Abstract
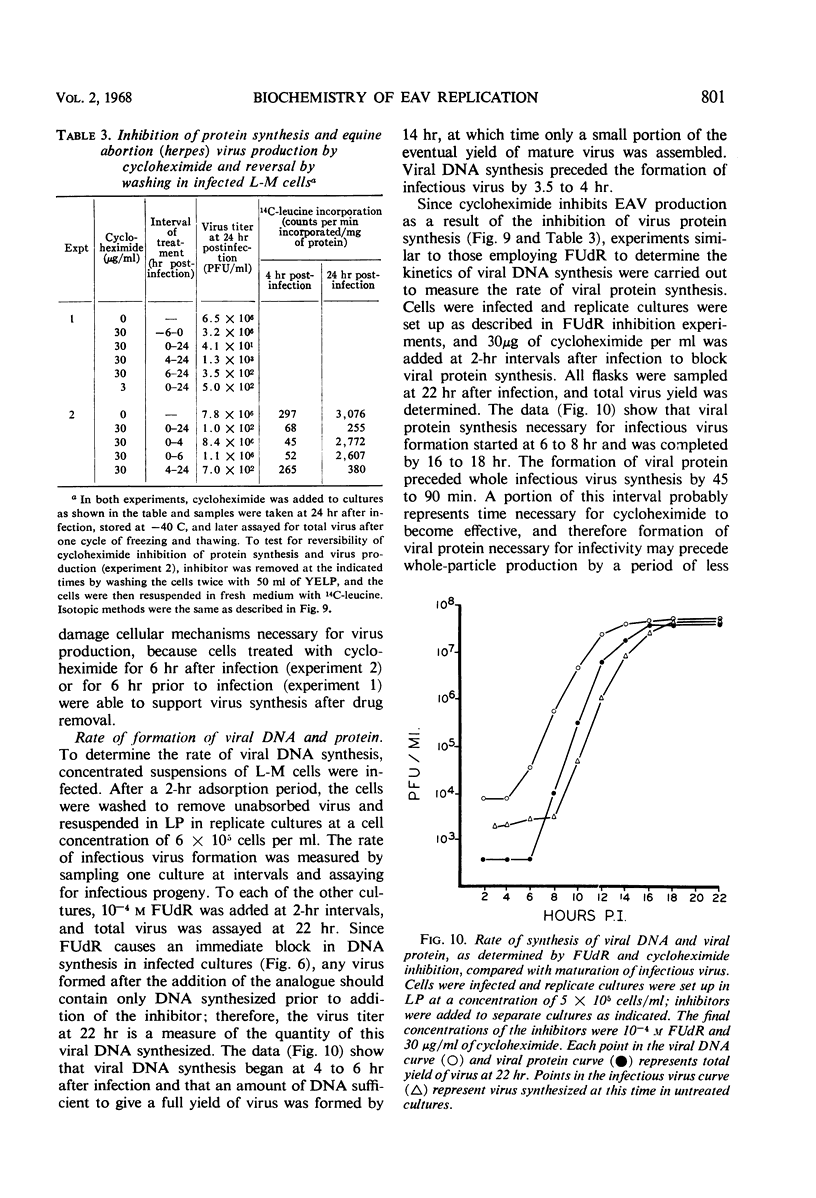
Infection of exponential-phase suspension cultures of mouse fibroblast cells (L-M) with equine abortion virus (EAV) resulted in inhibition of cell growth and marked alterations in host metabolic processes. The synthesis of deoxyribonucleic acid (DNA) and ribonucleic acid was inhibited within 4 hr after infection and was suppressed by more than 90% by the time of maximal virus replication (14 to 18 hr). The overall rate of protein synthesis, however, was similar in uninfected and virus-producing cells as determined by measurements of net protein and isotope incorporation. The time course of viral DNA and protein synthesis and assembly into mature virus was determined with the inhibitors 5-fluorodeoxyuridine (FUdR) and cycloheximide, respectively. Thus, viral DNA synthesis was essentially completed at 14 hr, and viral protein and infectious virus synthesis was completed at 18 hr. Although the number of plaque-forming units (PFU) produced by FUdR-treated cells (103 to 104 PFU/ml) was at least 3 logs less than that produced by untreated cells, the yield of physical particles (as determined by electron microscopy) was approximately the same at 30 hr after infection. Besides being relatively non-infective, the particles produced in FUdR-treated cells appeared morphologically incomplete as they contained little or no nucleoid material.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARHELGER R. B., DARLINGTON R. W., RANDALL C. C. An electron microscopic study of equine abortion virus infection in hamster liver. Am J Pathol. 1963 Jun;42:703–713. [PMC free article] [PubMed] [Google Scholar]
- BEN-PORAT T., KAPLAN A. S. The synthesis and fate of pseudorabies virus DNA in infected mammalian cells in the stationary phase of growth. Virology. 1963 Jun;20:310–317. doi: 10.1016/0042-6822(63)90120-x. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello L. J., Ginsberg H. S. Inhibition of host protein synthesis in type 5 adenovirus-infected cells. J Virol. 1967 Oct;1(5):843–850. doi: 10.1128/jvi.1.5.843-850.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S., Flaks J. G., Barner H. D., Loeb M. R., Lichtenstein J. THE MODE OF ACTION OF 5-FLUOROURACIL AND ITS DERIVATIVES. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1004–1012. doi: 10.1073/pnas.44.10.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DARLINGTON R. W., RANDALL C. C. The nucleic acid content of equine abortion virus. Virology. 1963 Mar;19:322–327. doi: 10.1016/0042-6822(63)90071-0. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953 Oct;204(2):983–997. [PubMed] [Google Scholar]
- Darlington R. W., James C. Biological and morphological aspects of the growth of equine abortion virus. J Bacteriol. 1966 Jul;92(1):250–257. doi: 10.1128/jb.92.1.250-257.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington R. W., Moss L. H., 3rd Herpesvirus envelopment. J Virol. 1968 Jan;2(1):48–55. doi: 10.1128/jvi.2.1.48-55.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J. R., Ramsey F. K., Switzer W. P. Electron microscopy of cytomegalic inclusion disease of swine (inclusion body rhinitis). Am J Vet Res. 1965 Jul;26(113):939–947. [PubMed] [Google Scholar]
- GENTRY G. A., LAWSON L. A., RANDALL C. C. REPLICATION OF A DEOXYRIBONUCLEIC ACID VIRUS IN THYMINE-DEFICIENT MAMMALIAN CELLS. J Bacteriol. 1964 Nov;88:1324–1328. doi: 10.1128/jb.88.5.1324-1328.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GENTRY G. A., MORSE P. A., Jr, IVES D. H., GEBERT R., POTTER V. R. PYRIMIDINE METABOLISM IN TISSUE CULTURE CELLS DERIVED FROM RAT HEPATOMAS. II. THYMIDINE UPTAKE IN SUSPENSION CULTURES DERIVED FROM THE NOVIKOFF HEPATOMA. Cancer Res. 1965 May;25:509–516. [PubMed] [Google Scholar]
- Green M. Biosynthetic modifications induced by DNA animal viruses. Annu Rev Microbiol. 1966;20:189–222. doi: 10.1146/annurev.mi.20.100166.001201. [DOI] [PubMed] [Google Scholar]
- HAFF R. F. INHIBITION OF THE MULTIPLICATION OF PSEUDORABIES VIRUS BY CYCLOHEXAMIDE. Virology. 1964 Mar;22:430–431. doi: 10.1016/0042-6822(64)90036-4. [DOI] [PubMed] [Google Scholar]
- HAMADA C., KAPLAN A. S. KINETICS OF SYNTHESIS OF VARIOUS TYPES OF ANTIGENIC PROTEINS IN CELLS INFECTED WITH PSEUDORABIES VIRUS. J Bacteriol. 1965 May;89:1328–1334. doi: 10.1128/jb.89.5.1328-1334.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLMES I. H., WATSON D. H. AN ELECTRON MICROSCOPE STUDY OF THE ATTACHMENT AND PENETRATION OF HERPES VIRUS IN BHK21 CELLS. Virology. 1963 Sep;21:112–123. doi: 10.1016/0042-6822(63)90309-x. [DOI] [PubMed] [Google Scholar]
- HURLBERT R. B., POTTER V. R. A survey of the metabolism of orotic acid in the rat. J Biol Chem. 1952 Mar;195(1):257–270. [PubMed] [Google Scholar]
- Hyde J. M., Walkinshaw C. H. Ultrastructure of basidiospores and mycelium of Lenzites saepiaria. J Bacteriol. 1966 Oct;92(4):1218–1227. doi: 10.1128/jb.92.4.1218-1227.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Messenger RNA synthesis by a "coated" viral genome. Proc Natl Acad Sci U S A. 1967 Feb;57(2):314–320. doi: 10.1073/pnas.57.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOH P. C., PAYNE F. E. EFFECT OF 5-FLUORO-2'-DEOXYURIDINE ON THE SYNTHESIS OF VACCINIA VIRUS. Virology. 1965 Apr;25:575–584. doi: 10.1016/0042-6822(65)90085-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McCormick W., Penman S. Inhibition of RNA synthesis in HeLa and L cells by Mengovirus. Virology. 1967 Jan;31(1):135–141. doi: 10.1016/0042-6822(67)90017-7. [DOI] [PubMed] [Google Scholar]
- PLUMMER G., WATERSON A. P. Equine herpes viruses. Virology. 1963 Mar;19:412–416. doi: 10.1016/0042-6822(63)90083-7. [DOI] [PubMed] [Google Scholar]
- POLASA H., GREEN M. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION. 8. ANALYSIS OF PROTEIN SYNTHESIS. Virology. 1965 Jan;25:68–79. doi: 10.1016/0042-6822(65)90253-9. [DOI] [PubMed] [Google Scholar]
- RANDALL C. C. Adaptation of equine abortion virus to HeLa cells. Proc Soc Exp Biol Med. 1957 Jul;95(3):508–510. doi: 10.3181/00379727-95-23270. [DOI] [PubMed] [Google Scholar]
- RANDALL C. C., LAWSON L. A. Adaptation of equine abortion virus to Earle's L cells in serum-free medium with plaque formation. Proc Soc Exp Biol Med. 1962 Jul;110:487–489. doi: 10.3181/00379727-110-27558. [DOI] [PubMed] [Google Scholar]
- RANDALL C. C., WALKER B. M. DEGRADATION OF DEOXYRIBONUCLEIC ACID AND ALTERATION NUCLEIC ACID METABOLISM IN SUSPENSION CULTURES OF L-M CELLS INFECTED WITH EQUINE ABORTION VIRUS. J Bacteriol. 1963 Jul;86:138–146. doi: 10.1128/jb.86.1.138-146.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAZIN S., KNYSZYNSKI A., LIFSHITZ Y. NUCLEASES OF MYCOPLASMA. J Gen Microbiol. 1964 Aug;36:323–332. doi: 10.1099/00221287-36-2-323. [DOI] [PubMed] [Google Scholar]
- REISSIG M., KAPLAN A. S. The morphology of noninfective pseudorabies virus produced by cells treated with 5-fluorouracil. Virology. 1962 Jan;16:1–8. doi: 10.1016/0042-6822(62)90196-4. [DOI] [PubMed] [Google Scholar]
- Randall C. C., Gafford L. G., Gentry G. A., Lawson L. A. Lability of host-cell DNA in growing cell cultures due to Mycoplasma. Science. 1965 Sep 3;149(3688):1098–1099. doi: 10.1126/science.149.3688.1098. [DOI] [PubMed] [Google Scholar]
- SALZMAN N. P., SHATKIN A. J., SEBRING E. D. Viral protein and DNA synthesis in vaccinia virus-infected HeLacell cultures. Virology. 1963 Apr;19:542–550. doi: 10.1016/0042-6822(63)90049-7. [DOI] [PubMed] [Google Scholar]
- SALZMAN N. P. The rate of formation of vaccinia deoxyribonucleic acid and vaccinia virus. Virology. 1960 Jan;10:150–152. doi: 10.1016/0042-6822(60)90015-5. [DOI] [PubMed] [Google Scholar]
- SHATKIN A. J. The formation of vaccinia virus protein in the presence of 5-fluorodeoxyuridine. Virology. 1963 Jun;20:292–301. doi: 10.1016/0042-6822(63)90118-1. [DOI] [PubMed] [Google Scholar]
- SOEHNER R. L., GENTRY G. A., RANDALL C. C. SOME PHYSICOCHEMICAL CHARACTERISTICS OF EQUINE ABORTION VIRUS NUCLEIC ACID. Virology. 1965 Jul;26:394–405. doi: 10.1016/0042-6822(65)90003-6. [DOI] [PubMed] [Google Scholar]
- WILLIAMS R. C., KASS S. J., KNIGHT C. A. Structure of Shope papilloma virus particles. Virology. 1960 Sep;12:48–58. doi: 10.1016/0042-6822(60)90148-3. [DOI] [PubMed] [Google Scholar]
- Watanabe Y., Kudo H., Graham A. F. Selective inhibition of reovirus ribonucleic acid synthesis by cycloheximide. J Virol. 1967 Feb;1(1):36–44. doi: 10.1128/jvi.1.1.36-44.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems M., Penman S. The mechanism of host cell protein synthesis inhibition by poliovirus. Virology. 1966 Nov;30(3):355–367. doi: 10.1016/0042-6822(66)90114-0. [DOI] [PubMed] [Google Scholar]





