in 30 patients, distant in 40 patients, and both locoregional and distant in 6 patients.
The overall survival rate was 20.7%. There was no significant difference in the overall survival between the male and female sexes.
No significant difference was observed at overall survival between pT2 and pT3 in comparison with pathologic tumor invasion depth (pT).
Among the 116 patients, 76 had LN metastasis. The mean number of LNs dissected was 16.33. The median number of dissected nodes per patient was 14.5 (range, –43). There was no statistical difference in view of the disease-free survival time and common causes related to the number of dissected nodes (<10 and >10). There was no significant difference in the overall and disease-free survival times between patients with >10 and <10 removed LNs.
There was a significant difference in survival rates between patients with pN1 and pN2 nodes and those with pN0 had a different survival than patients with pN1/pN2 and pN3 nodes.
Histologic differentiation and cancer location made no significant difference in patient survival rate.
Dependence of patient survival rate on tumor length
The median tumor length was 4 cm (range, 1–10 cm). The tumor lengths of patients were divided into 3 subgroups: ≤3 cm, 3 to 6 cm, and ≥6 cm. There was a significant difference in the disease-free survival and overall survival rates between patients with tumors ≤3 cm and patients with tumors ≥6 cm and 3 to 6 cm (log-rank, P = 0.010; Fig. 1).
Fig. 1 .
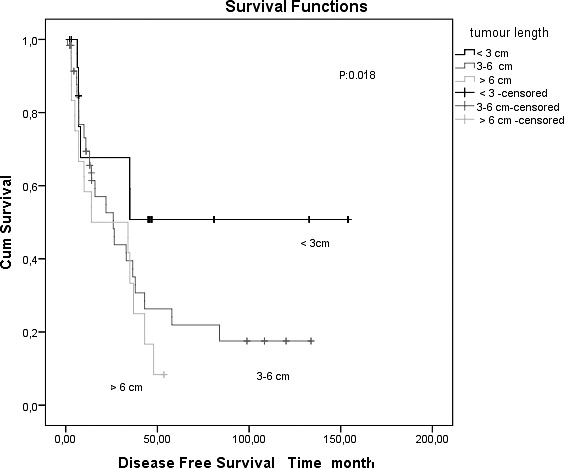
Kaplan-Meier survival curves in patients with resected esophageal carcinomas, comparing tumor length 3 cm or less and greater than 3 cm.
The overall 1-, 3-, and 5-year survival rates of patients with tumor length ≤3 cm were 68%, 51%, and 51%, respectively. The overall 1-, 3-, and 5-year survival rates of patients with tumor length >3 cm were 54%, 29%, and 11%, respectively. The survival rates were determined between tumor lengths ≤3 cm, 3 to 6 cm, and ≥6 cm, and they worsened with the increase in tumor length (Table 1).
The disease-free survival rates were found to be highly significantly different between the 3 subgroups according to tumor length (P = 0.018). Patients with tumor length ≤3 cm had significantly better survival rates.
The relationship between involved adventitia and tumor length
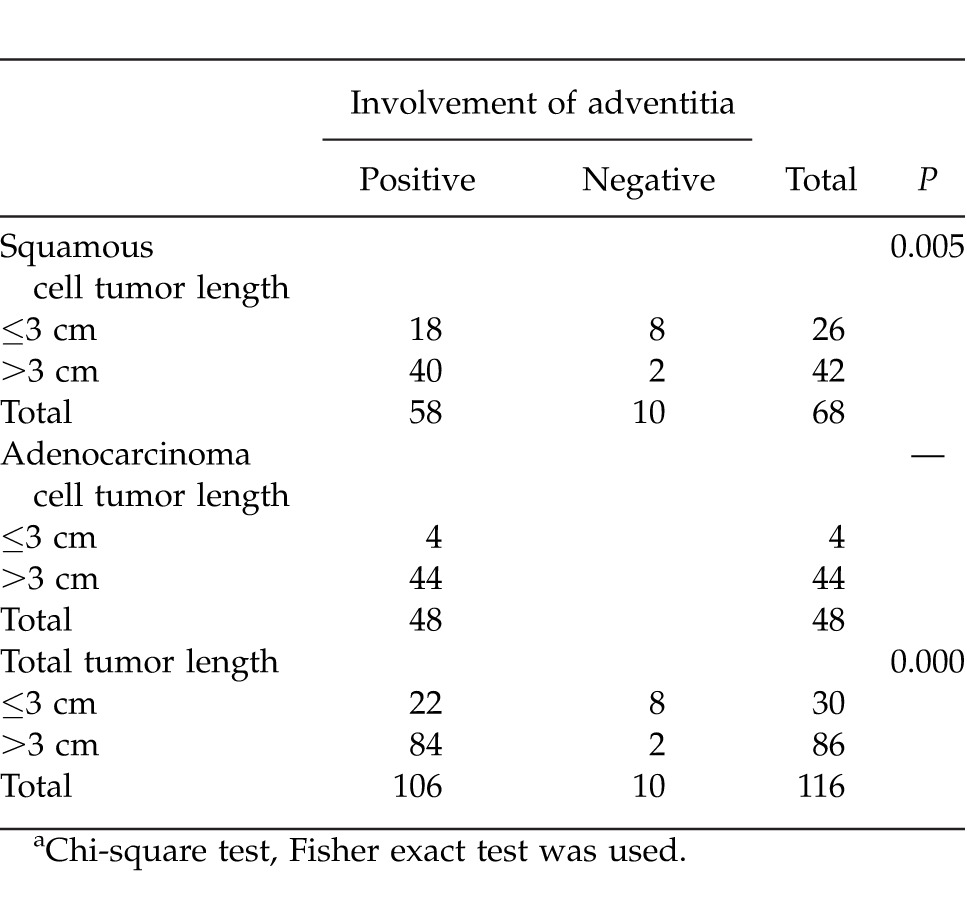
The adventitia invasion was T3 (involved adventitia) in 102 patients and T2 in 14 patients. The positive correlation was observed between adventitia involvement and tumor length >3 cm by χ2 cross-tabulation analyses (2-sided Fisher exact test, P = 0.005).
The adventitia invasion rates in patients with tumor length ≤3 cm were much lower than those of patients with tumor length >3 cm. The median tumor length was 4 cm in patients with involved adventitia and 2.6 cm in patients with adventitia that was not involved.
The relationship between tumor length and involved LNs
The median number of metastatic-positive LNs was 2 (range, 0–17). The relationship between tumor length and number of involved LNs was evaluated using the Kruskal-Wallis analysis method.
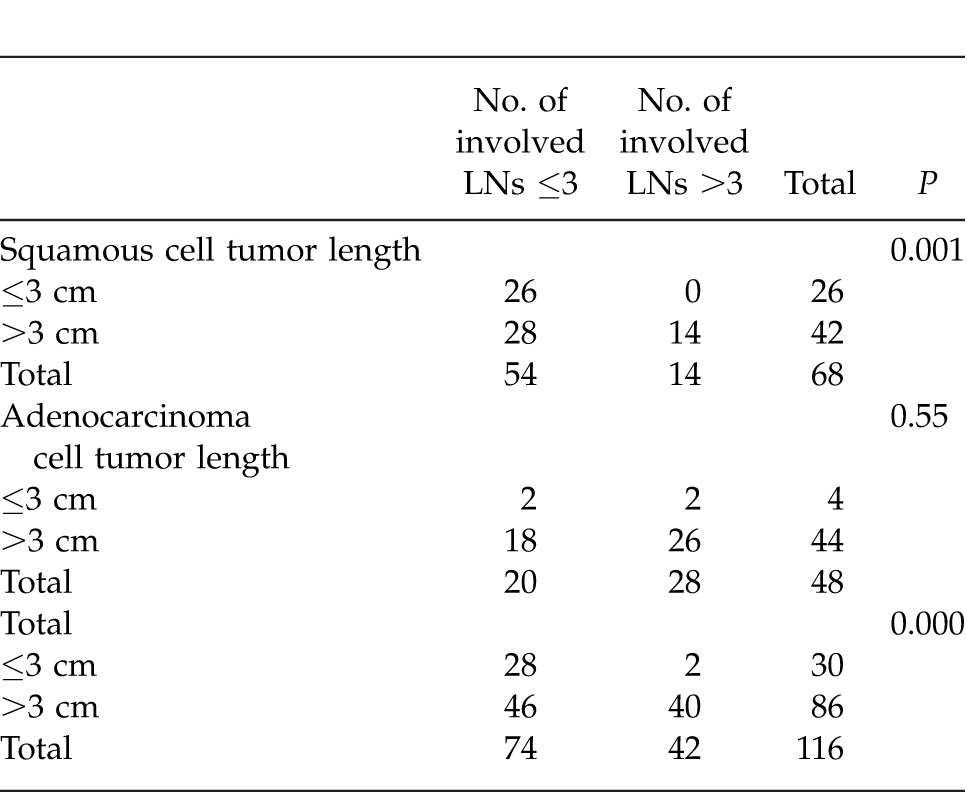
The involved LN rates in patients with tumor lengths ≤3 cm, 3 to 6 cm, and ≥6 cm were found to be significantly different (P < 0.001). The involved LN rate in patients with tumor length ≤3 cm was much lower than those of patients with tumor lengths 3 to 6 cm and ≥6 cm (P < 0.001). No significant difference was present in involved LN rates between 3 to 6 cm and ≥6 cm.
The prognostic influence of tumor length on disease-free survival
Multivariate analyses were performed using the Cox proportional hazards model, including sex, differentiation, dissected LNs, tumor location, and tumor length. Results indicated that the differentiation and tumor length were significant risk factors (Table 2). Patients with tumor length 3 to 6 cm had a worse prognosis compared with those patients with tumor length <3 cm. The disease-free survival rate in patients with tumor length 3 to 6 cm was fourfold worse than that of patients with tumor length ≤3 cm.
Table 2 .
Relationship between tumor depth of esophageal wall invasion and tumor length in patient with squamous cell and adenocell esophageal cancera
Discussion
Esophageal carcinoma is one of the most mortal malignancies worldwide. An effective and reasonable staging system of esophageal cancer is the essential precondition to assessing the appropriate treatments and predicted long-term survival.
Before 1987, esophageal tumor length ≤5 cm was categorized as T1 status and >5 cm as T2 status by the 1983 AJCC TNM staging system. Tumor length was replaced with depth of the esophageal wall invasion in the 1987 version of the AJCC TNM staging system.6 This may have been due to the revised TNM staging system, in which tumor length has been omitted since 1987 as a staging criterion.
The latest edition of the AJCC TNM staging system of esophageal cancer was published in 2009, in which adenocarcinoma and ESCC have been staged as 2 different types. Histologic grade, depth of tumor invasion, and the number of metastatic LNs are all independent staging factors for esophageal cancer.7,8
However, the prognostic role of tumor length in patients with esophageal cancer was ESCC, which is not mentioned.
Esophageal cancer is an extremely aggressive neoplasm that has been increasing in incidence in recent years. The overall survival rate of patients with esophageal cancer is <10% at 5 years9 because most patients with esophageal carcinomas are in an advanced stage of diagnosis.
Most patients will not be eligible for potentially curative resection at the time of presentation because of the unsatisfactory survival results with single-surgical approach or single-chemoradiotherapy modality, such as single-therapy modalities, especially clinical-stage T3.
Patients in the early stage of disease benefit from surgical approaches, whereas patients assigned to an intermediate stage can benefit from neoadjuvant therapy followed by restaging and surgical resection with curative intent. It is increasingly becoming standard care to offer neoadjuvant therapy modalities to patients with clinical-stage T3 or N1 esophagus cancer.10,11
Accurate staging of patients is important in view of treatment modalities in both the preoperative and postoperative terms. The newly diagnosed esophageal cancer patients should undergo a standardized staging routine before receiving operative or nonoperative therapy. In most institutions, noninvasive preoperative staging modalities are benefiting from endoscopic ultrasonography and PET-CT. PET-CT has low accuracy for staging locoregional disease, particularly in terms of the nodal status.12 Endoscopic ultrasonography is deemed as the most accurate technique for locoregional staging of invasive esophageal cancer, which remains weak in evaluating nodal status.13 However, the above-mentioned technologic and diagnostic modalities are not available everywhere.
The tumor length can be used as a prognostic factor to predict survival and reflect the disease stage, especially in ESCC. Several studies have evaluated the influence of tumor length on prognosis of esophageal carcinoma and reported a significant impact on the prognosis of both patients with adenocarcinoma and those with ESCC.2,4,5,13–16
The depth of tumor wall invasion is also a strong and independent prognostic factor for esophageal carcinomas. Based on the study results, tumor length was also a prognostic factor after controlling the factor of depth of the esophageal wall invasion. In our series, the adventitia invasion was T3 (involved adventitia) in 102 patients and T2 in 14 patients. Positive correlation was observed between adventitia involvement and tumor length >3 cm by χ2 cross-tabulation analyses (2-sided Fisher exact test, P = 0.005). Tumor length as predictive value was significant for T2 and T3 status in ESCC.
In our series, the 1-, 3-, and 5-year survival rates of patients with tumor lengths ≤3 cm, 3 to 6 cm, and ≥6 cm are displayed in the “Results” section. The survival rates of patients with tumor length ≤3 cm were significantly higher than those of the other 2 groups, especially in ESCC (Fig. 1 and Tables 1 and 3). A significant difference was detected at the 3-cm cutoff point on T status. Griffiths et al3 reported that patients with pathologic tumor length ≥3.5 cm had a poorer prognosis than those patients with shorter tumors after esophagectomy for cancer. This value for the cutoff point was similar to those in several previously reported results. It can be used as the cutoff value for further analyses.
Table 3 .
Multivariate prognostic analyses of disease-free survivala
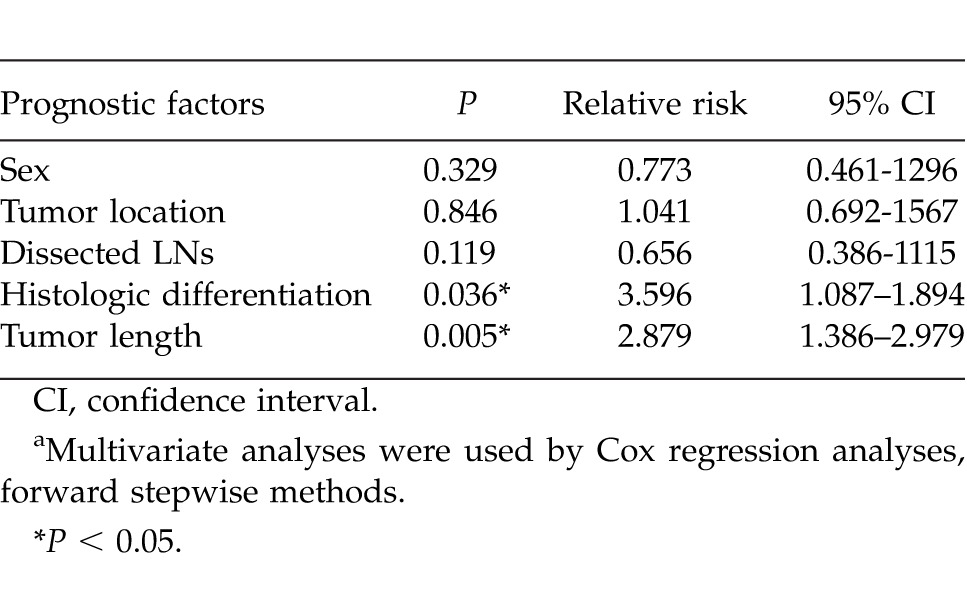
Several published studies indicated the impact of tumor length on long-term survival in patients with early-stage esophageal carcinoma.12,14,17 Our findings demonstrated that the predictive value of tumor length was significant for both T2 and T3 lesions in overall survival and disease-free survival. In our series, most patients were T3 according to the T status. Most patients were in the intermediate stage.
Eloubeidi et al2 has shown tumor length to be an independent prognostic factor in patients with esophageal cancer. Welch16 and Yendamuri et al.17 retrospectively evaluated 309 and 209 patients, and the authors concluded that tumor length was an independent predictor of survival for adenocarcinomas, excepting ESCC. Wu et al15 has suggested that tumor length serves as an independent predictor of long-term survival by the studies conducted on 582 patients with ESCC. Our findings demonstrated that tumor length is an independent prognostic factor for long-term survival according to the results for ESCC on multivariate analysis. But as a prognostic factor in our cohort, cancer location did not have any significant influence on survival in multivariate analysis.
LN status has been known to be a powerful independent prognostic factor for esophageal carcinomas. Gaur et al12 designed a clinical nomogram that consisted of a clinical tumor length to predict the presence of pathologic N and long-term survival difference in a small group of clinical T2 and N0 patients. Their results demonstrated that tumor length will be a good predictor in ESCC with N0. In our results, the involved LN rates in patients with tumor length ≤3 cm were much lower than those of patients with tumor length 3 to 6 cm and ≥6 cm in ESCC. Accordingly, tumor length has a significant impact on the survival of N0 and N1 patients. In order to assess the confounding effects of tumor length on N status, further prospective study is required.
Our findings demonstrated that patients with tumor length ≥3 cm had increasing adventitia involvement, which was in accordance with the metastatic LN ratio and poorer survival (Tables 2 and 4).
Table 4 .
Relationship between tumor length and number of involved LN≤3 and >3 in patient with squamous cell and adenocell esophageal cancer
Conclusion
The strength of the seventh edition of the AJCC TNM staging system is the new descriptors for the “N” and “M” classifications. Esophageal tumor length ≥3 cm was significantly associated with increasing tumor stage, worse lymph stage, increasing metastatic LN ratio, increasing overall TNM stage, and poor survival. The results of this study showed the requirement of consideration of tumor length as a prognostic grouping factor in ESCC.
According to the results, the measurement of tumor length could have additional advantages over the measurement of pathologic tumor depth invasion in predicting prognosis and treatment at preoperative, intraoperative, and postoperative terms. To confirm the prognostic role of tumor length in overall survival of ESCC patients, a prospective randomized study is necessary.
Moreover, the significance of adding tumor length for validation to be an additional criterion in the current TNM esophageal staging system can be suggested.
References
- 1.Lightdale CJ. Esophageal cancer. Am J Gastroenterol. 1999;94(1):20–29. doi: 10.1111/j.1572-0241.1999.00767.x. [DOI] [PubMed] [Google Scholar]
- 2.Eloubeidi MA, Desmond R, Arguedas MR, Reed CE, Wilcox CM. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S.: the importance of tumor length and lymph node status. Cancer. 2002;95(7):1434–1443. doi: 10.1002/cncr.10868. [DOI] [PubMed] [Google Scholar]
- 3.Griffiths EA, Brummell Z, Gorthi G, Pritchard SA, Welch IM. Tumor length as a prognostic factor in esophageal malignancy: univariate and multivariate survival analyses. J Surg Oncol. 2006;93(4):258–267. doi: 10.1002/jso.20449. [DOI] [PubMed] [Google Scholar]
- 4.Wu N, Pang LW, Chen ZM, Ma QY, Chen G. Tumour length is an independent prognostic factor of esophageal squamous cell carcinomas. Chin Med J. 2012;125(24):4445–4448. [PubMed] [Google Scholar]
- 5.Wang BY, Liu CY, Lin CH, Hsu PK, Hsu WH, Wu YC. Endoscopic tumor length is an independent prognostic factor in esophageal squamous cell carcinoma. Ann Surg Oncol. 2012;19(7):2149–2158. doi: 10.1245/s10434-012-2273-y. [DOI] [PubMed] [Google Scholar]
- 6.Beahrs OH, Myers MH. American Joint Committee on Cancer Manual for Staging of Cancer. 2nd ed. Philadelphia, PA: JB Lippincott;; 1983. eds. [Google Scholar]
- 7.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumors. 7th ed. Oxford, UK: Wiley-Blackwell Publishing Ltd;; 2010. eds. [Google Scholar]
- 8.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. American Joint Committee on Cancer (AJCC) Cancer Staging Manual. Chicago, IL: Springer Inc;; 2010. eds. [Google Scholar]
- 9.Parkin MD, Bray F, Ferlay J, Pisani P. Global cancer statistics. CA Cancer J Clin. 2002;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 10.Pennathur A, Luketich JD, Landreneau RJ, Ward J, Chiristie NA, Gibson MK, et al. Long-term results of a phase II trial of neoadjuvant chemotherapy followed by esophagectomy for locally advanced esophageal neoplasm. Ann Thorac Surg. 2008;85(6):1930–1936. doi: 10.1016/j.athoracsur.2008.01.097. discussion 1936–1937. [DOI] [PubMed] [Google Scholar]
- 11.Donignton JS, Miller DL, Allen MS, Deschamps C, Nichols FC, III, Pairolero PC. Tumor response to induction chemoradiation: influence on survival after esophagectomy. Eur J Cardiothorac Surg. 2003;24(4):631–636. doi: 10.1016/s1010-7940(03)00397-x. discussion 636–637. [DOI] [PubMed] [Google Scholar]
- 12.Gaur P, Sepesi P, Hofstetter WL, Correa AM, Bhutani MS, Vaporciyan AA, et al. A clinical nomogram predicting pathologic lymph node involvement in esophageal cancer patients. Ann Surg. 2010;252(4):611–617. doi: 10.1097/SLA.0b013e3181f56419. [DOI] [PubMed] [Google Scholar]
- 13.Luketich JD, Schauer P, Landreneau R, Nguyen N, Urso K, Ferson P, et al. Minimally invasive surgical staging is superior to endoscopic ultrasound in detecting lymph node metastases in esophageal cancer. J Thorac Cardiovasc Surg. 1997;114(5):817–823. doi: 10.1016/S0022-5223(97)70086-2. [DOI] [PubMed] [Google Scholar]
- 14.Bolton WD, Hofstetter WL, Francis AM, Correa AM, Ajani JA, Bhutani MS, et al. Impact of tumor length on long-term survival of pT1 esophageal adenocarcinoma. J Thorac Cardiovasc Surg. 2009;138(4):831–836. doi: 10.1016/j.jtcvs.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Wu YC, Wang BY, Goan YG, Hsu PK, Hsu WH. Tumor length as a prognostic factor in esophageal squamous cell carcinoma. Ann Thorac Surg. 2011;91(3):887–893. doi: 10.1016/j.athoracsur.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Welch IM, Griffiths EA, Brummell Z, Gorthi G, Pritchard SA. Tumor length as a prognostic factor in esophageal malignancy: univariate and multivariate survival analyses. J Surg Oncol. 2006;93(4):258–267. doi: 10.1002/jso.20449. [DOI] [PubMed] [Google Scholar]
- 17.Yendamuri S, Swisher SG, Correa AM, Hofstetter W, Ajani JA, Francis A. Esophageal tumor length is independently associated with long-term survival. Cancer. 2009;115(3):508–516. doi: 10.1002/cncr.24062. [DOI] [PubMed] [Google Scholar]


