Abstract
Background
Memantine has been used off-label to treat frontotemporal lobar degeneration (FTD). A previous 26 week open label study suggested a transient, modest benefit on neuropsychiatric symptoms as measured by the Neuropsychiatric Inventory (NPI).
Methods
We performed a randomized, parallel group, double blind, placebo controlled trial of 20 mg memantine taken orally daily for 26 weeks in FTD. Participants met Neary criteria for behavioral variant (bvFTD) or semantic dementia (SD) and had characteristic brain atrophy. Use of cholinesterase inhibitors was prohibited. The objective of the study was to determine whether memantine is an effective treatment for FTD. Individuals were randomized to memantine or matched placebo tablets in blocks of two and four. Primary endpoints were the change in total NPI score and Clinical Global Impression of Change (CGIC) scores after 26 weeks. Secondary outcomes included a neuropsychological battery, and other cognitive, global and activity of daily living measures. Clinicaltrials.gov identifier: NCT00545974
Findings
100 subjects were screened, 81 were randomized, 5 (6%) discontinued and 76 completed all visits. Enrollment numbers were lower than planned due to many subjects’ preference to take memantine or cholinesterase inhibitors off-label rather than participate in a clinical trial. 39 memantine and 42 placebo subjects entered the primary intent to treat analysis. There was no effect of memantine treatment on either the NPI (mean difference [MD] 2.2, 95%CI: −3.9, 8.3, p = 0.47) or CGIC (MD 0, 95%CI: −0.4, 0.4, p = 0.90) after 26 weeks of treatment. Memantine was generally well tolerated, however there were more frequent cognitive adverse events in the memantine group.
Interpretation
There was no benefit of memantine treatment in bvFTD or SD. These data do not support memantine use in FTD.
Funding
Forest Research Institute
Introduction
Frontotemporal lobar degeneration or frontotemporal degeneration (FTD) is a common cause of dementia in individuals who develop symptoms before age 65. FTD encompasses three core clinical syndromes, a behavioral variant frontotemporal dementia (bvFTD), and two primary progressive aphasias (PPA), semantic dementia (SD) and progressive nonfluent aphasia (PNFA). 1 BvFTD is the most common form of the disease and features prominent social and behavioral deficits as well as executive dysfunction. SD often begins as an aphasia, with progressive semantic knowledge loss, but also often features prominent behavioral abnormalities similar to bvFTD. 2 PNFA presents as a motor speech disorder with few other cognitive or behavioral impairments. There are no medications approved by the US Food and Drug Administration (FDA) to treat FTD and only a handful of randomized, placebo controlled trials have been conducted in FTD.3 Despite the lack of efficacy data supporting the use of medications approved for the treatment of Alzheimer’s disease (AD), such medications are frequently prescribed to FTD patients off-label in the US, with 55% of patients in a recent study using either an acetylcholinesterase inhibitor (AChI) or memantine. 4
Memantine is approved by the European Medicines Agency and the FDA for the treatment of moderate-severe AD and has also demonstrated beneficial effects in clinical trials of vascular dementia, Parkinson’s-related dementias and dementia of mixed etiologies reviewed in 5). Although the neuropathology and underlying neurotransmitter deficits are different in FTD than in AD, there is a scientific rationale for using memantine to treat FTD. First, memantine is believed to act as a non-competitive inhibitor of N-methyl D-aspartate (NMDA) receptors that may be over-activated in a variety of neurodegenerative diseases, including FTD. 5 Second, analyses of data from clinical trials of memantine in AD found clear benefits on a variety of abnormal behaviors as assessed by the Neuropsychiatric Inventory (NPI). 6 Since many of these behaviors are prominent features of FTD, memantine might also be predicted to improve these deficits. Third, a number of open label treatment studies in bvFTD and SD have demonstrated symptomatic improvements with memantine treatment. 7,8 In one of these studies, we found that initiation of memantine therapy was associated with a transient improvement in behavior as measured by the NPI 9 in bvFTD and SD subjects. 8 Since the transient improvement in NPI scores might have been attributable to a placebo effect or an effect of memantine treatment, the current study tested the hypothesis that memantine would improve or stabilize behavior as measured by the NPI and Clinical Global Impression Change (CGIC) 10 as compared to placebo after 26 weeks of therapy.
Methods
Subjects
Patients were recruited from nine US academic dementia research centers with expertise in the diagnosis of FTD including the University of California, San Francisco (UCSF) and Los Angeles (UCLA), the Mayo Clinic, Rochester and Jacksonville, Northwestern University Medical Center, Case Western Reserve Medical Center, University of North Carolina, Johns Hopkins University and the University of Pennsylvania. Study visits occurred between December, 2007 and May, 2012. Because this was a follow-up study to a 26 week open-label study of memantine that showed a similar pattern of changes in bvFTD and SD, but not PNFA, 9 the current study only included subjects with bvFTD or SD. 1 Individuals with FTD-motor neuron disease were included if motor impairments did not interfere with study procedures. Individuals had to be between 40–80 years of age and have a Mini-Mental State Exam (MMSE) score of ≥ 15 at screening. To exclude cases with slowly progressive bvFTD (bvFTD phenocopy) all subjects had a CT or MRI scan of brain within 24 months of randomization consistent with a diagnosis of bvFTD or SD. 11 All subjects had a reliable caregiver who could accompany them to study visits. Exclusion criteria included a diagnosis of PNFA, use of memantine, AChI, antipsychotic agents, valproate, lithium or benzodiazepines within four weeks prior to randomization. Use of AChI was prohibited due to potential confounding effects on memantine efficacy and reported adverse reactions in FTD. 12,13 If behavioral symptoms became difficult to control after the baseline visit, individuals were allowed to take an atypical antipsychotic medication (olanzapine, quetiapine or risperidone). Antidepressant use was allowed, if the dose had been stable for one month prior to randomization. Other exclusion criteria included evidence of disorders that preclude diagnosis of FTD. 1 Written informed consent was obtained from the subject and the subject’s caregiver in accordance with local IRB regulations. Clinicaltrials.gov identifier: NCT00545974.
Randomization and blinding
Subjects were randomized to memantine 10 mg twice daily or identical placebo tablets lacking memantine, that were packaged into kits (one per subject) of multiple blister packs (one week of treatment per pack). All subjects and study personnel were blinded to treatment assignment. Randomization codes were generated by an unblinded UCSF pharmacist (S.F.) using the Excel (Microsoft) random number generator in blocks of 2 and 4 subjects.
Study procedures
Each subject participated in six study visits over approximately 35 weeks. After the screening visit, a randomization/baseline visit occurred within 35 days, during which initial study medication was dispensed. Individuals were titrated to the full dose of 10 mg memantine or placebo taken orally twice daily, by 5 mg per week, reaching the full dose at week four. Subjects returned at weeks six, 12 and 26 (or early termination) for safety and efficacy assessments. In addition to the in-person visits, on weeks three, nine and 18, individuals received a phone call to assess adverse events and study medication compliance. After the week 26 visit, the study medication was stopped, and individuals returned for a 30 day off drug safety assessment. Compliance was assessed by counting study medication remaining in the blister packs. All outcome measures were assessed at baseline and week 26, with a subset of measures collected at weeks 6 and 12. Adverse events were grouped by Medical Dictionary for Regulatory Activities (MedRA) system organ class (www.meddramsso.com). Serious Adverse Events (SAEs) were defined as those leading to hospitalization or death.
Outcome measures
The primary outcomes were the NPI and CGIC. The NPI is a measure that assesses 12 neuropsychiatric abnormalities that reveals severe abnormalities in FTD. 9 The CGIC is a seven point categorical scale that gives a global impression of change from baseline. Secondary efficacy assessments included the clinical dementia rating sum of boxes (CDR-SB-FTD), with behavioral comportment, personality and language domains added to better capture FTD-related deficits; 14 the MMSE;8 the Functional Activities Questionnaire (FAQ) ; 15 Texas Functional Living Scale (TFLS) a performance-based assessment of capacity to perform ADLs;16 the Executive Interview (EXIT25), a neuropsychological composite to test executive function, 17 a modified Unified Parkinson’s Disease Rating Scale (UPDRS); 8 the time to initiation of antipsychotic therapy; and a neuropsychological battery, including a California Verbal Learning Test, category fluency, phonemic fluency, a 15 item Boston Naming Test (BNT), a modified Trails set-shifting task, backward digit span and the Digit Symbol as previously described. 14 Tertiary outcomes were the Zarit Burden Interview (ZBI), a 22 item questionnaire used to measure caregiver burden 18 and subject weight in Kg (since FTD patients often gain weight).
Sample Size Estimate
We based our sample size calculation on a comparison of changes in NPI from baseline to follow-up between the memantine treatment and placebo groups using a two sample t-test. We hoped to detect a medium effect size of half a standard deviation. 19 Standard power calculations for two sample t-tests (α = 0.05) with a standard deviation of 2.2 (half of 4.4 from 8) show that a sample of 65 per group would provide power greater than 80% power to detect this difference.
Statistical analysis
Primary and secondary outcomes were analyzed using an intent-to-treat (ITT) approach that included all subjects who received at least one dose of medication and had a post-baseline efficacy assessment. We used a repeated measures approach to assess the difference in changes over time in the repeated primary (NPI) and secondary outcomes between the memantine and placebo groups, that is, the time by treatment group interaction. Specifically, for each subject, we computed changes in outcomes between baseline and the 26 week follow up and assessed the magnitude of the difference in these changes using linear regression methods. Analyses were repeated using gender as a covariate. It was decided post-hoc to reduce the CGIC values to “improved, no change or worsened” due to the very few number of responses outside the middle 3 values. Week 26 CGIC values were compared using a Mann-Whitney U test. Exploratory analyses in each FTD subtype and observed cases (OC), subjects who completed all four efficacy visits, were conducted to investigate potential sources of bias in the ITT analyses. Finally, differences in outcome measures at individual time points were compared using least squares means with a two-sample t-test, and differences in adverse event frequencies were analyzed using Chi square tests. Analyses were performed using SAS 9.3 (SAS Inc., Cary, NC) or Stata 12 (StataCorp, College Station, TX).
Results
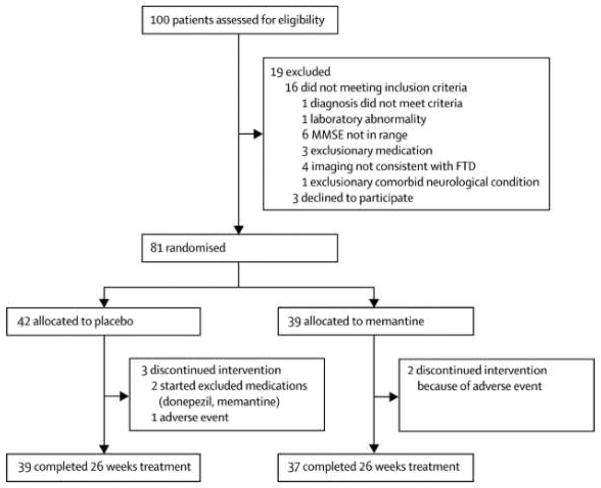
100 patients were assessed for eligibility and 81 patients (64 bvFTD and 17 SD) were randomly assigned to memantine (n=39) or placebo (n=42; Figure 1). Five patients (2 memantine, 3 placebo) discontinued treatment prior to the end of the study. The planned enrollment for the study was 140 subjects. Despite randomization, there was a greater percentage of men in the placebo group (Table 1; P=0.01). There were no other baseline differences in demographic variables, concomitant medication use, or outcome measures (Supplementary Data). 17 memantine and 13 placebo subjects took 100% of the study medication (p=0.24); for the remaining subjects, mean study medication compliance was 95.6% (95%CI: 92.3, 97.3) in the placebo group and 94.8% (93.0, 98.2) in the memantine group (p = 0.65).
Figure 1.
Patient flow diagram.
Table 1.
Baseline Characteristics
| Characteristics Mean (95% CI) | Placebo N = 42 | Memantine N = 39 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| bvFTD N = 33 | SD N = 9 | All N = 42 | bvFTD N = 31 | SD N = 8 | All N = 39 | |
| Men, (%)* | 28 (84.9) | 4 (44.4) | 32 (76.2) | 14 (45.2) | 5 (62.5) | 19 (48.7) |
| Age, y | 65.6 (62.8, 68.4) | 68.6 (63.4, 73.7) | 66.2 (63.8, 68.6) | 65.6 (62.7, 68.3) | 67.0 (62.5, 71.5) | 65.8 (63.5, 68.1) |
| Education, y | 15.4 (14.4, 16.4) | 15.0 (12.8, 17.2) | 15.3 (14.5, 16.2) | 15.7 (14.8, 16.7) | 15.8 (13.0, 18.5) | 15.7 (14.9, 16.6) |
| Disease duration, y | 3.5 (2.6, 4.4) | 2.8 (1.3, 4.3) | 3.3 (2.6, 4.1) | 3.0 (2.1, 4.0) | 2.8 (1.6, 3.9) | 3.0 (2.2, 3.7) |
| Weight, lbs. | 199.7 (183.8, 215.6) | 156.7 (143.0, 170.3) | 190.0 (176.3, 203.7) | 180.3 (166.0, 194.6) | 167.9 (135.4, 200.3) | 177.7 (165.1, 190.2) |
|
| ||||||
| Primary Outcomes | ||||||
| NPI | 22.2 (16, 28.3) | 18.6 (13.8, 23.4) | 21.5 (15.7, 27.3) | 21.1 (16,26.2) | 18.8 (15, 22.6) | 20.6 (15.8, 25.4) |
| CGI | 3.3 (3.1, 3.5) | 3.3 (3.2, 3.4) | 3.3 (3.1,3.5) | 3.5 (3.2, 3.8) | 3.4 (3.2,3.6) | 3.5 (3.2, 3.8) |
| Secondary Outcomes | ||||||
| CDR-SB-FTD | 4.8 (4.0, 5.6) | 3.0 (1.7, 4.3) | 4.4 (3.7, 5.1) | 5.8 (4.5, 7.1) | 3.8 (1.5, 6.0) | 5.4 (4.2, 6.5) |
| FAQ | 15.8 (13.2, 18.3) | 7.4 (0.9, 13.9) | 14.1 (11.6, 16.6) | 14.7 (11.9, 17.4) | 8.5 (1.1, 15.9) | 13.4 (10.8, 16.0) |
| TFLS | 40.2 (37.5, 42.9) | 42.1 (36.3, 47.9) | 40.6 (38.3, 43.0) | 38.3 (34.2, 42.4) | 43.8 (39.4, 48.1) | 39.4 (36.1, 42.8) |
| MMSE | 25.0 (23.7, 26.3) | 25.2 (21.3, 29.1) | 25.1 (23.8, 26.3) | 24.0 (22.1, 25.8) | 25.8 (22.7, 28.8) | 24.3 (22.8, 25.9) |
| EXIT25 | 17.2 (14.3, 20.1) | 16.7 (10.7, 22.6) | 17.1 (14.6, 19.6) | 17.0 (13.3, 20.7) | 14.0 (7.5, 20.5) | 16.3 (13.2, 19.4) |
| Letter fluency | 6.5 (5.0, 8.0) | 7.9 (4.2, 11.6) | 6.8 (5.4, 8.2) | 6.1 (4.3, 7.8) | 5.6 (4.1, 7.1) | 6.0 (4.6, 7.4) |
| Category fluency | 11.2 (5.8, 16.6) | 7.5 (1.3, 13.7) | 10.2 (6.2, 14.2) | 9.1 (5.8, 12.5) | 9.0 (4.5, 13.5) | 9.1 (6.6, 11.5) |
| Digit symbol | 37.8 (31.3, 44.3) | 45.0 (36.6, 53.4) | 39.3 (34.0, 44.7) | 34.2 (24.6, 43.7) | 55.8 (42.5, 69.0) | 38.6 (30.3, 46.9) |
| Digits backwards | 3.5 (3.0, 4.0) | 4.2 (3.0, 5.4) | 3.6 (3.2, 4.1) | 3.4 (2.8, 4.0) | 4.1 (3.2, 5.1) | 3.6 (3.0, 4.1) |
| Boston Naming Test | 12.2 (11.2, 13.2) | 6.2 (2.5, 10.0) | 10.8 (9.5, 12.2) | 12.9 (11.1, 14.7) | 7.9 (3.3, 12.4) | 11.9 (10.1, 13.6) |
| UPDRS | 3.2 (1.2, 5.1) | 3.4 (−1.2, 8.1) | 3.2 (1.5, 5.0) | 2.9 (0.4, 5.4) | 0.9 (−0.6, 2.3) | 2.4 (0.5, 4.3) |
| Tertiary Outcomes | ||||||
| ZBI 22 | 32.5 (27.8, 37.3) | 31.7 (24.7, 38.6) | 32.4 (28.5, 36.2) | 28.3 (23.0, 33.7) | 30.5 (18.6, 42.4) | 28.8 (24.1, 33.4) |
P values are for comparison between all placebo and all memantine subjects.
There were more men in the placebo group at baseline (p = 0.011, Chi square.)
Primary outcomes
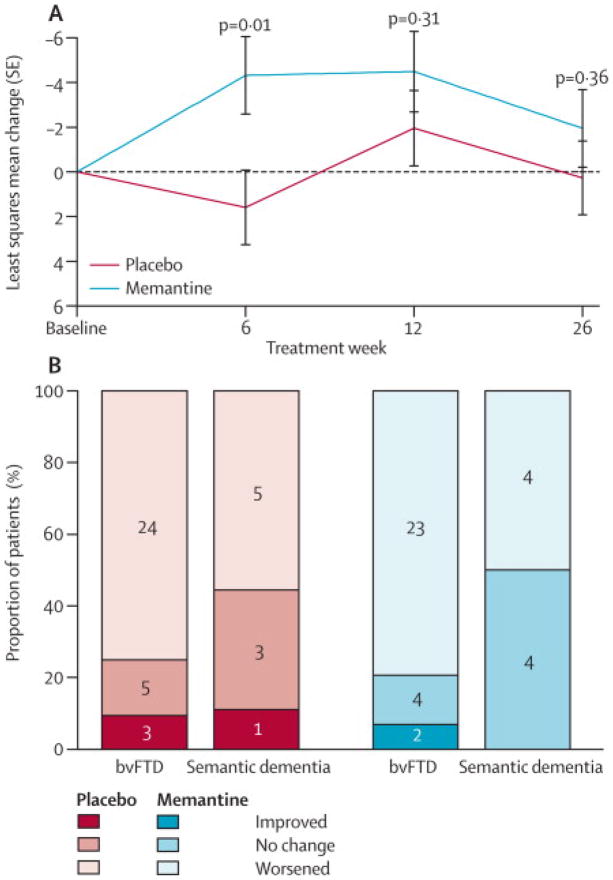
In the ITT analysis there were no differences between the memantine and placebo groups on the change in total NPI or CGIC scores after 26 weeks (Table 2). The mean difference in change in NPI score from baseline to week 26 was 2.2 (95%CI: −3.9, 8.3, P=0.47; Figure 2A). Adjusting for baseline gender differences post-hoc did not alter the result (Supplementary Table 1). The CGIC showed that at week 26, 27 subjects worsened, 8 remained stable and two improved in the memantine group, whereas 29 subjects worsened, 8 remained stable and four improved in the placebo group (p=0.90; Figure 2B).
Table 2.
Mean differences in longitudinal change from baseline
| Measure
| |||
|---|---|---|---|
| Difference | 95% CI | P value | |
| Primary outcomes | |||
| NPI | 2.2 | −3.9, 8.3 | 0.47 |
| CGIC | 0 | −0.4, 0.4 | 0.90 |
| Secondary outcomes Global | |||
| CDR-SB-FTD | 0 | −0.9, 0.9 | 0.99 |
| FAQ | −1.5 | −4.0, 1.0 | 0.23 |
| TFLS | 0.9 | −1.7, 3.5 | 0.49 |
| Cognitive | |||
| MMSE | 0.1 | −1.3, 1.5 | 0.69 |
| EXIT25 | −1.2 | −3.8, 1.4 | 0.34 |
| Boston Naming Test | 2.2 | 0.7, 3.6 | 0.004 |
| Category fluency | 0.4 | −1.7, 2.4 | 0.72 |
| Digits backwards | −0.3 | −0.8, 0.2 | 0.28 |
| Digit symbol | 8.1 | 1.1, 15.1 | 0.024 |
| Letter fluency | −0.2 | −1.5, 1.1 | 0.75 |
| Motor | |||
| UPDRS | −0.3 | −3.0, 2.4 | 0.83 |
| Tertiary outcome | |||
| ZBI 22 | 1.6 | −2.0, 5.3 | 0.38 |
Mean difference is placebo - memantine group.
Figure 2. Primary outcome variables.
(A) Change from baseline of total Neuropsychiatric Inventory (NPI) scores from the intent to treat population are shown with p values for a paired t test at each study visit. In the repeated measures analysis there was no group difference (p = 0.39). (B) Clinician’s Global Impression of Change (CGIC) values are shown at week 26 for n = 76 subjects who completed this visit. Only improved = “slightly improved (3)”, no change = “no change (4)” and Worsened = “slightly worsened (5)” are shown since no other values were recorded. Using a Mann Whitney test there was no difference in CGIC distributions (p = 0.90).
Secondary outcomes
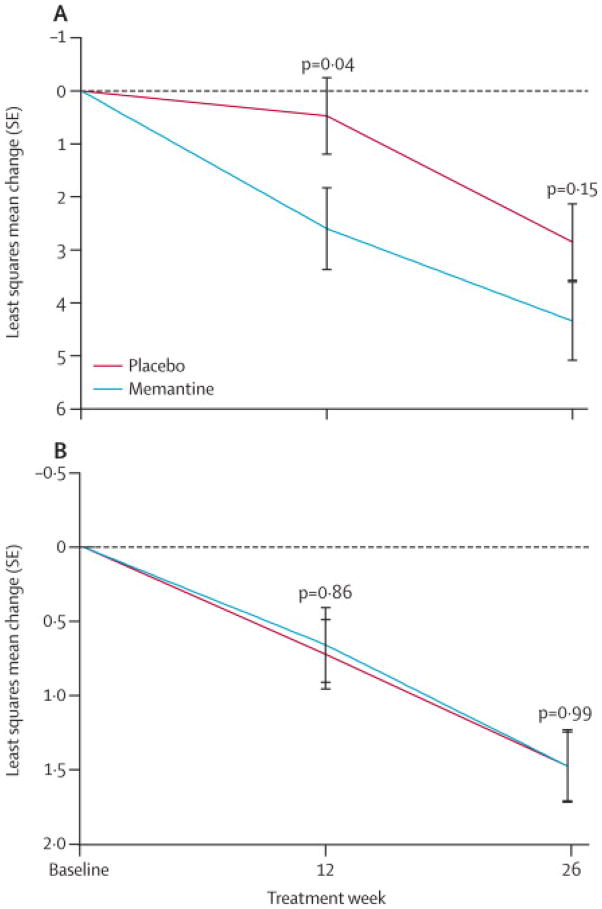
No treatment effect was observed on the functional outcome measures, the CDR-SB-FTD, FAQ and TFLS. CDR-SB-FTD scores increased similarly in both groups by 1.5 (0.8, 2.1) points over 26 weeks (Figure 3). Performance on the FAQ and TFLS declined similarly in the placebo and memantine groups (p=0.67).
Figure 3. Functional rating scales.
(A) Change from baseline Functional Activities Questionnaire (FAQ) scores in the intent to treat population. (B) Change from baseline in Clinical Dementia Rating sum of boxes (CDR-SB-FTD) scores.
The memantine group displayed worse neuropsychological performance than the placebo group on tests of naming (BNT) and processing speed (Digit Symbol; Figure 4, Table 2). There were no differences on other neuropsychological composite (MMSE and EXIT25), and individual test scores (Table 2). Consistent with the effects we observed on neuropsychological tests, there were numerically more cognitive AE’s (confusion, memory loss, language disorders; six vs. one; p= 0.056, supplementary table 4) in the memantine group than the placebo group, whereas the opposite was true for psychiatric AE’s (p=0.03). Two individuals experienced a SAE in the placebo group and one individual experienced two SAEs in the memantine group. SAEs were not judged to be treatment related. There were no differences in UPDRS or other safety assessments (Table 3). Since only three subjects began an antipsychotic medication during the study (Supplementary Data), time to antipsychotic use was not analyzed.
Figure 4. Neuropsychological tests.
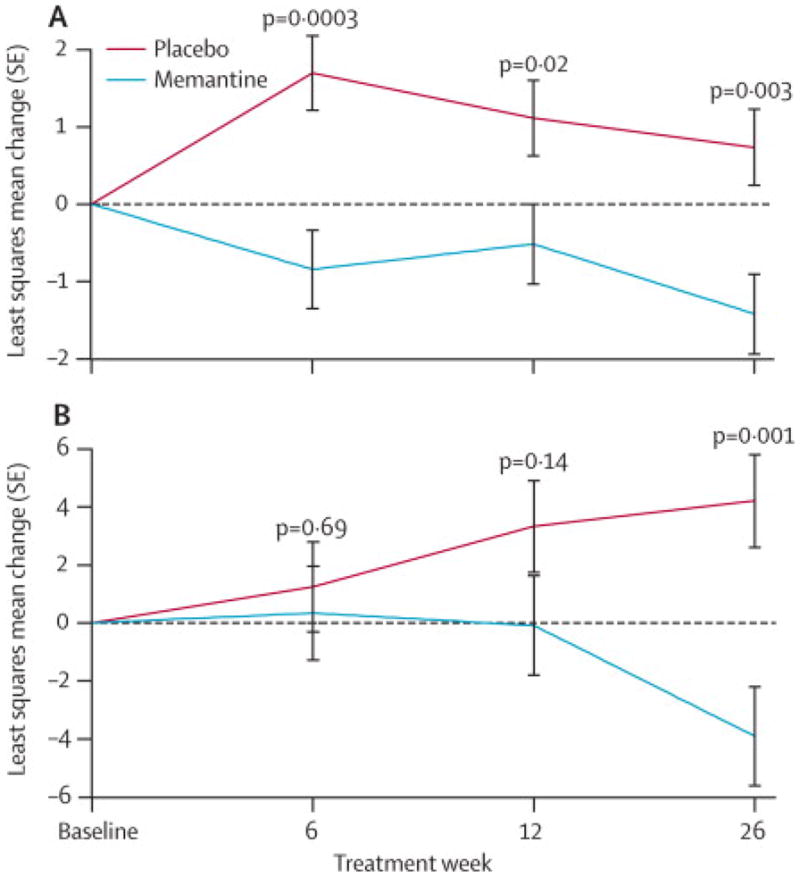
(A) Change from baseline modified Boston Naming Test (BNT). (B) Change from baseline Digit Symbol Substitution Test scores.
Table 3.
Adverse Event Summary
| Placebo | Memantine | |||
|---|---|---|---|---|
|
| ||||
| Body Class/Preferred Term | N | % | N | % |
| Body as Whole | ||||
| Fatigue | 1 | 2.4 | 1 | 2.6 |
| Cognitive Disorders | ||||
| Language Problems | 0 | 0.0 | 3 | 7.7 |
| Memory Loss | 0 | 0.0 | 2 | 5.1 |
| Gastrointestinal Disorders | ||||
| Diverticulitis | 2 | 4.8 | 0 | 0.0 |
| Nausea | 3 | 7.1 | 0 | 0.0 |
| Injury | ||||
| Abrasion | 0 | 0.0 | 2 | 5.1 |
| Fall | 2 | 4.8 | 5 | 12.8 |
| Nervous System Disorders | ||||
| Back Pain | 0 | 0.0 | 2 | 5.1 |
| Dizziness | 2 | 4.8 | 2 | 5.1 |
| Headache | 3 | 7.1 | 1 | 2.6 |
| Psychiatric Disorders | ||||
| Agitation | 2 | 4.8 | 0 | 0.0 |
| Behavioral Rigidity | 1 | 2.4 | 1 | 2.6 |
| Inappropriate Sexual Behavior | 4 | 9.5 | 0 | 0.0 |
| Insomnia | 4 | 9.5 | 0 | 0.0 |
| Obsessive Compulsive Symptoms | 1 | 2.4 | 2 | 5.1 |
| Somnolence | 1 | 2.4 | 1 | 2.6 |
| Renal and Urinary Disorders | ||||
| Urinary Tract Infection | 0 | 0.0 | 2 | 5.1 |
| Urinary Frequency | 1 | 2.4 | 1 | 2.6 |
| Respiratory Disorders | ||||
| Upper Respiratory Infection | 0 | 0.0 | 2 | 5.1 |
| Skin and Subcutaneous Tissue Disorders | ||||
| Rash | 1 | 2.4 | 1 | 2.6 |
Adverse events (AEs, all severities combined), occurring in two or more individuals (N), in either group combined, and percent of ITT population (%) in each group. AEs occurring in only one individual are not shown. A complete list of adverse events is given in the Supplementary Materials (Supplementary Table 4).
Tertiary outcomes
There was no treatment effect on caregiver burden (ZBI, p=0.13) or change in weight.
Exploratory (post-hoc) analyses
Because we had previously observed a transient improvement in NPI scores in an open-label memantine treatment study, 8 we examined differences in NPI scores at individual time points and found a transient improvement (MD 5.9, 95%CI: 4.2, 7.6) at week six (p=0.01) that converged with changes in the placebo group at weeks 12 and 26 (p>0.30; Figure 2A).
We also investigated whether the effects we found on the BNT and digit symbol test were related to FTD subtype. When analyzed separately, BNT performance was worse in both the bvFTD and SD groups after 26 weeks (Supplementary Figure 1). On the Digit Symbol test, there was a small improvement in performance in the placebo group after 26 weeks of treatment, whereas the memantine group declined (MD: 8.1, 95%CI: 1.1, 15.1, P < 0.001; Figure 4B).
Discussion
We found no benefit of 20 mg daily memantine treatment in FTD on either of the primary outcome measures, the NPI or the CGIC, after 26 weeks of treatment. There was evidence of worse cognitive performance on tests of naming (BNT) and processing speed (Digit Symbol) associated with memantine treatment, and a trend towards more cognitive adverse events. However, the worse neuropsychological performance in the memantine group was not associated with a difference in the rate of decline in activities of daily living as measured by CDR-SB-FTD, FAQ and TFLS. Although memantine was safe and well tolerated in FTD, our results do not support a claim of benefit for memantine treatment in FTD patients. Since approximately 30% of bvFTD patients in the US take memantine,4 our findings have immediate public health implications.
Our results are similar to those from a recent 52 week randomized placebo controlled trial of memantine in 49 subjects with bvFTD that also demonstrated no benefit on the primary outcome, the Clinician’s Interview-Based Impression of Change (similar to the CGIC) or the NPI.20 Like the previous study, a major limitation of the current study was that we failed to enroll the planned number of subjects, which may have limited our ability to detect a treatment effect. This under-enrollment was due to many potential subjects’ preference to take memantine (and in many cases an AChI as well) rather than participate in a clinical trial during which they risked being randomized to placebo. Unfortunately, altering the enrollment criteria to allow use of these medications would have prevented us from testing our hypothesis that memantine might have benefit in the treatment of FTD. Instead, to improve recruitment, sites stressed equipoise regarding the efficacy of memantine when recruiting subjects. A second limitation of the study was the small size of the SD group, which limits the generalizability of our results to this FTD syndrome. Finally, since this trial was designed, a number of rating scales that better capture FTD-specific behaviors have been developed that might have been more sensitive to potential benefits of memantine than those we employed (reviewed in 21).
Despite these limitations, we believe that our study provides strong evidence that memantine is not an effective treatment for FTD. First, in an exploratory analysis, there was a transient improvement in NPI scores after six weeks of treatment that was similar in magnitude and time course to what we observed in a previous open-label treatment study (n= 34 bvFTD and SD patients) 8 suggesting that the pattern of changes observed on the NPI (Figure 2) did not arise by chance. Second, we conducted a study-level meta-analysis, combining six month CGIC data from the current study and 12 month CIBICplus data presented in the manuscript from the previous bvFTD clinical trial, 20 for a combined total of n=64 placebo and n=55 memantine cases. This meta-analysis found no difference between placebo and memantine on the combined global impression (MD = 0.082, 95%CI: −0.18, 0.34; P = 0.553). Third, we observed worse visuomotor and naming function in the memantine group in the pre-specified analyses (Table 2). Consistent with these findings, there was a greater number of cognitive adverse events in the memantine group (Table 3). Finally, the rate of decline in CDR-SB-FTD scores was identical in both groups, and numerically, FAQ scores appeared to decline more rapidly in the memantine group at week 12 (Figure 3), although this was an exploratory finding that should be interpreted with caution.
We found fewer psychiatric (behavioral) side effects in the memantine group than the placebo group (Table 3). The simplest explanation for the divergent effects of memantine we observed in this study would be that memantine had a general suppressive effect on attention and cognition that led to less distressing behavior as well a reduced ability to perform visuomotor processing and lexical retrieval tasks.
Our study suggests that FTD patients may respond differently to memantine than other forms of dementia, underscoring the importance of accurate diagnosis. In moderate-severe AD, memantine has demonstrated benefits on global and cognitive function alone or in combination with donepezil. 12 Although a pilot study of memantine in PPA (not differentiated by subtype) suggested a modest benefit of treatment on the Western Aphasia Battery, 22 some forms of PPA are due to underlying AD pathology which could explain this finding. Clinical trials of memantine for VaD also suggest a modest benefit on cognition in patients with mild to moderate impairment. 23 Two clinical trials of memantine in Parkinson’s-related dementia demonstrated efficacy for treatment of cognitive and behavioral symptoms.24,25 We speculate that the lack of benefit of memantine treatment in FTD could reflect a different pattern of neurotransmitter abnormalities in this disorder.3
This is the largest randomized placebo controlled trial conducted in FTD to date. In addition to the implications for the current treatment of FTD, we demonstrate that clinical trials are feasible in this disorder. Since approximately half of all FTD cases have underlying tau pathology, as in AD, it has been suggested that tau-directed therapeutics might eventually be used in both disorders.26 We found that the rate of decline as measured by the CDR-SB-FTD was approximately twice as fast as has been reported for the CDR-SB in AD. 27 The more rapid progression of FTD as compared to AD may allow for faster clinical trials in FTD than in AD to test the efficacy of therapies targeting proteins such as tau that are common to both disorders. 21 This study provides clear evidence of a lack of efficacy of memantine treatment for mild to moderate FTD, highlighting the urgent need to develop more effective FTD therapeutics.
Research in Context
Systematic review
We searched Pubmed using the following terms: “memantine,” and “frontotemporal dementia,” “semantic dementia,” “frontotemporal lobar degeneration,” “Pick’s,” “FTD,” “FTLD,” “primary progressive aphasia,” “PPA,” “corticobasal,” or “aphasia.” We included randomized, placebo-controlled trials in FTD or a related disorder that involved memantine. We identified one prior RCT in bvFTD21 and one in PPA (not differentiated by subtype). 22 We conducted a study-level meta-analysis, combining six month CGIC data from the current study and 12 month data from Table 4 from the previous bvFTD clinical trial 21, but not the PPA trial because it was not limited to SD, for a combined total of n=64 placebo and n=55 memantine cases. There was no difference between placebo and memantine on the combined global impression scores (MD = 0.082, 95%CI: −0.18, 0.34; p = 0.553, Mann-Whitney U).
Interpretation
This study confirms the lack of benefit of memantine for treatment of FTD.
Supplementary Material
Acknowledgments
This was an investigator-initiated study that was designed by the authors and managed by the UCSF Memory and Aging Center Clinical Trials Program. The study was funded by Forest Research Institute (FRI), the research arm of Forest Laboratories, the company that manufactures and markets memantine for treatment of Alzheimer’s Disease in the US, in response to a grant application from ALB. In addition to funding, FRI provided memantine and matched placebo tablets to the UCSF Investigational Pharmacy which created blister packs to improve compliance, monitored lot expiration and resupplied sites. FRI had no role in study design, data collection, analysis or interpretation. All data were available to and the manuscript was written by the corresponding author with assistance from other authors. FRI had no role in manuscript preparation.
We thank Drs. Heidi Kirsch and Michael Greicius and Mr. Ron Finley for their service on the Data Safety Monitoring Board.
Footnotes
Author Contributions and Disclosures
Adam L. Boxer, MD, PhD obtained funding, designed and supervised the study, and wrote the manuscript. Dr. Boxer has been a consultant for Plexikkon, Phloronol, Registrat-Mapi, Envivo, Neurophage, TauRx, Archer and Iperian, receives research support from Allon Therapeutics, Bristol Myers Squibb, EnVivo, Janssen, Forest, Pfizer and Genentech, and is funded by NIH grants R01AG038791, R01AG031278, the John Douglas French Foundation, the Alzheimer’s Drug Discovery Foundation, the Association for Frontotemporal Degeneration, the Silicon Valley Foundation, the Agouron Institute, the Tau Research Consortium and the Bluefield Project to Cure Frontotemporal Dementia.
David S. Knopman, MD participated in study design, enrolled subjects at his site, reviewed and made substantive comments on the manuscript. He serves as Deputy Editor for Neurology®; served on a Data Safety Monitoring Board for Lilly Pharmaceuticals; served as a consultant to TauRx, was an investigator in clinical trials sponsored by Baxter, Elan Pharmaceuticals, and Forest Pharmaceuticals; and receives research support from the NIH.
Daniel I. Kaufer, MD participated in study design, enrolled subjects at his site, reviewed and made substantive comments on the manuscript. He has no other relevant disclosures.
Murray Grossman, MD participated in study design, enrolled subjects at his site, reviewed and made substantive comments on the manuscript. He has no other relevant disclosures.
Chiadi Onyike, MD participated in study design, enrolled subjects at his site, reviewed and made substantive comments on the manuscript. He has no other relevant disclosures.
Neill Graf-Radford, MD participated in study design, enrolled subjects at his site, reviewed and made substantive comments on the manuscript. He has no other relevant disclosures.
Mario Mendez, MD participated in study design, enrolled subjects at his site, reviewed and made substantive comments on the manuscript. He has no other relevant disclosures.
Diana Kerwin, MD enrolled subjects at her site, reviewed and made substantive comments on the manuscript. She has no other relevant disclosures.
Alan Lerner, MD participated in study design, enrolled subjects at his site, reviewed and made substantive comments on the manuscript. He has no other relevant disclosures.
Chuang-Kuo Wu, MD participated in study design, enrolled subjects at his site, reviewed and made substantive comments on the manuscript. He has no other relevant disclosures.
Mary Koestler, PhD participated in study design, helped supervise study conduct and reviewed the manuscript. She has no other relevant disclosures.
Jill Shapira, RN participated in study design, helped supervise enrolment at her site and reviewed the manuscript. She has no other relevant disclosures.
Kathryn Sullivan, BS assisted with study conduct, data collection, cleaning and analysis. She has no relevant disclosures.
Kristen Klepac, BS assisted with study conduct, data collection, cleaning and analysis. She has no relevant disclosures.
Kristine Lipowski, BS assisted with subject recruitment and data collection. She has nothing to disclose
Jerin Ullah, MS performed the statistical analysis. She has no relevant disclosures.
Scott Fields, PharmD created the medication administration, randomization and blinding scheme, and supervised all aspects of study medication management. He has nothing to disclose.
Joel H. Kramer, PsyD participated in study design including selection of the neuropsychological tests and helped with their interpretation. He has no relevant disclosures.
Jennifer Merrilees, PhD participated in patient recruitment and evaluation, and reviewed the final manuscript.
John Neuhaus, PhD participated in study design including the statistical analysis plan. He supervised Ms. Ullah in her statistical analysis. He has nothing to disclose.
M. Marsel Mesulam, MD participated in patient recruitment and evaluation, reviewed the final manuscript and provided substantive feedback. He has nothing to disclose.
Bruce L. Miller, MD participated in conceptualization of the study, recruiting subjects and revising the manuscript. Dr. Miller serves as board member on the John Douglas French Alzheimer’s Foundation and Larry L. Hillblom Foundation, serves as a consultant for TauRx, Ltd., Allon Therapeutics, the Tau Consortium and the Consortium for Frontotemporal research, has received institutional support from Novartis, and is funded by NIH grants P50AG023501, P01AG019724, P50 AG1657303, and the state of CA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–1554. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 2.Rosen HJ, Allison SC, Ogar JM, et al. Behavioral features in semantic dementia vs other forms of progressive aphasias. Neurology. 2006;67:1752–1756. doi: 10.1212/01.wnl.0000247630.29222.34. [DOI] [PubMed] [Google Scholar]
- 3.Huey ED, Putnam KT, Grafman J. A systematic review of neurotransmitter deficits and treatments in frontotemporal dementia. Neurology. 2006;66:17–22. doi: 10.1212/01.wnl.0000191304.55196.4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu B, Ross L, Neuhaus J, et al. Off-Label Medication Use in Frontotemporal Dementia. American Journal Alzheimer’s Disease and Other Dementias. 2010;25:128–33. doi: 10.1177/1533317509356692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalia LV, Kalia SK, Salter MW. NMDA receptors in clinical neurology: excitatory times ahead. Lancet Neurol. 2008;7:742–755. doi: 10.1016/S1474-4422(08)70165-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cummings JL, Schneider E, Tariot PN, Graham SM. Behavioral effects of memantine in Alzheimer disease patients receiving donepezil treatment. Neurology. 2006;67:57–63. doi: 10.1212/01.wnl.0000223333.42368.f1. [DOI] [PubMed] [Google Scholar]
- 7.Swanberg MM. Memantine for behavioral disturbances in frontotemporal dementia: a case series. Alzheimer Dis Assoc Disord. 2007;21:164–166. doi: 10.1097/WAD.0b013e318047df5d. [DOI] [PubMed] [Google Scholar]
- 8.Boxer AL, Lipton AM, Womack K, et al. An Open Label Study of Memantine in Three Subtypes of Frontotemporal Lobar Degeneration. Alzheimer Dis Assoc Disord. 2009;23:211–217. doi: 10.1097/WAD.0b013e318197852f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48:S10–16. doi: 10.1212/wnl.48.5_suppl_6.10s. [DOI] [PubMed] [Google Scholar]
- 10.Schneider LS, Olin JT, Doody RS, et al. Validity and reliability of the Alzheimer’s Disease Cooperative Study-Clinical Global Impression of Change. The Alzheimer’s Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11 (Suppl 2):S22–32. doi: 10.1097/00002093-199700112-00004. [DOI] [PubMed] [Google Scholar]
- 11.Kipps CM, Nestor PJ, Dawson CE, Mitchell J, Hodges JR. Measuring progression in frontotemporal dementia: implications for therapeutic interventions. Neurology. 2008;70:2046–2052. doi: 10.1212/01.wnl.0000313366.76973.8a. [DOI] [PubMed] [Google Scholar]
- 12.Tariot PN, Farlow MR, Grossberg GT, Graham SM, McDonald S, Gergel I. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. Jama. 2004;291:317–324. doi: 10.1001/jama.291.3.317. [DOI] [PubMed] [Google Scholar]
- 13.Mendez MF, Shapira JS, McMurtray A, Licht E. Preliminary findings: behavioral worsening on donepezil in patients with frontotemporal dementia. Am J Geriatr Psychiatry. 2007;15:84–87. doi: 10.1097/01.JGP.0000231744.69631.33. [DOI] [PubMed] [Google Scholar]
- 14.Knopman DS, Kramer JH, Boeve BF, et al. Development of methodology for conducting clinical trials in frontotemporal lobar degeneration. Brain. 2008;131:2957–2968. doi: 10.1093/brain/awn234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37:323–329. doi: 10.1093/geronj/37.3.323. [DOI] [PubMed] [Google Scholar]
- 16.Cullum CM, Saine K, Chan LD, Martin-Cook K, Gray KF, Weiner MF. Performance-Based instrument to assess functional capacity in dementia: The Texas Functional Living Scale. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:103–108. [PubMed] [Google Scholar]
- 17.Royall DR, Mahurin RK, Gray KF. Bedside assessment of executive cognitive impairment: the executive interview. J Am Geriatr Soc. 1992;40:1221–1226. doi: 10.1111/j.1532-5415.1992.tb03646.x. [DOI] [PubMed] [Google Scholar]
- 18.Bedard M, Molloy DW, Squire L, Dubois S, Lever JA, O’Donnell M. The Zarit Burden Interview: a new short version and screening version. Gerontologist. 2001;41:652–657. doi: 10.1093/geront/41.5.652. [DOI] [PubMed] [Google Scholar]
- 19.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, N.J: L. Erlbaum Associates; 1988. [Google Scholar]
- 20.Vercelletto M, Boutoleau-Bretonniere C, Volteau C, et al. Memantine in behavioral variant frontotemporal dementia: negative results. Journal of Alzheimer’s disease : JAD. 2011;23:749–759. doi: 10.3233/JAD-2010-101632. [DOI] [PubMed] [Google Scholar]
- 21.Boxer AL, Gold M, Huey E, et al. The advantages of frontotemporal degeneration drug development (part 2 of frontotemporal degeneration: The next therapeutic frontier) Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2012 doi: 10.1016/j.jalz.2012.03.003. epub. Oct. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson NA, Rademaker A, Weintraub S, Gitelman D, Wienecke C, Mesulam M. Pilot trial of memantine in primary progressive aphasia. Alzheimer disease and associated disorders. 2010;24:308. doi: 10.1097/WAD.0b013e3181cf468d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kavirajan H, Schneider LS. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: a meta-analysis of randomised controlled trials. Lancet neurology. 2007;6:782–792. doi: 10.1016/S1474-4422(07)70195-3. [DOI] [PubMed] [Google Scholar]
- 24.Aarsland D, Ballard C, Walker Z, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet neurology. 2009;8:613–618. doi: 10.1016/S1474-4422(09)70146-2. [DOI] [PubMed] [Google Scholar]
- 25.Emre M, Tsolaki M, Bonuccelli U, et al. Memantine for patients with Parkinson’s disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet neurology. 2010;9:969–977. doi: 10.1016/S1474-4422(10)70194-0. [DOI] [PubMed] [Google Scholar]
- 26.Boxer AL, Gold M, Huey E, et al. Frontotemporal degeneration, the next therapeutic frontier: Molecules and animal models for frontotemporal degeneration drug development. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2012 doi: 10.1016/j.jalz.2012.03.002. epub, Oct. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Williams MM, Storandt M, Roe CM, Morris JC. Progression of Alzheimer’s disease as measured by Clinical Dementia Rating Sum of Boxes scores. Alzheimers Dement. 2012 doi: 10.1016/j.jalz.2012.01.005. epub, Aug. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.