Abstract
Objective
Bidis are hand-rolled cigarettes commonly smoked in South Asia and are marketed to Western populations as a safer alternative to conventional cigarettes. This study examined the association between bidis and other forms of tobacco use and cancer incidence in an urban developing country population.
Methods
Using data from the large, well-characterized Mumbai cohort study, adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were computed from Cox proportional hazards regression models in order to compare the relative effect of various forms of tobacco use on cancer incidence.
Results
During 649,228 person-years of follow-up 1,267 incident cancers occurred in 87,222 male cohort members. Incident oral cancer in bidi smokers (HR = 3.55; 95% CI = 2.40,5.24) was 42% higher than in cigarette smokers (HR = 2.50;95% CI = 1.65,3.78). For all respiratory and intrathoracic organs combined, the increase was 69% (HR = 5.54; 95% CI = 3.46,8.87 vs. HR = 3.28; 95% CI = 1.99,5.39); for lung and larynx, the increases were 35 and 112%, respectively. Smokeless tobacco use was associated with cancers of the lip, oral cavity, pharynx, digestive, respiratory, and intrathoracic organs.
Conclusions
Despite marketing claims to the contrary, we found that smokeless tobacco use and bidi smoking are at least as harmful as cigarette smoking for all incident cancers and are associated with increased risk of oral and respiratory/intrathoracic cancers.
Keywords: Public health, Disease incidence, Cancer, Tobacco, Cohort study
Introduction
Cancer causes more deaths than AIDS, tuberculosis, and malaria combined. One in eight deaths worldwide is due to cancer. It is the second leading cause of death in economically developed countries (following cardiovascular diseases) and the third leading cause of death in developing countries (following cardiovascular and diarrheal diseases) [1-3]. The burden of cancer is increasing in developing countries as childhood mortality and deaths from infectious diseases decline and more people live to older ages. Other, as yet undetermined, factors also may be contributing to apparent increases in incidence [4-7].
Despite their potential for being among the most preventable of chronic diseases, more than 12 million new cancer cases were diagnosed in 2008, of which many more than half occurred in economically developing countries [8]. The corresponding estimate for total cancer deaths was 7.6 million (about 20,000 cancer deaths a day), of which 62% occur in economically developing countries. The global case fatality rate in economically developing countries (70%) is much higher than rates in developed countries (54%). Largely due to the emergence of an epidemic of tobacco-related cancers, by 2,050, the global burden is expected to grow to 27 million new cancer cases, most of which will occur in developing countries [8].
As a cause of human cancer, tobacco use is uniquely important. In some populations, in recent decades tobacco has caused about half of all cancer deaths in men, and a smaller, but increasing, proportion of cancer deaths in women [9]. We have estimated that in this Mumbai population 41.6% of male and 20.7% of female cancer deaths could be attributed to their tobacco usage [10].
There is no entirely safe way to use tobacco, regardless of whether it is smoked, chewed, applied on gums and teeth, or mixed with other ingredients [8, 10]. Among all types of tobacco use, cigarettes account for the largest share of manufactured tobacco products in the world (96% of total value of sales). However, other methods of smoking and chewing tobacco are practiced widely throughout the world, especially in South and Southeast Asia [11, 12]. Some of these are being exported to the United States and other Western countries [13, 14]. Unlike most other countries, in India the most common form of smoking tobacco is in the form of bidis—conical, “home-made cigarettes” consisting of a small amount of (~0.2 g) flaked tobacco hand-wrapped in a dried temburni (Dio-spyros melonoxylon) leaf and tied with string. Every year, around 750 billion to 1.2 trillion bidis are produced and consumed in India, accounting for nearly half (~48%) of tobacco consumption and making them, much more popular than conventional cigarettes (which account for ~14% of tobacco consumption) [15]. Despite the small amount of tobacco they contain, bidis can deliver more tar and carbon monoxide than manufactured cigarettes [16, 17] and therefore carry a greater risk of cancer, cardiovascular, and lung disease [10, 18, 19]. Tobacco cultivation in India began in the late seventeenth century, [18, 20] and bidis were first created when tobacco workers took left over tobacco and rolled it in leaves for smoking [18]. Currently, most bidis are made by women in their homes. India’s Ministry of Labor estimates that over 4 million people (of which ~10% were children) in India are employed producing bidis [18]. The bidi industry is loosely regulated, and workers are constantly exposed to tobacco dust and other harmful particles [20, 21]. A roller can make about 1,000 bidis a day (handle 225–450 g of tobacco flake per day) and is paid a government-set minimum wage of 40 rupees (~USD $ 1) per 1,000 bidis rolled [15, 20]. Studies have shown that cotinine levels in the bodily fluids of bidi workers are elevated, even among those who do not use tobacco [21]. Bidis are very inexpensive in India, costing as little as 4 rupees (~USD $ 0.10) on average for a pack of 25 bidis. Smoking of bidis in India tends to be associated with lower social standing [15]. Without acknowledgment of the potential risk, they are now being marketed in the West with claims of being a “safer” and “more natural” alternative to cigarettes [22]. Concomitantly, an alarming increase in bidi smoking among youth in the USA has been reported in several surveys [18, 23-28].
Because of their potential carcinogenic effect and widespread use in South Asia and increase in marketing in the West, this study was designed to compare and contrast the effect of bidi smoking and use of smokeless tobacco products (also common in India) on incidence of various types of cancers through a large, carefully designed and conducted cohort study in Mumbai, Maharashtra, India.
Materials and methods
Recruitment
Mumbai (formerly Bombay) is a large, densely populated cosmopolitan city. It is divided into three parts: the main city, suburbs, and extended suburbs. This cohort study was conducted in the main city of Mumbai. Smoking is extraordinarily rare among Indian women, [18] including those in the Mumbai cohort study (MCS) [10]. Therefore, this analysis was restricted only to men, a total of 88,658 of whom aged C35 years were recruited into the cohort during 1991–1997. The voters’ lists were used as the sampling frame. These lists provided name, age, sex, and address of all individuals aged ≥18 years and were grouped in polling stations comprising 1,000–1,500 voters. We excluded polling stations that served the upper-middle class and upper-class housing complexes (based on pilot study experiences); as such, complexes were not accessible to us because of security issues (i.e., they were essentially “gated communities”). To be eligible, subjects had to be resident of the study area and have no evident cancer at the time of recruitment. Data were collected using a structured questionnaire administered in face-to-face interviews in the subject’s home. The study satisfied all the criteria with regard to the ethical treatment of human subjects, especially those formulated by the Indian Council of Medical Research. Details regarding the recruitment procedures and survey instrument have been described earlier [10, 29].
Follow-up
An active house-to-house follow-up was conducted (during 1997–2003) after an average of 5.5 years from administration of the initial survey at the time of recruitment. The field investigators were instructed to revisit each person. If the person was alive and available, a face-to-face re-interview was conducted. If the person was reported to have died, the date and place of death were recorded as accurately as feasible. Permanent migration from the study area was considered as withdrawal from the study, and the date of migration was noted. Follow-up details have been described earlier [10, 29-31].
Cancer incidence in the population-based cancer registry Mumbai
Cancer incidence is defined as the occurrence of new cancer cases in a defined population during a specified time period. For any year, incidence is based on those cancers registered and first diagnosed between 1 January and 31 December of that year.
The population-based cancer registry (PBCR) of Mumbai was established in June 1963, as a unit of the Indian Cancer Society situated at Mumbai. It was the first such registry in India. Information is obtained on all cancer patients registered in 150 government hospitals/institutions and private hospitals or nursing homes in Mumbai who are under the care of specialists (surgeons, physicians, pathologists, radiologists, and gynecologists). Cases under code ‘0 =’ (benign) or ‘1’ (uncertain whether benign or malignant borderline malignancy) or ‘2’ (carcinoma in situ) are not included. Patients, in whom cancer has been ruled out or has not been diagnosed, are also omitted. Cancer cases for which the death certificate is the only source of information, however, are included. The coding system devised by the World Health Organization using code number C00-97 as published in the manual of the International Classification of Diseases, Injuries, and Cause of Death (10th revision) is used.
A study published by Yeole [32], using data for years 1964–1997 from seven consecutive volumes (II to VIII) of Cancer Incidence of Five Continents published by IARC, concluded that the data collected by PBCR Mumbai meets international standards for completeness and reliability.
Linking the MCS database with PBCR incidence database
Information on all incident cases aged ≥35 years reported during years 1991–2003 were abstracted from PBCR database. The most common variables available between the two databases, MCS and PBCR, that were used to establish the link between two databases, were Name, Gender, Age, Postal Pincode, Religion, and Mother tongue. During the matching process, we found that the address of the individual is the key field for identifying and confirming a match. As the PBCR database did not have an electronically inputted address field, the project staff of MCS computerized the address field for all of the abstracted records for years 1991–2003 in PBCR cancer incidence database. Most of the matching was done manually, as the special record linkage program (Rec-Link) developed by IARC does not support long text variables such as address that were required to provide reliable and accurate match. For obtaining reliable matches through the Rec-Link program, the name field in both databases was split into three fields; first name (individual’s name), middle name (generally father’s or husband’s name), and last name (surname). Because no specific order was followed to record the name of an individual in MCS, various combinations of name fields (3 by 3) between two datasets were used along with a few other common variables such as age, gender, and Pincode to obtain probable matches. Then, all the probable matches were manually checked and compared for address fields between two datasets. Based on the address field, the matches were finally confirmed. We also found a few exact matches (around 80) outside the MCS area, but within Mumbai, and for those a 10% random check was performed. Their status, i.e., having emigrated outside the study area but still residing within Mumbai (mostly they were older people staying with their children or other relatives at different locations in Mumbai), was found to be consistent during random check. Therefore, they were included in the final analytic dataset.
Thus, all the common matched cases found in either or both of these two databases constitute the numerator. The denominator was calculated as the number of person-years of observation up to 31 December 2003, the cutoff date for calculation of person-time of observation or the date of endpoint ascertainment (defined as the date of expiry, date of cancer incidence, re-interview, or migration), whichever occurred earlier.
Tobacco use
Respondents were broadly classified as having never used tobacco, or being a current or former user of smokeless tobacco only, or being a current or former smoker (which also may include the use of smokeless tobacco products in addition to smoking). Thus, different categories of tobacco use were mutually exclusive. The proportion of past users was small, 4.5% (2.8% of smokers and 1.7% of smokeless tobacco users) compared to 69.3% current users;[29] thus, they were combined with current users.
The most common form of tobacco smoking in India is bidi smoking. In this cohort, bidi smoking was reported by 11.9% (compared to 9.9% who reported cigarette smoking). Some men reported bidi as well as cigarette smoking (1.4%); as most were predominantly bidi smokers, they were combined with bidi smoking. Details of smokeless tobacco use in this cohort and various tobacco habits prevalent in India are available elsewhere [10, 29-31, 33].
Other covariates
As described in previous work [10, 29-31, 34], additional covariates include body mass index [BMI = weight(kg)/height(m)2], classified into six categories from extremely thin (BMI < 16.0 kg/m2) to obese (BMI ≥ 30.0 kg/m2); education (as a proxy for socioeconomic status), an important predictor of health and tobacco use, was classified as illiterate (no formal schooling), primary school (up to 5 years of education), middle school (6–8 years), secondary school (9–12 years), and college (any); age, in 10-year intervals beginning at 35 years (the lowest age of recruitment into the cohort); mother tongue, an indicator of state of origin because states in India are delineated according to language (and language is associated with other health-related behaviors, such as diet); and religion (because of its association with health-related behaviors).
Statistical analysis
Of the total 88,658 recruited men during 1991–1997, 87,222 were available for this analysis. Person-years of follow-up were calculated by using the date of recruitment through 31 December 2003 or the date of endpoint ascertainment (defined as the date of expiry, date of cancer incidence, re-interview, or migration), whichever occurred earlier. In instances where the exact date of death or migration was not available, the midpoint between date of recruitment and the date of ascertainment was used. For cancer cases obtained from linking two datasets, the midpoint (30 June) of the year in which a cancer was first diagnosed was used as the incidence date. Age-adjusted incidence rates were calculated by using the overall 5-year age-specific person-years as weights (i.e., the direct method). Multivariate analysis was performed by using Cox proportional hazards regression modeling [35]. The response variable, cancer incidence (case), was a dichotomous variable (“yes” or “no”), and the time to event (or censoring) was regarded as a continuous variable. Age, education, religion, mother tongue, tobacco use, and BMI were fitted as independent variables in the final Cox proportional hazards model [35].
Results
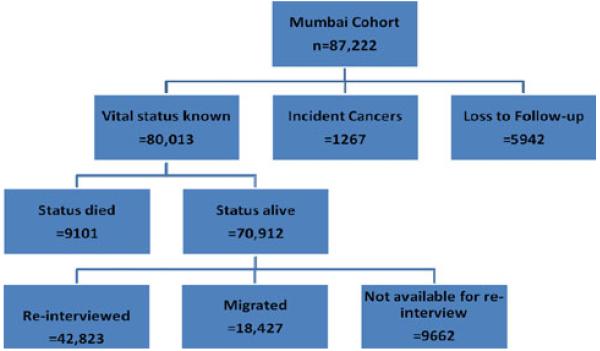
As documented in our previous work, this cohort consists predominantly of individuals of middle and lower socioeconomic status (SES; Table 1) [10, 29, 30, 34]. There was a high rate of tobacco use, both in smoking (mainly bidi smoking) as well as in smokeless form. Total person-years of observations were 649,228. The average period of follow-up to either event (cancer) or censoring (confirmation of no cancer) was 7.4 years (Tables 1 and 2, Fig. 1). Overall cancer incidence was relatively low compared to European and American populations, about 195 cases/100,000/year and about 141 cases/100,000/year in never-tobacco users (Table 2). As would be expected, compared to never-users the rates were somewhat higher in smokeless tobacco users (180 cases/100,000/year) and much higher in smokers (270 cases/100,000/year).
Table 1.
Summary of descriptive data, Mumbai cohort study, Mumbai, Maharashtra, India, 1991–2003
| Persons (n = 87,222) | % (total = 100) | Cancer cases (n = 1,267) | % (total = 100) | |
|---|---|---|---|---|
| Age (years) | ||||
| 35–39 | 8,345 | 9.6 | 58 | 4.6 |
| 40–44 | 7,010 | 8.0 | 89 | 7.0 |
| 45–49 | 23,263 | 26.7 | 179 | 14.1 |
| 50–54 | 14,074 | 16.1 | 171 | 13.5 |
| 55–59 | 10,608 | 12.2 | 189 | 14.9 |
| 60–64 | 9,574 | 11.0 | 204 | 16.1 |
| 65–69 | 6,583 | 7.5 | 173 | 13.7 |
| 70 and Up | 7,765 | 8.9 | 204 | 16.1 |
| Education | ||||
| Illiterate | 14,770 | 16.9 | 170 | 13.4 |
| Primary | 32,983 | 37.8 | 573 | 45.2 |
| Middle | 25,752 | 29.5 | 354 | 27.9 |
| Secondary | 8,162 | 9.4 | 98 | 7.7 |
| College | 5,555 | 6.4 | 72 | 5.7 |
| Religion | ||||
| Hindu | 67,023 | 76.8 | 1,030 | 81.3 |
| Muslim | 13,943 | 16.0 | 82 | 6.5 |
| Buddhist | 3,705 | 4.2 | 87 | 6.9 |
| Christian | 2,182 | 2.5 | 55 | 4.3 |
| Others | 369 | 0.4 | 13 | 1.0 |
| Mother tongue | ||||
| Marathi | 48,596 | 55.7 | 901 | 71.1 |
| Hindi | 14,627 | 16.8 | 85 | 6.7 |
| Gujarati | 8,400 | 9.6 | 148 | 11.7 |
| Urdu | 7,648 | 8.8 | 37 | 2.9 |
| South Indian | 7,774 | 8.9 | 94 | 7.4 |
| Others | 177 | 0.2 | 2 | 0.2 |
| BMI (kg/m2)a | ||||
| Normal | 54,385 | 62.4 | 749 | 59.1 |
| Thin | 8,004 | 9.2 | 141 | 11.1 |
| Very thin | 3,473 | 4.0 | 87 | 6.9 |
| Extremely thin | 3,760 | 4.3 | 75 | 5.9 |
| Overweight | 15,285 | 17.5 | 185 | 14.6 |
| Obese | 2,315 | 2.7 | 30 | 2.4 |
| Tobacco users | ||||
| Never | 26,179 | 30.0 | 273 | 21.5 |
| Smokeless | 33,682 | 38.6 | 476 | 37.6 |
| Smoker | 27,361 | 31.4 | 518 | 40.9 |
BMI body mass index = weight(kg)/height(m)2; BMI categories were defined by as follows (all units kg/m2): extremely thin (BMI < 16.0); very thin (BMI 16.0–<17.0); thin (BMI 17.0–<18.5); normal (BMI 18.5–<25.0); overweight (BMI 25.0–<30.0); and obese (BMI ≥ 30.0)
Table 2.
Overall cancer incidence, adjusted HRs, and 95% CIs, by tobacco use: Mumbai cohort study, Mumbai, Maharashtra, India, 1991–2003
| Men | Person-years | Incident cases | HRsa (95% CIs) |
|---|---|---|---|
| Never-users | 193,661 | 273 | 1.0 |
| Smokeless (all) | 263,621 | 476 | 1.20 (1.03–1.41) |
| Frequency (per day)d | |||
| 1–5 | 171,160 | 318 | 1.17 (0.99–1.40) |
| 6–10 | 70,833 | 123 | 1.12 (0.90–1.40) |
| 11–15 | 14,607 | 27 | 1.34 (0.90–2.01) |
| >=16 | 6,841 | 8 | 1.09 (0.77–1.54) |
| Duration (years)d | |||
| 1–10 | 32,553 | 38 | 0.85 (0.42–1.73) |
| 11–20 | 42,721 | 49 | 1.08 (0.79–1.48) |
| >=21 | 187,637 | 387 | 1.18 (1.00–1.40) |
| Types of smokeless | |||
| Mishri | 32,588 | 59 | 1.21(0.91–1.62) |
| Mishri plusa | 91,913 | 186 | 1.18 (0.96–1.46) |
| Betelquid | 82,297 | 127 | 1.10 (0.89–1.36) |
| Other tobacco | 49,416 | 89 | 1.31 (1.02–1.68) |
| Areca nut | 7,407 | 15 | 1.25 (0.74–2.10) |
| Smoker (all) | 191,946 | 518 | 2.00 (1.72–2.32) |
| Frequency (per day)d | |||
| 1–5 | 49,592 | 109 | 1.61 (1.29–2.02) |
| 6–10 | 52,645 | 159 | 2.19 (1.80–2.68) |
| 11–15 | 29,355 | 79 | 1.97 (1.53–2.55) |
| >=16 | 60,322 | 171 | 2.11 (1.73–2.57) |
| Duration (years)d | |||
| 1–10 | 19,300 | 27 | 1.35 (0.91–2.02) |
| 11–20 | 39,642 | 69 | 1.71 (1.30–2.24) |
| >=21 | 132,930 | 422 | 2.11 (1.80–2.47) |
| Types of smoking | |||
| Bidic | 106,796 | 295 | 2.11 (1.77–2.53) |
| Frequency (per day)d | |||
| 1–5 | 17,756 | 45 | 1.85 (1.34–2.57) |
| 6–10 | 22,699 | 64 | 2.11 (1.59–2.81) |
| 11–15 | 21,377 | 55 | 1.95 (1.44–2.64) |
| >=16 | 44,933 | 131 | 2.29 (1.83–2.87) |
| Duration (years)d | |||
| 1–10 | 7,758 | 13 | 1.60 (1.07–2.39) |
| 11–20 | 18,135 | 28 | 1.70 (0.97–2.99) |
| >=21 | 80,846 | 254 | 2.23 (1.83–2.70) |
| Cigarette | 85,150 | 223 | 1.92 (1.60–2.29) |
| Frequency (per day)d | |||
| 1–5 | 31,836 | 64 | 1.50 (1.14–1.97) |
| 6–10 | 29,946 | 95 | 2.26 (1.79–2.87) |
| 11–15 | 7,979 | 24 | 2.09 (1.38–3.18) |
| >=16 | 15,389 | 40 | 1.76 (1.26–2.46) |
| Duration (years)d | |||
| 1–10 | 11,542 | 14 | 1.14 (0.66–1.95) |
| 11–20 | 21,507 | 41 | 1.82 (1.30–2.54) |
| >=21 | 52,084 | 168 | 2.00 (1.64–2.43) |
Hazard ratios (RRs) were adjusted for age, education, religion, mother tongue, and BMI
May include mishri plus other smokeless tobacco products
May include smokers of both bidis and cigarettes
The numbers may not add up to the total due to few missing values
Fig. 1.
Flow diagram of incident cancers and house-to-house follow-up of the study subjects
Fully adjusted hazard ratios (HRs) along with their 95 percentile confidence intervals (95% CIs) are presented in Table 2 (for all cancer sites combined) and Table 3 (for various site-specific cancers) using never-users as reference group. For all sites combined (Table 2), the hazard ratios for both bidi and cigarette smokers appear to be elevated and the point estimate for bidi smokers was about 10% higher than that of cigarette smokers. For lip, oral cavity, and pharyngeal cancers (Table 3), bidis were associated with an increase in risk of about a 40% over that of cigarettes. The largest increase in risk among these anatomic sites was observed for cancers of the gum. Although use of tobacco products increased risk over all cancers of the digestive tract, specific types of use were more weakly associated with individual anatomic sites. Smoking of both bidis and cigarettes was associated with increased esophageal and pancreatic cancer incidence, while each type of habit also was associated individually with increased risk of esophageal cancer. In contrast, cancers of the respiratory and intrathoracic organs appeared to be greatly elevated (by about 69%) in bidi smokers relative to cigarette smokers. Laryngeal cancers appeared to be most affected, followed by bronchus and lung. In addition to smoking, smokeless tobacco use, also, was found to be associated with lip, oral cavity, and pharyngeal cancers, cancers of digestive organs (mainly esophagus and liver and intrahepatic bile ducts), and cancers of the respiratory and intrathoracic organs.
Table 3.
Men cancer site-specific incidence, adjusted HRs, and 95% CIs, by tobacco use: Mumbai cohort study, Mumbai, Maharashtra, India, 1991–2003
| Cancer sitea | Never-user | Smokeless | Smoker | Bidib | Cigarette | |
|---|---|---|---|---|---|---|
| All cancer sites | n | 273 | 476 | 518 | 295 | 223 |
| HR (95% CI) | 1.0 | 1.20 (1.03–1.41) | 2.00 (1.72–2.32) | 2.11 (1.77–2.53) | 1.92 (1.60–2.29) | |
| Lip, oral cavity and pharynx (C00–14) |
n | 44 | 107 | 136 | 89 | 47 |
| HR (95% CI) | 1.0 | 1.48 (1.03,2.13) | 3.03 (2.14,4.29) | 3.55 (2.40,5.24) | 2.50 (1.65,3.78) | |
| Tongue (C01, 02) |
n | 14 | 19 | 32 | 18 | 14 |
| HR (95% CI) | 1.0 | 0.85 (0.42,1.74) | 2.34 (1.23,4.45) | 1.92 (0.91,4.05) | 2.41 (1.14,5.08) | |
| Gum (C03) |
n | 3 | 11 | 12 | 10 | 2 |
| HR (95% CI) | 1.0 | 2.05 (0.55,7.64) | 3.94 (1.09,14.26) | 6.46 (1.64,25.40) | 1.52 (0.25,9.18) | |
| Mouth (C04,5,6) |
n | 15 | 22 | 22 | 17 | 5 |
| HR (95% CI) | 1.0 | 0.78 (0.40,1.54) | 1.27 (0.65,2.50) | 1.79 (0.84,3.82) | 0.67 (0.24,1.85) | |
| Pyriform sinus (C12) |
n | 8 | 24 | 25 | 16 | 9 |
| HR (95% CI) | 1.0 | 1.97 (0.85,4.58) | 2.96 (1.31,6.68) | 2.97 (1.20,7.36) | 3.06 (1.16,8.05) | |
| Digestive organs (C15–26) |
n | 76 | 159 | 121 | 61 | 60 |
| HR (95% CI) | 1.0 | 1.43 (1.07,1.90) | 1.70 (1.27,2.28) | 1.55 (1.07,2.23) | 1.83 (1.30,2.58) | |
| Esophagus (C15) |
n | 7 | 36 | 40 | 22 | 18 |
| HR (95% CI) | 1.0 | 3.65 (1.59,8.38) | 5.75 (2.54,12.98) | 5.46 (2.23,13.58) | 5.72 (2.38,13.76) | |
| Stomach (C16) |
n | 16 | 31 | 20 | 9 | 11 |
| HR (95% CI) | 1.0 | 1.28 (0.67,2.43) | 1.21 (0.62,2.37) | 0.98 (0.41,2.33) | 1.51 (0.69,3.29) | |
| Colon (C18) |
n | 12 | 20 | 14 | 8 | 6 |
| HR (95% CI) | 1.0 | 1.22 (0.57,2.61) | 1.33 (0.60,2.94) | 1.24 (0.47,3.30) | 1.14 (0.42,3.07) | |
| Rectum (C20) |
n | 18 | 13 | 6 | 2 | 4 |
| HR (95% CI) | 1.0 | 0.48 (0.22,1.02) | 0.40 (0.15,1.01) | 0.24 (0.05,1.11) | 0.57 (0.19,1.70) | |
| Liver and intrahepatic bile ducts (C22) |
n | 9 | 31 | 16 | 9 | 7 |
| HR (95% CI) | 1.0 | 2.35 (1.08,5.10) | 2.00 (0.87,4.61) | 2.43 (0.88,6.72) | 1.96 (0.72,5.31) | |
| Pancreas (C25) |
n | 5 | 15 | 17 | 7 | 10 |
| HR (95% CI) | 1.0 | 1.95 (0.68,5.54) | 3.86 (1.40,10.66) | 2.92 (0.85,10.02) | 4.66 (1.58,13.71) | |
| Respiratory and intrathoracic organs (C30–39) |
n | 27 | 64 | 114 | 76 | 38 |
| HR (95% CI) | 1.0 | 1.71 (1.08,2.73) | 4.30 (2.80,6.60) | 5.54 (3.46,8.87) | 3.28 (1.99,5.39) | |
| Larynx (C32) |
n | 9 | 26 | 40 | 28 | 12 |
| HR (95% CI) | 1.0 | 1.86 (0.85,4.06) | 4.33 (2.07,9.05) | 6.10 (2.72,13.69) | 2.87 (1.21,6.85) | |
| Bronchus and lung (C34) |
n | 17 | 34 | 65 | 41 | 24 |
| HR (95% CI) | 1.0 | 1.59 (0.87,2.90) | 4.05 (2.35,7.00) | 4.69 (2.56,8.59) | 3.48 (1.86,6.52) | |
| Male genital organ (C60–63) |
n | 33 | 26 | 24 | 11 | 13 |
| HR (95% CI) | 1.0 | 0.71 (0.41,1.23) | 0.96 (0.56,1.65) | 0.77 (0.38,1.59) | 1.16 (0.60,2.23) | |
| Prostate (C61) |
n | 30 | 20 | 20 | 9 | 11 |
| HR (95% CI) | 1.0 | 0.63 (0.35,1.15) | 0.91 (0.51,1.63) | 0.73 (0.33,1.61) | 1.13 (0.56,2.27) | |
| Urinary tract (C64–68) |
n | 15 | 23 | 27 | 14 | 13 |
| HR (95% CI) | 1.0 | 1.17 (0.59,2.31) | 1.99 (1.04,3.81) | 1.79 (0.81,3.94) | 2.05 (0.96,4.35) | |
| Bladder (C67) |
n | 10 | 15 | 20 | 9 | 11 |
| HR (95% CI) | 1.0 | 1.19 (0.52,2.76) | 2.16 (0.98,4.73) | 1.62 (0.61,4.25) | 2.78 (1.16,6.64) | |
| Ill-defined, secondary and unspecified sites (C76–80) |
n | 15 | 23 | 31 | 19 | 12 |
| HR (95% CI) | 1.0 | 1.13 (0.57,2.23) | 2.10 (1.12,3.96) | 2.72 (1.30,5.71) | 1.83 (0.85,3.93) | |
| Unspecified site C80 |
n | 11 | 17 | 29 | 17 | 12 |
| HR (95% CI) | 1.0 | 1.12 (0.51,2.48) | 2.65 (1.30,5.41) | 3.17 (1.39,7.25) | 2.50 (1.09,5.70) | |
| Lymphoid, haematopoietic and related tissue (C81–96) |
n | 40 | 44 | 37 | 14 | 23 |
| HR (95% CI) | 1.0 | 0.75 (0.48,1.18) | 1.06 (0.67,1.67) | 0.77 (0.40,1.47) | 1.33 (0.79,2.22) | |
| Non-Hodgkin’s (C83,85) | n | 12 | 22 | 18 | 6 | 12 |
| HR (95% CI) | 1.0 | 1.33 (0.64,2.76) | 1.84 (0.87,3.86) | 1.12 (0.39,3.16) | 2.41 (1.08,5.41) | |
| All others | n | 22 | 26 | 25 | 9 | 16 |
| HR (95% CI) | 1.0 | 0.94 (0.51,1.71) | 1.27 (0.70,2.30) | 0.97 (0.42,2.24) | 1.84 (0.96,3.54) | |
| Other skin (C44) | n | 1 | 2 | 9 | 5 | 4 |
| HR (95% CI) | 1.0 | 1.85 (0.16,21.53) | 10.12 (1.24,82.95) | 9.90 (1.03,95.57) | 12.38 (1.36,112.71) |
Hazard ratios (HRs) were adjusted for age, education, religion, mother tongue, and BMI; also shown is the 95% confidence interval of the HR obtained by fitting cox proportional hazards model
Name of the anatomic cancer site along with ICD code number
May include smokers of both bidis and cigarettes
There was a dose–response relationship (Table 2) for frequency (1–5 vs. > 5 per day) and for duration (≤ 20 vs. > 20 in years) of smoking. Although the proportion of bidi smokers who smoke more than five bidis per day (83%) was higher than the corresponding proportion in cigarettes smokers who smoke more than five cigarettes (63%), there was no statistical difference observed in HRs across various categories of frequency of habit per day. Similar results also were observed for duration of habits for these two smoking forms.
Discussion
The results of the MCS are not consistent with the claims that smoking bidis is a safer alternative to smoking cigarette. As we demonstrated, bidi smoking appeared to be atleast as harmful as cigarette smoking. Furthermore, for certain anatomic sites, the risks were considerably elevated in both bidi smokers and smokeless tobacco users, and the point estimate was even higher than observed in cigarette smokers. Most troubling, these are the very sites which drive cancer rates up in developing countries and among the poor in developed countries [36-39]. We also found that smokeless tobacco use was associated with all cancers combined, and cancer of some specific sites. The weakness related to loss of follow-up is small in relation to the benefit of testing the effect of bidi smoking in a large cohort, representing individuals using tobacco in many forms, especially bidi. Also, loss to follow-up was not related to exposure, misclassification was minimal for the primary exposure, and all data were collected prospectively [10, 30,31, 34].
Bidis and smokeless tobacco products, manufactured in India, Bangladesh, Nepal, Pakistan, and to a lesser extent in other Asian countries, account for the largest proportion of tobacco consumption in India; and are exported to several countries [18, 40-43]. According to the International Labor Organization, in 1997-1998, 1.1 million kg of bidis worth US $ 6.5 million were exported to 36 countries, of which about half were exported to the United Arab Emirates, followed by the United States, Singapore, Afghanistan, Saudi Arabia and Panama [41]. There is recent evidence of increases in prevalence of bidi consumption from different parts of the world, including the United States [13, 14, 18,20, 23-28].
In recent years, multinational cigarette companies have shifted their focus to the huge market in developing countries, especially vulnerable groups such as youth and women. Meanwhile, local products, such as bidis from India, kreteks from Indonesia, and herbal cigarettes from China, are being promoted in developed countries such as the United States. Some reasons for the increasing popularity of bidis are their availability in different flavors that appeal to youth, lower prices than commercially available cigarettes, easy accessibility, trendy image, and false notions that bidis have fewer or no health risks. Thus, they are seen as a “cool” and “safe” alternative to regular cigarettes [23-28]. Advertising bidis and other tobacco products as safe alternatives is particularly concerning. Unlike other tobacco products in India, bidis have not carried even a minimal text warning, until very recently (31 May 2009).
Bidis differ from cigarettes in a number of ways, including the manner in which they are smoked. If bidis are not puffed at least twice a minute, they will extinguish on their own meaning they must be re-lit. This necessitates deeper, more frequent inhalation, which is harder on the lungs than smoking a conventional cigarette [17]. The average smoker puffs a cigarette nine times versus puffing a bidi 28 times [33, 42]. Like conventional cigarettes, bidi smoke contains particulate matter; however, they do not have filters. Bidis may have higher amounts of chemicals such as phenol (250 vs. 150 μg), hydrogen cyanide (903 vs. 445 μg), benzopyrenes, carbon monoxide (7.7 vs. 3.5 vol%), and ammonia (284 vs. 180 μg) [17, 33]. Although bidis contain less tobacco than cigarettes (approximately 20% less), the nicotine content of the tobacco is higher [16,17]. One study revealed that bidis contain 21.2 mg/g of nicotine compared to 16.3 mg/g for commercial cigarettes and 13.5 mg/g for unfiltered cigarettes [17, 18]. Also, smoke from a bidi can contain 3–5 times as much nicotine as that from conventional cigarette [18, 25]. Health officials in the West should take note of these results as there is no reason to believe that they cannot be generalized. Due to population growth in developing countries and increases in female tobacco use, especially smoking, global prevalence of smoking and the overall number of tobacco users has been increasing rapidly [1, 18, 20, 23-28, 33, 42]. In this context, bidis and smokeless tobacco products should not be viewed as a safer alternative to cigarettes.
These results, the first ever from a longitudinal study on bidi smoking, smokeless tobacco use, and cancer incidence, add importantly to a base of scientific studies in India that have confirmed that both bidi smoking and smokeless tobacco use are no less hazardous than cigarette smoking [10, 30, 43]. The International Agency for Research on Cancer (IARC) found bidi smoke to be carcinogenic almost two decades ago [44]. IARC recognizes a strong association between bidi smoking and cancer at various sites in humans, with significant trends associated with the duration of bidi smoking and number of bidis smoked [45]; similar association was also observed in this cohort.
Cancer affects all communities worldwide, but there are marked differences in the prevalence and types of cancers among communities. While the total cancer burden remains highest in affluent societies, less developed economies are closing the gap very rapidly. A major reason why cancer mortality rates are falling in the United States is that so many people have quit smoking cigarettes. Along with economic expansion, developing countries have begun to experience changes in lifestyles similar to those that occurred in advanced economies over the past couple of generations, and these may result in much higher cancer rates. Even though much of the focus is on cancers related to affluence, e.g., particularly cancers of the breast, colon, and prostate, tobacco-related cancers will continue to be a major driving force [9, 36, 38]. Therefore, our findings regarding bidis have relevance both in India and in other places to which they are being actively exported, such as the United States.
Traditionally, tobacco control programs primarily have focused on reducing cigarette consumption. Effective strategies are now needed to expand the focus of tobacco control programs to all types of tobacco use, including bidis [9-11, 18, 26-28, 42]. Countries that adopted comprehensive tobacco control programs with a mix of interventions (including bans on tobacco advertising, strong warnings on packages, controls on the use of tobacco in indoor locations, high taxes on tobacco products, and health education and smoking cessation programs) have had considerable success in decreasing the prevalence of cigarette smoking [9, 46]. A similar policy framework with a mix of interventions will have to be adapted to the local requirements and effectively implemented in India and other Southeast Asian countries where bidi use is highly prevalent, as well as in countries like the United States where the bidi market is relatively new and expanding. However, in the United States bidis are treated like conventional cigarettes and they are taxed at the same rate and are required to have a tax stamp and carry a surgeon general’s warning [20, 27]. A study conducted in San Francisco reported that about 4 in 10 packs of bidis did not contain the required warning labels and 7 in 10 did not carry the tax stamp [20]. However, 2006 statistics on bidi usage shows that 2.9% of high school students in the United States take part in bidi smoking to 1.4% of adults 18–24 years old [25]. As some bidis are flavored, it is important to mention that both the United States [47] and Canada [48] have banned flavored tobacco, except mentholated products. The adoption of comprehensive legislation on tobacco control by India, The Cigarettes and Other Tobacco Products (Prohibition of advertisement and regulation of trade, commerce, production, supply and distribution) Act, 2003, is an important step forward in this direction. Strong enforcement of the provisions of this comprehensive act on the other products in addition to cigarettes, such as bidis and various smokeless products, is urgently required.
Acknowledgments
The authors appreciate the help and input of Cathy Backinger (National Cancer Institute, Bethesda, MD, USA), Paolo Boffetta (International Agency for Research on Cancer (IARC), Lyon, France), Thomas Glynn (American Cancer Society, Atlanta, GA, USA), Alan Lopez (University of Queensland, Australia), D. M. Parkin (Queen Mary University of London, UK), Richard Peto (Clinical Trial Service Unit of the University of Oxford, Oxford, United Kingdom) and R. Sankaranarayanan (the International Agency for Research on Cancer, Lyon, France) in the conduct of the study. The authors also are grateful for the cooperation of the Municipal Corporation of Greater Mumbai (BMC) in providing access to information on cause of death. This work was partly supported by funding from: the International Agency for Research on Cancer, Lyon, France (Collaborative Research Agreement DEP/89/12); the Clinical Trial Service Unit of the University of Oxford, Oxford, United Kingdom; the World Health Organization Geneva, Switzerland; and Narotam Sekhsaria Foundation, Mumbai, India. Dr. Hébert was supported by grant 1 U01 CA114601 from the National Cancer Institute, Center to Reduce Cancer Health Disparities, and a USIA Fulbright Senior Research Fellowship for the 2008–2009 academic year through the US Educational Foundation in India.
Footnotes
Conflict of interest None declared.
Contributor Information
Mangesh S. Pednekar, Healis, Sekhsaria Institute for Public Health, Navi Mumbai, Maharashtra, India
Prakash C. Gupta, Healis, Sekhsaria Institute for Public Health, Navi Mumbai, Maharashtra, India; Department of Epidemiology and Biostatistics, Arnold School of Public Health, University of South Carolina, Columbia, SC, USA
Balkrishna B. Yeole, Indian Cancer Society, Bombay Population-Based Cancer Registry, Mumbai, Maharashtra, India
James R. Hébert, Healis, Sekhsaria Institute for Public Health, Navi Mumbai, Maharashtra, India; Department of Epidemiology and Biostatistics, Arnold School of Public Health, University of South Carolina, Columbia, SC, USA; South Carolina Statewide Cancer Prevention and Control Program, University of South Carolina, Columbia, SC, USA
References
- 1.Lopez AD, Mathers CD. Measuring the global burden of disease and epidemiological transitions: 2002–2030. Ann Trop Med Parasitol. 2006;100(5-6):481–499. doi: 10.1179/136485906X97417. [DOI] [PubMed] [Google Scholar]
- 2.Murray CJL, Lopez AD, Black R, Mathers CD, Shibuya K, Ezzati M, Salomon JA, Michaud CM, Walker N, Vos T. Global burden of disease 2005: call for collaborators. Lancet. 2007;370(9582):109–110. doi: 10.1016/S0140-6736(07)61064-2. [DOI] [PubMed] [Google Scholar]
- 3.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJL. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. [see comment] Lancet. 2006;367(9524):1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 4.McMichael A. Planetary overload: global environmental change and the health of the human species. Cambridge University Press; Cambridge: 1993. [Google Scholar]
- 5.McMichael AJ. Global environmental change and human population health: a conceptual and scientific challenge for epidemiology. Int J Epidemiol. 1993;22(1):1–8. doi: 10.1093/ije/22.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Hiatt RA, Breen N. The social determinants of cancer: a challenge for transdisciplinary science. Am J Prev Med. 2008;35(2 Suppl):S141–S150. doi: 10.1016/j.amepre.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall KL, Feng AX, Moser RP, Stokols D, Taylor BK. Moving the science of team science forward: collaboration and creativity. Am J Prev Med. 2008;35(2 Suppl):S243–S249. doi: 10.1016/j.amepre.2008.05.007. [see comment] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia M, Jemal A, Ward E, Center M, Hao Y, Siegel R, Thun M. American Cancer Society; Atlanta: 2007. Global cancer facts and figures 2007. [Google Scholar]
- 9.Morgan GD, Backinger CL, Leischow SJ. The future of tobacco-control research. Cancer Epidemiol Biomark Prev. 2007;16:1077–1080. doi: 10.1158/1055-9965.EPI-06-0928. [DOI] [PubMed] [Google Scholar]
- 10.Pednekar MS. Acta Universitatis Tamperensis 1340. Tampere University Press; Tampere: 2008. The impact of tobacco use and/or body composition on adult mortality in urban developing country population. 2008. Available at url: http://www.acta.uta.fi/pdf/978-951-44-7431-6.pdf. [Google Scholar]
- 11.Gupta PC, Ray CS. Smokeless tobacco and health in India and South Asia. Respirology. 2003;8:419–431. doi: 10.1046/j.1440-1843.2003.00507.x. [DOI] [PubMed] [Google Scholar]
- 12.Jayant K, Deo MG. Oral cancer and cultural practices in relation to betel quid and tobacco chewing and smoking. Cancer Detect Prev. 1986;9(3-4):207–213. [PubMed] [Google Scholar]
- 13.Tobacco exports up by 7.4% in April. Times of India. (PTI), May 31, 2010, 04.33 pm IST accessed on June 2010 available at url: http://www.timesofindia.indiatimes.com/business/india-business/Tobacco-exports-up-by-74-in-April/articleshow/5994747.cms.
- 14. [April 28, 2010 access on June 2010];India’s tobacco exports up 29% in FY2010 personal finance magazine—Moneylife. available at url: http://www.moneylife.in/article/8/5082.html.
- 15.India, The tax treatment of bidis [Accessed on May 2010];International Union Against Tuberculosis and Lung Disease, January 2008. available at url: http://www.tobaccofreeunion.org/files/44.pdf.
- 16.Malson JL, Pickworth WB. Bidis–hand-rolled, Indian cigarettes: effects on physiological, biochemical and subjective measures. Pharmacol Biochem Behav. 2002;72(1-2):443–447. doi: 10.1016/s0091-3057(02)00709-8. [DOI] [PubMed] [Google Scholar]
- 17.Malson JL, Sims K, Murty R, Pickworth WB. Comparison of the nicotine content of tobacco used in bidis and conventional cigarettes. Tobacco Control. 2001;10(2):181–183. doi: 10.1136/tc.10.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta PC, Asma S. Bidi smoking and public health, 2008, Ministry of Health and Family Welfare, Government of India. New Delhi: 2008. [Google Scholar]
- 19.Rahman M, Sakamoto J, Fukui T. Bidi smoking and oral cancer: a meta-analysis. Int J Cancer. 2003;106(4):600–604. doi: 10.1002/ijc.11265. [DOI] [PubMed] [Google Scholar]
- 20.Yen KL, Hechavarria E, Bostwick SB. Bidi cigarettes: an emerging threat to adolescent health. Arch Pediatr Adolesc Med. 2000;154(12):1187–1189. doi: 10.1001/archpedi.154.12.1187. [DOI] [PubMed] [Google Scholar]
- 21.Mahimkar MB, Bhisey RA. Occupational exposure to bidi tobacco increases chromosomal aberrations in tobacco processors. Mutat Res. 1995;334(2):139–144. doi: 10.1016/0165-1161(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 22.Malson JL, Lee EM, Moolchan ET, Pickworth WB. Nicotine delivery from smoking bidis and an additive-free cigarette. Nicotine Tobacco Res. 2002;4(4):485–490. doi: 10.1080/1462220021000018498. [DOI] [PubMed] [Google Scholar]
- 23.Celebucki C, Turner-Bowker DM, Connolly G, Koh HK. Bidi use among urban youth—Massachusetts. MMWR. 1999;48(36):796–799. [PubMed] [Google Scholar]
- 24.Fisher L. Bidis-the latest trend in US teen tobacco use. Cancer Causes Control. 2000;11:577–578. doi: 10.1023/a:1008991500413. [DOI] [PubMed] [Google Scholar]
- 25.Delnevo CD, Pevzner ES, Hrywna M, Lewis MJ. Bidi cigarette use among young adults in 15 states. Prev Med. 2004;39(1):207–211. doi: 10.1016/j.ypmed.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 26.Deckers SK, Farley J, Heath J. Tobacco and its trendy alternatives: implications for pediatric nurses. Critical Care Nurs Clin N Am. 2006;18:95–104. doi: 10.1016/j.ccell.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Ribisl KM, Kim AE, Williams RS. Web sites selling cigarettes: how many are there in the USA and what are their sales practices? Tobacco Control. 2001;10:352–359. doi: 10.1136/tc.10.4.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanski SE, Prokhorov AV, Klein JD. Youth and tobacco. Minerva Pediatrica. 2004;56:553–565. [PubMed] [Google Scholar]
- 29.Gupta PC. Survey of sociodemographic characteristics of tobacco use among 99, 598 individuals in Bombay, India using handheld computers. Tob Control. 1996;5(2):114–120. doi: 10.1136/tc.5.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gupta PC, Pednekar MS, Parkin DM, Sankarnarayanan R. Tobacco associated mortality in Mumbai (Bombay) India: results of the Bombay cohort study. Int J Epidemiol. 2005;34(6):1395–1402. doi: 10.1093/ije/dyi196. [DOI] [PubMed] [Google Scholar]
- 31.Pednekar MS, Gupta PC. Prospective study of smoking and tuberculosis in India. Prev Med. 2007;44(6):496–498. doi: 10.1016/j.ypmed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Yeole BB. An assessment of improvement in reliability and completeness of Mumbai cancer registry data from 1965–1997. Asian Pac J Cancer Prev Bangkok. 2001;2:225–232. [PubMed] [Google Scholar]
- 33.Gupta PC, Hamner JE, Murti PR, editors. Oxford University Press; Oxford: 1992. Proceedings of an international symposium on control of tobacco-related cancers and other diseases: proceedings of an international symposium in Bombay, India, 15–19 Jan 1990; pp. 25–46. [Google Scholar]
- 34.Pednekar MS, Gupta PC, Shukla HC, Hebert JR. Association between tobacco use and body mass index in urban Indian population: implications for public health in India. BMC Public Health. 2006;6:70. doi: 10.1186/1471-2458-6-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hosmer DW, Lemeshow S. Applied logistic regression. Wiley; New York City: 1989. [Google Scholar]
- 36.Thrasher JF, Johnson TP. International Agency for Research on Cancer. Lyon, France: 2008. IARC handbooks of cancer prevention: tobacco control. Volume 12. Methods for evaluating tobacco control policies: developing and assessing comparable questions in cross-cultural survey research on tobacco. [Google Scholar]
- 37.Chu KC, Miller BA, Springfield SA. Measures of racial/ethnic health disparities in cancer mortality rates and the influence of socioeconomic status. J National Med Assoc. 2007;99(10):1092–1104. [PMC free article] [PubMed] [Google Scholar]
- 38.Day TA, Chi A, Neville B, Hebert JR. Prevention of head and neck cancer. Curr Oncol Rep. 2005;7(2):145–153. doi: 10.1007/s11912-005-0041-x. [DOI] [PubMed] [Google Scholar]
- 39.Ward E, Jemal A, Cokkinides V, Singh GK, Cardinez C, Ghafoor A, Thun M. Cancer disparities by race/ethnicity and socioeconomic status. Ca Cancer J Clin. 2004;54(2):78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 40.World Health Organization. India . Tobacco or Health: a global status report. Geneva: 1997. [Google Scholar]
- 41.International Labor Organization . Employment trends in the tobacco sector: challenges and prospects. Geneva: 2003. Appendix: special cases: bidi and kretek; pp. 85–93. Report for discussion at the tripartite meeting on the future of employment in the Tobacco Sector. [cited 2004 Jun 5] Available from: http://www.ilo.org/public/englishdialogue/sectortechmeet/tmets03/tmets-r.pdf. [Google Scholar]
- 42.Reddy KS, Gupta PC. Report on tobacco control in India. Ministry of Health and Family Welfare, Government of India; New Delhi: 2004. [Google Scholar]
- 43.Ray CS, Gupta PC, de Beyer J. Research on tobacco in India (including betel quid and areca nut). Health, Nutrition and Population (HNP) discussion paper. Economics of tobacco control Paper no. 9 World Bank. 2003:33–167. [Google Scholar]
- 44.International Agency for Research on Cancer . IARC Monographs on the evaluation of carcinogenic risk of chemicals to humans—tobacco smoking. Vol. 38. IARC, Lyon: 1986. [PubMed] [Google Scholar]
- 45.International Agency for Research on Cancer . IARC Monographs on the evaluation of carcinogenic risk of chemicals to humans—tobacco smoking and tobacco smoke. Vol. 83. IARC, Lyon: 2002. Available from: http://www.monographs.iarc.fr/htdocs/monographsvol83/01-smoking.html. [Google Scholar]
- 46.World Health Organization . The world health report, 2002. Geneva: 2002. Reducing risks, promoting healthy life; p. 225. [DOI] [PubMed] [Google Scholar]
- 47.US FDA [access on May 13, 2010];first tobacco action, bans flavors. available at url: http://www.reuters.com/article/idUSN2236998020090922.
- 48. [accessed on May 13, 2010];Canada bans fruit-flavored cigarettes. available at url: http://www.reuters.com/article/idUSTRE5975GD20091008?loomia_ow=t0:s0:a49:g43:r1:c1.000000:b30639980:z0.