Identification of Rb1 induction in chronic asymptomatic HIV-1 infection as a mediator of apoptosis resistance in monocytes, in association with protective autophagy.
Keywords: inflammation, autophagy, chemokines, p53, p21, Beclin
Abstract
We have previously described an antiapoptotic steady-state gene expression profile in circulating human monocytes from asymptomatic viremic HIV+ donors, but the mechanism associated with this apoptosis resistance remains to be fully elucidated. Here, we show that Rb1 activation is a dominant feature of apoptosis resistance in monocytes exposed to HIV-1 in vivo (as measured ex vivo) and in vitro. Monocytes from asymptomatic viremic HIV+ individuals show a positive correlation between levels of hypophosphorylated (active) Rb1 and VL in conjunction with increases in other p53-inducible proteins associated with antiapoptosis regulation, such as p21 and PAI-1 (SERPINE1), when compared with circulating monocytes from uninfected donors. Monocytes exposed in vitro to HIV-1 R5 isolates but not X4 isolates showed lower caspase-3 activation after apoptosis induction, indicating a role for the CCR5 signaling pathway. Moreover, monocytes exposed to R5 HIV-1 or MIP-1β induced Rb1 and p21 expression and an accumulation of autophagy markers, LC3 and Beclin. The inhibition of Rb1 activity in HIV-1 R5 viral-exposed monocytes using siRNA led to increased apoptosis sensitivity, thereby confirming a central role for Rb1 in the antiapoptotic phenotype. Our data identify Rb1 induction in chronic asymptomatic HIV-1 infection as a mediator of apoptosis resistance in monocytes in association with protective autophagy and contributing to monocyte survival during immune activation and/or HIV-1 viremia.
Introduction
HIV-induced apoptosis is primarily responsible for the selective depletion of CD4+ T cells as a hallmark of progression to AIDS. CD4+ T cell loss is a consequence of productive infection and bystander cell death, facilitated by the cross-linking of uninfected cells to CD4 on the T cell surface of infected cells, as well as by soluble gp-120 [1, 2], the principal T cell apoptosis-inducing protein encoded by HIV-1. Unlike CD4 T cells, macrophages isolated from HIV-1+ individuals remain refractory to the cytopathic effect of systemic HIV-1 infection, facilitating their potential role to sustain immune activation or serve as long-term viral reservoirs [3–5]. Indeed, as a result of their ability to harbor various opportunistic infections, such as Mycobacterium avium complex or Pneumocystis carinii, as well as their longevity during a time when lymphoid tissue is functionally impaired and activated [6], monocytes/macrophages have been the focus of studies aimed at determining the precise mechanisms that promote their survival during immune activation and chronic HIV-1 infection. Interestingly, monocytes acutely exposed to M-tropic strains of HIV-1 in vitro exhibit reduced susceptibility to apoptotic induction following exposure to Fas ligand or heavy metal stimulation, suggesting that productive infection is not a prerequisite for their conditioning [7]. Moreover, binding and signaling through the chemokine receptor CCR5 have been shown to be sufficient to confer an apoptosis-resistant phenotype illustrating the potential for redundant regulation between viral and host-mediated immune activation [7].
p53 is the most-studied tumor-suppressor protein associated with the regulation of apoptosis. p53 is a major checkpoint protein that regulates the cellular response to intrinsic and extrinsic stress stimuli, acting as a switch to either induce cell cycle arrest, thus facilitating DNA repair, or to activate apoptosis in the event of irreparable DNA damage. Several studies have documented p53 activation in CD4+ T cells during HIV infection as a contributing mechanism that promotes apoptosis [8]. The significant up-regulation in the expression of activated p53 in CD4+ T cells, as well as the proapoptotic p53-dependent gene PUMA, has been interpreted as a major contributing mechanism of CD4 T cell loss during chronic HIV-1 infection [8–10]. While monocytes/macrophages exposed to HIV-1 in vitro can up-regulate p53-associated proteins coupled with increased survival, such as p21 [11, 12], recent data show that apoptosis-inducing PUMA is also expressed in circulating monocytes from HIV-infected viremic subjects, although there are a lack of data indicating its direct role in apoptosis regulation [7].
The tumor-suppressor Rb1 is part of a signal transduction pathway activated downstream of p53 signaling [13]. Rb1 can inhibit progression through the G1 phase of the cell cycle [14], a function that is accomplished largely by the modulation of other cellular transcription factors [15–17]. Rb1 is required for coordinating cell cycle exit, with tissue-specific gene expression occurring during terminal differentiation [18, 19]. Importantly, expression of functional Rb1 with concomitant overexpression of WT p53 can overcome p53-dependent apoptosis, as shown in HeLa cells [20] and in HTB9 human bladder carcinoma cells [21]. Autophagy is a lysosomoal degradation pathway that is generally considered a prosurvival mechanism protecting cells under stress, such as nutrient deprivation, oxidative stress, and pathogen infection [22, 23]. Although autophagy can function as a tumor-suppression mechanism, it has a more prominent role in sustaining cell viability in cells undergoing long-term metabolic stress conditions. It is implicated in development [24], differentiation [22], innate and adaptive immunity during viral infection [25], and aging and cell death [26]. Rb1 has been shown to play an important role in the regulation of autophagy induction [27], with some studies connecting autophagy with chemoresistance during cancer therapy [28–30]. No study to date has addressed the expression or function of Rb1 in primary monocytes or in HIV-1 infection.
In the present study, we tested the hypothesis that the decreased sensitivity to apoptosis—observed in circulating monocytes in asymptomatic chronic-infected subjects or monocytes acutely exposed to HIV-1—is the result of monocyte expression of Rb1 as a transcriptional mediator of antiapoptotic regulation over p53 activation.
MATERIALS AND METHODS
Donors, cell subset isolation, and HIV-1 isolates
PBMCs were isolated from untreated, asymptomatic, HIV-infected viremic donors with no clinical evidence of active comorbidities or from ART-treated, suppressed donors from the Jonathan Lax Immune Disorder Clinic (Philadelphia FIGHT, Philadelphia, PA, USA). The cohort analyzed consisted of 55 donors, of whom 16 were uninfected, 15 were HIV-positive and ART-naïve, and 24 were suppressed on ART. For inclusion, CD4 T cells counts were required to be >200 cells/mm3 [median of 473 cells/mm3; IQR (first quartile, third quartile) 47.5 (435.5, 483)] and VL, >10,000 copies/ml [median of 13,468 copies/ml; IQR (first quartile, third quartile) 46,798 (5802, 52,600)]. ART-treated and -suppressed donors were defined as having an undetectable VL of <48 copies by PCR at the time of analysis and for a minimum period of 4 months preceding the time of blood draw. Age- and gender-matched healthy, HIV-negative donors were identified from The Wistar Institute Blood Donor Program (Philadelphia, PA, USA). Informed consent was obtained before any blood donation, as approved by the Institutional Review Board from The Wistar Institute and Philadelphia FIGHT. PBMCs were separated by Ficoll-Paque (Amersham Pharmacia Biotech, Uppsala, Sweden) density gradient separation, and monocytes were isolated by negative selection following column purification (Miltenyi Biotec, Auburn, CA, USA), per the manufacturer's instructions, yielding >90% purity. Functional assays were performed in isolated monocytes cultured for 36 h with live BAL (R5 HIV or HIVR5), JAGO (HIVR5), 3B (X4 HIV or HIVX4), and NL43 (HIVX4), using 50 ng/ml (based on p24 Gag antigen content; obtained from the University of Pennsylvania Center for AIDS Research Viral/Molecular Core, Philadelphia, PA, USA), 1 μg MIP-1β (PeproTech, Rocky Hill, NJ, USA), or 1 μg RANTES (PeproTech). Maraviroc was obtained from the U.S. NIH Reference Reagent Program (Bethesda, MD, USA).
Transcriptome data
The transcriptome data discussed in this publication can be accessed from the National Center for Biotechnology Information's GEO and are accessible through GEO Series Accession Number GSE14542 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc).
Quantitative real-time PCR
Total RNA was isolated from 106 cells using the RNeasy mini kit (Qiagen, Valencia, CA, USA). PCR was performed in a 20-μl reaction volume in an ABI Prism 7000 (Applied Biosystems, Foster City, CA, USA), using 5 μl cDNA prepared from 0.5 μg total RNA, prepared using iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA, USA), and diluted 1:5 in RNAse-free water. PCR product specificity was assessed by melting curve analysis. The expression levels for p53, primer sequence: 5′-CCG CAG TCA GAT CCT AGC G-3′ (forward), 5′-AAT CAT CCA TTG CTT GGG ACG-3′ (reverse); Rb1, primer sequence: 5′-TTG GTG TAC CGG AAA GTG ACA-3′ (forward), 5′-CTG CCT TGC TAG GTT AGG GAA-3′ (reverse); and cyclin-dependent kinase inhibitor 1A, primer sequence: 5′-CTG CCC AAG CTC TAC CTT CC-3′ (forward), 5′-CCC GCA GTA TCT TGC CTC C-3′ (reverse) were determined relative to that in the reference sample—in our case, Human Spleen Total RNA (Ambion, Life Technologies, Grand Island, NY, USA). β-Actin, primer sequence: 5′-TTC CTG GGG ATG GAG TC-3′ (forward), 5′-CAG GTC TTT GCG GAT GTC-3′ (reverse) was selected as an added internal control for the amount of cDNA in each assay.
Apoptosis induction and caspase-3 assay
Intracellular caspase-3 staining was performed as described previously [7]. Briefly, after incubation in the presence or absence of 20 μM CdCl2 (Sigma-Aldrich, St. Louis, MO, USA), an established apoptosis inducer, for 16 h, 106 cells (total PBMC or enriched monocytes) were stained with CD14-allophycocyanin (BD Biosciences, San Jose, CA, USA) and 7-AAD (BD Biosciences) and incubated for 15 min at room temperature. Cells were then fixed and permeabilized using BD Cytofix/Cytoperm permeabilization solution (BD Biosciences) and stained with intracellular FITC-active caspase-3 antibody (BD Biosciences) for 30 min at 4°C. PBMCs (100,000) or 25,000 monocytes were acquired on a CyAn flow cytometer (Dako, Carpinteria, CA, USA) and analyzed for active caspase-3 expression.
Intracellular flow cytometry measurements
For the detection of constitutive levels of p53, Rb1, and p21, 1 million cells were stained with surface antibodies inclusive of CD14. Cells were washed once with FACS buffer (1× PBS with 0.1% BSA and 0.02% NaN3) and fixed using 1 ml 1× BD FACS lysing solution (BD Biosciences) for 10 min at room temperature. Cells were then permeabilized using 1 ml Perm III buffer (BD Biosciences) and incubated for 30 min on ice. Cells were subsequently divided and incubated with antibodies against p53 (BD Biosciences), p53ser15 (Cell Signaling Technology, Danvers, MA, USA), underphosphorylated Rb1 (BD Biosciences), or p21 (Calbiochem, Gibbstown, NJ, USA). All antibodies were titrated to determine the appropriate saturating concentration. Isotype-matched, nonreactive mAb were used in each staining experiment to determine gates for positive events in monocyte subsets in all experiments. A total of 100,000 events was acquired and analyzed on a CyAn flow cytometer (Dako).
Western blot
Whole cell extracts were prepared by adding 100 μl RIPA lysis buffer (Pierce Biotechnology, Rockford, IL, USA) to isolated monocytes. Cells were incubated at 4°C for 30 min. Precleared lysates were mixed with 4× SDS loading buffer containing 1 μM DTT and heated to 100°C for 5 min. Lysates were resolved on precast 4–15% Tris-HCl gels (Bio-Rad Laboratories), and proteins were subsequently transferred to PVDF membranes (Bio-Rad Laboratories), blocked with 1% BSA in PBS with 0.02% v/v Tween-20, and detected with primary antibodies: goat anti-human p21 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-human Rb1 (Santa Cruz Biotechnology), mouse anti-human PAI-1 (BD Biosciences), rabbit polyclonal to PUMA (Abcam, Cambridge, MA, USA), LC3A/B antibody (Cell Signaling Technology), or rabbit polyclonal to Beclin (Cell Signaling Technology). Secondary HRP-conjugated anti-mouse IgG (BD Biosciences) or anti-rabbit IgG (Cell Signaling Technology) was used, respectively. Blots were developed using the ECL Plus kit (GE Healthcare Life Sciences, Piscataway, NJ, USA). Anti-β-actin antibody (Santa Cruz Biotechnology) was used for normalization. Band intensity was quantified using the LAS-3000 luminescent imaging system (Fujifilm, Valhalla, NY, USA). For reprobing when indicated, blots were stripped with Restore stripping buffer (Pierce Biotechnology) for 15 min at room temperature and reblocked for 1 h.
siRNA transfection
Monocyte transfections were conducted as described previously [31]. Briefly, dsRNA SMARTpool oligonucleotides, purchased from Dharmacon (Lafayette, CO, USA), were suspended at a final concentration of 1 μg/μl in Tris-EDTA buffer. Transfections were performed with a nucleofector device using the Amaxa human monocyte nucleofector kit (Lonza, Walkersville, MD, USA). Freshly isolated monocytes were suspended in human monocyte nucleofector solution at a final concentration of 10 × 106 cells/100 μl, mixed with 5 μg siRNA, and transferred into Amaxa-certified cuvettes. Cells were pulsed using the Y-01 or Y-001 program of the Amaxa nucleofector and immediately added to 1 ml prewarmed media using pipettes provided in the Amaxa kit. The transfected monocytes were allowed to recover for 1 h in a humidified 37°C/5% CO2 chamber and subsequently incubated in the presence or absence of BAL or JAGO (150 ng p24 antigen) for 36 h before being analyzed for caspase-3 activation following CdCl2 stimulation. Knockdown efficiency was confirmed by real-time RT-PCR and Western blot.
Statistical analysis
Variable distributions were analyzed using nonparametric tests as a result of a non-normality distribution of variables. Between-group comparisons were performed using the Kruskal-Wallis test to compare variables among the groups, and if significant, Wilcoxon rank-sum test is then applied to detect the difference between groups. Correlation analysis was done using the Spearman correlation test. All statistical analyses were performed using R, version 2.10.0 (Institute for Statistics and Mathematics, Vienna, Austria). A P value of <0.05 (see brackets) was considered significant.
RESULTS
Circulating monocytes from chronically HIV-infected patients are resistant to apoptosis and show Rb1 and p21 activation
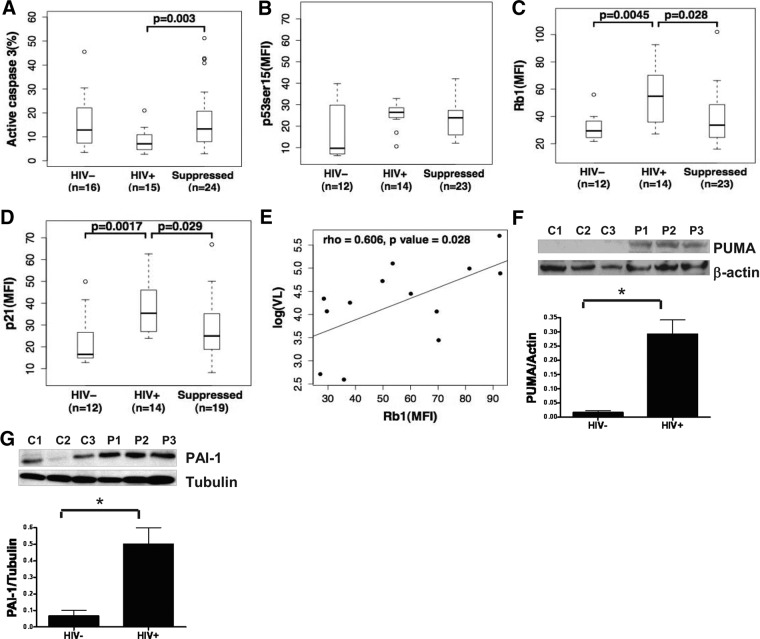
To validate our previous data showing a resistance to apoptosis, together with overexpression of p53-associated genes that are known regulators of apoptosis [7], we sought to determine whether p53 protein expression and activation were increased in circulating monocytes from untreated viremic HIV-infected individuals concurrently with an observed apoptosis resistance as measured ex vivo. We compared activation-induced apoptosis in monocytes isolated from untreated viremic HIV-infected individuals with those from uninfected controls. As shown in Fig. 1A for this cohort and consistent with our prior observations, monocytes from viremic HIV-infected subjects showed resistance to apoptotic stimulation. Interestingly, monocytes from the same donor group showed increased expression of p53ser15, with significantly higher levels of hypophosphorylated (active) Rb1 and p21 by multicolor flow cytometry (Fig. 1B–D). Furthermore, we also measured protein expression for p53-associated proteins PUMA and PAI-1, known to impact apoptosis regulation, and observed that both were up-regulated in monocytes from viremic HIV-infected donors when compared with monocytes from uninfected control subjects (Fig. 1F and G). As a result of the strong, positive correlation observed between Rb1 and log10 VL (Fig. 1E), we hypothesized that Rb1 expression would be decreased in the presence of ART unless activated by therapy itself. We found that ART-treated subjects showed significantly increased monocyte sensitivity to apoptosis (Fig. 1A), while also significantly reducing monocyte levels of Rb1 and p21 expression (Fig. 1C and D). Interestingly, we observed an increase in apoptosis sensitivity in conjunction with retention of p53ser15 expression in ART-treated subjects (Fig. 1B), which is consistent with antiapoptosis effects associated with p21 and Rb1, independently of factors that may be up-regulating p53ser15 in the presence of viral suppression. Taken together, our findings stress that in chronically viremic, asymptomatic HIV-infected individuals with CD4 counts above 200 cells/mm3, p53-mediated changes in monocyte gene expression are associated with VL and may contribute to apoptosis resistance in association with an increase in Rb1.
Figure 1. Circulating monocytes from HIV-infected patients are resistant to apoptosis and show change in p53 activation, Rb1, and p21.
(A) Monocytes obtained from HIV− (n=16) and HIV+ (n=15) and suppressed (n=24) donors incubated with 20 μM CdCl2 overnight (16 h). Apoptosis induction was determined by using flow cytometry to detect active caspase-3. p53ser15 (B), Rb1 (C), and p21 (D) expression in CD14+ monocytes from HIV− donors (n=12) as compared with HIV+ donors (n=14) and suppressed donors (n=23). MFI, Mean fluorescence intensity. (E) Scatter plot images with Rb1 expression levels in CD14+ cells are shown plotted against each donor's log10 VL. Rb1 levels correlated positively with VL. Pearson's correlation coefficient and P value are listed. Monocytes were enriched from PBMCs from HIV− (C; n=6) and HIV+ donors (P; n=6) and lysed, and PUMA (F) and PAI-1 (G) were detected by Western blotting. Data shown are representative of six viremic donors tested. *denotes P < 0.05.
CCR5 but not CXCR4 signaling activates an antiapoptotic monocyte response in association with induced changes in p53, p21, and Rb1
The finding that signaling through CCR5, following receptor engagement by R5 HIV or natural CCR5 ligands (RANTES, MIP-1β, or MIP-1α), was sufficient to protect cells from CdCl2-induced apoptosis [7] raised the question as to whether signaling through CXCR4 might also be associated with an enrichment of p53-associated gene expression and more significantly, elevated Rb1. We therefore addressed the role of CCR5 or CXCR4 in monocytes isolated from healthy control donors exposed in vitro for 36 h to R5 HIV, RANTES, X4 HIV, or SDF-1α prior to apoptosis induction with CdCl2. Results showed that caspase-3 activation was significantly lower in R5 HIV-exposed monocytes when compared with X4 HIV-exposed (Fig. 2A and B) or SDF-1α-treated monocytes (data not shown). Treatment of monocytes with R5 HIV-1 or X4 HIV-1 resulted in down-regulation of CCR5 and CXCR4, respectively (Fig. 2C and D). This observation reinforces the interpretation that CCR5 and CXCR4 chemokine receptors are down-regulated after exposure to HIV-1 R5 or X4, respectively, yet only HIV-1 R5 results in a reduction of induced apoptosis frequency. Having confirmed that the antiapoptotic effect of HIV-1 through coreceptor binding is restricted to R5 viruses, we next measured the extent to which R5 HIV exposure might regulate p53 gene expression, as well as intracellular levels of p53-associated proteins p21 and Rb1. Having established that CXCR4 engagement had no effect on p53ser15 activation (data not shown), we focused on treatment of monocytes with R5 HIV or MIP-1β, showing a clear induction of p53 (Fig. 3A) and Rb1 (Fig. 3B) gene expression concurrently with higher p21 and Rb1 protein expression (Fig. 3C). Of interest, a more potent effect was observed in the modulation of gene and protein expression by 50 ng/ml HIV R5 isolate exposure than by 1 μg/ml MIP-1β exposure, suggesting that particle membrane stability, as a result of binding or CD4 ligation, may potentiate effects otherwise observed by using soluble host CCR5 ligands. We confirmed the role of CCR5 in mediating these changes by the reversal of protein expression for p21 (Fig. 3D) when cells were treated with R5 HIV in the presence of Maraviroc, a small molecule CCR5 inhibitor. Taken together, these data support a role for CCR5 ligation by HIV-1 exposure or immune activation that can mediate the induction of p53 gene expression and Rb1 activation as corroborated by the positive association between monocyte Rb1 activation and VL described above.
Figure 2. CCR5 but not CXCR4 signaling activates an antiapoptotic monocyte response.

Freshly isolated monocytes from 12 healthy HIV− donors were incubated at 37°C/5% CO2 in a humidified chamber in the presence or absence of infectious 50 ng/ml R5 HIV-1 (A) or 50 ng/ml X4 HIV-1 (B). Cells were harvested after 36 h and challenged with 100 μM CdCl2 for 5 h. Apoptosis was measured by flow cytometry using an antibody to detect active caspase-3. 7-AAD was used for dead cell exclusion. P values for paired analysis are shown. Effects of X4 HIV-1 (C) and R5 HIV-1 (D) on CXCR4 and CCR5 expression, respectively.
Figure 3. CCR5 engagement modulates Rb1 activation and induces an autophagic response in monocytes.

Monocytes were incubated in the presence or absence of 50 ng/ml R5 HIV-1 or 1 μg/ml MIP-1β and harvested after 36 h for real-time RT-PCR analysis of total RNA performed with p53-specific (A) and Rb1-specific (B) primers, and data were normalized to β-actin. The experiment was repeated three times. Whole cell lysates were prepared and analyzed by SDS-PAGE, followed by immunoblotting with anti-Rb1 and anti-p21 antibody (C). (D) Monocytes were pretreated with (right panel) or without (left panel) 15 μM Maraviroc (MRV) and then exposed to R5 HIV-1. p21 expression was detected after 36 h and analyzed by flow cytometry (data representative of two separate experiments). (E) Cell lysates prepared as in C but probed with anti-LC3A/B antibody (left panel) or anti-Beclin antibody (right panel). β-Actin was used as a protein loading control. The experiment is repeated in three additional donors. *P < 0.05 (see brackets).
Rb1 overexpression in HIV-exposed monocytes induces autophagic responses
To further support a role for Rb1-mediated monocyte survival, we sought to determine whether the ability of Rb1 to positively regulate autophagy [27] might be corroborated independently in HIV-1-exposed monocytes. We compared the expression of the microtubule-associated protein LC3A/B (I/II) in monocytes exposed in vitro to R5 HIV or MIP-1β and observed an increased expression of LC3B(II) (Fig. 3E, left panel), while reconfirming a concurrent decreased sensitivity to apoptosis induction in the same donors (data not shown). Furthermore, Beclin, which is a critical activator of autophagy, whose overexpression correlates with protection against Sindbis virus-induced neuronal apoptosis [25], was also found to be up-regulated in R5 HIV or MIP-1β-exposed monocytes (Fig. 3E, right panel), although the levels of induction were not as high in all donors tested when combined as was observed with LC3B expression. Taken together, these observations support the involvement of Rb1 activation and its induced regulation of autophagy proteins as contributing to a state of greater monocyte survival following HIV-1 exposure.
Inhibition of Rb1 rescues sensitivity to apoptosis induction in otherwise resistant, HIV-1-exposed monocytes
Based on the described dominant role of Rb1 over p53-induced apoptosis effects [20, 21], the overexpression of activated Rb1 in association with in vivo viremia, and its induction following in vitro exposure to R5 HIV-1, we sought to provide direct evidence supporting the role of Rb1 on apoptosis regulation by use of RNA interference against the gene encoding Rb1. Monocytes were treated with Rb1 antisense oligonucleotides in vitro to suppress Rb1 expression in cells that were subsequently exposed to R5 HIV-1 and challenged for apoptosis induction. We confirmed suppression of Rb1 expression by real-time RT-PCR and Western blotting (Fig. 4A). Monocytes not exposed to R5 HIV-1 yet transfected with Rb1 antisense-specific oligonucleotides showed equal sensitivity to CdCl2-induced apoptosis as cells transfected with a nontargeting control siRNA (Fig. 4B), consistent with a lack of Rb1 activation in non-CCR5-ligated monocytes (data not shown). After coculture with R5 HIV-1, however, cells treated with Rb1 antisense oligonucleotides consistently showed a greater than threefold higher level of apoptosis as compared with cells that were transfected with a nontargeting siRNA control construct, which showed the expected trend toward a decrease in apoptosis sensitivity (Fig. 4B and C). Conversely to Rb1 inhibition increasing apoptosis sensitivity, a two- to threefold decrease in apoptosis was observed when monocytes were transfected with TP53-specific antisense oligonucleotides to knock down p53 expression in R5 HIV-1-unexposed and -exposed monocytes as compared with the siControl, supporting the presence of pro- and antiapoptotic mechanisms within monocytes (Fig. 4D). To further support TP53 inhibition as increasing monocyte survival, a similar increase in caspase-3 induction was observed upon monocyte exposure to PFT-α, a chemical inhibitor of p53 (Fig. 4D). Collectively, our data support the finding that CCR5-mediated signaling can cause monocyte apoptosis resistance during chronic HIV-1 viremia by up-regulating Rb1 in spite of a concurrent increase in proapoptotic p53.
Figure 4. Inhibition of Rb1 rescues sensitivity to apoptotic stimulation.
Monocytes from uninfected donors were treated with negative-control siRNA or Rb1 siRNA. Knockdown efficiency was confirmed by real-time RT-PCR and Western blot (A). (B) Cells with target or control siRNAs against Rb1 were then incubated in the absence (upper panels) or presence (lower panels) of R5 HIV-1 and subsequently challenged with 100 μM CdCl2 for 5 h. Apoptosis induction was determined by detecting active caspase 3 by flow cytometry. Results were reproduced in monocytes from five different donors (C). (D) Cells with siRNAs against TP53 (left) or treated in the absence or presence of PFT-α (right) were incubated with (lower panels) or without (upper panels) R5 HIV-1. Apoptosis induction was determined by detecting active caspase 3 by flow cytometry.
DISCUSSION
We present the first evidence of increased Rb1 expression in association with greater viability of monocytic cells following apoptosis induction during chronic HIV infection as a significant potential in vivo mechanism for monocyte/macrophage persistence during ongoing HIV replication. Our ex vivo observations on circulating monocytes and in vitro induction data following R5 HIV exposure of monocytes are consistent with the retinoblastoma tumor suppressor gene (Rb1) playing a role in the normal coordination of cell proliferation versus death, as reported previously [32], and with its ability to negatively regulate p53-mediated apoptosis [20, 21]. Indeed, inducible p53 in conjunction with Rb1 expression in monocytes following HIV-1 viremia may help explain the transcriptional program underlying an increase in cellular survival under conditions for stress and inflammation in vivo [33]. A role for Rb1 to protect against apoptosis is supported by several observations of ectopic expression of Rb1 in other cell systems acting to inhibit apoptosis triggered by p53 [20], radiation [34], E2F1 [35], myocyte differentiation [36], or ceramide [37]. Rb1 may also be a determinant of p21 activity, as induction of p53-mediated apoptosis is enhanced by a lack of p21 expression—p21 is an upstream regulator of Rb1 [36, 38]. Furthermore, reports that identify PAI-2 as an Rb1-binding protein that promotes the cytoprotective capacity of Rb1 by blocking Rb1 degradation, thus promoting the antiapoptotic activity of Rb1 [39], may indicate a similar role for the detected, increased levels of PAI-1 in monocytes where up-regulation of Rb1 and PAI-1 proteins is noted (Fig. 1). Our in vitro and ex vivo data strongly support a role for Rb1 in apoptotic resistance in HIV disease. While a clear induction of Rb1 was noted ex vivo in circulating monocytes from viremic subjects, as measured by intracellular staining, acute induction of Rb1 in monocytes exposed to R5 HIV in vitro showed variable baseline/induction levels supportive of induction but also indicative that chronic viremia may be a more uniform microenvironment for Rb1 expression in monocytes versus an acute in vitro model setting. Furthermore, it is acknowledged that a direct reversal of apoptosis resistance via Rb1 inhibition in monocytes from HIV-infected patients would be required to definitively support our conclusions. In addition, further analysis of the in vivo characteristics of predominant HIV-1 coreceptor use between CCR5 or CXCR4 could address whether Rb1 modulation can be selectively biased to coreceptor use or whether it is independent of additional host factor interactions with CCR5 (chemokine expression per immune activation).
In the framework of HIV disease, p53 has been identified as a key player in mediating the depletion of lymphoid cells with a major role for up-regulation of PUMA as a proapoptotic p53 target gene overexpressed in lymphocytes [8]. It is of interest to note that apoptosis induction was decreased upon siRNA targeting of TP53 and also following chemical inhibition of p53 transactivation by PFT-α (Fig. 4D), suggesting that although under “steady-state” viremia, monocytes have apoptosis-inducing (i.e., PUMA) and apoptosis-inhibiting mediators (i.e., p21/Rb1), Rb1 is having a dominant role in determining cell survival when all p53-induced proteins are expressed. Given the role of Rb1 as an antiapoptotic cofactor through its direct association with death-inducing proteins, such as p53 [20, 21] and p84N5 [40], we interpret that its function might involve a direct interaction with the proapoptotic p53. It also remains to be determined whether p53 or Rb1 might regulate downstream gene expression that would alter zinc homeostasis, which was identified recently as a mediator of the antiapoptosis response in HIV-exposed monocytes [41]. Data from subjects with viral suppression with ART showed that the p53 activation is still maintained, yet p21/Rb1 induction is decreased to suggest that either ART can induce p53 activation by independent mechanisms to lower CCR5 interaction or that reversal of p53 activation is less sensitive to viral suppression than the induction of p21/Rb1. The effects of ART suppression on decreasing Rb1 and p21 expression are consistent with their antiapoptotic role during viremia, as also evidenced in vitro. Further experiments are needed to determine whether apoptosis regulation during ART is associated with sustained p53ser15 and PUMA expression or a decrease in PAI-1 expression. Lastly, given the recent evidence in in vivo SIV disease that shows higher monocyte turnover occurring during end-stage disease [42], further investigation will be needed to define what changes in Rb1 expression may occur with advanced HIV disease progression (lower CD4 counts or presence of comorbidities), as our present study does not address this question.
While our data address the steady-state apoptosis sensitivity of circulating monocytes, several other antiapoptosis mediators have been described within infected macrophages associated with interactions with HIV-1 envelope [41, 43] and/or HIV accessory proteins Tat and Nef, known to interfere with the DNA-binding and -signaling functions of p53 [44, 45] or Vpr, a potent cell cycle arrest protein [46]. In addition to its housekeeping role, autophagy has been shown to prolong cell survival, particularly under conditions of stress [47]. Although our data suggest a strong, autophagic response to HIV-1 exposure that coincides with elevated Rb1 levels, further study will be required to establish whether the modulation of autophagy in HIV-1-exposed monocytes is a direct consequence of Rb1 activation in these cells.
In summary, our data support a model that HIV-exposed monocytes in HIV infection in vivo express p53-mediated apoptosis-inducing (i.e., PUMA) and apoptosis-inhibiting mechanisms (i.e., p21/Rb1) at the same time with Rb1, exerting a dominant role in determining cell survival when expressed (Fig. 5). The preconditioning of monocytes/macrophages to have greater survival capacity through CCR5 interactions may provide for a general R5 HIV-1 effect on the monocyte compartment that would be expected to support in vivo persistence of monocyte/macrophage innate inflammation and infected cell populations at the time of viral exposure/infection and during asymptomatic chronic infection.
Figure 5. Model for Rb1-mediated monocyte apoptosis regulation following CCR5 ligation.
Figure illustrating the possible relationship between HIV binding to monocyte surface and the functional outcome associated with Rb1 activation.
ACKNOWLEDGMENTS
This study was supported by U.S. National Institutes of Health grant A1047760, The Philadelphia Foundation, and The Wistar Institute funds from the Commonwealth Universal Research Enhancement Program, Pennsylvania Department of Health CURE Program, and Wistar Cancer Center support grant (P30 CA010815). We thank Dr. Fareeda Shaheen (Penn Center for AIDS Research), Wistar Genomics Facility, Jeffery Faust (Wistar Flow Cytometry Facility), James Hayden and Frederick Keeney (Wistar Microscopy Facility) for help with experiments, Wistar Phleobotomist Deborah Davis, Philadelphia FIGHT staff Kenneth Lynn and Craig Carty for blood-donor recruitment.
Footnotes
- 7-AAD
- 7-amino-actinomycin D
- ART
- antiretroviral therapy
- CdCl2
- cadmium chloride
- GEO
- Gene Expression Omnibus
- HIVR5
- CCR5-tropic HIV strain
- HIVX4
- CXCR4-tropic HIV strain
- IQR
- interquartile range
- LC3
- light chain 3
- p53ser15
- phospho-p53ser15
- PAI-1
- plasminogen activator inhibitor-1
- PFT-α
- pifithrin-α
- Philadelphia FIGHT
- Philadelphia Field Initiation Group for HIV Trials
- PUMA
- p53-up-regulated modulator of apoptosis
- R5
- CCR5-tropic
- Rb1
- retinoblastoma protein
- SDF
- stromal cell-derived factor
- siControl
- small interfering control
- siRNA
- small interfering RNA
- TP53
- tumor protein p53
- VL
- viral load
- X4
- CXCR4-tropic
AUTHORSHIP
B.G. designed and performed experiments and analyzed and interpreted data. A.D.R. contributed to preliminary experiments. X.Y. performed statistical analysis. J.K. and K.M. contributed clinical samples. R.G.C. contributed reagents. L.S. supervised the microarray project. L.J.M. assisted in the design of experiments and supervised the project. B.G. and L.J.M. wrote the manuscript.
REFERENCES
- 1. Banda N. K., Bernier J., Kurahara D. K., Kurrie R., Haigwood N., Sekaly R. P., Finkel T. H. (1992) Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J. Exp. Med. 176, 1099–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Holm G. H., Zhang C., Gorry P. R., Peden K., Schols D., De Clercq E., Gabuzda D. (2004) Apoptosis of bystander T cells induced by human immunodeficiency virus type 1 with increased envelope/receptor affinity and coreceptor binding site exposure. J. Virol. 78, 4541–4551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fauci A. S. (1988) The human immunodeficiency virus: infectivity and mechanisms of pathogenesis. Science 239, 617–622 [DOI] [PubMed] [Google Scholar]
- 4. Brown C. R., Czapiga M., Kabat J., Dang Q., Ourmanov I., Nishimura Y., Martin M. A., Hirsch V. M. (2007) Unique pathology in simian immunodeficiency virus-infected rapid progressor macaques is consistent with a pathogenesis distinct from that of classical AIDS. J. Virol. 81, 5594–5606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Igarashi T., Brown C., Endo Y., Buckler-White A., Plishka R., Bischofberger N., Hirsch V., Martin M. A. (2001) Macrophages are the principal reservoir and sustain high virus loads in rhesus macaques after the depltion of CD4+ T cells by a highly pathogenic simian immunodeficiency virus/HIV type 1 chimera (SHIV): implications for HIV-1 infections of humans. Proc. Natl. Acad. Sci. USA 98, 658–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Orenstein J. M., Fox C., Wahl S. M. (1997) Macrophages as a source of HIV during opportunistic infections. Science 276, 1857–1861 [DOI] [PubMed] [Google Scholar]
- 7. Giri M. S., Nebozyhn M., Raymond A., Gekonge B., Hancock A., Creer S., Yousef M., Foulkes A. S., Mounzer K., Shull J., Silvestri G., Kostman J., Collman R. G., Showe L., Montaner L. J. (2009) Circulating monocytes in HIV-1-infected viremic subjects exhibit an antiapoptosis gene signature and virus- and host-mediated apoptosis resistance. J. Immunol. 182, 4459–4470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perfettini J.-L., Roumier T., Castedo M., Larochette N., Boya P., Raynal B., Lazar V., Ciccosanti F., Nardacci R., Penninger J., Piacentini M., Kroemer G. (2004) NF-κB and p53 are the dominant apoptosis-inducing transcription factors elicited by the HIV-1 envelope. J. Exp. Med. 199, 629–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castedo M., Perfettini J.-L., Piacentini M., Kroemer G. (2005) p53—a pro-apoptotic signal transducer involved in AIDS. Biochem. Biophys. Res. Commun. 331, 701–706 [DOI] [PubMed] [Google Scholar]
- 10. Salomoni P., Cossarizza A. (2004) HIV: no PUMA no death? Cell Death Differ. 11, 691–692 [DOI] [PubMed] [Google Scholar]
- 11. Vazquez N., Greenwell-Wild T., Marinos N. J., Swaim W. D., Nares S., Ott D. E., Schubert U., Henklein P., Orenstein J. M., Sporn M. B., Wahl S. M. (2005) Human imunodeficiency virus type 1-induced macrophage gene expression includes the p21 gene, a target for viral regulation. J. Virol. 79, 4479–4491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coberley C. R., Kohler J. J., Brown J. N., Oshier J. T., Baker H. V., Popp M. P., Sleasman J. W., Goodenow M. M. (2004) Impact on genetic networks in human macrophages by a CCR5 strain of human immunodeficiency virus type 1. J. Virol. 78, 11477–11486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harrington E. A., Bruce J. L., Harlow E., Dyson N. (1998) pRB plays an essential role in cell cycle arrest induced by DNA damage. Proc. Natl. Acad. Sci. USA 95, 11945–11950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goodrich D. W., Wang N. P., Qian Y. W., Lee E. Y.-H.P., Lee W. H. (1991) The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle. Cell 67, 293–302 [DOI] [PubMed] [Google Scholar]
- 15. Bagchi S., Weinmann R., Raychaudhuri P. (1991) The retinoblastoma protein copurifies with E2F-1, an E1A-regulated inhibitor of the transcription factor E2F. Cell 65, 1063–1072 [DOI] [PubMed] [Google Scholar]
- 16. Flemington E. K., Speck S. H., Kaelin W. G., Jr., (1993) E2F-1-mediated transactivation is inhibited by complex formation with the retinoblastoma susceptibility gene product. Proc. Natl. Acad. Sci. USA 90, 6914–6918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weintraub S. J., Prater C. A., Dean D. C. (1992) Retinoblastoma protein switches the E2F site from positive to negative element. Nature 358, 259–261 [DOI] [PubMed] [Google Scholar]
- 18. Classon M., Harlow E. (2002) The retinoblastoma tumor suppressor in development and cancer. Nat. Rev. Cancer 2, 910–917 [DOI] [PubMed] [Google Scholar]
- 19. Lui H., Dibling B., Spike B., Dirlam A., Macleod K. (2004) New roles for the RB tumor suppressor protein. Curr. Opin. Genet. Dev. 14, 55–64 [DOI] [PubMed] [Google Scholar]
- 20. Haupt Y., Rowan S., Oren M. (1995) p53-mediated apoptosis in HeLa cells can be overcome by excess pRB. Oncogene 10, 1563–1571 [PubMed] [Google Scholar]
- 21. Shinohara H., Zhou J., Yoshikawa K., Yazumi S., Ko K., Yamaoka Y., Mizukami T., Yoshida T., Akinaga S., Tamaoki T., Motoda H., Benedict W. F., Takahashi R. (2000) Retinoblastoma protein-initiated cellular growth arrest overcomes the ability of cotransfected wild-type p53 to induce apoptosis. Br. J. Cancer 83, 1039–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wirawan E., Vanden Berghe T., Lippens S., Agostinis P., Vandenabeele P. (2012) Autophagy: for better or for worse. Cell Res. 22, 43–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moreau K., Luo S., Rubinsztein D. C. (2010) Cytoprotective roles for autophagy. Curr. Opin. Cell Biol. 22, 206–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Clarke P. G. (1990) Developmental cell death: morphological diversity and multiple mechanisms. Anat. Embryol. (Berl) 181, 195–213 [DOI] [PubMed] [Google Scholar]
- 25. Liang X. H., Kleeman L. K., Jiang H. H., Gordon G., Goldman J. E., Berry G., Herman B., Levine B. (1998) Protection against fatal Sindbis virus encephalitis by Beclin, a novel Bcl-2-interacting protein. J. Virol. 72, 8586–8596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Duprez L., Wirawan E., Vanden Berghe T., Vandenabeele P. (2009) Major cell death pathways at a glance. Microbes Infect. 11, 1050–1062 [DOI] [PubMed] [Google Scholar]
- 27. Jiang H., Martin V., Gomez-Manzano C., Johnson D.G., Alonso M., White E., Xu J., McDonnell T.J., Shinojima N., Fueyo J. (2010) The RB-E2F1 pathway regulates autophagy. Cancer Res. 70, 7882–7893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bhutia S. K., Kegelman T. P., Das S. K., Azab B., Su Z.-Z., Lee S.-G., Sarkar D., Fisher P. B. (2010) Astrocyte elevated gene-1 induces protective autophagy. Proc. Natl. Acad. Sci. USA 107, 22243–22248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levine B., Kroemer G. (2008) Autophagy in the pathogenesis of disease. Cell 132, 27–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang L., Yu Y., Kang R., Yang M., Xie M., Wang Z., Tang D., Zhao M., Liu L., Zhang H., Cao L. (2012) Up-regulated autophagy by endogenous HMGB1 promotes chemoresistance in leukemia cells. Leuk. Lymphoma 53, 315–322 [DOI] [PubMed] [Google Scholar]
- 31. Malik M., Chen Y.-Y., Kienzle M. F., Tomkowicz B. E., Collman R. G., Ptasznik A. (2008) Monocyte migration and LFA-1-mediated attachment to brain microvascular endothelia is regulated by SDF-1 α through Lyn kinase. J. Immunol. 181, 4632–4637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goodrich D. W., Lee W. H. (1993) Molecular characterization of the retinoblastoma susceptiblity gene. Biochim. Biophys. Acta 1155, 43–61 [DOI] [PubMed] [Google Scholar]
- 33. Chau B., Wang J. Y. (2003) Coordinated regulation of life and death by RB. Nat. Rev. Cancer 3, 130–138 [DOI] [PubMed] [Google Scholar]
- 34. Haas-Kogan D. A., Kogan S. C., Levi D., Dazin P., T'Ang A., Fung Y. K., Israel M. A. (1995) Inhibition of apoptosis by the retinoblastoma gene product. EMBO J. 14, 461–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hsieh J. K., Fredersdorf S., Kouzarides T., Martin K., Lu X. (1997) E2F1-induced apoptosis requires DNA binding but not transactivation and is inhibited by the retinoblastoma protein through direct interaction. Genes Dev. 11, 1840–1852 [DOI] [PubMed] [Google Scholar]
- 36. Wang J., Guo K., Wills K. N., Walsh K. (1997) Rb functions to inhibit apoptosis during myocyte differentiation. Cancer Res. 57, 351–354 [PubMed] [Google Scholar]
- 37. McConkey D. J., Goodrich D., Bucana C., Klostergaard J. (1996) The human retinoblastoma gene product suppresses ceramide-induced apoptosis in human bladder tumor cells. Oncogene 13, 1693–1700 [PubMed] [Google Scholar]
- 38. Waldman T., Lengauer C., Kinzler K. W., Vogelstein B. (1996) Uncoupling of S phase and mitosis induced by anticancer agents in cells lacking p21. Nature 381, 713–716 [DOI] [PubMed] [Google Scholar]
- 39. Tonnetti L., Netzel-Arnett S., Darnell G. A., Hayes T., Buzza M. S., Anglin I. E., Suhrbier A., Antalis T. M. (2008) Serpin B2 protection of retinoblastoma protein from calpain enhances tumor cell survival. Cancer Res. 68, 5648–5657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Doostzadeh-Cizeron J., Evans R., Yin S., Goodrich D. W. (1999) Apoptosis induced by the nuclear death domain protein p84N5 is inhibited by association with Rb protein. Mol. Biol. Cell 10, 3251–3261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Raymond A. D., Gekonge B., Giri M. S., Hancock A., Papasavvas E., Chehimi J., Kossenkov A. V., Nicols C., Yousef M., Mounzer K., Shull J., Kostman J., Showe L., Montaner L. J. (2010) Increased metallothionein gene expression, zinc, and zinc-dependent resistance to apoptosis in circulating monocytes during HIV viremia. J. Leukoc. Biol. 88, 589–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hasegawa A., Liu H., Ling B., Borda J. T., Alvarez X., Sugimoto C., Vinet-Oliphant H., Kim W.-K., Williams K. C., Ribiero R. M., Lackner A. A., Veazey R. S., Kuroda M. J. (2009) The level of monocyte turnover predicts disease progression in the macaque model of AIDS. Blood 114, 2917–2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Swingler S., Mann A., Zhou J., Swingler C., Stevenson M. (2007) Apoptotic killing of HIV-1-infected macrophages is subverted by the viral envelope glycoprotein. PLoS Pathog. 3, 1281–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Greenway A. L., McPhee D. A., Allen K., Johnstone R., Holloway G., Mills J., Azad A., Sankovich S., Lambert P. (2002) Human immunodeficiency virus type 1 Nef binds to tumor suppressor p53 and protects cells against p53-mediated apoptosis. J. Virol. 76, 2692–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Choi H.-J., Smithgall T. E. (2004) HIV-1 Nef promotes survival of TF-1 macrophages by inducing Bcl-Xl expression in an extracellular signal-regulated kinase-dependent manner. J. Biol. Chem. 279, 51688–51696 [DOI] [PubMed] [Google Scholar]
- 46. Amini S., Khalili K., Sawaya B. E. (2004) Effect of HIV-1 Vpr on cell cycle regulators. DNA Cell Biol. 23, 249–260 [DOI] [PubMed] [Google Scholar]
- 47. Mizushima N., Levine B., Cuervo A. M., Klionsky D. J. (2008) Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]