Abstract
Bacterial therapies possess many unique mechanisms for treating cancer that are unachievable with standard methods. Bacteria can specifically target tumors, actively penetrate tissue, are easily detected and can controllably induce cytotoxicity. Over that last decade, Salmonella, Clostridium and other genera have been shown to control tumor growth and promote survival in animal models. In this Innovation article I propose that synthetic biology techniques can be used to solve many of the key challenges associated with bacterial therapies such as toxicity, stability and efficiency; and can be used to tune their beneficial features, allowing the engineering of ‘perfect’ cancer therapies.
Introduction
Bacteria have unique capabilities that make them well-suited as ‘perfect’ anticancer agents. Because their genetics can be easily manipulated, bacteria can be engineered to overcome the limitations that hamper current cancer therapies. Many current treatments, including chemotherapy and radiation, are toxic to normal tissue and cannot completely destroy all cancer cells1. Three major causes of these problems are incomplete tumor targeting, inadequate tissue penetration and limited toxicity to all cancer cells1–3. These drawbacks prevent effectual treatment and are associated with increased morbidity and mortality.
Using a top-down engineering approach, the ideal cancer therapy can be envisioned: it would be tiny programmable robot factories (Figure 1A) that specifically target tumors, are selectively cytotoxic to cancer cells, are self-propelled, are responsive to external signals, can sense the local environmental and are externally detectable. Specific targeting would permit the use of more toxic molecules without systemic effects. Self-propulsion would enable penetration into tumor regions that are inaccessible to passive therapies. Responsiveness to external signals would enable precise control of the location and timing of cytotoxicity. Sensing the local environment would permit “smart,” responsive therapies that can make decisions about where and when drugs are administered. Finally, the ability to be externally detected would provide critical information about the state of the tumor, the success of localization and the efficacy of treatment.
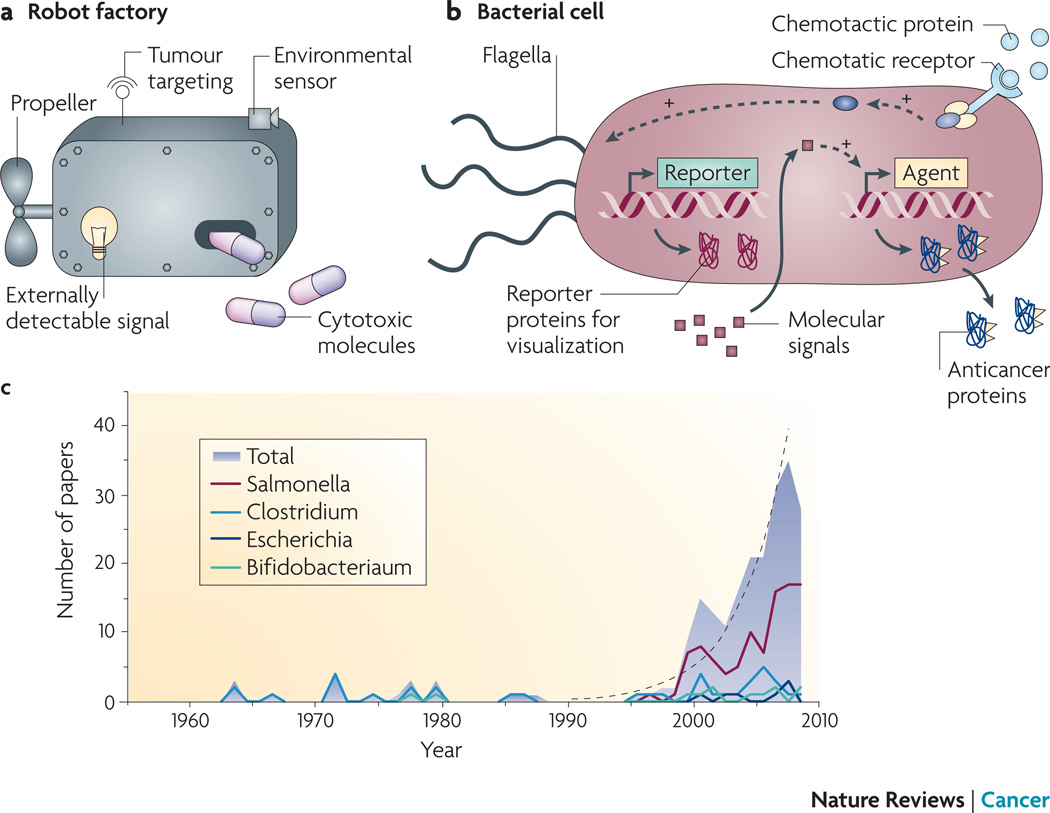
Figure 1. Bacteria are the optimal robot factory cancer therapies.
A) The perfect cancer therapy would be able to perform six important functions: target tumors, produce cytotoxic molecules, self-propel, respond to triggering signals, sense the local environment and produce externally detectable signals. B) Bacteria have biological mechanisms to perform these functions: gene translation machinery to produce anticancer proteins (green); flagella to chemotax,;specific gene promoter regions to respond to molecular signals (purple cubes);chemotaxis receptors (orange);and 5) machinery to produce detectable molecules (red). C) The number of papers describing bacterial anti-cancer therapies has grown exponentially (black line) since the mid-1990s.
Bacteria can be viewed as these perfect robot therapies because they have biological mechanisms to perform all of the ideal functions mentioned above (Figure 1B. Over the last century, many genera of bacteria have been shown to preferentially accumulate in tumors, including Salmonella4, Escherichia5 , Clostridium6–7 and Bifidobacterium8. Caulobacter9, Listeria10–11, Proteus12 and Streptococcus13 have also been investigated as anticancer agents. For propulsion and sensing, bacteria have flagella that enable tissue penetration14 and chemotactic receptors that direct chemotaxis towards molecular signals in the tumor microenvironment15–16. For example, the TAR receptor detects aspartate secreted by viable cancer cells and the TRG receptor promotes migration towards ribose in necrotic tissue16. Selective cytotoxicity can be engineered by transfection with genes for therapeutic molecules, including toxins17–19, cytokines20–21, tumor antigens22 and apoptosis inducing factors23–27. External control can be achieved using gene promoter strategies that respond to small molecules17, 28–29 or radiation23, 26–27, 30. Bacteria can also be detected using light5, 31–32, magnetic resonance imaging (MRI)33 or positron emission tomography (PET)34–36. Finally and most importantly, the ease of genetically manipulating bacteria is the feature that will have the greatest effect on therapy development because it enables precise tuning and limitless functional combinations.
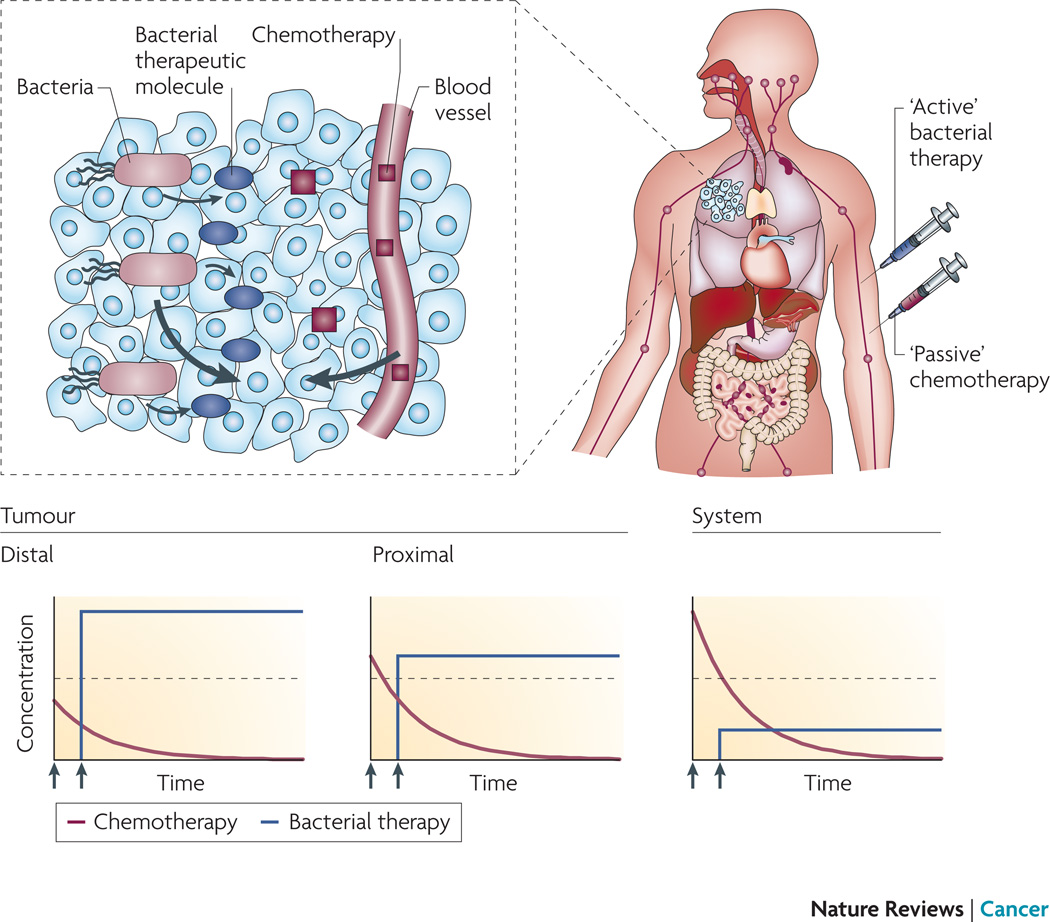
Once fully implemented and tested, the unique capabilities of bacterial therapies will change the way cancer is treated. Manufacture of drugs within tumors would beneficially shift temporal drug concentration profiles compared to intravenous administration (Figure 2). Because bacteria can migrate and accumulate far from vasculature, more of the therapeutic would be present in distal regions for longer periods of time compared to small molecules that only diffuse passively. Intratumoral production would be more toxic to cancer tissue and less toxic to normal tissue. This inversion of drug localization would eliminate tumors from the inside out, and would have the simultaneous effects of increasing efficacy and decreasing damage to normal tissue.
Figure 2. The transport properties of bacterial therapies produce preferable drug concentration profiles.
When injected systemically, bacteria (red syringe, green organisms), specifically accumulate in tumors and migrate to distal regions far from vasculature (brown cells). These distal regions are typically hypoxic and hypoglycemic and contain quiescent and necrotic cells. Once triggered (small red arrows), bacteria begin to produce therapeutic molecules (red ovoids) that diffuse (large red arrows) into viable tissue (clear cells). Systemically injected (small blue arrows), passive chemotherapeutic molecules (blue cubes) diffuse into tumor tissue from blood vessels (large blue arrows). The concentration of bacterially produced molecules (red lines) is greatest in distal tumor regions and would remain constant as long as expression of these proteins continues. The concentration of chemotherapeutic molecules is greatest in systemic blood and drops as it is cleared by the liver or kidneys. Based on these profiles, bacterially produced molecules will be more cytotoxic (dotted line) in the distal regions of tumors and less systemically toxic. The profile of passive molecules is less favorable, with more systemic toxicity and less efficacy deep in tissue.
To date many different bacterial strategies have been implemented in animal models (Tables 1 and 2) and some human trials have been carried out (Table 3). Using these strategies, many researchers have observed experimental success, with reduced tumor volume, increased survival and treatment of metastatic disease (Table 1). Success has also been shown treating multiple tumor sites (Table 1); the most notable is pancreatic cancer13, 37, for which new targeted treatments could dramatically improve the poor current prognosis of less than 25% five-year survival. Since the mid 1990’s, the number of published bacterial therapy papers has increased with a doubling time of 2.5 years (Figure 1C). This rapid rise has been driven almost entirely by increasing use of Salmonella as a delivery vector (Figure 1C). This Innovation article will describe many of the advances that have fuelled this enthusiasm including, specific bacterial targeting of tumors; intratumoral penetration; native bacterial cytotoxicity; expression of anticancer agents; gene triggering strategies; and detection of bacterial therapies.
Table 1.
Efficacy of bacterial therapies and strategies in animal models
| A. Strategies showing tumor regression and/or increased survival | |
| Native bacterial toxicity | |
| Bifidobacterium | 8 |
| Caulobacter | 9 |
| Clostridium | 6, 62, 115–117 |
| Escherichia | 118 |
| Listeria | 10–11 |
| Proteus | 12 |
| Salmonella | 4, 37, 46, 48, 64–67, 119–122 |
| Streptococcus | 13 |
| Combination with other therapies | |
| Clostridium | 14, 107–110 |
| Escherichia | 18 |
| Salmonella | 52, 123–125 |
| Agents with control of expression | |
| Salmonella | 19, 23 |
| Expression of Anticancer Agents | |
| Escherichia | 18 |
| Salmonella | 20–22, 24, 74–79, 81–82, 126 |
| Gene Transfer | |
| Salmonella | 44, 51, 88–95 |
| RNAi | |
| Salmonella | 96 |
| Prodrug cleavage | |
| Clostridium | 47, 127–129 |
| Salmonella | 130–134 |
| B. Strategies that reduced metastatic burden or prevented metastasis formation | |
| Native bacterial toxicity | |
| Salmonella | 121, 135–136 |
| Expression of Anticancer Agents | |
| Escherichia | 18 |
| Salmonella | 20–21, 24, 74–76, 93 |
| C. Sites targeted showing either tumor regression or increased survival | |
| Breast cancer | 48, 131 |
| Colon cancer | 131 |
| Hepatocellular carcinoma | 64 |
| Melanoma | 130–131 |
| Neuroblastoma | 78 |
| Pancreatic cancer | 13, 37 |
| Prostate cancer | 96 |
| Spinal cord glioma | 122 |
Table 2.
Bacterial strategies
| A. Expressed anticancer agents | ||
|---|---|---|
| Cytotoxic agents | ||
| Cytolysin A (ClyA, HlyE) | 17–19 | |
| Fas Ligand | 24 | |
| TNFα | 25–27 | |
| TRAIL | 23 | |
| Cytokines | ||
| CCL21 | 21 | |
| Interleukin 2 (IL-2) | 72–73, 75–80 | |
| Interleukin 18 (IL-18) | 74 | |
| LIGHT | 20 | |
| Antigens and antibodies | ||
| C-Raf | 22 | |
| CtxB-PSA fusion protein | 60 | |
| CPV-OmpA fusion protein | 81 | |
| NY-ESO-1 tumor antigen | 82 | |
| Single chain HIF-1α antibodies | 83 | |
| B. Genetic transfer | ||
| Cytotoxic and antiangiogenic agents | ||
| Endostatin | 44 | |
| Thrombospondin-1 | 51 | |
| TRAIL and Smac | 88 | |
| Cytokines and growth factors | ||
| Interleukin 12 (IL-12) | 89–91 | |
| GM-CSF | 90 | |
| Flt3 Ligand | 92 | |
| Tumor antigens | ||
| α-fetoprotein (AFP) | 95 | |
| Flk-1 | 93–94 | |
| Gene silencing (shRNA) | ||
| Stat3 | 96 | |
| Bcl2 | 97 | |
| C. Gene triggering strategies | ||
| Signal | Promoter | |
| γ-irradiation | pRecA | 23, 26–27, 30 |
| L-arabinose | pBAD | 17, 28–29 |
| Oxygen (FNR) | FF+20* | 19 |
| HIP-1 | 101 | |
| pflE and ansB | 103 | |
| Salicylate | XylS2-dependent Pm promoter | 99 |
| D. Combinations with other treatments | ||
| Anti-vascular agents | 14, 123 | |
| Chemotherapeutic drugs | 14, 51, 108, 110 | |
| Heat shock proteins | 125 | |
| Heavy metals | 107 | |
| Radiation | 18, 109, 124 | |
| E. Imaging strategies | ||
| Bioluminescence | 5, 17, 104–106 | |
| Fluorescence | 5, 31–32 | |
| Magnetic resonance (MRI) | 33, 137 | |
| Positron Emission (PET) | 34–36 | |
Table 3.
Published human trials using bacterial cancer therapies
| Strain | Cancer type | n | Responses | Ref. |
|---|---|---|---|---|
| C. butyricum M-55 | Squamous cell carcinoma, metastatic, malignant neuroma, leiomyosarcoma, melanoma, sinus carcinoma |
5 | Oncolysis (3) | 63 |
| C. butyricum M-55 | Vascular glioblastoma | 49 | Oncolysis | 138 |
|
S. typhimurium VNP20009 |
Metastatic melanoma and renal cell carcinoma |
25 | Focal tumor colonization (3) | 55 |
|
S. typhimurium VNP20009 |
Metastatic melanoma | 4 | Tumor biopsy culture positive for VNP20009 (1) |
54 |
|
S. typhimurium VNP20009 TAPET-CD |
Squamous cell carcinoma, adenocarcinoma |
3 | Intratumoral bacterial colonization (2) |
56 |
Bacterial targeting of tumors
One of the major advantages of bacterial therapies for cancer is the ability to specifically target tumors. The mechanisms of bacterial accumulation in tumors differ depending on oxygen tolerance. Obligate anaerobes (e.g. Clostridium, and Bifidobacterium) cannot survive in oxygen and injected bacterial spores can only germinate in anoxic regions of tumors38–39. Completely deoxygenated tissue is unique to tumors and is not present in most other organs of the body. Obligate anaerobes are therefore highly effective at accumulating in the large hypoxic regions of tumors14. This absolute specificity was demonstrated early by Malmgren et al. who injected Clostridium into tumor-bearing mice and showed that only the mice with tumors died from the infection7.
Facultative anaerobes (e.g. Salmonella and Escherichia) use a more complex set of mechanisms to target tumors. Five interacting mechanisms are thought to control the accumulation of facultative anaerobes in tumors: entrapment of bacteria in the chaotic vasculature of tumors40, flooding into tumors following inflammation41, chemotaxis toward compounds produced by tumors15–16, preferential growth in tumor-specific microenvironments15, 31, and protection from clearance by the immune system42. These mechanisms enable Salmonella to accumulate in tumors at ratios greater than 1000:1 compared to organs rich in reticuloendothelial cells (such as the liver and spleen) and even greater in other organs40, 43–45.
When injected systemically, Salmonella attach to the walls of tumor vasculature with a low but measurable frequency (∼0.035% of bacteria in the blood)40. In addition, the number of bacteria that adhere is dependent on blood velocity, suggesting that hemodynamics play an important role in the initial interaction of bacteria with tumors40. Similarly, the accumulation of Salmonella is associated with an influx of blood into tumors, caused by an immunologically induced rise in the blood concentration of tumor necrosis factor-α (TNFα)41. This mechanism would be reduced for attenuated msbB− strains that elicit much lower (∼10%) TNFα levels46. The production of TNFα immediately after injection therefore has contradictory effects; it promotes accumulation in tumors but is also the primary cause of bacterial toxicity due to septic shock46. This dependence on an immune response to promote targeting could also reduce the utility of repeated dosing with bacteria, which is a limitation that does not affect bacteria delivered as spores47.
In in vitro tumor models, Salmonella identify and penetrate tumors by detecting and chemotaxing towards small molecule gradients of serine, aspartate and ribose15–16. In addition, the growth rate of Salmonella is greater in in vitro tumors when dying cells are present15, a phenomenon which is also observed in animal tumor models40–41, 46. The importance of this mechanism for promoting accumulation is supported by the increased tumor specificity of auxotrophic Salmonella that require leucine and arganine, which are nutrients derived from dying tumor tissue31, 48.
Because tumors are immune-privileged environments49, bacteria can replicate unimpeded by the macrophage and neutrophil clearance mechanisms that normally serve to eliminate them50. In this way, the immune system plays a complicated role in bacteriolytic therapy; it provides a mechanism to guide bacterial accumulation, but also impedes dispersion and efficacy. The interaction between bacteria and the immune system also works in reverse; many bacterial therapies sensitize the immune system to induce tumor clearance51–52.
Intratumoral penetration
Intratumoral targeting is an essential characteristic of an optimized cancer therapy (Figure 1). Compared to normal tissue, tumors have chaotic vasculature and large intercapillary distances, impeding delivery of therapeutic molecules3, 53. This reduces therapeutic efficacy by creating cellular regions that have low drug concentrations and reduced nutrient supply1, 3. Low levels of oxygen and glucose create quiescent cells that are unresponsive to chemotherapeutics designed to target rapidly growing cells. Proper intratumoral targeting enables drug delivery directly to these distal, unresponsive cells that are far from tumor vasculature (Figure 2). In this way, the metabolic heterogeneity of tumors is both a blessing and a curse; molecular gradients reduce therapeutic efficacy but also create unique environments that can be targeted.
Motility is the key feature of bacterial therapies that enables intratumoral targeting. Bacteria can actively swim away from vasculature and penetrate deep into tumor tissue (Figure 2). Because bacteria are complex living organisms that can acquire energy from their environment, their transport is not entropically limited. This contrasts to the concentration of passive molecules, which drops with distance from vasculature. Because bacteria are self-propelled, their density can be higher far from the vascular source. It has been shown that bacteria that can disperse throughout tumor tissue have a greater ability to regress tumors14. Salmonella have also been shown to chemotax towards molecules produced by dying tumor tissue15–16. Salmonella contain chemoreceptors that sense small molecules in the local environment. For example, using knockouts, it has been shown that the aspartate receptor intiates chemotaxis towards viable tumor tissue; the serine receptor induces tissue penetration; and the ribose receptor directs migration toward necrotic tissue16.
In addition to intrinsic motility, the host immune system plays a critical role in preventing bacterial dissemination throughout tumors. Neutrophils have been shown to prevent bacteria from spreading from necrotic into viable tumor tissue50. This containment is one possible reason that attenuated Salmonella had limited success reducing tumor growth in human trials54–56. Depleting host neutrophils increases tumor bacterial densities and enables spread throughout viable tumor tissue50.
Native bacterial cytotoxicity
Many successful experiments have shown that the natural toxicity of bacteria is sufficient to regress tumors (Table 1). Native bacterial cytotoxicity is caused by sensitization of the immune system and competition for nutrients42. Although some organisms naturally produce toxins, these are typically removed to prevent pathogenicity14. Much early work on bacterial therapies relied on natural toxicity because direct genetic modification was not possible. The ability of bacteria to regress tumors has been recognized since the early 1800’s57. In the time before strict antiseptic technique, tumor regression was occasionally observed following severe bacterial infection57. This observation led to the development of Coley’s toxin, a bacterial extract that stimulates a general immune response57–59. Because of this early success, this approach persists in many contemporary strategies20, 60 that are similarly designed to stimulate immune responses (Table 2). The idea that living bacteria could be anticancer therapeutic agents was first advanced in the middle of the 20th century6–7. The increased availability of antibiotics and the discovery that tumors contain anoxic regions61 spurred multiple investigations6, 62 which showed that Clostridium, an obligate anaerobe, could regress tumors in mice (Table 1). There was sufficient enthusiasm to initiate a small clinical trial, and oncolysis was observed in three out of five patients following injection with C. butyricum63 (Table 3).
More recently, Salmonella has been tested for its anti-cancer properties4, 46, and similar to Clostridium, Salmonella is naturally cytotoxic and has been shown to regress tumors when administered alone (Table 1A). Immunosensitization is one of the key mechanisms of Salmonella cytotoxicity; accumulation of S. choleraesuis in tumors induces neutrophil infiltration and antitumor immune responses64. When investigated in human trials, Salmonella with a modified lipid-A (strain VNP200009) was found to be non-toxic and tumor colonization was observed55. In dogs administered VNP200009, colonization was also observed and complete cure was seen in 4 of the 35 animals65. There is also potential that Salmonella could be delivered orally to reduce toxicity. Following oral administration in mice, Salmonella preferentially accumulated in tumors and maintained its anticancer effects66 with very low toxicity67. Oral delivery may be different in humans, where bacterial escape from the gut into the circulation occurs less often than in mice68.
Expression of anticancer agents
Another advantage of bacterial anticancer agents is that they can be genetically modified to increase their effectiveness. Many strategies have been employed (Tables 1, 2) and two major mechanisms have been studied: the direct expression of proteins that have physiological activities against tumors and transfer of eukaryotic expression vectors into infected cancer cells. For both of these mechanisms, three categories of anticancer agents have been investigated: cytotoxic agents that directly kill cancer cells, cytokines that stimulate immune cells to kill cancer cells, and tumor antigens that sensitize the immune system against cancer cells. Prodrug strategies have been reviewed previously69–70 and will not be discussed here.
Cytotoxic agents
Bacterial toxins are the most obvious cytotoxic agents because these genes are native to bacterial physiology. Cytolysin A (ClyA or HlyE) is a bacterial toxin that acts by forming pores in mammalian cell membranes and inducing apoptosis18–19. ClyA is a native bacterial protein that is ready transported to the bacterial surface and secreted without modification17–18. Multiple groups have shown that treating mice with E. coli or S. typhimurium expressing ClyA reduces tumor growth17–19.
Three of the cytotoxic agents are members of TNFα family: FAS ligand (FASL), TNF-related apoptosis-inducing ligand (TRAIL) and TNFα23–27. These proteins selectively induce apoptosis via death receptor pathways, which activate caspase-8 and caspase-3, an important apoptotic mediator23. All three are selectively cytotoxic to cancer cells compared to normal cells23–24. FASL specifically induces apoptosis in cells that possess the FAS receptor24. TNFα and TRAIL have been shown to be cytotoxic towards colon, breast, lung, prostate, renal, ovarian, bladder, glioma and pancreatic tumors23, 71. When systemically administered as protein drugs, all three members of this family have two deficiencies that are overcome by bacterial delivery: hepatotoxicity and a short circulatory half-life23, 25–27. Producing these proteins in situ would maintain a higher continual concentration in tumors compared to delivery to the circulatory system (Figure 2), and would reduce the systemic toxicity associated with their administration as small molecules. FASL is also immunologically active: it attracts tumor rejecting granulocytes, induces interleukin (IL23) production by dendritic cells and stimulates proliferation of T cells — three mechanisms that may culminate in specific killing of cancer cells24.
Cytokines
Bacteria can also be engineered to deliver specific cytokines that have anti-tumor effects (Table 2). Cytokines induce immune cells to clear tumors by stimulating multiple mechanisms such as immune cell activation, proliferation and migration. When administered as a small molecule, IL2 activates the cytolytic function of natural killer (NK) and lymphokine-activated killer cells72 and promotes lymphocyte proliferation73. Similar to IL2, IL18 (also known as IFNγ-inducing factor) induces T and NK cell proliferation and enhances their production of cytokines74. IL18 also suppresses angiogenesis by inhibiting fibroblast growth74. CCL21 controls migration of immune cells and may prevent tumor-induced immunosuppression21. LIGHT (also known as TNFSF14 and HVEM-L) is a TNF-family cytokine homologous to lymphotoxin that induces dendritic cell (DC) growth20.
IL2 is the most extensively studied bacterially delivered cytokine72–73, 75–80. Reports describing IL2 delivery by Salmonella were the first to suggest that this genus could be effectively used as an anticancer agent73, 80. Oral administration of Salmonella expressing IL2 has been shown to function prophylactically and prevent tumor formation79. Despite multiple anticancer effects, IL2 and IL18 have had limited success as chemotherapeutics because of severe systemic toxicity72–74. Similar to the TNFα-family agents, local production of these cytokines within tumors would limit toxicity while stimulating tumor-infiltration by lymphocytes72. Treatment with Salmonella expressing LIGHT or CCL21 has been shown to induce leukocyte and neutrophil infiltration and inhibit tumor growth20–21.
Tumor-specific antigens and antibodies
The expression of tumor-specific antigens is another bacterial strategy that utilizes the host immune system (Table 2). It functions by sensitizing immune cells and preventing the formation of tumors that present those antigens22, 60, 81–82. For example, RAF1 (also known as c-RAF) is a transcription factor upregulated in many tumors22; prostate-specific antigen (PSA) is upregulated in many prostate tumors60; and NY-ESO-1 (also known as CTG1B) is a germ cell protein often expressed by tumor cells82. To induce a more efficient immune response, PSA has been fused to cholera toxin subunit B (CtxB), a mucosal adjuvant60. Alternately, a non-specific immune response can be induced by the expression of a potent antigen, e.g. canine parvovirus (CPV)81. To facilitate interaction with immune cells, different protein secretion systems have been employed: for example, RAF1 and CtxB-PSA were fused to the α-hemolysin secretion signal22, 60 and CPV was bound to OmpA, a membrane protein that forms outer membrane vesicles81. Because these strategies rely on a systemic immune response, it is not necessary for these antigens to be expressed in tumors82. Also, because the response is retained by the immune system, these bacterial therapies could be used for prevention or as treatment vaccines.
Alternatively, bacteria can be engineered to express single chain antibodies to inhibit proteins necessary for tumor cell function. For example, C. novyi has been modified to express single chain antibodies that bind the hypoxia inducible factor 1α (HIF1α) antigen83. HIF1α is an important target because it is associated with resistance to radiotherapy and chemotherapy and poor clinical outcome83. Preliminary studies have shown that bacterially produced antibodies bind the HIF1α epitope83.
Gene transfer
The ability of therapeutic bacteria to transfer genetic material to mammalian cells was first reported in 1995, when it was shown that Shigellae could transfer plasmid DNA into baby hamster kidney cells84. Soon after, it was shown that Salmonella could also be used for trans-kingdom DNA transfer85–86. These reports generated significant enthusiasm for using bacteria (specifically Salmonella) to transfer the genes for cytotoxic and immunological agents into cancer cells (Table 2). Compared to direct expression, this approach has benefits as well as drawbacks. Gene transfer, which utilizes more permanent mammalian systems, may produce stronger, more stable expression. However, expression of the transferred genes may be harder to control87; expression could be limited by poor transfer efficiency; transferred genes may be heterogeneously distributed in tissues; and the genes could transfer to tissues other than those they are targeted towards.
Many of the same strategies have been attempted with gene transfer as with direct expression: cytotoxic agents, cytokines and tumor antigens (Table 2). Two early reports describe the transfer of the anti-angiogenic genes, endostatin44 and thrombospondin 151, which kill tumors by preventing new blood vessel formation and cutting off the nutrient supply44. Although direct administration of endostatin to cancer patients showed only minimal antitumor activity, transfer of endostatin from Salmonella reduced microvessel density, decreased VEGF expression, and slowed tumor growth in mice44. Using a similar strategy as direct expression, reduction of tumor growth was shown by transferring the genes encoding TRAIL and SMAC (also known as DIABLO) into tumor cells from Salmonella88.
The anti-tumor effects of three cytokines and growth factors have been explored by bacterial gene transfer: IL1289–91, granulocyte/macrophage colony-stimulating factor (GM-CSF)90, and Fms-like tyrosine kinase ligand (FLT3L)92. Similar to bacterially expressed cytokines, these molecules stimulate NK, T and DC cells89–91. In addition, IL12 induces IFN-γ production and GM-CSF activates neutrophils and macrophages to lyse tumor cells90. When expressed together, IL12 and GM-CSF significantly reduce tumor growth in mice, while limiting the systemic toxicity associated with systemic cytokine injection90.
The transfer of genes for two tumor antigens has been shown to be effective at reducing tumor growth in mouse models: α-fetoprotein (AFP) and vascular endothelial growth factor receptor 2 (VEGFR2, also known as FLK1)93–95. Antibodies against AFP, an embryonic protein overexpressed in hepatocellular carcinoma and not present in normal adult tissue, prevents formation of liver and colon tumors95. VEGFR-2 is an endothelial cell receptor that controls angiogenesis and antibodies against VEGFR2 have been shown to prevent angiogenesis and tumor growth in glioblastoma94 and lung cancer93 models.
Gene silencing
A complementary strategy to bacterial induction of gene expression is gene silencing. Silencing is achieved by transferring plasmids encoding small hairpin RNAs (shRNA) from Salmonella into cancer cells96–97. Gene-specific shRNAs are processed by the enzyme Dicer into small interfering double-stranded RNAs (siRNAs) that induce the degradation of target mRNAs96. To date, two genes have been silenced using this technique, signal transducer and activator of transcription 3 (Stat3)96 and Bcl297. Both factors inhibit apoptosis and STAT3 promotes cancer cell growth; overexpression of these factors has been associated with many tumor types, including prostate cancer and malignant melanoma96–97. Silencing of Stat3 has been shown to prevent prostate tumor and metastasis formation in mice97.
Gene triggering strategies
Control of gene expression is critical for managing the timing and location of drug production. Incorporation of specific promoter sequences upstream of genes that encode anticancer proteins enables control of transcription by external signals. Precise triggering of expression can be used to induce greater intratumoral effects while minimizing systemic toxicity23. Some gene products require tighter control than others; for example, cytotoxic molecules and cytokines that are known to be toxic cannot be constitutively expressed but tumor-specific antigens do not need to be expressed in tumors and so tight control of the genes expressing these antigens is not necessary82.
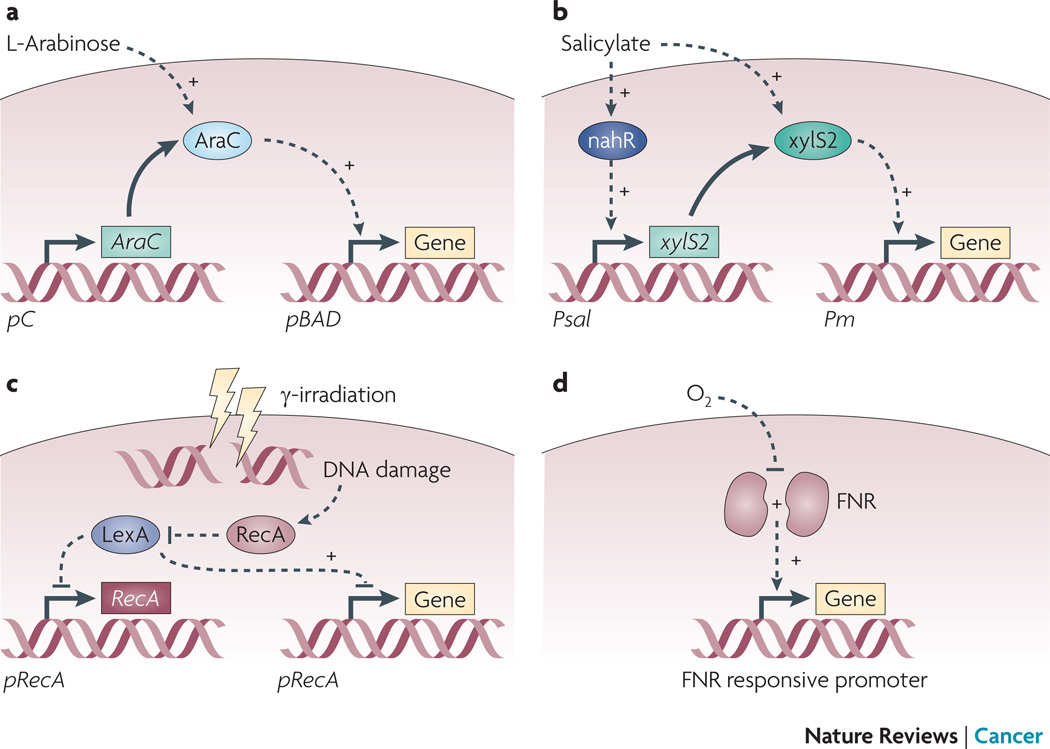
There are two categories of gene triggering strategies: extracellular triggers and environmental sensors (Table 2). Three external triggers have been investigated: l-arabinose, salicylate and γ-irradiation (Figure 3). The pBAD system utilizes the regulatory protein AraC to respond to extracellular l-arabinose17, 28–29 and is very tightly regulated98. The salicylate system is also tightly regulated and its cascade amplifies gene expression, producing induction ratios of 20–150 fold in vitro99. Both L-arabinose and salicylate are suitable and non-toxic biological triggers. In mouse models, it has been shown that intravenous administration of l-arabinose can activate gene expression in colonized tumors29.
Figure 3. Gene triggering systems.
A) The pBAD system, which responds to extracellular l-arabinose, contains two components: the arabinose sensitive protein AraC and the pBAD promoter. Constitutively expressed regulator AraC induces transcription by binding to the pBAD promoter. AraC is a positive and negative regulator of pBAD: it activates transcription in the presence of arabinose and represses transcription in its absence. B) The salicylate cascade system utilized a two salicylate-sensitive regulator proteins, nahR and xylS2 to maintain tight regulation. In the presence of salicylate, nahR activates transcription from the promoter Psal, leading to the expression of XylS2. XylS2, which is also sensitive to salicylate, activates transcription from the promoter PmC) The RecA system senses γ-irradiation, which causes DNA damage. This damage activates RecA, which induces autoproteolysis of LexA. Transcription is induced when LexA, a repressor of the recA promoter, releases from DNA. Feed-forward regulation increases the RecA concentration when the system is active. D) The FNR system turns on in hypoxic environments. The absence of oxygen promotes dimerization of FNR, which induces transcription. Multiple promoters bind FNR, including FF+20*, HIP-1, pflE and ansB.
The RecA mechanism utilizes γ-irradiation as a trigger of gene expression (Figure 3) and is based on the SOS DNA repair system23, 26–27, 30. Irradiation has a major advantage over molecular triggers because it can directly penetrate tumor tissue and is not restricted by diffusion limitations2. γ-irradiation causes DNA damage and activates the protein RecA23, which promotes autoproteolysis of the repressor LexA. The lysis of LexA, a repressor of the recA promoter, induces gene expression. This system is amplified by self induction of RecA when LexA is cleaved. To reduce basal expression and increase radiation responsiveness an extra Cheo box has been incorporated into the recA promoter, which has been shown to increase expression ten-fold100.
To date, all environmental triggering strategies have been designed to sense hypoxia using the fumarate and nitrate reduction (FNR) regulator (Figure 3)19, 101. FNR is an oxygen-responsive transcription factor naturally present in Salmonella19, 101–102. In the absence of oxygen, iron-sulfide clusters induce the formation of FNR homodimers that bind to specific DNA sequences and promote transcription19, 101. In the presence of oxygen, the clusters and FNR homodimers disassemble, reducing transcription. Two artificial promoters have been developed that contain FNR-binding sites: FF+20* 19 and hypoxia inducible promoter-1 (HIP1101; Table 2). These two promoters were created by random19 and directed101 mutagenesis to amplify expression in hypoxia and reduce expression in normoxia19. To identify bacterial promoters that could be used in environmental triggering strategies, Arrach et al. develped a reporter system that they tested in tumor-bearing mice103. The two most active promoters, pflE and ansB, both contained FNR-binding sites and are known to be oxygen dependent103. These experiments did, however, identify other promoters that were not oxygen dependent and may rely on alternative environmental triggers.
Detection
Being able to locate colonized bacteria is clinically important because it enables the detection of obscured tumors and metastases. Four different strategies have been implemented to identify bacteria in tumors: bioluminescence, fluorescence, magnetic resonance and positron emission (Table 2). Bioluminescent bacteria are generated by transformation with plasmids containing the luxCDABE operon from Photobacterium leiognathi5, 17, 104–106, and fluorescent bacteria are generated by transformation with plasmids containing the gene for green fluorescent protein (GFP)5, 31–32. Both of these mechanisms have proven to be very efficient at identifying tumors in mice using whole mouse imaging5, 17, 31–32, 104–106. These light-based mechanisms may have limited clinical application, however, because of the poor penetration of visible light through tissue.
Alternately, magnetotactic bacteria could be injected and detected by MRI. For example, Magnetospirillum magneticum produces magnetite (Fe3O4) particles and has been shown to accumulate in tumors33. For improved tumor targeting, the genes for magnetite production could be transferred into other bacterial strains33. Two different methods that have been used to detect bacteria with PET are expression of an exogenous viral tyrosine kinase34–35 and reliance on endogenous protein kinases36. When herpes simplex thymidine kinase (HSV1-TK) is expressed in Salmonella, it selectively phosphorylates and traps the detectable marker 2’-fluoro-1-β-d-arabino-furanosyl-5-iodouracil (FIAU)35. Alternately, the endogenous protein kinases of E. coli Nissle 1917 have been shown to phosphorylate and trap [18F]-2’-Fluoro-2’deoxy-1-β-d-arabino-furanosyl-5-ethyl-uracil ([18F]-FEAU)36. Both these methods have successfully been shown to identify bacteria accumulated in mouse tumors34–36.
Conclusions and future perspectives
Recently, many experiments have shown that bacterial therapies can successful regress tumors and promote survival in mice. However, numerous challenges remain before bacteria can be used in the clinic, including limited drug production, intrinsic bacterial toxicity, targeting efficiency, genetic instability and combination with other therapies. Tuning drug production is necessary to synthesize drugs at high enough concentrations to induce therapeutic effects but not so high that they cause systemic toxicity (see Figure 2). Controlling bacterial toxicity will be critical to ensure safety and permit regulatory approval. Both Clostridium and Salmonella have been shown to be non-pathogenic in multiple animal species46, 65 and in human trials54–56, 63, but any retained virulence could be problematic for immunocompromised late-stage cancer patients. Variable targeting efficiency could lead to poor efficacy for large groups of patients and will affect which sites could be effectively treated with bacteria. Targeting efficacy will also play a large role in the treatment of metastatic disease because, to be effective, bacteria will have to colonize a high percentage of distal sites. Genetic instability is a potential problem because mutations could create ineffective or harmful phenotypes. The rate of mutation will specify the upper time limit that bacterial colonies could be allowed to remain in tumors. Finally, determining the correct combination of bacteria and other cancer therapies (Tables 1 and 2)14, 18, 107–110 will be critical for creating strategies that can completely clear tumors and metastases. Solving these challenges could overcome the limitations that have previously been seen in the clinic54–56 (Table 3): reduced toxicity will increase the maximum-tolerated dose; improved targeting will increase tumor colonization; and efficient drug production will promote tumor regression.
All these challenges can be addressed using synthetic biology techniques. Rates of protein drug production can be optimized by manipulating multiple factors111, including gene copy number, promoter strength, optimized codons, bacterial metabolism, mRNA secondary structure112 and synthetic ribosome binding sites113. Both toxicity and targeting are affected by the immune response following injection and innate bacterial virulence. Determining which virulence factors are essential for targeting and which introduce unnecessary toxicity can be achieved by screening knockouts of the pathogenicity genes that, for example, enable evasion of the immune system, induce uptake into cells, promote intracellular replication and stimulate cytokine synthesis114. Other targeting mechanisms can be enhanced by genetic manipulation of endogenous chemoreceptors16, selective control of bacterial proliferation in tumors, and strategies to avoid sequestration by neutrophils. Similarly, genetic stability could be enhanced by incorporating engineered genes on the bacterial chromosome and limiting homologous recombination and horizontal gene transfer.
This moment in history is a turning point for bacterial therapies. The preliminary proof-of-concept experiments have demonstrated the vast capacity of bacteria for treating cancer and illustrated the large number of effective tools that these robot factories possess. The ultimate bacterial therapy will consist of a collection of strains designed for specialized purposes rather than a single perfect strain. Successful treatment could utilize these strains cooperatively and in combination with molecular chemotherapy: a detectable facultative anaerobe could be used for diagnosis; an engineered immunogenic stain could be used to sensitize the immune system; an obligate anaerobe could be used to treat inoperable primary tumors; and a motile Salmonella strain that controllably produces a cytotoxic agent could be used to treat diffuse tumors and metastatic disease. All bacterial therapies will be in used in combination with other therapeutics (Tables 1 and 2)14, 18, 107–110, which will have a synergistic effect: small molecules would kill cancer cells close to blood vessels and bacteria would kill cells far from vessels (Figure 2). The greatest strength of bacterial therapies is their genetic flexibility, which enables tuning for individualized therapy, targeting to multiple tumor sites and precise control of cytotoxicity. Once perfected, anticancer bacteria are expected to be an essential clinical tool, which can perform functions unachievable by other therapies, and can detect, prevent, and treat tumors and metastases.
Acknowledgements
This work was partly supported by the US National Institutes of Health, National Cancer Institute grant CA120825.
References
- 1.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 2.St Jean AT, Zhang MM, Forbes NS. Bacterial therapies: completing the cancer treatment toolbox. Current Opinion in Biotechnology. 2008;19:511–517. doi: 10.1016/j.copbio.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jain RK. The next frontier of molecular medicine: delivery of therapeutics. Nat Med. 1998;4:655–657. doi: 10.1038/nm0698-655. [DOI] [PubMed] [Google Scholar]
- 4.Pawelek JM, Low KB, Bermudes D. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 1997;57:4537–4544. [PubMed] [Google Scholar]
- 5.Yu YA, et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004;22:313–320. doi: 10.1038/nbt937. [DOI] [PubMed] [Google Scholar]
- 6.Parker RC, Plummer HC, Siebenmann CO, Chapman MG. Effect of histolyticus infection and toxin on transplantable mouse tumors. Proc Soc Exp Biol Med. 1947;66:461–467. doi: 10.3181/00379727-66-16124. [DOI] [PubMed] [Google Scholar]
- 7.Malmgren RA, Flanigan CC. Localization of the vegetative form of Clostridium tetani in mouse tumor following intravenous spore administration. Cancer Res. 1955;15:473–478. [PubMed] [Google Scholar]
- 8.Kohwi Y, Imai K, Tamura Z, Hashimoto Y. Antitumor effect of Bifidobacterium infantis in mice. Gann. 1978;69:613–618. [PubMed] [Google Scholar]
- 9.Bhatnagar PK, Awasthi A, Nomellini JF, Smit J, Suresh MR. Anti-tumor effects of the bacterium Caulobacter crescentus in murine tumor models. Cancer Biology & Therapy. 2006;5:485–491. doi: 10.4161/cbt.5.5.2553. [DOI] [PubMed] [Google Scholar]
- 10.Pan ZK, Weiskirch LM, Paterson Y. Regression of established B16F10 melanoma with a recombinant Listeria monocytogenes vaccine. Cancer Research. 1999;59:5264–5269. [PubMed] [Google Scholar]
- 11.Kim SH, Castro F, Paterson Y, Gravekamp C. High Efficacy of a Listeria-Based Vaccine against Metastatic Breast Cancer Reveals a Dual Mode of Action. Cancer Research. 2009;69:5860–5866. doi: 10.1158/0008-5472.CAN-08-4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arakawa M, Sugiura K, Reilly HC, Stock CC. Oncolytic effect of Proteus mirabilis upon tumor-bearing animals. 2. Effect on transplantable mouse and rat tumors. Gann. 1968;59:117. [PubMed] [Google Scholar]
- 13.Maletzki C, Linnebacher M, Kreikemeyer B, Emmrich J. Pancreatic cancer regression by intratumoural injection of live Streptococcus pyogenes in a syngeneic mouse model. Gut. 2008;57:483–491. doi: 10.1136/gut.2007.125419. [DOI] [PubMed] [Google Scholar]
- 14.Dang LH, Bettegowda C, Huso DL, Kinzler KW, Vogelstein B. Combination bacteriolytic therapy for the treatment of experimental tumors. Proc Natl Acad Sci U S A. 2001;98:15155–15160. doi: 10.1073/pnas.251543698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasinskas RW, Forbes NS. Salmonella typhimurium specifically chemotax and proliferate in heterogeneous tumor tissue in vitro. Biotechnology and Bioengineering. 2006;94:710–721. doi: 10.1002/bit.20883. [DOI] [PubMed] [Google Scholar]
- 16.Kasinskas RW, Forbes NS. Salmonella typhimurium lacking ribose chemoreceptors localize in tumor quiescence and induce apoptosis. Cancer Research. 2007;67:3201–3209. doi: 10.1158/0008-5472.CAN-06-2618. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen VH, et al. Genetically engineered Salmonella typhimurium as an imageable therapeutic probe for cancer. Cancer Res. 2010;70:18–23. doi: 10.1158/0008-5472.CAN-09-3453. [DOI] [PubMed] [Google Scholar]
- 18.Jiang SN, et al. Inhibition of Tumor Growth and Metastasis by a Combination of Escherichia coli-mediated Cytolytic Therapy and Radiotherapy. Mol Ther. 2010 doi: 10.1038/mt.2009.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryan RM, et al. Bacterial delivery of a novel cytolysin to hypoxic areas of solid tumors. Gene Ther. 2009;16:329–339. doi: 10.1038/gt.2008.188. [DOI] [PubMed] [Google Scholar]
- 20.Loeffler M, Le’Negrate G, Krajewska M, Reed JC. Attenuated Salmonella engineered to produce human cytokine LIGHT inhibit tumor growth. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:12879–12883. doi: 10.1073/pnas.0701959104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loeffler M, Le’Negrate G, Krajewska M, Reed JC. Salmonella typhimurium engineered to produce CCL21 inhibit tumor growth. Cancer Immunology Immunotherapy. 2009;58:769–775. doi: 10.1007/s00262-008-0555-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gentschev I, et al. Use of a recombinant Salmonella enterica serovar Typhimurium strain expressing C-Raf for protection against C-Raf induced lung adenoma in mice. BMC Cancer. 2005;5:15. doi: 10.1186/1471-2407-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganai S, Arenas RB, Forbes NS. Tumour-targeted delivery of TRAIL using Salmonella typhimurium enhances breast cancer survival in mice. Br J Cancer. 2009;101:1683–1691. doi: 10.1038/sj.bjc.6605403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loeffler M, Le’Negrate G, Krajewska M, Reed JC. Inhibition of tumor growth using salmonella expressing Fas ligand. J Natl Cancer Inst. 2008;100:1113–1116. doi: 10.1093/jnci/djn205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Theys J, et al. Stable Escherichia coli-Clostridium acetobutylicum shuttle vector for secretion of murine tumor necrosis factor alpha. Applied and Environmental Microbiology. 1999;65:4295–4300. doi: 10.1128/aem.65.10.4295-4300.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nuyts S, et al. Increasing specificity of anti-tumor therapy: cytotoxic protein delivery by non-pathogenic clostridia under regulation of radio-induced promoters. Anticancer Res. 2001;21:857–861. [PubMed] [Google Scholar]
- 27.Nuyts S, et al. Radio-responsive recA promoter significantly increases TNFalpha production in recombinant clostridia after 2 Gy irradiation. Gene Ther. 2001;8:1197–1201. doi: 10.1038/sj.gt.3301499. [DOI] [PubMed] [Google Scholar]
- 28.Loessner H, et al. Remote control of tumour-targeted Salmonella enterica serovar Typhimurium by the use of L-arabinose as inducer of bacterial gene expression in vivo. Cell Microbiol. 2007;9:1529–1537. doi: 10.1111/j.1462-5822.2007.00890.x. [DOI] [PubMed] [Google Scholar]
- 29.Stritzker J, et al. Tumor-specific colonization, tissue distribution, and gene induction by probiotic Escherichia coli Nissle 1917 in live mice. Int J Med Microbiol. 2007;297:151–1562. doi: 10.1016/j.ijmm.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Nuyts S, et al. The use of radiation-induced bacterial promoters in anaerobic conditions: a means to control gene expression in clostridium-mediated therapy for cancer. Radiat Res. 2001;155:716–723. doi: 10.1667/0033-7587(2001)155[0716:tuorib]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 31.Zhao M, et al. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci U S A. 2005;102:755–760. doi: 10.1073/pnas.0408422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffman RM, Zhao M. Whole-body imaging of bacterial infection and antibiotic response. Nature Protocols. 2006;1:2988–2994. doi: 10.1038/nprot.2006.376. [DOI] [PubMed] [Google Scholar]
- 33.Benoit MR, et al. Visualizing Implanted Tumors in Mice with Magnetic Resonance Imaging Using Magnetotactic Bacteria. Clinical Cancer Research. 2009;15:5170–5177. doi: 10.1158/1078-0432.CCR-08-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tjuvajev J, et al. Salmonella-based tumor-targeted cancer therapy: tumor amplified protein expression therapy (TAPET (TM)) for diagnostic imaging. Journal of Controlled Release. 2001;74:313–315. doi: 10.1016/s0168-3659(01)00340-6. [DOI] [PubMed] [Google Scholar]
- 35.Soghomonyan SA, et al. Positron emission tomography (PET) imaging of tumor-localized Salmonella expressing HSV1-TK. Cancer Gene Therapy. 2005;12:101–108. doi: 10.1038/sj.cgt.7700779. [DOI] [PubMed] [Google Scholar]
- 36.Brader P, et al. Escherichia coli Nissle 1917 facilitates tumor detection by positron emission tomography and optical imaging. Clinical Cancer Research. 2008;14:2295–2302. doi: 10.1158/1078-0432.CCR-07-4254. [DOI] [PubMed] [Google Scholar]
- 37.Nagakura C, et al. Efficacy of a genetically-modified Salmonella typhimurium in an orthotopic human pancreatic cancer in nude mice. Anticancer Res. 2009;29:1873–1878. [PubMed] [Google Scholar]
- 38.Lambin P, et al. Colonisation of Clostridium in the body is restricted to hypoxic and necrotic areas of tumours. Anaerobe. 1998;4:183–188. doi: 10.1006/anae.1998.0161. [DOI] [PubMed] [Google Scholar]
- 39.Minton NP. Clostridia in cancer therapy. Nature Reviews Microbiology. 2003;1:237–242. doi: 10.1038/nrmicro777. [DOI] [PubMed] [Google Scholar]
- 40.Forbes NS, Munn LL, Fukumura D, Jain RK. Sparse initial entrapment of systemically injected Salmonella typhimurium leads to heterogeneous accumulation within tumors. Cancer Research. 2003;63:5188–5193. [PubMed] [Google Scholar]
- 41.Leschner S, et al. Tumor Invasion of Salmonella enterica Serovar Typhimurium Is Accompanied by Strong Hemorrhage Promoted by TNF-alpha. Plos One. 2009;4 doi: 10.1371/journal.pone.0006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sznol M, Lin SL, Bermudes D, Zheng LM, King I. Use of preferentially replicating bacteria for the treatment of cancer. J Clin Invest. 2000;105:1027–1030. doi: 10.1172/JCI9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clairmont C, et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J Infect Dis. 2000;181:1996–2002. doi: 10.1086/315497. [DOI] [PubMed] [Google Scholar]
- 44.Lee CH, Wu CL, Shiau AL. Endostatin gene therapy delivered by Salmonella choleraesuis in murine tumor models. Journal of Gene Medicine. 2004;6:1382–1393. doi: 10.1002/jgm.626. [DOI] [PubMed] [Google Scholar]
- 45.Zheng LM, et al. Tumor amplified protein expression therapy: Salmonella as a tumor-selective protein delivery vector. Oncology Research. 2000;12:127–135. doi: 10.3727/096504001108747602. [DOI] [PubMed] [Google Scholar]
- 46.Low KB, et al. Lipid A mutant Salmonella with suppressed virulence and TNFalpha induction retain tumor-targeting in vivo. Nat Biotechnol. 1999;17:37–41. doi: 10.1038/5205. [DOI] [PubMed] [Google Scholar]
- 47.Theys J, et al. Repeated cycles of Clostridium-directed enzyme prodrug therapy result in sustained antitumour effects in vivo. Br J Cancer. 2006;95:1212–1219. doi: 10.1038/sj.bjc.6603367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao M, et al. Targeted therapy with a Salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Research. 2006;66:7647–7652. doi: 10.1158/0008-5472.CAN-06-0716. [DOI] [PubMed] [Google Scholar]
- 49.Streilein JW. Unraveling immune privilege. Science. 1995;270:1158–1159. doi: 10.1126/science.270.5239.1158. [DOI] [PubMed] [Google Scholar]
- 50.Westphal K, Leschner S, Jablonska J, Loessner H, Weiss S. Containment of tumor-colonizing bacteria by host neutrophils. Cancer Research. 2008;68:2952–2960. doi: 10.1158/0008-5472.CAN-07-2984. [DOI] [PubMed] [Google Scholar]
- 51.Lee CH, Wu CL, Shiau AL. Systemic administration of attenuated Salmonella choleraesuis carrying thrombospondin-1 gene leads to tumor-specific transgene expression, delayed tumor growth and prolonged survival in the murine melanoma model. Cancer Gene Therapy. 2005;12:175–184. doi: 10.1038/sj.cgt.7700777. [DOI] [PubMed] [Google Scholar]
- 52.Lee CH, Wu CL, Tai YS, Shiau AL. Systemic administration of attenuated Salmonella choleraesuis in combination with cisplatin for cancer therapy. Molecular Therapy. 2005;11:707–716. doi: 10.1016/j.ymthe.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 53.Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465. [PubMed] [Google Scholar]
- 54.Heimann DM, Rosenberg SA. Continuous intravenous administration of live genetically modified salmonella typhimurium in patients with metastatic melanoma. J Immunother. 2003;26:179–180. doi: 10.1097/00002371-200303000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Toso JF, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. Journal of Clinical Oncology. 2002;20:142–152. doi: 10.1200/JCO.2002.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nemunaitis J, et al. Pilot trial of genetically modified, attenuated Salmonella expressing the E. coli cytosine deaminase gene in refractory cancer patients. Cancer Gene Ther. 2003;10:737–744. doi: 10.1038/sj.cgt.7700634. [DOI] [PubMed] [Google Scholar]
- 57.Hall SS. A commotion in the blood : life, death, and the immune system. New York: Henry Holt; 1997. [Google Scholar]
- 58.Coley WB. Contribution to the knowledge of sarcoma. Ann. Surgery. 1891;14:199–220. doi: 10.1097/00000658-189112000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nauts HC, Swift WE, Coley BL. The treatment of malignant tumors by bacterial toxins as developed by the late William B. Coley, MD, reviewed in the light of modern research. Cancer Research. 1946;6:205–216. [PubMed] [Google Scholar]
- 60.Fensterle J, et al. Cancer immunotherapy based on recombinant Salmonella enterica serovar Typhimurium aroA strains secreting prostate-specific antigen and cholera toxin subunit B. Cancer Gene Ther. 2008;15:85–93. doi: 10.1038/sj.cgt.7701109. [DOI] [PubMed] [Google Scholar]
- 61.Mottram JC. Factors of importance in radiosensitivity of tumors. Br J Radiol. 1936;9:606–614. [Google Scholar]
- 62.Möse JR, Möse G. Oncogenesis by clostridia. I. Activity of Clostridium butyricum (M-55) and other nonpathogenic clostridia against the Ehrlich carcinoma. Cancer Res. 1964;24:212–216. [PubMed] [Google Scholar]
- 63.Carey RW, Holland JF, Whang HY, Neter E, Bryant B. Clostridial oncolysis in man. Europ J Cancer. 1967;3:37–46. [Google Scholar]
- 64.Lee CH, Wu CL, Shiau AL. Salmonella choleraesuis as an anticancer agent in a syngeneic model of orthotopic hepatocellular carcinoma. International Journal of Cancer. 2008;122:930–935. doi: 10.1002/ijc.23047. [DOI] [PubMed] [Google Scholar]
- 65.Thamm DH, et al. Systemic administration of an attenuated, tumor-targeting Salmonella typhimurium to dogs with spontaneous neoplasia: Phase I evaluation. Clinical Cancer Research. 2005;11:4827–4834. doi: 10.1158/1078-0432.CCR-04-2510. [DOI] [PubMed] [Google Scholar]
- 66.Jia LJ, et al. Oral delivery of tumor-targeting Salmonella for cancer therapy in murine tumor models. Cancer Science. 2007;98:1107–1112. doi: 10.1111/j.1349-7006.2007.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen G, et al. Oral delivery of tumor-targeting Salmonella exhibits promising therapeutic efficacy and low toxicity. Cancer Science. 2009;100:2437–2443. doi: 10.1111/j.1349-7006.2009.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bermudes D, Low B, Pawelek J. Cancer Gene Therapy. 2000:57–63. [Google Scholar]
- 69.Hedley D, Ogilvie L, Springer C. Carboxypeptidase-G2-based gene-directed enzyme-prodrug therapy: a new weapon in the GDEPT armoury. Nat Rev Cancer. 2007;7:870–879. doi: 10.1038/nrc2247. [DOI] [PubMed] [Google Scholar]
- 70.Brown JM, Wilson WR. Exploiting tumour hypoxia in cancer treatment. Nature Reviews Cancer. 2004;4:437–447. doi: 10.1038/nrc1367. [DOI] [PubMed] [Google Scholar]
- 71.Walczak H, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 72.Barbe S, et al. Secretory production of biologically active rat interleukin-2 by Clostridium acetobutylicum DSM792 as a tool for anti-tumor treatment. FEMS Microbiol Lett. 2005;246:67–73. doi: 10.1016/j.femsle.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 73.Saltzman DA, et al. Attenuated Salmonella typhimurium containing interleukin-2 decreases MC-38 hepatic metastases: a novel anti-tumor agent. Cancer Biother Radiopharm. 1996;11:145–153. doi: 10.1089/cbr.1996.11.145. [DOI] [PubMed] [Google Scholar]
- 74.Loeffler M, Le’Negrate G, Krajewska M, Reed JC. IL-18-producing Salmonella inhibit tumor growth. Cancer Gene Therapy. 2008;15:787–794. doi: 10.1038/cgt.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sorenson BS, Banton KL, Frykman NL, Leonard AS, Saltzman DA. Attenuated Salmonella typhimurium with interleukin 2 gene prevents the establishment of pulmonary metastases in a model of osteosarcoma. J Pediatr Surg. 2008;43:1153–1158. doi: 10.1016/j.jpedsurg.2008.02.048. [DOI] [PubMed] [Google Scholar]
- 76.Sorenson BS, Banton KL, Frykman NL, Leonard AS, Saltzman DA. Attenuated Salmonella typhimurium with IL-2 gene reduces pulmonary metastases in murine osteosarcoma. Clin Orthop Relat Res. 2008;466:1285–1291. doi: 10.1007/s11999-008-0243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Al-Ramadi BK, et al. Potent anti-tumor activity of systemically-administered IL-2-expressing Salmonella correlates with decreased angiogenesis and enhanced tumor apoptosis. Clinical Immunology. 2009;130:89–97. doi: 10.1016/j.clim.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 78.Barnett SJ, et al. Attenuated Salmonella typhimurium invades and decreases tumor burden in neuroblastoma. J Pediatr Surg. 2005;40:993–997. doi: 10.1016/j.jpedsurg.2005.03.015. discussion 997−8. [DOI] [PubMed] [Google Scholar]
- 79.Feltis BA, et al. Liver and circulating NK1.1(+)CD3(-) cells are increased in infection with attenuated Salmonella typhimurium and are associated with reduced tumor in murine liver cancer. Journal of Surgical Research. 2002;107:101–107. doi: 10.1006/jsre.2002.6428. [DOI] [PubMed] [Google Scholar]
- 80.Saltzman DA et al. Antitumor mechanisms of attenuated Salmonella typhimurium containing the gene for human interleukin-2: a novel antitumor agent? J Pediatr Surg. 1997;32:301–306. doi: 10.1016/s0022-3468(97)90198-6. [DOI] [PubMed] [Google Scholar]
- 81.Lee SR, et al. Multi-Immunogenic Outer Membrane Vesicles Derived from a MsbB-Deficient Salmonella enterica Serovar Typhimurium Mutant. Journal of Microbiology and Biotechnology. 2009;19:1271–1279. [PubMed] [Google Scholar]
- 82.Nishikawa H, et al. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J Clin Invest. 2006;116:1946–1954. doi: 10.1172/JCI28045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Groot AJ, et al. Functional antibodies produced by oncolytic clostridia. Biochemical and Biophysical Research Communications. 2007;364:985–989. doi: 10.1016/j.bbrc.2007.10.126. [DOI] [PubMed] [Google Scholar]
- 84.Sizemore DR, Branstrom AA, Sadoff JC. Attenuated Shigella as a DNA delivery vehicle for DNA-mediated immunization. Science. 1995;270:299–302. doi: 10.1126/science.270.5234.299. [DOI] [PubMed] [Google Scholar]
- 85.Darji A, et al. Oral somatic transgene vaccination using attenuated S. typhimurium. Cell. 1997;91:765–775. doi: 10.1016/s0092-8674(00)80465-1. [DOI] [PubMed] [Google Scholar]
- 86.Weiss S, Chakraborty T. Transfer of eukaryotic expression plasmids to mammalian host cells by bacterial carriers. Curr Opin Biotechnol. 2001;12:467–472. doi: 10.1016/s0958-1669(00)00247-0. [DOI] [PubMed] [Google Scholar]
- 87.Palffy R, et al. Bacteria in gene therapy: bactofection versus alternative gene therapy. Gene Ther. 2006;13:101–105. doi: 10.1038/sj.gt.3302635. [DOI] [PubMed] [Google Scholar]
- 88.Fu W, Chu L, Han XW, Liu XY, Ren DM. Synergistic antitumoral effects of human telomerase reverse transcriptase-mediated dual-apoptosis-related gene vector delivered by orally attenuated Salmonella enterica Serovar Typhimurium in murine tumor models. Journal of Gene Medicine. 2008;10:690–701. doi: 10.1002/jgm.1191. [DOI] [PubMed] [Google Scholar]
- 89.Li YH, et al. Prophylaxis of tumor through oral administration of IL-12 GM-CSF gene carried by live attenuated salmonella. Chinese Science Bulletin. 2001;46:1107–1112. [Google Scholar]
- 90.Li YH, et al. Oral cytokine gene therapy against murine tumor using attenuated Salmonella typhimurium. International Journal of Cancer. 2001;94:438–443. doi: 10.1002/ijc.1489. [DOI] [PubMed] [Google Scholar]
- 91.Qi H, Li YH, Zheng SB. [Oral gene therapy via live attenuated Salmonella leads to tumor regression and survival prolongation in mice] Nan Fang Yi Ke Da Xue Xue Bao. 2006;26:1738–1741. [PubMed] [Google Scholar]
- 92.Yoon WS, Choi WC, Sin JI, Park YK. Antitumor therapeutic effects of Salmonella typhimurium containing Flt3 Ligand expression plasmids in melanoma-bearing mouse. Biotechnology Letters. 2007;29:511–516. doi: 10.1007/s10529-006-9270-9. [DOI] [PubMed] [Google Scholar]
- 93.Zuo SG, et al. Orally administered DNA vaccine delivery by attenuated Salmonella typhimurium targeting fetal liver kinase 1 inhibits murine Lewis lung carcinoma growth and metastasis. Biol Pharm Bull. 2010;33:174–182. doi: 10.1248/bpb.33.174. [DOI] [PubMed] [Google Scholar]
- 94.Feng K, et al. Anti-angiogenesis effect on glioma of attenuated Salmonella typhimurium vaccine strain with flk-1 gene. J Huazhong Univ Sci Technolog Med Sci. 2004;24:389–391. doi: 10.1007/BF02861875. [DOI] [PubMed] [Google Scholar]
- 95.Chou CK, Hung JY, Liu JC, Chen CT, Hung MC. An attenuated Salmonella oral DNA vaccine prevents the growth of hepatocellular carcinoma and colon cancer that express alpha-fetoprotein. Cancer Gene Therapy. 2006;13:746–752. doi: 10.1038/sj.cgt.7700927. [DOI] [PubMed] [Google Scholar]
- 96.Zhang L, et al. Intratumoral delivery and suppression of prostate tumor growth by attenuated Salmonella enterica serovar typhimurium carrying plasmid-based small interfering RNAs. Cancer Research. 2007;67:5859–5864. doi: 10.1158/0008-5472.CAN-07-0098. [DOI] [PubMed] [Google Scholar]
- 97.Yang N, Zhu X, Chen L, Li S, Ren D. Oral administration of attenuated S. typhimurium carrying shRNA-expressing vectors as a cancer therapeutic. Cancer Biol Ther. 2008;7:145–151. doi: 10.4161/cbt.7.1.5195. [DOI] [PubMed] [Google Scholar]
- 98.Guzman LM, Belin D, Carson MJ, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Royo JL, et al. In vivo gene regulation in Salmonella spp. by a salicylate-dependent control circuit. Nature Methods. 2007;4:937–942. doi: 10.1038/nmeth1107. [DOI] [PubMed] [Google Scholar]
- 100.Nuyts S, et al. Insertion or deletion of the Cheo box modifies radiation inducibility of Clostridium promoters. Appl Environ Microbiol. 2001;67:4464–4470. doi: 10.1128/AEM.67.10.4464-4470.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mengesha A, et al. Development of a flexible and potent hypoxia-inducible promoter for tumor-targeted gene expression in attenuated Salmonella. Cancer Biology & Therapy. 2006;5:1120–1128. doi: 10.4161/cbt.5.9.2951. [DOI] [PubMed] [Google Scholar]
- 102.Strauch KL, Lenk JB, Gamble BL, Miller CG. Oxygen regulation in Salmonella typhimurium. J Bacteriol. 1985;161:673–680. doi: 10.1128/jb.161.2.673-680.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Arrach N, Zhao M, Porwollik S, Hoffman RM, McClelland M. Salmonella promoters preferentially activated inside tumors. Cancer Res. 2008;68:4827–4832. doi: 10.1158/0008-5472.CAN-08-0552. [DOI] [PubMed] [Google Scholar]
- 104.Min JJ, et al. Noninvasive real-time imaging of tumors and metastases using tumor-targeting light-emitting Escherichia coli. Molecular Imaging and Biology. 2008;10:54–61. doi: 10.1007/s11307-007-0120-5. [DOI] [PubMed] [Google Scholar]
- 105.Min JJ, Nguyen VH, Kim HJ, Hong YJ, Choy HE. Quantitative bioluminescence imaging of tumor-targeting bacteria in living animals. Nat Protoc. 2008;3:629–636. doi: 10.1038/nprot.2008.32. [DOI] [PubMed] [Google Scholar]
- 106.Cheng CM, et al. Tumor-targeting prodrug-activating bacteria for cancer therapy. Cancer Gene Therapy. 2008;15:393–401. doi: 10.1038/cgt.2008.10. [DOI] [PubMed] [Google Scholar]
- 107.Gericke D, Engelbart K. Oncolysis by Clostridia .2. Experiments on tumor spectrum with variety of Clostridia in combination with heavy metal. Cancer Research. 1964;24:217. [PubMed] [Google Scholar]
- 108.Dang LH, et al. Targeting vascular and avascular compartments of tumors with C. novyi-NT and anti-microtubule agents. Cancer Biology & Therapy. 2004;3:326–337. doi: 10.4161/cbt.3.3.704. [DOI] [PubMed] [Google Scholar]
- 109.Bettegowda C, et al. Overcoming the hypoxic barrier to radiation therapy with anaerobic bacteria. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15083–15088. doi: 10.1073/pnas.2036598100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cheong I, et al. A bacterial protein enhances the release and efficacy of liposomal cancer drugs. Science. 2006;314:1308–1311. doi: 10.1126/science.1130651. [DOI] [PubMed] [Google Scholar]
- 111.Voigt CA. Genetic parts to program bacteria. Curr Opin Biotechnol. 2006;17:548–557. doi: 10.1016/j.copbio.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 112.Pfleger BF, Pitera DJ, Smolke CD, Keasling JD. Combinatorial engineering of intergenic regions in operons tunes expression of multiple genes. Nat Biotechnol. 2006;24:1027–1032. doi: 10.1038/nbt1226. [DOI] [PubMed] [Google Scholar]
- 113.Salis HM, Mirsky EA, Voigt CA. Automated design of synthetic ribosome binding sites to control protein expression. Nat Biotechnol. 2009;27:946–950. doi: 10.1038/nbt.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ohl ME, Miller SI. Salmonella: a model for bacterial pathogenesis. Annu Rev Med. 2001;52:259–274. doi: 10.1146/annurev.med.52.1.259. [DOI] [PubMed] [Google Scholar]
- 115.Engelbart K, Gericke D. Oncolysis by Clostridia .V. Transplanted tumors of hamster. Cancer Research. 1964;24:239. [PubMed] [Google Scholar]
- 116.Thiele EH, Boxer GE, Arison RN. Oncolysis by Clostridia .3. Effects of Clostridia + chemotherapeutic agents on rodent tumors. Cancer Research. 1964;24:222. [PubMed] [Google Scholar]
- 117.Mohr U, Boldingh WH, Behagel HA, Emminger A. Oncolysis by a new strain of Clostridium. Cancer Research. 1972;32:1122. [PubMed] [Google Scholar]
- 118.Weibel S, Stritzker J, Eck M, Goebel W, Szalay AA. Colonization of experimental murine breast tumours by Escherichia coli K-12 significantly alters the tumour microenvironment. Cellular Microbiology. 2008;10:1235–1248. doi: 10.1111/j.1462-5822.2008.01122.x. [DOI] [PubMed] [Google Scholar]
- 119.Luo X, et al. Antitumor effect of VNP20009, an attenuated Salmonella, in murine tumor models. Oncol Res. 2001;12:501–508. doi: 10.3727/096504001108747512. [DOI] [PubMed] [Google Scholar]
- 120.Rosenberg SA, Spiess PJ, Kleiner DE. Antitumor effects in mice of the intravenous injection of attenuated Salmonella typhimurium. Journal of Immunotherapy. 2002;25:218–225. doi: 10.1097/01.CJI.0000014623.45316.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao M, et al. Monotherapy with a tumor-targeting mutant of Salmonella typhimurium cures orthotopic metastatic mouse models of human prostate cancer. Proc Natl Acad Sci U S A. 2007;104:10170–10174. doi: 10.1073/pnas.0703867104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kimura H, et al. Targeted therapy of spinal cord glioma with a genetically modified Salmonella typhimurium. Cell Prolif. 2010;43:41–48. doi: 10.1111/j.1365-2184.2009.00652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jia LJ, et al. Enhanced therapeutic effect by combination of tumor-targeting Salmonella and endostatin in murine melanoma model. Cancer Biol Ther. 2005;4:840–845. doi: 10.4161/cbt.4.8.1891. [DOI] [PubMed] [Google Scholar]
- 124.Platt J, et al. Antitumour effects of genetically engineered Salmonella in combination with radiation. European Journal of Cancer. 2000;36:2397–2402. doi: 10.1016/s0959-8049(00)00336-1. [DOI] [PubMed] [Google Scholar]
- 125.Shilling DA, et al. Salmonella typhimurium stimulation combined with tumour-derived heat shock proteins induces potent dendritic cell anti-tumour responses in a murine model. Clin Exp Immunol. 2007;149:109–116. doi: 10.1111/j.1365-2249.2007.03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Al-Ramadi BK, et al. Attenuated bacteria as effectors in cancer immunotherapy. Ann N Y Acad Sci. 2008;1138:351–357. doi: 10.1196/annals.1414.036. [DOI] [PubMed] [Google Scholar]
- 127.Liu SC, Minton NP, Giaccia AJ, Brown JM. Anticancer efficacy of systemically delivered anaerobic bacteria as gene therapy vectors targeting tumor hypoxia/necrosis. Gene Therapy. 2002;9:291–296. doi: 10.1038/sj.gt.3301659. [DOI] [PubMed] [Google Scholar]
- 128.Liu SC, et al. Optimized Clostridium-directed enzyme prodrug therapy improves the antitumor activity of the novel DNA cross-linking agent PR-104. Cancer Research. 2008;68:7995–8003. doi: 10.1158/0008-5472.CAN-08-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dubois L, et al. Efficacy of gene therapy-delivered cytosine deaminase is determined by enzymatic activity but not expression. Br J Cancer. 2007;96:758–761. doi: 10.1038/sj.bjc.6603624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jazowiecka-Rakus J, Szala S. Antitumour activity of Salmonella typhimurium VNP20047 in B16(F10) murine melanoma model. Acta Biochimica Polonica. 2004;51:851–856. [PubMed] [Google Scholar]
- 131.Friedlos F, et al. Attenuated Salmonella targets prodrug activating enzyme carboxypeptidase G2 to mouse melanoma and human breast and colon carcinomas for effective suicide gene therapy. Clinical Cancer Research. 2008;14:4259–4266. doi: 10.1158/1078-0432.CCR-07-4800. [DOI] [PubMed] [Google Scholar]
- 132.Fu W, et al. Synergistic antitumor efficacy of suicide/ePNP gene and 6-methylpurine 2'-deoxyriboside via Salmonella against murine tumors. Cancer Gene Therapy. 2008;15:474–484. doi: 10.1038/cgt.2008.19. [DOI] [PubMed] [Google Scholar]
- 133.Fu W, Lan HK, Liang SH, Gao T, Ren DM. Suicide gene/prodrug therapy using salmonella-mediated delivery of Escherichia coli purine nucleoside phosphorylase gene and 6-methoxypurine 2 '-deoxyriboside in murine mammary carcinoma 4T1 model. Cancer Science. 2008;99:1172–1179. doi: 10.1111/j.1349-7006.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mei S, Theys J, Landuyt W, Anne J, Lambin P. Optimization of tumor-targeted gene delivery by engineered attenuated Salmonella typhimurium. Anticancer Res. 2002;22:3261–3266. [PubMed] [Google Scholar]
- 135.Hayashi K, et al. Cancer metastasis directly eradicated by targeted therapy with a modified Salmonella typhimurium. J Cell Biochem. 2009;106:992–998. doi: 10.1002/jcb.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hayashi K, et al. Systemic targeting of primary bone tumor and lung metastasis of high-grade osteosarcoma in nude mice with a tumor-selective strain of Salmonella typhimurium. Cell Cycle. 2009;8:870–875. doi: 10.4161/cc.8.6.7891. [DOI] [PubMed] [Google Scholar]
- 137.Dresselaers T, et al. Non-invasive 19F MR spectroscopy of 5-fluorocytosine to 5-fluorouracil conversion by recombinant Salmonella in tumours. Br J Cancer. 2003;89:1796–1801. doi: 10.1038/sj.bjc.6601345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Heppner F, Mose JR. The liquefaction (oncolysis) of malignant gliomas by a non pathogenic Clostridium. Acta Neurochir (Wien) 1978;42:123–125. doi: 10.1007/BF01406639. [DOI] [PubMed] [Google Scholar]