Abstract
Sexual diversity of ADG in Harderian gland of golden hamster was demonstrated on TLC. Female ADG contained iso- and anteiso-branched acyl and alkyl components, but male ADG contained only straight chain ones, which suggested the hormonal control of the expression of acyl-CoA dehydrogenases in the catabolism of BCAA. Acyl-CoA dehydrogenases were not expressed in the absence of testosterone, and then isovaleryl-CoA, 2-methylbutyryl-CoA, and isobutyryl-CoA accumulated, and acted as primers for the synthesis of iso- and anteiso-branched fatty acids. The incorporation of [U-14C] leucine into lipids was monitored by TLC. The cholesterol fraction was labeled in males but not in female, which means that cholesterol was not produced from BCAA in female gland due to the lack of expression of acyl-CoA dehydrogenases. We monitored the behavior of male hamsters toward female gland lipids, and found slightly greater attractiveness in female ones than that in male ones although the difference was not significant. Considering the lifestyle of golden hamster in nature, we propose a hypothesis that the lipids from the Harderian gland of golden hamster serve as a pheromone to declare their territory and to seek the mate with good congeniality.
Keywords: Harderian gland, golden hamster, sexual dimorphism, acyl-CoA dehydrogenase, androgen receptor, pheromone
Golden hamster was discovered in 1930 in Syria as a mother with twelve youngsters. All of the present golden hamsters in the world are the descendants of this family. Golden hamster is a solitary animal to live alone without making a group, and only come together for mating. It must be housed individually when kept as pets. They have a fixed action pattern including scent-marking, and a pheromone has been thought to deliver their message between individuals to mate. We isolated a lipid from the Harderian gland of golden hamster, and characterized the chemical nature of this lipid having sexual diversity, and also clarified the molecular basis of this character.
Discovery and definition of Harderian gland
In 1694 Swiss anatomist Johan Jakob Harder reported a gland under the name of “Glandula nova lachrymalis”, which was found in the orbit of fallow deer but was different from the nictitans gland.1) Same type of gland was discovered in many animals, not only in mammals but also in birds, reptiles, and amphibians. This gland is well developed in rodents (rat, mouse, golden hamster, Mongolian gerbil, guinea pig, etc.), lagomorphs (rabbit, pika) and cetaceans, and may have been lost secondarily in chiroptera, primates, terrestrial carnivores, peris sodactyls, etc. Later the ocular glands in the inner canthus of the eye became generally regarded as the Harderian gland, and the distinction between the new gland discovered by Harder and nictitans gland was forgotten. Nowadays the definition of Harderian gland is as follows.2) The mammalian Harderian gland is an ocular gland that has tubuloalveolar endopieces (tubular alveoli) and secretes lipids by a merocrine mechanism. It occupies a considerable part of the orbit and is located around the posterior half of the eyeball. Histologically this gland has a single layer of columnar epithelial cells surrounding the lumen and a single secretary duct opens near the third eyelid in the inner canthus, and it is covered with an endothelium of the well-developed orbital venous sinus.
Function of Harderian gland
Harderian gland is an exocrine gland, and has several functions as a source of lubrication and protection for the cornea by lipids, or photoprotective role by porphyrin.3) A new porphyrin, named harderoporphyrin, was isolated and characterized from the rat Harderian gland by Kennedy4) and subsequently shown to occur in the female hamster as well.5) A Harderian gland-derived polypeptide growth factor has been characterized by Yokoyama et al.6), 7) It might prove extremely insightful to examine porphyrins and the differentiation of the Harderian gland in a cell culture system. Using cultured cells of Harderian gland, we proved that the porphyrins and lipids were secreted separately from glandular cells, and that the lipid-porphyrin complex was formed in the glandular lumina.8)
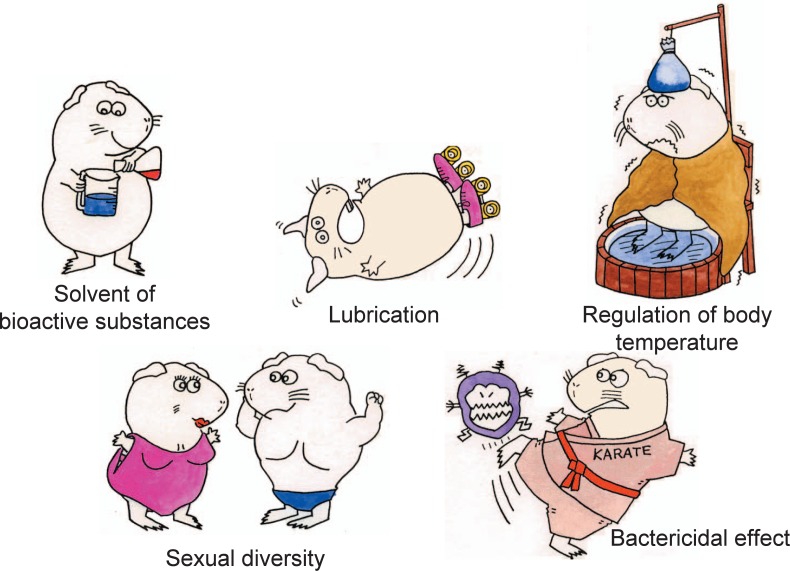
Harderian gland contains about 20% lipid by weight, and the main secretory product is lipids.7) As illustrated in Fig. 1,9) the excreted lipids from Harderian gland act as lubricants10), 11) and probably as the solvent for pheromones or other biologically active substances2), 12), 13). Yokoyama et al. purified a novel growth factor, which was also dissolved in the excretory lipids.6) Secretory lipids also serve a thermoregulation function in gerbils.14), 15) The lipids spread on the pelage act to insulate the animal against cold and wetness, and the lipids and pigments (porphyrins) darken the pelage and increase radiation absorption. Sandbathing removes excessive amount of pelage lipids, and lowers the body temperature.16) Also, removal of Harderian gland or fur lipids can result in an increased heat loss as well as water flux to and from the skin.17) Removal of Harderian materials from the pelage by sandbathing aids osmoregulation through lowering the rate of insensible perspiration. 18) Water and fat contents of Harderian gland were determined in the exophthalmic animals.19) An increase in water content was observed but no change occurred in the lipid content. In a transient exophthalmos induced by exposure to high-intensity illumination, the lipid content in the Harderian gland decreased significantly while the water content increased in accordance with the weight increase of this gland.20) In toads, Harderian gland of only females contained numerous lipid droplets.21) This finding suggested a pheromonal function, which might influence the sexual behavior of the male. A bactericidal effect has been attributed to unsaturated glycerin ethers in the rabbit Harderian gland.22)
Fig. 1.
Putative functions of golden hamster Harderian glands.
Sexual dimorphism of Harderian gland
Sexual dimorphism is one of the characteristics of the Harderian gland. This phenomenon was observed in several animals when we started the present project, but the golden hamster was the only species in which a true sexual dimorphism of the Harderian gland has been established. That was why we selected this animal as an experimental model for the elucidation of molecular basis of sex dimorphism in the Harderian gland.
Histochemical observations revealed a distinct sexual dimorphism of the Harderian gland of golden hamsters.23) Bucana et al. recognized several structural differences between the Harderian glands of male and female golden hamsters, the most characteristic being the presence of tubular clusters in the secretory cells of the male and the absence of these in the female. Woolley and Worley demonstrated that treatment with sex hormones or gonadectomy can reverse the sex difference in pigmentation, as well as vacuole size, of the hamster Harderian gland.24) Payne et al. showed that several naturally occurring androgens were able to prevent the transformation of the Harderian gland to the female type in castrated males.12)
Golden hamster as an experimental animal
The first description of the golden hamster was in 1833, and a stuffing of one body was made in 1839. Until 1930, it was assumed that the golden hamster was extinct as a species. Then, in 1930, near the town of Allepo in Syria, a Hebrew zoologist by the name of Israel Aharoni, came across a nest containing a small rodent with twelve babies that he could not identify. He carefully placed the family into a container and had them taken to the Hebrew University in Jerusalem. There they were identified as golden hamsters and, under laboratory conditions, encouraged to breed. As a result, all the golden hamsters, kept as pets in the world today, was descended from the mother and babies found in Syria back in 1930.25) It was never again been found in the wild.
Sexual diversity of lipids in the Harderian gland of golden hamster
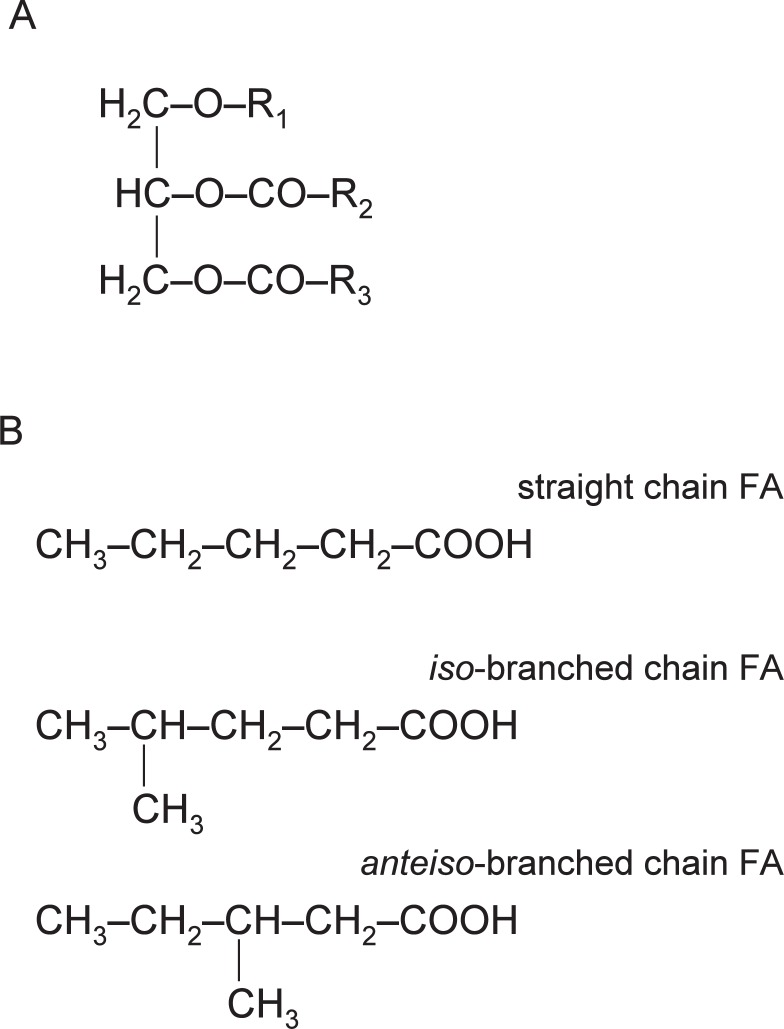
The total lipids in the Harderian gland accounted for about 20% of the fresh tissue. When the lipid components of the hamster Harderian gland were studied by Lin and Nadakavukaren,11) thin layer chromatography (TLC) findings suggested the presence of 1-alkyl-2,3-diacylglycerol (ADG) (Fig. 2). About 92% of the total lipid consisted of ADG, trace amounts of free cholesterol and phospholipids being also present.
Fig. 2.
Structures of 1-alkyl-2,3-diacylglycerol (ADG) and fatty acids in the golden hamster Harderian gland.
A, R1- is an alkyl residue; R2CO- and R3CO- are acyl residues. B, Fatty acids form ester linkages at the number 2 and 3 positions of glycerol, to make up the acyl portion of ADG. Fatty alcohols, formed from the corresponding fatty acid, form ether linkages at the number 1 position of glycerol, to make up the alkyl portion of ADG. Methyl branching residues at the (n-1) position in iso-branched chain fatty acids and the (n-2) position in anteiso-branched chain fatty acids.
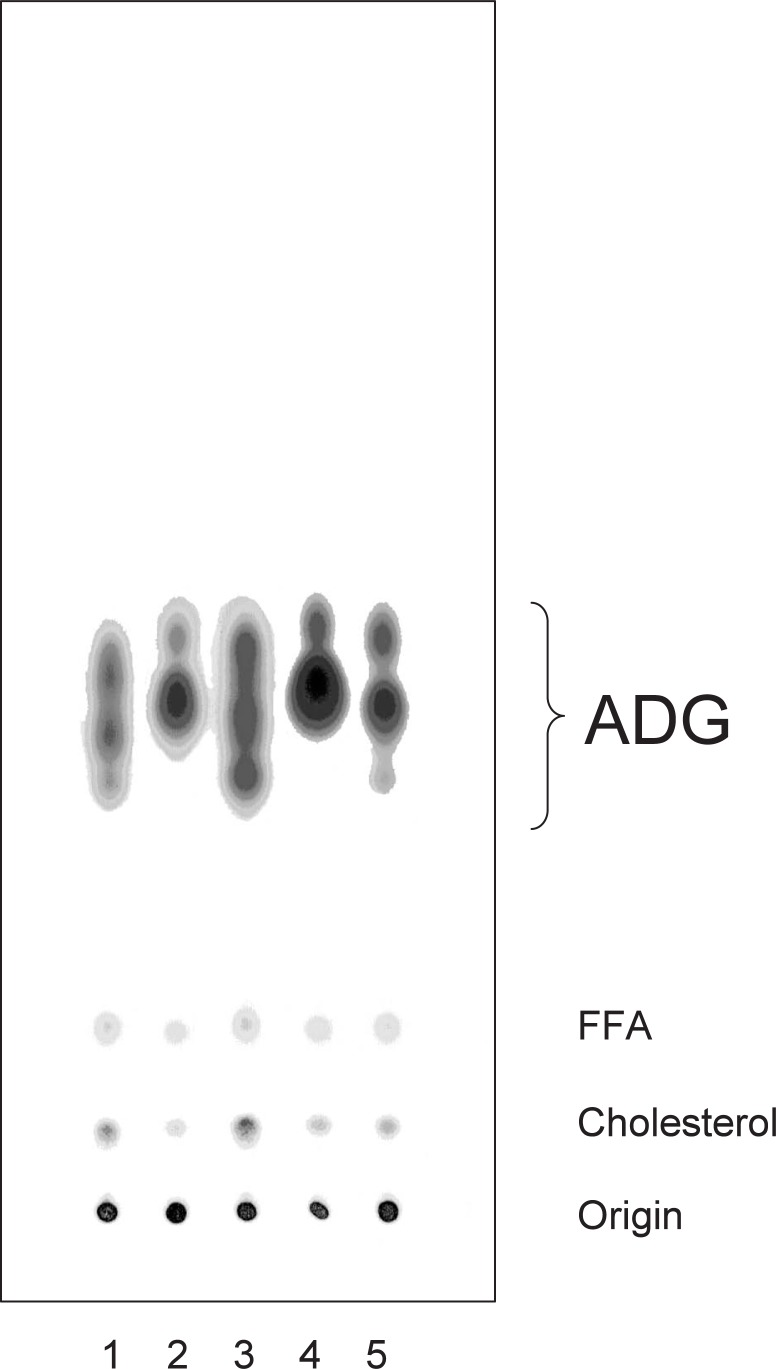
The diversity of ADG according to gender was clearly demonstrated on thin layer plates by us as shown in Fig. 3.26) ADG (F, lane 1) from females gave two subfractions, F-1 (lane 2) and F-2 (lane 3), and that from males (M, lane 4) was separated into three subfractions, M-1 (lane 5), M-2 (lane 6), and M-3 (lane 7).
Fig. 3.
Thin-layer chromatogram of lipid in Harderian glands of golden hamsters.
The Harderian glands of golden hamsters were obtained and they were kept overnight in 5 ml of chloroform/methanol (2:1, v/v) at room temperature. The solvents were evaporated off under N2 gas. Small portions of the lipids were dissolved in chloroform-methanol (2:1, v/v) and ADGs and their subfractions were prepared as described by Seyama et al. (1995). The lipid fractions were then spotted on a silica gel 60 plate (Merck, Darmstadt) and the plate was developed with hexane-diethyl ether-acetic acid (80:20:1, v/v/v). The spots were located by heating the plate at 120 °C after spraying it with 20% sulfuric acid. Samples: lane 1, crude lipid from glands of females (F); lane 2, purified female ADG (F-1); lane 3, purified female ADG (F-2); lane 4, crude lipid from glands of males (M); lane 5, purified male ADG (M-1); lane 6, purified male ADG (M-2); lane 7, purified male ADG (M-3). ADG, 1-alkyl-2,3-diacylglycerol; Chol, cholesterol; Orig, origin.
The fatty acid compositions of these lipids were first reported by Lin and Nadakavukaren in 1981 and showed significant sexual differences.11) The major fatty acids in glands of females were palmitic acid (C16:0) and oleic acid (C18:1), which accounted for more than 70%, whereas the lipids in glands of males had a greater proportion of fatty acids with a chain length ranging from C10 to C15. They suggested that the sexual difference of ADG in this gland comprised the presence and absence of unsaturated fatty acids. They analyzed fatty acids of ADG by gas liquid chromatography (GLC), but the identification has been questioned and incorrect as pointed out by Murawski et al. in 199127) who reported the presence of iso-branched fatty acids instead of unsaturated fatty acids. But they did not identify anteiso-branched fatty acids also present in ADG from female gland.
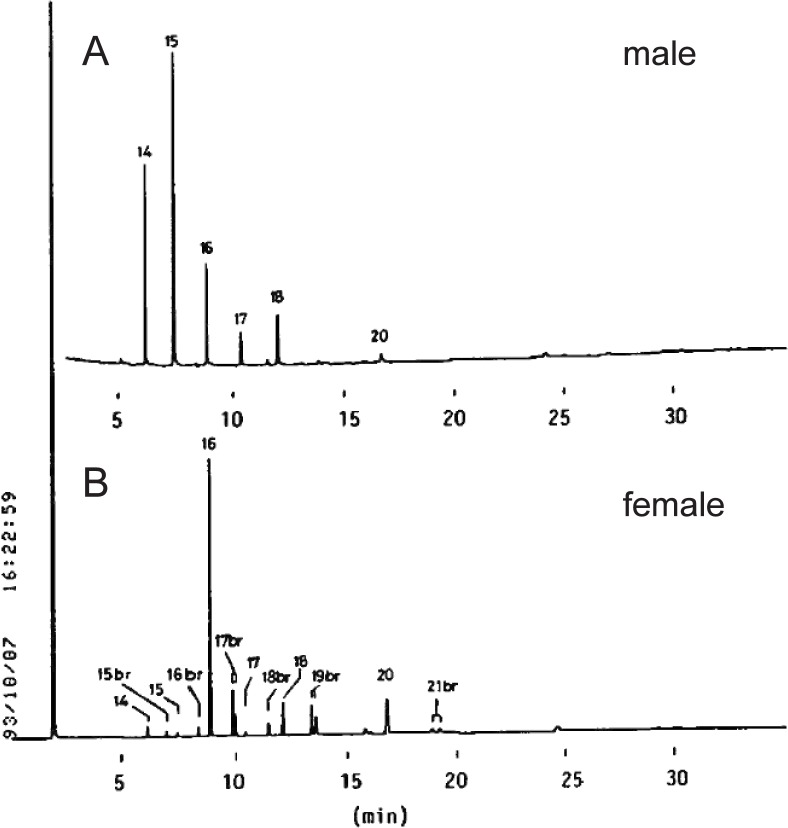
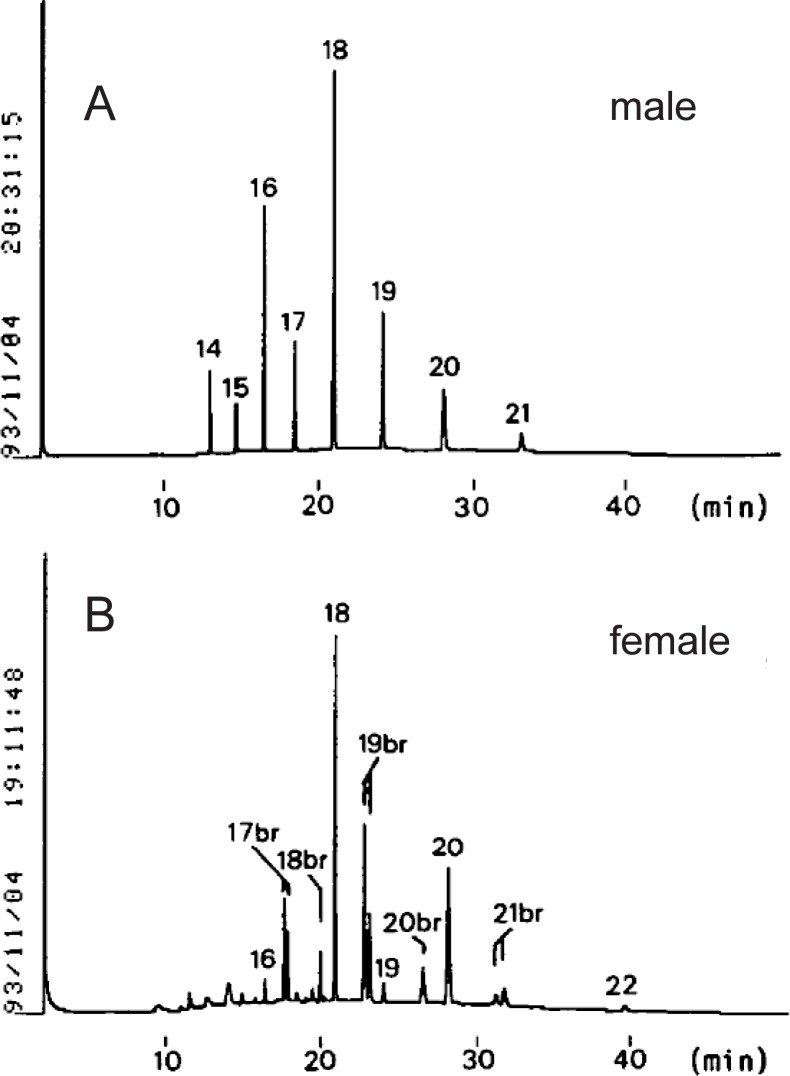
When we examined the fatty acid and alkyl compositions of ADG in the Harderian gland of golden hamster in 1995, we detected anteiso- in addition to iso-branched fatty acids and alkyl residues in the glands of female but not those of male. Unsaturated components were absent in both fatty acids and alkyl residue from male and female glands. The fatty acid composition of the male ADG (M) was simple, containing straight chain fatty acids ranging from 12 to 20, as shown in Fig. 4.26) Both even and odd-numbered acids were found, the major one being 15:0. On the other hand, the ADG from female contained 16:0 as a major component, together with a large amount of branched-chain acids ranging from 14 to 21. The branching resided mostly at the iso- and anteiso-positions. The iso-branched acids were eluted earlier than the corresponding anteiso-branched acids on GLC. Odd-numbered fatty acids (15:0, 17:0, 19:0, 21:0) had iso- and anteiso-branching, but even-numbered (16:0, 18:0) ones had only iso-branching.
Fig. 4.
Gas chromatogram of fatty acid methyl esters of ADG from Harderian glands of golden hamsters.
(A) M; (B) F. Samples were prepared as described by Seyama et al. (1995). Numbers (14–21) indicate the chain length of each peak; “14” means 14:0. The abbreviation, “br” means branched chain acid. Column: 0.28 mm × 25 m fused silica column coated with OV-1. The carrier gas was N2, at the flow rate of 1 ml/min. The column temperature was kept at 180 °C for 1 min and then increased to 250 °C at the rate of 5 °C/min.
Alkyl compositions were also analyzed by GLC, as isopropyridene derivatives, as shown in Fig. 5.26) The alkyl composition of the male ADG (M) contained straight aliphatic chains ranging from 14 to 21, both odd- and even-numbered, the major one being 18:0. On the other hand, the ADG from females had a large amount of iso- and anteiso-branching comprising almost half the alkyl residues (45%) (Fig. 5).26) Even-numbered alkyl residues contained iso-branching, and odd-numbered ones both iso- and anteiso-branching.
Fig. 5.
Gas chromatogram of isopropylidene derivatives of alkylglycerol derived from ADG.
(A) M; (B) F. Numbers (14–21) indicate the chain length of each peak; “14” means 14:0. The abbreviation, “br” means branched chain alkyl residue. Column: 0.28 mm × 25 m fused silica column coated with OV-1. The carrier gas was N2, at the flow rate of 1 ml/min. The column temperature was kept at 180 °C for 1 min and then increased to 250 °C at the rate of 5 °C/min.
These observations are of interest with regard to hormonal control of the expression of acyl-CoA dehydrogenases (isovaleryl-CoA dehydrogenase and 2-methyl-branched chain acyl-CoA dehydrogenase) in the catabolism of branched chain amino acids (BCAA); valine, leucine, and isoleucine.
Previously branched chain fatty acids were also detected in ADG from the Harderian gland of guinea pig.28) In this case, the alkyl and acyl moieties of ADG consisted of saturated aliphatic chains ranging from 14 to 21 carbon atoms and from 15 to 26 carbon atoms, respectively. About 60 mol% of them had methyl branches, which located at the even-numbered carbon atoms. Mono- and dimethyl branched chains accounted for most of the branched chains. The aliphatic chains contained methyl branches especially in the alkyl chains at the 1-position and the acyl chains at the 2-position. Iso type chains were not found. This is the major difference between the ADG from guinea pig Harderian gland and that from golden hamster ones. The fatty acid synthase from guinea pig Harderian gland produced many odd-numbered and methyl-branched fatty acids in the presence of methylmalonyl-CoA.29) Thus produced methyl branched fatty acids appeared in phospholipids of guinea pig Harderian gland.30), 31) It has been demonstrated that fatty acids with methyl branches at the even-numbered carbon atoms are synthesized by the condensation of methylmalonyl-CoA by fatty acid synthase preparations from the uropygial gland and the liver of geese,32), 33) the liver of rat33) and chicken,34) and the adipose tissue of sheep.34) So the mechanism to synthesize branched chain fatty acids is species specific.
Presence of propionic, hexanoic, and octanoic acids at the 3-position of ADG
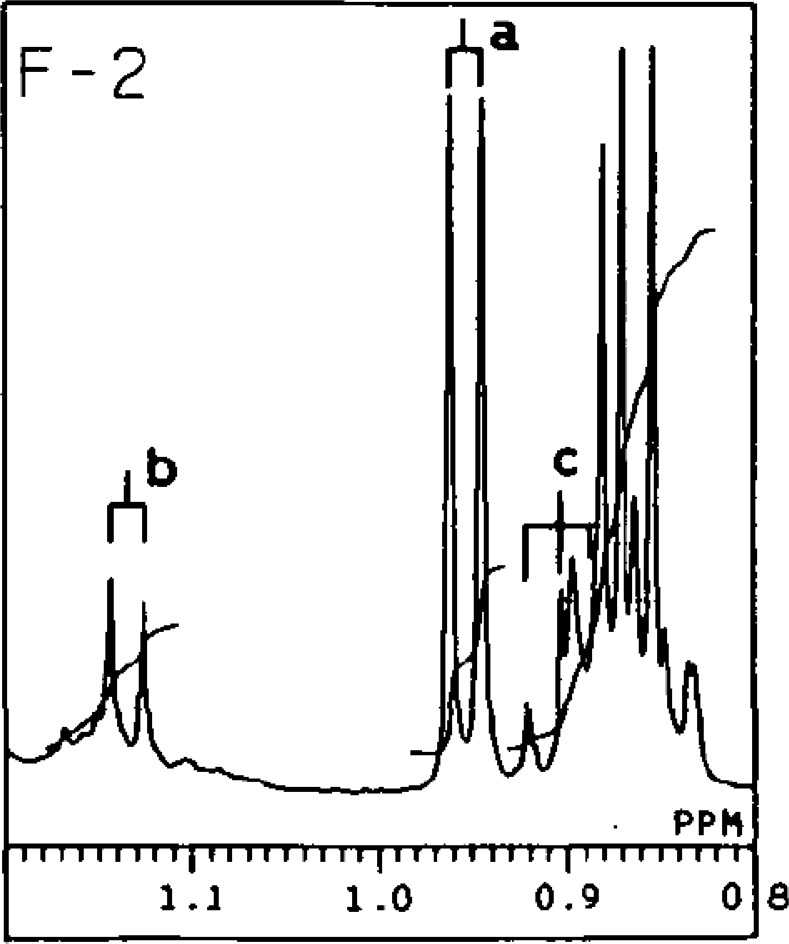
Although GLC of fatty acids is an established sensitive and reliable method, it is not suitable to detect short chain fatty acids. When a sample contains short chain fatty acids as in the present case (less than 8 carbon numbers), the fatty acid methyl esters will be lost during sample processing, such as during extraction and evaporation, or overlooked on gas chromatography. Nuclear magnetic resonance (NMR) spectral analysis of ADG provided an explanation as to the different behaviors of these five fractions on TLC (Fig. 3, lane 2, 3, 5, 6, 7). Propionic, hexanoic, and octanoic acids were esterified at the 3-position of M-3 (Fig. 3, lane 7). Such short chain fatty acids explain the different behavior on TLC of M-3, which was distinguishable from M-1 and M-2. The NMR spectra of intact ADG, F-2 in Fig. 6,26) for example, revealed the presence of isovaleric and 2-methylbutyric acids at the 3-position of the glycerol moiety. The large doublet signal (a) (δH 0.98, J = 7 Hz) in the 1H NMR spectrum was assigned to the methyl group of isovaleric acid. The small doublet (b) (δH 1.14, J = 7 Hz) and triplet (c) (δH 0.90, J = 7 Hz) were expected to be those of 2-methylbutyric acid.35) The proton signals in Fig. 626) suggested that the 3-position of F-2 was mainly substituted by isovaleric and 2-methylbutyric acids, both putative precursors in the biosynthesis of iso- and anteiso-type fatty acids.
Fig. 6.
1H NMR spectra of the methyl signal regions of intact ADG subfraction (F-2 in Fig. 3).
The 1H NMR spectrum was measured in a CDCl3 solution, with tetramethylsilane as an internal standard, with a GX-400 spectrometer (JEOL, Tokyo) at 400 MHz.
The presence of such short chain fatty acids also explains the different behaviors of F-1 and F-2 on TLC. These unusual components were not detectable on conventional GLC. We strongly recommend the use of both procedures (GLC and NMR) when examining a lipid which contains short chain components. As M-3 and F-2 contained short chain fatty acids which could not be determined by GLC, the GLC profile in Fig. 426) do not indicate the true fatty acid composition of these lipids. We are attempting to quantify such a wide range of acids, from C3 to C20 by above mentioned method. The NMR analysis indicated that the 3-position of glycerol in M-3 was occupied mostly by short chain acids. In the case of M-1, M-2, and F-1, the NMR spectra revealed that there were no short chain acids at this position.26)
Variety of primers in fatty acid synthesis in the Harderian gland
The fatty acid synthase requires primer and elongating C-2 unit for the activity. Usually former is malonyl-CoA and latter is acetyl-CoA, and the products are even-numbered straight chain fatty acids.36) The presence of even- and odd-numbered straight chain fatty acids in the male Harderian gland suggested the presence of acetyl-CoA (C2) and propionyl-CoA (C3) as primers of fatty acid synthesis in the cytosol. Even-numbered fatty acids were produced with the former primer (2+2n), and odd-numbered fatty acids with the latter primer (3+2n). In the case of the female ADG, even-numbered (4+2n) iso-branched acids, and odd-numbered (5+2n) iso- and anteiso-branched acids were detected, suggesting the presence of isobutyryl-CoA (C4), isovaleryl-CoA (C5), and 2-methylbutyryl-CoA (C5) as primers, respectively. These CoAs are intermediates of the BCAA, valine, leucine, and isoleucine, respectively. iso- and anteiso-branched fatty acids were isolated and characterized by Weitkamp,37)i.e., skin surface lipids of man,38) dog,39) sheep,40) and rodent.41) These branched chain acids are derived from the BCAA, valine, leucine, and isoleucine.42)
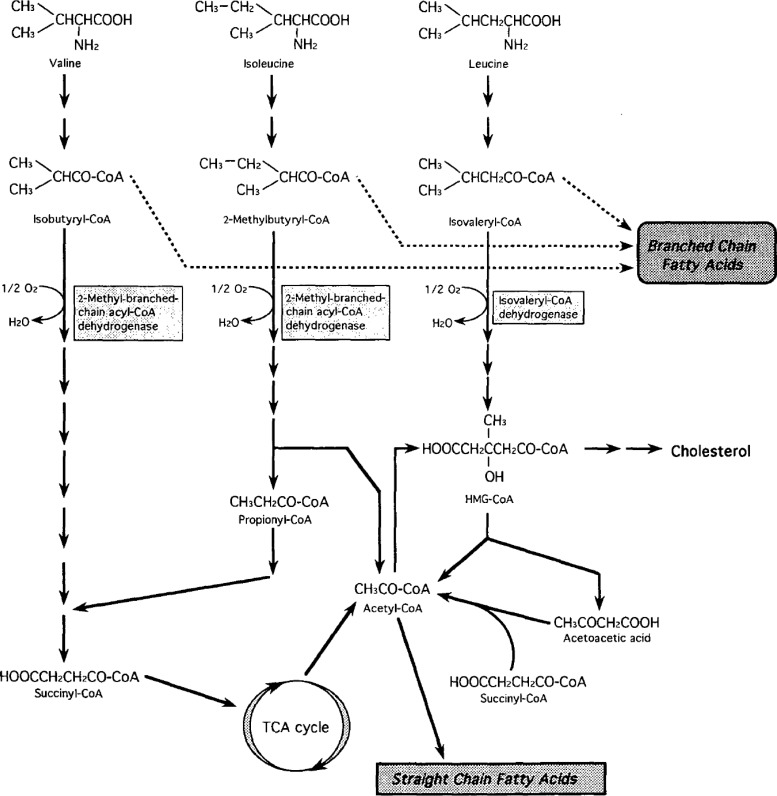
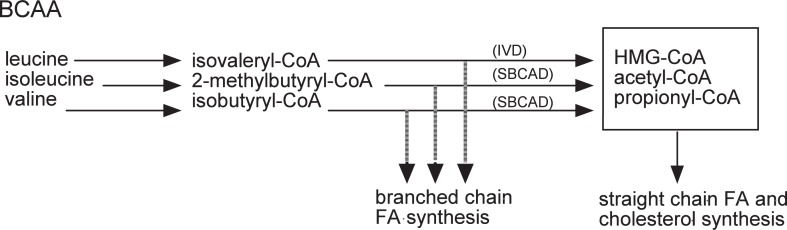
In the catabolism of these amino acids, isobutyryl-CoA from valine and 2-methylbutyryl-CoA from isoleucine are catabolized further by 2-methyl-branched-chain acyl-CoA dehydrogenase, and isovaleryl-CoA from leucine is catabolized by isovaleryl-CoA dehydrogenase43) (Fig. 7).26) When these enzymes are inactive, the accumulated short chain acyl CoA serves as a primer for the production of even-numbered iso-acids from isobutyryl-CoA, odd-numbered iso-acids from isovaleryl-CoA, and odd-numbered anteiso-acids from 2-methylbutyryl-CoA.44) The fatty acid composition of the ADG from females suggested such a situation. When these enzymes are active, valine is degraded to succinyl-CoA, leucine to acetoacetate and acetyl-CoA, and isoleucine to acetyl-CoA and propionyl-CoA. Acetyl-CoA and propionyl-CoA are primers of even- and odd-carbon number normal acids in fatty acid synthesis. This was the situation observed in the Harderian gland of male golden hamsters. The amount of iso- and anteiso-branched chain fatty acids in a male gland was minute, as shown in Fig. 4,26) but was detectable, as the peak on the gas chromatogram and the total amount reached about 2%. The same was held for the alkyl composition of male ADG. These findings suggested that a small part of isobutyryl-CoA, 2-methylbutyryl-CoA, and isovaleryl-CoA was also used as a primer for fatty acid synthesis in the male gland. These observations led to the idea that a sex hormone, like testosterone, affects the metabolism of BCAA at the step of 2-methyl-branched-chain acyl-CoA dehydrogenase and isovaleryl-CoA dehydrogenase, and brings about dramatic sexual diversity in the lipid composition in the glands of male and female golden hamsters.
Fig. 7.
Catabolic pathway of the branched chain amino acids; valine, leucine, and isoleucine.
Specific incorporation of the isotope labels from BCAA into the aliphatic chains of ADG
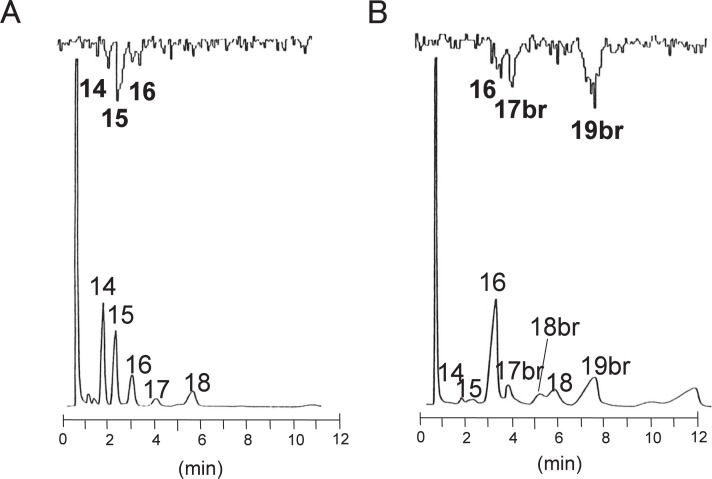
Hamsters were injected intraperitoneally with [U-14C] leucine and thereafter ADG in the gland was eluted. The derivatives of the alkyl and acyl groups were obtained and analyzed by radio-GLC. As for the acyl groups, straight-C14, -C15, and -C16 chains were labeled in males (Fig. 8A),45) and branched-C17 and -C19 chains were labeled in females (Fig. 8B).45) Additionally, a straight-C16 chain was also labeled in female glands but the radioactivity was quite low in spite of the large amount detected in the total fraction on GLC. These data clearly show that the BCAA metabolites were efficiently incorporated into the branched aliphatic chains in female ADG.45)
Fig. 8.
Radio-GLC analysis of fatty acid methyl esters prepared from ADG of golden hamster Harderian glands.
Golden hamsters were injected with 25 μCi of [U-14C]leucine and glands were obtained at 6 h after injection. Lipids in the glands were obtained and ADG was eluted. The acyl groups of ADG were extracted as fatty acid methyl esters. They were dissolved in hexane and then analyzed with a Gas Chromatograph GC-6AM (Shimadzu). The column effluent was passed through an Aloka peak analyzer radio-gas chromatograph system (Aloka). The voltage of the flame ionization detector (FID) was plotted upward and radio-activity was plotted downward. A, male; B, female. Each number indicates the chain length of each peak. “br” means branched chain.
As for the alkyl groups, straight-C16 and -C18 chains were labeled in males, and branched-C17 and -C19 chains were labeled in females.
Androgenic control of ADG synthesis
Male hamster Harderian glands have both large and small lipid droplets and entirely straight-chain alkyl and acyl groups, whereas females have only small lipid droplets and have both straight- and branched-chain alkyl and acyl groups.26), 46) The size and appearance of lipid droplets are controlled by androgens.46)
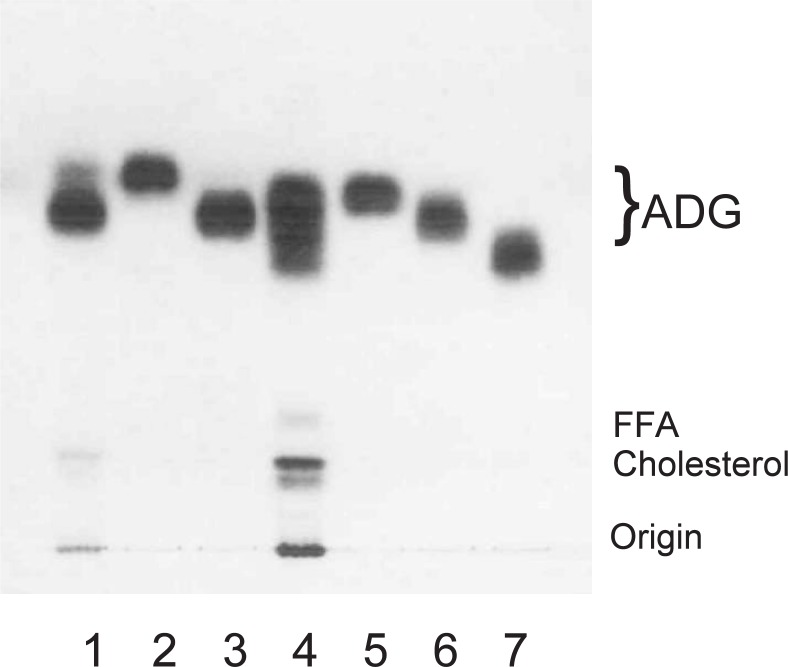
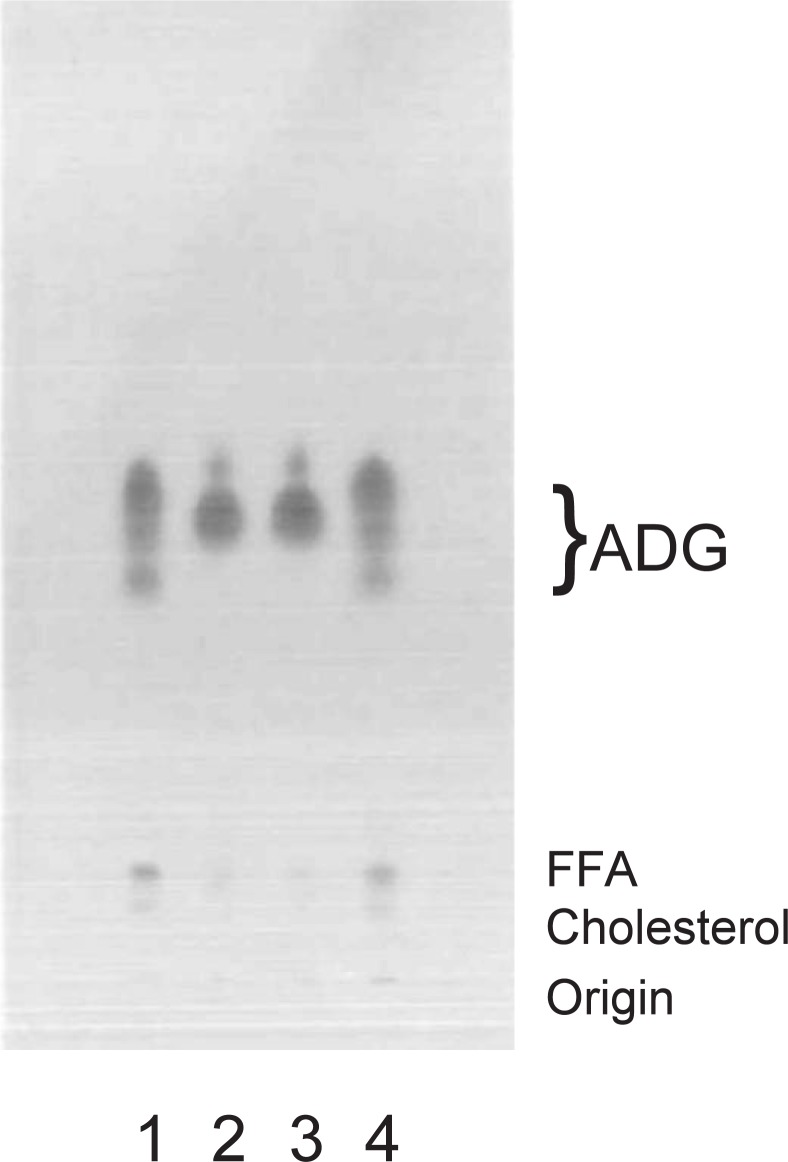
The primary endocrine control of Harderian ADG appeared to be due to androgens. Thin layer chromatograms (Fig. 947)) from lipid extracts of intact male hamsters (lane 1) showed ADGs as an elongated spots. Castrated male hamsters (lane 2) showed two distinct spots, similar to those seen intact female. Intact female hamsters (lane 3) showed two distinct spots, a faint upper spot and a lower intense spot. ADGs of female hamsters supplied with exogenous testosterone (lane 4) showed TLC patterns indistinguishable from those of intact males, with an elongated triplet spot.
Fig. 9.
Thin-layer chromatogram of lipid in Harderian glands of golden hamsters.
A silica gel 60 plate (Merck, Darmstadt) was developed with hexane-diethyl ether-acetic acid (80:20:1, v/v/v). The spots were located by heating the plate at 120 °C after spraying it with 20% sulfuric acid. Lane 1, crude lipid from glands of intact males; lane 2, castrated males; lane 3, intact females; lane 4, testosterone-treated females. ADG: 1-alkyl-2,3-diacylglycerol; FFA: free fatty acid.
Castrated males had a female pattern on TLC and showed branched chain alkyl and acyl groups. Amongst the straight chain alkyl groups, C18:0 and C20:0 predominated, and of the straight chain fatty acids, C16:0 was in greatest abundance. Female hamsters treated with exogenous testosterone, on the other hand, showed male TLC patterns and entirely straight chain alkyl and acyl groups; C18:0 and C19:0 were the main alkyl groups and C15:0, C14:0 were the main fatty acids. These observations suggested that the testosterone inhibited the production of branched chain fatty acids in the Harderian gland of golden hamster by inducing the activity of isovaleryl-CoA dehydrogenase and 2-methyl branched-chain acyl-CoA dehydrogenase as previously mentioned (Fig. 726)).
Androgens appear to be primarily responsible for dimorphic features of the Harderian gland in golden hamsters. Thus, cell types, organelles, porphyrin concentrations, and N-acetyltransferase activity are all regulated by androgens; castration transforms these features in males to the female pattern and testosterone has the opposite effect in female. 48)–54)
Harderian glands of golden hamsters of both sexes appear to respond to androgen in a manner similar to accessory sex glands. In accessory sex glands, testosterone binds to the androgen receptor and stimulates androgenic characteristics by its effect on the genome. Branched chain alkyl and acyl groups were present only in a low testosterone environment. The enzymes, isovaleryl-CoA dehydrogenase (which catabolized the branched chain amino acid leucine) and 2-methyl-branched chain acyl-CoA dehydrogenase (which catabolized isoleucine and valine), were inactive in the absence of testosterone. In these circumstances, isovaleryl-CoA, 2-methylbutyryl-CoA, and isobutyryl-CoA accumulate, and these act as primers for the synthesis of odd-numbered iso-branched, odd-numbered anteiso-branched, and even-numbered iso-branched fatty acids, respectively.
Odd chain-length fatty acids, particularly C15:0, are also characteristic of ADGs in a testosterone-rich environment. Odd chain-length fatty acids are synthesized when propionyl-CoA is a primer for fatty acid synthesis.55) This propionyl-CoA may also be a by-product of valine or isoleucine catabolism or may originate elsewhere.
In subfraction of M3 of male ADGs, propionic, hexanoic, and octanoic acids are present, and in subfraction F2 of female ADGs, isovaleric and methyl butyric acids are present.26) It would be interesting to know whether androgens influence these features.
Thus, androgens are essential to the control of the composition of ADGs in the golden hamster Harderian gland. However, reduction in serum androgen levels in male hamsters by a natural means (i.e., short photoperiods) did not mimic castration in leading to a female pattern of lipid droplet size.46)
Effect of photoperiod in modulating the androgenic control of ADG composition
Female hamsters in long days have alkyl groups with long saturated straight chains (C18:0 and C20:0) and methyl-branched chains (even and odd chain length iso-branched and odd chain length anteiso-branched chains).56) Acyl groups in females in long days are mostly long straight chains (C16:0) and methyl-branched chains. In females, short photoperiods led to reductions in the proportions of methylbranched chains and changes in the proportions of straight chain alkyl and acyl groups; these changes were prevented by pinealectomy. The total proportion of branched-chain acyl groups was reduced from 25 to 18% in short days. Changes to branched-chain acyl groups included increase to iso- and anteiso-branched C15 and reductions to iso-branched C17, C18, and C19, and anteiso-branched C19.
Male hamsters with intact gonads, maintained in long days, had no methyl-branched chain alkyl or acyl groups; saturated straight chains were generally shorter than those of females and the odd chain length saturated C15:0 acyl-group was common. Short photoperiods did not significantly alter the composition of male ADG. Castrated male hamsters in long days showed distinctively female phenotype, with long straight chains and methyl-branched alkyl and acyl groups. Castrated males in short days showed a mixture of male and female characteristics: shorter straight chain alkyl and acyl groups, a total absence of methyl-branched alkyl groups, and the presence of methyl-branched acyl groups.
These results and those of other studies suggested that testosterone controls the enzymes isovaleryl acyl-CoA dehydrogenase and 2-methyl branched-chain acyl-CoA dehydrogenase; in the absence of these enzymes, the primers for the synthesis of methyl-branched chain fatty acids are produced. Our results indicate that this control is modulated by short photoperiods perhaps due to reduced prolactin levels.
Long days are stimulatory for many features of the physiology of golden hamsters, and are generally regarded as the “control” condition in experiments. In these conditions, animals are reproductively competent. Serum levels of gonadotrophins, prolactin, sex steroids, thyroxin, and other hormones are high; gonads are active; mating occurs; and, since (in nature) long days occur during spring and summer, survival of the offspring is optimal.57)
Short photoperiods are features of the autumn and winter. Golden hamsters maintained in short days become reproductively quiescent. Serum levels of gonadotrophins, prolactin, sex steroids, thyroxin, and other hormones are low; testes, uteri, and accessory sex glands atrophy; and estrous cycles cease. These effects probably prevent reproduction from occurring during winter, when survival of the offspring would be doubtful. Short photoperiod-mediated changes in the physiology of golden hamsters are controlled by the pineal gland and it’s hormone melatonin; pinealectomy prevents these effect. 57)
It is also suggested that characteristics of male-type ADG are better adapted to conditions of autumn and winter than are those of female-type ADG.
Cholesterol synthesis from BCAA in male but not female Harderian gland
We investigated the incorporation profile of branched chain amino acids (BCAA) metabolites, which are the source of branched chain acyl-CoAs in the Harderian gland of golden hamster, and deduced the enzymes which cause the sexual differences in the lipid composition in this gland.45)
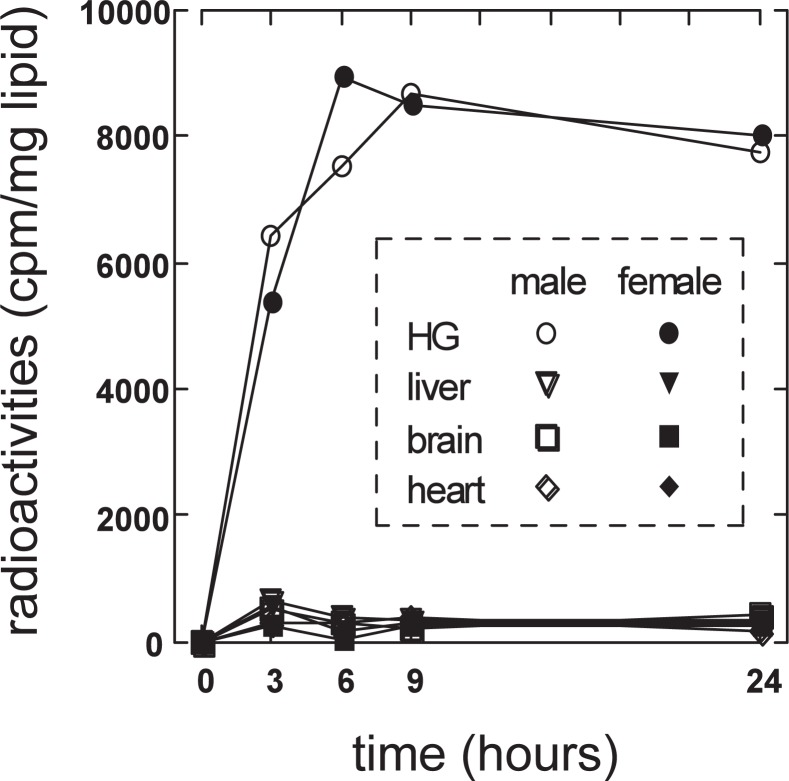
The radioactivity incorporated into lipids was monitored after the incorporation of [U-14C] leucine into peritoneum of golden hamsters (Fig. 1045)). The leucine metabolites were efficiently incorporated into the lipids in the Harderian gland in both sexes as compared with those in other tissues such as liver, heart, and brain. The level of tracer incorporation reached the maximum after 6 to 9 h and then decreased gradually. Similar results were obtained when [U-14C] isoleucine or [U-14C] valine was injected.
Fig. 10.
The incorporation of radioactivity into lipids in tissues of golden hamsters after [U-14C]leucine injection.
Golden hamsters were injected intraperitoneally with 2.5 μCi of [U-14C]leucine (Du Pont, Boston, MA, USA) diluted with Dulbecco’s phosphate-buffered saline (D-PBS). Three, 6, 9 and 24 h after injection, Harderian gland, liver, brain, and heart were obtained under anesthesia with sodium pentobarbital. They were kept overnight in 5 ml of chloroform/methanol (2:1, v/v) at room temperature and then lipids were extracted in the liquid phase. Five hundred micro liters of this fraction was evaporated under N2 gas, and then dissolved in 2 ml of Clear-sol I (Nacalai Tesque, Kyoto). Radioactivity in the solution was measured with a liquid scintillation spectrometer (Tri-Carb 15000; Packard Instruments, Downers Grove, IL, USA).
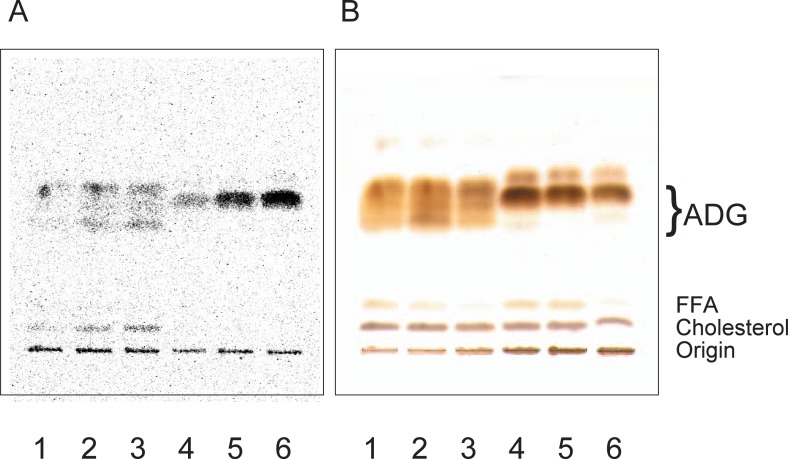
Lipid extracts were obtained from glands at 1, 3, and 6 h after injection, developed on TLC plates, and then analyzed by autoradiography. Fig. 1145) shows a representative autoradiogram when [U-14C] leucine was injected. The most strongly labeled fraction was ADG both in male and females, and the incorporation of the isotope into the ADG fraction reached the maximal level at 6 h after injection. But the radioactivity incorporation profiles (A) differed with the gender, like those observed of the total ADG detected by H2SO4 (B). The cholesterol fraction was labeled in males, but not in females, although a total cholesterol spot was detected in both sexes.
Fig. 11.
Thin-layer chromatogram of lipids in the Harderian glands of golden hamsters.
Golden hamsters were injected with 2.5 μCi of [U-14C]leucine into peritoneum. Lipids in the gland were obtained and developed on a TLC plate. A, a representative autoradiogram of a plate. B, H2SO4 detection of lipids on the same plate in panel A. Lipids obtained at 1 h after injection were spotted on lanes 1 and 4, at 3 h on lanes 2 and 5, and at 6 h on lanes 3 and 6. Lanes 1 to 3 contained male lipids, while lanes 4 to 6 contained female lipids. “ADG” and “FFA” indicate the positions of 1-alkyl-2,3-diacylglycerol and free fatty acids, respectively.
When [U-14C] isoleucine or [U-14C] valine was injected instead of leucine, the radioactivity in the lipid fraction reached the maximum level at 6 h after injection. The TLC results were quite the same as those of leucine injection. Namely, sexual differences in the ADG profile were observed both on autoradiography and H2SO4 detection, and male ADG gave 3 spots, while female ADG gave 2 spots. Sexual dimorphism was also observed in the cholesterol profile and radioactivity was detected only in male (data not shown).
These results showed that cholesterol was not produced from BCAA in female glands, and thus some enzymes for the production of cholesterol were thought to be regulated in the Harderian gland of golden hamster. As indicated in Fig. 12,45) branched chain acyl-CoAs are metabolized to acetyl-CoA or propionyl-CoA in male glands where the androgen level is high, and they are consequently incorporated into straight chain fatty acids. While in the female gland where the androgen level is low, BCAAs are metabolized to branched chain acyl-CoAs, but they would not be efficiently metabolized further, and they were thought to be used as the primer of fatty acid synthesis (as indicated by broken lines in Fig. 1245)). This hypothesis is consistent with the sexual difference observed in the isotope incorporation into the cholesterol fraction (Fig. 1145)), because 3-hydroxy-3-methylglutaryl-CoA, known as the source of cholesterol, would not be produced from BCAAs when branched chain acyl-CoAs were not catabolized (Fig. 1245)).
Fig. 12.
The metabolism of branched chain amino acids, and the synthesis of fatty acids and cholesterol.
Isovaleryl-CoA derived from leucine is catalyzed initially by isovaleryl-CoA dehydrogenase (IVD). 2-methylbutyryl-CoA and isobutyryl-CoA derived from isoleucine and valine, respectively, are both catalyzed initially by short/branched chain acyl-CoA dehydrogenase (SBCAD).
Noninvasive lipid analysis using exudates from the Harderian gland
We developed a unique bioassay system of androgen receptor activity in the Harderian gland.58) This gland excretes a large amount of lipids through an only one orifice. The fatty acid composition of the exudates reflects the activity of androgen receptor in the gland. We take an aliquot of lipid with glass capillary under a light anesthesia by ether, and plot it directly on thin layer plate (Fig. 13). This method is a noninvasive monitoring system for the assay of androgen effect. It is easy to operate and sensitive enough.
Fig. 13.
Thin-layer chromatogram of secreted fluid from the Harderian glands of golden hamsters.
Golden hamsters (8 weeks old) were subcutaneously injected with 1 mg/kg of testosterone daily. After 1 week they were anesthetized with pentobarbital and irritated with diethyl ether. They immediately excreted fluids from the Harderian gland onto the eyelid and about 1 μl of them were collected using a 5 μl glass capillary (Drummond). The fluid was directly spotted on a silica gel 60 plate (Merck, Darmstadt) and the plate was developed with hexane-diethyl ether-acetic acid (80:20:1, v/v/v). The lipids in the fluid were located by heating the plate at 120 °C after spraying it with 20% sulfuric acid. Samples: lane 1, intact male; lane 2, castrated male; lane 3, castrated male treated with testosterone; lane 4, intact female; lane 5, female treated with testosterone. ADG: 1-alkyl-2,3-diacylglycerol; Chol: cholesterol; Orig: origin.
Lane 1 shows a typical male type ADG, and after castration it changes to female type with two spots as shown in lane 2. Seven days after administration of TE to the castrated male, the exudates shows male type profile similar to that in lane 1. Female exudates show female profile as in lane 4, and it changes to male type profile at seven days after an administration of TE. It is not necessary to kill the animal as before, and we can continue the observation with the same body in this noninvasive method. This monitoring system is applicable, for example, to the screening of androgen inhibitor.
Hormonal regulation of acyl-CoA dehydrogenases
Acyl-CoA dehydrogenases play important roles in the lipid metabolism. By now, six enzymes are identified. Very long chain acyl-CoA dehydrogenase (VLCAD), long chain acyl-CoA dehydrogenase (LCAD), medium length acyl-CoA dehydrogenase (MCAD), and short chain acyl-CoA dehydrogenase (SCAD) regulate fatty acid β-oxidation in liver, while isovaleryl-CoA dehydrogenase (IVD) and short branched chain acyl-CoA dehydrogenase (SBCAD) metabolize branched chain acyl-CoAs and prevent the formation of branched chain fatty acids (Fig. 7,26)Fig. 1245)).
Harderian gland is an exocrine organ, which produces enormous lipids. In golden hamster, male gland contains only straight chain fatty acids while female gland contains about 50% of branched chain ones. It implies that IVD and SBCAD may be regulated by sex hormones in this gland. Here, we investigated the androgenic regulation of IVD, SBCAD, and androgen receptor (AR) in this gland. We also speculated the biological significance of lipids secreted from this gland.
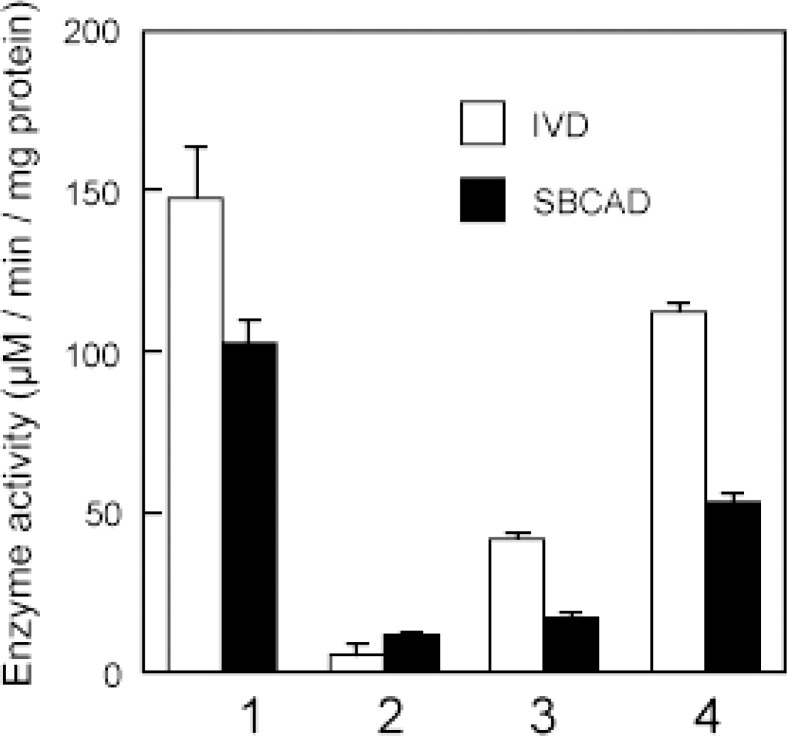
Male hamsters were castrated at 8 weeks old, and 2 weeks later they were subcutaneously injected with testosterone enanthate (TE). Mitochondrial fraction was obtained from glands. It was incubated with each substrate (isovaleryl-CoA or isobutyryl-CoA) and decrease of absorbance was monitored for the determination of IVD and SBCAD activities.59) IVD and SBCAD activities were observed in male gland (lane 1), but it was very low in female (lane 2) (Fig. 14). These activities were induced time-dependently in female gland after subcutaneous TE injection (lane 3, 3rd day; lane 4, 8th day) (Fig. 14). The same induction was also observed in castrated male glands (data not shown).
Fig. 14.
Effects of testosterone enanthate on IVD and SBCAD activities in the Harderian glands of golden hamsters. Golden hamsters (8 weeks old) were injected subcutaneously with 5 mg/kg of testosterone enanthate and Harderian glands were obtained after 0, 3, and 8 days after injection. The activities of IVD and SBCAD in the glands were analyzed by the method described by Ikeda and Tanaka (1988). Briefly, the glands were homogenized with a homogenizer and mitochondrial fractions were pelleted by 5,000 × g for 10 min. They were sonicated 10 times for 10 sec with 1 min rest on ice using a sonifier (Ultraschallprozessor; TUV, Brandenburg, Berlin, Germany), and then they were centrifuged at 20,000 × g for 20 min. A 150 μl portion of supernatants were mixed with 50 μl of 1 mM FAD (Sigma), 50 μl of phenazine methosulfate (Wako), 200 μl of 0.123 mM 2,6-dichlorophenolindophenol (DCIP; Wako) in a 1 cm light path semimicrocuvett. The reaction was started by adding 50 μl of 1 mM isovaleryl-CoA (Sigma) for IVD substrate or 2-methylbutyryl-CoA (Sigma) for SBCAD substrate. Bleaching of DCIP at 600 nm wavelength was monitored for 1 min. Enzyme activity was expressed as reduction of DCIP per mg protein per minute.
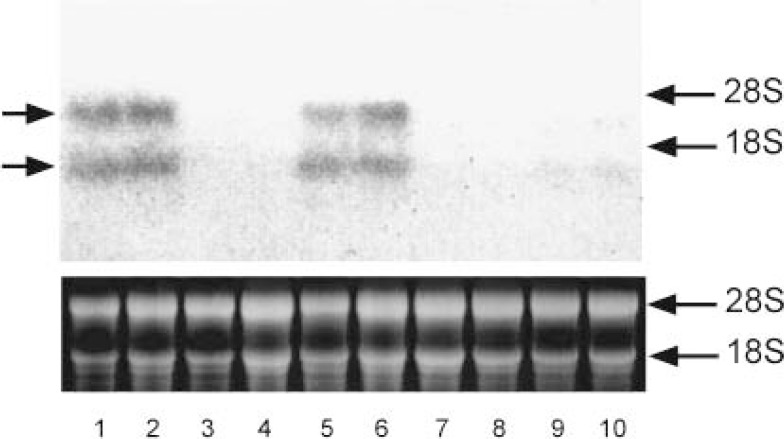
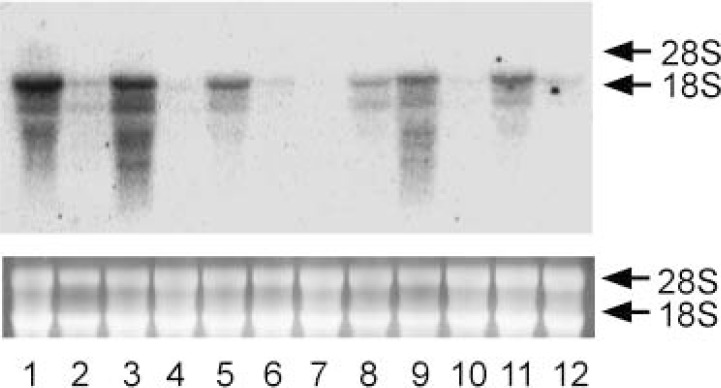
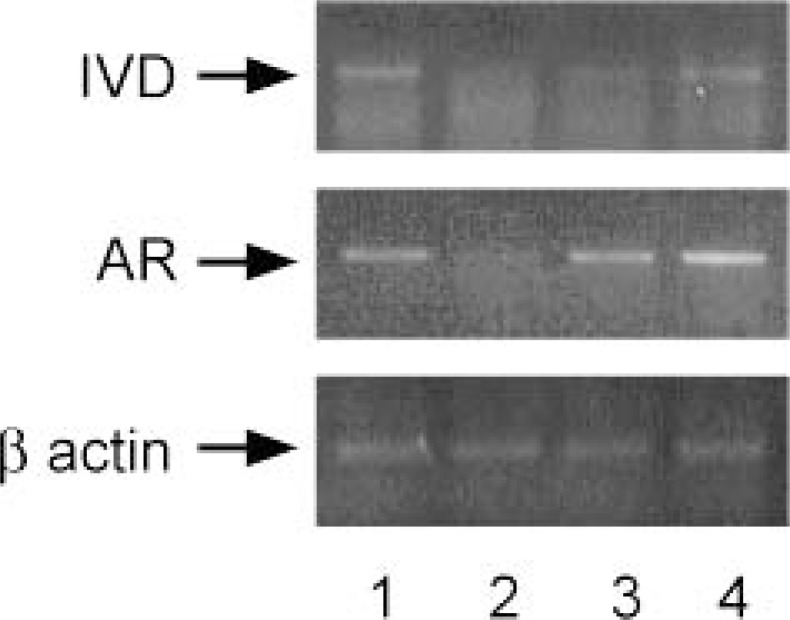
Total RNA was prepared from male hamster Harderian glands and used for reverse transcriptase-polymerase chain reaction (RT-PCR) using oligonucleotide containing partial sequence of golden hamster cDNA.60) The amplified cDNA was ligated into plasmids and the inserted sequence was determined. Northern blotting showed that IVD mRNA was detected in male gland (lanes 1 and 2), but was not in female (lanes 7 and 8) or castrated male gland (lane 3 and 4) (Fig. 15). Treatment of androgen (both testosterone and testosterone enanthate) induced IVD mRNA expression greatly in female (lanes 9 and 10) and castrated male gland (lanes 5 and 6) (Fig. 15). Tissue distribution of IVD mRNA was also analyzed and only Harderian gland showed a marked sexual difference (lane 1, male; lane 7, female) (Fig. 16). From RT-PCR analysis, it was suggested that the expression of IVD and AR mRNA was induced by androgen as soon as 3 days after TE injection (Fig. 17).
Fig. 15.
Northern blot hybridization analysis of IVD mRNA contents in the Harderian glands of golden hamsters and effect of testosterone enanthate (TE) on their expression.
Total RNA was prepared from Harderian glands of golden hamsters and mRNA of IVD was measured by Northern blot hybridization analysis. A 30 μg portion of total RNA was put on each lane. The positions of ribosomal RNA are indicated on the right. Bands of ribosomal RNA stained by ethidium bromide (bottom, the sizes shown on the right) demonstrate equal amounts of total RNA par lane. The blot shows a typical result of a series of experiments. Lanes 1 and 2, male; lanes 3 and 4, castrated male; lanes 5 and 6, castrated male treated with 5 mg/kg TE, lanes 7 and 8, female; lanes 9 and 10, female treated with 5 mg/kg TE. TE, testosterone enanthate.
Fig. 16.
Expression of IVD in various tissues of golden hamsters.
Total RNA was prepared from each tissue of golden hamster and mRNA of IVD was measured by Northern blot hybridization analysis. A 30 μg portion of total RNA was put on each lane. Bands of ribosomal RNA stained by ethidium bromide (bottom, the sizes shown on the right) demonstrate equal amounts of total RNA per lane. The blot shows a typical result of a series of experiments. Lanes 1 to 6, male; lanes 7 to 12, female; lanes 1 and 7, Harderian gland, lanes 2 and 8, liver; lanes 3 and 9, kidney; lanes 4 and 10, brain; lanes 5 and 11, heart; lanes 6 and 12, thigh muscle.
Fig. 17.
Effect of androgen on the expression of IVD and androgen receptor (AR) in the Harderian gland of golden hamsters. Golden hamsters (8 weeks old) were injected subcutaneously with 5 mg/kg of testosterone enanthate and Harderian glands were obtained after indicated time. Total RNA was extracted from them and mRNA was amplified by reverse transcription-polymerase chain reaction (RT-PCR) with specific primers for IVD, androgen receptor (AR), and beta-actin. Representative ethidium bromide-stained gels are shown. Castration of male hamsters was done 2 weeks before injection. Lane 1, male; lanes 2 to 4, castrated male; lane 2, 0 day; lane 3, 3 days, lane 4, 7 days after injection.
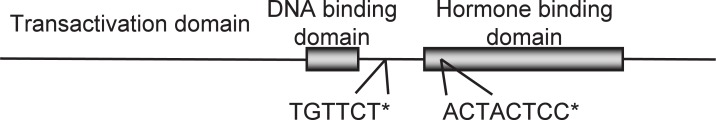
We also determined the hamster AR cDNA sequence and found that it contains quite homologous sequences with self regulatory elements of human AR within the coding region (Fig. 18).61), 62) There are two specific regions, namely the DNA binding region and hormone binding region.
Fig. 18.
Deduced structure of golden hamster AR cDNA.
The sequence of golden hamster AR cDNA was determined and the structure was deduced by comparing sequences with human AR cDNA reported by Dai and Burnstein (1996).61)
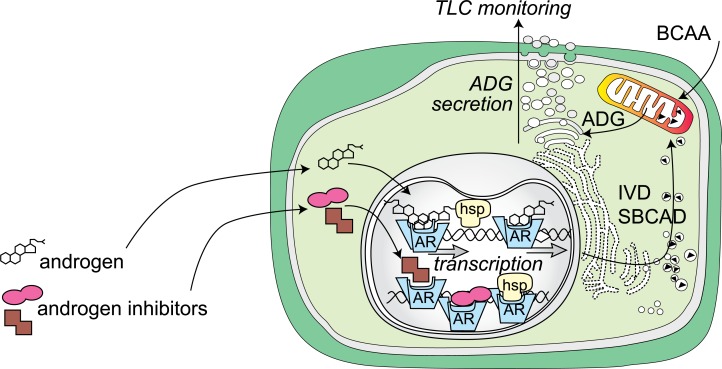
These observations strongly suggested the presence of hormonal regulation mechanism in the Harderian gland of golden hamster (Fig. 19). When the androgen receptor in the nucleus is activated by androgen, it stimulates a gene expression through interaction with androgen response elements, which are associated with androgen target genes, namely IVD and SBCAD genes in this case. After transcription and translation process, mature IVD and SBCAD proteins are anchored in the mitochondria, then catalyze the degradation of branched chain amino acids (BCAA) to acetyl-CoA or propionyl-CoA. So in the male gland, straight chain fatty acids are produced under the stimulation by androgen. In female gland on the other hand, due to the lack of androgen, the catabolism of BCAA is stopped at the level of isovaleryl-CoA or isobutyryl-CoA, 2-methylbutyryl-CoA, and branched chain fatty acids are produced afterwards. Our noninvasive TLC method is adequate to monitor such a situation in the cells without killing the animal.
Fig. 19.
A schematic diagram of androgenic control of IVD and SBCAD expression in golden hamster Harderian gland. BCAA: branched chain amino acid; hsp: heat-shock protein.
Monitoring attractiveness of lipids secreted from the Harderian glands
Harderian gland signals are important for the female in her display of sexual interest. Previously Payne63) showed that female glands itself contained attractive substances for male. Harriman and Thiessen observed that female Mongolian gerbils apparently use olfactory cues from the males to guide their approach behaviors (perceptive behaviors), and that female direct fewer perceptive behaviors toward males who are Harderianectomized.18) Many other social behaviors may be modified and synchronized by chemosignals from the Harderian glands.
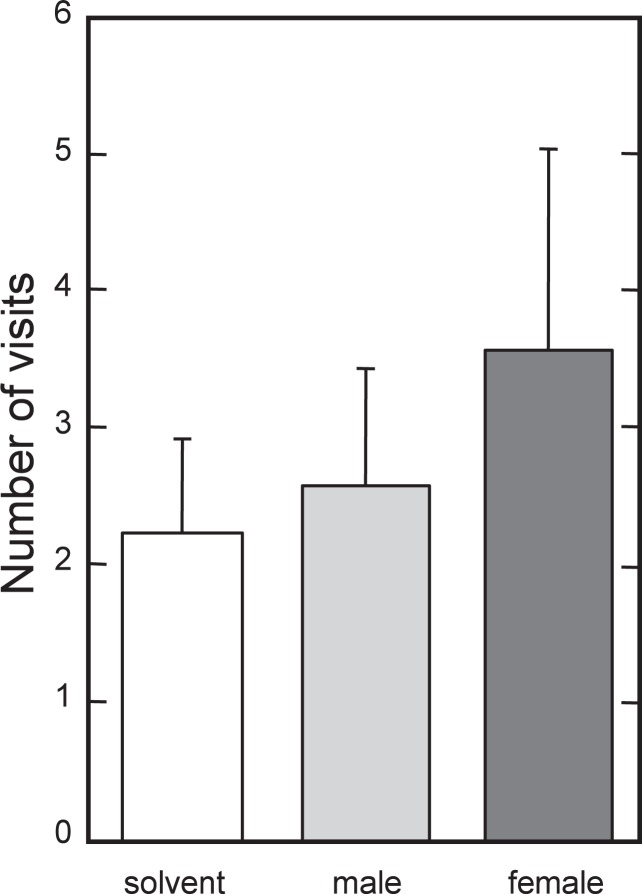
In our experiments, lipids were applied on an SEP-PAC Silica column (Waters) and ADG (the most abundant lipids in the gland) was eluted. Lipids from male or female glands were spotted and dried on a plate coated with metal (2 cm in diameter). Male hamsters were placed in a polycarbonate cage (20 × 36 × 20 cm) covered with a sheet of white paper. Three metal plates spotted with solvent alone, male or female lipids were put on the sheet. The number of times each plate was sniffed was counted within 5 minutes. Attractiveness of lipids in the gland was also monitored and slight but not significant attractiveness for male hamsters was observed in female lipids (Fig. 20).60), 62) We could find slightly greater attractiveness in female one than that of male but the difference was not significant. However it may still be possible that some insoluble substances which may conjugate with branched chain lipids may act as an attractant for males.
Fig. 20.
Attractiveness of lipids extracted from golden hamster Harderian glands.
Harderian glands were obtained from ten female golden hamsters (8 weeks old) under anesthesia with sodium pentobarbital. They were kept overnight in 5 ml of chloroform/methanol (2:1, v/v) at room temperature and then lipids were extracted in the liquid phase. Small portion of this fraction was evaporated under N2 and lipid content was determined. The liquid phase containing 10 mg of lipids was spotted on each plate coated with aluminum sheet (2 cm in diameter). Male hamsters were placed in a polycarbonate cage (width, 20 cm, depth, 36 cm, height, 20 cm) with a sheet of 3 MM paper put on the bottom. Three metal plates spotted with solvent alone, male or female lipids were placed at random on the bottom sheet. The number of times each plate was sniffed was counted within 5 minutes.
Role of lipids secreted from the Harderian gland of golden hamster
The lipid secreted from the Harderian gland is not a triacylglycerol (TG) but an ADG. The function of TG is to store energy in the cell, and that of ADG is to work outside of the body after secretion from the gland. The acyl and alkyl residues of ADG from the Harderian gland of golden hamster have peculiar characteristics: odd and even numbered chains in male, and iso- and anteiso-branched chains in female. It is necessary to consider the character of this animal to understand the real function of this lipid in nature.
It is well known that the golden hamster has no habit to live in a group as a family, but keep their own territory alone. In spring, it comes out from a cave, and looks around a partner for a while, not for life, copulates, and then breeds babies usually around twelve in number. Father leaves soon, but mother gives the breast to her babies for a while. They disperse on the field separately within few months.
Considering such lifestyle, we propose a hypothesis that the lipid exudates from the Harderian gland serves as a pheromone to declare their individual territory and to seek the mate with a good congeniality. The sexual difference of the ADG will be a signal of such pheromone, and the profile of acyl and alkyl group of ADG will be used to differentiate their affinity.
Androgen induced IVD and SBCAD activities in the Harderian gland of golden hamster. This induction was tissue-specific and was preceded by the induction of AR in the gland. As the lipid composition in other organs such as liver and heart shows no sexual difference in golden hamster, induction of these two enzymes should require some tissue specific transcriptional activator or co-regulator. Analysis of transcriptional regulatory domains of these genes may provide important information for this molecular mechanism. Previously Payne63) showed that female glands itself contained attractive substances for male. Because lipids share the largest volume of the gland and also because female gland contains branched chain fatty acids, a kind of which is supposed to have pheromonal function, we monitored the behavior of male hamsters toward female gland lipids. We could find slightly greater attractiveness in female one than that in male one but the difference was not significant. However it may still possible that some insoluble substances which may conjugate with branched chain lipids may act as an attractant for males. The next project is to seek their receptor, probably at the vomeronasal organ of the golden hamster.
Acknowledgments
The authors wish to thank the coworkers whose names appear in the references. We would especially like to acknowledge Drs. Shunichiro Kubota (The University of Tokyo, Graduate School of Arts and Sciences), Hideaki Otsuka (Hiroshima University, Faculty of Pharmacy), Azumi Hida (Showagakuin Junior College), Paul Pevet (University of Louis Pasteur), Gerald Buzzell (United Arab Emirates University), for their helpful collaboration in this project.
Abbreviations:
- TLC
thin layer chromatography
- ADG
1-alkyl-2,3-diacylglycerol
- GLC
gas liquid chromatography
- BCAA
branched chain amino acid
- NMR
nuclear magnetic resonance
- VLCAD
very long chain acyl-CoA dehydrogenase
- LCAD
long chain acyl-CoA dehydrogenase
- MCAD
medium chain acyl CoA dehydrogenase
- SCAD
short chain acyl-CoA dehydrogenase
- IVD
isovaleryl-CoA dehydrogenase
- SBCAD
short branched chain acyl-CoA dehydrogenase
- AR
androgen receptor
- TE
testosterone enanthate
- RT-PCR
reverse transcriptase-polymerase chain reaction
- TG
triacylglycerole
Profile
Yousuke Seyama was born in 1941, and started his research career in 1966 as a graduate student in the laboratory of Professor Tamio Yamakawa at Faculty of Medicine, the University of Tokyo. He worked two years from 1969 to 1971 as a research associate at Harvard Medical School and Massachusetts General Hospital in the laboratory of Professor Herman Kalckar, studying the reaction mechanism of UDP-galactose 4-epimerase. In 1973, he received PhD degree from the University of Tokyo. He was appointed Associate at Faculty of Medicine, the University of Tokyo in 1972, Associate Professor in 1979, and Professor in 1983. In 2001 he was promoted to Professor at Ochanomizu University, Faculty of Human Life and Environmental Sciences. After retirement from Ochanomizu University in 2006, he moved to National Institution for Academic Degrees and University Evaluation, as a Visiting Professor.
His research has been focused on lipid biochemistry, especially on genetic disease named cerebrotendinous xanthomatosis (CTX), Harderian gland lipid, bioactive lipids like leukotriene, PAF receptor, and also on glycolipids. He clarified the regulation mechanism of fatty acid synthesis using deuterium labeled substrates followed by mass spectrometric analysis. In regard to CTX, he developed novel methods to quantify cholestanol concentration in plasma, to analyze defective enzyme, and finally he determined the mutations in several CTX families.
Toward these establishments, he was awarded Prize for Young Biochemists from Japanese Biochemical Society in 1978, Inoue Science Prize from Inoue Science Foundation in 1988, Academic Award of Japanese Society of Nutrition and Food Science in 1998, and Award from Japanese Society for Biomedical Mass Spectrometry in 2004.
He has served as Vice President of Japanese Biochemical Society from 1995 to 1996 and from 1999 to 2000, President of Japanese Society of Nutrition and Food Science from 2000 to 2002, President of Japanese Conference on the Biochemistry of Lipids (JCBL) from 1999 to 2004, ordinary and corresponding member of the Steering Committee of International Conferences on the Biochemistry of Lipids (ICBL) from 1989 to 2005. He is an honorary member of Japanese Biochemical Society and Japanese Conference on the Biochemistry of Lipids. He has served as Editorial Board of Journal of Biochemistry, Japanese Journal of Nutrition and Food Science, and Journal of Food Science and Nutrition.

References
- 1).Harder, J.J. (1694) Glandula nova lachrymalis una ductu excretorio in cervis damis. Acta Erudit. Publ. Lipsiae, 49–52 [Google Scholar]
- 2).Sakai, T. (1981) The mammalian Harderian gland: morphology, biochemistry, function and phylogeny. Arch. Histol. Jpn. 44, 299–333 [DOI] [PubMed] [Google Scholar]
- 3).Spike, R., Payne, A.P. and Moore, M. (1992) Porphyrins and their possible significance in Harderian glands. InHarderian Glands (eds. Webb, S., Hoffman, R.A., Puig-Domingo, M. and Reiter, R.). Springer-Verlag, Berlin Heidelberg, pp. 165–193 [Google Scholar]
- 4).Kennedy, G.Y. (1970) Harderoporphyrin: a new porphyrin from the Harderian glands of the rat. Comp. Biochem. Physiol. 36, 21–36 [DOI] [PubMed] [Google Scholar]
- 5).Spike, R.C., Payne, A.P., Thompson, G.G. and Moore, M.R. (1990) High-performance liquid chromatographic analyses of porphyrins in hamster Harderian glands. Biochim. Biophys. Acta 1034, 1–3 [DOI] [PubMed] [Google Scholar]
- 6).Yokoyama, Y., Kano, K., Kaji, K. and Seyama, Y. (1989) Purification and characterization of a growth factor from guinea pig Harderian gland. J. Biol. Chem. 264, 17058–17063 [PubMed] [Google Scholar]
- 7).Yokoyama, Y., Kano, K., Kaji, K., Shirama, K., Matsuda, Y., Namiki, H. and Seyama, Y. (1992) Effect of Harderian gland-derived growth factor on the growth of cornea stromal cells. Cornea 11, 380–385 [DOI] [PubMed] [Google Scholar]
- 8).Park, S.H., Satoh, Y., Kumagai, S. and Seyama, Y. (1996) Localization of porphyrin in mouse Harderian glands. Arch. Histol. Cytol. 59, 189–195 [DOI] [PubMed] [Google Scholar]
- 9).Seyama, Y., Kasama, T., Yasugi, E., Park, S.-H. and Kano, K. (1992) Lipids in Harderian glands and their significance. InHarderian Glands (eds. Webb, S., Hoffman, R.A., Puig-Domingo, M. and Reiter, R.). Springer-Verlag, Berlin Heidelberg, pp. 195–217 [Google Scholar]
- 10).Cohn, S.A. (1955) Histochemical observations on the Harderian gland of the albino mouse. J. Histochem. Cytochem. 3, 342–353 [DOI] [PubMed] [Google Scholar]
- 11).Lin, W.L. and Nadakavukaren, M.J. (1981) Harderian gland lipids of male and female golden hamsters. Comp. Biochem. Physiol. 70B, 627–630 [DOI] [PubMed] [Google Scholar]
- 12).Payne, A.P., McGadey, J., Moore, M.R. and Thompson, G.G. (1977) Androgenic control of the Harderian gland in the male golden hamster. J. Endocrinol. 75, 73–82 [DOI] [PubMed] [Google Scholar]
- 13).Sakai, T. and Yohro, T. (1981) A histological study of the Harderian gland of Mongolian gerbils, Meriones meridianus. Anat. Rec. 200, 259–270 [DOI] [PubMed] [Google Scholar]
- 14).Thiessen, D.D. (1977) Thermoenergetic and the evolution of pheromone communication. InProgress in psychobiology and physiological psychology (eds. Sprague, J.M. and Epstein, A.N.). vol. 7, Academic Press, New York, pp. 91–191 [Google Scholar]
- 15).Thiessen, D.D. (1988) Body temperature and grooming in the Mongolian gerbil. Ann. NY Acad. Sci. 525, 27–39 [DOI] [PubMed] [Google Scholar]
- 16).Kittrell, E.M.W. and Thiessen, D.D. (1981) Does removal of the Harderian gland affect the physiology of the Mongolian gerbil, Meriones unguiculatus? Physiol. Psychol. 9, 299–304 [Google Scholar]
- 17).Harlow, H.J. (1984) The influence of Harderian gland removal and fur lipid removal on heat loss and water flux to and from the skin of muskrats, Ondotra zibethicus. Physiol. Zool. 57, 349–356 [Google Scholar]
- 18).Harriman, A.E. and Thiessen, D.D. (1983) Removal of Harderian exudates by sandbathing contributes to osmotic balance in Mongolian gerbils. Physiol. Behav. 31, 317–323 [DOI] [PubMed] [Google Scholar]
- 19).Smelser, G.K. (1943) Water and fat content of orbital tissues of guinea pigs with experimental exophthalmos produced by extracts of the anterior pituitary gland. Am. J. Physiol. 140, 308–315 [Google Scholar]
- 20).Johnson, D.D., Rudeen, P.K. and O’Steen, W.K. (1979) Photically induced experimental exophthalmos: role of Harderian and pituitary glands. Invest. Ophthalmol. Visual. Sci. 18, 1280–1285 [PubMed] [Google Scholar]
- 21).Minucci, S., Chieffi Baccari, G., Di Matteo, L. and Chieffi, G. (1989) A sexual dimorphism of the Harderian gland of the toad, Bufo viridis. Basic Appl. Histochem. 33, 299–310 [PubMed] [Google Scholar]
- 22).Kuehnel, W. (1971) Struktur und Cytochemie der Hardershen Druese von Kaninchen. Z. Zellforsch. Mikrosk. Anat. 119, 384–404 [PubMed] [Google Scholar]
- 23).Bucana, C.D. and Nadakavukaren, M.J. (1972) Fine structure of the hamster Harderian gland. Z. Zellforsch. Mikrosk. Anat. 129, 178–187 [DOI] [PubMed] [Google Scholar]
- 24).Woolley, G.W. and Worley, J. (1954) Sexual dimorphism in the Harderian gland of the hamster (Cricetus auratus). Anat. Rec. 118, 416–417 [Google Scholar]
- 25).Fulton, G.P. (1968) The golden hamster in biomedical research, Iowa State University Press, Ames, IA [Google Scholar]
- 26).Seyama, Y., Otsuka, H., Ohashi, K., Vivien-Roels, B. and Pevet, P. (1995) Sexual dimorphism of lipids in Harderian glands of golden hamsters. J. Biochem. 117, 661–670 [DOI] [PubMed] [Google Scholar]
- 27).Murawski, U., Platte, C. and Scott, A. (1991) A triple methyl-branched alkoxylipid as the main component of Harderian gland lipids in the golden hamster (Mesocricetus auratus). Endocrinolgia 38, 275–278 [Google Scholar]
- 28).Yamazaki, T., Seyama, Y., Otsuka, H., Ogawa, H. and Yamakawa, T. (1981) Identification of alkyldiacylglycerols containing saturated methyl branched chains in the Harderian gland of guinea pig. J. Biochem. 89, 683–691 [DOI] [PubMed] [Google Scholar]
- 29).Seyama, Y., Otsuka, H., Kawaguchi, A. and Yamakawa, T. (1981) Fatty acid synthetase from the Harderian gland of guinea pig: biosynthesis of methyl-branched fatty acids. J. Biochem. 90, 789–797 [DOI] [PubMed] [Google Scholar]
- 30).Seyama, Y., Ohashi, K., Imamura, T., Kasama, T. and Otsuka, H. (1983) Branched chain fatty acids in phospholipids of guinea pig Harderian gland. J. Biochem. 94, 1231–1239 [DOI] [PubMed] [Google Scholar]
- 31).Seyama, Y., Ohashi, K., Yasugi, E. and Otsuka, H. (1984) Fatty acid composition of cardiolipin from guinea pig Harderian gland. J. Biochem. 96, 1639–1643 [DOI] [PubMed] [Google Scholar]
- 32).Buckner, J.S. and Kolattukudy, P.E. (1975) Lipid biosynthesis in the sebaceous glands: synthesis of multibranched fatty acids from methylmalonyl-Coenzyme A in cell-free preparations from the uropygial gland of goose. Biochemistry 14, 1774–1782 [DOI] [PubMed] [Google Scholar]
- 33).Buckner, J.S., Kolattukudy, P.E. and Rogers, L. (1978) Synthesis of multimethyl-branched fatty acids by avian and mammalian fatty acid synthetase and its regulation by malonyl-CoA decarboxylase in the uropygial gland. Arch. Biochem. Biophys. 186, 152–163 [DOI] [PubMed] [Google Scholar]
- 34).Scaife, J.R., Wahle, K.W. and Garton, G.A. (1978) Utilization of methylmalonate for the synthesis of branched-chain fatty acids by preparations of chicken liver and sheep adipose tissue. Biochem. J. 176, 799–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).Hoffman, R.A., Johnson, L.B. and Reiter, R.J. (1985) Harderian glands of golden hamsters: temporal and sexual differences in immunoreactive melatonin. J. Pineal Res. 2, 161–168 [DOI] [PubMed] [Google Scholar]
- 36).Seyama, Y and Kawaguchi, A. (1987) Fatty acid synthesis and the role of pyridine nucleotides. InPyridine Nucleotide Coenzyme (eds. Dolphin, D., Poulson, R. and Avramovic, O.), Vol. 2B, John Wiley & Sons, Inc, New York, pp. 381–431 [Google Scholar]
- 37).Weitkamp, A.W. (1945) The acidic constituents of Degras. A new method of structure elucidation. J. Am. Chem. Soc. 67, 447–454 [Google Scholar]
- 38).Haahti, E. (1961) Major lipid constituents of human skin surface with special reference to gaschromatographic methods. Scand. J. Clin. Lab. Invest. 13, 1–108 [PubMed] [Google Scholar]
- 39).Wheatley, V.R. and Sher, D.W. (1961) Studies of the lipids of dog skin. I. The chemical composition of dog skin lipids. J. Invest. Dermatol. 36, 169–170 [DOI] [PubMed] [Google Scholar]
- 40).Downing, D.T., Kranz, Z.H. and Murray, K.H. (1960) Studies in waxes. XIV. An investigation of hydrolysed wool wax by gas chromatography. Aust. J. Chem. 13, 80–94 [Google Scholar]
- 41).Wheatley, V.R. (1957) The composition of the sebum of some common rodents. Biochem. J. 65, 36–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42).Velick, S.F. and English, J. (1945) The synthesis and configuration of d-14-methylpalmitic acid and its identity with the natural acid from wool fat. J. Biol. Chem. 160, 473–480 [PubMed] [Google Scholar]
- 43).Tanaka, K., Ikeda, Y., Matsubara, Y. and Hyman, D.B. (1987) Molecular basis of isovaleric acidemia and medium-chain acyl-CoA dehydrogenase deficiency. Enzyme 38, 91–107 [DOI] [PubMed] [Google Scholar]
- 44).Grigor, M.R., Dunckley, G.G. and Purves, H.D. (1970) The synthesis of the branched-chain fatty acids of rat skin surface lipid. Biochim. Biophys. Acta 218, 389–399 [DOI] [PubMed] [Google Scholar]
- 45).Hida, A., Uchijima, Y. and Seyama, Y. (1998) Sexual differences in branched chain amino acid metabolism into fatty acids and cholesterol in Harderian gland of golden hamster. J. Biochem. 124, 648–653 [DOI] [PubMed] [Google Scholar]
- 46).Buzzell, G.R., Blank, J.L., Vaughan, M.K. and Reiter, R.J. (1995) Control of secretory lipid droplets in the Harderian gland by testosterone and the photoperiod: comparison of two species of hamsters. Gen. Comp. Endocrinol. 99, 230–238 [DOI] [PubMed] [Google Scholar]
- 47).Seyama, Y., Hida, A., Hayashi, S. and Buzzell, G.R. (1996) Androgenic control of 1-alkyl-2,3-diacylglycerol in the Harderian gland of the golden hamster, Mesocricetus auratus. J. Biochem. 119, 799–804 [DOI] [PubMed] [Google Scholar]
- 48).Hoffman, R.A. (1971) Influence of some endocrine glands, hormones and blinding on the histology and porphyrins of the Harderian glands of golden hamsters. Am. J. Anat. 132, 463–478 [DOI] [PubMed] [Google Scholar]
- 49).Lin, W. and Nadakavukaren, M.J. (1979) The androgenic effect on the fine structure on the Harderian gland in the male hamster. Cell Tissue Res. 198, 119–127 [DOI] [PubMed] [Google Scholar]
- 50).Marrufo, B., Menendez-Pelaez, A., Buzzell, G.R., Gonzalez-Brito, A. and Reiter, R.J. (1989) 5α-Dihydrotestosterone administration converts indolamine metabolism and porphyrin content of the female Syrian hamster Harderian gland to the male type. Proc. Soc. Exp. Biol. Med. 192, 192–195 [DOI] [PubMed] [Google Scholar]
- 51).Buzzell, G.R., Menendez-Pelaez, A., Hoffman, R.A., Vaughan, M.K. and Reiter, R.J. (1990) N-acetyltransferase activity in the Harderian glands of the Syrian hamster, Mesocricetus auratus, is regulated by androgens and by hormones of the pituitary-thyroid axis. J. Endocrinol. 127, 59–67 [DOI] [PubMed] [Google Scholar]
- 52).Buzzell, G.R., Menendez-Pelaez, A., Chlumecky, V. and Reiter, R. J. (1991) Gender differences and time course of castration-induced changes in porphyrins, indoles, and proteins in the Harderian glands of the Syrian hamster. Can. J. Physiol. Pharmacol. 69, 1814–1818 [DOI] [PubMed] [Google Scholar]
- 53).Buzzell, G.R., Hoffman, R.A., Vaughan, M.K. and Reiter, R. J. (1992) Hypophysectomy prevents the castration-induced increase in porphyrin concentrations in the Harderian glands of the male golden hamster: a possible role for prolactin. J. Endocrinol. 133, 29–35 [DOI] [PubMed] [Google Scholar]
- 54).Menendez-Pelaez, A., Buzzell, G.R., Gonzalez-Brito, A. and Reiter, R.J. (1990) Androgenic control of N-acetyltransferase activity in the Harderian glands of the Syrian hamster is mediated by 5 alpha-dihydrotestosterone. J. Cell Biochem. 42, 95–100 [DOI] [PubMed] [Google Scholar]
- 55).Smith, S. (1994) The animal fatty acid synthase: one gene, one polypeptide, seven enzymes. Faseb J. 8, 1248–1259 [PubMed] [Google Scholar]
- 56).Buzzell, G.R., Hida, A., Fu, S. and Seyama, Y. (1997) Effect of the photoperiod in modulating the androgenic control of 1-alkyl-2,3-diacylglycerol composition in the Harderian gland of the golden hamster, Mesocricetus auratus. J. Exp. Zool. 277, 99–105 [PubMed] [Google Scholar]
- 57).Reiter, R.J. (1980) The pineal and its hormones in the control of reproduction in mammals. Endocr. Rev. 1, 109–131 [DOI] [PubMed] [Google Scholar]
- 58).Uchijima, Y., Hida, A., Ozawa, S. and Seyama, Y. (1998) Androgenic control of acyl-CoA dehydrogenase and its utilization for assaying androgenic activity. Chem. Phys. Lipids 94, 176 [Google Scholar]
- 59).Ikeda, Y. and Tanaka, K. (1988) Isovaleryl-CoA dehydrogenase from rat liver. Methods in Enzymology 166, 374–389 [DOI] [PubMed] [Google Scholar]
- 60).Matsubara, Y., Indo, Y., Naito, E., Ozasa, H., Glassberg, R., Vockley, J., Ikeda, Y., Kraus, J. and Tanaka, K. (1989) Molecular cloning and nucleotide sequence of cDNAs encoding the precursors of rat long chain acyl-coenzyme A, short chain acyl-coenzyme A, and isovaleryl-coenzyme A dehydrogenases. Sequence homology of four enzymes of the acyl-CoA dehydrogenase family. J. Biol. Chem. 264, 16321–16331 [PubMed] [Google Scholar]
- 61).Dai, J.L. and Burnstein, K.L. (1996) Two androgen response elements in the androgen receptor coding region are required for cell-specific up-regulation of receptor messenger RNA. Mol. Endocrinol. 10, 1582–1594 [DOI] [PubMed] [Google Scholar]
- 62).Uchijima, Y., Hida, A., Kubota, S. and Seyama, Y. (2000) Androgenic regulation of lipid metabolism in golden hamster Harderian gland. Chem. Phys. Lipids 107, 77–78 [Google Scholar]
- 63).Payne, A.P. (1979) The attractiveness of Harderian gland smears to sexually naive and experienced male golden hamsters. Anim. Behav. 27, 897–904 [DOI] [PubMed] [Google Scholar]