Abstract
Synovial sarcoma is a deadly malignancy with limited sensitivity to traditional cytotoxic chemotherapy. SS18-SSX fusion oncogene expression characterizes human synovial sarcomas and drives oncogenesis in a mouse model. Elevated expression of BCL2 is considered a consistent feature of the synovial sarcoma expression profile. Our objective was to evaluate the expression of apoptotic pathway members in synovial sarcomas and interrogate the impact of modulating SS18-SSX expression on this pathway. We show in human and murine synovial sarcoma cells that SS18-SSX increases BCL2 expression, but represses other anti-apoptotic genes, including MCL1 and BCL2A1. This repression is achieved by directly suppressing expression via binding through ATF2 to the cyclic AMP response element in the promoters of these genes and recruiting TLE1/Groucho. The suppression of these two anti-apoptotic pathways silences the typical routes by which other tumors evade BH3-domain peptidomimetic pharmacotherapy. We show that mouse and human synovial sarcoma cells are sensitive in vitro to ABT-263, a BH3-peptidomimetic, much more so than are other tested cancer cell lines. ABT-263 also enhances the sensitivity of these cells to doxorubicin, a traditional cytotoxic chemotherapy used for synovial sarcoma. We also demonstrate the capacity of ABT-263 to stunt synovial sarcomagenesis in vivo in a genetic mouse model. These data recommend pursuit of BH3-peptidomimetic pharmacotherapy in human synovial sarcomas.
Keywords: synovial sarcoma, apoptosis, chemotherapy, targeted therapy, mouse model
INTRODUCTION
Synovial sarcoma is a soft-tissue malignancy with a predilection for adolescents and young adults [1]. Approximately half of patients diagnosed with synovial sarcoma will die from disease, that portion much higher in those who present with or develop metastasis. Targeted therapeutic strategies are both lacking and greatly needed.
Synovial sarcomas bear a characteristic chromosomal translocation between chromosomes 18 and X, generating a fusion oncogene comprised of the promoter and 5′ coding sequence of SS18 (previously called SYT) through its tenth exon and the 3′ coding sequence of an SSX gene, SSX1, SSX2, or SSX4 [1]. Expression of the human SS18-SSX2 cDNA in certain tissues induces synovial sarcomagenesis in mice [2, 3]. The fusion oncoprotein functions as a bridge between ATF2, bound to cyclic AMP response elements (CREs) in the promoters of target genes, and TLE1, which recruits histone deacetylase and the polycomb group repressor complex, resulting in epigenetic gene silencing [4].
A high level of BCL2 is considered part of the definitional synovial sarcoma expression signature [5]. Nearly all synovial sarcomas will stain for high levels of BCL2 by immunohistochemistry [6, 7]. Although apoptosis pathways have not been thoroughly investigated to define a tumor-specific role for BCL2 in synovial sarcoma, BCL2 is suspected to contribute to resistance to cytotoxic chemotherapies [8]. Anti-sense knock-down of BCL2 was previously noted to sensitize synovial sarcoma cells to doxorubicin-induced apoptosis [9].
A new class of compounds antagonizes BCL2 family members more generally by mimicking the BH3 domain, through which BCL2, BCL-xL, and BCL-w act to suppress downstream activators of apoptosis [10]. ABT-263, an orally available BH3 domain peptidomimetic was found to be safe and marginally effective against follicular cell lymphoma and small cell lung cancer in phase I trials [11, 12]. A primary challenge to its efficacy has been the tendency of cancers to up-regulate alternate anti-apoptotic genes, unaffected by ABT-263. Specifically, MCL1 is the most common route of escape from ABT-263, with BCL2A1 as another means of evasion [13, 14].
We show that the biology inherent to synovial sarcomagenesis suppresses these escape routes, enabling tumor sensitivity to this new class of pharmaceuticals.
RESULTS AND DISCUSSION
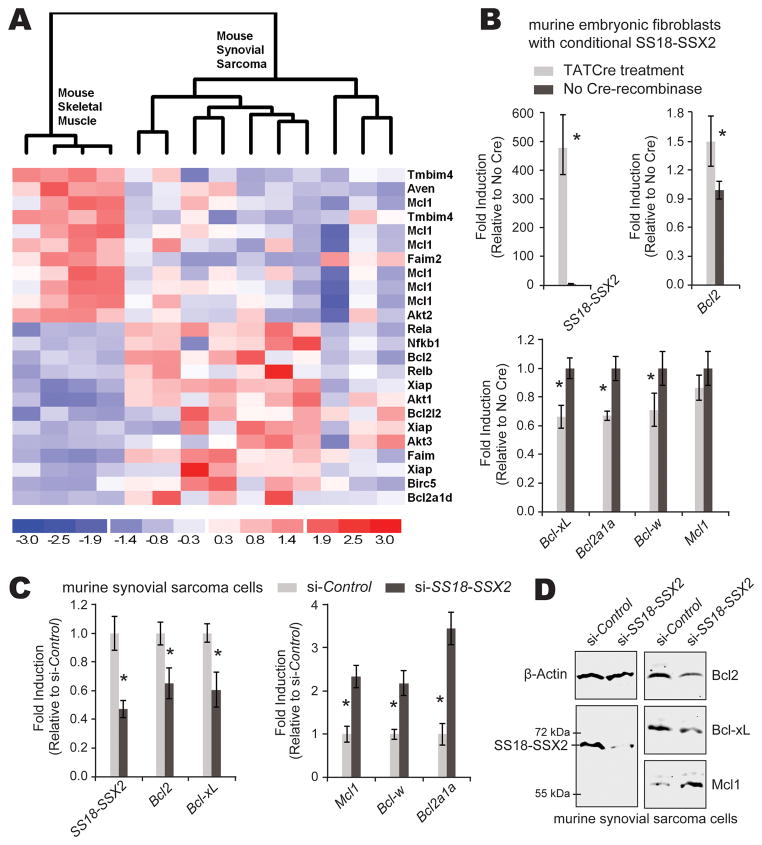
In order to investigate apoptotic pathway members in synovial sarcomas, we assembled a list of genes directly involved in apoptosis by combining related Kegg pathway and Gene Ontology gene lists and then dividing them into anti- and pro-apoptotic components (Supplemental Table 1). We interrogated published and publicly available mouse synovial sarcoma expression profiles from the Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo, profiles GSE6461 and GSE14469 [2, 3],) using D-chip software (www.dichip.org) [15] with an alpha of 0.05 as the criterion for stringency (Fig. 1A and Supplemental Figure 1A). While Bcl2 expression was elevated in tumors, more striking was the unusual balance of apoptotic pathways, with up-regulation of many pro-apoptotic members and the consistent down-regulation of Mcl1 (Fig. 1A and Supplemental Figure 1A). Similar patterns were confirmed by comparing 29 human synovial sarcomas from GSE20196 [16] to 5 human mesenchymal stem cell control samples from GSE26272 [17] (Supplemental Figures 1B and C). An additional analysis compared 16 human synovial sarcomas to 21 human malignant fibrous histiocytomas (soft-tissue sarcomas without SS18-SSX expression) from the prior study set GDS2763 [18]. In this tumor-to-tumor comparison, BCL2 had 2.7-fold higher expression (t-test p = 0.0002) and MCL1 1.7-fold lower expression (p = 0.002) in the synovial sarcomas.
Figure 1. Anti-apoptotic genes in a mouse model of synovial sarcoma.
(A) Reanalysis of expression arrays from two previously published models of synovial sarcoma in mice according to an anti-apoptotic gene list shows skeletal muscle control samples (left) compared to synovial sarcomas induced by expression of SS18-SSX2 initiated by two different promoters of Cre-recombinase, Myf5 and Rosa26 (5 each). Mcl1 represents 6 of the significantly down-regulated microarray probes. (B) E14.5 mouse embryonic fibroblasts heterozygous for conditionally activated SS18-SSX2 were induced by exposure to TATCre protein or control conditions in vitro. After 48 hours, this resulted in increased Bcl2 expression and decreases in other BH3-domain genes by SYBR green quantitative PCR from RT-PCR-generated cDNAs. Mcl1 down-regulation was not statistically significant (p = 0.2, Student’s t-test). (C) Cells derived from mouse synovial sarcomas were transfected with anti-SS18-SSX2 siRNA or control scrambled siRNA, achieving knockdown of SS18-SSX2, downregulation of Bcl2 and Bcl-xL and upregulation of Mcl1, Bcl-w, and Bcl2a1a, all measured by SYBR green qPCR from cDNAs generated from total RNA procured 48 hours after application of siRNA. (D) Western confirms the same directional changes in protein levels for SS18-SSX2, Bcl2, BclxL, and Mcl1 72 hours following siRNA knockdown of SS18-SSX2 in mouse synovial sarcoma cells. Each experiment was performed with a sample of at least 3 and independently repeated. (All charts depict mean with standard deviation error bars; Student’s t-test confirmed p-value < 0.05 in each comparison marked with an asterisk).
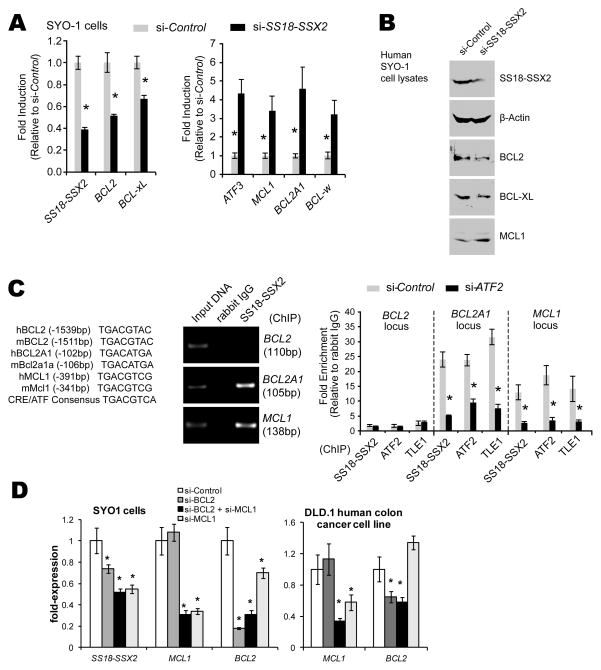
The strong consistency of high BCL2 levels in synovial sarcomas prompted the hypothesis that BCL2 expression is regulated by the SS18-SSX fusion oncogene. The promoter of the BCL2 gene in both the human and mouse genomes bears a CRE with a conserved and typical ATF binding sequence. Some genes with promoters bearing CREs have been shown to be directly suppressed by SS18-SSX binding to the promoter via ATF2 and recruiting TLE1 [4]. Such suppression, if active, would achieve downregulation, rather than the upregulation of BCL2 noted in synovial sarcomas. In order to investigate the control exerted by SS18-SSX2 on expression of BCL2, we performed both overexpression of SS18-SSX2 in naive cells and knockdown of the fusion oncogene in synovial sarcoma cells. Mouse embryonic fibroblasts (MEFs) heterozygous for conditionally activatable SS18-SSX2 in the Rosa26 locus were harvested from day 14.5 embryos, dissociated in trypsin, and plated in Dulbecco’s modified Eagle medium (DMEM) with 10% fetal bovine serum (FBS). Cre-mediated recombination to activate SS18-SSX2 expression in these MEFs was achieved by the application of 5 μM TATCre protein in DMEM [19]. When induced to express SS18-SSX2, the MEFs showed increased levels of Bcl2 by quantitative polymerase chain reaction (qPCR) following reverse transcriptase (RT) PCR from total RNA harvested at 48 hours (Fig. 1B). For the opposite experiment, thoracic cage tumors from our previously reported mouse model of synovial sarcoma were harvested from 15-week-old mice, dissociated in collagenase, hyaluronidase, and DNase, then plated in DMEM with 10% FBS. Short interfering RNA (siRNA) was applied using the Lipofectamine RNAiMax reagent (Sigma) as previously described [4, 20]. Bcl2 levels decrease in response to siRNA against SS18-SSX2 transcripts versus scrambled siRNA controls in cells derived from mouse synovial sarcomas (Fig. 1C and D). This suggests that the fusion oncoprotein promotes Bcl2 expression. For a human correlate, we used the SYO1 cell line, derived from a human synovial sarcoma expressing SS18-SSX2, kindly provided by Akira Kawai (National Cancer Center Hospital, Tokyo)[21]. Similar decrease in BCL2 expression was noted in the SYO1 cells upon siRNA directed against SS18-SSX2 (Fig. 2A and B). To determine whether or not SS18-SSX2 and its repressor complex could be identified at the putative ATF2 binding site in the BCL2 promoter cyclic AMP response element (CRE), we performed chromatin immunoprecipitation (ChIP) in SYO1 cells using antibodies directed against each known member of the complex, SS18-SSX2, ATF2, and TLE1 (Fig. 2C). This ChIP identified none of these components bound to the BCL2 CRE (Fig. 2C and D). From these data we conclude that BCL2 transcriptional regulation by SS18-SSX2 either uses a distinct mechanism or is indirectly impacted by SS18-SSX2 suppressing another BCL suppressor.
Figure 2. SS18-SSX2 in a human synovial sarcoma cell line also directly suppresses expression of MCL1 and BCL2A1 and can be identified at their promoters.
(A) SYBR green qPCR of cDNAs made from total RNA isolated from the SYO-1 human synovial sarcoma cell line 48 hours following siRNA directed against SS18-SSX2 or scrambled control confirms downregulation of BCL2 and BCL-xL and upregulation of MCL1, BCL2A1, and BCL-w (ATF3 is a “positive” control, known to be directly suppressed by SS18-SSX2 in this cell line). (B) Western confirms the same directional changes at the protein level, from extracts obtained 72 hours post-application of the siRNA. (C) The ATF binding sequence in the cyclic AMP response element (CRE) in the promoters of each of the genes in question shows perfect cross-species homology between mouse and human. However, chromatin immunoprecipitation (ChIP) with an antibody directed against the SS18-SSX junction identifies that CRE bound at the MCL1 and BCL2A1 promoters, but not at BCL2. Further, ChIP with an antibody directed against each of the members of the expression-suppressing complex identifies each at the same promoters, but released from the promoter 72 hours following siRNA directed against ATF2, the DNA-binding member of the complex. Each experiment was performed with a sample of at least 3 and independently repeated. (D) By 48 hours following application of siRNA directed against scrambled control, BCL2, MCL1, or both BCL2 and MCL1, expression levels of targets were confirmed to be knocked down in both SYO1 cells and the DLD.1 human colon cancer cell line. No off target effects were noted in DLD.1 cells, but BCL2 was noted to be downregulated upon application of siRNA against MCL1 in SYO1 cells. (All results are presented as mean +/− standard deviation. All comparisons with p-values < 0.05 by Student’s t-test are indicated with an asterisk.)
Given the consistent downreglation of Mcl1/MCL1 in synovial sarcoma expression profiles (Fig. 1A, Supplemental Figure 1B), we investigated the effects of SS18-SSX2 on this and other members of the mitochondrial apoptosis pathway. Specifically, levels of Bcl2a1a decreased in MEFs following induction of the expression of SS18-SSX2 (Fig. 1B). Mcl1 levels also trended toward downregulation in that setting, but not significantly (Fig. 1B and Supplemental Figure 2). In mouse synovial sarcoma cells, both Mcl1 and Bcl2a1a levels increased markedly upon knock-down of SS18-SSX2 using siRNA (Fig. 1C and D). Similarly, knockdown of SS18-SSX2 in the human SYO1 cell line produced increased levels of both MCL1 and BCL2A1 (Fig. 2A). These data suggest that the expression of both MCL1 and BCL2A1 may be suppressed by the expression of SS18-SSX2 in the tumors. However, the fact that the impact of SS18-SSX2 on the expression of Mcl1 in the MEFs was not similarly significant suggests that the transcriptional regulation by SS18-SSX2 of Mcl1 must be context-dependent. In similar fashion, a change in SS18-SSX2 expression demonstrated the opposite effect on Bcl-xL in the tumor and MEF settings, also displaying context-dependence. Context-dependent transcriptional regulation fits well into the paradigm of the known epigenetic mechanism of recruiting polycomb group proteins and HDACs to the cyclic AMP response elements (CREs) of target genes.
Both MCL1 and BCL2A1 have conserved CREs with putative ATF binding sites (Fig. 2C). In order to ascertain if these genes are directly suppressed by SS18-SSX2, we performed ChIP in the SYO1 cells. Indeed, each known member of the suppressive complex was identified as bound to the CRE in the promoters of both genes (Fig. 2C). Further, siRNA directed against ATF2, the DNA-binding member of the complex, released the complex from the CRE in each promoter (Fig. 2C). These data confirm MCL1 and BCL2A1 as novel direct targets of SS18-SSX-driven repression in the synovial sarcoma context using ATF2 promoter binding.
Observing that BCL2 and MCL1 specifically have opposite responses to a change in the expression level of SS18-SSX2 in synovial sarcoma cells, we asked whether either MCL1 or BCL2 might play a role in indirectly regulating the expression of the other, as a negative feedback loop for the mitochondrial anti-apoptotic pathway. In knock-down of either gene, response of the other was modest at best in SYO1 cells (Fig. 2D). The observed modest decrease in BCL2 expression following siRNA directed against MCL1 may have derived from inadvertent suppression of SS18-SSX2 itself. No change in the non-targeted gene expression was noted in another human cancer cell line, suggesting that these anti-apoptotic genes may have some subtle relationship in the synovial sarcoma cells specifically. We also observed that knock-down of either gene, or the combination did not discernibly change the growth and proliferation of the SYO1 cells. This fits with prior observations that Genasense, an antisense oligonucleotide pharmaceutical directed against BCL2, enhanced sensitivity to doxorubicin, but was not cytotoxic or growth suppressive as a single agent [9]. There are likely other anti-apoptotic genes that fill-in for BCL2’s downregulation, but MCL1 itself is an unlikely candidate in these tumors.
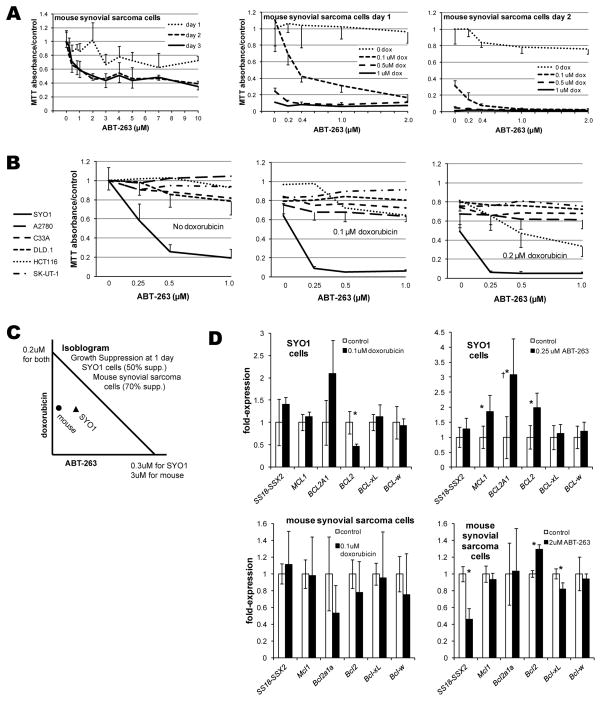
Cancers that have evaded BH3-domain peptidomimetic pharmacotherapy have primarily utilized either MCL1 or BCL2A1 as means of avoiding apoptosis in the setting of drug-antagonized BCL2, BCL-xL, and BCL-w [13, 14]. New appreciation that both MCL1 and BCL2A1 are direct targets of SS18-SSX-driven suppression suggests that synovial sarcomas might be susceptible to BH3-domain peptidomimetic pharmacotherapy. In order to test this hypothesis, cells derived from our mouse model of synovial sarcoma were plated in 96-well plates. One, two, or three days following the addition of varied concentrations of ABT-263 or DMSO vehicle, cells were washed and incubated with 5mg/mL Thiazolyl Blue Tetrazolium Bromide (Sigma). Mouse synovial sarcoma cells were modestly sensitive to the BH3-domain peptidomimetic, but only after 2 days (Fig. 3A). Because the most clinically desirable effect of pharmacologic blockade of the anti-apoptotic members of the mitochondrial pathway would be to enhance sensitivity to traditional cytotoxic chemotherapy, the concentrations of ABT-263 and doxorubicin were varied independently in a similar proliferation assay. ABT-263 enhanced the growth-suppression of doxorubicin at nanomolar concentrations (Fig. 3A).
Figure 3. ABT-263 inhibits synovial sarcoma cell lines in vitro.
(A) Increasing concentrations of ABT-263 as a single agent modestly decrease proliferation/survival of mouse synovial sarcoma cells in vitro as determined by a proliferation assay, but only meaningfully by 2 days following application. Even nanomolar concentrations of ABT-263 enhanced the cytotoxicity of doxorubicin in these mouse synovial sarcoma cells. (B) The SYO1 human synovial sarcoma cell line displayed marked sensitivity to nanomolar concentrations of ABT-263 as single agent or in combination with doxorubicin in proliferations assays performed 1 day after drug application. Side-by-side comparison to 5 other human cancer cell lines demonstrated their poor sensitivity to ABT-263 as a single agent or in combination with doxorubicin, except for HCT116, a colon cancer cell line. (C) Isobologram for 50% suppression of growth and proliferation of SYO1 cells after one day of ABT-263 and/or doxorubicin and 70% suppression of mouse synovial sarcoma cells demonstrate synergism between these drugs for both cell lines. (D) Most changes in gene expression following application of doxorubicin or ABT-263 to SYO1 cells or mouse synovial sarcoma cells are not significant. †While the expression of BCL2A1 increases significantly following application of ABT-263 to SYO1 cells, it remains at a barely detectable level. Each proliferation assay had a sample size of 8 in each drug concentration at each time point following application, expression following drug applications had 6 replicates. All results are presented as means with standard deviation error bars. Asterisks denote p-values < 0.05 by Student’s t-test.
To confirm that these quantitative assays were highlighting true changes in cytotoxicity, rather than metabolic rate alone, cell counts of apparently adherent, viable cells were performed one day following treatments, immediately prior to application of the MTT assay reagents. Indeed, cell counts corroborated the suspicion that the quantitative proliferation assay changes derived from cell growth and survival, not simply changes in metabolism (Supplemental Fig. 3).
To rule out the possibility that the observed sensitivity to ABT-263 derived from non-specific toxicity alone, MTT assays were performed simultaneously comparing the human SYO1 synovial sarcoma cell line to five other human cancer cell lines, all treated with identical dosing combinations (Fig. 3B). Response of any kind was discernible in only one other cell line, and that only a partial response and only in combination with doxorubicin. From this we concluded that the synovial sarcoma responsiveness to ABT-263 cannot be attributed to nonspecific toxicity alone, but rather some targeted effect. With regard to the interaction between ABT-263 and doxorubicin, it fell on the synergistic side of the isobologram generated for each (Fig. 3C).
We next evaluated the expression of mitochondrial anti-apoptotic genes following administration of ABT-263 or doxorubicin. The greater sensitivity of SYO1 cells to ABT-263 required that a very low dose of ABT-263 be applied, in order to have cells remaining after treatment from which RNA might be isolated. In cells that evaded the cytotoxicity for a full day, expression of BCL2, MCL1 and BCL2A1 was elevated (Fig. 3D). True to the epigenetic mechanism of SS18-SSX2-mediated gene suppression, neither MCL1 nor BCL2A1 completely loses the capacity to respond to other factors that control expression. However, each is suppressed sufficiently to render the response in most cells insufficient to support full evasion of apoptosis. The levels of BCL2A1 are so low in SYO1 cells, that even after tripling in expression, it remains 3 orders of magnitude lower in its expression than BCL2 itself.
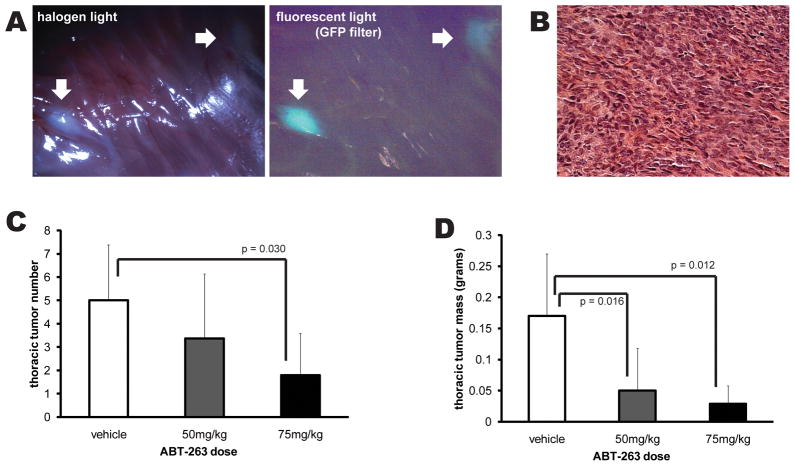
The natural history of mice expressing the human cDNA SS18-SSX2 in cells that express Myf5-Cre includes consistent tumor formation by the age of 12 weeks. The most frequent site of disease is the intercostals muscles of the thoracic cage, where 3 to 8 tumors are typically detectable by that age. These then grow over the ensuing weeks. With approval of the university institutional animal care and use committee, mice were treated beginning in the thirteenth week with three-times weekly doses of either 2.5 μL/gram body mass DMSO vehicle or the same with ABT-263 dissolved at the concentration of 20 μg/μL (for 50 mg/kg dosing regimen) or 30 μg/μL (for 75 mg/kg dosing). Mice were euthanized at 15 weeks of age. The thoracic cage of each mouse was dissected of all muscles but the intercostals. Tumors identified grossly and confirmed by GFP fluorescence were counted, excised, and weighed (Fig. 4A). Histology then confirmed that each mass measured matched the typical appearance of a synovial sarcoma in this model (Fig. 4B). Experimental and control mice from the same litters were used for the studies. As a single agent in this setting, ABT-263 reduced the number of tumors at the higher dose, and the total mass of tumors per mouse thoracic cage at both doses compared to control vehicle (Fig. 4). ABT-263 is often dosed orally in other mouse models, but could not be in these mice due to typically severe kyphosis (a non-tumor phenotype of their genetic manipulation) affecting esophageal access. Even accounting for enhanced bioavailability via the intraperitoneal administration, the doses we used are much lower than the typical 75mg/kg body mass daily for three weeks. Further, we did not observe any toxicity in other tissues from the doses used. One mouse in the higher dose group became moribund before the last treatment, but this cannot strictly be attributed to any drug toxicity, as this naturally occurs at a relatively high background rate in these mice. Given the modest sensitivity of the cells derived from similar mouse synovial sarcomas to ABT-263 as a single agent, this marked in vivo responsiveness suggests that the drug may inhibit evasion of cell-to-cell or immune-mediated induction of apoptosis, rather than cell intrinsic alone.
Figure 4. ABT-263 inhibits synovial sarcoma in vivo in a genetic mouse model.
Mice bearing conditional expression of the SS18-SSX2 human cDNA from the Rosa26 locus, activated by Myf5-Cre, develop synovial sarcomas most consistently in the intercostal muscles of the thoracic cage, detectable by 12 weeks of age. (A) Mice treated with intraperitoneal injection of either DMSO or DMSO with ABT-263 for three doses weekly during weeks 13, 14, and 15 of life were euthanized at 15 weeks for visual and GFP-fluorescence assessment of the thoracic cage (white arrows), followed by measurement of the mass of the tumors themselves. (B) Histology confirmed the characteristic synovial sarcoma appearance of each mass counted and weighed. (C) Treatment of mice with 75mg/kg/dose (n = 5) of ABT-263 decreased the total number of thoracic cage masses identified (by Student’s t-test). (D) Treatment with either 50mg/kg/dose (n=8) or the higher dose resulted in statistically significant reduction of total tumor mass compared to vehicle control (n = 7). All experimental mice derived from the same two parents, with controls and treatment mice in each of the three litters used. All results are presented as means with standard deviation error bars.
These data provide a molecular rationale for treatment of synovial sarcomas with this new class of BH3-domain peptidomimetics. Further, they illustrate the capacity of a genetic mouse model of a human cancer, not only as a platform for preclinical evaluation of treatment strategies, but also as tool for deciphering pathways important in mediating their molecular effects. Given the relatively small numbers of human synovial sarcomas treated at any single referral sarcoma unit, there is an urgent need to develop molecularly targeted therapies that have a high chance of efficacy in the clinic prior to initiating clinical trials. If drugs or regimens and their biology can be credentialed in an in vivo synovial sarcomagenesis model, therapeutic strategies may be prioritized; more effective clinical trials may be performed.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the support of the Paul Nabil Bustany Fund for Synovial Sarcoma Research, the Huntsman Cancer Foundation, and career development support from National Cancer Institute (NIH) K08CA138764. This work was also supported by grants from the Canadian Cancer Society Research Institute (Grant #018355) and the Terry Fox Foundation and CIHR Institute of Cancer (TFF 105265). We thank Matt Hockin, PhD, at the University of Utah for producing the TAT-Cre protein.
Footnotes
CONFLICTS OF INTEREST
The authors declare no competing interests financial or otherwise related to the work reported above. Specifically, none of the authors has any relationship whatsoever with the pharmaceutical company preparing ABT-263 for market. We purchased the drug from another source.
References
- 1.Ladanyi M, Antonescu CR, Leung DH, Woodruff JM, Kawai A, Healey JH, et al. Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: a multi- institutional retrospective study of 243 patients. Cancer Res. 2002;62(1):135–40. [PubMed] [Google Scholar]
- 2.Haldar M, Hancock JD, Coffin CM, Lessnick SL, Capecchi MR. A conditional mouse model of synovial sarcoma: insights into a myogenic origin. Cancer Cell. 2007;11(4):375–88. doi: 10.1016/j.ccr.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Haldar M, Hedberg ML, Hockin MF, Capecchi MR. A CreER-based random induction strategy for modeling translocation-associated sarcomas in mice. Cancer Res. 2009;69(8):3657–64. doi: 10.1158/0008-5472.CAN-08-4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su L, Sampaio AV, Jones KB, Pacheco M, Goytain A, Lin SC, et al. Deconstruction of the SS18-SSX Fusion Oncoprotein Complex: Insights into Disease Etiology and Therapeutics. Cancer Cell. 2012 doi: 10.1016/j.ccr.2012.01.010. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hirakawa N, Naka T, Yamamoto I, Fukuda T, Tsuneyoshi M. Overexpression of bcl-2 protein in synovial sarcoma: a comparative study of other soft tissue spindle cell sarcomas and an additional analysis by fluorescence in situ hybridization. Hum Pathol. 1996;27(10):1060–5. doi: 10.1016/s0046-8177(96)90284-1. [DOI] [PubMed] [Google Scholar]
- 6.Sun B, Sun Y, Wang J, Zhao X, Wang X, Hao X. Extent, relationship and prognostic significance of apoptosis and cell proliferation in synovial sarcoma. Eur J Cancer Prev. 2006;15(3):258–65. doi: 10.1097/01.cej.0000198896.02185.68. [DOI] [PubMed] [Google Scholar]
- 7.Knosel T, Heretsch S, Altendorf-Hofmann A, Richter P, Katenkamp K, Katenkamp D, et al. TLE1 is a robust diagnostic biomarker for synovial sarcomas and correlates with t(X;18): analysis of 319 cases. Eur J Cancer. 2010;46(6):1170–6. doi: 10.1016/j.ejca.2010.01.032. [DOI] [PubMed] [Google Scholar]
- 8.Mancuso T, Mezzelani A, Riva C, Fabbri A, Dal Bo L, Sampietro G, et al. Analysis of SYT-SSX fusion transcripts and bcl-2 expression and phosphorylation status in synovial sarcoma. Lab Invest. 2000;80(6):805–13. doi: 10.1038/labinvest.3780085. [DOI] [PubMed] [Google Scholar]
- 9.Joyner DE, Albritton KH, Bastar JD, Randall RL. G3139 antisense oligonucleotide directed against antiapoptotic Bcl-2 enhances doxorubicin cytotoxicity in the FU-SY-1 synovial sarcoma cell line. J Orthop Res. 2006;24(3):474–80. doi: 10.1002/jor.20087. [DOI] [PubMed] [Google Scholar]
- 10.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68(9):3421–8. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 11.Wilson WH, O’Connor OA, Czuczman MS, LaCasce AS, Gerecitano JF, Leonard JP, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11(12):1149–59. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi L, Camidge DR, Ribeiro de Oliveira M, Bonomi P, Gandara D, Khaira D, et al. Phase I study of Navitoclax (ABT-263), a novel Bcl-2 family inhibitor, in patients with small-cell lung cancer and other solid tumors. J Clin Oncol. 2011;29(7):909–16. doi: 10.1200/JCO.2010.31.6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yecies D, Carlson NE, Deng J, Letai A. Acquired resistance to ABT-737 in lymphoma cells that up-regulate MCL-1 and BFL-1. Blood. 2010;115(16):3304–13. doi: 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tahir SK, Wass J, Joseph MK, Devanarayan V, Hessler P, Zhang H, et al. Identification of expression signatures predictive of sensitivity to the Bcl-2 family member inhibitor ABT-263 in small cell lung carcinoma and leukemia/lymphoma cell lines. Mol Cancer Ther. 2010;9(3):545–57. doi: 10.1158/1535-7163.MCT-09-0651. [DOI] [PubMed] [Google Scholar]
- 15.Li C, Wong WH. Model-based analysis of oligonucleotide arrays: expression index computation and outlier detection. Proc Natl Acad Sci U S A. 2001;98(1):31–6. doi: 10.1073/pnas.011404098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakayama R, Mitani S, Nakagawa T, Hasegawa T, Kawai A, Morioka H, et al. Gene expression profiling of synovial sarcoma: distinct signature of poorly differentiated type. Am J Surg Pathol. 2010;34(11):1599–607. doi: 10.1097/PAS.0b013e3181f7ce2c. [DOI] [PubMed] [Google Scholar]
- 17.Liu TM, Guo XM, Tan HS, Hui JH, Lim B, Lee EH. Zinc-finger protein 145, acting as an upstream regulator of SOX9, improves the differentiation potential of human mesenchymal stem cells for cartilage regeneration and repair. Arthritis Rheum. 2011;63(9):2711–20. doi: 10.1002/art.30430. [DOI] [PubMed] [Google Scholar]
- 18.Nakayama R, Nemoto T, Takahashi H, Ohta T, Kawai A, Seki K, et al. Gene expression analysis of soft tissue sarcomas: characterization and reclassification of malignant fibrous histiocytoma. Mod Pathol. 2007;20(7):749–59. doi: 10.1038/modpathol.3800794. [DOI] [PubMed] [Google Scholar]
- 19.Joshi SK, Hashimoto K, Koni PA. Induced DNA recombination by Cre recombinase protein transduction. Genesis. 2002;33(1):48–54. doi: 10.1002/gene.10089. [DOI] [PubMed] [Google Scholar]
- 20.Su L, Cheng H, Sampaio AV, Nielsen TO, Underhill TM. EGR1 reactivation by histone deacetylase inhibitors promotes synovial sarcoma cell death through the PTEN tumor suppressor. Oncogene. 2010;29(30):4352–61. doi: 10.1038/onc.2010.204. [DOI] [PubMed] [Google Scholar]
- 21.Kawai A, Naito N, Yoshida A, Morimoto Y, Ouchida M, Shimizu K, et al. Establishment and characterization of a biphasic synovial sarcoma cell line, SYO-1. Cancer Lett. 2004;204(1):105–13. doi: 10.1016/j.canlet.2003.09.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.