Abstract
Approximately 30 to 50% of people suffering from Gilles de la Tourette Syndrome (GTS) also fulfill diagnostic criteria for obsessive-compulsive disorder (OCD). Despite this high degree of comorbidity, very few studies have addressed the question of obsessive-compulsive symptoms (OCS) in GTS patients using specific brain event-related potentials (ERP) responses. The aim of the current study was to quantify neurocognitive aspects of comorbidity, using ERPs. Fourteen adults with GTS (without OCD) were compared to a group of 12 participants with GTS and comorbid obsessive-compulsive symptoms (GTS+OCS), to a group of 15 participants with OCD and to a group of 14 control participants without neurological or psychiatric problems. The P200 and P300 components were recorded during a visual counting oddball task. Results showed intact P200 amplitude in all groups, whilst the P300 amplitude was affected differentially across groups. The P300 oddball effect was reduced in participants in both OCD and GTS+OCS groups in the anterior region. However, the P300 oddball effect was significantly larger in participants of the GTS group compared to all other groups, mostly in the parietal region. These findings suggest that adults with GTS are characterized by enhanced working memory updating processes and that the superimposition of OCS can lead to a reduction of these processes. The discrepancy between our findings and results obtained in previous studies on GTS could reflect the modulating effect of OCS on late ERP components.
Keywords: Comorbidity, Obsessive-compulsive disorder, Oddball effect, P200, P300, Tourette syndrome
1. Introduction
Gilles de la Tourette Syndrome (GTS) is a complex neuropsychiatric disorder with childhood onset, diagnosed in the presence of multiple motor and phonic tics. Epidemiological studies have noted that 30 to 50% of the GTS population is affected by obsessive-compulsive disorder (OCD), which is considered to be part of a spectrum of GTS symptomatology that extends beyond the tics disorder (Marcus and Kurlan, 2001). OCD is characterized by recurrent intrusive thoughts accompanied by repetitive, seemingly purposeful behaviors, sufficiently severe to interfere with daily functioning. The frequent comorbidity between GTS and OCD, along with the behavioral similarities between them, has lead to propose that they might share common genetic or neurophysiological bases. This hypothesis finds support from studies showing that some forms of OCD constitute distinct expressions of the genetic alteration related to GTS (Pauls, 1992; Pauls et al., 1995, 1986; Sheppard et al., 1999). Brain imaging investigations also suggest that both GTS and OCD could be provoked by a default in inhibitory functions, caused by a metabolic reduction in basal ganglia structures projecting to frontal regions, involving either the prefrontal and primary motor cortices in GTS, or the lateral orbitofrontal and anterior cingulate cortices in OCD (Mink, 2001; Saxena et al., 1998; Sheppard et al., 1999).
Neuropsychological studies consistently showed attentional disinhibition in both clinical populations (Channon et al., 1992; Georgiou et al., 1995, 1996; Lavoie et al., 2007; Tata et al., 1996; Tolin et al., 2002). In order to extend findings on neuropsychological variables, several studies have recorded Event-Related Potentials (ERPs), which provide indications about cerebral activation in synchrony with cognitive events such as attention, memory (Linden, 2005) and motor functions (Hackley and Valle-Inclan, 2003). Other recent reviews showed that ERPs are useful neurocognitive markers, for instance, in Alzheimer’s disease (Olichney and Hillert, 2004), brain injury (Lavoie et al., 2004; Mazzini, 2004) and various psychiatric disease (Banaschewski and Brandeis, 2007; Ford, 1999).
ERP investigations on GTS and OCD have mostly studied the P200 and the P300 components. Recorded in a classical oddball paradigm, the P300 component has been known to represent stimulus evaluation and categorization, along with working memory updating processes (see Polich, 2007 for a detailed review). The P200 component is less well understood, but it has been mainly related to attention allocation and vigilance (e.g. Wolach and Pratt, 2001). In previous studies, a reduction in P300 amplitude has been consistently observed in GTS (Johannes et al., 1997, 2001b) and in OCD (Kim et al., 2003; Miyata et al., 1998; Sanz et al., 2001; Towey et al., 1994), pointing towards a decrease in memory updating processes in both disorders. Nevertheless, these P300 patterns were elicited in slightly different experimental procedures, so nuancing homogeneous interpretation of their functional significance across groups. In GTS, working memory processes have been found to be affected in complex attentional performance while in OCD, anomalies appeared non specifically across a wide spectrum of cognitive demands (Kim et al., 2003; Mavrogiorgou et al., 2002; Miyata et al., 1998; Morault et al., 1998; Sanz et al., 2001; Towey et al., 1994).
Following on from previous results, several questions remain. What is the sensitivity of specific ERP components to GTS versus OCD symptoms? What is the contribution of other symptoms such as depression and anxiety that often co-occur with GTS and OCD? What is the influence of comorbid obsessive-compulsive symptoms (OCS) on the ERP profile of adults suffering from GTS? Despite acknowledgement that impulsive and obsessive-compulsive symptoms might influence ERPs in GTS groups (van Woerkom et al., 1988, 1994), this influence has never been characterized and controlled directly.
The current study aimed at investigating specific ERP responses in adults suffering from GTS and at exploring the influence of obsessive-compulsive symptoms (OCS) on ERP components. In the current study, we analyze the P200 and P300 components in clinical groups diagnosed with GTS, OCD and an additional group sharing both GTS and obsessive-compulsive symptoms (GTS +OCS). Based on previous ERP studies (Asahi et al., 1993; Van de Wetering et al., 1985; van Woerkom et al., 1988, 1994), we predict that both GTS and OCD groups will show a reduced P200 amplitude. We also hypothesize that the GTS group will demonstrate a normal P300 oddball effect. Following past studies showing smaller amplitude in OCD participants during an oddball task (Sanz et al., 2001), we hypothesize that the presence of OCS will attenuate the P300 amplitude. Consequently, we expect the OCD group to demonstrate a reduced P300 compared to the three other groups. If OCD and GTS have additive effects, we expect the GTS+OCS group to present a reduced P300 compared to the non-OCD group with GTS. Their P300 should nevertheless be similar or larger compared to the OCD group.
2. Methods
2.1. Participants
All participants (n=55) were recruited through local newspapers or from staff members of the Lafontaine hospital (control group). Fourteen control participants were matched for age, education and gender (Table 1) to a sample of fourteen GTS, fifteen OCD and twelve GTS+OCS participants. They all had normal visual acuity (Snellen notation system: ≥11). The current study was part of a larger program aiming to study a specialized cognitive-behavioral treatment (CBT) adapted for either tics or OCD. The pre-CBT diagnosis was made by a certified psychiatrist (E.S. for the GTS group or C.T. for the pure OCD group) and a clinical psychologist (supervised by K.O.). The GTS group and the GTS+OCS group primarily fulfilled the diagnostic criteria for GTS (307.23), while the OCD group fulfilled the diagnostic criteria for obsessive-compulsive disorder (300.3) (DSM IV-TR: American Psychiatric Association, 2000).
Table 1.
Demographic data
| Control (n=14) A |
GTS (n=14) B |
GTS+OCS (n=12) C |
OCD (n=15) D |
ANOVA a
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | p | Tukey | |
| Age (years) | 37 | 13 | 32 | 10 | 38 | 12 | 37 | 13 | n.s. | - |
| Education (years) | 15 | 2 | 15 | 3 | 15 | 3 | 14 | 3 | n.s. | - |
| Gender (M/F) | 43/57 | 50/50 | 75/25 | 40/60 | n.s. | - | ||||
| Raven intelligence (percentiles) | 72 | 31 | 75 | 20 | 71 | 27 | 80 | 18 | n.s. | - |
A Kruskall-Wallis was applied to the non-parametric gender data.
2.2. Inclusion-exclusion criteria
Exclusion criteria for all participants was the presence of another diagnosis than GTS or OCD as primary disorders on axis I or any other diagnosed problem on axis II, III or IV of the DSM IV-TR (American Psychiatric Association, 2000). None of the control participants showed identifiable psychiatric or neurological disease. Participants currently receiving any other form of behavioral or cognitive treatment were excluded. However, they were included even if they received medications (see list of medication on Table 3) for their tic or OCD symptoms, on the condition that they had been stabilized for at least three months. The study was approved by the local ethics committee and all participants gave their written informed consent.
Table 3.
Medication taken by some of the participants of the OCD and GTS+OCS groups
| Medication categories
| |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antidepressants | Antidepressants Anti-obsessional | Anxiolytic | Anti-psychotic | Stimulant | Anti-convulsive | ||||||||||||||
|
| |||||||||||||||||||
| Bupropion | Citalopram | Lithium* | Trazodone | Venlafaxine† | Paroxetine | Clomipramine | Fluoxetine | Sertraline | Fluvoxamine | Lorazepam | Bromazepam | Quetiapine | Risperidone | Pimozide | Olanzapine | Methylphenidate | Clonazepam | Topiramate | |
| GTS+OCS
| |||||||||||||||||||
| 1 | • | • | |||||||||||||||||
| 2 | • | • | • | ||||||||||||||||
| 3 | • | ||||||||||||||||||
| 4 | • | • | |||||||||||||||||
| 5 | • | ||||||||||||||||||
| 6 | • | • | |||||||||||||||||
|
| |||||||||||||||||||
| OCD
| |||||||||||||||||||
| 1 | • | • | • | ||||||||||||||||
| 2 | • | • | |||||||||||||||||
| 3 | • | ||||||||||||||||||
| 4 | • | • | |||||||||||||||||
| 5 | • | ||||||||||||||||||
| 6 | • | ||||||||||||||||||
| 7 | • | • | |||||||||||||||||
| 8 | • | • | |||||||||||||||||
| 9 | • | ||||||||||||||||||
| 10 | • | ||||||||||||||||||
Antidepressant-Antimania
Antidepressant-anxiolytic.
2.3. Clinical assessment
GTS symptoms severity was assessed with the Tourette Syndrome Global Scale (TSGS: Harcherik et al., 1984). The TSGS was administered by the clinician to the GTS and GTS+OCS groups. The first TSGS factor rates the nature of the tic (i.e. motor or phonic), while the second scale rates the tic complexity. A third scale assesses functional impairment, including behavioral, learning, motor restlessness and occupational problems. The inter-rater reliability of the TSGS global score was found to be very good (k=0.77, p<.001). Convergent validity of the motor and phonic tic factors showed strong correlations with the corresponding Yale global tic severity scale, with correlations ranging from r=0.86 to r=0.91 (Leckman et al., 1989). The OC symptoms severity was evaluated with the self-rated Yale-Brown Obsessive-Compulsive Scale (Y-BOCS: Goodman et al., 1989a) for the three clinical groups, while both self and clinician-rated Y-BOCS (Goodman et al., 1989b) was administered in the pure OCD group. With our sample of OCD, the reliability between self and clinician-rated global scores were good (α=0.71) with no statistical differences between both evaluations (p=.11). Only the self-rated Y-BOCS will be reported in the current study. Other studies confirm the validity and reliability of the scales (internal consistency=0.91–0.94, r=0.90) (Y-BOCS: Goodman et al., 1989a; Steketee, 1994; Taylor, 1995). The self-rated Padua inventory (Sanavio, 1988) was also administered to all groups and consists in a 60-item inventory of obsessions and compulsions. The total scale (α=0.95) and the subscales (α=0.75–0.91) are also reliable. The Beck Anxiety Inventory (BAI: Beck et al., 1988) was administered and consists in a 21-items anxiety symptoms checklist rating the symptom intensity for the last week on a 0–3 scale (α=0.91). For the presence of depression, the Beck Depression Inventory (BDI: Beck et al., 1961), which consist in a 21-item relative to depression (α=0.91). The Personality Diagnostic Questionnaire-4th Edition (PDQ-4) was administered for detection of personality disorders (α=0.50 – 0.71), consistent with the DSM-IV (Hyler, 1994; Rodgers et al., 2004; Wilberg et al., 2000). Finally, the Anxiety Disorders structured Interview Schedule for DSM-IV (ADIS-IV: Brown et al., 1994) was administered by a psychologist to assess comorbid anxiety disorders.
2.4. Experimental assessment and recordings
2.4.1. Visual oddball paradigm
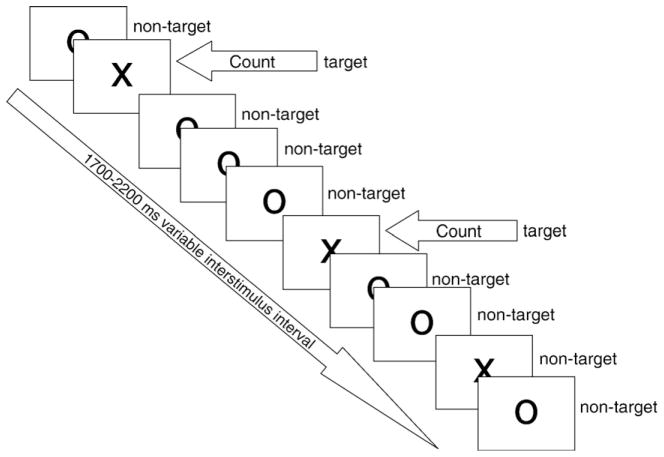
The counting visual oddball task was used in order to circumvent the potential problem of motor-related potentials that are known to have an attenuating effect on the P300 amplitude (Kok, 1988; Salisbury et al., 2001). The sequence of stimuli is exemplified in Fig. 1. Participants were seated in a dimly lit room with their head at a distance of 90 cm from the monitor. The stimulus display comprised a total of 200 black stimuli presented on a white background, representing either the letters «X» or «O» in Arial font (size=48). The letter «O» represented the non-target stimulus (p=.80) and the letter «X» the target stimulus (p=.20). Participants had to count mentally the target stimuli and report their exact number (n=40) at the end of the experiment. Stimuli were presented randomly for 100 ms each at the center of a monitor screen (Viewsonic SVGA 17 inches flat screen monitor). Inter stimulus interval randomly ranged from 1700 to 2200 ms.
Fig. 1.
Illustration of the visual counting oddball task used in the current study.
2.4.2. Electrophysiological recordings
All electrophysiological signals were acquired through an analog amplifier (SA Instrumentation Inc, San Diego). Electroencephalogram (EEG) was recorded from 26 tin electrodes, referenced to linked mastoids with impedance kept below 5 KΩ. Electrodes, mounted in a nylon cap (ElectroCap International, Eaton, Ohio), were placed according to the guidelines for standard electrode position nomenclature (American EEG Society, 1994). EEG recordings were continuously sampled at 250 Hz and amplified with a calibrated gain of ±10,000 with high-low pass filter settings at 0.01 and 30 Hz respectively. Electro-oculogram (EOG) was recorded from 4 bipolar electrodes placed horizontally at the outer canthus of each eye and vertically at infra- and supra-orbital position on the right eye, in line with the pupil when looking straight ahead. Stimuli presentation and data acquisition were both controlled by a data acquisition program (InstEP Systems, Montréal, Canada) running on two Pentium PCs.
2.4.3. EEG and ERP signal extraction
EOG artifacts contaminating the EEG signal were corrected offline using a dynamic regression in the frequency domain (Woestenburg method: InstEP-TALO). Remaining epochs exceeding 100 μV and clippings due to amplifiers saturation were eliminated during the averaging procedure. Raw signals were automatically averaged offline, time-locked to the stimulus onset, in a time window from 100 milliseconds before until 800 milliseconds after stimulus onset, for each stimulus category (target and non-target). A minimum amount of 20 trials were included in each category. The P200 and P300 components were scored baseline-to-peak, defined as the most positive peak comprised between 100 to 300 ms and 300 to 1000 ms post stimulus onset respectively.
2.5. Statistical analysis
Several one-way analyses of variance (ANOVA) were performed on age, education and non-verbal intelligence (Raven matrices), Beck Depression Inventory (BDI), Beck Anxiety Inventory (BAI), Padua inventory and Y-BOCS scores. TSGS scores were analyzed using a t-test comparing GTS and GTS+OCS groups. Gender was analyzed using the Kruskall-Wallis, non parametric test.
P200 and P300 amplitude and latency were analyzed separately using repeated-measures analyses of variance (MAN-OVA). Subsequently, a separate Multivariate Analysis of Covariance (MANCOVA) was applied on ERP amplitude and latency data considering BDI, BAI and medication status as covariates. The analysis comprised a between-groups factor including four levels (GTS, OCD, GTS + OCS and control groups), and the following within-groups factors: Condition, with two levels (target, non-target); Hemisphere, with two levels (left, right); Region, with two levels (anterior, posterior) and Electrodes, with five levels. The electrodes were divided in four quadrant as left anterior (F3, F7, FC3, FT7 and C3), left posterior (CP3, TP7, P3, T5 and O1), right anterior (F4, F8, FC4, FT8, and C4) and right posterior (CP4, TP8, P4, T6 and O2). The significance level was set at 5% (two-tailed). Finally, linear regression analyses were calculated between P300 component amplitude and OCD (Padua) or tic severity (TSGS).
3. Results
3.1. Demographic and clinical results
No group difference was observed regarding age, education level, gender and non-verbal intelligence (Table 1). However, group differences were observed on clinical questionnaires and interviews (Table 2). The independent group t-test comparison of the TSGS global scores between the GTS and the GTS+OCS groups revealed no significant differences regarding their tic severity (p=.57) as well as their behavioral (p=.30) subscales.
Table 2.
Clinical symptoms description and severity distribution
| Control (n=14) A
|
GTS (n=14) B
|
GTS+OCS (n=12) C
|
OCD (n=15) D
|
ANOVAa
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | Mean | s.d. | % | Mean | s.d. | % | Mean | s.d. | % | Mean | s.d. | p | Tukey | |
| GTS severity (TSGS global) | 0 | 0 | 22 | 11 | 28 | 21 | 0 | 0 | ** | A vs C | ||||
| Absence | 100 | - | - | 0 | 0 | 100 | - | - | A vs B | |||||
| Mild | 0 | - | - | 57 | 50 | 0 | - | - | B vs D | |||||
| Moderate | 0 | - | - | 43 | 17 | 0 | - | - | C vs D | |||||
| Severe | 0 | - | - | 0 | 25 | 0 | - | - | ||||||
| Extreme | 0 | - | - | 0 | 8 | 0 | - | - | ||||||
| OCD severity (Padua) | 13 | 10 | 24 | 11 | 60 | 10 | 98 | 37 | *** | A vs D | ||||
| 1st quartile (0–16) | 71 | 21 | 0 | 0 | A vs C | |||||||||
| 2nd quartile (17–44) | 29 | 72 | 0 | 0 | B vs D | |||||||||
| 3rd quartile (45–74) | 0 | 7 | 75 | 13 | C vs D | |||||||||
| 4th quartile (75 +) | 0 | 0 | 25 | 87 | ||||||||||
| OCD severity (Y-BOCS self-rated) | - | - | - | 5 | 6 | 18 | 8 | 25 | 6 | ** | B vs D | |||
| Absence | - | - | - | 57 | 0 | 0 | C vs D | |||||||
| Mild | - | - | - | 43 | 8 | 0 | B vs C | |||||||
| Moderate | - | - | - | 0 | 25 | 40 | ||||||||
| Severe | - | - | - | 0 | 42 | 33 | ||||||||
| Extreme | - | - | - | 0 | 25 | 27 | ||||||||
| Depression severity (BDI) | 3 | 3 | 4 | 5 | 12 | 6 | 25 | 12 | *** | A vs D | ||||
| Absence | 93 | 86 | 42 | 0 | A vs C | |||||||||
| Mild | 7 | 14 | 42 | 33 | B vs D | |||||||||
| Moderate | 0 | 0 | 17 | 40 | C vs D | |||||||||
| Severe | 0 | 0 | 0 | 27 | ||||||||||
| Anxiety severity (BAI) | 2 | 2 | 9 | 8 | 17 | 13 | 24 | 14 | *** | A vs D | ||||
| Very Low | 100 | 93 | 67 | 53 | A vs D | |||||||||
| Moderate | 0 | 7 | 17 | 33 | C vs D | |||||||||
| Potential problem | 0 | 0 | 17 | 13 | ||||||||||
| Personality disorders (PDQ-4) | 12 | 8 | 19 | 9 | 40 | 13 | 41 | 12 | *** | A vs D | ||||
| 1st quartile (0–13) | 69 | 33 | 0 | 0 | A vs C | |||||||||
| 2nd quartile (14–28) | 31 | 50 | 17 | 14 | B vs C | |||||||||
| 3rd quartile (29–39) | 0 | 17 | 33 | 43 | B vs D | |||||||||
| 4th quartile (40 +) | 0 | 0 | 50 | 43 | ||||||||||
Note:
=p<.01;
=p<.001
Y-BOCS: Yale-Brown Obsession-Compulsion Scale; BDI: Beck Depression Inventory; BAI: Beck Anxiety Inventory; TSGS: Tourette Syndrome Global Scale; PDQ-4: Personality Diagnostic Questionnaire-4th Edition.
The ADIS-IV revealed the occurrence of anxiety disorders as a secondary trouble in the GTS group, including social phobia (n=1), specific phobia (n=2) and generalized anxiety disorders (n=1). This was also true for the GTS+OCS group where anxiety disorders were found such as OCD (n=5), panic disorders (n=1) and generalized anxiety disorders (n=2). Comparison between the three clinical groups revealed significant differences on the Y-BOCS self-rated global score (F[2,38]=39.66, p<.001). The post hoc multiple comparison test showed that the OCD group had significantly higher OC global scores than the GTS+OCS (p<.05) and GTS (p<.001). The GTS+OCS group had higher OC global scores than the GTS (p<.001). Consistent with the Y-BOCS, group differences were also significant on the Padua inventory (F[3,51]=47.35, p<.001). The post hoc multiple comparison test revealed that the OCD group had significantly higher global scores than the GTS+OCS (p<.001), GTS (p<.001) and the control group respectively (p<.001). The GTS+OCS group had also higher OC global scores than the GTS (p<.001) and the control groups (p<.005), but no significant discrepancy appear between the GTS and the control group (p=.98) in OC symptom. Participants of the OCD group also obtained significantly higher depression scores at the BDI (F[3,51]=27.38; p<0.001) than the other three groups, along with significantly higher anxiety scores at the BAI (F[3,51]=13.64; p<0.001) than the GTS and the control group respectively. Participants of the GTS+OCS group also obtained significantly higher scores than the control group (p<0.01) on the BAI. Finally, the global score of the PDQ-4 questionnaire showed significant group differences in personality traits (F[3,51]=24.29; p<0.001). Post hoc multiple comparison tests revealed significant differences between OCD and GTS (p<0.001), between OCD and control (p<0.01) and between GTS+OCS and control (p<0.01) groups. There were no significant differences between OCD and GTS+OCS (p= .99) or between GTS and control (p=.38) groups. Few participants in the OCD (n=4) and GTS+OCS (n=1) groups showed significant personality disorders (higher global score than 50). This was mainly due to their higher scores (larger than 4) in the obsession-compulsion and depressive mood subscales. In sum, the OCD and GTS+OCS groups were the much symptomatic regarding symptoms of anxiety, depression and personality disorders, while the pure GTS group was more comparable to the control group on these clinical dimensions.
3.2. Electrophysiological results
3.2.1. P200 component
The P200 component peaked at an average latency of 208 milliseconds (ms) post stimulus. Significant condition main effect (F[1,51] = 6.15; p <0.05) and condition by region interaction effect (F[1,51]=13.52; p<0.005) were observed on the P200 latency. In the posterior region, the latency was significantly delayed in response to non-target (214 ms) compared to target (195 ms) stimuli while in the anterior area, it was similar across conditions (210 ms vs 211 ms). The amplitude of the P200 component was significantly larger for target than non-target stimuli. Regardless of group attribution, the effect was more important in the right anterior region, leading to a significant condition by hemisphere by region interaction (F[1,51]= 7.37; p<0.01). No group differences were detected with the P200 component.
3.2.2. P300 component
The P300 component peaked at an average latency of 450 ms post stimulus. The P300 latency was delayed in the anterior (474 ms) compared to the posterior (429 ms) region, as revealed by a significant region main effect (F[1,51]=29.09; p<0.001). Analysis of the P300 amplitude revealed significant group (F[3,51]=6.45; p<0.001) and condition (F[1,51]=219.87; p<0.001) main effects, along with group by condition (F[3,51]= 4.88; p<0.01) and group by condition by region (F[3,51]=3.05; p <0.05) interaction effects. This group by condition by region interaction remained significant after covarying separately for depression (F[3,50]=2.94; p<0.05), anxiety (F[3,50]=3.21; p<0.05) and medication status (F[3,50]=2.98; p<0.05). In order to further investigate this three way interaction, ANOVAs were conducted separately for anterior and posterior regions and confirmed the larger group by condition interaction in the posterior (F[3,51]=6.03; p<0.005) than in the anterior (F[3,51]=3.53; p<0.05) region.
3.2.2.1. Anterior P300 amplitude
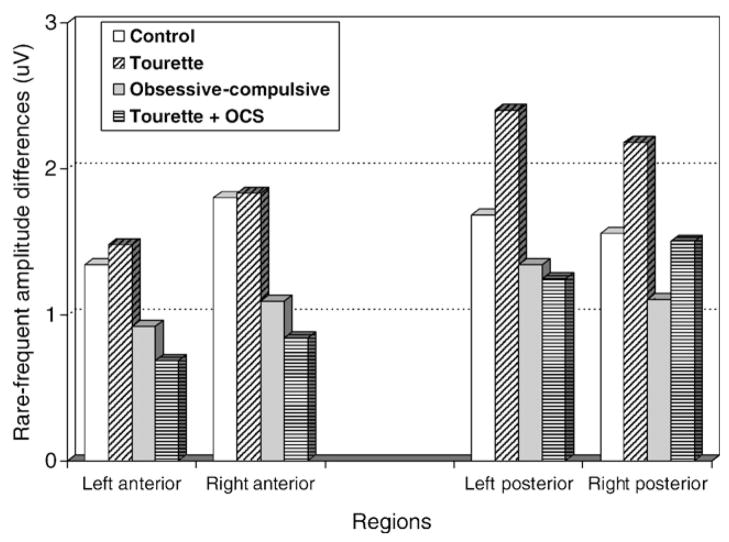
Analysis within the anterior region was applied to the target and non-target conditions separately and revealed significant group differences only in the target condition (F[3,51]= 6.52; p<0.005). Between-group comparisons revealed that participants in the GTS group demonstrated a significantly larger P300 amplitude than participants in the GTS+OCS (p<0.01) and OCD (p<0.01) groups. Partial comparisons of each group with the control group further revealed significant group main effects between control and OCD (F[1,27]=7.19; p<0.05) groups and between control and GTS + OCS (F[1,24]= 5.55; p<0.05) groups. Comparisons between control and GTS groups and also between OCD and GTS+OCS groups failed to demonstrate significant difference. Therefore, both OCD and GTS+OCS groups showed smaller P300 anterior amplitude to the target stimuli than the other two groups. (Figs. 2 and 3). A linear regression analysis showed that increased OCS scores at the Padua inventory had an attenuating effect on the P300 amplitude (R2 =0.13) at the right anterior region (F[1,53]=7.65, p<.01).
Fig. 2.
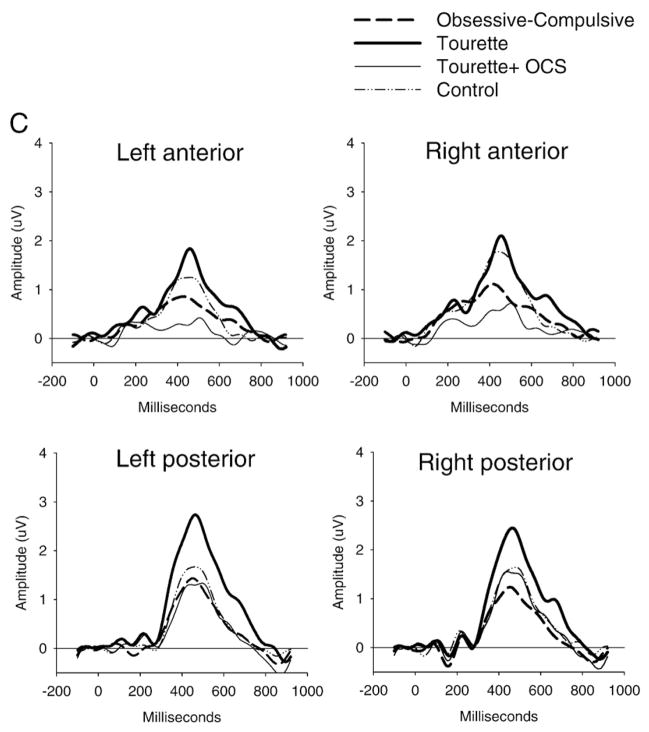
In the anterior region, the oddball effect (rare minus frequent) was found to be reduced in the OCD and GTS+OCS groups compared to the GTS and control groups. In the posterior region, the oddball effect was found to be increased in the GTS group compared to the three other groups.
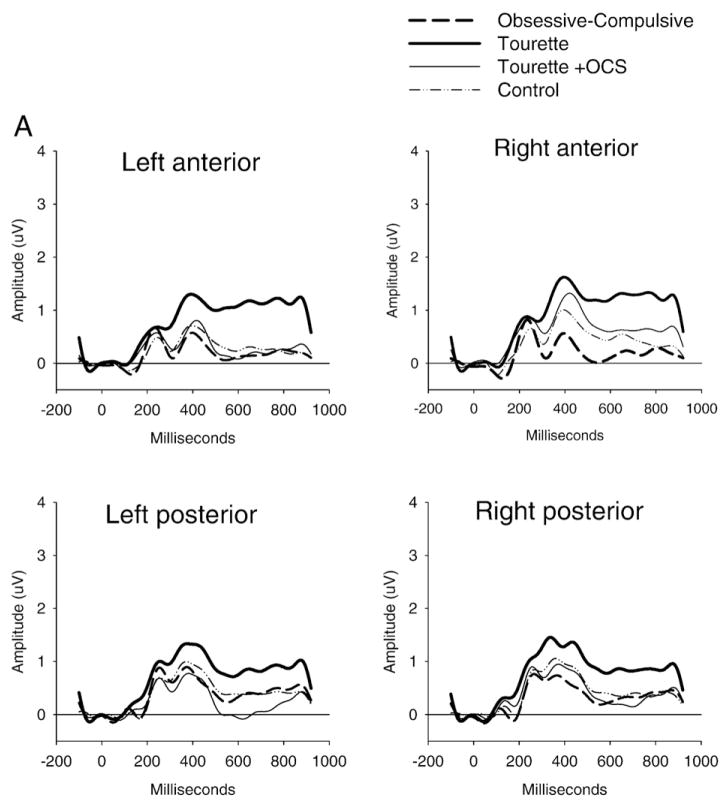
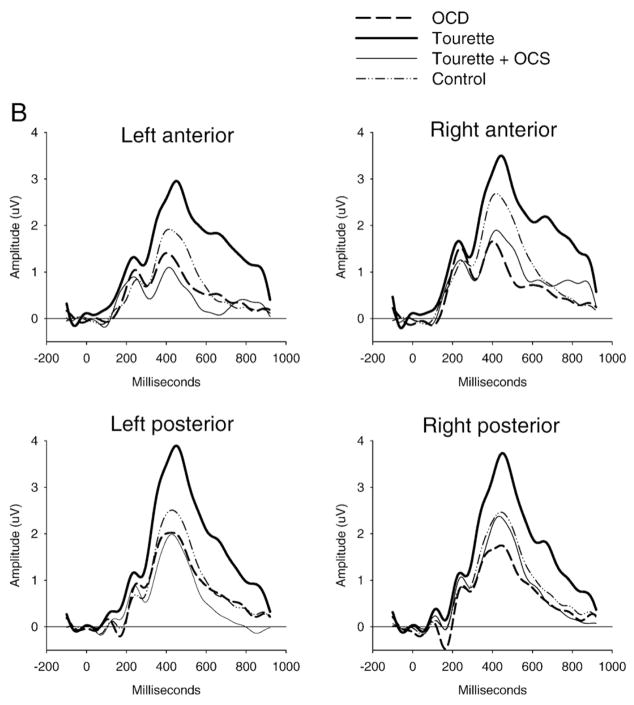
Fig. 3.
Stimulus-locked ERP waveforms to non-target (Panel A) and target stimuli (Panel B) and the difference between target and non-target stimuli (oddball effect: Panel C). The P300 component (around 450 ms) showed increased posterior amplitude in the GTS group and reduced anterior amplitude in the OCD and GTS+OCS groups for the target stimuli only, giving rise to an increased or decreased oddball effect respectively.
3.2.2.2. Posterior P300 amplitude
As for the anterior P300, analysis within the posterior region applied to the target and non-target stimuli separately revealed significant group differences only in the target condition (F[3,51]=9.49; p<0.001). Between-group comparisons revealed that participants in the GTS group demonstrated a significantly larger P300 amplitude to target amplitude than participants in the control (p<0.01), GTS+OCS (p<0.01) and OCD (p<0.001) groups respectively, these three groups not being significantly different from each other. However, post-hoc comparisons between each group and the control group, further revealed a significant main effect of group between the control and the OCD (F[1,27]=4.36; p<0.05) group. There was no significant difference between control and GTS+OCS groups. So, GTS group had larger P300 posterior amplitude to the target stimuli than the three other groups. (Figs. 2 and 3). A linear regression analysis showed that increased tic symptom severity predicted larger target P300 amplitude (R2 =0.12) especially in the right posterior region (F [1,53]=7.14, p<.05). However, increased OCS severity at the Padua inventory was associated with a reduced effect on the P300 amplitude (R2 =0.11), at right posterior regions (F[1,53]= 6.51, p<.05).
4. Discussion
4.1. Summary of main findings
The current study aimed at investigating cognitive processing and electrocortical functioning in adults suffering from GTS and at exploring the influence of obsessive-compulsive symptoms (OCS) on P200 and P300 components. Across all clinical groups, our results showed a normal P200 component, whilst the P300 component was clearly affected by the presence of clinical symptoms. Participants suffering from OCD or from GTS+OCS showed a reduced P300 oddball effect, mainly in the right anterior region, but otherwise did not differ significantly from each other. These two groups also showed significantly greater symptom severity regarding depression and anxiety when compared with both GTS and control groups. The target P300 amplitude was negatively correlated with obsessive-compulsive and depressive symptoms. Conversely, participants suffering from GTS showed a larger P300 oddball effect, the target P300 amplitude being positively correlated with tic symptom severity.
4.2. Influence of obsessive-compulsive symptoms on ERP profile
Participants suffering from OCS, either in addition to GTS or with OCD, demonstrated similar results. They showed no P200 amplitude difference compared to the control group and, as we first expected, obtained a significantly smaller P300 oddball effect in the right anterior region compared to the GTS and control groups.
Our findings regarding the P200 component were in line with previous results observed by Miyata and collaborators (1998), who found no difference on the P200 amplitude or latency in the OCD group. Available data about the functional significance of the P200 component considers it as an index of vigilance (Ferrara et al., 2002). Consequently, these results argue in favor of intact vigilance and focal attention processes in OCD. However, as previously mentioned, other P200 results in this population has been found to be incongruent with ours (Asahi et al., 1993). Further studies would thus be needed in order to better characterize that component with OCD groups.
Concerning the P300 component, more consistent results were found. The profile showed a significant negative relationship between the P300 oddball effect and OCS severity, where an augmentation in the level of symptom severity was associated with a linear reduction of the oddball effect. Johannes and collaborators (1997) observed a target P300 amplitude reduction in groups characterized as «pure» GTS, but who actually showed a clinical profile very similar to our GTS+OCS group. Similar results were previously obtained with pure OCD groups (Beech et al., 1983; Ciesielski et al., 1981; Malloy et al., 1989; Sanz et al., 2001; Towey et al., 1994). Nonetheless, these earlier studies didn’t mention the relationship between P300 component and symptom intensity. Based on these results, we conclude that the intensity of OCS has a strong attenuating effect on the P300 and on the specific cognitive processes subtending it. From a neurocognitive perspective, the P300 amplitude has been related to contextual memory updating (Donchin, 1981) and response certainty (Kutas et al., 1977). Concurrently, several studies reported memory impairments and response uncertainty as features often appearing in the OCD population (O’Connor et al., 2005). Poorer performance has been observed on tests of logical memory (Sher et al., 1984), short-term (Boone et al., 1991; Christensen et al., 1992) and long-term (Zitterl et al., 2001) visual memory in participants with OCD. It has also been proposed that uncertainty and low confidence level could impede information processing in patients with OCD and mediate their memory difficulties. Repetitive thoughts and actions characterizing OCD could occur because of repeated neural signals indicating that they have not been adequately carried out (Pitman, 1987). The resulting uncertainty could impact on memory functions and, consequently, on the capacity to inhibit thoughts and actions (Zitterl et al., 2001).
Our findings highlighted the influence of OCS on processes, mediated through the right prefrontal cortical regions, which are hypothesized to be involved in memory inhibition mechanisms (Depue et al., 2007). Indeed, it has been found that people suffering from OCD may feel the need to remember features in too much detail, or adopt a sequential rather than a comprehensive approach to pattern recognition and organization (Savage et al., 1999) when performing even a simple oddball task. Anomaly in certainty or memory inhibition mechanisms could influence information processing and on working memory updating processes, as reflected by the anterior P300 amplitude attenuation.
4.3. Effect of Gilles de la Tourette syndrome on ERP profile
Our results suggest that adults suffering from GTS would present relatively intact focal attention (P200), coupled with overactive working memory updating processes, as reflected by an enhanced P300 oddball effect compared to the other clinical groups and the control group. At first sight, these results do not entirely support previous interpretations brought in similar ERP studies.
Previous ERP studies focusing on GTS have reported a P200 amplitude (van Woerkom et al., 1988, 1994) and latency (van Woerkom et al., 1988) decrease to non-target frequent stimuli, which have been interpreted as indicating a disturbance in arousal and focal attention toward non-relevant stimuli (van Woerkom et al., 1988, 1994). However, these studies included only the non-target stimuli in their discussion of the P200. Since results about target stimuli were not considered, interpretation of results in terms of a specific attention misallocation could be misleading.
More importantly, methodological shortcomings regarding clinical comorbidity need to be taken into account before explaining the discrepancies between our results and previous findings. Indeed, absence of P300 amplitude or latency differences in GTS (Johannes et al., 2001b, 2002; van Woerkom et al., 1988) has been reported in earlier investigations, which could be ascribed to the influence of comorbid OCD in the sample of participants. Previous ERP studies either included GTS participants suffering from mild to severe OCS or provided no information about the presence of such symptoms in their sample. With a high comorbidity rate between GTS and OCD, this leaves a strong possibility for the presence of OCS among their participants. It is very likely that OCS have an influence on the ERP profile of people affected primarily by GTS. Consequently, we propose that the relatively intact P300 amplitude that has been observed in most ERP studies could reflect this influence. As mentioned earlier, OCS would have a moderating or even an attenuating effect on the potentially intact P300 of GTS participants, canceling out the enhancing effect of GTS symptoms on this component.
Another important clinical issue is whether GTS+OCD is etiologically related to either GTS, OCD or whether GTS+OCD represent a more severe expression of GTS or OCD. According to earlier studies, GTS+OCD represent a more severe phenotype, compared to pure GTS or OCD and appear to be more closely linked to GTS than OCD on the dimension of repetitive behavior (Cath et al., 2001) and other psychopathological comorbidity profile such as bipolar disorders, social phobia, body dismorphic disorders and attention deficit hyperactivity disorders (Coffey et al., 1998). Neuropsychological results obtained from a semantic Simon task revealed that the pure GTS and pure OCD groups were consistently disadvantaged in the more cognitively demanding conditions compared to matched controls (Rankins et al., 2006). But the results of Rankins et al. also revealed more similarities between pure GTS and GTS+OCD than with OCD per se, on measure of cognitive inhibition. Our results seem partially at odd with these previous studies of GTS and OCD, which examined the phenotypes of these patients. The discrepancy found between the conclusion of these studies and our findings can be ascribed partly to our sample selection and to the nature of the task. From a purely clinical point of view, our group of GTS+OCS resembled more an OCD group than a GTS group, particularly regarding obsessive-compulsive symptoms, anxiety, depression and personality characteristics, which distinguishes their comorbidity profile from both Cath et al. and Coffey et al. studies. Another distinct factor was the nature of our task and corresponding measures, which did not necessarily activate and record the same cognitive processes as in Rankins et al. In fact, our P300 oddball effect seemed to follow a pattern congruent with the symptom profile, with the GTS+OCS showing more similarity to the OCD group than to the pure GTS group. The reduced P300 oddball effect, showed negative correlation with OCD symptoms and this seems consistent with brain imaging studies showing a reduced volume of left and right orbitofrontal cortices, also negatively correlated with OCD symptoms severity (Atmaca et al., 2007).
But why exactly do GTS group show a larger P300 oddball effect, in contrast to the groups showing more OC symptoms? We propose that the larger P300 amplitude observed in the GTS group could be attributable to overactivation of specialized cortical areas. Hyperactivation of the dorsolateral region has been demonstrated to be involved in GTS by past fMRI studies (Peterson et al., 2001). The magnitude of signal change in the dorsolateral prefrontal cortex was found to be positively correlated with tic severity and its greater activation was also found to be associated with regulation of performance in complex attentional tasks in participants with GTS (Marsh et al., 2007).
Other evidence has suggested that GTS patients show dysfunctional noradrenergic regulation, which translates into a state of overarousal, as reflected by elevated cerebrospinal fluid corticotropin-releasing factor (Chappell et al., 1996; Sandyk and Bamford, 1988). The noradrenergic system is involved in regulating working memory and attentional functions in prefrontal cortex (see Ramos and Arnsten, 2007). Several studies with primates have demonstrated that the locus coeruleus-norepi-nephrine neurons (LC-NE) were selectively activated by infrequent visual target stimuli such as in the oddball task (Aston-Jones et al., 1994; Rajkowski et al., 1994). In human, the administration of clonidine, an adrenergic agonist, diminishes LC neuron firing as well as NE release, which showed a clear reduction of an effect on the P300 amplitude (Halliday et al., 1994). With GTS patients, clonidine administration significantly improve symptoms, which provides indirect support for involvement of central noradrenergic systems in tic expression (Lichter and Jackson, 1996). Overactivity of the noradrenergic system, as reflected by the larger P300 amplitude in parietal areas in individuals with GTS may be caused, at least in part, by a difficulty engaging regulatory systems in order to control a task that requires selective attention (Marsh et al., 2007; Peterson et al., 1998). In sum, implication of dorsolateral prefrontal cortex and noradrenergic system could suggest that the larger P300 oddball effect observed in the GTS group reflect a higher state of arousal, which narrows the amount of attention available for task performance (Kok, 1990; Polich, 2007; Pribram and McGuinness, 1975).
4.4. Possible influences of confounding variables
The first possible confounding factor is related to the posterior P300 amplitude, which could have been influenced by motor response. In order to control for the fact that motor activity often overlap and sometimes distort the P300 response (Kok, 1988; Salisbury et al., 2001), we employed a counting oddball paradigm, a procedure often utilized in similar ERP investigations (Potts, 2004; Watson et al., 2005). However, a potential problem associated with the counting task was the absence of any quantifiable reaction time to insure that patients were paying attention to the task. Our results showed that all subjects were able to count the number of target accurately. As a result, the anomalies in the P300 amplitude cannot be attributed to a problem in motor execution overlapping with the cognitive processes at study or to a lower level of vigilance and attention on tasks.
The presence of significantly more anxious, depressed and abnormal personality traits in our OCD group could have contributed to the results. For instance, it has been reported that major depression is related to P300 amplitude reduction (Anderer et al., 2002; Blackwood et al., 1987; Gangadhar et al., 1993; Roschke and Wagner, 2003) and one could argue that this could have contributed to the amplitude reduction observed in participants with OCD compared to the GTS and control groups. However, covariance analysis revealed that group differences remained statistically significant after depressive symptoms had been introduced as a covariable.
Third, participants in the OCD group were also taking more medication than participants of the GTS and control groups. Medication included antidepressant medication, some of which are known to treat OCD symptoms (Table 3). However, this medication have been found to have a normalizing effect on the P300 amplitude (Blackwood et al., 1987; Gangadhar et al., 1993), which could have neutralized the effect of depressive symptoms. Since participants of the OCD group demonstrated clinically significant symptoms despite the medication, we suggest that the P300 oddball effect decrease reflected a genuine influence of obsessive-compulsive symptomatology.
Finally, another limitation was our small group sample size which could have reduced the power and the extent of our conclusions. However, our sample size compares to earlier samples in the ERP field, which used GTS patients samples at n=6 (Van de Wetering et al., 1985), n=10 (Johannes et al., 2001a,b, 2003), n=12 (Johannes et al., 1997) and n=24 (van Woerkom et al., 1994). The same applies with ERP studies of OCD patients with various sample sizes from a n=8 (Di Russo et al., 2000), n=9 (Gehring et al., 2000), n=13 (Morault et al., 1997), n=15 (Sanz et al., 2001) and n=21 (Morault et al., 1998). Future research would ideally recruit larger samples and compare GTS+OCS with other comorbid groups such as highly anxious, depressive or hyperactive GTS patients.
5. Conclusions
The current study investigated the impact of OCS comorbidity in Tourette patients on specific brain ERP responses. The presence of OCS influences the ERP profile of adults suffering from GTS by decreasing the electro-cortical component associated with working memory updating processes. On the other hand, GTS is mainly characterized by a larger P300 reflecting enhanced working memory updating processes, which could be congruent with overactivation of cortical areas involved in both the control of tics and in the regulation of performance. In sum, our results highlight the importance of considering OCS and other comorbid conditions as an important confounding variable in future studies of GTS.
Acknowledgments
This work was supported by a Canadian Institutes of Health Research (CIHR) operating grant (57936), a Fonds pour la Recherche en Santé du Québec (FRSQ), clinical research grant (5271) and the laboratory infrastructure from the Fernand-Seguin research center. Geneviève Thibault was supported by a Ph.D. fellow from the CIHR (139370). We wish to express our gratitude to Marie-Claude Pélissier, Frederic Aardema, Anick Laverdure and Ariane Fontaine for research coordination and clinical screening, to Martine Germain and Nerly Jeudin for electro-physiological recordings, to Véronique Labelle and Tina Imbriglio, for technical assistance and Sophie Lecours, Maria-Teresa Hernandez, Cathy Léveillé and Anne-Marie Daoust for the neuropsychometric testing. We are also grateful to Melodee Mograss who made the English corrections. Last but not the least, we thank all participants for their participation in this study.
Abbreviations
- GTS
Gilles de la Tourette syndrome
- OCD
obsessive-compulsive disorder
- ERP
event-related potential
- OCS
obsessive-compulsive symptoms
- BDI
Beck depression inventory
- BAI
Beck anxiety inventory
- TSGS
Tourette syndrome global scale
- Y-BOCS
Yale-Brown obsessive-compulsive scale
References
- American EEG Society. Guideline thirteen: guidelines for standard electrode position nomenclature. J Clin Neurophysiol. 1994;11:111–3. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington DC: American Psychiatric Association; 2000. Text revision. [Google Scholar]
- Anderer P, Saletu B, Semlitsch HV, Pascual-Marqui RD. Structural and energetic processes related to P300: LORETA findings in depression and effects of antidepressant drugs. Methods Find Exp Clin Pharmacol. 2002;24:85–91. Suppl D. [PubMed] [Google Scholar]
- Asahi K, Ogura C, Hirano K, Nageishi Y. Endogenous event-related potentials in obsessive character. Jpn J Psychiatry Neurol. 1993;47(1):63–9. doi: 10.1111/j.1440-1819.1993.tb02031.x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J Neurosci. 1994;14(7):4467–80. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atmaca M, Yildirim H, Ozdemir H, Tezcan E, Poyraz AK. Volumetric MRI study of key brain regions implicated in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(1):46–52. doi: 10.1016/j.pnpbp.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Banaschewski T, Brandeis D. Annotation: what electrical brain activity tells us about brain function that other techniques cannot tell us - a child psychiatric perspective. J Child Psychol Psychiatry. 2007;48(5):415–35. doi: 10.1111/j.1469-7610.2006.01681.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56(6):893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beech HR, Ciesielski KT, Gordon PK. Further observations of evoked potentials in obsessional patients. Br J Psychiatry. 1983;142:605–9. doi: 10.1192/bjp.142.6.605. [DOI] [PubMed] [Google Scholar]
- Blackwood DH, Whalley LJ, Christie JE, Blackburn IM, St Clair DM, McInnes A. Changes in auditory P3 event-related potential in schizophrenia and depression. Br J Psychiatry. 1987;150:154–60. doi: 10.1192/bjp.150.2.154. [DOI] [PubMed] [Google Scholar]
- Boone KB, Ananth J, Philpott L, Kaur A, Djenderedjian A. Neuropsychological characteristics of nondepressed adults with obsessive-compulsive disorder. Neuropsychiatry Neuropsychol Behav Neurol. 1991;4(2):96–109. [Google Scholar]
- Brown TA, DiNardo PA, Barlow DH. Anxiety disorders interview schedule for DSM-IV. Boulder, CO: Graywind Publications; 1994. [Google Scholar]
- Cath DC, Spinhoven P, van Woerkom TC, van de Wetering BJ, Hoogduin CA, Landman AD, et al. Gilles de la Tourette’s syndrome with and without obsessive-compulsive disorder compared with obsessive-compulsive disorder without tics: which symptoms discriminate? J Nerv Ment Dis. 2001;189(4):219–28. doi: 10.1097/00005053-200104000-00003. [DOI] [PubMed] [Google Scholar]
- Channon S, Flynn D, Robertson MM. Attentional deficits in Gilles de la Tourette syndrome. Neuropsychiatry Neuropsychol Behav Neurol. 1992;5:170–7. [Google Scholar]
- Chappell P, Leckman J, Goodman W, Bissette G, Pauls D, Anderson G, et al. Elevated cerebrospinal fluid corticotropin-releasing factor in Tourette’s syndrome: comparison to obsessive compulsive disorder and normal controls. Biol Psychiatry. 1996;39(9):776–83. doi: 10.1016/0006-3223(95)00221-9. [DOI] [PubMed] [Google Scholar]
- Christensen KJ, Kim SW, Dysken MW, Hoover KM. Neuropsychological performance in obsessive-compulsive disorder. Biol Psychiatry. 1992;31(1):4–18. doi: 10.1016/0006-3223(92)90003-i. [DOI] [PubMed] [Google Scholar]
- Ciesielski KT, Beech HR, Gordon PK. Some electrophysiological observations in obsessional states. Br J Psychiatry. 1981;138:479–84. doi: 10.1192/bjp.138.6.479. [DOI] [PubMed] [Google Scholar]
- Coffey BJ, Miguel EC, Biederman J, Baer L, Rauch SL, O’Sullivan RL, et al. Tourette’s disorder with and without obsessive-compulsive disorder in adults: are they different? J Nerv Ment Dis. 1998;186(4):201–6. doi: 10.1097/00005053-199804000-00001. [DOI] [PubMed] [Google Scholar]
- Depue BE, Curran T, Banich MT. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science. 2007;317(5835):215–9. doi: 10.1126/science.1139560. [DOI] [PubMed] [Google Scholar]
- Di Russo F, Zaccara G, Ragazzoni A, Pallanti S. Abnormal visual event-related potentials in obsessive-compulsive disorder without panic disorder or depression comorbidity. J Psychiatr Res. 2000;34(1):75–82. doi: 10.1016/s0022-3956(99)00030-8. [DOI] [PubMed] [Google Scholar]
- Donchin E. Presidential address, 1980. Surprise!...Surprise? Psychophysiology. 1981;18(5):493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Ferrara M, De Gennaro L, Ferlazzo F, Curcio G, Cristiani R, Bertini M. Topographical changes in N1-P2 amplitude upon awakening from recovery sleep after slow-wave sleep deprivation. Clin Neurophysiol. 2002;113(8):1183–90. doi: 10.1016/s1388-2457(02)00146-3. [DOI] [PubMed] [Google Scholar]
- Ford JM. Schizophrenia: the broken P300 and beyond. Psychophysiology. 1999;36(6):667–82. [PubMed] [Google Scholar]
- Gangadhar BN, Ancy J, Janakiramaiah N, Umapathy C. P300 amplitude in non-bipolar, melancholic depression. J Affect Disord. 1993;28(1):57–60. doi: 10.1016/0165-0327(93)90077-w. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychol Sci. 2000;11(1):1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Georgiou N, Bradshaw JL, Phillips JG, Bradshaw JA, Chiu E. The Simon effect and attention deficits in Gilles de la Tourette’s syndrome and Huntington’s disease. Brain. 1995;118:1305–18. doi: 10.1093/brain/118.5.1305. [DOI] [PubMed] [Google Scholar]
- Georgiou N, Bradshaw JL, Phillips JG, Chiu E. The effect of Huntington’s disease and Gilles de la Tourette’s syndrome on the ability to hold and shift attention. Neuropsychologia. 1996;34(9):843–51. doi: 10.1016/0028-3932(95)00170-0. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Delgado P, Heninger GR, et al. The Yale-Brown obsessive compulsive scale. II. Validity. Arch Gen Psychiatry. 1989a;46(11):1012–6. doi: 10.1001/archpsyc.1989.01810110054008. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, et al. The Yale-Brown obsessive compulsive scale. I. Development, use, and reliability. Arch Gen Psychiatry. 1989b;46(11):1006–11. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Hackley SA, Valle-Inclan F. Which stages of processing are speeded by a warning signal? Biol Psychol. 2003;64(1–2):27–45. doi: 10.1016/s0301-0511(03)00101-7. [DOI] [PubMed] [Google Scholar]
- Halliday R, Naylor H, Brandeis D, Callaway E, Yano L, Herzig K. The effect of D-amphetamine, clonidine, and yohimbine on human information processing. Psychophysiology. 1994;31(4):331–7. doi: 10.1111/j.1469-8986.1994.tb02441.x. [DOI] [PubMed] [Google Scholar]
- Harcherik DF, Leckman JF, Detlor J, Cohen DJ. A new instrument for clinical studies of Tourette’s syndrome. J Am Acad Child Psych. 1984;23(2):153–60. doi: 10.1097/00004583-198403000-00006. [DOI] [PubMed] [Google Scholar]
- Hyler SE. Personality Questionnaire PDQ-41. New York: New York State Psychiatric Institute; 1994. [Google Scholar]
- Johannes S, Weber A, Muller-Vahl KR, Kolbe H, Dengler R, Munte TF. Event-related brain potentials show changed attentional mechanisms in Gilles de la Tourette syndrome. Eur J Neurol. 1997;4:152–61. doi: 10.1111/j.1468-1331.1997.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Mantey M, Nager W, Rada D, Muller-Vahl KR, et al. Altered inhibition of motor responses in Tourette syndrome and Obsessive-Compulsive disorder. Acta Neurol Scand. 2001a;104:36–43. doi: 10.1034/j.1600-0404.2001.00308.x. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Nager W, Muller-Vahl KR, Dengler R, Munte TF. Electrophysiological measures and dual-task performance in Tourette syndrome indicate deficient divided attention mechanisms. Eur J Neurol. 2001b;8(3):253–60. doi: 10.1046/j.1468-1331.2001.00199.x. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Nager W, Muller-Vahl KR, Dengler R, Munte TF. Excessive action monitoring in Tourette syndrome. J Neurol. 2002;249(8):961–6. doi: 10.1007/s00415-002-0657-9. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Nager W, Rada D, Muller-Vahl KR, Emrich HM, et al. Tourette syndrome and obsessive-compulsive disorder: event-related brain potentials show similar mechanisms of frontal inhibition but dissimilar target evaluation processes. Behav Neurol. 2003;14:9–17. doi: 10.1155/2003/326468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Kang SS, Youn T, Kang DH, Kim JJ, Kwon JS. Neuropsychological correlates of P300 abnormalities in patients with schizophrenia and obsessive-compulsive disorder. Psychiatry Res. 2003;123(2):109–23. doi: 10.1016/s0925-4927(03)00045-3. [DOI] [PubMed] [Google Scholar]
- Kok A. Overlap between P300 and movement-related-potentials: a response to Verleger. Biol Psychol. 1988;27(1):51–8. doi: 10.1016/0301-0511(88)90005-1. [DOI] [PubMed] [Google Scholar]
- Kok A. Internal and external control: a two-factor model of amplitude change of event-related potentials. Acta Psychol (Amst) 1990;74(2–3):203–36. [PubMed] [Google Scholar]
- Kutas M, McCarthy G, Donchin E. Augmenting mental chronometry: the P300 as a measure of stimulus evaluation time. Science. 1977;197(4305):792–5. doi: 10.1126/science.887923. [DOI] [PubMed] [Google Scholar]
- Lavoie ME, Dupuis F, Johnston KM, Leclerc S, Lassonde M. Visual p300 effects beyond symptoms in concussed college athletes. J Clin Exp Neuropsychol. 2004;26(1):55–73. doi: 10.1076/jcen.26.1.55.23936. [DOI] [PubMed] [Google Scholar]
- Lavoie ME, Thibault G, Stip E, O’Connor KP. Memory and executive functions in adults with Gilles de la Tourette syndrome and chronic tic disorder. Cogn Neuropsychiatry. 2007;12(2):165–81. doi: 10.1080/13546800600826371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, et al. The yale global tic severity scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psych. 1989;28(4):566–73. doi: 10.1097/00004583-198907000-00015. [DOI] [PubMed] [Google Scholar]
- Lichter DG, Jackson LA. Predictors of clonidine response in Tourette syndrome: implications and inferences. J Child Neurol. 1996;11(2):93–7. doi: 10.1177/088307389601100205. [DOI] [PubMed] [Google Scholar]
- Linden DE. The p300: where in the brain is it produced and what does it tell us? Neuroscientist. 2005;11(6):563–76. doi: 10.1177/1073858405280524. [DOI] [PubMed] [Google Scholar]
- Malloy P, Rasmussen S, Braden W, Haier RJ. Topographic evoked potential mapping in obsessive-compulsive disorder: evidence of frontal lobe dysfunction. Psychiatry Res. 1989;28(1):63–71. doi: 10.1016/0165-1781(89)90198-4. [DOI] [PubMed] [Google Scholar]
- Marcus D, Kurlan R. Tics and its disorders. Neurol Clin. 2001;19(3):735–58. viii. doi: 10.1016/s0733-8619(05)70043-8. [DOI] [PubMed] [Google Scholar]
- Marsh R, Zhu H, Wang Z, Skudlarski P, Peterson BS. A developmental fMRI study of self-regulatory control in Tourette’s syndrome. Am J Psychiatry. 2007;164(6):955–66. doi: 10.1176/appi.ajp.164.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavrogiorgou P, Juckel G, Frodl T, Gallinat J, Hauke W, Zaudig M, et al. P300 subcomponents in obsessive-compulsive disorder. J Psychiatr Res. 2002;36 (6):399–406. doi: 10.1016/s0022-3956(02)00055-9. [DOI] [PubMed] [Google Scholar]
- Mazzini L. Clinical applications of event-related potentials in brain injury. Phys Med Rehabil Clin North Am. 2004;15(1):163–75. doi: 10.1016/s1047-9651(03)00101-3. [DOI] [PubMed] [Google Scholar]
- Mink JW. Basal ganglia dysfunction in Tourette’s syndrome: a new hypothesis. Pediatr Neurol. 2001;25(3):190–8. doi: 10.1016/s0887-8994(01)00262-4. [DOI] [PubMed] [Google Scholar]
- Miyata A, Matsunaga H, Kiriike N, Iwasaki Y, Takei Y, Yamagami S. Event-related potentials in patients with obsessive-compulsive disorder. Psychiatry Clin Neurosci. 1998;52(5):513–8. doi: 10.1046/j.1440-1819.1998.00427.x. [DOI] [PubMed] [Google Scholar]
- Morault PM, Bourgeois M, Laville J, Bensch C, Paty J. Psychophysiological and clinical value of event-related potentials in obsessive-compulsive disorder. Biol Psychiatry. 1997;42(1):46–56. doi: 10.1016/S0006-3223(96)00228-4. [DOI] [PubMed] [Google Scholar]
- Morault P, Guillem F, Bourgeois M, Paty J. Improvement predictors in obsessive-compulsive disorder. An event-related potential study. Psychiatry Res. 1998;81(1):87–96. doi: 10.1016/s0165-1781(98)00091-2. [DOI] [PubMed] [Google Scholar]
- O’Connor KP, Aardema F, Pélissier MC. Beyond reasonable doubt. Chichester: John Wiley & Sons Ltd; 2005. [Google Scholar]
- Olichney JM, Hillert DG. Clinical applications of cognitive event-related potentials in Alzheimer’s disease. Phys Med Rehabil Clin North Am. 2004;15 (1):205–33. doi: 10.1016/s1047-9651(03)00103-7. [DOI] [PubMed] [Google Scholar]
- Pauls DL. The genetics of obsessive compulsive disorder and Gilles de la Tourette’s syndrome. Psychiatr Clin North Am. 1992;15(4):759–66. [PubMed] [Google Scholar]
- Pauls DL, Towbin KE, Leckman JF, Zahner GE, Cohen DJ. Gilles de la Tourette’s syndrome and obsessive-compulsive disorder. Evidence supporting a genetic relationship. Arch Gen Psychiatry. 1986;43(12):1180–2. doi: 10.1001/archpsyc.1986.01800120066013. [DOI] [PubMed] [Google Scholar]
- Pauls DL, Alsobrook JP, II, Goodman W, Rasmussen S, Leckman JF. A family study of obsessive-compulsive disorder. Am J Psychiatry. 1995;152(1):76–84. doi: 10.1176/ajp.152.1.76. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Anderson AW, Zhang H, Gatenby JC, Lacadie CM, et al. A functional magnetic resonance imaging study of tic suppression in Tourette syndrome. Arch Gen Psychiatry. 1998;55(4):326–33. doi: 10.1001/archpsyc.55.4.326. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Staib L, Scahill L, Zhang H, Anderson C, Leckman JF, et al. Regional brain and ventricular volumes in Tourette syndrome. Arch Gen Psychiatry. 2001;58(5):427–40. doi: 10.1001/archpsyc.58.5.427. [DOI] [PubMed] [Google Scholar]
- Pitman RK. A cybernetic model of obsessive-compulsive psychopathology. Compr Psychiatry. 1987;28(4):334–43. doi: 10.1016/0010-440x(87)90070-8. [DOI] [PubMed] [Google Scholar]
- Polich J. Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol. 2007;118(10):2128–48. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts GF. An ERP index of task relevance evaluation of visual stimuli. Brain Cogn. 2004;56(1):5–13. doi: 10.1016/j.bandc.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Pribram KH, McGuinness D. Arousal, activation, and effort in the control of attention. Psychol Rev. 1975;82(2):116–49. doi: 10.1037/h0076780. [DOI] [PubMed] [Google Scholar]
- Rajkowski J, Kubiak P, Aston-Jones G. Locus coeruleus activity in monkey: phasic and tonic changes are associated with altered vigilance. Brain Res Bull. 1994;35(5–6):607–16. doi: 10.1016/0361-9230(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007;113(3):523–36. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankins D, Bradshaw JL, Georgiou-Karistianis N. The semantic Simon effect in Tourette’s syndrome and obsessive-compulsive disorder. Brain Cogn. 2006;61(3):225–34. doi: 10.1016/j.bandc.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Rodgers R, Callahan S, Chabrol H. Revision of the translation of certain items in the French version of PDQ-4 (Personality Diagnostic Questionnaire, Hyler, 1994) Encephale. 2004;30(4):408–9. [PubMed] [Google Scholar]
- Roschke J, Wagner P. A confirmatory study on the mechanisms behind reduced P300 waves in depression. Neuropsychopharmacology. 2003;28:S9–S12. doi: 10.1038/sj.npp.1300139. Suppl 1. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Rutherford B, Shenton ME, McCarley RW. Button-pressing affects P300 amplitude and scalp topography. Clin Neurophysiol. 2001;112 (9):1676–84. doi: 10.1016/s1388-2457(01)00607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanavio E. Obsessions and compulsions: the Padua inventory. Behav Res Ther. 1988;26(2):169–77. doi: 10.1016/0005-7967(88)90116-7. [DOI] [PubMed] [Google Scholar]
- Sandyk R, Bamford CR. Heightened cortisol response to administration of naloxone in Tourette’s syndrome. Int J Neurosci. 1988;39(3–4):225–7. doi: 10.3109/00207458808985707. [DOI] [PubMed] [Google Scholar]
- Sanz M, Molina V, Martin-Loeches M, Calcedo A, Rubia FJ. Auditory P300 event related potential and serotonin reuptake inhibitor treatment in obsessive-compulsive disorder patients. Psychiatry Res. 2001;101(1):75–81. doi: 10.1016/s0165-1781(00)00250-x. [DOI] [PubMed] [Google Scholar]
- Savage CR, Baer L, Keuthen NJ, Brown HD, Rauch SL, Jenike MA. Organizational strategies mediate nonverbal memory impairment in obsessive-compulsive disorder. Biol Psychiatry. 1999;45(7):905–16. doi: 10.1016/s0006-3223(98)00278-9. [DOI] [PubMed] [Google Scholar]
- Saxena S, Brody AL, Schwartz JM, Baxter LR. Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry. 1998;(35):26–37. Suppl. [PubMed] [Google Scholar]
- Sheppard DM, Bradshaw JL, Purcell R, Pantelis C. Tourette’s and comorbid syndromes: obsessive compulsive and attention deficit hyperactivity disorder. A common etiology? Clin Psychol Rev. 1999;19(5):531–52. doi: 10.1016/s0272-7358(98)00059-2. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Mann B, Frost RO. Cognitive dysfunction in compulsive checkers: further explorations. Behav Res Ther. 1984;22(5):493–502. doi: 10.1016/0005-7967(84)90053-6. [DOI] [PubMed] [Google Scholar]
- Steketee G. Behavioural assessment and treatment planning with obsessive-compulsive disorder. Behav Ther. 1994;25:613–33. [Google Scholar]
- Tata PR, Leibowitz JA, Prunty MJ, Cameron M, Pickering AD. Attentional bias in obsessional compulsive disorder. Behav Res Ther. 1996;34(1):53–60. doi: 10.1016/0005-7967(95)00041-u. [DOI] [PubMed] [Google Scholar]
- Taylor S. Assessment of obsessions and compulsions: reliability, validity, and sensitivity to treatment effects. Clin Psychol Rev. 1995;15:261–97. [Google Scholar]
- Tolin DF, Hamlin C, Foa EB. Directed forgetting in obsessive-compulsive disorder: replication and extension. Behav Res Ther. 2002;40(7):793–803. doi: 10.1016/s0005-7967(01)00062-6. [DOI] [PubMed] [Google Scholar]
- Towey JP, Tenke CE, Bruder GE, Leite P, Friedman D, Liebowitz M, et al. Brain event-related potential correlates of overfocused attention in obsessive-compulsive disorder. Psychophysiology. 1994;31(6):535–43. doi: 10.1111/j.1469-8986.1994.tb02346.x. [DOI] [PubMed] [Google Scholar]
- Van de Wetering BJ, Martens CM, Fortgens C, Slaets JP, van Woerkom TC. Late components of the auditory evoked potentials in Gilles de la Tourette syndrome. Clin Neurol Neurosurg. 1985;87(3):181–6. doi: 10.1016/0303-8467(85)90004-6. [DOI] [PubMed] [Google Scholar]
- van Woerkom TC, Fortgens C, Rompel-Martens CM, Van de Wetering BJ. Auditory event-related potentials in adult patients with Gilles de la Tourette’s syndrome in the oddball paradigm. Electroencephalogr Clin Neurophysiol. 1988;71(6):443–9. doi: 10.1016/0168-5597(88)90048-2. [DOI] [PubMed] [Google Scholar]
- van Woerkom TC, Roos RA, van Dijk JG. Altered attentional processing of background stimuli in Gilles de la Tourette syndrome: a study in auditory event-related potentials evoked in an oddball paradigm. Acta Neurol Scand. 1994;90(2):116–23. doi: 10.1111/j.1600-0404.1994.tb02690.x. [DOI] [PubMed] [Google Scholar]
- Watson TD, Azizian A, Berry S, Squires NK. Event-related potentials as an index of similarity between words and pictures. Psychophysiology. 2005;42(4):361–8. doi: 10.1111/j.1469-8986.2005.00295.x. [DOI] [PubMed] [Google Scholar]
- Wilberg T, Dammen T, Friis S. Comparing personality diagnostic questionnaire-4+ with Longitudinal, Expert, All Data (LEAD) standard diagnoses in a sample with a high prevalence of axis I and axis II disorders. Compr Psychiatry. 2000;41(4):295–302. doi: 10.1053/comp.2000.0410295. [DOI] [PubMed] [Google Scholar]
- Wolach I, Pratt H. The mode of short-term memory encoding as indicated by event-related potentials in a memory scanning task with distractions. Clin Neurophysiol. 2001;112(1):186–97. doi: 10.1016/s1388-2457(00)00501-0. [DOI] [PubMed] [Google Scholar]
- Zitterl W, Urban C, Linzmayer L, Aigner M, Demal U, Semler B, et al. Memory deficits in patients with DSM-IV obsessive-compulsive disorder. Psychopathology. 2001;34(3):113–7. doi: 10.1159/000049292. [DOI] [PubMed] [Google Scholar]