Abstract
The development of highly potent chiral discrimination methods that solve the problems of the diastereomer method, in which it is impossible to discriminate the diastereomers having chiral centers separated by more than four bonds, is described. On the basis of the results obtained, a new hypothesis, Induced Chiral Fields that the achiral reversed phase can provide chiral fields depending on the structures of the eluents, is proposed to explain the significant results of separation of the diastereomers derived from newly developed chiral and fluorescent labeling reagents and optical isomers by reversed-phase HPLC, which was hitherto impossible.
Keywords: chiral discrimination, helically chiral diastereomer, Induced Chiral Fields, reversed-phase HPLC, fluorescent and chiral labeling reagent, modified Mosher’s reagent
Introduction
Since the discovery of enantiomerism by L. Pasteur in 1848,1) discrimination of optical isomers has been among the major subjects in science.
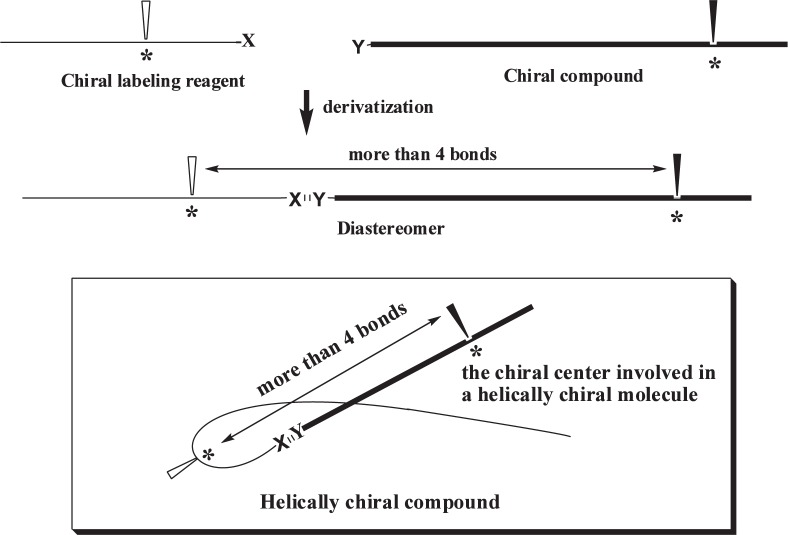
The most reliable and widely used method for the discrimination of optical isomers is the diastereomer method. However, the diastereomer method has a problem in that it is impossible to discriminate diastereomers having chiral centers separated by more than four bonds. The problem has been assumed to be intrinsic to the diastereomer method and is very difficult to solve.
The following hypothesis has been proposed as a solution to the problem: if diastereomers were provided with a helically chiral conformation by the chirality of the chiral labeling reagent, the other chiral center in the diastereomers would be involved in the helically chiral molecule (no longer the chiral center remote from the other chiral center) and, therefore, would be discriminated by some means (Fig.1).2)–4)
Fig. 1.
A solution of the problem by helically chiral diastereomers.
A study to examine the validity of the hypothesis has been pursued. On the basis of the results obtained, a new hypothesis, “Induced Chiral Fields namely that the achiral reversed phase can provide chiral fields depending on the substrates,” is proposed to explain the significant results of separation of the diastereomers derived from new chiral labeling reagents and optical isomers by reversed-phase HPLC, which was hitherto impossible.
Results and discussion
1. Design of fluorescent and chiral labeling reagents for the discrimination of chiral carboxylic acids that provide diastereomers with a helically chiral conformation as the preferred one by the gauche effect and the ability of the reagents for chiral discrimination
2-(Anthracene-2,3-dicarboximido)propanol (1) and 1-(anthacene2,3-dicarboximi-do)-2-propanol (2) and their O-triflates (3, 4) were designed as fluorescent and chiral labeling reagents for chiral carboxylic acids (Fig. 2)3),4); it was expected that the preferred conformation of their esters would be the helically chiral gauche-trans (gt) as the result of the gauche effect5) between the oxygen atom of ester and the nitrogen atom of imido group and the gt conformation could be further stabilized by CH-πinteraction (Fig. 3).6) The anthracenedicarboximido group is for both highly sensitive fluorometry and anisotropy for 1H-NMR study (Fig. 2).
Fig. 2.

Fluorescent and chiral labeling reagents that are expected to provide helically chiral diastereomers by the gauche effect.
Fig. 3.
Expected preferred conformation of the esters of the fluorescent and chiral labeling reagents.
Chiral carboxylic acids can be labeled with the reagent 1–4 to give diastereomers, for example 5 in Fig. 3. The preferred conformation of 5 will be gt among the three possible conformations due to the gauche effect between the ester oxygen atom and the imido nitrogen atom. The gt conformer is a helically chiral molecule. Thus, the diastereomer could be provided with a helical chiral conformation by the chirality of the labeling reagent, and the other chiral center in the diastereomer originated from the chiral carboxylic acid is the one involved in the helically chiral molecule (no longer the chirality remote from the other chiral center irrespectively of the distance between the two chiral centers) and therefore could be discriminated by some means (Figs. 1 and 3).
The enantiomers of anteiso fatty acids (C4–C13) were separated by means of reversed-phase HPLC and detected at the femto (10−15) mole level by fluorometry. There is a rule in the elution order between (R) and (S)-configuration of branched methyl groups, namely, that the diastereomers of (S)-reagents with (S)-fatty acids having a chiral center at an even number carbon will elute faster than the diastereomers of (S)-reagents with (R)-fatty acids and that the elution order will change with those at an odd number carbon.3),4)
However, the enantiomers of C14 anteiso fatty acid (12-methyltetradecanoic acid) could not be separated as the diastereomers of these reagents by HPLC. Therefore, the HPLC discrimination of higher anteiso fatty acids was not studied.
The chirality of hydroxyl carboxylic acids could also be discriminated by labeling with these reagents and normal-phase HPLC. The labeling reaction of hydroxyl carboxylic acids with O-triflate reagents (3, 4) can be performed in an SN2 manner using tetraethyl ammonium carbonate (TEAC) as the base in CH3CN or DMF at room temperature without the formation of either intra- or intermolecular esters of substrates. It should be noted that the labeling with 4 proceeds with complete inversion of the stereochemistry of 4 (Fig. 3).
Esters and lactones are labeled with 3 or 4 in one pot, first by treatment of them with TEAC in MeOH and then by evaporation of MeOH followed by treatment with 3 or 4 in CH3CN (or DMF), and the diastereomers are separated by means of normal-phase HPLC. For example, all four stereoisomers of beraprost sodium7) and 3-hydroxy-4-methyloctanoic acid8) (Fig. 4) were separated by labeling with 4.
Fig. 4.

Structures of hydroxycarboxylic acids that have plural asymmetric centers.
On the other hand, by means of 1H-NMR, the (R)- and (S)-stereochemistries of the branched methyl groups of methyl-branched carboxylic acids can be discriminated up to C11-methyl branching, which indicates that the anisotropy of the anthracene ring of these reagents can reach up to the C11-methyl group (Fig. 5).3),4)
Fig. 5.
Side view of the gauche-trans conformer of the ester of a methyl branched long chain carboxylic acid with a fluorescent and chiral labeling reagent.
The conformational analysis of p-methoxycinnamate of 1 by both exciton CD and 1H-NMR studies showed that the proportion of gauche-trans : trans-gauche : gauche-gauche conformations was ca 77 : 21 : 2 (Fig. 6 and Table 1).8)–10)
Fig. 6.
CD spectrum of (S)-2-(2,3-anthracenedicarboximido) propyl p-methoxycinnnamate and the vicinal coupling constants of protons the reagent.
Table 1.
The population of gt, tg, and gg by 1H-NMR

The results suggested that the chiral labeling reagent that provides the helically chiral gauche conformer in 100% will give better chiral discrimination.
2. Design of the fluorescent and chiral labeling reagents that provide diastereomers with a single helically chiral gauche conformer in 100% and ability of the reagents for chiral discrimination
The gt conformation of the diastereomers derived from reagents 1,2,3, and 4 by the gauche effect could be fixed by forming a ring as shown in Fig. 7. In this way, the fluorescent and chiral labeling reagents 6 for chiral carboxylic acid and 7 for chiral alcohol were prepared.11),12) It should be noted that the gauche effect is not necessary for either 6 or 7 to provide helically chiral gauche diastereomers.
Fig. 7.
Cyclohexane reagents that have a fixed chiral gauche conformation.
Enantiomers of anteiso fatty acids (C4–C28) were separated by C30 reversed-phase HPLC as the diastereomers labeled with 6.11),12) There is a rule regarding the elution order, namely, that the elution order of the fatty acids having a methyl group at C4–C11 is the same as that of diastereomers with the reagents 1, 2, 3, 4, but the elution order will change at the fatty acid having a methyl group at C12.12) A similar turning point in the elution order was observed at C10 with the diastereomers derived from (1R, 2R)-naphtharene-2′,3′-dicarboximidocyclohexanol, which is one benzene ring smaller than 6, and methyl branched fatty acids.13)
The change of the elution order indicated that the chiral discrimination mechanism will be different for diastereomers having the branched methyl group over the anthracene ring and those having the branched methyl group beyond the anthracene ring.
It should be noted that ODS (C18) can separate the enantiomers up to C20 anteiso fatty acid (18-methylicosanoic acid) but not the enantiomers of higher anteiso fatty acids.12) These results suggested that the chain length of the reversed phase played an important role in the discrimination and that the methylene chains of the diastereomers and those of the reversed-phases fully interacted with each other.
Reagent 6 can discriminate the four stereoisomers of 4,8,12-trimethyltridecanoic acid (8),4) 2,6-dimethyloctane-1,8-dioic acid (9),15) and cyclo-propanecarboxylic acid (10).15) However, 6 can not completely discriminate all eight stereoisomers of 4,8,12,16-tetramethylheptadecanoic acid (11).4)
Fig. 8.
Strucures of chiral carboxylic acids.
In this case as well, up to the C11-branched methyl group of fatty acid can be discriminated by means of 1H-NMR as the diastereomers derived from 6.
Enantiomers of anteiso fatty alcohols (C4–C28) (12) were separated by C30-reversed-phase HPLC as the diastereomers derived from 7.11)–13)
The enantiomers of chiral secondary alcohols up to C30 with methylene chains that are different by only one carbons at the asymmetric carbon (13),16) secondary alcohols with a branched methyl group on any carbon of the shorter methylene chain of 13 (14),16) and all four stereoisomers of secondary alcohols with a branched methyl group (15)17) could be discriminated by labeling with 7 and reversed-phase HPLC. However, 7 cannot completely discriminate all eight stereoisomers of 4,8,12,16-tetramethylheptadecanol (16). The structures of 12, 13, 14, 15, and 16, and their representative HPLC chromatograms are shown in Figs. 9 and 10.
Fig. 9.
Strucures of chiral alcohols.
Fig. 10.

Representative HPLC chromatograms of (1R,2R)-2-(anthracene-2,3-dicarboximido)cyclohexane carboxylic acid derivatives of chiral secondary alcohols.
One of the characteristics of the anthracenedicarboximido reagents is that they give crystalline derivatives that are suitable for X-ray studies. The X-ray structure of the (1R, 2R)-7 ester of (S)-11-docosanol (17) is shown in Fig. 11. In Fig. 11, the shorter methylene chain of 17 is laid over the anthracenedicarboximido group to show that the preferred conformation (the 1,3-syn relationship between the carbonyl oxygen and hydrogen of the secondary alcohol and s-trans between the carbonyl oxygen and the α-hydrogen on the cyclohexane ring) continues to have a crystalline structure.
Fig. 11.

X-ray structure of (R)-11-docosanoyl (1S,2S)-2-(anthracene-2,3-dicarboximido)cyclohexane carboxylate.
The reagent 7 could act as a modified Mosher’s reagent18) for 1H-NMR study but could be used for a longer distance than a Mosher reagent. The 1H-NMR spectra of the 7-tridecanol ester of (1R, 2R)-7 is shown in Fig. 12. The signals of the terminal methyl groups appeared at different positions that were more shielded than those of 7-tridecanol. The results indicated that both methyl groups have a chance to come over the anthraceneimido group by rotation. Judging from the preferred conformation, the more shielded methyl signal could be assigned to that of the pro-S methylene chain. The assignment was confirmed by the N.O.E. between the methyl group and the protons of the anthracene ring (Fig. 12).
Fig. 12.
N.O.E between the protons of the terminal methyl group and those of anthrace ring of (R)-7-tridecanoyl (1R,2R)-2-(anthracene-2,3-dicarboximido)cyclohexane carboxylate.
The 1H-NMR spectra of the (S)-5-undecanol ester of (1R, 2R)-7 (18) are shown in Fig. 13. The terminal methyl groups have different chemical shifts that are more shielded than those of (S)-5-undecanol. Judging from the preferred conformation of 18, the more shielded methyl signal could be assigned to that of the C5-terminal. The assignment was confirmed by the study of the HOHAHA spectrum of 18 conducted by Bax and Davis.18) Thus, the methyl signal at 0.52 ppm, which appeared faster than the other one by irradiation of the signal of alcohol methine proton at 4.75 ppm, was assigned to the methyl group of shorter methylene chain.
Fig. 13.
HOHAHA spectrum of (S)-6-dodecanoyl (1R,2R)-2-(anthracene-2,3-dicarboximido)cyclohexane carboxylate by irradiation of the alcohol proton.
The 1H-NMR spectra of the (1R, 2R)-7 amide of (R)- and (S)-2-heptylamine (19) are shown in Fig. 14. In this case as well, the spectra showed that the preferred conformation of 19 is the 1,3-syn relation between the carbonyl oxygen and the methine hydrogen of the amine and the s-trans between the carbonyl oxygen and the α-hydrogen on the cyclohehane ring, which is similar to that of the 7-esters of secondary alcohols. Thus, the absolute configuration of chiral amines can be determined by the 1H-NMR of their 7-amides.20)
Fig. 14.
1H-NMR spectra of (S)-heptyl (1R, 2R)-2-(anthracene-2,3-dicarboximido)cyclohexane carboxamide (A) and its (R)-isomer (B).
Thus, 7 is a very useful reagent for chiral discrimination by 1H-NMR.
3. Design of sugar labeling reagents
It is known that 1,3,4,6-tetra-O-acetyl-2-deoxy-2-phthalimido-β-D-gluopyranose (20) gives β-glycoside selectively by Lewis-acid catalyzed glycosidation of alcohols.21)
In order to examine the effect of the polarity of the labeling reagents on the reversed-phase HPLC separation, 1,3,4,6-tetra-O-acetyl-2-deoxy-2-(anthracene-2,3-dicarboximido)-β-D-glucopyranose (21), its O-benzoyl (22), and the O-methyl analog (23) were prepared as fluorescent and chiral labeling reagents (Fig. 15).22)
Fig. 15.

Structures of 1,3,4,6-tetra-O-acetyl-2-deoxy-2-phthalimido-β-D-glucopyranose (20) and sugar reagents.
The HPLC chromatograms of the 4,8,12,16-tetrametylheptadecanoyl glycosides of 21, 22, and 23, together with that of the 16 ester of 7, are shown in Fig. 16. The sugar regents 21 and 23 can discriminate all eight stereoisomers of 16, but 7 cannot.
Fig. 16.
HPLC chromatograms of the diastereomers of 4,8,12,16-tetramethyl heptadecanol labeled with the cyclohexane reagent and sugar reagents.
The most remarkable difference between 7 and the sugar reagents is their polarity. Thus, the difference in their ability for HPLC separation could be explained as follows.
The non-polar diastereomers derived from 7 and 16 will interact with the methylene chains of the reversed phase in two ways, as shown in Fig. 17; one is cyclohexane part of the molecule directed to the mobile phase, and the other is the cyclohexane part directed to silica gel (upside down). The mechanism of chiral discrimination between the two ways will be different. Therefore, good separation of diastereomers cannot be attained.
Fig. 17.
Difference of the mode of interaction with the reversed phase between derivatives labeled with the nonpolar cyclohexane reagent and those labeled with polar sugar reagent.
On the other hand, the polar diastereomers derived from sugar reagents and 16 will interact with the reversed phase mostly in a way in which the polar sugar part is directed to the mobile phase. Thus, the more orderly interaction of the polar diastereomer with the reversed phase will result in the separation of all eight diastereomers.
In addition, it should be noted that 21 can separate all eight stereoisomers of α-tocopherol (Fig. 18).23)
Fig. 18.
HPLC chromatogram of stereoisomers of α-tocopherol labeled with 1,3,4,6-tetra-O-acetyl-2-anthracene-2′,3′-dicarboximido-2-deoxy-β-D-glucopyranose.
4. Proposal of Induced Chiral Fields
The separation of the diastereomers by reversed-phade HPLC can usually be explained by the difference of the three-dimentional shapes between diastereomers.
The separation of diastereomers up to C11 methyl branched anteiso-carboxylic acids could be understood to be in this category.
However, it is very difficult to identify the difference between the shapes of the diastereomers derived from 7 and those of the enantiomers of C29-anteiso fatty acid well enough to achieve separation by reversed-phase HPLC. Furthermore, the results described above suggest that the methylene chains of the diastereomer and those of the reversed-phase fully interact with each other. Taking these results into account and in order to attract the interest of scientific interest toward the mechanism of the separation, I would like to propose here a hypothesis, “Induced Chiral Fields,” to explain the significant results of separation of diastereomers by reversed-phase HPLC.
The hypothesis of Induced Chiral Fields proposes that the interaction of large helically chiral molecules having a long methylene chain, such as the diastereomer derived from 7 and C29-anteiso fatty acids, and an anthracenecarboximido group with the methylene chains of reversed-phase chirally bends or twists the methylene chains of the reversed-phase to create the appearance of new chiral fields. (Fig. 19). The rate at which the diastereomers move from the chiral fields to the achiral methylene chains of the reversed-phase to create new Induced Chiral Fields is different in the (R)- and (S)-stereochemistry of the branched methyl groups in the diastereomers. Thus, the diastereomers are separated by reversed-phase HPLC.
Fig. 19.
Induced Chiral Fields.
Furthermore, the Induced Chiral Fields hypothesis proposes that the achiral reversed phase could be the chiral phase depending on the structure of substrate.
Conclusion
Highly potent chiral discrimination methods for both 1H-NMR and HPLC that have solved the problems assumed to be intrinsic to the diastereomer method were developed, and a hypothesis to explain the significant separation of diastereomers by reversed phase was proposed.
I hope that the study presented in this paper is a contribution to the advance of chiral discrimination.
The work described in this paper was achieved through the remarkable effort of many researchers, whose names are listed in the references below.
References
- 1).Pasteur, L. (1848) Ann. Chim. Phys. 24, 442 [Google Scholar]
- 2).Akasaka, K., Ohrui, H. and Meguro, H. (1993) Analyst 118, 765–768 [Google Scholar]
- 3).Akasaka, K., Meguro, H. and Ohrui, H. (1997) Tetrahedron Lett. 6853–6856 [Google Scholar]
- 4).Ohrui, H. (2004) BUNSEKI KAGAKU 53, 805–815 [Google Scholar]
- 5).Wolfe, S. (1972). Accounts. Chem. Res. 5, 102–111 [Google Scholar]
- 6).Nishio, M. and Morita, M. (1989) Tetrahedron 45, 7201–7245 [Google Scholar]
- 7).Akasaka, K., Ohrui, H., Meguro, H. and Umetsu, T. (1997) Anal. Sciences 13, 461–466 [Google Scholar]
- 8).Ohrui, H. (1998) J. Syn. Org. Chem. Jp. 56, 591 [Google Scholar]
- 9).Akasaka, K., Imaizumi, K. and Ohrui, H. (1999) BUNSEKI KAGAKU 48, 1085–1094 [Google Scholar]
- 10).Akasaka, K., Imaizumi, K. and Ohrui, H. (1998) Enantiomer 3, 169–174 [Google Scholar]
- 11).Ohrui, H., Terashima, H., Imaizumi, K. and Akasaka, K. (2002) Proc. Jpn. Acad., Ser. B 78, 69–72 [Google Scholar]
- 12).Akasaka, K. and Ohrui, H. (2004) Biosci. Biotechnol. Biochem. 68, 153–158 [DOI] [PubMed] [Google Scholar]
- 13).Imaizumi, K., Terashima, H., Akasaka, K. and Ohrui, H. (2003) Anal. Sciences 19, 1243–1249 [DOI] [PubMed] [Google Scholar]
- 14).Nakai, T., Yajima, A., Akasaka, K., Kaihoku, T., Ohtaki, M., Nukada, T., Ohrui, H. and Yabuta, G. (2005) Biosci. Biotechnol. Biochem. 69, 2401–2408 [DOI] [PubMed] [Google Scholar]
- 15).Tashiro, T., Akasaka, K., Ohrui, H., Fattorusso, E. and Mori, K. (2002) Eur. J. Org. Chem. 3659 [Google Scholar]
- 16).Ohtaki, T., Akasaka, K., Kabuto, C. and Ohrui, H. (2005) Chirality 17, S172–S176 [DOI] [PubMed] [Google Scholar]
- 17).Mori, K., Ohtaki, T., Ohrui, H., Berkebile, D.R. and Carlson, D.A. (2004) Eur. J. Org. Chem. 1089–1096 [Google Scholar]
- 18).Dale, J.A. and Mosher, H.S. (1973) J. Am. Chem. Soc. 95, 512–519 [Google Scholar]
- 19).Bax, A. and Davis, D.G. (1985) J. Magn. Reson. 65, 355–360 [Google Scholar]
- 20).Akasaka, K., Ohtaki, T., Kabuto, K., Kitahara, T. and Ohrui, H. (2005) Biosci. Biotechnol. Biochem. 69, 2002–2004 [DOI] [PubMed] [Google Scholar]
- 21).Lemieux, R.U., Annas, S.Z. and Chung, B.Y. (1982) Can. J. Chem. 60, 58–62 [Google Scholar]
- 22).Ohrui, H., Kato, R., Kodaira, T., Shimizu, H., Akasaka, K. and Kitahara, T. (2005) Biosci. Biotechnol. Biochem. 69, 1054–1057 [DOI] [PubMed] [Google Scholar]
- 23).Kodaira, T. (2006) Master thesis of Tohoku University [Google Scholar]