Abstract
The surgical approach to benign, metastatic, and some low-grade malignant tumors is often difficult due to their typically precarious locations. This article presents a series of cases where intraoperative stealth navigation was used to treat periarticular tumors. The use of paired point imaging with image fusion has made approaching tumors through an accurate and minimally invasive technique a viable option for the treatment of a subset of musculoskeletal tumors.
Conventional resection of periarticular and pelvic tumors of bone usually requires an extensive surgical approach to adequately visualize the tumor and protect the nearby neurovascular structures. When tumors in periarticular locations are encountered, dislocation of the affected joint may be necessary, putting periarticular and subchondral bone at risk for osseous necrosis and articular surfaces at risk of mechanical insult at the time of dislocation. While arthroscopic techniques may enable a minimally invasive mode of surgical approach in certain anatomic locations, this is not always feasible and can add additional operative time and morbidity to the patient. This article describes 5 cases where locally aggressive tumors in challenging periarticular anatomic locations were treated in a minimally invasive manner with the assistance of image fusion and paired-point registration.
Materials and Methods
Intraoperative cone beam 3-dimensional imaging with navigation was used to perform real-time treatment of 5 periarticular lesions. All cases were performed through a minimally invasive approach with paired-point imaging and were completed as outpatient procedures.
Intraoperative navigation surgery consisted of the following standard general procedure: Patients were positioned on a radiolucent carbon table. First, the reflective array was fixed to the operative bone with percutaneous approach by using either a single 1.6-mm Kirschner wire or a single static array. Patient-to-image registration was performed by paired-point registration using intraoperative computed tomography (CT) images (O-arm navigation system; Medtronic, Minneapolis, Minnesota) and K-wire. Then, a 2- to 3-cm longitudinal incision was made, centered over previously placed K-wire. The approach was completed with blunt dissection, paying special attention to prevent injury to the neurovascular structures at risk at each anatomic site. The tumor site was verified using a probe under navigation guidance. Once the tumor location was confirmed, the K-wire was removed and tumor resection was performed using the appropriate modality, without wide soft tissue dissection under navigation guidance.
Case Reports
Patient 1
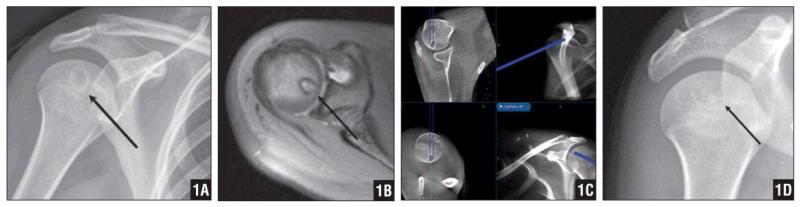
A 17-year-old girl presented with right shoulder pain of 1 month’s duration with active and passive range of motion (ROM) and no history of trauma. Plain radiographs revealed an epiphyseal lesion in the right humeral head (Figure 1A). Magnetic resonance imaging (MRI) was performed to further characterize the lesion (Figure 1B). Patient age, epiphyseal location of the tumor, and symptoms led to the favored diagnosis of chondroblastoma. Due to the deep subchondral location of the tumor within, the navigation-guided technique was planned to minimize the morbidity of an open approach.
Figure 1.
Plain Radiograph Showing an Epiphyseal Lesion in the Right Humeral Head (A). MRI Demonstrating a Benign Lesion Consistent with a Chondroblastoma (B). Real-Time Navigation of the Lesion with the Guide Wire (C). Four-Month Follow-Up Radiograph Demonstrating Resolution of the Lesion. The Patient Was Symptom Free (D).
After routine preoperative consent, anesthesia induction, and preparation of the patient’s shoulder, the O-arm navigation system was set up in the usual manner. The reference was placed in the humeral diaphysis, with 2 percutaneous drill pins placed anteriorly. The O-arm was used to confirm appropriate position. The instruments, including the probe, drill, and curette, were then registered. A 5-mm incision was made laterally over the greater tuberosity, and soft tissue was bluntly dissected to the humerus. The 0.25-in drill was registered and advanced into the humeral head, using dynamic navigation to access the lesion (Figure 1C). The location of the drill was confirmed on all the planes in the navigation system, and the instrument was withdrawn (Figure 1C). The residual specimen from the flutes of the drill bit was sent to pathology as fresh specimen.
The lesion was then aggressively curetted with registered curettes to assure that a wide curettage was completed without violating the articular surface. As is our standard approach after percutaneous curettage of a periarticular tumor, a radiofrequency ablation probe was deployed into the lesion and used to ablate any residual microscopic disease and expand the margin without generating thermal necrosis of the articular cartilage. The final pathology report confirmed the diagnosis of chondroblastoma.
The patient’s pain resolved and shoulder function recovered with no pain or limitation of ROM noted at 1-month follow-up. Her postoperative course was uneventful. Bony regeneration of the osteolytic/chondroblastic lesion was noted at 4-month follow-up (Figure 1D). Her Musculoskeletal Tumor Society Functional Score improved from 22 preoperatively to 28 after surgical intervention.
Patient 2
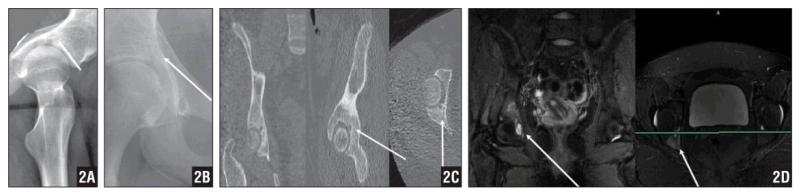
A 16-year-old girl presented after undergoing plain pelvic radiography at a local clinic. She reported pain in the right buttock area and right inguinal area of 3 months’ duration. She reported no trauma or sports injury history. Plain radiographs suggested an osteolytic lesion in her right acetabulum that raised suspicion for an osteoid osteoma (Figures 2A, B). Computed tomography and MRI revealed a lytic right acetabular tumor without the characteristic nidus seen in a classic osteoid osteoma (Figures 2C, D).
Figure 2.
Lateral Plain Radiograph of the Hip Demonstrating an Osteolytic Lesion in the Patient’s Right Acetabulum, Which Raised Suspicion for an Osteoid Osteoma (A). AP Radiograph of the Lesion (B). CT Scan Revealing a Lytic Right Acetabular Tumor Without the Characteristic Nidus Seen in a Classic Osteoid Osteoma (C). An MRI not Characteristic of an Osteoid Osteoma (D).
The patient was placed prone on the radiolucent flat-top operating table. The stealth reference probe was placed on the left posterior superior iliac spine through a percutaneous stab incision. The O-arm was then used to generate a CT image. Once the tumor was located and the navigation system was merged, the probe was used to find the appropriate location for the percutaneous skin incision. After the guide pin was advanced into the lesion, the wound was serially dilated. After navigating the probes and other instruments used for curettage, the lesion was curetted without invading the hip joint. Diagnostic tissue was felt necessary because of the atypical presentation in this case. After curettage, a registered radiofrequency ablation probe was deployed into the lesion. Computed tomography scan confirmed the location of the probe. The final pathology report confirmed the diagnosis of osteoid osteoma.
As is our standard protocol for osteoid osteomas, we used the radiofrequency ablation probe in the CT scanner with anesthesia. As this patient had a pelvic osteoid osteoma and was obese, it was felt that she was a better candidate in the operative suite with less radiation exposure to her, as many spins were likely going to be needed for localization in the CT suite alone. Paired-point imaging necessitated only 2 spins of the scanner.
The patient recovered well, with no ROM limitation postoperatively. Her Musculoskeletal Tumor Society Functional Score improved from 20 preoperatively to 28 postoperatively. The patient did well for 8 months after the index procedure but developed hip pain consistent with recurrence of the disease. She underwent repeat radiofrequency ablation probing using the same procedure with the navigation technique. She recovered well and was pain free 6 months after revision surgery.
Patient 3
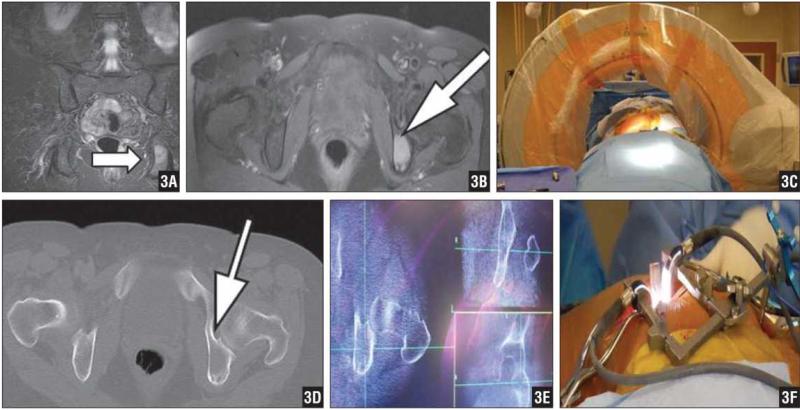
A 54-year-old woman with a history of pheochromocytoma diagnosed 12 years earlier presented with buttock pain. She underwent adrenalectomies and resections of metastatic disease from her abdomen prior to presentation. She was referred to our clinic due to a suspicious osteolytic lesion that was noted in her left ischium (Figures 3A, B).
Figure 3.
Coronal (A) and Axial (B) Gadolinium-Enhanced MRIs Demonstrating a Left Ischial Lesion Suggestive of Metastatic Pheochromocytoma. Intraoperative Photograph of CT Setup Whereby the Posterior Superior Iliac Spine Was Identified and a Percutaneous Incision Was Made to Deploy the Navigation Reference Frame (C). Preoperative CT Image of the Ischial Lesion (D). Triplane Intraoperative CT Images of the Ischial Lesion (E). Intraoperative Photograph of Minimally Invasive Access Portal to Excise the Lesion in This Morbidly Obese Patient (F).
Due to the deep nature of her tumor, the operation was planned with the navigation-assisted technique for more accurate localization and less soft tissue dissection. She was placed prone on the OSI flat-top operative table. The posterior superior iliac spine was identified and a percutaneous incision made to deploy the navigation reference frame. Intraoperative CT was obtained (Figures 3C–F). A longitudinal skin incision was made over the ischium and carried down through subcutaneous tissue. The tumor was then thoroughly curetted. The radiofrequency ablation probe was deployed, and a short ablation was performed.
The postoperative course was uneventful. The final pathology report confirmed the diagnosis of pheochromocytoma. Her Musculoskeletal Tumor Society Functional Score improved from 20 preoperatively to 29 at last follow-up.
Patient 4
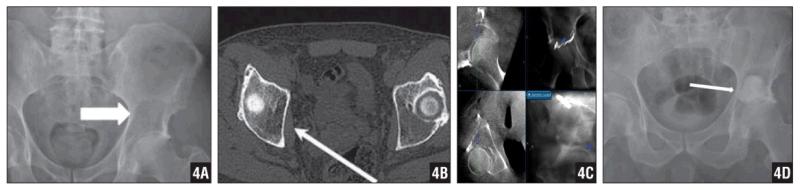
A 71-year-old man presented with worsening right hip pain. A CT scan revealed multiple lytic lesions consistent with multiple myeloma. Laboratory examination showed an increase in kappa light chain protein to 1280 (normal range, 74–295) and a kappa-to-lambda ratio of 33.5 (normal range, 1.3–2.7), which is compatible with the diagnosis of multiple myeloma. Full-body CT scan revealed a large lytic lesion in the left supracetabular area (Figures 4A, B). His Musculoskeletal Tumor Society Functional Score was 19. As an alternative to total hip arthroplasty, the patient elected to have the lesion curetted and replaced with cement.
Figure 4.
AP Pelvis Radiograph (A) and CT Scan (B) Revealing a Large Lytic Lesion in the Left Supra-Acetabular Region. Percutaneous Navigation and Curettage of the Lesion (C). Postoperative AP Pelvis Radiograph Revealing Deployed Cement. While 100% Fill of the Cavity Was not Obtained, the Intraoperative Strategy Was to Mechanically Augment the Dome, Which Was Achieved. The Patient Experienced No Pain Postoperatively (D).
The patient was placed in the right lateral decubitus position. The navigation reference frame was fixed to the iliac crest through a small stab incision. Intraoperative CT and navigation were completed in the standard fashion. A 4-cm longitudinal skin incision was made and taken down to the posterior aspect of acetabulum under navigation guide (splitting the gluteus maximus and elevating the medius and minimus tendons). An extensive curettage was performed, and the navigation system was used to confirm that the lesion was excised without invading hip joint (Figure 4C). Radio-opaque dye was used to assure that there was adequate curettage without violation of the articular surface into the joint. Compressed Gelfoam (Pharmacia & Upjohn, Kalamazoo, Michigan) was inserted and compressed on the roof of the acetabulum. Antibiotic-impregnated cement was then packed into the lesion. Intraoperative CT scan performed after cementation confirmed that there was no extrusion of cement into the joint and articular surface (Figure 4D). The pathology report confirmed the diagnosis of myeloma. The patient started weight-bearing and ROM exercises immediately postoperatively, and had an uneventful postoperative course. His Musculoskeletal Tumor Society Functional Score was 26 out of 30 at last follow-up.
Patient 5
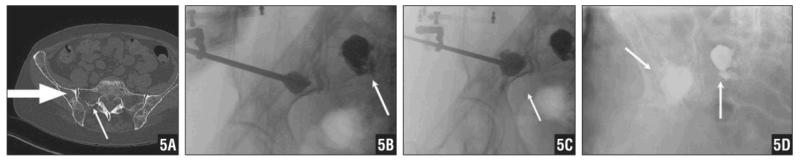
A 64-year-old woman presented with a 4-year history of multiple myeloma. She began to have right inguinal and buttock pain 1 year prior to presentation. Computed tomography scan of her pelvis revealed a large lytic lesion of her right ilium superior to the acetabular dome that extended to the sacroiliac joint, as well as a lesion in the right sacral ala (Figure 5A). It was recommended by her myeloma specialist to avoid irradiating the pelvis despite an impending fracture and symptomatic lesions; therefore, operative intervention was elected.
Figure 5.
CT Scan of the Patient’s Pelvis Revealing a Large Lytic Lesion of Her Right Ilium Superior to the Acetabular Dome that Extends to the Sacroiliac Joint. A Lesion in the Right Sacral Ala Is also Noted (A). Intraoperative Fluoroscopy Available via the CT Scanner Demonstrating Percutaneous Cementation of the Sacral (B) and Supra-Acetabular (C) Lesions Following Void Creation via Balloon Tamps. Postoperative AP Radiograph of the Pelvis (D).
The patient was placed in the left lateral decubitus position. The navigation reference frame was placed on her right iliac crest through a stab incision. A CT scan was obtained by O-arm in the usual fashion for navigation. A small stab wound was then performed and navigated down to the sacrum ala behind the posterior superior iliac spine for a direct posterior approach. The registered radiofrequency ablation probe was deployed, and short ablation was performed.
A kyphoplasty balloon (Kyphon, Inc, Sunnyvale, California) was then placed into the appropriate position and confirmed on navigation with radio-opaque dye. The myelomatous space was expanded with the kyphoplasty balloon and confirmed under fluoroscopic guidance. Polymethylmethacrylate was then delivered into the space with interval confirmation that it was not extruded into the joint. Then, the navigated K-wire and drill were used to locate the lesion in the posterior column of right iliac bone. Using the technique previously described, a separate stab wound was made and carefully dissected down to bone. Another CT scan was obtained for navigation. Radiofrequency ablation probing was then performed. The kyphoplasty balloon was used to create a potential space (Figure 5B). Bone cement was introduced under fluoroscopic guidance (Figures 5C, D). Another CT scan was performed to confirm the cement was appropriately deployed.
The patient started ROM exercises immediately postoperatively, with an uneventful postoperative course. Her Musculoskeletal Tumor Society Functional Score was 28 out of 30 at last follow-up.
Results
All of the patients in this case series had an excellent Musculoskeletal Tumor Society Functional Score (range, 26–29) postoperatively. Basic demographic data and postoperative results are listed in the Table. Average follow-up was 8.8 months (range, 6–16 months). Musculoskeletal Tumor Society Functional Scores were satisfactory in all patients. Near-complete relief of preoperative symptoms was obtained in all patients. Excellent 3-D multiplanar visualization of the tumor was obtained in all patients, and minimal invasive approaches were completed successfully by using intraoperative navigation guidance. No surgery-related complications were encountered during any of the procedures. Average intraoperative blood loss was 78 mL (range, 10–100 mL). Average operative time was 92 minutes (range, 65–125 minutes).
Only 1 of our patients (patient 2) developed local tumor recurrence within the follow-up period. She was treated successfully with repeat radiofrequency ablation probing under intraoperative navigation guidance via the same minimally invasive approach as her index procedure. The patient recovered with no complications and no tumor recurrence at last follow-up.
Discussion
Navigation-assisted surgery is an emerging technology. In recent years, the accuracy and usefulness of intraoperative navigation has been evaluated in orthopedic surgeries. It was initially described and implemented in intracranial applications in neurosurgery.1 The initial reports with regard to orthopedic surgery focused primarily on total joint arthroplasty, trauma surgery, and spine surgery, specifically for pedicle screw placement.2-7 Reports exist of its use in musculoskeletal tumor surgery, although most of them focus on the treatment of osteoid osteoma and usually consist of percutaneous radiofrequency ablation.8,9 Navigation has also been used for resection and reconstruction of tumors with the presumption that it may reduce local recurrence rates and enhance outcomes by preserving adjacent joints.10-12 To our knowledge, no study documents the efficacy of intraoperative navigation in minimally invasive periarticular bone tumor excision.
The main advantage of using intraoperative navigation is to provide intraoperative guidance with high accuracy. We applied this advantage to the treatment of our patients. Accurate intraoperative guidance made it possible to operate on these difficult locations without conventional wide exposures and approaches. Using intraoperative navigation, surgeons are able to accurately localize the tumor site. Tumor excision can then be achieved with minimal skin incision and minimal soft tissue detachment.
The question as to whether each of these cases could have been managed with image-guided ablation alone is important. In patient 1, where we were confident of the diagnosis, concern existed that a prolonged ablation in this area might damage the articular cartilage of the proximal humerus. We also were able to more thoroughly curettage the area prior to ablation with incredible accuracy with a curette that was precisely navigated. In patient 2, we aimed to confirm the diagnosis, as the presentation was sufficiently atypical that tissue diagnosis was warranted prior to the treatment of the confirmed osteoid osteoma diagnosis, albeit benign. Although she suffered from tumor recurrence months after her index procedure, the patient was treated with repeat curettage and radiofrequency ablation probing with intraoperative navigation guidance. She recovered well without complications or recurrence noted. Inadequate curettage and ablation in her index procedure may have been the cause of the recurrence.
In patient 3, the referring oncologist felt this was the only site of active disease, desired as complete a resection as possible, and asked that we curettage in an extended manner rather than simply percutaneously ablate the lesion.13,14 In patient 4, the acetabulum was structurally compromised, and cement augmentation was chosen over hip arthroplasty. As in spinal cementation, it is safer to create a void in which to place the cement than to place it under pressure.15,16 Furthermore, our goal was to obtain a congruent fill along the subchondral bone, which necessitated a mini-open approach to excavate the tumor. Lastly, in patient 5, for the same reason, we applied a kyphoplasty balloon into the supra-acetabular and sacral lesion to create a void, and cementoplasty was then performed smoothly through percutaneous stab wounds.
The purpose of this case series was to present a relatively novel application of an existing but relatively new and emerging technology. The immediate postoperative course in each of the cases was free of any complications, and each patient was treated as an outpatient. Long-term clinical follow-up will be necessary to establish whether these techniques are equivalent to their open counterparts in terms of oncologic tumor control. In selected cases, intraoperative navigation surgery allows precise access with less sacrifice of soft tissue compared to conventional open excision.
One possible shortcoming of using this technique is the possibility of inadequate tumor removal because of relative less invasive approach and wound exposure. We believe this could be improved with more clinical experiences. Although we had no control group, we believe the blood loss (average, 78 mL) and operation time (average, 92 minutes) in our series fell in a reasonable range. While we feel this is an ideal indication for the application of this technology, we consider that its application may be further expanded in the best interest of the patient, by minimizing morbidity, and to society, by cost containment. Regarding the latter, if a more aggressive approach had been undertaken, hospitalization and recovery may have been longer. With the exception of the probes, reference pins, and O-arm cover, all of the technology applied here is reusable. With increased efficiency with the use of these devices, and prospective data evaluating the outcomes of patients in whom these techniques are used, this technology may become a standard in the treatment of musculoskeletal disease.
Table 1.
Demographic Data and Postoperative Results
|
Patient
No./Sex/Age, y |
Tumor Location | Tumor Type |
Incision Size, mm |
MSTS
|
Blood Loss, mL |
Operative Time, min |
Follow-up, mo |
|
|---|---|---|---|---|---|---|---|---|
| Preop | Postop | |||||||
| 1/F/17 | Right humeral head |
Chondroblastoma | 5 | 22 | 28 | 10 | 75 | 8 |
| 2/F/16 | Right acetabulum |
Osteoid osteoma | 15 | 20 | 28 | 10 | 65 | 16 |
| 3/F/54 | Left ischium | Pheocromocytoma | 30 | 20 | 29 | 120 | 90 | 7 |
| 4/M/71 | Left acetabulum | Multiple myeloma | 30 | 19 | 26 | 150 | 105 | 6 |
| 5/F/64 | Right ilium | Multiple myeloma | 3 | 20 | 28 | 100 | 125 | 7 |
| Right sacrum ala | Multiple myeloma | 3 | ||||||
References
- 1.Golfinos JG, Fitzpatrick BC, Smith LR, Spetzler RF. Clinical use of a frameless stereotactic arm: results of 325 cases. J Neurosurg. 1995;83(2):197–205. doi: 10.3171/jns.1995.83.2.0197. doi:10.3171/jns.1995.83.2.0197. [DOI] [PubMed] [Google Scholar]
- 2.Amiot LP, Lang K, Putzier M, Zippel H, Labelle H. Comparative results between conventional and computer-assisted pedicle screw installation in the thoracic, lumbar, and sacral spine. Spine (Phila Pa 1976) 2000;25(5):606–614. doi: 10.1097/00007632-200003010-00012. doi:10.1097/00007632-200003010-00012. [DOI] [PubMed] [Google Scholar]
- 3.Dutton AQ, Yeo SJ, Yang KY, Lo NN, Chia KU, Chong HC. Computer-assisted minimally invasive total knee arthroplasty compared with standard total knee arthroplasty. A prospective, randomized study. J Bone Joint Surg Am. 2008;90(1):2–9. doi: 10.2106/JBJS.F.01148. doi:10.2106/JBJS.F.01148. [DOI] [PubMed] [Google Scholar]
- 4.Easley M, Chuckpaiwong B, Cooperman N, et al. Computer-assisted surgery for subtalar arthrodesis. A study in cadavers. J Bone Joint Surg Am. 2008;90(8):1628–1636. doi: 10.2106/JBJS.G.00513. doi:10.2106/JBJS.G.00513. [DOI] [PubMed] [Google Scholar]
- 5.Jolles BM, Genoud P, Hoffmeyer P. Computer-assisted cup placement techniques in total hip arthroplasty improve accuracy of placement. Clin Orthop Relat Res. 2004;(426):174–179. doi: 10.1097/01.blo.0000141903.08075.83. doi:10.1097/01.blo.0000141903.08075.83. [DOI] [PubMed] [Google Scholar]
- 6.Kahler DM. Navigated long-bone fracture reduction. J Bone Joint Surg Am. 2009;91(Suppl 1):102–107. doi: 10.2106/JBJS.H.01286. doi:10.2106/JBJS.H.01286. [DOI] [PubMed] [Google Scholar]
- 7.Zwingmann J, Konrad G, Kotter E, Südkamp NP, Oberst M. Computer-navigated iliosacral screw insertion reduces malposition rate and radiation exposure [published online ahead of print November 26, 2008] Clin Orthop Relat Res. 2009;467(7):1833–1838. doi: 10.1007/s11999-008-0632-6. doi:10.1007/s11999-008-0632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajasekaran S, Kamath V, Shetty AP. Intraoperative Iso-C three-dimensional navigation in excision of spinal osteoid osteomas. Spine (Phila Pa 1976) 2008;33(1):E25–29. doi: 10.1097/BRS.0b013e31815e6308. doi:10.1097/BRS.0b013e31815e6308. [DOI] [PubMed] [Google Scholar]
- 9.Yang WT, Chen WM, Wang NH, Chen TH. Surgical treatment for osteoid osteoma—experience in both conventional open excision and CT-guided mini-incision surgery. J Chin Med Assoc. 2007;70(12):545–550. doi: 10.1016/S1726-4901(08)70058-3. doi:10.1016/S1726-4901(08)70058-3. [DOI] [PubMed] [Google Scholar]
- 10.Cho HS, Kang HG, Kim HS, Han I. Computer-assisted sacral tumor resection. A case report. J Bone Joint Surg Am. 2008;90(7):1561–1566. doi: 10.2106/JBJS.G.00928. doi:10.2106/JBJS.G.00928. [DOI] [PubMed] [Google Scholar]
- 11.Wong KC, Kumta SM, Chiu KH, et al. Computer assisted pelvic tumor resection and reconstruction with a custom-made prosthesis using an innovative adaptation and its validation. Comput Aided Surg. 2007;12(4):225–232. doi: 10.3109/10929080701536046. [DOI] [PubMed] [Google Scholar]
- 12.Wong KC, Kumta SM, Chiu KH, Antonio GE, Unwin P, Leung KS. Precision tumour resection and reconstruction using image-guided computer navigation. J Bone Joint Surg Br. 2007;89(7):943–947. doi: 10.1302/0301-620X.89B7.19067. doi:10.1302/0301-620X.89B7.19067. [DOI] [PubMed] [Google Scholar]
- 13.Mishra AK, Agarwal G, Kapoor A, Agarwal A, Bhatia E, Mishra SK. Catecholamine cardiomyopathy in bilateral malignant pheochromocytoma: successful reversal after surgery. Int J Cardiol. 2000;76(1):89–90. doi: 10.1016/s0167-5273(00)00363-6. doi:10.1016/S0167-5273(00)00363-6. [DOI] [PubMed] [Google Scholar]
- 14.Shinoto M, Hasuo K, Aibe H, et al. Percutaneous osteoplasty for hypervascular bone metastasis [published online ahead of print January 8, 2009] Radiat Med. 2008;26(10):603–608. doi: 10.1007/s11604-008-0277-0. doi:10.1007/s11604-008-0277-0. [DOI] [PubMed] [Google Scholar]
- 15.Denaro L, Longo UG, Denaro V. Vertebroplasty and kyphoplasty: reasons for concern? Orthop Clin North Am. 2009;40(4):465–471. doi: 10.1016/j.ocl.2009.05.004. doi:10.1016/j.ocl.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 16.Taylor RS, Taylor RJ, Fritzell P. Balloon kyphoplasty and vertebroplasty for vertebral compression fractures: a comparative systematic review of efficacy and safety. Spine (Phila Pa 1976) 2006;31(23):2747–2755. doi: 10.1097/01.brs.0000244639.71656.7d. doi:10.1097/01.brs.0000244639.71656.7d. [DOI] [PubMed] [Google Scholar]