Abstract
Objectives
To perform a systematic review of randomized controlled trials to determine whether prevention or slowing of progression of chronic kidney disease would translate into improved mortality, and if so, the attributable risk due to CKD itself on mortality.
Background
CKD is associated with increased mortality. This association is largely based on evidence from the observational studies and evidence from randomized controlled trials is lacking.
Methods
We searched Ovid, Medline and Embase for RCTs in which an intervention was given to prevent or slow the progression of CKD and mortality was reported as primary, secondary or adverse outcomes were eligible and selected. For the first phase, pooled relative risks for renal endpoints were assessed. For the second phase, we assessed the effect on mortality in trials of interventions that definitively reduced CKD endpoints.
Results
Among 52 studies selected in first phase, only renin-angiotensin-aldosterone-system blockade vs. placebo (n = 18 trials, 32,557 participants) met the efficacy criteria for further analysis in the second phase by reducing renal endpoints 15 to 27% compared to placebo. There was no difference in all-cause mortality (RR 0.99, 95% CI 0.92 to 1.08) or CV death (RR 0.97, 95% CI 0.78 to 1.21) between the treatment and control groups in these trials. There was sufficient statistical power to detect a 9% relative risk reduction in all-cause mortality and a 14% relative risk reduction in cardiovascular mortality.
Conclusions
Firm evidence is lacking that prevention of CKD translates into reductions in mortality. Larger trials with longer follow-up time are needed to determine the benefit of CKD prevention on survival.
Introduction
Chronic kidney disease (CKD) represents an increasing burden on health care systems worldwide. The prevalence of CKD has increased over the past several years. It is currently estimated that 17% of people in the United States have CKD, and worldwide the prevalence is 23–36% in people aged ≥64 [1], [2]. Over the past several years, it has been generally accepted in the medical literature and community that CKD is independently associated with premature mortality [3]–[5].
In order to confirm that a nontraditional factor, such as CKD, is a causal risk factor for mortality, the following conditions should be met: (i) biological plausibility as to why the factor may promote premature mortality; (ii) demonstration that the mortality risk increases with severity of CKD; (iii) demonstration of an association between the CKD and mortality in observational studies; and (iv) demonstration in placebo-controlled clinical trials that treatment of CKD decreases mortality. There is an abundance of evidence for first three conditions, however, the veracity of the last condition is largely unproven.[6]–[10] Randomized controlled trials eliminate the possibility that other conditions such as diabetes and hypertension, which cause CKD, confound the observed association between CKD and mortality. This is with the recognition though that an intervention’s effects may be complex, impacting an outcome such as mortality through many potential pathways one of which may be CKD prevention. A sufficient amount of time would also need to elapse since an intermediate endpoint such as CKD was prevented, before one would expect to see a reduction in subsequent attributable deaths.
Furthermore, even evidence from recent observational studies also questions the causal relationship between decreased glomerular filtration rate (GFR) and mortality. Garg et al. demonstrated the risk for cardiovascular events and death in people who donate a kidney were no higher in the first decade after transplantation than in matched non-donors [11]. Wald et al. used a large Ontario database to perform a propensity score matched cohort analysis and found that survivors of acute kidney injury that required dialysis had a significantly elevated risk for development of end stage renal disease (adjusted hazard ratio 3.2) but all-cause mortality rates were not elevated (adjusted hazard ratio 0.95) [12].
With this background, we performed a systematic review and meta-analysis of randomized controlled trials (RCTs) to determine whether interventions that are efficacious for reducing the incidence or progression of CKD result in a commensurate reduction in mortality (cardiovascular or all-cause).
Methods
We used a standardized protocol to search the published literature and identify trials for our analysis.
Literature Sources and Search Terms
We performed an exhaustive search and evaluation of peer-reviewed research published between 1948 and July 2011, including Ovid, MEDLINE and Scopus (EMBASE). We used many search terms and filters that include “exp renal insufficiency, chronic”, “hypertension, renal”, “proteinuria”, “diabetic nephropathies”, “disease progression”, “survival analysis”, “treatment outcomes”, “mortality.mp.” and “randomized controlled trials”. The search was limited to randomized controlled trials that studied human subjects without language restriction. All efforts were made to obtain the English translation of the trials published in non-English languages.
Study Selection and Data Collection Process
We included trials with both CKD and non-CKD participants. We included trials that reported CKD outcomes along with mortality as primary, secondary or adverse outcomes. All included trials had a mean/median follow up time of at least 1 year and a sample size of at least 100 total participants. Two authors independently reviewed the references and resolved disagreements by discussion. The authors analyzed the quality of reporting by using the Cochrane Collaboration’s tool for assessing risk of bias: randomization method, allocation concealment, blinding of participants, staff and assessors, selective reporting (for renal endpoints), and description of withdrawals. [13] After careful assessment, we included a total of 52 trials for data abstraction.
Data Abstraction
We used a standardized data abstraction form for description of the trial, such as the title of the study, the authors, year of publication, country, trial design, number of participants and their baseline characteristics. We also abstracted the type of intervention and the incidence of primary and secondary outcomes in the control and treatment groups.
Outcome Measures
The renal endpoints included the following: doubling of serum creatinine (sCr), End Stage Renal Disease (ESRD) defined as initiation of dialysis or renal transplant, composite of doubling of serum creatinine or ESRD, albuminuria/proteinuria (incidence, progression, regression). The non-renal endpoints were cardiovascular (CV) death and all-cause mortality.
Statistical Analysis
Trials were grouped by type of intervention and then were analyzed in two phases. In the first phase of the analysis, pooled relative risks (RR) for each of the renal endpoints for each treatment vs. control were computed with Mantel-Haenszel statistics. Interstudy heterogeneity was calculated using the Chi2 method and the I2 statistic. A priori, we decided that if the pooled upper bound of the 95% confidence interval (CI) was <1 and at least 3 individual studies demonstrated efficacy for the renal endpoints as evidenced by an upper bound of the 95% CI <1, then the intervention was deemed “efficacious for prevention of incident or progressive CKD” and advanced to the second phase. We combined the trials evaluating either angiotensin converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARBs) as one intervention called “RAAS blockade”. In the second phase, the association between the renoprotective intervention and all-cause and cardiovascular mortality was assessed. The results were considered significant with 2-sided α error <0.05. All the results are reported with 95% CIs. Statistical calculations and graphs were made using the Review Manager (RevMan), Version 5.1. Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2011.
In addition, for the second phase, we performed sensitivity analyses that examined the pooled relative risk of CV death and all-cause mortality by pooling only the trials within the type of intervention that demonstrated efficacy for the treatment for the CKD outcome. We also explored the diversity in study results and possible association of certain covariates to hard outcomes by performing sub-group analyses and by using meta-regression. For each meta-regression and subgroup analysis, only studies for which the factor of interest was available were included in the analysis. The statistical significance was determined by the proportion of variability explained by each study level characteristic and from the size of residual variance [14]. Best-fit lines in meta-regression graphs were estimated by generalized estimating equations using estimates from meta-regression models as the input values and were weighted by the variance of each estimate [15]. Meta-regression analyses were conducted in SAS 9.3 and R 2.15.0.
Results
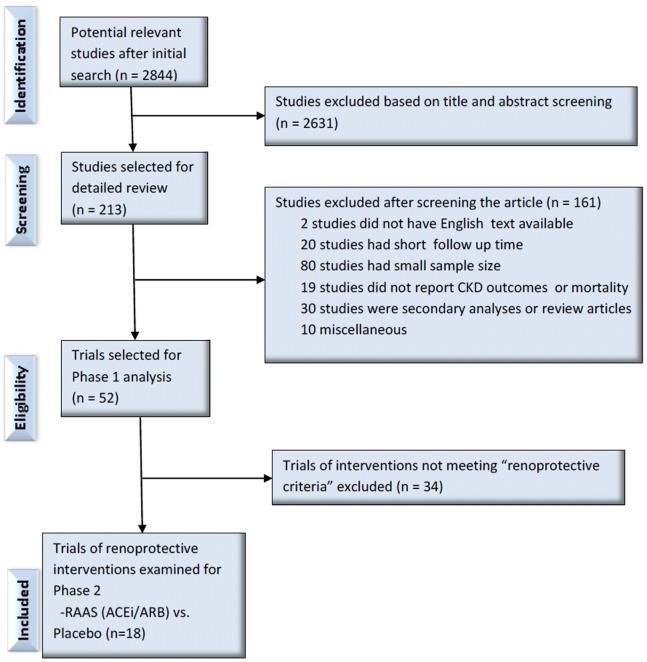
Our search identified 2844 citations for the first phase. Based on title and abstract review, we excluded 2631 citations. After a detailed review of 213 citations we further excluded 161 for various reasons. Among those, 20 trials had follow up time less than a year, 80 trials had small sample size, 19 trials did not report CKD outcomes or mortality, 30 citations were review articles or secondary analyses and 10 were excluded based on miscellaneous reasons (Figure 1). We also were unable to obtain full text in English and excluded those 2 trials. After a careful and thorough review, we included 52 trials in phase 1 [16]–[70]. The characteristics of these trials are listed in Table 1.
Figure 1. Flow Diagram of Study Selection Process.
Table 1. Baseline Study Characteristics of All Studies in Phase 1.
| STUDY | Sample | Follow up | Age (y) | Male | Clinical | Baseline Renal Function Indicators | Co-Morbidities (%) | Country | |||||
| Size | in years (mean/median) | mean | (%) | Setting | sCr eGFR/CrCl MicroAlb MacroAlb mg/dl ml/min/1.73 m2 (%) (%) | DM HTN CVD | |||||||
| UKPDS34 1998 | 753 | 10.7 | 53 | 46 | T2DM | 0.9 (0.7–1.1) | – | 0 | 2.2 | 100 | UK | ||
| UKPDS33 1998 | 3867 | 10 | 53.3 | 61 | T2DM | 0.9 (0.7–1.1) | – | 0 | 1.9 | 100 | UK | ||
| Rachmani R et al 2004 | 141 | 7.7 | 57.1 | 49 | T2DM, HTN | – | 106±9.5 | 100 | 0 | 100 | 100 | 0 | Israel |
| DCCT 1993 | 1441 | 6.5 | 27 | 53 | IDDM | – | 128±30 | 0 | 0 | 100 | USA, Canada | ||
| Kumamoto 1995 | 110 | 6 | 49 | 44.5 | T2DM | – | – | 46 | 0 | 100 | 0 | 0 | Japan |
| VADT 2009 | 1791 | 5.6 | 60.4 | 97 | T2DM, HTN | 1.0±0.2 | – | – | – | 100 | 72 | 40 | USA |
| 4S | 3842 | 5.5 | 60 | 69 | CHD, CKD, DM | 1.1±0.2 | 66.5±8.4 | – | – | 3.5 | 31 | 100 | Europe, USA |
| AFCAPS-TexCAPS 2010 | 4994 | 5.3 | 60 | 81.7 | HLP | 1.1±0.2 | 71±12 | – | – | 2 | 28.5 | 0 | USA |
| ADVANCE 2008 | 11140 | 5 | 66 | 57.5 | T2DM, CVD, HTN | 0.9±0.3 | – | 26.8 | 3.6 | 100 | 68.5 | 32 | Multicontinental |
| Mauer M, et al 2009 | 256 | 5 | 29.9 | 46 | T1DM | – | 129±20 | 0 | 0 | 100 | 0 | USA, Canada | |
| SHARP 2011 | 9270 | 4.9 | 62 | 63 | CKD | – | 26.6±13 | 38 | 42 | 23 | 100 | 15 | Multicontinental |
| ALLHAT_BP 2005 | 33357 | 4.8 | 67 | 52.3 | HTN, CVD, T2DM, CKD | – | 76±10 | – | – | 39 | 100 | 26 | USA, Canada |
| ALLHAT_LIPID 2008 | 10060 | 4.8 | 66.6 | 51.2 | HTN, CVD, T2DM, CKD | – | 78.5±19 | – | – | 35.1 | 100 | 36.6 | USA, Canada |
| TRANSCEND 2009 | 5926 | 4.6 | 66.9 | 57 | CVD, T2DM | 1.0±0.3 | – | 10.3 | 0 | 35.7 | 76.4 | 74.5 | Multicontinental |
| ONTARGET 2008 | 25620 | 4.6 | 66.4 | 73 | CVD, T2DM | 1.1±0.2 | – | 13.2 | – | 38 | 69 | 85 | Multicontinental |
| HOPE 2000 | 3577 | 4.5 | 65.4 | 63 | CVD, T2DM | 1.0±0.3 | – | 32 | 0 | 98 | 56 | 60 | Europe, Canada |
| BENEDICT-B 2011 | 281 | 4.5 | 62.3 | 73.7 | HTN, T2DM | 0.9±0.2 | – | 100 | 0 | 100 | 100 | – | Multicontinental |
| ADVANCE 2007 | 11140 | 4.3 | 66 | 57 | T2DM, CVD | 0.9±0.3 | 26 | 4 | 100 | 32 | Multicontinental | ||
| DIRECT 2009 | 3322 | 4 | 40.3 | 54.6 | T1DM | 1.0±0.2 | – | 0 | 0 | 100 | 23 | – | Multicontinental |
| DIABHYCAR 2004 | 4912 | 4 | 65.1 | 69.8 | T2DM | 1.0±0.2 | – | 74 | 26 | 100 | 55.7 | 24.4 | Multicontinental |
| Fogari R et al 2002 | 309 | 4 | 62 | 56 | T2DM, HTN | 1.0±0.5 | 89.6±14.1 | 100 | 0 | 100 | 100 | 0 | Italy |
| Facchini F et al 2003 | 191 | 3.9 | 59.5 | 50.5 | T2DM, CKD | 1.8±0.6 | 63±30 | 0 | 100 | 100 | USA | ||
| Steno Type 2 1999 | 160 | 3.8 | 55.1 | 74 | T2DM | 0.8±0.1 | 117±24.5 | 100 | 0 | 100 | 20 | Denmark | |
| BENEDICT 2004 | 1204 | 3.6 | 62 | 52.7 | HTN, T2DM | 0.9±0.2 | – | 0 | 0 | 100 | 100 | – | Multicontinental |
| ACCORD 2010 | 10251 | 3.5 | 62 | T2DM, CVD | 0.9 (0.7–1.0) | 90 (76–105) | 26.5 | 6.5 | 100 | USA, Canada | |||
| RENAAL 2001 | 1513 | 3.4 | 60 | 63 | T2DM, HTN, CKD | 1.9±0.5 | – | 0 | 100 | 100 | 96 | 11 | Multicontinental |
| ROADMAP 2011 | 4447 | 3.2 | 57.7 | 46.1 | T2DM | 0.8±0.2 | 84.9±17.2 | 0 | 0 | 100 | 24.8 | Multicontinental | |
| CASE-J 2008 | 4703 | 3.2 | 63.8 | 55.1 | HTN, T2DM, CVD, CKD | – | – | – | – | 43 | 100 | 43.2 | Japan |
| AIPRI 1996 | 583 | 3 | 51 | 72 | CKD | 2.1±0.6 | 42.6±11.1 | 82 | Multicontinental | ||||
| CSG 1993 | 409 | 3 | 34.5 | 53 | IDDM, HTN | 1.3±0.4 | 81.5±40.5 | 100 | 75.5 | USA | |||
| AASK 2001 | 1094 | 3 | 54.3 | 61 | CKD with HtnNS, CVD | 1.9±0.7 | 46.4±13.4 | – | 33 | 0 | 100 | 52.5 | USA |
| Hannedouche T et al 1994 | 100 | 3 | 51 | 53 | CKD, HTN | 3.0±0.1 | – | 0 | 100 | 0 | 100 | 0 | France |
| ACCOMPLISH 2010 | 11506 | 2.9 | 70 | 64 | CVD, HTN, DM, CKD | 1.3±0.3 | 63.8±14 | 35 | 19 | 59.7 | 100 | 100 | Multicontinental |
| ACTION I 2004 | 690 | 2.8 | 39 | 59.3 | T1DM, CKD | 1.6±0.4 | 60±29 | 0 | 100 | 100 | 83 | USA, Canada | |
| DIVINe 2010 | 252 | 2.7 | 60.4 | 74.5 | DM, CKD, HTN | 1.5±1.0 | 54±27 | 0 | 100 | 82 | 93.7 | 31 | Canada |
| Ruggenenti 1999 | 186 | 2.7 | 49.7 | 75 | HTN, CKD | 2±0.1 | 46.5±18.2 | 0 | 100 | 0 | 82 | – | Italy |
| IDNT 2001 | 1148 | 2.6 | 58.9 | 66.3 | T2DM, HTN, CKD | 1.6±0.5 | – | 0 | 100 | 100 | 100 | 28.6 | Multicontinental |
| ATLANTIS 2000 | 134 | 2 | 40 | 45 | IDDM, HTN | – | 104±26 | 100 | 0 | 100 | – | – | UK, Ireland |
| Laffel L, et al 1995 | 143 | 2 | 32.7 | 50.3 | T1DM | 1.1±0.2 | 80±21.5 | 100 | 0 | 100 | 0 | 0 | USA, Canada |
| MCSG 1996 | 235 | 2 | 32 | 52 | IDDM | 0.9±0.2 | 95±35.5 | 100 | 0 | 100 | 0 | 0 | Multicontinental |
| IRMA 2 2001 | 590 | 2 | 58 | 68 | T2DM, HTN, CVD | 1.0±0.1 | 109±2 | 100 | 0 | 100 | 100 | 26.6 | Multicontinental |
| Stefoni S et al 1996 | 189 | 2 | 53.2 | 67 | CKD, HTN | 2.3±0.1 | 40.8±1.5 | 0 | 100 | 0 | Italy | ||
| SURE 2009 | 205 | 2 | 66 | 66.5 | T2DM, HTN, CKD | 100 | 96 | 15 | China | ||||
| Val-HeFT 2009 | 5010 | 1.9 | 62 | 77 | CVD, CHF, CKD, DM | – | 59.8±10.5 | – | – | 33.7 | 7.5 | 45 | USA |
| Goicoechea M et al 2010 | 113 | 1.9 | 71.8 | CKD | 1.8±0.5 | 40±11.9 | 37.5 | 27.5 | Spain | ||||
| REIN 2 2005 | 335 | 1.5 | 53.7 | 75 | CKD | 2.7±1.1 | 35±18.3 | 0 | 100 | 0 | Italy | ||
| REIN 1997 | 166 | 1.3 | 49.3 | 78 | HTN, CKD | 2.4±0.9 | 38.8±18.2 | 0 | 100 | 0 | 87 | Italy | |
| BEAM 2011 | 227 | 1 | 66.8 | 56 | T2DM, CKD | 2.0±0.5 | 32.4±6.9 | 29 | 34 | 100 | USA | ||
| CAP-KD 2009 | 479 | 1 | 63 | 33 | CKD | 2.6±1.0 | 22.3±10.8 | Japan | |||||
| DIAL 2004 | 180 | 1 | 59 | 73 | T2DM, HTN | 0.8±0.2 | – | 100 | 0 | 100 | 100 | 4 | Italy |
| GUARD 2008 | 304 | 1 | 57.7 | 65.4 | T2DM, HTN | – | 90.6 (46.2–177.5) | 100 | 100 | USA | |||
| NESTOR 2004 | 570 | 1 | 60 | 64 | T2DM, HTN | – | 92.5±29.3 | 100 | 0 | 100 | 100 | Multicontinental | |
We pooled studies by type of intervention and assessed the effects on renal endpoints (Table 2). Of all the interventions, only RAAS blockade (ACEi or ARB) vs. placebo consistently reduced renal endpoints as evidenced by a pooled upper bound of the 95% confidence interval (CI) <1 with at least 3 individual studies having an upper bound of the 95% CI <1. Thus, RAAS blockade was deemed “efficacious for prevention of incident or progressive CKD” and advanced to the second phase of our analysis. There were 18 trials of RAAS blockade vs. placebo [17], [18], [21], [24], [29], [31], [40], [47]–[51], [53], [54], [57], [69], [70]. Two of these trials had two treatment arms which were compared with the placebo separately. [54], [57] We report these data as two separate comparisons vs. placebo within each of these two trials, thus the total comparisons would be equal to 20 in the subsequent analyses.
Table 2. Studies included in Phase I Analysis grouped by Intervention.
| ACEi and/or ARB vs Placebo (N = 32557) | ACEi vs ACEi plus ARB |
| • Hope 2000 | • ONTARGET 2008 |
| • DIABHYCAR 2004 | ACEi vs Beta Blocker (N = 100) |
| • AIPRI 1996 | • Hannedouche T, et al 1994 |
| • CSG 1993 | ACEi vs CCB (N = 1274) |
| • Ruggenenti 1999 | • AASK 2001 |
| • ATLANTIS 2000 | • DIAL 2004 |
| • Laffel L, et al 1995 | ACEi vs Diuretic (N = 33927) |
| • MCSG 1996 | • NESTOR 2004 |
| • REIN 1997 | • ALLHAT_BP 2005 |
| • TRANSCEND 2009 | Allopurinol vs Control (N = 113) |
| • DIRECT 2009 | • Goicoechea M, et al 2010 |
| • RENAAL 2001 | Bardoxolone vs Placebo (N = 227) |
| • ROADMAP 2011 | • BEAM 2011 |
| • IRMA2 2001 | CCB vs Placebo (N = 2352) |
| • Val-HeFT 2009 | • BENEDICT 2004 |
| • Mauer M, et al 2009 | CCB vs Diuretic (N = 33357) |
| • BENEDICT 2004 | • ALLHAT_BP 2005 |
| • IDNT 2001 | |
| Intensive vs Conventional Glycemic Control (N = 29353)¥ | Conventional therapy vs Conventional plus AST-120 therapy for CKD (N = 479) |
| • UKPDS34 1998 | • CAP-KD 2009 |
| • UKPDS33 1998 | CCB vs ARB (N = 5851) |
| • DCCT 1993 | • CASE-J 2008 |
| • Kumamoto 1995 | • IDNT 2001 |
| • VADT 2009 | Intensive vs Conventional BP Control (N = 335) |
| • ADVANCE 2008 | • REIN 2 2005 |
| • ACCORD 2010 | Ibopamine vs Control (N = 189) |
| Usual vs Structured Care of CKD (N = 506) * | • Stefoni S, et al 1996 |
| • Rachmani R, et al 2004 | Statin vs Placebo (N = 18896) * |
| • Steno Type 2 1999 | • 4S |
| • SURE 2009 | • AFCAPS-TexCAPS 2010 |
| ACEi plus CCB vs ACEi (N = 509) | • ALLHAT_LIPID 2008 |
| • BENEDICT-B 2011 | Statin plus Ezetimibe vs Placebo (N = 9270) |
| • Fogari R, et al 2002 | • SHARP 2011 |
| ACEi plus CCB vs Placebo (N = 1204) | Pimagedline (AGE Inhibitor) vs Placebo (N = 690) |
| • BENEDICT 2004 | • ACTION I 2004 |
| ACEi plus CCB vs ACEi plus Diuretic (N = 11810) | CR-LIPE Diet vs Control (N = 191) |
| • ACCOMPLISH 2010 | • Facchini F, et al 2003 |
| • GUARD 2008 | Vitamin B vs Placebo (N = 252) |
| ACEi plus CCB vs CCB (N = 309) | • DIVINe 2010 |
| • Fogari R, et al 2002 | |
| ACEi plus Diuretics vs Placebo (N = 11140) | |
| • ADVANCE 2007 |
Micro and macroalbuminuria were the only renal outcomes improved by intervention. No beneficial effect of intervention reported for other renal outcomes (doubling of creatinine, ESRD), thus not forwarded to phase II analysis.
No beneficial effects seen for renal outcomes by this intervention thus not forwarded to phase II analysis.
ACEi Angiotensin converting enzyme inhibitor; ARB Angiotensin receptor blocker; CCB Calcium channel blocker; AST-120 an oral adsorbent agent; AGE Advanced glycation endproduct; CR-LIPE carbohydrate restricted low iron available polyphenol enriched diet.
A total of 32,557 patients were enrolled in these 18 RAAS trials. Study populations include adults with a mean age of participants 50.8 years. Approximately 62% were male. The median follow up time in these trials was 3 years with a range of 1.3 to 4.6 years. About 83% trials were in the setting of diabetes mellitus and 6 trials (33%) included patients with CKD (stage III or more and/or macroalbuminuria) [18], [22], [23], [25], [30], [31], [35], [39], [41], [43], [46], [53], [55], [60], [66], [67], [70]. The overall quality of studies included was good. All but 4 trials had low risk of selection bias due to random sequence generation although majority did not mention any specific methods for allocation concealment. Performance and detection biases were also low. About 25% trials had high risk of reporting bias due to selective reporting (Figures S1A and B).
Renal Endpoints
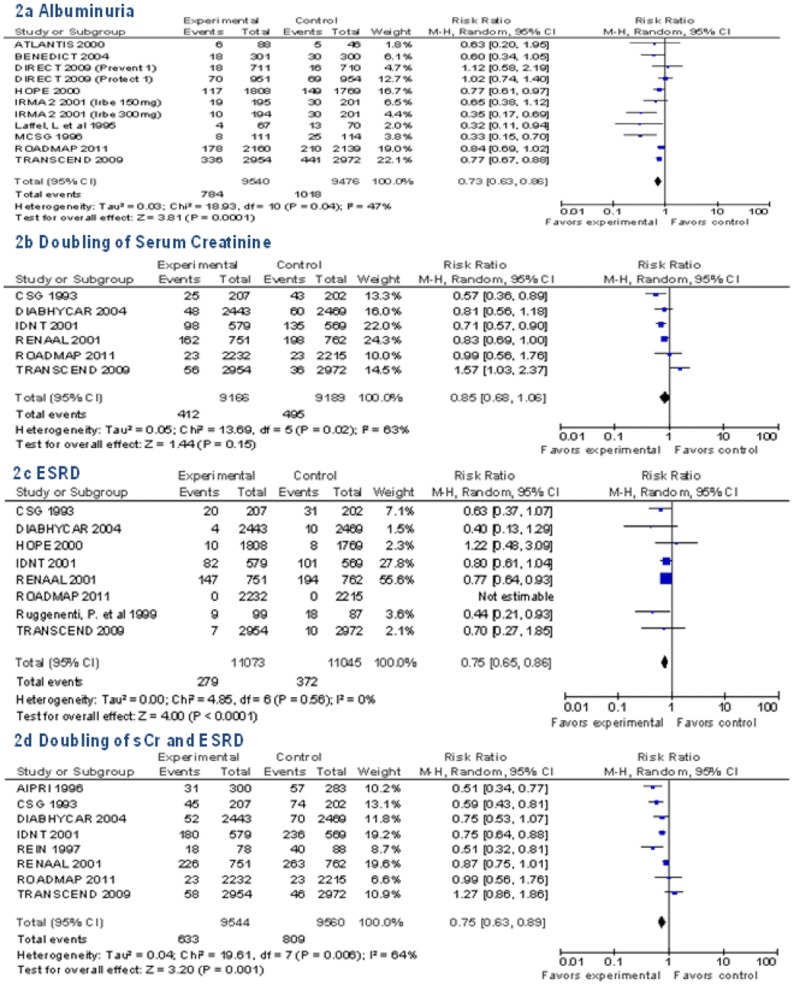
RAAS blockade vs. placebo was effective for the following renal endpoints: incident albuminuria was reduced by 27% (n = 11 trials, pooled RR 0.73 [95% CI 0.63 to 0.86], risk difference (RD) −3 [95% CI −5 to −1], I2 = 47%); ESRD by 25% (n = 8 trials, RR 0.75, [95% CI 0.65 to 0.86], RD −1 [95% CI −2 to 0], I2 = 0%); the composite endpoint of doubling of sCr and ESRD by 25% (n = 8 trials, RR 0.75 [95% CI 0.63 to 0.89], RD −4 [95% CI −6 to −2], I2 = 94%). There was a non-significant trend toward benefit for the endpoint of doubling of sCr (n = 6 trials, RR 0.85 [95% CI 0.68 to 1.06], RD −1 [95% CI −3 to 0], I2 = 90%), as shown in Figure 2.
Figure 2. Pooled Relative Risk for Renal Endpoints in Renin-Angiotensin-Aldosterone-System (RAAS) Trials.
Mortality
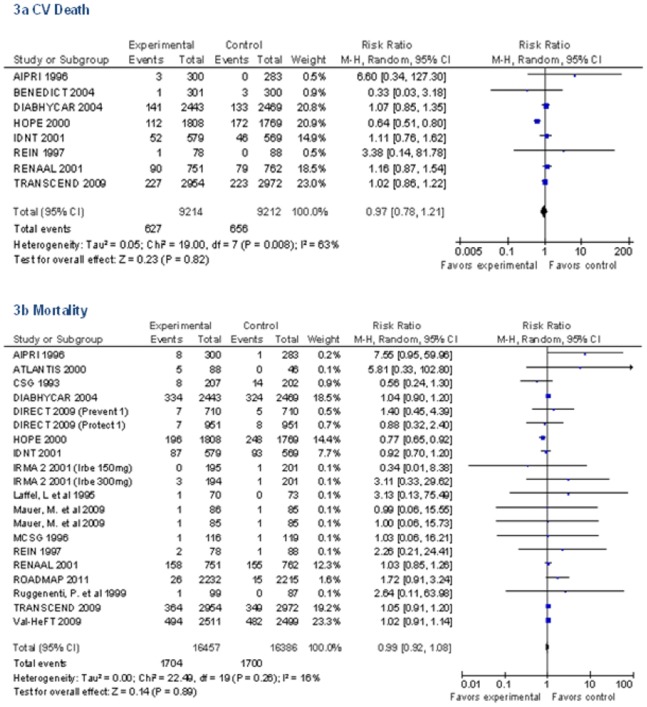
Only eight RAAS trials reported on the outcome CV death (Figure 3a and Table 3). There were 627 deaths (6.8%) in treatment group vs. 656 deaths (7.1%) in control group (RR 0.97 [95% CI 0.78 to 1.21], RD 0 [95% CI −1 to 1], I2 = 67%). All 18 RAAS trials (20 total comparisons due to multiple arms in two trials) reported all-cause mortality (Figure 3b and Table 3). There were 1704 deaths (10.4%) in the RAAS treatment group vs. 1700 deaths (10.4%) in the control group (RR 0.99 [95% CI 0.92 to 1.08], RD 0 [95% CI 0 to 1], I2 = 29%). We had at least 80% power to detect a 14% relative risk reduction (RRR) in CV death and a 9% RRR in all-cause mortality at 2 sided alpha of 0.05 assuming baseline event rate in controls to be 7.1% and 10.4% respectively (Table 3).
Figure 3. Pooled Relative Risk for Mortality in RAAS Trials.
Table 3. Incidence and power calculations for effect size in RAAS Trials.
| Outcome | No. of studies | Sample Size | Incidence in Control Group n (%) | Incidence in Treatment Group n (%) | RR (95% CI) | Required RR for 80% power | Power With Current Event Rate |
| CV Death | 8 | 18426 | 656 (7.1) | 627 (6.8) | 0.97 (0.78, 1.21) | 0.86 | 8% |
| All-cause Mortality | 20 | 32843 | 1700 (10.4) | 1704 (10.4) | 0.99 (0.92, 1.08) | 0.91 | 5% |
Abbreviations: RAAS = Renin angiotensin aldosterone system; CV = cardiovascular; RR = relative risk.
Sensitivity Analyses
We examined mortality only in the positive trials that demonstrated a statistically significant benefit for the renal endpoints. 8 trials reported on progressive renal disease as doubling of sCr and ESRD. 6 out of these 8 trials were protective for progressive renal disease (RR 0.70 [95% CI 0.59 to 0.82], RD −10 [95% CI −18 to −1], I2 = 94%), yet, in these 6 positive trials for CKD, there was no benefit in terms of all-cause mortality (RR 1.01 [95% CI 0.87 to 1.17], RD 1 [95% CI −1 to 2], I2 = 32%) or CV death (n = 5 trials, RR 1.11 [95% CI 0.95 to 1.31], RD 1 [95% CI 0 to 2], I2 = 0%). 11 trials reported on albuminuria out of which 6 were protective for albuminuria (RR 0.68 [95% CI 0.55 to 0.84], RD −4 [95% CI −7 to −2], I2 = 74%), however in those 6 positive trials, there was no benefit for all-cause mortality (RR 1.02 [95% CI 0.77 to 1.35], RD 0 [95% CI −1 to 1], I2 = 74%). Only two of these 6 trials reported CV death.
In order to further explore the causal relationship between the CKD and mortality within the group of RAAS vs. placebo trials, we performed several sub-group analyses and meta-regression by study-level variables and their association with the RR for mortality. When study-level variables were examined categorically, we found a non-significant trend towards reduction in mortality via RAAS blockade by length of follow up time, such that there was absolutely no benefit seen for studies with follow-up time <2 years and 2 to 4 years, but there was a suggestion of protection with follow-up time >4 years (RR 0.91 [95% CI 0.71 to 1.17], RD −1 [95% CI −3 to 1], I2 = 61%). However, only 4 trials had median follow-up greater than 4 years, and the statistical heterogeneity for this subgroup was high. There was no substantial differences in the pooled estimates when stratified by sample size (<1500 vs. >1500 participants), by mean GFR of study participants (>60 vs. ≤60 ml/min/1.73m2), by studies that enrolled participants exclusively with diabetes vs. other trials, by the proportion of participants in each trial with albuminuria (Table 4). Meta-regression was used to explore the association between three continuous study level variables and the risk of death: A) ARR of ESRD and doubling of sCr between RAAS blockade vs. placebo; B) ARR of albuminuria by RAAS blockade vs. placebo; C) and overall study sample size. None of the three study level characteristics explained a significant proportion of variability in the risk of death. ARR of albuminuria explained 13% of the variability, sample size explained 11%, and ARR of ESRD and doubling of sCr explained 3% of the variability (Figure S2).
Table 4. Sub analyses of pooled relative risk of mortality.
| Stratification | Number | Pooled RR | I2 | |
| Studies | Participants | (95% CI) | ||
| Follow up | ||||
| ≤2 years | 7 | 6,278 | 1.03 (0.92–1.15) | 0% |
| >2–4 years | 9 | 16,520 | 1.03 (0.90–1.18) | 34% |
| >4 years | 4 | 9,759 | 0.91 (0.71–1.17) | 58% |
| Sample Size | ||||
| <1500 | 10 | 3,816 | 0.93 (0.73–1.19) | 0% |
| >1500 | 8 | 28,707 | 1.00 (0.90–1.10) | 44% |
| eGFR | ||||
| >60 ml/min/1.73 m2 | 14 | 25,251 | 0.97 (0.86–1.08) | 19% |
| ≤60 ml/min/1.73 m2 | 4 | 7,272 | 1.04 (0.89–1.21) | 25% |
| Diabetes | ||||
| No | 4 | 11,685 | 1.04 (0.92–1.18) | 26% |
| Yes | 14 | 20,838 | 0.96 (0.85–1.07) | 13% |
| Albuminuria | ||||
| None | 5 | 8,110 | 1.39 (0.87–2.22) | 0% |
| up to 33% | 2 | 9,503 | 0.91 (0.67–1.22) | 86% |
| >33% | 8 | 8,908 | 1.02 (0.92–1.14) | 0% |
| Microalbuminuria | 7 | 15,584 | 0.96 (0.82–1.12) | 43% |
| Macroalbuminuria | 3 | 2,827 | 1.00 (0.85–1.17) | 0% |
Abbreviations: RR = relative risk; I2 = heterogeneity; eGFR = estimated glomerular filtration rate.
Discussion
In this systematic review and meta-analysis, we sought to examine if the risk reduction in renal endpoints in randomized clinical trials also leads to a decrease in mortality. Despite the fact that RAAS blockade was beneficial for reduction in several renal endpoints (albuminuria, doubling of sCr and ESRD), we could not obtain firm evidence that renal benefit also translated into improved mortality, either all-cause or CV death.
These results are contrary to those observed in observational studies. A large systematic review and meta-analysis of 41 studies showed that point estimate for the unadjusted relative risk of mortality in patients with CKD (vs. those without CKD) exceeded 1.0 in 40 studies and was significant in 93% cohorts. The overall pooled relative risk for mortality for CKD vs. no CKD was 1.77 (95% CI 1.33 to 2.34). [10] Meta-regression analyses revealed an increasing risk of mortality with decreasing renal function. These findings, though observational, would imply that mortality can be reduced when there is a reduction in CKD outcomes. However, despite examining the outcomes in RCTs containing over 32,000 participants in trials of RAAS blockade vs. placebo, we could not confirm the implications from the observed associations between CKD and mortality.
We had enough statistical power to detect differences in mortality and CV death of 9 to 14%. However, the point estimates for all-cause mortality and CV death were very close to 1, making it unlikely that larger sample size resulting in more statistical power would have demonstrated a meaningful reduction in mortality. Thus, our findings leave a few possibilities that warrant careful consideration: i) CKD is causal for mortality and CV mortality but the duration of follow-up of the trials was not sufficiently long enough to witness the reduction in these outcome, ii) the effect size on CKD by RAAS blockade was too small to translate into a reduction in mortality, iii) the etiologic fraction of CKD for mortality and CV mortality is too small and requires larger sample size, iv) the event rate of mortality and CV mortality was too low; v) CKD is associated with mortality although a direct causal pathway between them is not existent.
Was the length of follow-up in the trials not sufficiently long enough to witness a beneficial effect on mortality? The median follow up time in the trials included in our meta-analysis was 3 years for the RAAS trials. When we examined mortality by doing the sub-analysis of studies stratified by the median duration of follow-up, we observed a non-significant trend towards reduced mortality in the 4 studies with median follow-up >4 years. Recently, the data from two large CKD intervention trials with prolonged follow-up (22 years in The Diabetes Control and Complications Trial/Epidemiology of Diabetes Intervention and Complications [DCCT/EDIC study] and 12 years in African American Study of Kidney Disease [AASK study]) were published [71], [72]. The AASK collaborative group reported a 24% reduction in doubling of sCr and ESRD in patients with urine protein to creatinine ratio (PCR) of >0.22 at 12 years but there was no improvement for mortality (Hazard Ratio 0.98, 95% CI 0.65 to 1.46). Data from DCCT/EDIC study showed that intensive glycemic control resulted in a 46% reduction in the endpoint of impaired eGFR, but there was no significant difference in mortality (HR 0.88, 95% CI 0.54 to 1.42). These two studies, despite very long follow up time, could not demonstrate that improvement in CKD outcomes manifested in a reduction in mortality, although by themselves, they were underpowered to detect a difference in mortality.
Was the effect size afforded by RAAS blockade for the renal outcomes too small to translate into reduction of mortality? In the RCTs herein, the relative risk reduction for renal outcomes was 15 to 27%, and the absolute risk reduction only ranged from 1 to 4%. Even if the mortality benefit completely paralleled that for CKD, then the largest absolute difference in mortality between the 2 interventions can only be 4%. However, the benefits of renal protection are not transmitted 100% towards the benefit of the hard outcome. This relates to the concept of etiologic fraction. The “etiologic fraction” or “attributable risk exposed” of CKD for non-renal outcomes lies somewhere between 0 to 99%. Using the data from meta-analysis by Tonelli et al. [10] the attributable proportion in the total population (Apt) of CKD for death is 34.1%, (Table 5) which means a 4% reduction in the risk of CKD will translate into a 1.3% reduction in all-cause mortality.
Table 5. Calculation for Attributable Proportion of CKD for All-cause Mortality.
| Mortality | No Mortality | Total | |
| CKD | a 24420 | b 173104 | 197524 |
| No CKD | c 29437 | d 928376 | 957813 |
| Total | 53857 | 1101480 | Grand Total (a+b+c+d) 1155337 |
APt = [(Rt–Ru)/Rt]×100.
APt is Attributable Proportion of mortality due to CKD in the total population.
Rt is the prevalence of mortality in the total population = a+c/a+b+c+d.
Ru is the prevalence of mortality in the unexposed (without CKD) population = c/c+d.
Rt = 53857/1155337 = 4.66%.
Ru = 29437/957813 = 3.07%.
APt = 34.1%.
The 4th possibility relates to the event rate of the hard outcomes in the trials. In order to appreciate a sizeable effect on an outcome, the event rate needs to be of a certain magnitude in order to influence it. The mortality rate in the RCTs ranged from 0.0 [47] to 21.65 [19]. Observational studies have demonstrated a mortality rate as high 6.8/100 person-years in patients with CKD [73]. Due to insufficient data to perform meta-regression in order to explore the association of event rate in studies with the risk of mortality, we are unable to conclude if a higher event rate would have explained the present lack of mortality benefit.
The last possibility is that CKD may not be directly causal for CV death and overall mortality. This notion although seems provocative and contrary to common belief in the nephrology community, to disregard it completely as a heresy may not be the right approach. Another way to look at this association would be to see the increased mortality in patients in which CKD is induced or incidence is increased iatrogenically. On the contrary, Lau, et al. have shown the renal cell carcinoma patients undergoing radical nephrectomy were at a 3-fold higher risk of developing CKD when compared to those who underwent nephron sparing surgery. Yet there was no difference in mortality in the two groups at 10 years [74]. Moreover, Wald, et al. used propensity-based methods to match patients with and without severe AKI. After a median follow up time of 3 years, they demonstrated that those who experienced AKI had a 3-fold increased risk of ESRD. Nevertheless, despite the fact that there were 3 times as many patients who developed ESRD compared to the no AKI group, there was absolutely no difference in all-cause mortality [12]. The discordant association between CKD risk and mortality, in both directions, provides some equipoise around the assumption of a direct causal pathway between CKD and mortality. We as clinicians and researchers have witnessed several times over the past few years that approaching the targets based on surrogate markers has not translated into clinical improvement. Recent, well designed trials to evaluate the effect of reduction of proteinuria did not show any significant improvement in hard outcomes [75], [76]. The same is true for anemia showing no improvement in clinical outcomes with correction of hemoglobin as well as HDL in patients with CVD [77]–[81]. The “independent associations” from observational studies may be confounded by other patient factors such as hypertension, dyslipidemia and diabetes that coexist with CKD. These established cardiovascular risk factors may be responsible for increased mortality witnessed in patients with CKD.
Conclusions
We have definitive evidence to demonstrate a reduction in CKD endpoints by RAAS blockade. However, we still lack conclusive evidence that the reduction in CKD endpoints will translate into a meaningful reductions in CV and all-cause mortality. To date, evidence from RCTs is not sufficient to fulfill the most important condition to prove the causality between CKD and mortality. However, the results of our analysis cannot be generalized to other interventions that may reduce or prevent CKD. For example, at the present time, there is insufficient data published for some interventions, such as allopurinol and alkali therapy, in CKD patients and the effect on mortality [82]–[84]. We suggest that unless more potent and efficacious agents for prevention or treatment of CKD are discovered (to increase the effect size), then RCTs of RAAS blockade (or other similarly effective agents) will need to be performed with longer duration of follow-up time than typically performed in CKD trials (at least 4 years or more of follow-up) in order to prove that direct causal relationships between CKD and mortality exist. Until then, we are unclear whether CKD shares the same company as other surrogate and non-causal endpoints in nephrology, such as anemia and vitamin D deficiency. [78], [79], [81], [85].
Supporting Information
Risk of Bias Summary for each included trial (1A). Risk of Bias Graph presented as percentage across all included trials (1B).
(TIF)
Meta-regression Analysis of Association between Study Level Covariates and Risk of All-cause Mortality.
(TIF)
PRISMA 2009 Checklist.
(DOC)
Funding Statement
The authors have no support or funding to report.
References
- 1. CDC (2007) Prevalence of chronic kidney disease and associated risk factors–United States, 1999–2004. MMWR Morb Mortal Wkly Rep 56: 161–165. [PubMed] [Google Scholar]
- 2. Zhang QL, Rothenbacher D (2008) Prevalence of chronic kidney disease in population-based studies: systematic review. BMC Public Health 8: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. NKF (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 39: S1–266. [PubMed] [Google Scholar]
- 4. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305. [DOI] [PubMed] [Google Scholar]
- 5. Ruilope LM, Salvetti A, Jamerson K, Hansson L, Warnold I, et al. (2001) Renal function and intensive lowering of blood pressure in hypertensive participants of the hypertension optimal treatment (HOT) study. J Am Soc Nephrol 12: 218–225. [DOI] [PubMed] [Google Scholar]
- 6. Foley RN, Parfrey PS, Sarnak MJ (1998) Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32: S112–119. [DOI] [PubMed] [Google Scholar]
- 7. Levey AS, Beto JA, Coronado BE, Eknoyan G, Foley RN, et al. (1998) Controlling the epidemic of cardiovascular disease in chronic renal disease: what do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis 32: 853–906. [DOI] [PubMed] [Google Scholar]
- 8. Levin A, Singer J, Thompson CR, Ross H, Lewis M (1996) Prevalent left ventricular hypertrophy in the predialysis population: identifying opportunities for intervention. Am J Kidney Dis 27: 347–354. [DOI] [PubMed] [Google Scholar]
- 9. Sarnak MJ, Levey AS (2003) Schoolwerth AC, Coresh J, Culleton B, et al (2003) Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Hypertension 42: 1050–1065. [DOI] [PubMed] [Google Scholar]
- 10. Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, et al. (2006) Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol 17: 2034–2047. [DOI] [PubMed] [Google Scholar]
- 11. Garg AX, Meirambayeva A, Huang A, Kim J, Prasad GV, et al. (2012) Cardiovascular disease in kidney donors: matched cohort study. BMJ 344: e1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wald R, Quinn RR, Luo J, Li P, Scales DC, et al. (2009) Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302: 1179–1185. [DOI] [PubMed] [Google Scholar]
- 13.Higgins JP, Altman D, Sterne JA (2011) Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT GS, editor. Cochrane Handbook for Systematic Reviews of Interventions version 510.
- 14. van Houwelingen HC, Arends LR, Stijnen T (2002) Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 21: 589–624. [DOI] [PubMed] [Google Scholar]
- 15. Zeger SL, Liang KY, Albert PS (1988) Models for longitudinal data: a generalized estimating equation approach. Biometrics 44: 1049–1060. [PubMed] [Google Scholar]
- 16. DCCT (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 17. MCSG (1996) Captopril reduces the risk of nephropathy in IDDM patients with microalbuminuria. The Microalbuminuria Captopril Study Group. Diabetologia 39: 587–593. [DOI] [PubMed] [Google Scholar]
- 18. GISEN (1997) Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Lancet 349: 1857–1863. [PubMed] [Google Scholar]
- 19. UKPDS34 (1998) Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352: 854–865. [PubMed] [Google Scholar]
- 20. UKPDS33 (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352: 837–853. [PubMed] [Google Scholar]
- 21. HOPE (2000) Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators.[Erratum appears in Lancet 2000 Sep 2; 356(9232): 860]. Lancet 355: 253–259. [PubMed] [Google Scholar]
- 22. Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, et al. (2001) Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA 285: 2719–2728. [DOI] [PubMed] [Google Scholar]
- 23. Akizawa T, Asano Y, Morita S, Wakita T, Onishi Y, et al. (2009) Effect of a carbonaceous oral adsorbent on the progression of CKD: a multicenter, randomized, controlled trial.[Erratum appears in Am J Kidney Dis. 2010 Mar; 55(3): 616]. Am J Kidney Dis 54: 459–467. [DOI] [PubMed] [Google Scholar]
- 24. Anand IS, Bishu K, Rector TS, Ishani A, Kuskowski MA, et al. (2009) Proteinuria, chronic kidney disease, and the effect of an angiotensin receptor blocker in addition to an angiotensin-converting enzyme inhibitor in patients with moderate to severe heart failure. Circulation 120: 1577–1584. [DOI] [PubMed] [Google Scholar]
- 25. Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, et al. (2011) The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. The Lancet 377: 2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bakris GL, Sarafidis PA, Weir MR, Dahlöf B, Pitt B, et al. (2010) Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a randomised controlled trial. The Lancet 375: 1173–1181. [DOI] [PubMed] [Google Scholar]
- 27. Bakris GL, Toto RD, McCullough PA, Rocha R, Purkayastha D, et al. (2008) Effects of different ACE inhibitor combinations on albuminuria: results of the GUARD study. Kidney International 73: 1303–1309. [DOI] [PubMed] [Google Scholar]
- 28. Barnett AH (2005) Preventing renal complications in diabetic patients: the Diabetics Exposed to Telmisartan And enalaprIL (DETAIL) study. Acta Diabetologica 42 Suppl 1S42–49. [DOI] [PubMed] [Google Scholar]
- 29.Bilous R, Chaturvedi N, Sjolie AK, Fuller J, Klein R, et al.. (2009) Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes: three randomized trials. Annals of Internal Medicine 151: 11–20, W13–14. [DOI] [PubMed]
- 30. Bolton WK, Cattran DC, Williams ME, Adler SG, Appel GB, et al. (2004) Randomized trial of an inhibitor of formation of advanced glycation end products in diabetic nephropathy. American Journal of Nephrology 24: 32–40. [DOI] [PubMed] [Google Scholar]
- 31. Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, et al. (2001) Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. New England Journal of Medicine 345: 861–869. [DOI] [PubMed] [Google Scholar]
- 32. Chan JC, So WY, Yeung CY, Ko GT, Lau IT, et al. (2009) Effects of structured versus usual care on renal endpoint in type 2 diabetes: the SURE study: a randomized multicenter translational study. Diabetes Care 32: 977–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dalla Vestra M, Pozza G, Mosca A, Grazioli V, Lapolla A, et al. (2004) Effect of lercanidipine compared with ramipril on albumin excretion rate in hypertensive Type 2 diabetic patients with microalbuminuria: DIAL study (diabete, ipertensione, albuminuria, lercanidipina). Diabetes, Nutrition & Metabolism - Clinical & Experimental 17: 259–266. [PubMed] [Google Scholar]
- 34. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, et al. (2009) Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360: 129–139. [DOI] [PubMed] [Google Scholar]
- 35. Facchini FS, Saylor KL (2003) A low-iron-available, polyphenol-enriched, carbohydrate-restricted diet to slow progression of diabetic nephropathy. Diabetes 52: 1204–1209. [DOI] [PubMed] [Google Scholar]
- 36. Fan FH, Xie D, Zhang X, Ping YC, Wei RZ, et al. (2007) Renoprotection of Optimal Antiproteinuric Doses (ROAD) study: A randomized controlled study of benazepril and losartan in chronic renal insufficiency. Journal of the American Society of Nephrology 18: 1889–1898. [DOI] [PubMed] [Google Scholar]
- 37. Fogari R, Preti P, Zoppi A, Rinaldi A, Corradi L, et al. (2002) Effects of amlodipine fosinopril combination on microalbuminuria in hypertensive type 2 diabetic patients. American Journal of Hypertension 15: 1042–1049. [DOI] [PubMed] [Google Scholar]
- 38. Gaede P, Vedel P, Parving HH, Pedersen O (1999) Intensified multifactorial intervention in patients with type 2 diabetes mellitus and microalbuminuria: the Steno type 2 randomised study. Lancet 353: 617–622. [DOI] [PubMed] [Google Scholar]
- 39. Goicoechea M, De Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, et al. (2010) Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clinical Journal of the American Society of Nephrology 5: 1388–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haller H, Ito S, Izzo JL Jr, Januszewicz A, Katayama S, et al. (2011) Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. New England Journal of Medicine 364: 907–917. [DOI] [PubMed] [Google Scholar]
- 41. Hannedouche T, Landais P, Goldfarb B, el Esper N, Fournier A, et al. (1994) Randomised controlled trial of enalapril and beta blockers in non-diabetic chronic renal failure. BMJ 309: 833–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Heerspink HJL, Ninomiya T, Perkovic V, Woodward M, Zoungas S, et al. (2010) Effects of a fixed combination of perindopril and indapamide in patients with type 2 diabetes and chronic kidney disease. European Heart Journal 31: 2888–2896. [DOI] [PubMed] [Google Scholar]
- 43. House AA, Eliasziw M, Cattran DC, Churchill DN, Oliver MJ, et al. (2010) Effect of B-vitamin therapy on progression of diabetic nephropathy: a randomized controlled trial. JAMA 303: 1603–1609. [DOI] [PubMed] [Google Scholar]
- 44. Huskey J, Lindenfeld J, Cook T, Targher G, Kendrick J, et al. (2009) Effect of simvastatin on kidney function loss in patients with coronary heart disease. Findings from the Scandinavian Simvastatin Survival Study (4S). Atherosclerosis 205: 202–206. [DOI] [PubMed] [Google Scholar]
- 45. Ismail-Beigi F, Craven T, Banerji MA, Basile J, Calles J, et al. (2010) Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial.[Erratum appears in Lancet. 2010 Oct 30; 376(9751): 1466]. Lancet 376: 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kendrick J, Shlipak MG, Targher G, Cook T, Lindenfeld J, et al. (2010) Effect of lovastatin on primary prevention of cardiovascular events in mild CKD and kidney function loss: a post hoc analysis of the Air Force/Texas Coronary Atherosclerosis Prevention Study. American Journal of Kidney Diseases 55: 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Laffel LM, McGill JB, Gans DJ (1995) The beneficial effect of angiotensin-converting enzyme inhibition with captopril on diabetic nephropathy in normotensive IDDM patients with microalbuminuria. North American Microalbuminuria Study Group. American Journal of Medicine 99: 497–504. [DOI] [PubMed] [Google Scholar]
- 48. Lewis EJ, Hunsicker LG, Bain RP, Rohde RD (1993) The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 329: 1456–1462. [DOI] [PubMed] [Google Scholar]
- 49. Lewis EJ, Hunsicker LG, Clarke WR, Berl T, Pohl MA, et al. (2001) Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. New England Journal of Medicine 345: 851–860. [DOI] [PubMed] [Google Scholar]
- 50.Mann JFE, Schmieder RE, Dyal L, McQueen MJ, Schumacher H, et al.. (2009) Effect of telmisartan on renal outcomes: a randomized trial.[Summary for patients in Ann Intern Med. 2009 Jul 7; 151(1): I28; PMID: 19451555]. Annals of Internal Medicine 151: 1–10, W11–12. [DOI] [PubMed]
- 51. Marre M, Lievre M, Chatellier G, Mann JFE, Passa P, et al. (2004) Effects of low dose ramipril on cardiovascular and renal outcomes in patients with type 2 diabetes and raised excretion of urinary albumin: randomised, double blind, placebo controlled trial (the DIABHYCAR study).[Erratum appears in BMJ. 2004 Mar 20; 328(7441): 686]. BMJ 328: 495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marre M, Puig JG, Kokot F, Fernandez M, Jermendy G, et al. (2004) Equivalence of indapamide SR and enalapril on microalbuminuria reduction in hypertensive patients with type 2 diabetes: the NESTOR Study. Journal of Hypertension 22: 1613–1622. [DOI] [PubMed] [Google Scholar]
- 53. Maschio G, Alberti D, Janin G, Locatelli F, Mann JF, et al. (1996) Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. New England Journal of Medicine 334: 939–945. [DOI] [PubMed] [Google Scholar]
- 54. Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, et al. (2009) Renal and retinal effects of enalapril and losartan in type 1 diabetes. New England Journal of Medicine 361: 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ogihara T, Nakao K, Fukui T, Fukiyama K, Ueshima K, et al. (2008) Effects of candesartan compared with amlodipine in hypertensive patients with high cardiovascular risks: candesartan antihypertensive survival evaluation in Japan trial. Hypertension 51: 393–398. [DOI] [PubMed] [Google Scholar]
- 56. Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, et al. (1995) Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 28: 103–117. [DOI] [PubMed] [Google Scholar]
- 57. Parving HH, Lehnert H, Brochner-Mortensen J, Gomis R, Andersen S, et al. (2001) The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 345: 870–878. [DOI] [PubMed] [Google Scholar]
- 58. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, et al. (2008) Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358: 2560–2572. [DOI] [PubMed] [Google Scholar]
- 59. Patel A, MacMahon S, Chalmers J, Neal B, Woodward M, et al. (2007) Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 370: 829–840. [DOI] [PubMed] [Google Scholar]
- 60. Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, et al. (2011) Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med 365: 327–336. [DOI] [PubMed] [Google Scholar]
- 61. Rachmani R, Slavachevski I, Berla M, Frommer-Shapira R, Ravid M (2005) Teaching and motivating patients to control their risk factors retards progression of cardiovascular as well as microvascular sequelae of Type 2 diabetes mellitus- a randomized prospective 8 years follow-up study. Diabetic Medicine 22: 410–414. [DOI] [PubMed] [Google Scholar]
- 62. Rahman M, Baimbridge C, Davis BR, Barzilay J, Basile JN, et al. (2008) Progression of Kidney Disease in Moderately Hypercholesterolemic, Hypertensive Patients Randomized to Pravastatin Versus Usual Care: A Report From the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). American Journal of Kidney Diseases 52: 412–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Rahman M, Pressel S, Davis BR, Nwachuku C, Wright JT Jr, et al. (2005) Renal outcomes in high-risk hypertensive patients treated with an angiotensin-converting enzyme inhibitor or a calcium channel blocker vs a diuretic: a report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Archives of Internal Medicine 165: 936–946. [DOI] [PubMed] [Google Scholar]
- 64. Ruggenenti P, Fassi A, Ilieva AP, Bruno S, Iliev IP, et al. (2004) Preventing microalbuminuria in type 2 diabetes. N Engl J Med 351: 1941–1951. [DOI] [PubMed] [Google Scholar]
- 65. Ruggenenti P, Fassi A, Ilieva AP, Iliev IP, Chiurchiu C, et al. (2011) Effects of verapamil added-on trandolapril therapy in hypertensive type 2 diabetes patients with microalbuminuria: the BENEDICT-B randomized trial. Journal of Hypertension 29: 207–216. [DOI] [PubMed] [Google Scholar]
- 66. Ruggenenti P, Perna A, Loriga G, Ganeva M, Ene-Iordache B, et al. (2005) Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): Multicentre, randomised controlled trial. Lancet 365: 939–946. [DOI] [PubMed] [Google Scholar]
- 67. Stefoni S, Mosconi G, La Manna G, Bonomini V, Mioli V, et al. (1996) Low-dosage ibopamine treatment in progressive renal failure: a long-term multicentre trial. American Journal of Nephrology 16: 489–499. [DOI] [PubMed] [Google Scholar]
- 68. Yusuf S, Teo KK, Pogue J, Dyal L, Copland I, et al. (2008) Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med 358: 1547–1559. [DOI] [PubMed] [Google Scholar]
- 69. O’Hare P, Bilbous R, Mitchell T, O’Callaghan CJ, Viberti GC (2000) Low-dose ramipril reduces microalbuminuria in type 1 diabetic patients without hypertension: results of a randomized controlled trial. Diabetes Care 23: 1823–1829. [DOI] [PubMed] [Google Scholar]
- 70. Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, et al. (1999) Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 354: 359–364. [DOI] [PubMed] [Google Scholar]
- 71. Appel LJ, Wright JT Jr, Greene T, Agodoa LY, Astor BC, et al. (2010) Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med 363: 918–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, et al. (2011) Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 365: 2366–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dalrymple LS, Katz R, Kestenbaum B, Shlipak MG, Sarnak MJ, et al. (2011) Chronic kidney disease and the risk of end-stage renal disease versus death. J Gen Intern Med 26: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lau WK, Blute ML, Weaver AL, Torres VE, Zincke H (2000) Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc 75: 1236–1242. [DOI] [PubMed] [Google Scholar]
- 75. Parving HH, Brenner BM, McMurray JJ, de Zeeuw D, Haffner SM, et al. (2012) Cardiorenal end points in a trial of aliskiren for type 2 diabetes. N Engl J Med 367: 2204–2213. [DOI] [PubMed] [Google Scholar]
- 76. Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK (2008) Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 358: 2433–2446. [DOI] [PubMed] [Google Scholar]
- 77. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, et al. (2011) Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 365: 2255–2267. [DOI] [PubMed] [Google Scholar]
- 78. Drueke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, et al. (2006) Normalization of hemoglobin level in patients with chronic kidney disease and anemia. New England Journal of Medicine 355: 2071–2084. [DOI] [PubMed] [Google Scholar]
- 79. Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, De Zeeuw D, et al. (2009) A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. New England Journal of Medicine 361: 2019–2032. [DOI] [PubMed] [Google Scholar]
- 80. Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, et al. (2012) Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med 367: 2089–2099. [DOI] [PubMed] [Google Scholar]
- 81. Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, et al. (2006) Correction of anemia with epoetin alfa in chronic kidney disease. New England Journal of Medicine 355: 2085–2098. [DOI] [PubMed] [Google Scholar]
- 82. Kabul S, Shepler B (2012) A review investigating the effect of allopurinol on the progression of kidney disease in hyperuricemic patients with chronic kidney disease. Clin Ther 34: 2293–2296. [DOI] [PubMed] [Google Scholar]
- 83.Roderick P, Willis NS, Blakeley S, Jones C, Tomson C (2007) Correction of chronic metabolic acidosis for chronic kidney disease patients. Cochrane Database Syst Rev: CD001890. [DOI] [PMC free article] [PubMed]
- 84. Susantitaphong P, Sewaralthahab K, Balk EM, Jaber BL, Madias NE (2012) Short- and long-term effects of alkali therapy in chronic kidney disease: a systematic review. Am J Nephrol 35: 540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA 307: 674–684. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Risk of Bias Summary for each included trial (1A). Risk of Bias Graph presented as percentage across all included trials (1B).
(TIF)
Meta-regression Analysis of Association between Study Level Covariates and Risk of All-cause Mortality.
(TIF)
PRISMA 2009 Checklist.
(DOC)