Abstract
Adult neurogenesis is a unique form of plasticity found in the hippocampus, a brain region key to learning and memory formation. While many external stimuli are known to modulate the generation of new neurons in the hippocampus, little is known about the local circuitry mechanisms that regulate the process of adult neurogenesis. The neurogenic niche in the hippocampus is highly complex and consists of a heterogeneous population of cells including interneurons. Because interneurons are already highly integrated into the hippocampal circuitry, they are in a prime position to influence the proliferation, survival, and maturation of adult-generated cells in the dentate gyrus. Here we review the current state of our understanding on the interplay between interneurons and adult hippocampal neurogenesis. We focus on activity- and signaling-dependent mechanisms, as well as research on human diseases that could provide better insight into how interneurons in general might add to our comprehension of the regulation and function of adult hippocampal neurogenesis.
Keywords: hippocampus, dentate gyrus, subgranular zone, interneuron, GABA, reelin, NPAS3, apoE, SDF-1
INTRODUCTION
Adult neurogenesis is a form of structural and functional plasticity seen in only a few regions in the mammalian brain, such as the hippocampus [1]. The hippocampus is integral to learning and memory formation [e.g. 2,3,4], and evidence suggests that adult hippocampal neurogenesis is important in learning, memory, and other hippocampal functions [e.g. 5]. While many factors such as stress [6], age [7], environmental enrichment [8], voluntary exercise [9] and exposure to drugs of abuse [10,11,12,13] affect the process of adult hippocampal neurogenesis, the local circuitry mechanisms mediating these changes remain elusive. Identification of these mechanisms is important if we are to harness the novel neuroplasticity of adult hippocampal neurogenesis to help repair the injured, diseased, or aged brain [e.g. 14,15,16].
The dentate gyrus (DG) of the hippocampus contains a neurogenic niche, the subgranular zone (SGZ), which is inhabited by a heterogeneous population of cells and cellular elements (Fig 1A, 1B). The niche is a nursery of sorts, hosting those cells in the process of adult hippocampal neurogenesis (Fig 1A): Type-1 neural stem cells (NSCs), actively-dividing progenitors (Type-2, Type-3), and immature and mature DG granule neurons [17,18,19], all of which are surrounded by vasculature [20]. Several publications have examined the relationship of adult-generated and embryonic-generated excitatory glutamatergic granule cell (GC) neurons with the vasculature [20,21,22], and have clearly demonstrated a complex but intriguing crosstalk between these elements of the niche.
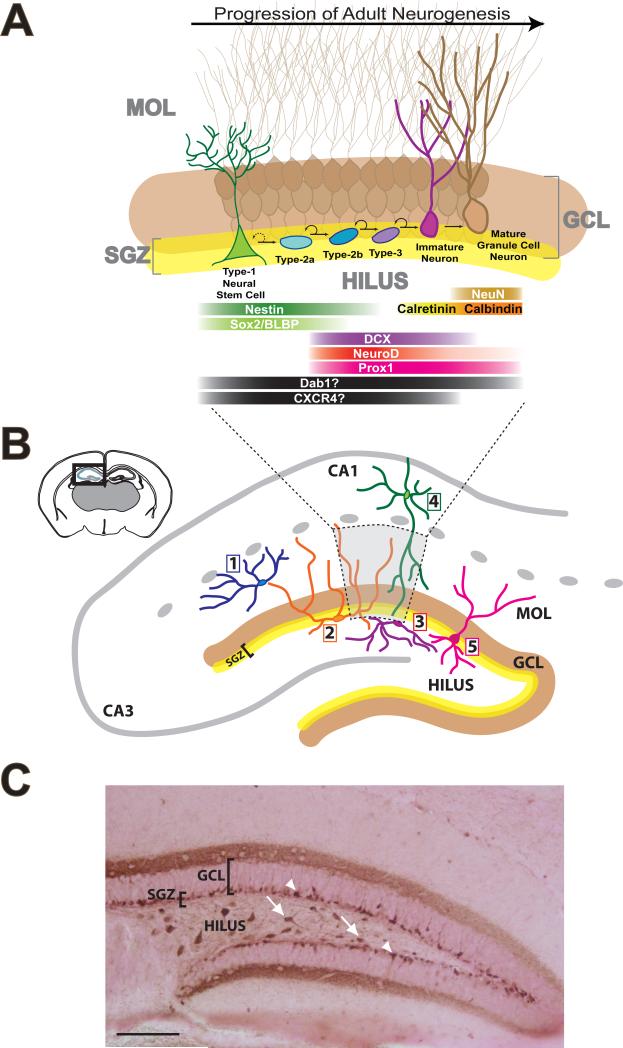
Figure 1. An overview of hippocampal neurogenesis and dentate gyrus (DG) interneurons.
(A) Simplified schematic of the stages of adult neurogenesis [modeled after: 16] depicting the presumed generation of adult generated hippocampal neurons in the subgranular zone (SGZ, yellow area). The remaining regions of the dentate gyrus labeled include the molecular layer (MOL), granule cell layer (GCL, brown), and hilus. In the SGZ, Type-1 cells (green) give rise to proliferating cells (blue and darker purple) to immature (magenta) and mature (brown) dentate gyrus granule cells which extend their dendritic process up into MOL and their axons to the hippocampal CA3 region (not shown). The SGZ is often defined as a zone that includes the innermost portion of the GCL bordering the hilus and approximately two cell body thicknesses into the hilus [97,172,177,178,179]. Depicted below the schematic of the cellular progression of adult neurogenesis in the SGZ and GCL are various proteins expressed in cells at each stage of neurogenesis [e.g. 17,34,180]. As noted in the text, Nestin and Sox2/BLBP are expressed in Type-1 cells; DCX, NeuroD, and Prox1 are expressed in progenitors; and Calretinin, Calbindin, NeuN are expressed in immature and mature granule cell neurons. Relevant to this review on interneurons, there is some evidence that Dab1 and CXCR4 are expressed in adult-generated cells and neurons [48,87,95,107]. However, as this evidence is not firm or confirmed for a particular stage of neurogenesis, expression of these proteins is indicated by a question mark. As described in the text, it will be important to determine the expression of Apoer2 and Vldlr and other interneuron- and reelin-relevant proteins during the process of adult neurogenesis. The dotted lines below (A) indicate that the stages of neurogenesis depicted occur within the dentate gyrus proper, which is showed in lower magnification in (B). (B) Upper left and smaller schematic in (B) depict a coronal section through a mouse brain with the hippocampus outlined [modeled after: 14]. Larger schematic in (B) depicts the enlarged DG and the regions of the DG of interest when discussing adult hippocampal neurogenesis. It also shows the hippocampal CA1 and CA3 regions for reference. The larger schematic in (B) also highlights five different types of hippocampal interneurons with close proximity to and likely innervation of the SGZ and neurogenic niche. Note the large cell bodies of the MOPP interneurons (molecular layer perforant path cell, #1, blue); the HICAP interneurons (hilar commissural-associational pathway related cell, #2, orange); the HIPP interneurons (hilar perforant path-associated cell, #3, purple); the L-M interneurons (s. lacunosum/s. moleculare cells, #4, green); and a typical DG basket cell (also called pyramidal basket cell, #5, pink) [23,25,26,37]. The grey region outlined with the dotted line identifies the sample area of the neurogenic niche depicted in (A). When the adult-generated cells and neurons shown in (A) are considered in conjunction with the 5 types of hippocampal interneurons shown in (B), we hope it is apparent that there is significant opportunity for hippocampal interneurons to influence adult hippocampal neurogenesis. Interneurons, for example, are poised to release GABA near the cell bodies and dendrites of cells in many stages of adult hippocampal neurogenesis. (C) Photomicrograph of the DG of a wildtype mouse stained for calretinin via colorimetric immunohistochemistry. Calretinin+ cell bodies in the SGZ represent maturing granule cells (indicated by arrowheads) [181]. Calretinin+ cell bodies in the hilus represent interneurons and mossy cells (indicated by arrows). Calretinin+ terminals in the inner MOL [e.g. 182,183] represent fibers from a few different regions: associated and commissural hilar mossy cell projections (reviewed in [184]), afferents from the supramammillary nucleus of the hypothalamus [134,185,186], and an undetermined contribution from maturing adult-generated cells in the SGZ/GCL [181]. Scale bar=~200μm.
However, the DG and SGZ niche also host many other cells whose relationship with NSCs, progenitor cells, and adult-generated neurons is much less studied. These other niche elements include excitatory hilar mossy cells, many classes of interneurons (which are generally inhibitory), and glial cells [23,24]. Interneurons are particularly interesting to consider for their regulation of adult hippocampal neurogenesis based on their proximity to adult neural stem cells and their progenitors in the SGZ (Fig 1B, 1C) [23]. Many hippocampal interneurons reside within the DG and have dendritic and axonal projections limited by the boundaries of the dentate gyrus (e.g. Fig 1). However, the projections of hippocampal interneurons are highly complex [23]. In fact, there is evidence that the axons of interneurons in the CA1 region of the hippocampus traverse the hippocampal fissure and also innervate the DG [25,26]. This raises the interesting possibility that activity of “downstream” hippocampal regions like CA3 or CA1 could feedback onto and regulate the process of adult neurogenesis in the “upstream” DG (Fig 1B) [e.g. 26,27]. Even with this complex integration of interneurons into the hippocampal circuitry and proposed interneuron regulation of general synaptic activity in the hippocampus (both reviewed in [28]), interneurons have received surprisingly little attention in regards to how they influence the neurogenic niche and the generation of new neurons in the adult brain.
For this review, we mined the existing literature linking interneurons to adult hippocampal neurogenesis. Our search reveals that most publications fall into one of two main branches. The first branch of research is concentrated on activity-dependent interneuronal regulation of adult hippocampal neurogenesis. We consider this branch to include work on how GABA (γ-aminobutyric acid) and other neurotransmitters that are released from interneurons influence adult hippocampus neurogenesis. The second branch of research focuses on cell signaling-based interneuron regulation of adult hippocampal neurogenesis. We consider this branch to include signaling molecules such as reelin, apolipoprotein E (apoE), stromal cell-derived factor-1 (SDF-1, also called CXCL12) and its receptor C-X-C chemokine receptor 4 (CXCR4), and basic helix-loop-helix (bHLH)-PAS transcription factor, neuronal PAS domain protein 3 (NPAS3). There are some molecules that are represented in both branches, such as SDF-1/CXCR4. However, for the sake of this review we consider direct neurotransmitter effects in the activity-dependent branch and signaling molecules under the signaling-based branch.
We found many publications that could fall into these two branches, and discussion of these studies form the bulk of this review. However, it is useful to say from the outset that in contrast to these publications, we found only a handful of papers that address another question of obvious interest: are new interneurons themselves generated in the adult hippocampus? It appears possible in other brain regions, as adult-generated GABAergic interneurons are evident in both the striatum and neocortex [29]. Likewise, evidence suggests that adult-generated GABAergic interneurons may also exist in the hippocampus [30,31,32,33], although additional work is needed. If production of new hippocampal interneurons during adulthood proves true, it adds yet another layer to the complexity of brain plasticity and the regulation of both synaptic activity and adult neurogenesis.
Given that it remains controversial as to whether new hippocampal interneurons are formed during adulthood [e.g. 1,34], here we restrict our review to the two complementary branches of research that are less controversial: interneuron activity-dependent regulation of adult hippocampal neurogenesis, and interneuron cell signaling-dependent regulation of adult hippocampal neurogenesis.
INTERNEURON ACTIVITY-DEPENDENT REGULATION OF ADULT NEUROGENESIS IN THE SGZ
Prior to discussing what is known about activity-dependent mechanisms relevant to interneurons that may contribute to the process of adult hippocampal neurogenesis, it is useful to stress the heterogeneity of DG interneurons. The subclasses of interneurons that reside within the hippocampus are remarkably diverse, and there are various factors to take into consideration when classifying interneurons: location, morphology, target fields, and expression of proteins like parvalbumin, calretinin (Fig 1C), and calbindin [23,35]. For example, interneurons whose soma or neurites are in close proximity to the SGZ and the neurogenic niche include the molecular layer perforant path interneuron (MOPP), the hilar commissural-associational pathway related interneuron (HICAP), the hilar perforant path-associated interneuron (HIPP), the s. lacunosum/s. moleculare (L-M) interneuron, and basket cells (Fig 1B) [23,36]. Even within a given interneuron class there is striking diversity; for example, there are at least five classes of hippocampal basket cells alone [37], and only one type, the DG pyramidal basket cell is highlighted in Fig 1B. This diversity aside, most hippocampal interneurons share a common characteristic: they are GABAergic, producing and releasing this largely inhibitory neurotransmitter [35]. Thus, the major portion of this first section on activity-dependent regulation of neurogenesis as it relates to interneurons, will focus on GABA, with only a small portion at the end to highlight non-GABAergic research.
GABA
A few groups have undertaken the task of exploring the role of GABA in mediating both developmental as well as adult neurogenesis. During embryonic and early postnatal development, newly-generated neurons follow a distinct sequence of events through which they form their synaptic connections within the developing neurocircuitry (reviewed in [38]). Initially the neurons are “silent”, meaning that they have no spontaneous or evoked postsynaptic currents to any of the commonly applied agents (e.g. GABA, NMDA, AMPA, glycine). However, upon the formation of GABAergic synapses (within 12 days of retroviral labeling in the postnatal mouse brain [39]), they become sensitive to depolarization by GABA, followed by the development of glutamatergic inputs, and lastly a switch to hyperpolarization by GABA, a sign of neuronal maturity. Adult-generated neurons in the DG SGZ go through an almost identical progression of steps initiating synaptic connectivity with the surrounding and preexisting hippocampal circuitry [40]. This stepwise maturation and integration closely synchronizes with the progressive stages of the process of adult neurogenesis, beginning with Type-1 neural stem cells which give rise to transiently amplifying progenitor cells (Type-2), followed by immature neurons and eventual maturation to fully integrated DG granule cells (reviewed in [41]).
Type-1 cells in the SGZ of the hippocampus are the putative neural stem cells (NSCs), which are thought to be the “source” cells of the process of adult neurogenesis [19,42]. They are often identified by their unique radial glial-like morphology and expression of markers such as Nestin, Sox2, and BLBP [34]. While Type-1 cells divide infrequently [43], there is some indication that perhaps proliferation of Type-1 cells is hippocampal activity-dependent. For example, Type-1 cell number increases after kainate-induced seizures in mice [44]. Interestingly, laboratory animals given drugs to induce seizures also have fewer DG interneurons [45]. However, whether Type-1 cells and hippocampal interneurons are sensitive to the same seizure-related stimulus or if a loss of interneuron activity or signaling increases Type-1 cell division has yet to be investigated (Fig 2). It is also still unclear whether Type-1 cells specifically express functional GABA receptors, which would make them able to respond to extrasynaptic GABA in the DG [46,47,48,49]. Only one study published by Wang et al. [47] was able to show a Type-1 cell response to GABAA receptor agonist muscimol and the inhibition of a GABA-mediated response by the GABAA receptor antagonist bicuculline. It is intriguing to consider whether GABAergic interneurons, which innervate the SGZ, GCL, and molecular layer of the DG [23], regulate the rare or slow proliferation of these radial-glial like Type-1 cells. There is minimally anatomical support for such a relationship; Type-1 cells retain their cell bodies in the SGZ and extend their processes into the outer GCL and inner molecular layers [50], all regions with significant interneuron innervation (Fig 1B) [23].
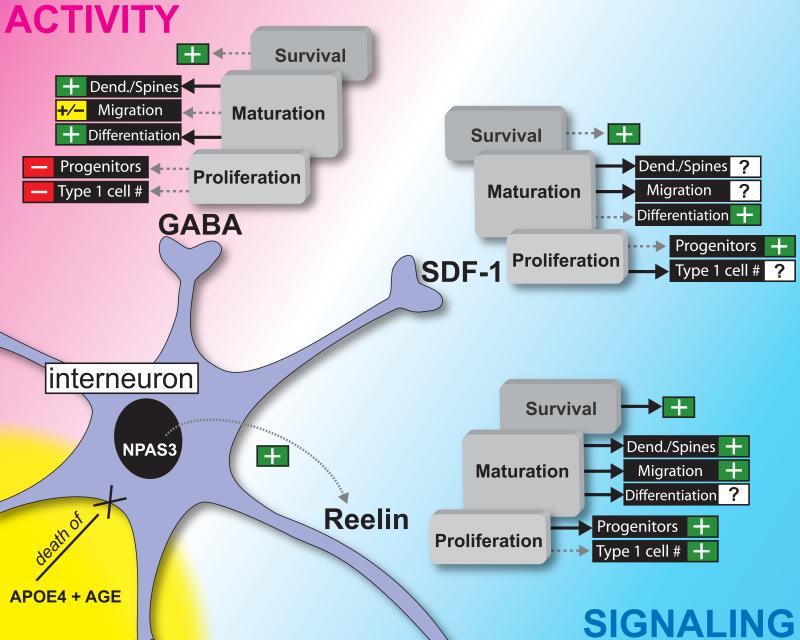
Figure 2. A summary of the key interneuronal players, both activity-(neurotransmitter dependent) and signaling-dependent, which play a role in the regulation of adult hippocampal neurogenesis.
Solid lines indicated findings that are supported by one or more publication. Dotted gray lines indicate findings that are suggested in the published literature, but that warrant more in-depth research. Question marks indicate areas in the field that have not yet been explored. +/- indicates either a positive influence (+) or negative (-) influence, respectively, of the specified neurotransmitter/protein on a given stage of neurogenesis (proliferation, maturation, survival). The “Dend./Spines” subcategory refers to an effect on either dendritogenesis or spine formation, or both. GABA: GABA released from interneurons has distinct effects on SGZ/GCL cells in the stages of proliferation, maturation, and survival. For example, evidence shows a correlation among the increase in neuronal activity in seizures, the decrease in interneuron number, and the increase in the number of Type-1 cells in the hippocampal SGZ [44,45]. This suggests that GABA may be inhibiting Type-1 number, but more work is needed to clarify this. Likewise, recent evidence suggests that constitutive deletion of select GABAA receptor subunits increases SGZ cell proliferation [53]. This indicates that GABA may exert a negative effect on the proliferation of SGZ progenitors. This same publication showed a GABAA receptor subunit-dependent alteration in cell migration towards the outer GCL, with constitutive deletion of one subunit decreasing the migration away from the SGZ and deletion of another subunit increasing the migration away from the SGZ [53]. A series of studies has shown that phasic GABA inputs raise intracellular Ca2+ levels, which initiate NeuroD expression, a transcription factor necessary for the differentiation and survival of adult-generated cells in the SGZ [46,57,58,59]. This indicates that GABA has a positive influence on differentiation and survival, although more work is needed to clarify the effect on survival specifically. These results coincide with other studies exploring the activation/inactivation of GABARs, concluding that GABA positively influences dendritogenesis [49,53,65]. Reelin: Reelin released from interneurons appears to positively influence cells across all stages of hippocampal neurogenesis. For example, decreased developmental levels of reelin expression result in fewer Type-1 cells in adulthood [86,87,88,89]. However, the effect of reelin overexpression on Type-1 cell number remains unknown. Overexpression of reelin increases proliferation of progenitors and also plays a role in neuronal migration and dendritic spine hypertrophy [94]. While there is no direct evidence that reelin influences differentiation of adult-generated cells, there is a significant effect of reelin overexpression on the survival of adult-generated cells with age [94]. Inducible interneuron-specific ablation of reelin in adulthood is needed to complement the current research on reelin overexpression. SDF-1: Less is known about the role of SDF-1 signaling in hippocampal neurogenesis, but some messages have emerged. For example, it has not yet been investigated how Type-1 cell number and adult-generated cell migration and dendritogenesis are affected by SDF-1. However, it is proposed that SDF-1 may increase adult neurogenesis; administration of an antagonist against the SDF-1 receptor, CXCR4, results in a decrease in adult-generated GCs in the DG [108]. Likewise, concomitant release of SDF-1 with GABA from DG interneurons enhances GABAergic inputs on progenitors and immature neurons, and therefore has the potential to increase intracellular Ca2+ levels positively affecting differentiation and adult-generated cell survival in the SGZ through NeuroD expression [46,48,57,58,59]. NPAS3: While there is no direct link showing that the transcription factor NPAS3 regulates reelin expression, NPAS3 KO mice show decreased levels of reelin and decreased neurogenesis [84,109]. This is additional correlative evidence that reelin in general promotes adult hippocampal neurogenesis. APOE4/AGE: While more work is needed on the role of the Alzheimer's risk factor apoE4 in regulating the interneuron/neurogenesis relationship, it is notable that expression of apoE4 significantly decreases interneuron number with age. Thus, apoE4 and age may work in concert to decrease interneuron number, which would influence adult neurogenesis by disrupting the various interneuron influences on adult neurogenesis that are depicted in this figure [104,105,106].
Significantly more data have been published regarding the impact of GABA on the maturation of Type-2 cells, the transiently amplifying progenitors that arise from the Type-1 putative neural stem cells (Fig 1A). Based on expression of particular antigens, the Type-2 cell population can be divided into “younger” Type-2a progenitors expressing Nestin and “older” Type-2b progenitors expressing Nestin and the immature neuronal marker doublecortin (DCX; Fig 1A), indicating a transition into the neuronal lineage [51,52]. The heterogeneity of Type-2 cells is also supported by analysis of their electrophysiological properties. One subset of Type-2 cells is referred to as “silent”, responding to tonic but not phasic GABA, while the other subset is referred to as “GABA-only”, depolarized by phasic GABA but not yet responding to glutamatergic stimuli [46,49]. Retroviral labeling of adult-generated cells with GFP also supports this heterogeneity, where GFP-labeled cells show responses to tonic GABA as early as 3 days-post-infection (dpi) and phasic GABA at 7dpi [40,49]. While it is tempting to consider Type-2a Nestin+ cells “silent” and the Type-2b Nestin+/DCX+ cells responsive to “GABA-only”, this has not yet been shown. In fact, the specific GABAergic electrophysiological properties of Type-2a and -2b cells have yet to be directly studied.
There is some evidence that proliferation of these progenitor cells may be influenced by tonic GABA in the adult hippocampus [53]. In this study, Duveau et al. show that there is decreased proliferation of cells in the dentate gyrus of mice constitutively lacking the GABAA receptor subunit α4, a subunit responsible for regulating tonic GABA inputs [53,54]. Likewise, adult-generated cell migration may be negatively affected in the GABAA receptor subunit α4 KO animals [53]. However, whether these data are a result of entire animal KO of the receptor subunit or specific deletion in the adult generated cells still needs to be investigated (Fig 2). This is an ideal question for application of the novel cell-specific deletion or manipulation of genes that has become more widely used in recent years [55,56].
As adult-generated cells differentiate into mature DG GCLs, they not only respond to tonic GABA but also receive phasic GABAergic inputs. This phasic input is important because it depolarizes the maturing cells and elevates their intracellular Ca2+ ([Ca2+]i) levels via activation of voltage-gated calcium channels [57]. This increase in [Ca2+]i has been shown to stimulate the expression of NeuroD [46,57], a transcription factor necessary for the survival and differentiation of adult-generated cells in the SGZ [58,59]. In this activity-dependent manner, GABAergic interneurons in the existing hippocampal circuitry have the power to regulate the differentiation of adult-generated DG cells (Fig 2). However, it is important to note that NeuroD is expressed in cells that retain proliferative capacity [58]. Although GABA released from interneurons can stimulate differentiation of Type-2 cells, the effect of GABA on proliferation of these cells, as with the Type-1 cells, is yet to be explored.
The process of adult neurogenesis continues its progression with the transition of Type-2 cells to Type-3 cells and immature neurons (Fig 1A) [18]. While still mitotically active, Type-3 cells lose Nestin expression (becoming Nestin-) and retain expression of immature neuronal markers such as DCX and NeuroD, confirming their neuronal lineage and earning them the status of neuroblast [51]. The terminology in the literature is mixed on this point, with neuroblasts being considered either immature neurons or the precursors to immature neurons. However, most studies agree on the definition of an immature neuron: an SGZ cell that is DCX+/NeuroD+ and perhaps NeuN+ or Prox1+ (markers of mature granule neurons) that has begun to extend its processes into the outer 2/3 of the molecular layer [60]. Because dendritic propagation and maturation is not dependent on exit from the cell cycle [61], dendritic arborization and thus synaptogenesis follows a heterogeneous timeline. Some information is published on the timeline of the formation of dendrites, spines, synapses, and axon guidance [39,62,63], but much about these processes remains unknown. For example, it is not clear if or how arborization and synaptogenesis are synchronized with each other or across cells. This uncertainty aside, it is still interesting that GABA appears to regulate the maturation of adult-generated DG neuron processes (reviewed in [64]). For example, the immature DG neurons of mice given a GABAA receptor antagonist have shorter dendrites and decreased spine density [65]. In contrast, the immature DG neurons of mice given a GABAA receptor agonist have longer dendrites [49]. Of course, systemic administration of a compound (particularly GABA compounds, which can have robust behavioral effects) does not prove that hippocampal GABAergic interneurons are responsible for dendritic regulation. This could, for example, be the result of a change in circuitry or behavior that influences SGZ neurogenesis (Fig 2). Likewise, while the pool of hippocampal GABA is largely interneuronal [35,66], it has been suggested that ambient GABA may be released from other sources such astrocytes [67,68,69]. However, the concept of GABAergic interneurons regulating adult neurogenesis is intriguing, and has some support in the fact that stimulation of a certain type of interneuron, the hippocampal basket cell, enhances tonic currents in adult-generated DG cells [49]. Additional studies with local application of GABA compounds or other more sophisticated ways to locally manipulate or stimulate GABAergic interneurons coupled with tracking adult hippocampal neurogenesis are warranted.
The influence of other neurotransmitters
The potential regulation of SGZ neurogenesis by GABA and perhaps GABAergic interneurons is most concentrated on the “early” stages on neurogenesis. However, “later” stages of neurogenesis, when immature neurons are making synapses in CA3, are also influenced by other neurotransmitters. For example, it is during the initial synaptogenic period when adult-generated cells are approximately 14-18 days old, that the first excitatory glutamatergic inputs are received [39,40,49]. This last synaptogenic step is followed by a switch from depolarizing to hyperpolarizing GABA [49] and the completion of adult-generated cell integration into the existing hippocampal circuitry. When fully integrated in the DG circuitry, the adult-generated neurons are able to glutamatergically regulate interneuron activity [70]. Thus, a dynamic pattern of influence emerges: GABAergic interneurons potentially influence the complex, multi-step process of SGZ neurogenesis, and the resulting adult-generated neurons can then in turn regulate the activity of GABAergic interneurons.
As we have stressed above and as is nicely reviewed elsewhere [23,28], most DG interneurons are GABAergic, and most DG GABA neurotransmission comes from DG or hippocampal interneurons. However, there are cholinergic interneurons in the DG as well, and it is worth considering how these ChAT+ interneurons (named after the enzyme responsible for synthesizing acetylcholine (ACh)) with their broadly-branching dendritic trees [71,72] might influence the process of neurogenesis. However, since the vast majority of the cholinergic input into the hippocampus and DG comes from the forebrain [72,73,74], it remains challenging to determine whether DG cholinergic interneurons have any direct impact on SGZ neurogenesis. Interestingly, lesion of forebrain cholinergic neurons impairs SGZ neurogenesis, leading to decreased proliferation and survival of adult-generated cells [75,76]. While such studies show that SGZ neurogenesis is sensitive to cholinergic signaling – potentially through Ach-stimulated release of GABA from hippocampal interneurons [77] – it is still unclear what role the resident cholinergic interneurons, optimally located within the DG, may play in the regulation of the process of adult SGZ neurogenesis.
While GABA and ACh are secreted by interneurons [35], and have the potential to directly modulate adult neurogenesis in the hippocampus (reviewed above and in [27]), there are an enormous number of factors that could indirectly regulate interneuron function and interneuron regulation of neurogenesis. For example, the neurotransmitter serotonin can excite CA1 hippocampal interneurons that project to the DG [26]. Disruption of serotonin signaling has a profound effect on SGZ neurogenesis [78,79,80,81,82]. These correlative data with serotonin are reminders that regulation of interneuron activity in the hippocampus, indirectly via other neurotransmitters apart from GABA, is another important mechanism by which interneurons regulate SGZ neurogenesis.
As reviewed above, various neurotransmitters have been implicated in regulating hippocampal interneuron activity both directly (GABA, perhaps ACh) and indirectly (serotonin) to modulate adult neurogenesis in the SGZ. However, interneurons secrete various proteins and participate in numerous signaling pathways that may also have the ability to regulate adult neurogenesis [48,83,84]. Therefore, the second half of this review will focus on signaling proteins and mechanisms, which involve hippocampal interneurons and regulate adult neurogenesis in the DG.
INTERNEURON SIGNALING-DEPENDENT REGULATION OF ADULT NEUROGENESIS IN THE SGZ
While much attention has been given to GABAergic regulation of adult hippocampal neurogenesis, it is also important to address proteins and signaling pathways involving interneurons that may play a role in regulating the generation of new DG granule cells in the adult hippocampus. These factors include reelin, apoE, SDF-1, and NPAS3, and are discussed below.
REELIN
Reelin is a very large extracellular matrix glycoprotein expressed by Cajal-Retzius cells during development. Reelin plays an integral role in neuronal migration and the development of the brain (reviewed in [85]), and its disruption results in a robust developmental phenotype. Mutant mice lacking functional reelin, termed reeler mice, have an inversion of cortical layers and a highly disorganized hippocampus in which a significant portion of DG radial-glial cells have prematurely differentiated into astrocytes [86,87,88,89]. Interestingly, these same radial-glial cells, whose number is decreased in the reeler mice, are the putative Type-1 NSCs in the SGZ (Fig 2). Likely because of this, reeler mice show decreased neurogenesis in the adult hippocampus and in other regions of adult neurogenesis as well [39,90]. Although loss of reelin has been implicated in disrupting adult hippocampal neurogenesis in reeler mice, the phenotype is likely a secondary effect of the developmental defects and not a result of the loss of function of the reelin protein solely in the adult hippocampus. Unfortunately, because reeler mice have such a prominent developmental phenotype, it is difficult to glean the precise function of the protein in the adult brain just from examination of these mutant mice.
Fortunately, other experimental approaches have been employed to explore the role of reelin in the adult brain and several of these studies have relevance for SGZ neurogenesis. For example, immunohistochemical studies have revealed that during adulthood reelin is expressed by a subset of GABAergic hippocampal interneurons including basket cells in the hilus of the DG [91]. Behavioral and electrophysiological studies strongly suggest that reelin signaling is important for synaptic plasticity and hippocampal-based learning and memory [92,93]. Recently, a study published by the Soriano group has taken an interesting tactic in genetically overexpressing reelin in the adult mouse forebrain [94]. They find increased reelin expression increases adult neurogenesis and regulates neuronal migration and synaptic density (Fig 2). While the study does not explore the specific mechanism by which reelin regulates adult neurogenesis, the authors propose that reelin may alter the cell cycle properties of the transiently amplifying progenitors (probably Type-2 cells) and increase the survival of DCX+ immature neurons (Fig 2). This is supported by the increased number of DCX+ labeled immature neurons in older mice that overexpress reelin, which is in sharp contrast to wildtype mice where adult neurogenesis decreases with age [7].
Another useful approach to dissecting the role of reelin in regards to adult neurogenesis is to focus on the signaling molecules that comprise the reelin signaling cascade. For example, the signaling molecule Disabled 1 (Dab1) is an integral part of the intracellular portion of the reelin signaling pathway, and is found in both hippocampal radial-glial cells and neural progenitors [87,95]. However much remains unknown in regards to reelin signaling and adult hippocampal neurogenesis. For example, it is undetermined whether neural progenitors and immature neurons show reelin-induced phosphorylation of Dab1 (Fig 1B). It has also not yet been shown whether the hippocampal adult-generated cells even express the reelin receptors Apoer2 and Vldlr (Fig 1B). If they do, this would suggest that adult hippocampal neurogenesis can be directly responsive to the reelin produced by DG interneurons [96].
If adult-generated cells and neurons are able to directly respond to reelin signaling – and more careful anatomical work is needed before we can say that with certainty – there are numerous questions that one could ask. For example, since reeler mice have such a profound migrational effect during development, it would be interesting to explore how reelin affects the migration of adult-generated hippocampal neurons. The majority of adult-generated granule neurons remain in the inner third of the hippocampal DG granule cell layer as they mature [40,97]. It is possible that reelin controls this very restricted migration; particularly in light of work showing that adult-generated GCs in transgenic animals overexpressing reelin have aberrant migration, with adult-generated neurons spread throughout the GCL [94]. Additionally, seizure-induced loss of reelin-producing interneurons correlates with the seizure-induced ectopic migration of DG granule neurons [95,98]. These studies indicate that a very precise level or location of reelin expression may be required to maintain an organized DG GCL. Based on these published data, the field would obviously benefit from the generation of reelin conditional and inducible knockout mouse lines and viruses that would allow cell-specific production or disruption of reelin signaling related genes. Such tools would allow the exploration of the mechanisms underlying how interneurons may modulate not only proliferation and survival of adult-generated hippocampal neurons, but also their migration through reelin signaling.
APOE
ApoE is a protein that has critical functions in lipid transport [e.g. 99,100]. ApoE in the brain is produced mainly by astrocytes [101] and it has the ability to bind to reelin receptors [99]. Although hippocampal interneurons likely do not produce apoE in a healthy brain [102,103], in the mouse, interneurons appear to be highly susceptible to age-related effects of the human apoE4 isoform [104], the major risk factor for late-onset Alzheimer's disease (AD) (reviewed in [105]). More specifically, the number of hilar GABAergic interneurons significantly decreases with age in the human apoE4 knock-in mice when compared to wildtype mice [104,106]. It is because of these detrimental effects of apoE4 on the interneurons – and the subsequent loss of GABAergic inputs onto adult progenitor cells in the SGZ – that there is a significant decrease in adult neurogenesis in the apoE4 knock-in mouse hippocampus [106] (Fig 2). This promising link between decreased adult neurogenesis and AD is addressed later in this review.
SDF-1 and CXCR4
One of the most intriguing links between interneurons and adult hippocampal neurogenesis comes from studies on SDF-1, a chemokine expressed in postnatal DG interneurons [107]. More specifically, SDF-1 is found in the vesicles of basket cell terminals and colocalizes with GABA-containing synaptic vesicles [48,107]. The receptor for SDF-1, CXCR4, is expressed by cells in the early and later stages of adult hippocampal neurogenesis, namely the Type-2 cells and immature neurons, but not mature DG GCs [48,107]. Thus, the SDF-1/CXCR4 ligand/receptor coupling offers a presumably direct route for interneurons to influence adult hippocampal neurogenesis (Fig 2). Interestingly, concomitant release of SDF-1 with GABA from the DG interneurons enhances GABAergic inputs on Nestin+ Type-2 progenitors and DCX+ immature neurons [48]. This is in keeping with work showing that GABAergic activity plays an important role in regulating adult neurogenesis [e.g. 27]. It is also of note that i.c.v. administration of a CXCR4 antagonist decreases adult-generated mature granule cell number in the rat [108]. Functionally, the CXCR4 antagonist also has the ability to impair long-term memory formation in animals housed in an enriched environment, a stimulus known to increase adult neurogenesis [8,108]. Of course, additional work is needed, for example, to examine how SDF-1 from endothelial cells might also fit into this regulation scheme [48]; whether inducible deletion of CXCR4 from adult-generated SGZ cells blocks the GABAergic regulation; and whether a similar SDF-1/CXCR4 coupling regulates neurogenesis in other regions of postnatal neurogenesis. However, the recent publications with SDF-1 signaling noted above, shed much needed light on how DG interneurons might signal to regulate neurogenesis and relevant hippocampal function as well.
NPAS3
The bHLH-PAS transcription factor NPAS3 is expressed by GABAergic interneurons in the adult hippocampus [84]. Mice deficient for NPAS3 show decreased hippocampal levels of both the fibroblast growth factor receptor 1 (FGFR1) [109], a growth factor receptor implicated in affecting adult neurogenesis [110,111], and reelin [84], without grossly influencing interneuron number [84]. Interestingly, they also show impaired SGZ neurogenesis [109]. However, whether this impairment is a result of disrupted FGF or reelin signaling or from some other phenotype (such as small body size in general) has yet to be conclusively determined (Fig 2).
HUMAN DISEASE, INTERNEURONS, AND ADULT NEUROGENESIS
Some very interesting clues about the interplay between hippocampal interneurons and neurogenesis have emerged from research on human disease and disorders. Here we briefly review what key diseases – AD, schizophrenia, seizure disorders, and psychiatric disorders like depression and addiction and animal models of these disorders – can reveal about the relationship between interneurons and neurogenesis. Also, while ageing is not a disorder per se, the process of ageing is relevant to the onset of many of these disorders, and therefore is also discussed.
Alzheimer's disease and schizophrenia
Alzheimer's disease, a neurodegenerative disorder, and schizophrenia, a neuropsychiatric disorder, are quite distinct in their neuropathology [e.g. 112,113]. However, both AD and schizophrenia are marked by abnormal hippocampal structure and disrupted hippocampal neurogenesis [e.g. 14,114,115,116,117], and both disorders are also correlatively associated with the interneuronal protein reelin [e.g. 118]. Both Alzheimer's disease animal models and schizophrenia patients display decreased numbers and function of hippocampal interneurons [104,119,120]. In addition, reelin binds apoE4, one of the major risk factors for late-onset Alzheimer's disease [e.g. 121]. Highly relevant for this section on research from human disease is a recent paper that studied mice genetically modified to express apoE4 [106]. ApoE4 mice have decreased interneuron number and impaired hippocampal neurogenesis (an interesting combination of enhanced proliferation but impaired survival and dendritic arborization) (Fig 2). Most notably for this review, apoE4 reduces GABAergic signaling onto adult-generated neurons and disrupts neurogenesis, a defect that can be reversed by potentiation of GABAergic signaling [106]. Reelin signaling can also modulate the phosphorylation state of the microtubule stabilizing protein tau, which when hyperphosphorylated initiates the formation of neurofibrillary tangles, a major pathological constituent of the disease [122]. It appears that the elimination of the Tau protein from apoE4 knock-in mice rescues the demise of GABAergic interneurons in the hippocampus as well as the deficits in hippocampal-dependent learning and memory [104]. Post mortem human tissue analysis reveals decreased reelin levels in the hippocampi of schizophrenic patients [123], and mice heterozygous for reelin show similar results in sensorimotor gating tests as those seen in schizophrenics [120,124]. It is also important to mention that NPAS3, like reelin, has been related as a genetic link to schizophrenia, as it is mutated in a family with schizophrenia [125]. Taken together, these studies underscore the utility of the interneuron/neurogenesis hypothesis when considering the trajectory and potential treatment strategies for disorders like Alzheimer's disease and schizophrenia. Also, when considered with other work cited above [e.g. 46,49,65], many of these studies add to the growing evidence that GABAergic interneurons may regulate hippocampal neurogenesis and that this regulation is functionally important.
Seizure disorders
Epilepsy is a seizure disorder, and neuropathological examination of the brains of epileptic humans reveals dispersion of the granule cell layer of the DG and abnormal sprouting of axonal fibers in humans [126,127,128]. Animal models of seizure disorders show a similar dispersion of the DG GCL, and notably aberrant adult neurogenesis post-seizure [128,129], in keeping with the disrupted neurogenesis seen in the epileptic human brain [e.g. 130,131,132,133]. In addition to these changes in neurogenesis, there is a small change in number, distribution, and connectivity of some hippocampal interneurons in the human epileptic brain [e.g. 130,134,135]. There is evidence that the aberrant dispersion of cells in the human GCL in patients with temporal lobe epilepsy shares an inverse relationship with reelin expression, likewise, reelin expressing interneurons are sensitive to seizures [95,128]. These data further confirm that the scientific community may find clues to which mechanisms regulate adult neurogenesis in the hippocampus by further exploring epilepsy and seizure animal models. For example, it would be useful to identify whether reelin signaling is more important for maintaining dentate structure or neuronal number.
Stress-related disorders and depression
Stress was among the first stimuli shown to decrease proliferation in the adult hippocampal SGZ [6], and since that time has become appreciated as one of the most potent regulators of neurogenesis [e.g. 136]. Stress hormones, like corticosterone, are likely responsible for stress-induced disruption of SGZ neurogenesis [e.g. 137,138,139,140]. In addition, adult-generated hippocampal cells and neurons appear to be directly responsive to stress hormones since, for example, corticosterone receptors are evident on cells in discrete stages of adult neurogenesis [e.g. 141]. However, research has shown that stress can also influence interneuron structure and function, which in turn might influence adult neurogenesis. For example, hippocampal parvalbumin interneurons may be sensitive to chronic stress, as tree shrews undergoing long-term psychosocial stress show decreased parvalbumin-immunoreactivity in the hippocampus [142]. However, whether this loss of immunoreactivity is due to loss of GABAergic interneurons or a loss of parvalbumin expression is still unclear. Furthermore, while many publications show that stress can result in altered hippocampal interneurons population number and/or diversity balance [e.g. 142,143,144], this may be stress- and species-specific [e.g. 145]. Regardless, a direct effect of stress on hippocampal interneurons is certainly feasible since hippocampal interneurons express receptors that allow them to respond to stress hormones [e.g. 146,147]. Therefore, if GABAergic interneuron function is negatively affected by most stressors, it could help clarify how stress decreases adult neurogenesis in the hippocampus (Fig 2).
There is also functional relevance for this line of thinking about stress effects on adult neurogenesis. For example, chronic mild stress (CMS) paradigms are generally used to generate models of depression in laboratory animals (reviewed in [148]). CMS models show a disruption of the GABAergic system in the brain [149], replicating human data showing that depressed individuals have decreased GABAergic function (reviewed in [150]). While decreased adult neurogenesis is not currently implicated as a cause for depression, adult neurogenesis may be required for antidepressant function [e.g. 15,151,152]. Interestingly, Sahay and Hen have proposed that the non-heterogeneous distribution of different classes of interneurons across that septo-temporal axis of the hippocampus maybe be partially responsible for the varied effects of antidepressants on adult neurogenesis in the dorsal and ventral hippocampus (reviewed in [153]).
Addiction
It is well known that many drugs of abuse, such as opiates and psychostimulants, have a profound effect on adult neurogenesis in the hippocampus [e.g. 13,154]. However, the mechanism of how drugs of abuse influence SGZ neurogenesis is unknown. While researchers are exploring the likely possibility that drugs of abuse act via circuit-level changes and perhaps direct effects on progenitor cells, it is also possible that drugs of abuse directly or indirectly influence interneuron function, which in turn alters SGZ neurogenesis. There is evidence for some direct action of drugs of abuse on hippocampal interneurons. For example, interneurons express receptors for a major ingredient in tobacco, nicotine (the nicotinic ACh receptor [155,156,157]), the active ingredient in marijuana, delta-9-tetrahydrocannabinol (Δ9-THC) (the cannabinoid receptor 1 [157,158]), and for morphine, heroin, and other opiates (the mu opioid receptor [159,160]). Activation of the cannabinoid receptor 1 and mu opioid receptor on hippocampal interneurons decreases GABAergic activity (as reviewed in [161,162]), which could then lead to decreased GABAergic influence on SGZ neurogenesis. While the effects of drugs of abuse on the GABAergic activity of interneurons residing near and innervating the neurogenic niche could be involved in the changes seen in adult neurogenesis after drug exposure and the progression of addiction, additional research in this realm is warranted. Since stress is a predisposing factor in relapse to drug-taking [e.g. 154,163] and hippocampal interneurons express receptors for both drugs of abuse and stress hormones [146,147], one useful future line of research might focus on how stress-induced changes in interneuron function alter both neurogenesis and relapse to drug-taking or drug-seeking.
Age
While ageing is not a disorder or a disease, there are several disorders whose occurrence increases with age, such as Alzheimer's disease and depression [e.g. 164,165]. Therefore it is useful to note findings from ageing research that have relevance to understanding the interplay between interneurons and hippocampal neurogenesis. For example, there is a correlation between the age-induced decline in interneurons immunoreactive for GAD67 (glutamic acid decarboxylase; the enzyme that catalyzes the production of GABA) and the decline in adult neurogenesis. Specifically, there are 41% fewer GAD+ interneurons in middle aged rats (15 months) and 50% fewer in aged rats (23 months) compared to adult rats (7 months) [166]. Likewise, adult hippocampal neurogenesis, as measured by the number of DG GC cells immunoreactive for the mitotic marker BrdU given 4-6 weeks earlier, significantly decreases in the middle-aged (12 months) and aged (27 months) rat. Adult hippocampal neurogenesis drops to about 10-30% of adult (6 month) rat levels, and interestingly, there is no significant difference between middle-aged and aged animals [7]. These data bring up an attractive correlation: in middle-age there is a significant deficit in both GABA-producing interneurons and adult hippocampal neurogenesis in the DG. Of course, a decrease in GAD67 expressing interneurons is not the only change that occurs in the neurogenic niche with age. For example, neurotrophic factors such as VEGF also decrease with age [167,168], and this decrease and other age-dependent changes in the niche could contribute to age-induced decrease in adult hippocampal neurogenesis. Regardless, an age-dependent decrease in adult hippocampal neurogenesis (as a result of any mechanism) is particularly intriguing in light of the association with neurodegenerative diseases such as Alzheimer's disease.
CONCLUSION
Correlative evidence shows that interneurons play a substantial activity-dependent role in modulating adult neurogenesis in the mammalian hippocampus. GABAergic activity has the ability to promote adult-generated cell maturation and differentiation in the GCL. Various signaling elements, such as apoE or SDF-1 and its receptor CXCR4, regulate interneuron GABAergic activity and thus adult-neurogenesis in an activity-dependent manner. On the other hand, the interneuron-mediated reelin pathway exerts a primarily signaling-dependent effect on the generation of new granule cells in the hippocampus. While we have made strides in understanding this interplay between interneurons and neurogenesis, many questions remain unanswered (Fig 2). For example, the direct effect of GABAergic activity on proliferation of various neuronal progenitors still needs to be addressed, and more work is needed to verify that this is indeed a direct effect of GABAergic interneurons and not a more widespread circuit level influence. We also need a better understanding of what receptors and signaling components are evident on which stages of adult-generated neurons in order to really appreciate how signals from interneurons might influence the dynamic process of adult hippocampal neurogenesis. This would also allow us to inducibly manipulate these signaling cascades by, for example, conditionally knocking out key receptors for reelin on cells once they reach a particular stage of adult hippocampal neurogenesis as has been done for other molecular regulators of adult hippocampal neurogenesis [e.g. 50,169]. We also need greater understanding of how interneuron influence might vary among species, strains, and even anatomically along the septotemporal axis of the hippocampus [170,171,172]. The latter is particularly critical given the distinct function of the hippocampus along the septotemporal axis [153,173]. Finally, while here we have focused on how interneurons might regulate adult hippocampal neurogenesis (Fig 2), it is important to remember that once the adult-generated cells themselves are differentiated, they in turn could regulate interneuron signaling and perhaps number. Given this complexity of interactions, the dynamic nature of adult hippocampal neurogenesis, and the diversity of hippocampal interneurons, the question of the interplay between interneurons and neurogenesis would certainly benefit from the insight that computer modeling of neurogenesis and its regulation might provide [e.g. 174,175,176]. It would also be beneficial for the field if more computer simulations of neurogenesis began to take into account the likely robust influence of hippocampal interneurons on the process of neurogenesis.
While the field is beginning to grasp potential outlets with which to pursue identification of the local circuitry and related molecular mechanisms regulating adult hippocampal neurogenesis, we are still in search of an answer. Clearly, many human diseases and disorders show interplay between hippocampal interneurons and adult neurogenesis. Therefore in addition to the lines of research proposed above, we propose that better scrutiny of human diseases and animal models of these diseases will be intellectually profitable in allowing us to better understand the mechanisms by which interneurons can influence neural stem cell and progenitor proliferation, survival, and maturation and their putative impact on hippocampal function.
Acknowledgements
The authors would like to acknowledge the past figures of Dr. Jessica Ables, Dr. Nathan DeCarolis, and Aparna Sankararaman for inspiring the figures in this review. This work was supported by grants to AJE from the National Institutes of Health and in particular grants from the National Institute on Drug Abuse (R01DA016765, R01DA016765-07S1, K02DA023555, R21DA023701) and grants from the National Alliance for Research on Schizophrenia and Depression and NASA. IM is supported by a postdoctoral fellowship on a Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research T32 Training Grant (T32DA 007290) from NIDA. The content is solely the responsibility of the authors and does not necessarily represent the official views of these granting organizations.
REFERENCES
- 1.Kempermann G. Neural Stem Cells. Adult Neurogenesis 2: Stem Cells and Neuronal Development in the Adult Brain. 2 ed. Oxford University Press; New York: 2011. pp. 51–106. [Google Scholar]
- 2.Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–713. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–130. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- 4.Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 5.Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 6.Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci U S A. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. Journal of Neuroscience. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 9.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 10.Mandyam CD, Wee S, Crawford EF, Eisch AJ, Richardson HN, et al. Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biol Psychiatry. 2008;64:958–965. doi: 10.1016/j.biopsych.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. Journal of Neuroscience. 2010;30:304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noonan MA, Choi KH, Self DW, Eisch AJ. Withdrawal from cocaine self- administration normalizes deficits in proliferation and enhances maturity of adult- generated hippocampal neurons. Journal of Neuroscience. 2008;28:2516–2526. doi: 10.1523/JNEUROSCI.4661-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canales JJ. Adult neurogenesis and the memories of drug addiction. Eur Arch Psychiatry Clin Neurosci. 2007;257:261–270. doi: 10.1007/s00406-007-0730-6. [DOI] [PubMed] [Google Scholar]
- 14.DeCarolis NA, Eisch AJ. Hippocampal neurogenesis as a target for the treatment of mental illness: a critical evaluation. Neuropharmacology. 2010;58:884–893. doi: 10.1016/j.neuropharm.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.David DJ, Wang J, Samuels BA, Rainer Q, David I, et al. Implications of the functional integration of adult-born hippocampal neurons in anxiety-depression disorders. Neuroscientist. 2010;16:578–591. doi: 10.1177/1073858409360281. [DOI] [PubMed] [Google Scholar]
- 16.Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Ming GL, et al. Adult neurogenesis, mental health, and mental illness: hope or hype? Journal of Neuroscience. 2008;28:11785–11791. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 18.Kempermann G, Jessberger S, Steiner B, Kronenberg G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004;27:447–452. doi: 10.1016/j.tins.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 19.Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. Journal of Neuroscience. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Arguello AA, Fischer SJ, Schonborn JR, Markus RW, Brekken RA, et al. Effect of chronic morphine on the dentate gyrus neurogenic microenvironment. Neuroscience. 2009;159:1003–1010. doi: 10.1016/j.neuroscience.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heine VM, Zareno J, Maslam S, Joels M, Lucassen PJ. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci. 2005;21:1304–1314. doi: 10.1111/j.1460-9568.2005.03951.x. [DOI] [PubMed] [Google Scholar]
- 23.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 24.Kosaka T, Hama K. Three-dimensional structure of astrocytes in the rat dentate gyrus. J Comp Neurol. 1986;249:242–260. doi: 10.1002/cne.902490209. [DOI] [PubMed] [Google Scholar]
- 25.Lacaille JC, Schwartzkroin PA. Stratum lacunosum-moleculare interneurons of hippocampal CA1 region. I. Intracellular response characteristics, synaptic responses, and morphology. Journal of Neuroscience. 1988;8:1400–1410. doi: 10.1523/JNEUROSCI.08-04-01400.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMahon LL, Kauer JA. Hippocampal interneurons are excited via serotonin- gated ion channels. J Neurophysiol. 1997;78:2493–2502. doi: 10.1152/jn.1997.78.5.2493. [DOI] [PubMed] [Google Scholar]
- 27.Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity- dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Houser CR. Interneurons of the dentate gyrus: an overview of cell types, terminal fields and neurochemical identity. Prog Brain Res. 2007;163:217–232. doi: 10.1016/S0079-6123(07)63013-1. [DOI] [PubMed] [Google Scholar]
- 29.Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng X, Li Y, Huang Y, Feng X, Feng G, et al. Pulse labeling and long-term tracing of newborn neurons in the adult subgranular zone. Cell Res. 2011;21:338–349. doi: 10.1038/cr.2010.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu S, Wang J, Zhu D, Fu Y, Lukowiak K, et al. Generation of functional inhibitory neurons in the adult rat hippocampus. Journal of Neuroscience. 2003;23:732–736. doi: 10.1523/JNEUROSCI.23-03-00732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rietze R, Poulin P, Weiss S. Mitotically active cells that generate neurons and astrocytes are present in multiple regions of the adult mouse hippocampus. J Comp Neurol. 2000;424:397–408. [PubMed] [Google Scholar]
- 33.Aguirre AA, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J Cell Biol. 2004;165:575–589. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kempermann G, Song H, Gage FH. Neurogenesis in the Adult Hippocampus. In: Gage FH, Kempermann G, Song H, editors. Adult Neurogenesis. Cold Spring Harbor Laboratory Press.; Cold Spring Harbor: 2008. pp. 159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maccaferri G, Lacaille JC. Interneuron Diversity series: Hippocampal interneuron classifications--making things as simple as possible, not simpler. Trends Neurosci. 2003;26:564–571. doi: 10.1016/j.tins.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 36.Han ZS, Buhl EH, Lorinczi Z, Somogyi P. A high degree of spatial selectivity in the axonal and dendritic domains of physiologically identified local-circuit neurons in the dentate gyrus of the rat hippocampus. Eur J Neurosci. 1993;5:395–410. doi: 10.1111/j.1460-9568.1993.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 37.Ribak CE, Seress L. Five types of basket cell in the hippocampal dentate gyrus: a combined Golgi and electron microscopic study. J Neurocytol. 1983;12:577–597. doi: 10.1007/BF01181525. [DOI] [PubMed] [Google Scholar]
- 38.Ben-Ari Y. Excitatory actions of gaba during development: the nature of the nurture. Nat Rev Neurosci. 2002;3:728–739. doi: 10.1038/nrn920. [DOI] [PubMed] [Google Scholar]
- 39.Zhao C, Teng EM, Summers RG, Jr., Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. Journal of Neuroscience. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esposito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, et al. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. Journal of Neuroscience. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duan X, Kang E, Liu CY, Ming GL, Song H. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008;18:108–115. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, et al. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Filippov V, Kronenberg G, Pivneva T, Reuter K, Steiner B, et al. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol Cell Neurosci. 2003;23:373–382. doi: 10.1016/s1044-7431(03)00060-5. [DOI] [PubMed] [Google Scholar]
- 44.Huttmann K, Sadgrove M, Wallraff A, Hinterkeuser S, Kirchhoff F, et al. Seizures preferentially stimulate proliferation of radial glia-like astrocytes in the adult dentate gyrus: functional and immunocytochemical analysis. Eur J Neurosci. 2003;18:2769–2778. doi: 10.1111/j.1460-9568.2003.03002.x. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi M, Buckmaster PS. Reduced inhibition of dentate granule cells in a model of temporal lobe epilepsy. Journal of Neuroscience. 2003;23:2440–2452. doi: 10.1523/JNEUROSCI.23-06-02440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 47.Wang LP, Kempermann G, Kettenmann H. A subpopulation of precursor cells in the mouse dentate gyrus receives synaptic GABAergic input. Mol Cell Neurosci. 2005;29:181–189. doi: 10.1016/j.mcn.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 48.Bhattacharyya BJ, Banisadr G, Jung H, Ren D, Cronshaw DG, et al. The chemokine stromal cell-derived factor-1 regulates GABAergic inputs to neural progenitors in the postnatal dentate gyrus. Journal of Neuroscience. 2008;28:6720–6730. doi: 10.1523/JNEUROSCI.1677-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, et al. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ables JL, Decarolis NA, Johnson MA, Rivera PD, Gao Z, et al. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. Journal of Neuroscience. 2010;30:10484–10492. doi: 10.1523/JNEUROSCI.4721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steiner B, Klempin F, Wang L, Kott M, Kettenmann H, et al. Type-2 cells as link between glial and neuronal lineage in adult hippocampal neurogenesis. Glia. 2006;54:805–814. doi: 10.1002/glia.20407. [DOI] [PubMed] [Google Scholar]
- 52.Lagace DC, Whitman MC, Noonan MA, Ables JL, DeCarolis NA, et al. Dynamic contribution of nestin-expressing stem cells to adult Neurogenesis. Journal of Neuroscience. 2007;27:12623–12629. doi: 10.1523/JNEUROSCI.3812-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Duveau V, Laustela S, Barth L, Gianolini F, Vogt KE, et al. Spatiotemporal specificity of GABA(A) receptor-mediated regulation of adult hippocampal neurogenesis. Eur J Neurosci. 2011;34:362–373. doi: 10.1111/j.1460-9568.2011.07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- 55.Johnson MA, Ables JL, Eisch AJ. Cell-intrinsic signals that regulate adult neurogenesis in vivo: insights from inducible approaches. BMB Rep. 2009;42:245–259. doi: 10.5483/bmbrep.2009.42.5.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dhaliwal J, Lagace DC. Visualization and genetic manipulation of adult neurogenesis using transgenic mice. Eur J Neurosci. 2011;33:1025–1036. doi: 10.1111/j.1460-9568.2011.07600.x. [DOI] [PubMed] [Google Scholar]
- 57.Deisseroth K, Singla S, Toda H, Monje M, Palmer TD, et al. Excitation-neurogenesis coupling in adult neural stem/progenitor cells. Neuron. 2004;42:535–552. doi: 10.1016/s0896-6273(04)00266-1. [DOI] [PubMed] [Google Scholar]
- 58.Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, et al. Neurod1 is essential for the survival and maturation of adult-born neurons. Nature Neuroscience. 2009;12:1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miyata T, Maeda T, Lee JE. NeuroD is required for differentiation of the granule cells in the cerebellum and hippocampus. Genes & Development. 1999;13:1647–1652. doi: 10.1101/gad.13.13.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Overstreet LS, Hentges ST, Bumaschny VF, de Souza FS, Smart JL, et al. A transgenic marker for newly born granule cells in dentate gyrus. Journal of Neuroscience. 2004;24:3251–3259. doi: 10.1523/JNEUROSCI.5173-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Plumpe T, Ehninger D, Steiner B, Klempin F, Jessberger S, et al. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 2006;7:77. doi: 10.1186/1471-2202-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, et al. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nature Neuroscience. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toni N, Teng EM, Bushong EA, Aimone JB, Zhao CM, et al. Synapse formation on neurons born in the adult hippocampus. Nature Neuroscience. 2007;10:727–734. doi: 10.1038/nn1908. [DOI] [PubMed] [Google Scholar]
- 64.Sernagor E, Chabrol F, Bony G, Cancedda L. GABAergic control of neurite outgrowth and remodeling during development and adult neurogenesis: general rules and differences in diverse systems. Front Cell Neurosci. 2010;4:11. doi: 10.3389/fncel.2010.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sun B, Halabisky B, Zhou Y, Palop JJ, Yu G, et al. Imbalance between GABAergic and Glutamatergic Transmission Impairs Adult Neurogenesis in an Animal Model of Alzheimer's Disease. Cell Stem Cell. 2009;5:624–633. doi: 10.1016/j.stem.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Glykys J, Mody I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol. 2007;582:1163–1178. doi: 10.1113/jphysiol.2007.134460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jow F, Chiu D, Lim HK, Novak T, Lin S. Production of GABA by cultured hippocampal glial cells. Neurochem Int. 2004;45:273–283. doi: 10.1016/j.neuint.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 68.Liu QY, Schaffner AE, Chang YH, Maric D, Barker JL. Persistent activation of GABA(A) receptor/Cl(−) channels by astrocyte-derived GABA in cultured embryonic rat hippocampal neurons. J Neurophysiol. 2000;84:1392–1403. doi: 10.1152/jn.2000.84.3.1392. [DOI] [PubMed] [Google Scholar]
- 69.Kozlov AS, Angulo MC, Audinat E, Charpak S. Target cell-specific modulation of neuronal activity by astrocytes. Proc Natl Acad Sci U S A. 2006;103:10058–10063. doi: 10.1073/pnas.0603741103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scharfman HE, Kunkel DD, Schwartzkroin PA. Synaptic connections of dentate granule cells and hilar neurons: results of paired intracellular recordings and intracellular horseradish peroxidase injections. Neuroscience. 1990;37:693–707. doi: 10.1016/0306-4522(90)90100-i. [DOI] [PubMed] [Google Scholar]
- 71.Frotscher M, Schlander M, Leranth C. Cholinergic neurons in the hippocampus. A combined light- and electron-microscopic immunocytochemical study in the rat. Cell Tissue Res. 1986;246:293–301. doi: 10.1007/BF00215891. [DOI] [PubMed] [Google Scholar]
- 72.Frotscher M, Vida I, Bender R. Evidence for the existence of non-GABAergic, cholinergic interneurons in the rodent hippocampus. Neuroscience. 2000;96:27–31. doi: 10.1016/s0306-4522(99)00525-4. [DOI] [PubMed] [Google Scholar]
- 73.Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 74.Mesulam MM, Mufson EJ, Wainer BH, Levey AI. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience. 1983;10:1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- 75.Cooper-Kuhn CM, Winkler J, Kuhn HG. Decreased neurogenesis after cholinergic forebrain lesion in the adult rat. J Neurosci Res. 2004;77:155–165. doi: 10.1002/jnr.20116. [DOI] [PubMed] [Google Scholar]
- 76.Mohapel P, Leanza G, Kokaia M, Lindvall O. Forebrain acetylcholine regulates adult hippocampal neurogenesis and learning. Neurobiol Aging. 2005;26:939–946. doi: 10.1016/j.neurobiolaging.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 77.Ji D, Dani JA. Inhibition and disinhibition of pyramidal neurons by activation of nicotinic receptors on hippocampal interneurons. J Neurophysiol. 2000;83:2682–2690. doi: 10.1152/jn.2000.83.5.2682. [DOI] [PubMed] [Google Scholar]
- 78.Encinas JM, Vaahtokari A, Enikolopov G. Fluoxetine targets early progenitor cells in the adult brain. Proc Natl Acad Sci U S A. 2006;103:8233–8238. doi: 10.1073/pnas.0601992103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29:450–460. doi: 10.1038/sj.npp.1300320. [DOI] [PubMed] [Google Scholar]
- 80.Brezun JM, Daszuta A. Serotonin depletion in the adult rat produces differential changes in highly polysialylated form of neural cell adhesion molecule and tenascin-C immunoreactivity. J Neurosci Res. 1999;55:54–70. doi: 10.1002/(SICI)1097-4547(19990101)55:1<54::AID-JNR7>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 81.Radley JJ, Jacobs BL. 5-HT1A receptor antagonist administration decreases cell proliferation in the dentate gyrus. Brain Research. 2002;955:264–267. doi: 10.1016/s0006-8993(02)03477-7. [DOI] [PubMed] [Google Scholar]
- 82.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 83.Zhao S, Chai X, Frotscher M. Balance between neurogenesis and gliogenesis in the adult hippocampus: role for reelin. Dev Neurosci. 2007;29:84–90. doi: 10.1159/000096213. [DOI] [PubMed] [Google Scholar]
- 84.Erbel-Sieler C, Dudley C, Zhou Y, Wu X, Estill SJ, et al. Behavioral and regulatory abnormalities in mice deficient in the NPAS1 and NPAS3 transcription factors. Proc Natl Acad Sci U S A. 2004;101:13648–13653. doi: 10.1073/pnas.0405310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Curran T, D'Arcangelo G. Role of reelin in the control of brain development. Brain Res Brain Res Rev. 1998;26:285–294. doi: 10.1016/s0165-0173(97)00035-0. [DOI] [PubMed] [Google Scholar]
- 86.Weiss KH, Johanssen C, Tielsch A, Herz J, Deller T, et al. Malformation of the radial glial scaffold in the dentate gyrus of reeler mice, scrambler mice, and ApoER2/VLDLR-deficient mice. J Comp Neurol. 2003;460:56–65. doi: 10.1002/cne.10644. [DOI] [PubMed] [Google Scholar]
- 87.Forster E, Tielsch A, Saum B, Weiss KH, Johanssen C, et al. Reelin, Disabled 1, and beta 1 integrins are required for the formation of the radial glial scaffold in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:13178–13183. doi: 10.1073/pnas.202035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frotscher M, Haas CA, Forster E. Reelin controls granule cell migration in the dentate gyrus by acting on the radial glial scaffold. Cereb Cortex. 2003;13:634–640. doi: 10.1093/cercor/13.6.634. [DOI] [PubMed] [Google Scholar]
- 89.Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, et al. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 90.Won SJ, Kim SH, Xie L, Wang Y, Mao XO, et al. Reelin-deficient mice show impaired neurogenesis and increased stroke size. Exp Neurol. 2006;198:250–259. doi: 10.1016/j.expneurol.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 91.Pesold C, Impagnatiello F, Pisu MG, Uzunov DP, Costa E, et al. Reelin is preferentially expressed in neurons synthesizing gamma-aminobutyric acid in cortex and hippocampus of adult rats. Proc Natl Acad Sci U S A. 1998;95:3221–3226. doi: 10.1073/pnas.95.6.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weeber EJ, Beffert U, Jones C, Christian JM, Forster E, et al. Reelin and ApoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J Biol Chem. 2002;277:39944–39952. doi: 10.1074/jbc.M205147200. [DOI] [PubMed] [Google Scholar]
- 93.Beffert U, Weeber EJ, Durudas A, Qiu S, Masiulis I, et al. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 2005;47:567–579. doi: 10.1016/j.neuron.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 94.Pujadas L, Gruart A, Bosch C, Delgado L, Teixeira CM, et al. Reelin regulates postnatal neurogenesis and enhances spine hypertrophy and long-term potentiation. Journal of Neuroscience. 2010;30:4636–4649. doi: 10.1523/JNEUROSCI.5284-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gong C, Wang TW, Huang HS, Parent JM. Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. Journal of Neuroscience. 2007;27:1803–1811. doi: 10.1523/JNEUROSCI.3111-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hiesberger T, Trommsdorff M, Howell BW, Goffinet A, Mumby MC, et al. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of disabled-1 and modulates tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- 97.Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- 98.Parent JM, Elliott RC, Pleasure SJ, Barbaro NM, Lowenstein DH. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann Neurol. 2006;59:81–91. doi: 10.1002/ana.20699. [DOI] [PubMed] [Google Scholar]
- 99.Herz J, Beffert U. Apolipoprotein E receptors: linking brain development and Alzheimer's disease. Nat Rev Neurosci. 2000;1:51–58. doi: 10.1038/35036221. [DOI] [PubMed] [Google Scholar]
- 100.Mahley RW. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- 101.Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, et al. Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. Journal of Neuroscience. 2006;26:4985–4994. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Poirier J, Hess M, May PC, Finch CE. Astrocytic apolipoprotein E mRNA and GFAP mRNA in hippocampus after entorhinal cortex lesioning. Brain Res Mol Brain Res. 1991;11:97–106. doi: 10.1016/0169-328x(91)90111-a. [DOI] [PubMed] [Google Scholar]
- 103.Xu Q, Walker D, Bernardo A, Brodbeck J, Balestra ME, et al. Intron-3 retention/splicing controls neuronal expression of apolipoprotein E in the CNS. J Neurosci. 2008;28:1452–1459. doi: 10.1523/JNEUROSCI.3253-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, et al. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. Journal of Neuroscience. 2010;30:13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roses AD. Apolipoprotein E alleles as risk factors in Alzheimer's disease. Annu Rev Med. 1996;47:387–400. doi: 10.1146/annurev.med.47.1.387. [DOI] [PubMed] [Google Scholar]
- 106.Li G, Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, et al. GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell. 2009;5:634–645. doi: 10.1016/j.stem.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tran PB, Banisadr G, Ren D, Chenn A, Miller RJ. Chemokine receptor expression by neural progenitor cells in neurogenic regions of mouse brain. J Comp Neurol. 2007;500:1007–1033. doi: 10.1002/cne.21229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kolodziej A, Schulz S, Guyon A, Wu DF, Pfeiffer M, et al. Tonic activation of CXC chemokine receptor 4 in immature granule cells supports neurogenesis in the adult dentate gyrus. Journal of Neuroscience. 2008;28:4488–4500. doi: 10.1523/JNEUROSCI.4721-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pieper AA, Wu X, Han TW, Estill SJ, Dang Q, et al. The neuronal PAS domain protein 3 transcription factor controls FGF-mediated adult hippocampal neurogenesis in mice. Proc Natl Acad Sci U S A. 2005;102:14052–14057. doi: 10.1073/pnas.0506713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao M, Li D, Shimazu K, Zhou YX, Lu B, et al. Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biol Psychiatry. 2007;62:381–390. doi: 10.1016/j.biopsych.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 111.Ohkubo Y, Uchida AO, Shin D, Partanen J, Vaccarino FM. Fibroblast growth factor receptor 1 is required for the proliferation of hippocampal progenitor cells and for hippocampal growth in mouse. Journal of Neuroscience. 2004;24:6057–6069. doi: 10.1523/JNEUROSCI.1140-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- 113.Gimenez-Llort L, Blazquez G, Canete T, Johansson B, Oddo S, et al. Modeling behavioral and neuronal symptoms of Alzheimer's disease in mice: a role for intraneuronal amyloid. Neurosci Biobehav Rev. 2007;31:125–147. doi: 10.1016/j.neubiorev.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 114.Kobayashi K. Targeting the hippocampal mossy fiber synapse for the treatment of psychiatric disorders. Mol Neurobiol. 2009;39:24–36. doi: 10.1007/s12035-008-8049-5. [DOI] [PubMed] [Google Scholar]
- 115.Rothman SM, Mattson MP. Adverse stress, hippocampal networks, and Alzheimer's disease. Neuromolecular Med. 2010;12:56–70. doi: 10.1007/s12017-009-8107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Burger C. Region-specific genetic alterations in the aging hippocampus: implications for cognitive aging. Front Aging Neurosci. 2010;2:140. doi: 10.3389/fnagi.2010.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lazarov O, Marr RA. Neurogenesis and Alzheimer's disease: at the crossroads. Exp Neurol. 2010;223:267–281. doi: 10.1016/j.expneurol.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Knuesel I. Reelin-mediated signaling in neuropsychiatric and neurodegenerative diseases. Prog Neurobiol. 2010;91:257–274. doi: 10.1016/j.pneurobio.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 119.Takahashi H, Brasnjevic I, Rutten BP, Van Der Kolk N, Perl DP, et al. Hippocampal interneuron loss in an APP/PS1 double mutant mouse and in Alzheimer's disease. Brain Struct Funct. 2010;214:145–160. doi: 10.1007/s00429-010-0242-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Benes FM, Kwok EW, Vincent SL, Todtenkopf MS. A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry. 1998;44:88–97. doi: 10.1016/s0006-3223(98)00138-3. [DOI] [PubMed] [Google Scholar]
- 121.Kim J, Basak JM, Holtzman DM. The role of apolipoprotein E in Alzheimer's disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ohkubo N, Lee YD, Morishima A, Terashima T, Kikkawa S, et al. Apolipoprotein E and Reelin ligands modulate tau phosphorylation through an apolipoprotein E receptor/disabled-1/glycogen synthase kinase-3beta cascade. FASEB J. 2003;17:295–297. doi: 10.1096/fj.02-0434fje. [DOI] [PubMed] [Google Scholar]
- 123.Fatemi SH, Earle JA, McMenomy T. Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry. 2000;5:654–663. 571. doi: 10.1038/sj.mp.4000783. [DOI] [PubMed] [Google Scholar]
- 124.Barr AM, Fish KN, Markou A, Honer WG. Heterozygous reeler mice exhibit alterations in sensorimotor gating but not presynaptic proteins. European Journal of Neuroscience. 2008;27:2568–2574. doi: 10.1111/j.1460-9568.2008.06233.x. [DOI] [PubMed] [Google Scholar]
- 125.Kamnasaran D, Muir WJ, Ferguson-Smith MA, Cox DW. Disruption of the neuronal PAS3 gene in a family affected with schizophrenia. J Med Genet. 2003;40:325–332. doi: 10.1136/jmg.40.5.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Houser CR. Granule cell dispersion in the dentate gyrus of humans with temporal lobe epilepsy. Brain Res. 1990;535:195–204. doi: 10.1016/0006-8993(90)91601-c. [DOI] [PubMed] [Google Scholar]
- 127.Babb TL, Kupfer WR, Pretorius JK, Crandall PH, Levesque MF. Synaptic reorganization by mossy fibers in human epileptic fascia dentata. Neuroscience. 1991;42:351–363. doi: 10.1016/0306-4522(91)90380-7. [DOI] [PubMed] [Google Scholar]
- 128.Haas CA, Dudeck O, Kirsch M, Huszka C, Kann G, et al. Role for reelin in the development of granule cell dispersion in temporal lobe epilepsy. Journal of Neuroscience. 2002;22:5797–5802. doi: 10.1523/JNEUROSCI.22-14-05797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, et al. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.D'Alessio L, Konopka H, Lopez EM, Seoane E, Consalvo D, et al. Doublecortin (DCX) immunoreactivity in hippocampus of chronic refractory temporal lobe epilepsy patients with hippocampal sclerosis. Seizure. 2010;19:567–572. doi: 10.1016/j.seizure.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 131.Engel T, Schindler CK, Sanz-Rodriguez A, Conroy RM, Meller R, et al. Expression of neurogenesis genes in human temporal lobe epilepsy with hippocampal sclerosis. Int J Physiol Pathophysiol Pharmacol. 2011;3:38–47. [PMC free article] [PubMed] [Google Scholar]
- 132.Okamoto OK, Janjoppi L, Bonone FM, Pansani AP, da Silva AV, et al. Whole transcriptome analysis of the hippocampus: toward a molecular portrait of epileptogenesis. BMC Genomics. 2010;11:230. doi: 10.1186/1471-2164-11-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu YW, Curtis MA, Gibbons HM, Mee EW, Bergin PS, et al. Doublecortin expression in the normal and epileptic adult human brain. Eur J Neurosci. 2008;28:2254–2265. doi: 10.1111/j.1460-9568.2008.06518.x. [DOI] [PubMed] [Google Scholar]
- 134.Magloczky Z, Wittner L, Borhegyi Z, Halasz P, Vajda J, et al. Changes in the distribution and connectivity of interneurons in the epileptic human dentate gyrus. Neuroscience. 2000;96:7–25. doi: 10.1016/s0306-4522(99)00474-1. [DOI] [PubMed] [Google Scholar]
- 135.Siebzehnrubl FA, Blumcke I. Neurogenesis in the human hippocampus and its relevance to temporal lobe epilepsies. Epilepsia 49 Suppl. 2008;5:55–65. doi: 10.1111/j.1528-1167.2008.01638.x. [DOI] [PubMed] [Google Scholar]
- 136.Joels M, Karst H, Krugers HJ, Lucassen PJ. Chronic stress: implications for neuronal morphology, function and neurogenesis. Front Neuroendocrinol. 2007;28:72–96. doi: 10.1016/j.yfrne.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 137.Gass P, Kretz O, Wolfer DP, Berger S, Tronche F, et al. Genetic disruption of mineralocorticoid receptor leads to impaired neurogenesis and granule cell degeneration in the hippocampus of adult mice. EMBO Rep. 2000;1:447–451. doi: 10.1093/embo-reports/kvd088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kronenberg G, Kirste I, Inta D, Chourbaji S, Heuser I, et al. Reduced hippocampal neurogenesis in the GR(+/−) genetic mouse model of depression. Eur Arch Psychiatry Clin Neurosci. 2009;259:499–504. doi: 10.1007/s00406-009-0036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mayer JL, Klumpers L, Maslam S, de Kloet ER, Joels M, et al. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalises the corticosterone-induced reduction of adult hippocampal neurogenesis. J Neuroendocrinol. 2006;18:629–631. doi: 10.1111/j.1365-2826.2006.01455.x. [DOI] [PubMed] [Google Scholar]
- 140.Montaron MF, Piazza PV, Aurousseau C, Urani A, Le Moal M, et al. Implication of corticosteroid receptors in the regulation of hippocampal structural plasticity. Eur J Neurosci. 2003;18:3105–3111. doi: 10.1111/j.1460-9568.2003.03048.x. [DOI] [PubMed] [Google Scholar]
- 141.Garcia A, Steiner B, Kronenberg G, Bick-Sander A, Kempermann G. Age-dependent expression of glucocorticoid- and mineralocorticoid receptors on neural precursor cell populations in the adult murine hippocampus. Aging Cell. 2004;3:363–371. doi: 10.1111/j.1474-9728.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- 142.Czeh B, Simon M, van der Hart MG, Schmelting B, Hesselink MB, et al. Chronic stress decreases the number of parvalbumin-immunoreactive interneurons in the hippocampus: prevention by treatment with a substance P receptor (NK1) antagonist. Neuropsychopharmacology. 2005;30:67–79. doi: 10.1038/sj.npp.1300581. [DOI] [PubMed] [Google Scholar]
- 143.Arancibia S, Payet O, Givalois L, Tapia-Arancibia L. Acute stress and dexamethasone rapidly increase hippocampal somatostatin synthesis and release from the dentate gyrus hilus. Hippocampus. 2001;11:469–477. doi: 10.1002/hipo.1061. [DOI] [PubMed] [Google Scholar]
- 144.Seidel K, Helmeke C, Poeggel G, Braun K. Repeated neonatal separation stress alters the composition of neurochemically characterized interneuron subpopulations in the rodent dentate gyrus and basolateral amygdala. Dev Neurobiol. 2008;68:1137–1152. doi: 10.1002/dneu.20651. [DOI] [PubMed] [Google Scholar]
- 145.Holm MM, Nieto-Gonzalez JL, Vardya I, Henningsen K, Jayatissa MN, et al. Hippocampal GABAergic dysfunction in a rat chronic mild stress model of depression. Hippocampus. 2011;21:422–433. doi: 10.1002/hipo.20758. [DOI] [PubMed] [Google Scholar]
- 146.Williams TJ, Milner TA. Delta opioid receptors colocalize with corticotropin releasing factor in hippocampal interneurons. Neuroscience. 2011;179:9–22. doi: 10.1016/j.neuroscience.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hu W, Zhang M, Czeh B, Flugge G, Zhang W. Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology. 2010;35:1693–1707. doi: 10.1038/npp.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 149.Gronli J, Fiske E, Murison R, Bjorvatn B, Sorensen E, et al. Extracellular levels of serotonin and GABA in the hippocampus after chronic mild stress in rats. A microdialysis study in an animal model of depression. Behav Brain Res. 2007;181:42–51. doi: 10.1016/j.bbr.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 150.Brambilla P, Perez J, Barale F, Schettini G, Soares JC. GABAergic dysfunction in mood disorders. Mol Psychiatry. 2003;8:721–737. 715. doi: 10.1038/sj.mp.4001362. [DOI] [PubMed] [Google Scholar]
- 151.Castren E, Rantamaki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev Neurobiol. 2010;70:289–297. doi: 10.1002/dneu.20758. [DOI] [PubMed] [Google Scholar]
- 152.Tanti A, Belzung C. Open questions in current models of antidepressant action. Br J Pharmacol. 2010;159:1187–1200. doi: 10.1111/j.1476-5381.2009.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sahay A, Hen R. Adult hippocampal neurogenesis in depression. Nature Neuroscience. 2007;10:1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 154.Eisch AJ, Harburg GC. Opiates, psychostimulants, and adult hippocampal neurogenesis: Insights for addiction and stem cell biology. Hippocampus. 2006;16:271–286. doi: 10.1002/hipo.20161. [DOI] [PubMed] [Google Scholar]
- 155.Yakel JL, Shao Z. Functional and molecular characterization of neuronal nicotinic ACh receptors in rat hippocampal interneurons. Prog Brain Res. 2004;145:95–107. doi: 10.1016/S0079-6123(03)45006-1. [DOI] [PubMed] [Google Scholar]
- 156.Jones S, Yakel JL. Functional nicotinic ACh receptors on interneurones in the rat hippocampus. J Physiol. 1997;504(Pt 3):603–610. doi: 10.1111/j.1469-7793.1997.603bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Morales M, Hein K, Vogel Z. Hippocampal interneurons co-express transcripts encoding the alpha7 nicotinic receptor subunit and the cannabinoid receptor 1. Neuroscience. 2008;152:70–81. doi: 10.1016/j.neuroscience.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 159.Drake CT, Milner TA. Mu opioid receptors are extensively co-localized with parvalbumin, but not somatostatin, in the dentate gyrus. Neurosci Lett. 2006;403:176–180. doi: 10.1016/j.neulet.2006.04.047. [DOI] [PubMed] [Google Scholar]
- 160.Stumm RK, Zhou C, Schulz S, Hollt V. Neuronal types expressing mu- and delta-opioid receptor mRNA in the rat hippocampal formation. J Comp Neurol. 2004;469:107–118. doi: 10.1002/cne.10997. [DOI] [PubMed] [Google Scholar]
- 161.Hajos N, Freund TF. Distinct cannabinoid sensitive receptors regulate hippocampal excitation and inhibition. Chem Phys Lipids. 2002;121:73–82. doi: 10.1016/s0009-3084(02)00149-4. [DOI] [PubMed] [Google Scholar]
- 162.Drake CT, Chavkin C, Milner TA. Opioid systems in the dentate gyrus. Prog Brain Res. 2007;163:245–263. doi: 10.1016/S0079-6123(07)63015-5. [DOI] [PubMed] [Google Scholar]
- 163.Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101(Suppl 1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- 164.Gareri P, De Fazio P, De Sarro G. Neuropharmacology of depression in aging and age-related diseases. Ageing Res Rev. 2002;1:113–134. doi: 10.1016/s0047-6374(01)00370-0. [DOI] [PubMed] [Google Scholar]
- 165.Herrup K. Reimagining Alzheimer's disease--an age-based hypothesis. Journal of Neuroscience. 2010;30:16755–16762. doi: 10.1523/JNEUROSCI.4521-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Stanley DP, Shetty AK. Aging in the rat hippocampus is associated with widespread reductions in the number of glutamate decarboxylase-67 positive interneurons but not interneuron degeneration. J Neurochem. 2004;89:204–216. doi: 10.1111/j.1471-4159.2004.02318.x. [DOI] [PubMed] [Google Scholar]
- 167.Bernal GM, Peterson DA. Phenotypic and gene expression modification with normal brain aging in GFAP-positive astrocytes and neural stem cells. Aging Cell. 2011;10:466–482. doi: 10.1111/j.1474-9726.2011.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- 169.Lagace DC, Benavides DR, Kansy JW, Mapelli M, Greengard P, et al. Cdk5 is essential for adult hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2008;105:18567–18571. doi: 10.1073/pnas.0810137105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Piatti VC, Davies-Sala MG, Esposito MS, Mongiat LA, Trinchero MF, et al. The timing for neuronal maturation in the adult hippocampus is modulated by local network activity. Journal of Neuroscience. 2011;31:7715–7728. doi: 10.1523/JNEUROSCI.1380-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, et al. Adult-Born Hippocampal Neurons Are More Numerous, Faster Maturing, and More Involved in Behavior in Rats than in Mice. Journal of Neuroscience. 2009;29:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Snyder JS, Ramchand P, Rabbett S, Radik R, Wojtowicz JM, et al. Septo-temporal gradients of neurogenesis and activity in 13-month-old rats. Neurobiol Aging. 2011;32:1149–1156. doi: 10.1016/j.neurobiolaging.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, et al. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 174.Chambers RA, Potenza MN, Hoffman RE, Miranker W. Simulated apoptosis/neurogenesis regulates learning and memory capabilities of adaptive neural networks. Neuropsychopharmacology. 2004;29:747–758. doi: 10.1038/sj.npp.1300358. [DOI] [PubMed] [Google Scholar]
- 175.Becker S, Wojtowicz JM. A model of hippocampal neurogenesis in memory and mood disorders. Trends Cogn Sci. 2007;11:70–76. doi: 10.1016/j.tics.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 176.Aimone JB, Gage FH. Modeling new neuron function: a history of using computational neuroscience to study adult neurogenesis. Eur J Neurosci. 2011;33:1160–1169. doi: 10.1111/j.1460-9568.2011.07615.x. [DOI] [PubMed] [Google Scholar]
- 177.Gueneau G, Drouet J, Privat A, Court L. Differential radiosensitivity of neurons and neuroglia of the hippocampus in the adult rabbit. Acta Neuropathol. 1979;48:199–209. doi: 10.1007/BF00690520. [DOI] [PubMed] [Google Scholar]
- 178.Mandyam CD, Norris RD, Eisch AJ. Chronic morphine induces premature mitosis of proliferating cells in the adult mouse subgranular zone. J Neurosci Res. 2004;76:783–794. doi: 10.1002/jnr.20090. [DOI] [PubMed] [Google Scholar]
- 179.Olariu A, Cleaver KM, Shore LE, Brewer MD, Cameron HA. A natural form of learning can increase and decrease the survival of new neurons in the dentate gyrus. Hippocampus. 2005;15:750–762. doi: 10.1002/hipo.20097. [DOI] [PubMed] [Google Scholar]
- 180.Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, et al. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One. 2010;5:e8809. doi: 10.1371/journal.pone.0008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Brandt MD, Jessberger S, Steiner B, Kronenberg G, Reuter K, et al. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol Cell Neurosci. 2003;24:603–613. doi: 10.1016/s1044-7431(03)00207-0. [DOI] [PubMed] [Google Scholar]
- 182.Gulyas AI, Hajos N, Freund TF. Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J Neurosci. 1996;16:3397–3411. doi: 10.1523/JNEUROSCI.16-10-03397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gulyas AI, Miettinen R, Jacobowitz DM, Freund TF. Calretinin is present in non-pyramidal cells of the rat hippocampus--I. A new type of neuron specifically associated with the mossy fibre system. Neuroscience. 1992;48:1–27. doi: 10.1016/0306-4522(92)90334-x. [DOI] [PubMed] [Google Scholar]
- 184.Forster E, Zhao S, Frotscher M. Laminating the hippocampus. Nat Rev Neurosci. 2006;7:259–267. doi: 10.1038/nrn1882. [DOI] [PubMed] [Google Scholar]
- 185.Borhegyi Z, Leranth C. Distinct substance P- and calretinin-containing projections from the supramammillary area to the hippocampus in rats; a species difference between rats and monkeys. Exp Brain Res. 1997;115:369–374. doi: 10.1007/pl00005706. [DOI] [PubMed] [Google Scholar]
- 186.Magloczky Z, Acsady L, Freund TF. Principal cells are the postsynaptic targets of supramammillary afferents in the hippocampus of the rat. Hippocampus. 1994;4:322–334. doi: 10.1002/hipo.450040316. [DOI] [PubMed] [Google Scholar]