Abstract
Schizophrenia is associated with auditory processing impairments that could arise as a result of primary auditory cortex excitatory circuit pathology. We have previously reported a deficit in dendritic spine density in deep layer 3 of primary auditory cortex in subjects with schizophrenia. As boutons and spines can be structurally and functionally co-regulated, we asked whether the densities of intracortical excitatory or thalamocortical presynaptic boutons are also reduced. We studied 2 cohorts of subjects with schizophrenia and matched controls, comprising 27 subject pairs and assessed the density, number, and within-bouton vesicular glutamate transporter (VGluT) protein level of intracortical excitatory (VGluT1-immunoreactive) and thalamocortical (VGluT2-immunoreactive) boutons in deep layer 3 of primary auditory cortex using quantitative confocal microscopy and stereologic sampling methods. We found that VGluT1- and VGluT2-immunoreactive puncta densities and numbers were not altered in deep layer 3 of primary auditory cortex of subjects with schizophrenia. Our results indicate that reduced dendritic spine density in primary auditory cortex of subjects with schizophrenia is not matched by a corresponding reduction in excitatory bouton density. This suggests excitatory boutons in primary auditory cortex in schizophrenia may synapse with structures other than spines, such as dendritic shafts, with greater frequency. The discrepancy between dendritic spine reduction and excitatory bouton preservation may contribute to functional impairments of the primary auditory cortex in subjects with schizophrenia.
Keywords: VGluT1, VGluT2, primary auditory cortex, quantitative microscopy, stereology, postmortem human tissue
1. Introduction
Individuals with schizophrenia exhibit impairments in auditory processing, including pure tone frequency discrimination (Javitt et al. 2000; Leitman et al. 2010). Electrophysiological deficits observed during auditory processing tasks, such as reduction in the mismatch negativity (MMN) response to stimulus changes during a repetitive acoustic stimulus presentation (Javitt et al. 1994; Javitt et al. 2000; Naatanen and Kahkonen 2009), are also observed in schizophrenia. In addition to functional abnormalities, gray matter volume loss is consistently reported in the anatomical region of the primary auditory cortex in the superior temporal gyrus (STG) (McCarley et al. 1999; Rajarethinam et al. 2004) and Heschl’s gyrus (Hirayasu et al. 2000; Kasai et al. 2003; Salisbury et al. 2007; Takahashi et al. 2009) of subjects with schizophrenia and high-risk individuals.
Evidence suggests that alterations of excitatory, glutamatergic synapses could underlie the structural and functional abnormalities in the auditory cortex in schizophrenia. For example, the MMN response is disrupted by blocking the NMDA subtype of ionotropic glutamate receptor (Gunduz-Bruce et al. 2012; Javitt et al. 1996). In subjects with schizophrenia, spinophilin-immunoreactive (−IR) puncta density (a marker of dendritic spines) is reduced in the primary auditory cortex (Sweet et al. 2009), suggesting that the density of postsynaptic components of excitatory synapses in the auditory cortex is reduced. Trans-synaptic communication coordinates functional and structural changes between pre- and postsynaptic elements, as indicated by reports that postsynaptic alterations such as AMPA receptor removal (Ripley et al. 2011) or protein synthesis inhibition (McCann et al. 2007) lead to presynaptic bouton loss. Therefore, reduced dendritic spine density in schizophrenia may be indicative of a coordinated reduction in excitatory bouton density. Alternatively, if excitatory bouton density is unchanged, then this could indicate that the distribution of their postsynaptic targets is altered. For example, excitatory boutons may synapse with dendritic shafts with greater frequency, which occurs following spine loss in culture (Mateos et al. 2007; Woods et al. 2011). Here, we asked whether the density of excitatory boutons in deep layer 3 of primary auditory cortex is reduced in individuals with schizophrenia.
Glutamatergic inputs to the primary auditory cortex can be broadly classified into two groups: thalamocortical inputs primarily to layer 4 and deep layer 3 from the medial geniculate nucleus of the thalamus, and intracortical excitatory inputs originating within primary auditory cortex and from other cortical regions. It is important to distinguish between the two populations because alterations of excitatory synapses in the primary auditory cortex may have different functional consequences depending on whether thalamocortical boutons or intracortical excitatory boutons are affected. The two populations can be differentiated at the molecular level by the presence of different isoforms of the vesicular glutamate transporter (VGluT). Most reports indicate that VGluT1 is preferentially expressed in neurons in the cortex and hippocampus, and VGluT2 is preferentially expressed in neurons located in subcortical structures, including some nuclei of the thalamus (Fremeau, Jr. et al. 2001; Fremeau, Jr. et al. 2004a; Fremeau, Jr. et al. 2004b; Kaneko and Fujiyama 2002). Levels of VGluT protein have also been shown to be correlated with glutamate release and synaptic strength (Wilson et al. 2005), and so changes in within-bouton levels of these proteins may indicate functional impairments of these synapses.
We used quantitative fluorescence confocal microscopy to examine VGluT1- and VGluT2-immunoreactive puncta in primary auditory cortex of two cohorts of subjects, comprising a total of 27 subjects with schizophrenia and an equal number of matched controls. We found that densities and numbers of thalamocortical and intracortical excitatory boutons, and within-bouton VGluT protein levels were not altered in deep layer 3 of primary auditory cortex of subjects with schizophrenia. Taken together with our previous finding of reduced dendritic spine density in this region, our data suggest that excitatory boutons may aberrantly target non-spine postsynaptic structures, such as dendritic shafts. A shift in the distribution of excitatory synapses away from spines and onto dendritic shafts could alter excitatory circuit function in the primary auditory cortex.
2. Materials and Methods
2.1 Subjects and Animals
We studied two cohorts (Table 1 and Supplemental Table S1) of subjects diagnosed with schizophrenia or schizoaffective disorder and matched controls included in our previous studies (Dorph-Petersen et al. 2009; Moyer et al. 2012; Sweet et al. 2004; Sweet et al. 2007; Sweet et al. 2009). We also studied a cohort of four male macaque monkeys (Macaca fascicularis) chronically exposed to haloperidol decanoate, and four control macaques matched for sex and weight (Sweet et al. 2007). See Supplemental Methods for further description of subjects and tissue processing.
Table 1.
Summary of subject characteristics for cohorts 1 and 2. The same group of subjects was studied previously (Moyer et al. 2012). Each subject in cohorts 1 and 2 was previously matched to a normal comparison subject based on sex, and as closely as possible for age, postmortem interval (PMI), and group matched for handedness. There were no diagnostic group differences in age (t52=0.517, p=0.608) or PMI (t52=0.584, p=0.561) or in the distribution of handedness between diagnostic groups (χ1 2=1.46, p=0.314). Mean storage time did not differ between diagnostic groups (Cohort1: t28=0.040, p=0.968; Cohort 2: t22=0.596, p=0.557). Tobacco use at time of death was more frequent in the schizophrenia group compared to the control group (χ1 2=5.28, p=0.022).
| COHORT 1 | COHORT 2 | TOTAL | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Control | Schizophrenia | Control | Schizophrenia | Control | Schizophrenia | |
|
| ||||||
| N | 15 | 15 | 12 | 12 | 27 | 27 |
|
| ||||||
| Mean Age (SD) | 46.8 (8.3) | 47.6 (5.5) | 45.1 (12.9) | 47.3 (13.4) | 46.0 (10.4) | 47.4 (9.6) |
| Range | 27–64 | 38–63 | 19–65 | 25–71 | 19–65 | 25–71 |
|
| ||||||
| Sex (F/M) | 6/9 | 6/9 | 4/8 | 4/8 | 10/17 | 10/17 |
|
| ||||||
| Handedness (R/L/M/U) | 10/4/0/1 | 7/3/1/4 | 11/1/0/0 | 6/2/1/3 | 21/5/0/1 | 13/5/2/7 |
|
| ||||||
| PMI (SD) | 13.9 (5.5) | 15.9 (6.6) | 18.0 (6.6) | 17.9 (8.8) | 15.7 (6.3) | 16.8 (7.6) |
|
| ||||||
| Storage Time, mos (SD) | 168 (29) | 167 (26) | 111 (27) | 102 (30) | 142 (40) | 138 (43) |
|
| ||||||
| Illness Duration, yrs (SD) | 24.9 (5.6) | 22.1 (14.6) | 23.7 (10.6) | |||
| Range | 14–34 | 3–50 | 3–50 | |||
|
| ||||||
| Suicide, N (%) | 3 (20%) | 2 (17%) | 5 (19%) | |||
|
| ||||||
| Schizoaffective, N (%) | 3 (20%) | 4 (33%) | 7 (26%) | |||
|
| ||||||
| Living independently ATOD, N (%) | 7 (47%) | 2 (17%) | 9 (33%) | |||
|
| ||||||
| Alcohol/Substance abuse ATOD, N (%) | 9 (60%) | 7 (58%) | 16 (59%) | |||
|
| ||||||
| Antipsychotic ATOD, N (%) | 13 (87%) | 11 (92%) | 24 (89%) | |||
|
| ||||||
| Tobacco use ATOD (Y/N/U) | 3/4/8 | 9/2/4 | 4/6/2 | 8/3/1 | 7/10/10 | 17/5/5 |
Abbreviations: SD, standard deviation; F/M, female/male; R/L/A/U, right-handed/left-handed/ambidextrous/unknown; mos, months; yrs, years; N, number; ATOD, at time of death; Y/N/U, yes/no/unknown.
2.2 Immunohistochemistry
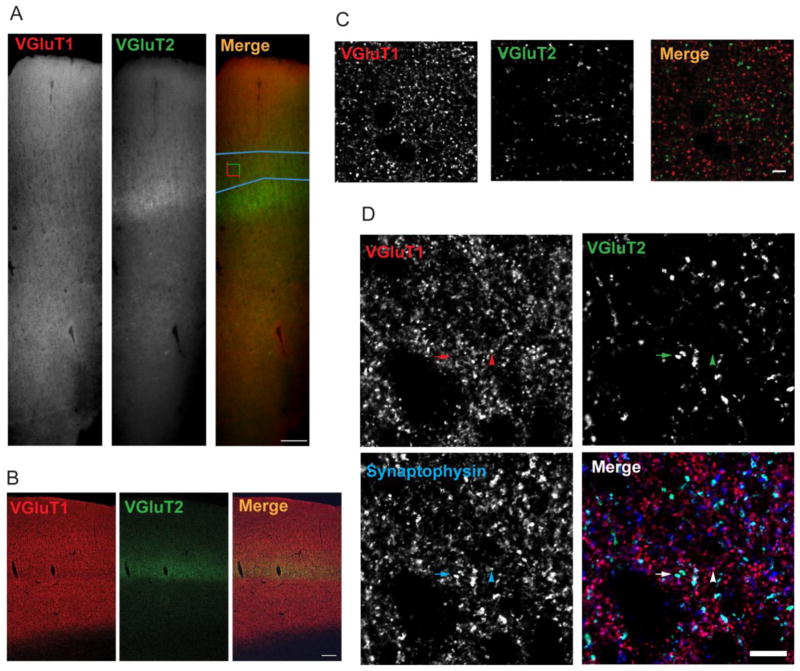
Auditory cortex containing tissue sections (3 from each subject in cohort 1 and 4 from each subject in cohort 2) from matched pairs were processed together in immunohistochemistry runs. VGluT1 was detected using a guinea pig anti-VGluT1 antibody (AB5905, Millipore, Billerica, MA), and VGluT2 was detected using a rabbit anti-VGluT2 antibody (V2514, HY-19; Sigma-Aldrich, St. Louis, MO). Although some evidence suggests that mRNA for these transporters may be co-expressed (De Gois et al. 2005; Graziano et al. 2008; Hackett et al. 2011), VGluT1 and 2 proteins demonstrate non-overlapping and complimentary patterns of expression throughout the brain. VGluT1 is the predominant vesicular glutamate transporter in corticocortical and corticothalamic neurons, while VGluT2 is the predominant transporter expressed in thalamocortical neurons (Fremeau, Jr. et al. 2001; Fremeau, Jr. et al. 2004a; Fremeau, Jr. et al. 2004b; Hackett et al. 2011). In monkey and human auditory cortex, we observed the expected pattern of VGluT1 immunoreactivity across all cortical layers, and an apparent increase in VGluT2 immunoreactivity in the thalamocortical termination zone in deep layer 3 and layer 4 (Figure 1A and 1B). In addition, we observed minimal colocalized labeling of puncta with these anti-VGluT1 and -VGluT2 antibodies, although both markers showed extensive colocalization with an antibody targeting the presynaptic bouton marker synaptophysin (Figure 1C and 1D). Also, we observed no colocalization of VGluT1- and VGluT2-immunoreactive (IR) puncta with a marker of inhibitory GABAergic presynaptic boutons, glutamate decarboxylase-65 (Moyer et al. 2012).
Figure 1.
Specificity of antibodies used to identify VGluT1- and VGluT2-immunoreactive puncta. A. VGluT1 and VGluT2 immunoreactivity across all cortical layers of primary auditory cortex of one human subject from cohort 2. Blue outline in “Merge” panel represents the deep layer 3 contour, and the counting frame represents a sampling site. Scale bar is 200 μm. B. VGluT1 and VGluT2 immunoreactivity across all cortical layers of primary auditory cortex of a macaque. Image was taken using a laser scanning confocal. Scale bar is 200 μm. C. The antibodies directed against VGluT1 and VGluT2 label two separate bouton populations with no observable colocalization of markers. Scale bar is 10 μm. D. The VGluT1 and VGluT2 antibodies demonstrate overlap with an antibody directed against synaptophysin, a protein found in all classical neurotransmitter releasing boutons. The arrowheads indicate a bouton that is immunoreactive for VGluT1 and synaptophysin and arrows indicate a bouton that is immunoreactive for VGluT2 and synaptophysin. Scale bar is 10 μm.
2.3 Quantification of VGluT1- and VGluT2-IR Puncta
VGluT1- and VGluT2-IR puncta within deep layer 3 of primary auditory cortex were quantified in this study using spinning disk confocal microscopy. Stereologic sampling, confocal imaging, and image processing were conducted as described previously (Moyer et al. 2012), and are described in the Supplemental Methods. Mean puncta fluorescence intensity, and mean density and number of puncta in deep layer 3 were determined for VGluT1- and VGluT2-IR puncta.
See Supplemental Methods for additional details of statistical analyses.
3. Results
3.1 VGluT1-IR Puncta
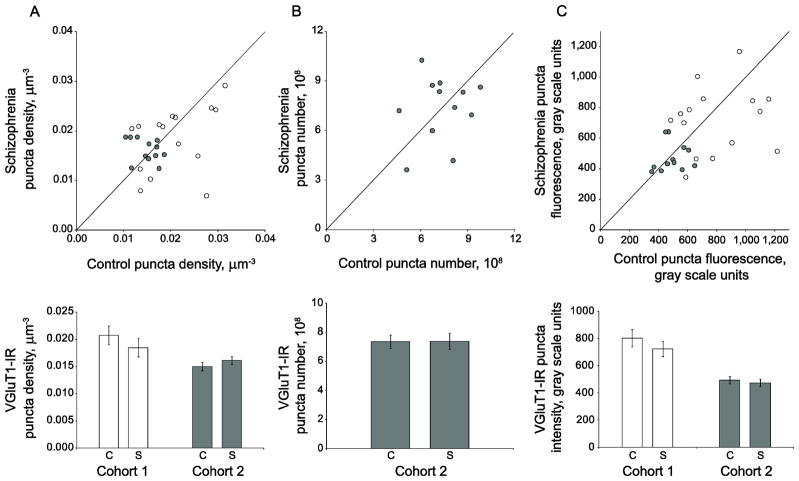
The density of VGluT1-IR puncta was not significantly different between subjects with schizophrenia and control subjects (F(1, 23.2)= 0.34, p=0.568, 95% CI: (−.001,.002)) (Figure 2A). Because we have previously determined the total volume of primary auditory cortex layer 3 for cohort 2 subjects (Dorph-Petersen et al. 2009), we were able to estimate the total number of VGluT1-IR puncta in primary auditory cortex deep layer 3 for the 12 subject pairs of cohort 2 and we found that the absolute number of VGluT1-IR puncta was not different in schizophrenia (F(1, 10) =0.003, p=0.956, 95% CI: (−1.62 × 108, 1.54 × 108)) (Figure 2B). Because of the differences in tissue processing methods, we were not able to estimate puncta number in subject pairs from cohort 1. We observed no significant alterations in primary auditory cortex deep layer 3 VGluT1-IR puncta fluorescence intensity (F(1, 149)= 0.42, p=0.518, 95% CI: (−.09,.18)), indicating that relative levels of within-bouton VGluT1 protein are not different between schizophrenia and control subjects (Figure 2C).
Figure 2.
VGluT1-IR puncta density, number, and mean fluorescence intensity are unaltered in deep layer 3 of primary auditory cortex of subjects with schizophrenia. A. (Top) VGluT1-IR puncta density for subjects in cohort 1 (open circles) and cohort 2 (gray circles). Reference line represents schizophrenia = control values, where points below the line indicate a pair where control > schizophrenia, and points above the line indicate schizophrenia > control. (Bottom) Diagnostic group mean puncta density for control (c) and schizophrenia (s) subjects in cohort 1 (open bars) and cohort 2 (gray bars). Error bars are +/− SEM. B. (Top) VGluT1-IR puncta number for subjects in cohort 2. Reference line represents schizophrenia = control values, where points below the line indicate a pair where control > schizophrenia, and points above the line indicate schizophrenia > control. (Bottom) Diagnostic group mean puncta number for control (c) and schizophrenia (s) subjects in cohort 2. Error bars are +/− SEM. C. (Top) VGluT1-IR puncta fluorescence intensity for subjects in cohort 1 (open circles) and cohort 2 (gray circles). Reference line represents schizophrenia = control values, where points below the line indicate a pair where control > schizophrenia, and points above the line indicate schizophrenia > control. (Bottom) Diagnostic group mean puncta fluorescence intensity for control (c) and schizophrenia (s) subjects in cohort 1 (open bars) and cohort 2 (gray bars). Error bars are +/− SEM.
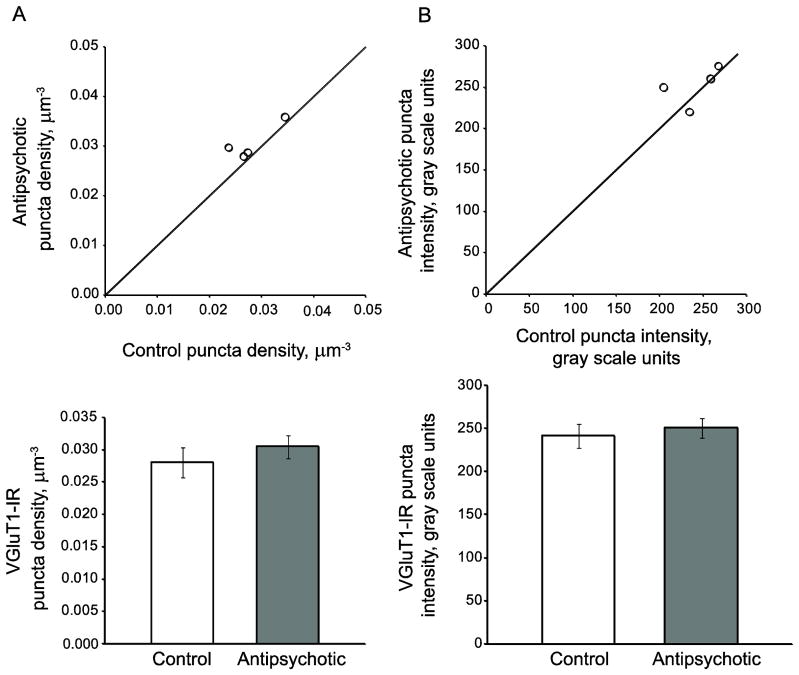
We observed no effect of chronic haloperidol exposure on density (F(1, 17.3)=0.95; p=0.343, 95% CI: (−.003,.01)) or intensity (F(1, 18.6)=0.36; p=0.554, 95% CI: (−.11,.21)), of VGluT1-IR puncta in deep layer 3 of primary auditory cortex of antipsychotic-exposed macaques (Figure 3).
Figure 3.
Chronic antipsychotic exposure does not alter VGluT1-IR puncta mean density or fluorescence intensity. (Top) VGluT1-IR puncta mean density (A) or fluorescence intensity (B) for monkey cohort. Reference line represents antipsychotic = control values, where points below the line indicate a pair where control > antipsychotic-exposed, and points above the line indicate antipsychotic-exposed > control. (Bottom) Group means for control (open bars) and antipsychotic-exposed (gray bars) animals. Error bars are +/− SEM.
3.2 VGluT2-IR puncta
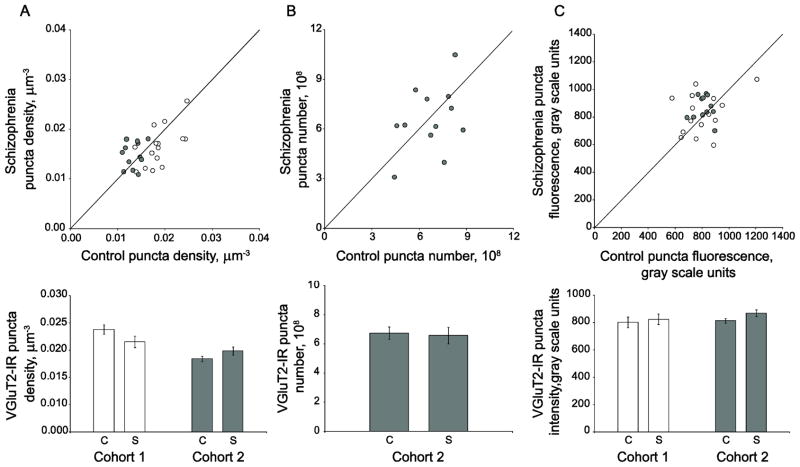
We observed no significant change in primary auditory cortex deep layer 3 VGluT2-IR puncta density (F(1, 23.3)= 0.97, p=0.335, 95% CI: (−.0003,.001)) in subjects with schizophrenia (Figure 4A). Similarly, we found that the absolute number of VGluT2-IR puncta was not altered in cohort 2 schizophrenia subjects relative to matched controls (F(1, 10) =0.153, p=0.704, 95% CI: (−1.11 × 108, 1.58 × 108)) (Figure 4B). There were also no diagnostic group differences in primary auditory cortex deep layer 3 VGluT2-IR puncta fluorescence intensity (F(1, 23.2)= 0.69, p=0.413, 95% CI: (−.09,.04)) (Figure 4C).
Figure 4.
VGluT2-IR bouton density, number, and mean fluorescence intensity are unaltered in deep layer 3 of primary auditory cortex of subjects with schizophrenia. A. (Top) VGluT2-IR puncta density for subjects in cohort 1 (open circles) and cohort 2 (gray circles). Reference line represents schizophrenia = control values, where points below the line indicate a pair where control > schizophrenia, and points above the line indicate schizophrenia > control. (Bottom) Diagnostic group mean puncta density for control (c) and schizophrenia (s) subjects in cohort 1 (open bars) and cohort 2 (gray bars) Error bars are +/− SEM. B. (Top) VGluT2-IR puncta number for subjects in cohort 2. Reference line represents schizophrenia = control values, where points below the line indicate a pair where control > schizophrenia, and points above the line indicate schizophrenia > control. (Bottom) Diagnostic group mean puncta number for control (c) and schizophrenia (s) subjects in cohort 2. Error bars are +/− SEM. C. (Top) Mean VGluT2-IR puncta fluorescence intensity for each schizophrenia-control subject pair in cohort 1 (open circles) and cohort 2 (gray circles). Reference line represents schizophrenia = control values, where points below the line indicate a pair where control > schizophrenia, and points above the line indicate schizophrenia > control. (Bottom) Diagnostic group mean puncta fluorescence intensity for control (c) and schizophrenia (s) subjects in cohort 1 (open bars) and cohort 2 (gray bars). Error bars are +/− SEM.
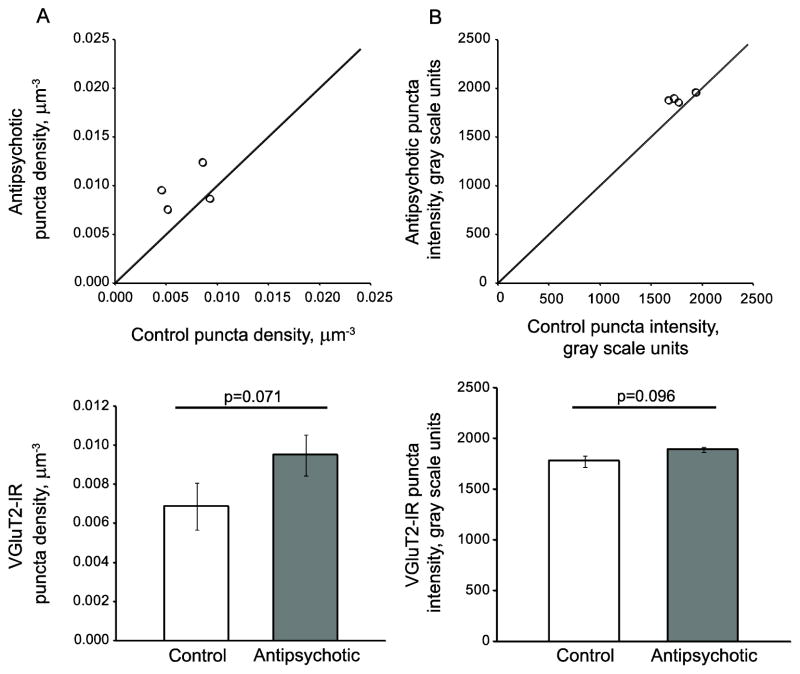
We observed trends toward increased VGluT2-IR puncta fluorescence intensity (F(1, 20.6)= 3.05, p=0.096, 95% CI: (−.02,.17)) and increased VGluT2-IR bouton puncta density (F(1, 20.5)=3.64, p=0.071, 95% CI: (−.0002,.003)) in haloperidol-exposed macaques, although neither reached statistical significance (Figure 5).
Figure 5.
Chronic antipsychotic exposure does not alter VGluT2-IR puncta mean density or fluorescence intensity. (Top) VGluT2-IR puncta mean density (A) or fluorescence intensity (B) for monkey cohort. Reference line represents antipsychotic = control values, where points below the line indicate a pair where control > antipsychotic-exposed, and points above the line indicate antipsychotic-exposed > control. (Bottom) Group means for control (open bars) and antipsychotic-exposed (gray bars) animals. Error bars are +/− SEM.
4. Discussion
Here we asked whether there are alterations in intracortical excitatory or thalamocortical boutons in deep layer 3 of the primary auditory cortex of subjects with schizophrenia. We report that neither VGluT1-IR intracortical excitatory nor VGluT2-IR thalamocortical boutons are altered in density or number. To the best of our knowledge, no studies have examined densities of these two bouton populations in schizophrenia, although the question of whether VGluT1 or VGluT2 mRNA or protein levels are altered has been asked. Conflicting studies of VGluT1 expression in schizophrenia report reduced mRNA in prefrontal cortex and hippocampus (Eastwood and Harrison 2005), no change in mRNA in the prefrontal cortex (Fung et al. 2011; Oni-Orisan et al. 2008), and increased mRNA and protein in anterior cingulate cortex (Oni-Orisan et al. 2008). Studies of VGluT2 expression in schizophrenia have found that mRNA and protein are unaltered in anterior cingulate and prefrontal cortices (Oni-Orisan et al. 2008), and that mRNA expression in inferior temporal cortex is reduced (Uezato et al. 2009). In the present study, we did not find VGluT1-or VGluT2-IR puncta fluorescence intensities to be altered, suggesting that within-bouton VGluT protein levels are unchanged in subjects with schizophrenia. This indicates that our ability to detect immunoreactive puncta was unimpaired by any changes in VGluT protein levels within presumptive boutons. This also suggests that excitatory boutons in the auditory cortex of subjects with schizophrenia are not impaired in terms of their VGluT protein content. However it remains possible that levels of other proteins involved in glutamate release are altered within excitatory boutons, as reductions in mRNA expression of other presynaptic release machinery genes have been reported in schizophrenia (Mirnics et al. 2000).
4.1 Possible implications of auditory cortex spine loss and excitatory bouton preservation
Together with our previous study (Sweet et al. 2009) we found that spine density is reduced in auditory cortex of subjects with schizophrenia but excitatory bouton density is not reduced. One interpretation of our result is that the primary auditory cortex in subjects with schizophrenia has a normal complement of excitatory boutons but that some of these lack postsynaptic contacts. Evidence of boutons which lack postsynaptic contacts has been reported in electron microscopy studies (Jones et al. 1997; Shepherd and Harris 1998) which suggests that boutons are stable in the absence of a post-synaptic contact. However, chronic in vivo imaging studies demonstrate varying rates of turnover for presynaptic boutons in the cortex (De Paola et al. 2006). This would suggest that boutons do not persist without postsynaptic contacts, but instead undergo formation and elimination such that a cross-sectional microscopy analysis will uncover a certain percentage of boutons which appear to not contact postsynaptic structures.
Probably the most parsimonious explanation for the observed deficit in dendritic spines but not excitatory boutons in the primary auditory cortex in schizophrenia is that a greater proportion of excitatory boutons form synapses with dendritic shafts rather than spines. This could result from a failure in the downregulation of shaft synapses relative to spine synapses during early development, as occurs during the first few postnatal weeks in the rat hippocampus (Boyer et al. 1998; Fiala et al. 1998). Alternatively, several lines of experimental evidence suggest that the prevalence of excitatory shaft synapses may increase when dendritic spines are lost. In the hippocampus, spine loss leads to an increase in the number of synapses occurring on dendritic shafts (Mateos et al. 2007), and an enrichment of PSD-95 in the dendritic shaft (Woods et al. 2011). Whether due to a developmental disturbance or to spine loss, it is tempting to speculate that individuals with schizophrenia may demonstrate an increased proportion of shaft synapses relative to spine synapses. Indeed, recent electron microscopy evidence has revealed an increased proportion of asymmetric synapses formed with dendritic shafts in the anterior cingulate cortex of subjects with schizophrenia (Barksdale et al. 2012). Future studies could examine if VGluT-IR boutons in the auditory cortex of subjects with schizophrenia demonstrate a relative increase in frequency of apposition to dendritic shaft postsynaptic densities, which would suggest an increased prevalence of shaft-targeting excitatory synapses. It would also be of interest to determine if expression of proteins which have been shown to mediate shaft synapse formation, such as GRIP1 and ephrinB3 (Aoto et al. 2007), are altered in this cortical region. Finally, emphasis should be placed on characterizing animal models of structural excitatory synapse pathology relevant to schizophrenia. For example, models of increased adolescent spine elimination such as the kalirin knockout mouse (Cahill et al. 2009), could be evaluated to determine whether excitatory boutons persist despite reduction in cortical spine density, and whether the proportions of shaft and spine synapses are altered.
The functional implications of a relative increase in shaft synapses concurrent with a reduction in spine synapses are not fully understood. Spines play important roles in segregating frequency inputs within the auditory cortex (Chen et al. 2011) and serve to normalize the magnitude of inputs occurring at different distances from the soma (Harnett et al. 2012). In addition there is a reduction in dendritic length and branching in the cortex of individuals with schizophrenia (Broadbelt et al. 2002; Glantz and Lewis 2000) that would be expected to alter excitatory input summation, EPSP magnitude (Jaslove 1992), summation of responses at the soma (Shepherd et al. 1989), and neuron firing rates (Tsay and Yuste 2004). Determining whether these alterations can account for impaired frequency discrimination and reduced auditory event related potentials observed in individuals with schizophrenia, however, will require analysis in animal models that recapitulate these structural changes.
4.2 Non-glutamatergic bouton populations in auditory cortex may be affected
Previously, we reported that the density of synaptophysin-IR puncta is reduced in deep layer 3 of primary auditory cortex of subjects with schizophrenia (Sweet et al. 2007). Synaptophysin is a vesicular protein that is found in classical neurotransmitter-releasing boutons (Navone et al. 1986); therefore, this finding could represent a relative density reduction of many different types of boutons. We have previously determined that the density of GAD65-IR puncta is unchanged in deep layer 3 of primary auditory cortex (Moyer et al. 2012), and here we report that the densities of presumptive intracortical and thalamocortical glutamatergic boutons are also unchanged. However, densities of other potential synaptophysin-IR bouton types have not yet been evaluated in primary auditory cortex in schizophrenia. For example, boutons of the chandelier cell population of inhibitory neurons, which appear to be affected in schizophrenia (Lewis 2011), have been shown to express only the 67 kDa isoform of GAD (Fish et al. 2011) and thus would not have been quantified in our previous study. Also, recent evidence suggests that a sub-population of inhibitory boutons originating from somatostatin-expressing interneurons, another population which has been implicated in the neuropathology of schizophrenia (Morris et al. 2008), may express only GAD67 (Rocco and Fish 2010). Finally, it is possible that the densities of non-glutamatergic, non-GABAergic boutons, such as cholinergic, serotonergic, or dopaminergic boutons, which are present in primary auditory cortex (Campbell et al. 1987), are reduced in schizophrenia.
4.3 Use of VGluTs as markers of intracortical excitatory and thalamocortical boutons
Several potential methodological limitations are important to consider. We identified presumptive intracortical and thalamocortical boutons on the basis of their VGluT1 and VGluT2 immunoreactivity. Most reports indicate complementary and non-overlapping expression of VGluT1 and VGluT2 in the neocortex and subcortical structures (Fremeau, Jr. et al. 2001; Fremeau, Jr. et al. 2004a; Fremeau, Jr. et al. 2004b; Kaneko and Fujiyama 2002). Congruent with these reports, we observed little to no colocalization of VGluT1 and VGluT2-immunoreactivity in human and monkey auditory cortex tissue sections (Figure 1D and 1E; also see (Sweet et al. 2010)). Based on this evidence, we conclude that the failure to observe differences in intracortical excitatory and thalamocortical bouton characteristics between control and schizophrenia subjects is not due to lack of specificity of the two bouton population markers. However, it should be noted that VGluT1 and VGluT2 can be colocalized in boutons in neocortical cultures and in layer 4 of the somatosensory cortex (De Gois et al. 2005; Graziano et al. 2008), and VGluT1 and VGluT2 mRNA expression may overlap in the medial geniculate nucleus (Hackett et al. 2011) (but see (Fremeau, Jr. et al. 2004b)), suggesting that both proteins could be coexpressed in thalamocortical projections to the auditory cortex. Therefore, it is not impossible that some VGluT1-IR puncta identified in our study could actually be thalamocortical rather than intracortical excitatory boutons. In addition, although the medial geniculate nucleus is likely to contribute the majority of subcortical inputs to the primary auditory cortex, it is possible that VGluT2-IR inputs from other subcortical structures are included in our presumptive thalamocortical bouton analyses. For example, the rodent primary auditory cortex receives input from the hypothalamus and the paraventricular nuclei of the thalamus (Budinger et al. 2008). It is possible that quantifying other subcortical excitatory inputs could have masked alterations which are specific to VGluT2-IR boutons originating from the auditory thalamus but are not present in VGluT2-IR boutons from other sources.
4.4 Conclusions
In summary, we report that intracortical excitatory and thalamocortical boutons are unaltered in their density, number, and level of VGluT protein in deep layer 3 of primary auditory cortex of individuals with schizophrenia. Thus, presynaptic excitatory boutons appear to be preserved in the primary auditory cortex, despite reduced density of postsynaptic dendritic spines. Further investigation is needed to understand whether excitatory boutons have intact expression of proteins influencing glutamate release and to evaluate the functional implications of a discrepancy between preservation of boutons and loss of spines. Future studies in postmortem human tissue and animal models will aid the development of treatments aimed at normalizing the function of excitatory circuitry in schizophrenia.
Supplementary Material
Acknowledgments
Funding body agreements and policies
This work was supported by Grants MH071533 (RAS), MH084053 (DAL), and MH085108 (KNF).
The authors would like to thank Dr. C. Sue Johnston for assistance with the clinical data, Mary Brady for assistance in generating the human cortical thickness micrograph, and Ruth Henteleff and the research staff of the Translational Neuroscience Program for excellent technical assistance.
Footnotes
Contributions
RAS, KNF, DAL, and CEM designed the study, contributed to data analyses and provided intellectual contributions. CEM and KMD carried out experiments and data analysis. JKA-A and ARS contributed to statistical design and analyses. K-AD-P contributed to stereological procedures and analyses. CEM wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
ARS is a statistical consultant to Janssen Research and Development. DAL currently receives investigator-initiated research support from Bristol-Myers Squibb, Curridium Ltd and Pfizer and in 2010-2012 served as a consultant in the areas of target identification and validation and new compound development to Bristol-Myers Squibb and Concert Pharmaceuticals. RAS currently serves as a consultant to Lilly, USA. CEM, KMD, KNF, JKA-A, and K-AD-P have no biomedical financial interests or potential conflicts of interest to disclose. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health, the National Institutes of Health, the Department of Veterans Affairs, or the United States Government.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Aoto J, Ting P, Maghsoodi B, Xu N, Henkemeyer M, Chen L. Postsynaptic ephrinB3 promotes shaft glutamatergic synapse formation. J Neurosci. 2007;27:7508–7519. doi: 10.1523/JNEUROSCI.0705-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barksdale KA, Roche JK, Lahti AC, Roberts RC. Synaptic and mitochondrial changes in the postmortem anterior cingulate cortex in schizophrenia. Neuroscience Meeting Planner. 2012 doi: 10.1016/j.schres.2015.07.016. Ref Type: Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer C, Schikorski T, Stevens CF. Comparison of hippocampal dendritic spines in culture and in brain. J Neurosci. 1998;18:5294–5300. doi: 10.1523/JNEUROSCI.18-14-05294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbelt K, Byne W, Jones LB. Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophr Res. 2002;58:75–81. doi: 10.1016/s0920-9964(02)00201-3. [DOI] [PubMed] [Google Scholar]
- Budinger E, Laszcz A, Lison H, Scheich H, Ohl FW. Non-sensory cortical and subcortical connections of the primary auditory cortex in Mongolian gerbils: bottom-up and top-down processing of neuronal information via field AI. Brain Res. 2008;1220:2–32. doi: 10.1016/j.brainres.2007.07.084. [DOI] [PubMed] [Google Scholar]
- Cahill ME, Xie Z, Day M, Photowala H, Barbolina MV, Miller CA, Weiss C, Radulovic J, Sweatt JD, Disterhoft JF, Surmeier DJ, Penzes P. Kalirin regulates cortical spine morphogenesis and disease-related behavioral phenotypes. Proc Natl Acad Sci U S A. 2009;106:13058–13063. doi: 10.1073/pnas.0904636106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell MJ, Lewis DA, Foote SL, Morrison JH. Distribution of choline acetyltransferase-, serotonin-, dopamine-beta-hydroxylase-, tyrosine hydroxylase-immunoreactive fibers in monkey primary auditory cortex. J Comp Neurol. 1987;261:209–220. doi: 10.1002/cne.902610204. [DOI] [PubMed] [Google Scholar]
- Chen X, Leischner U, Rochefort NL, Nelken I, Konnerth A. Functional mapping of single spines in cortical neurons in vivo. Nature. 2011;475:501–505. doi: 10.1038/nature10193. [DOI] [PubMed] [Google Scholar]
- De Gois S, Schafer MK, Defamie N, Chen C, Ricci A, Weihe E, Varoqui H, Erickson JD. Homeostatic scaling of vesicular glutamate and GABA transporter expression in rat neocortical circuits. J Neurosci. 2005;25:7121–7133. doi: 10.1523/JNEUROSCI.5221-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paola V, Holtmaat A, Knott G, Song S, Wilbrecht L, Caroni P, Svoboda K. Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron. 2006;49:861–875. doi: 10.1016/j.neuron.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Dorph-Petersen KA, Delevich KM, Marcsisin MJ, Zhang W, Sampson AR, Gundersen HJ, Lewis DA, Sweet RA. Pyramidal neuron number in layer 3 of primary auditory cortex of subjects with schizophrenia. Brain Res. 2009;1285:42–57. doi: 10.1016/j.brainres.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Decreased expression of vesicular glutamate transporter 1 and complexin II mRNAs in schizophrenia: further evidence for a synaptic pathology affecting glutamate neurons. Schizophr Res. 2005;73:159–172. doi: 10.1016/j.schres.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci. 1998;18:8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish KN, Sweet RA, Lewis DA. Differential Distribution of Proteins Regulating GABA Synthesis and Reuptake in Axon Boutons of Subpopulations of Cortical Interneurons. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Kam K, Qureshi T, Johnson J, Copenhagen DR, Storm-Mathisen J, Chaudhry FA, Nicoll RA, Edwards RH. Vesicular glutamate transporters 1 and 2 target to functionally distinct synaptic release sites. Science. 2004a;304:1815–1819. doi: 10.1126/science.1097468. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, Bellocchio EE, Fortin D, Storm-Mathisen J, Edwards RH. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31:247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004b;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Fung SJ, Sivagnanasundaram S, Weickert CS. Lack of change in markers of presynaptic terminal abundance alongside subtle reductions in markers of presynaptic terminal plasticity in prefrontal cortex of schizophrenia patients. Biol Psychiatry. 2011;69:71–79. doi: 10.1016/j.biopsych.2010.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Graziano A, Liu XB, Murray KD, Jones EG. Vesicular glutamate transporters define two sets of glutamatergic afferents to the somatosensory thalamus and two thalamocortical projections in the mouse. J Comp Neurol. 2008;507:1258–1276. doi: 10.1002/cne.21592. [DOI] [PubMed] [Google Scholar]
- Gunduz-Bruce H, Reinhart RM, Roach BJ, Gueorguieva R, Oliver S, D’Souza DC, Ford JM, Krystal JH, Mathalon DH. Glutamatergic modulation of auditory information processing in the human brain. Biol Psychiatry. 2012;71:969–977. doi: 10.1016/j.biopsych.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett TA, Takahata T, Balaram P. VGLUT1 and VGLUT2 mRNA expression in the primate auditory pathway. Hear Res. 2011;274:129–141. doi: 10.1016/j.heares.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harnett MT, Makara JK, Spruston N, Kath WL, Magee JC. Synaptic amplification by dendritic spines enhances input cooperativity. Nature. 2012;491:599–602. doi: 10.1038/nature11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, Snyderman D, Yurgelun-Todd D, Kikinis R, Jolesz FA, Shenton ME. Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry. 2000;57:692–699. doi: 10.1001/archpsyc.57.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaslove SW. The integrative properties of spiny distal dendrites. Neuroscience. 1992;47:495–519. doi: 10.1016/0306-4522(92)90161-t. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Shelley A, Ritter W. Associated deficits in mismatch negativity generation and tone matching in schizophrenia. Clin Neurophysiol. 2000;111:1733–1737. doi: 10.1016/s1388-2457(00)00377-1. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Steinschneider M, Schroeder CE, Arezzo JC. Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci U S A. 1996;93:11962–11967. doi: 10.1073/pnas.93.21.11962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC, Steinschneider M, Schroeder CE, Vaughan HG, Jr, Arezzo JC. Detection of stimulus deviance within primate primary auditory cortex: intracortical mechanisms of mismatch negativity (MMN) generation. Brain Res. 1994;667:192–200. doi: 10.1016/0006-8993(94)91496-6. [DOI] [PubMed] [Google Scholar]
- Jones TA, Klintsova AY, Kilman VL, Sirevaag AM, Greenough WT. Induction of multiple synapses by experience in the visual cortex of adult rats. Neurobiol Learn Mem. 1997;68:13–20. doi: 10.1006/nlme.1997.3774. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F. Complementary distribution of vesicular glutamate transporters in the central nervous system. Neurosci Res. 2002;42:243–250. doi: 10.1016/s0168-0102(02)00009-3. [DOI] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Onitsuka T, Spencer MH, Yurgelun-Todd DA, Kikinis R, Jolesz FA, McCarley RW. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60:766–775. doi: 10.1001/archpsyc.60.8.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman DI, Sehatpour P, Higgins BA, Foxe JJ, Silipo G, Javitt DC. Sensory deficits and distributed hierarchical dysfunction in schizophrenia. Am J Psychiatry. 2010;167:818–827. doi: 10.1176/appi.ajp.2010.09030338. [DOI] [PubMed] [Google Scholar]
- Lewis DA. The chandelier neuron in schizophrenia. Dev Neurobiol. 2011;71:118–127. doi: 10.1002/dneu.20825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos JM, Luthi A, Savic N, Stierli B, Streit P, Gahwiler BH, McKinney RA. Synaptic modifications at the CA3-CA1 synapse after chronic AMPA receptor blockade in rat hippocampal slices. J Physiol. 2007;581:129–138. doi: 10.1113/jphysiol.2006.120550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann CM, Nguyen QT, Santo NH, Lichtman JW. Rapid synapse elimination after postsynaptic protein synthesis inhibition in vivo. J Neurosci. 2007;27:6064–6067. doi: 10.1523/JNEUROSCI.0627-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME. MRI anatomy of schizophrenia. Biol Psychiatry. 1999;45:1099–1119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Marquez A, Lewis DA, Levitt P. Molecular characterization of schizophrenia viewed by microarray analysis of gene expression in prefrontal cortex. Neuron. 2000;28:53–67. doi: 10.1016/s0896-6273(00)00085-4. [DOI] [PubMed] [Google Scholar]
- Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer CE, Delevich KM, Fish KN, Asafu-Adjei JK, Sampson AR, Dorph-Petersen KA, Lewis DA, Sweet RA. Reduced glutamate decarboxylase 65 protein within primary auditory cortex inhibitory boutons in schizophrenia. Biol Psychiatry. 2012;72:734–743. doi: 10.1016/j.biopsych.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naatanen R, Kahkonen S. Central auditory dysfunction in schizophrenia as revealed by the mismatch negativity (MMN) and its magnetic equivalent MMNm: a review. Int J Neuropsychopharmacol. 2009;12:125–135. doi: 10.1017/S1461145708009322. [DOI] [PubMed] [Google Scholar]
- Navone F, Jahn R, Di Gioia G, Stukenbrok H, Greengard P, De Camilli P. Protein p38: an integral membrane protein specific for small vesicles of neurons and neuroendocrine cells. J Cell Biol. 1986;103:2511–2527. doi: 10.1083/jcb.103.6.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oni-Orisan A, Kristiansen LV, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Altered vesicular glutamate transporter expression in the anterior cingulate cortex in schizophrenia. Biol Psychiatry. 2008;63:766–775. doi: 10.1016/j.biopsych.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajarethinam R, Sahni S, Rosenberg DR, Keshavan MS. Reduced superior temporal gyrus volume in young offspring of patients with schizophrenia. Am J Psychiatry. 2004;161:1121–1124. doi: 10.1176/appi.ajp.161.6.1121. [DOI] [PubMed] [Google Scholar]
- Ripley B, Otto S, Tiglio K, Williams ME, Ghosh A. Regulation of synaptic stability by AMPA receptor reverse signaling. Proc Natl Acad Sci U S A. 2011;108:367–372. doi: 10.1073/pnas.1015163108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco BR, Fish KN. Differential GAD65 and GAD67 expression in somatostatin-positive axon terminals. Society for Neuroscience Annual Meeting; San Diego, CA. 2010. Ref Type: Abstract. [Google Scholar]
- Salisbury DF, Kuroki N, Kasai K, Shenton ME, McCarley RW. Progressive and interrelated functional and structural evidence of post-onset brain reduction in schizophrenia. Arch Gen Psychiatry. 2007;64:521–529. doi: 10.1001/archpsyc.64.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Harris KM. Three-dimensional structure and composition of CA3-->CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J Neurosci. 1998;18:8300–8310. doi: 10.1523/JNEUROSCI.18-20-08300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Woolf TB, Carnevale NT. Comparisons between Active Properties of Distal Dendritic Branches and Spines: Implications for Neuronal Computations. Journal of Cognitive Neuroscience. 1989;1:273–286. doi: 10.1162/jocn.1989.1.3.273. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Bergen SE, Sun Z, Marcsisin MJ, Sampson AR, Lewis DA. Anatomical evidence of impaired feedforward auditory processing in schizophrenia. Biol Psychiatry. 2007;61:854–864. doi: 10.1016/j.biopsych.2006.07.033. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Bergen SE, Sun Z, Sampson AR, Pierri JN, Lewis DA. Pyramidal cell size reduction in schizophrenia: evidence for involvement of auditory feedforward circuits. Biol Psychiatry. 2004;55:1128–1137. doi: 10.1016/j.biopsych.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Sweet RA, Fish KN, Lewis DA. Mapping Synaptic Pathology within Cerebral Cortical Circuits in Subjects with Schizophrenia. Frontiers in Human Neuroscience. 2010:4. doi: 10.3389/fnhum.2010.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet RA, Henteleff RA, Zhang W, Sampson AR, Lewis DA. Reduced dendritic spine density in auditory cortex of subjects with schizophrenia. Neuropsychopharmacology. 2009;34:374–389. doi: 10.1038/npp.2008.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Wood SJ, Yung AR, Soulsby B, McGorry PD, Suzuki M, Kawasaki Y, Phillips LJ, Velakoulis D, Pantelis C. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch Gen Psychiatry. 2009;66:366–376. doi: 10.1001/archgenpsychiatry.2009.12. [DOI] [PubMed] [Google Scholar]
- Tsay D, Yuste R. On the electrical function of dendritic spines. Trends Neurosci. 2004;27:77–83. doi: 10.1016/j.tins.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Uezato A, Meador-Woodruff JH, McCullumsmith RE. Vesicular glutamate transporter mRNA expression in the medial temporal lobe in major depressive disorder, bipolar disorder, and schizophrenia. Bipolar Disord. 2009;11:711–725. doi: 10.1111/j.1399-5618.2009.00752.x. [DOI] [PubMed] [Google Scholar]
- Wilson NR, Kang J, Hueske EV, Leung T, Varoqui H, Murnick JG, Erickson JD, Liu G. Presynaptic regulation of quantal size by the vesicular glutamate transporter VGLUT1. J Neurosci. 2005;25:6221–6234. doi: 10.1523/JNEUROSCI.3003-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods GF, Oh WC, Boudewyn LC, Mikula SK, Zito K. Loss of PSD-95 enrichment is not a prerequisite for spine retraction. J Neurosci. 2011;31:12129–12138. doi: 10.1523/JNEUROSCI.6662-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.