Abstract
Management of skin wound infections presents a serious problem in the clinic, in the community, and in both civilian and military clinical treatment centers. Staphylococcus aureus is one of the most common microbial pathogens in cutaneous wounds. Peptide-morpholino oligomer (PMO) conjugates targeted to S. aureus gyrase A mRNA have shown the ability to reduce bacterial viability by direct site-specific mRNA cleavage via RNase P. As a treatment, these conjugates have the added advantages of not being susceptible to resistance due to genetic mutations and are effective against drug resistant strains. While this strategy has proven effective in liquid culture, it has yet to be evaluated in an animal model of infected surface wounds. In the present study, we combined PMO conjugates with a thermoresponsive gel delivery system to treat full-thickness mouse cutaneous wounds infected with S. aureus. Wounds treated with a single dose of PMO conjugate displayed improved healing that was associated with increased epithelialization, reduced bacterial load, and increased matrix deposition. Taken together, our findings demonstrate the efficacy and flexibility of the PMO conjugate drug delivery system and make it an attractive and novel topical antimicrobial agent.
Keywords: Staphylococcus aureus, RNase P, skin wounds, PMOs
One of the most common combat wound pathogens is S. aureus, which also includes methicillin resistant strains (MRSA) (Murray, 2008; Murray et al., 2010). In the US, the rate of MRSA infections has increased steadily since the year 2000, increasing the cost of medical care and resulting in higher rates of morbidity and mortality (Klevens et al., 2007). In addition, genetic mutations leading to the development of antibiotic resistance have increased the difficulty in treating S. aureus infections (Daum, 2007).
New models and methods of treatment are currently being investigated to improve the efficacy of treatment of wounds infected by S. aureus and to combat drug resistance. These include adding antibacterial coatings to bandages, or replacing the standard antibiotics with therapeutic peptides or nanoparticulate drug delivery vehicles (Guthrie et al., 2012; Lee et al., 2011; Li et al., 2010; Martinez et al., 2009). Alternate avenues of therapy also include vaccines against S. aureus (Kim et al., 2010). However, the ability to directly target the genes that control replication, or drug resistance, could not only effectively reduce the bacterial load of S. aureus, but also limit the potential for off target effects.
RNase P is an essential enzyme that primarily cleaves transfer RNA (tRNA) (Lundblad and Altman, 2010). This conserved mechanism can be directed against specific mRNA by providing a complementary external guide sequence (EGS), which binds to the target mRNA and directs RNase P cleavage (Li et al., 1992). When given the proper EGS, RNase P is able to catalytically break down bacterial, viral, and eukaryotic mRNAs (Guerrier-Takada et al., 1995; Guerrier-Takada et al., 1997; Jiang et al., 2012; Trang et al., 2001; Yen et al., 2001). Currently, EGSs can be created using phosphorodiamidate morpholino oligonucleotides (MOs), conjugated with a cell-penetrating peptide (CPP) to improve intracellular transport, and then used to alter and kill both Gram positive and negative bacteria (Shen et al., 2009; Wesolowski et al., 2011). The improved conjugate system (PMO) has proven efficacy in inhibiting the development of Plasmodium falciparum, a parasite that causes malaria (Augagneur et al., 2012).
The effect of the conjugate on S. aureus growing in liquid culture was carried out as described previously for work on various bacterial species (Wesolowski et al., 2011). The conjugate was redesigned to target a S. aureus gyrA specific sequence (TGGCCAAGGT) (Table 1). In addition, a non-specific sequence targeting poly A sequences with significantly reduced efficacy against gyrA was used as a control conjugate. Specifically, the control conjugate had an eleven-mer poly dT (11-T) sequence and its effectiveness against S. aureus was 2-3 times less compared to the experimental conjugate.
Table 1.
Viability with different conjugates
| Strain | Drug resistance | Viability (2 μM) | MIC50 |
|---|---|---|---|
| S. aureus Newman RN4220 | Wild type | 9 × 10−3 | 2 μM |
| S. aureus ATCC43300* | Methicillin, oxacillin | 2 × 10−4 | < 2 μM |
Bacteria viabilities were assayed as described in (18). MIC50 were assayed by standard methods.
MRSA strain
In this study we have combined the targeted morpholino approach with a thermo-responsive gel delivery system in order to evaluate its efficacy in a murine cutaneous wound model, an established preclinical model for wound healing and treatment (Kyriakides and Bornstein, 2003; Kyriakides et al., 2009). GyrA and 11-T conjugates were evaluated in a mouse model of S. aureus infected skin wounds, in a procedure approved by Yale’ Institutional Animal Care and Use Committee, previously described in (Kyriakides et al., 2009). Under isofluorane anesthesia (Baxter Healthcare) we created matching, 6mm, full-thickness, dermal wounds on the flanks of C57Bl/6 mice using a punch biopsy device (Acupunch), and then infected the wound with 1×108 colony forming units (cfu) of S. aureus, Newman strain RN4220. The following day the conjugates were added to the open wound at a dose of 10 μM in a 20% solution of Pluronic F-127, a low-toxicity polymer with reverse thermal gelation properties (Gilbert et al., 1986). Wounds were covered with a sterile, transparent, film dressing (Tegaderm). Treatment groups were either gel with conjugate targeting the gyrA mRNA (7, 10, and 14 days), or gel with a control, non-specific conjugate (11-T, 7 and 10 days), or gel only (14 days).
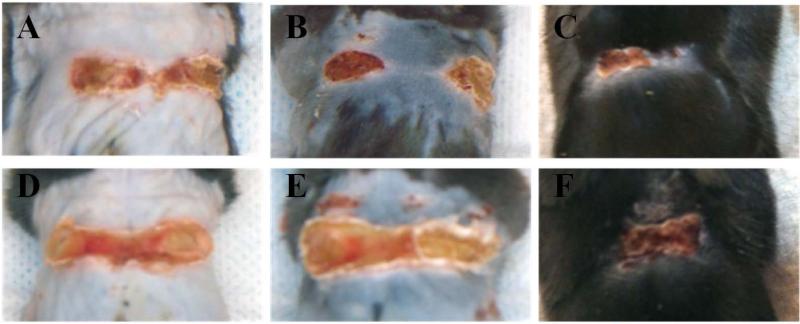
Based on superficial examination of wounds, mice that received the drug loaded gels showed accelerated closure when compared to the control mice (Figure 1). All wounds showed defined eschar formation under the bandage, which gradually sloughed off. Wounds that received the drug conjugate had a healthier appearance with smaller eschar formation and more rapid epithelialization.
Figure 1.
Conjugate delivery produced improved wound closure in S. aureus infected skin wounds. Wounds in both treated (A, B, C) and untreated (D, E, F) animals improved from 7 (A, D) to 14 (C, F) days, a time period sufficient for near-complete healing in uninfected animals (B, E are 10 days). Scabs formed shortly after wounding and sloughed off as healing progress. Eschar formation was more severe in untreated animals.
To further examine the wound healing process, the wounds were removed from the mouse flanks, fixed overnight in neutral buffered formalin, and prepared for histology. Paraffin sections were generated by the Yale Research Histology Service and selected sections were stained for H&E, Trichrome, and Gram stain using standard protocols. Stained tissue sections were imaged using a Zeiss Axio Imager A.1 and an AxioCam HRC camera and analyzed using Metamorph software (Molecular Devices).
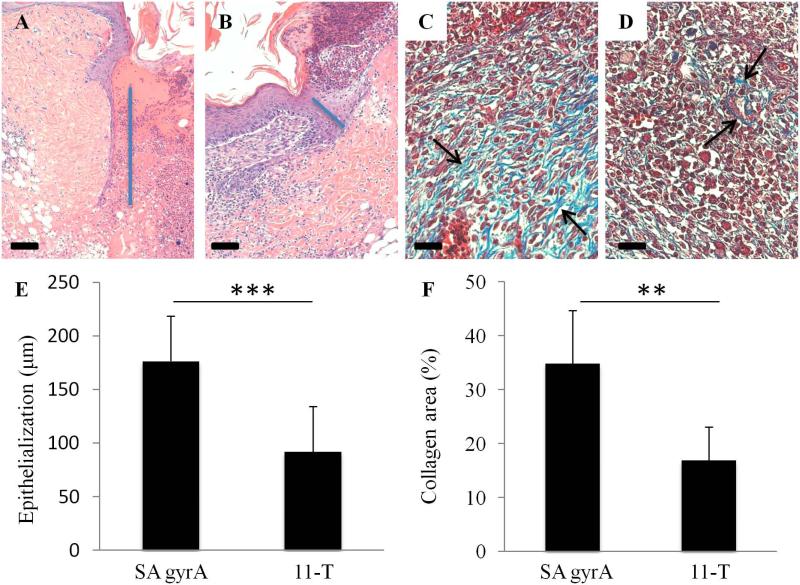
One of the final steps in the wound healing process is epithelialization, where epithelial cells divide and migrate from the edges of the wound towards the center (Singer and Clark, 1999). The migrating edge of the epithelial cell layer, which stretches from the wound surface to the basal surface, is called the epithelial tongue (indicated by blue line in Figure 2). Epithelial cell migration directly relates to the length of the epithelial tongue and subsequent wound closure. We measured the length of the epithelial tongue in H&E stained full-thickness wound tissue sections, from the center of the wound, and found that day 10 wounds treated with the peptide-morpholino conjugate showed increased epithelial tongue length, which correlates with the observations of improved wound closure.
Figure 2.
Conjugate drug treated wounds show improved epithelialization and increased mature collagen. The increased epithelial tongue (blue bar) length at 10 days shows improved migration and wound closure in treated (A) wounds when compared to control (B) wounds. There is a statistically significant increase in epithelial tongue length in wounds that received the drug (E, p < 0.001, average + SD). Trichrome stain showed a significantly increased amount of mature collagen (highlighted by arrows), as indicated by a light blue stain, in conjugate treated tissue (C). S. aureus infected wounds that received inactive conjugate (D) showed minimal mature collagen at day 10. Quantification of collagen positive area per high power field (F) confirmed the increase in collagen (p < 0.01). Means compared using Student's t-test. Scale bar is 100 μm in A and B and 50 μm in C and D.
Days after wounding, fibroblasts that have migrated to the wound site begin to replace the provisional matrix with a collagenous matrix (Singer and Clark, 1999). After 10 days, wounds treated with the gyrA PMO-CPP conjugate showed significantly increased collagen content (Figure 2), with collagen fibrils appropriately ordered in the wound bed. Untreated wounds showed a significant decrease in collagen content, which indicates delayed wound healing. Neither condition showed evidence of mature collagen at 7 days, indicating delayed healing as a consequence of S. aureus infection (Kyriakides et al., 1999).
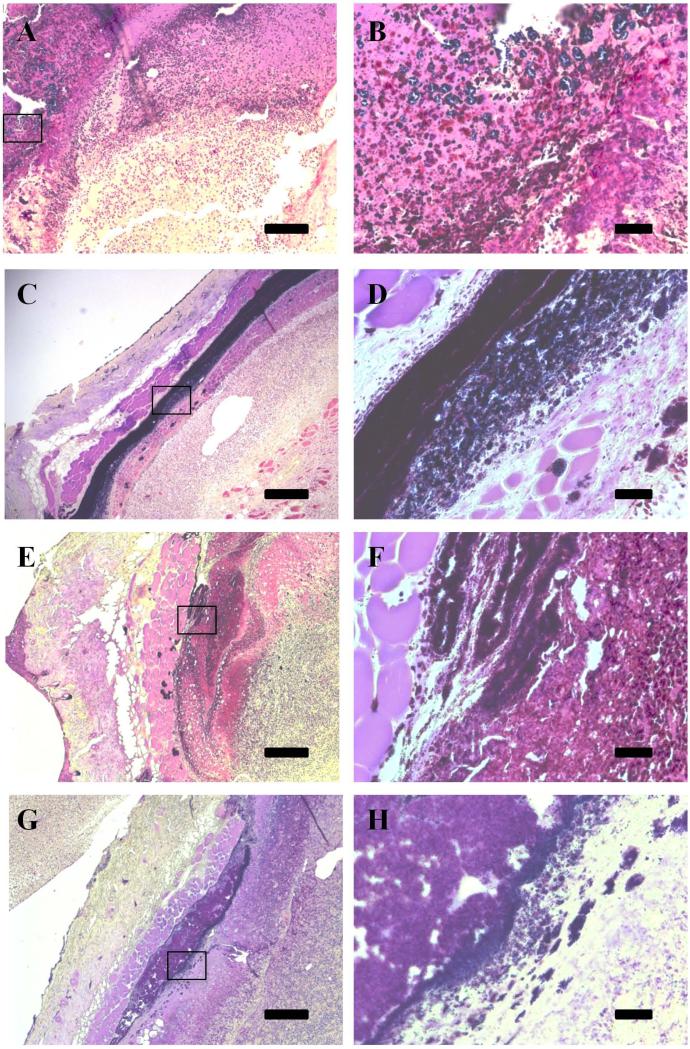
We have previously demonstrated the efficacy of our conjugate system in treating S. aureus in liquid culture (Wesolowski et al., 2011); here we demonstrated its ability in reducing S. aureus presence in an animal system. Gram stains were used to detect the presence of Gram positive bacteria in the healing skin wounds (Figure 3). Conjugate-treated skin wounds show a reduced presence of bacteria, indicated by a decreased amount of deep purple stain, when compared to the wounds that received control treatment. In particular, a dense layer of bacteria is clearly visible at seven days in the control (11-T treated) wounds (Fig. 3c).
Figure 3.
Gram stain shows reduced bacterial load in wounds receiving active conjugate. Treated wounds at 7 (A,B) and 10 days (E, F) show decreased gram positive staining when compared to control wounds at 7 (C, D) and 10 (G, H) days. Inset images (B, D, F, and H) highlight areas in the tissue where staphylococcal colonies are visible in the tissue. Scale is 200 μm for A, C, E, and G, and is 25 μm for B, D, F, and H.
Our targeted conjugate and thermoresponsive gel delivery system seems to compare favorably to antibacterial wound dressings that are currently under investigation (Martinez et al., 2009; Zahedi et al., 2010). Because the gel is able to fill the wound bed before hardening it provides a physical barrier that retains moisture. More importantly, the gel serves as a reservoir for a highly potent antimicrobial drug. We believe that our ability to provide direct antimicrobial contact with the wound bed is advantageous, especially for chronic or complex wounds. It should also be noted that an important limitation of this study was the use of a single dose of the drug. There is no known dose limiting toxicities associated with the targeted conjugate, and we anticipate that by increasing the frequency of administration and/or the dose, we could further improve wound healing by more effective reduction of the bacterial load.
Drug resistant strains of S. aureus have become an increasing problem, from the emergence of methicillin resistant strains 50 years ago to the appearance of vancomycin resistant strains in the past decade (Daum, 2007; Hirsch et al., 2008). Inhibition of gene expression via RNase P has been shown to target genes that confer drug resistance, and convert bacteria to a drug-sensitive phenotype (Guerrier-Takada et al., 1997). The PMO method has the added advantage of being resistant to single genetic mutation. The conjugate can recognize and direct the cleavage of mRNA with up to three nonconsecutive mutations with no decrease in antibacterial function (Shen et al., 2009). In the unlikely event that these mutations occur, an alternative 15 bp EGS can be used for targeting.
In summary, we have shown the first use of EGS-mediated gene silencing in an infected skin wound. The S. aureus -targeted, PMO-conjugate was effective at reducing bacteria and improving the re-epithelialization and healing of S. aureus -infected skin wounds. These effects were realized with a single dose of our thermoresponsive gel drug delivery system. Moreover, this drug delivery system could be used to treat other types of wounds and to fight infections associated with injury or burns, including those involving drug-resistant strains.
Acknowledgements
We thank the members of our laboratories for their assistance. This research was supported by NIH grant RO1GM072194 to TRK, a sub-award to SA from F. Liu (University of California, Berkeley), NIH R01AI041922-11A2, and NG was supported in part by a sub-award from COL A. Stojadinovic to SA, Henry M. Jackson Foundation W81XWH-08-2-0700.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Augagneur Y, Wesolowski D, Tae HS, Altman S, Ben Mamoun C. Gene selective mRNA cleavage inhibits the development of Plasmodium falciparum. Proc Natl Acad Sci U S A. 2012;109:6235–6240. doi: 10.1073/pnas.1203516109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357:380–390. doi: 10.1056/NEJMcp070747. [DOI] [PubMed] [Google Scholar]
- Gilbert JC, Hadgraft J, Bye A, Brookes LG. Drug Release from Pluronic F-127 Gels. International Journal of Pharmaceutics. 1986;32:223–228. [Google Scholar]
- Guerrier-Takada C, Li Y, Altman S. Artificial regulation of gene expression in Escherichia coli by RNase P. Proc Natl Acad Sci U S A. 1995;92:11115–11119. doi: 10.1073/pnas.92.24.11115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrier-Takada C, Salavati R, Altman S. Phenotypic conversion of drug-resistant bacteria to drug sensitivity. Proc Natl Acad Sci U S A. 1997;94:8468–8472. doi: 10.1073/pnas.94.16.8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie KM, Agarwal A, Tackes DS, Johnson KW, Abbott NL, Murphy CJ, Czuprynski CJ, Kierski PR, Schurr MJ, McAnulty JF. Antibacterial efficacy of silver-impregnated polyelectrolyte multilayers immobilized on a biological dressing in a murine wound infection model. Ann Surg. 2012;256:371–377. doi: 10.1097/SLA.0b013e318256ff99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch T, Spielmann M, Zuhaili B, Koehler T, Fossum M, Steinau HU, Yao F, Steinstraesser L, Onderdonk AB, Eriksson E. Enhanced susceptibility to infections in a diabetic wound healing model. BMC Surg. 2008;8:5. doi: 10.1186/1471-2482-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Gong H, Chen YC, Vu GP, Trang P, Zhang CY, Lu S, Liu F. Effective inhibition of cytomegalovirus infection by external guide sequences in mice. Proc Natl Acad Sci U S A. 2012;109:13070–13075. doi: 10.1073/pnas.1201620109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HK, Cheng AG, Kim HY, Missiakas DM, Schneewind O. Nontoxigenic protein A vaccine for methicillin-resistant Staphylococcus aureus infections in mice. J Exp Med. 2010;207:1863–1870. doi: 10.1084/jem.20092514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- Kyriakides TR, Bornstein P. Matricellular proteins as modulators of wound healing and the foreign body response. Thromb Haemost. 2003;90:986–992. doi: 10.1160/TH03-06-0399. [DOI] [PubMed] [Google Scholar]
- Kyriakides TR, Tam JW, Bornstein P. Accelerated wound healing in mice with a disruption of the thrombospondin 2 gene. J Invest Dermatol. 1999;113:782–787. doi: 10.1046/j.1523-1747.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- Kyriakides TR, Wulsin D, Skokos EA, Fleckman P, Pirrone A, Shipley JM, Senior RM, Bornstein P. Mice that lack matrix metalloproteinase-9 display delayed wound healing associated with delayed reepithelization and disordered collagen fibrillogenesis. Matrix Biol. 2009;28:65–73. doi: 10.1016/j.matbio.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Shin YP, Shin SH, Park S, Kim MH, Lee IH. Therapeutic efficacy of halocidin-derived peptide HG1 in a mouse model of surgical wound infection with methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2011;55:1296–1299. doi: 10.1128/AAC.00948-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhou Y, Dong K, Guo X. Potential therapeutic efficacy of a bactericidal-immunomodulatory fusion peptide against methicillin-resistant Staphylococcus aureus skin infection. Appl Microbiol Biotechnol. 2010;86:305–309. doi: 10.1007/s00253-009-2313-0. [DOI] [PubMed] [Google Scholar]
- Li Y, Guerrier-Takada C, Altman S. Targeted cleavage of mRNA in vitro by RNase P from Escherichia coli. Proc Natl Acad Sci U S A. 1992;89:3185–3189. doi: 10.1073/pnas.89.8.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad EW, Altman S. Inhibition of gene expression by RNase P. N Biotechnol. 2010;27:212–221. doi: 10.1016/j.nbt.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Martinez LR, Han G, Chacko M, Mihu MR, Jacobson M, Gialanella P, Friedman AJ, Nosanchuk JD, Friedman JM. Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infection. J Invest Dermatol. 2009;129:2463–2469. doi: 10.1038/jid.2009.95. [DOI] [PubMed] [Google Scholar]
- Murray CK. Epidemiology of infections associated with combat-related injuries in Iraq and Afghanistan. J Trauma. 2008;64:S232–238. doi: 10.1097/TA.0b013e318163c3f5. [DOI] [PubMed] [Google Scholar]
- Murray CK, Griffith ME, Mende K, Guymon CH, Ellis MW, Beckius M, Zera WC, Yu X, Co EM, Aldous W, Hospenthal DR. Methicillin-resistant Staphylococcus aureus in wound cultures recovered from a combat support hospital in Iraq. J Trauma 69 Suppl. 2010;1:S102–108. doi: 10.1097/TA.0b013e3181e44b57. [DOI] [PubMed] [Google Scholar]
- Shen N, Ko JH, Xiao G, Wesolowski D, Shan G, Geller B, Izadjoo M, Altman S. Inactivation of expression of several genes in a variety of bacterial species by EGS technology. Proc Natl Acad Sci U S A. 2009;106:8163–8168. doi: 10.1073/pnas.0903491106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- Trang P, Lee J, Kilani AF, Kim J, Liu F. Effective inhibition of herpes simplex virus 1 gene expression and growth by engineered RNase P ribozyme. Nucleic Acids Res. 2001;29:5071–5078. doi: 10.1093/nar/29.24.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski D, Tae HS, Gandotra N, Llopis P, Shen N, Altman S. Basic peptide-morpholino oligomer conjugate that is very effective in killing bacteria by gene-specific and nonspecific modes. Proc Natl Acad Sci U S A. 2011;108:16582–16587. doi: 10.1073/pnas.1112561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen L, Gonzalez-Zulueta M, Feldman A, Yuan Y, Fryer H, Dawson T, Dawson V, Kalb RG. Reduction of functional N-methyl-D-aspartate receptors in neurons by RNase P-mediated cleavage of the NR1 mRNA. J Neurochem. 2001;76:1386–1394. doi: 10.1046/j.1471-4159.2001.00153.x. [DOI] [PubMed] [Google Scholar]
- Zahedi P, Rezaeian I, Ranaei-Siadat SO, Jafari SH, Supaphol P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym Advan Technol. 2010;21:77–95. [Google Scholar]