Abstract
Gilles de la Tourette syndrome (GTS) is a neuropsychiatric disorder with childhood onset presenting with multiple fluctuating motor tics and one or more phonic tics. A significant proportion of people suffering from GTS are still symptomatic in adulthood and present other emotional and cognitive difficulties, along with motor problems that often accompany these comorbid conditions. The nature of these difficulties is still poorly understood and multiple comorbidities are often inadequately controlled. The current study investigates both stimulus evaluation and motor processing in GTS while controlling for comorbidity. Fifteen adults with GTS and 20 control participants were matched on gender, laterality and intelligence. The P300 component, the no-go anteriorization (NGA) as well as the stimulus and response-locked lateralized-readiness potentials (S-LRP, R-LRP) were elicited during a stimulus–response compatibility (SRC) paradigm. The standard version of the Stroop Color–Word Test (SCWT) was also administered. Reaction times showed that participants with GTS processed both the SRC and the SCWT more rapidly than the control group, while producing a delayed P300 peak latency. The GTS group also showed faster S-LRP onset in response to the incompatible and faster processing of interference in the SCWT. There was also a tendency toward a greater frontal shift of the NGA in the GTS group. The P300 latency showed that with GTS patients, stimulus evaluation occurs later whereas the overlapping pre-motor response selection processes occur faster. Our findings are congruent with a probable cortical motor over-activation hypothesis of GTS involving faster motor program selection in processing conflicting SR configuration.
Keywords: P300, NGA, Lateralized-readiness potential, Stimulus–response compatibility, Inhibition, Motor preparation, Stroop task, Pre-motor cortex, Supplementary motor area
1. Introduction
Gilles de la Tourette syndrome (GTS) is a debilitating neuropsychiatric disorder that carries significant social stigma. GTS is diagnosed on the basis of multiple fluctuating motor tics and one or more phonic tics (American Psychiatric Association, 2000). Symptoms usually begin during childhood, and at least 11% of people suffering from GTS remain fully symptomatic as adults (Bloch et al., 2006; Leckman et al., 1998). The manifestation of tics is part of a larger mosaic of collateral symptoms. Freeman et al. (2000) established that anger control problems, sleep difficulties, coprolalia, and self-injurious behavior attain high levels in individuals with GTS, particularly those with comorbidity. The most commonly reported comorbidity in GTS is attention-deficit/hyperactivity disorder (ADHD), which is also associated with reduced inhibition at multiple levels in the motor system (Hallett, 2001).
In addition to the numerous behavioral problems cited above, several neuropsychological studies discovered cognitive specificities in GTS such as a deficit in learning for mathematics and written language (Brookshire et al., 1994; Como, 2001), verbal fluency (Bornstein, 1991; Brookshire et al., 1994), fine motor coordination (Bornstein et al., 1983, 1991; Brookshire et al., 1994; Como, 2001; O’Connor et al., 2008) and a non-verbal memory deficit associated with a visuoperceptual integration difficulty in children (Harris et al., 1995; Schuerholz et al., 1996) and adults (Lavoie et al., 2007). Moreover, some studies proposed that GTS children achieved normal performances on tasks evaluating abstract concepts (Bornstein, 1990; Braun et al., 1993; Harris et al., 1995; Schuerholz et al., 1996), planning and response inhibition (Ozonoff and Jensen, 1999), and verbal fluency (Braun et al., 1993; Mahone et al., 2001), while, others proposed other types of executive function impairments (Sutherland et al., 1982; Bornstein et al., 1983; Baron-Cohen et al., 1994; Brookshire et al., 1994; Schuerholz et al., 1996). The lack of consistency in the neuropsychological results could be due to methodological problems considering that, in some cases, studies did not include a control group or did not control for the presence of comorbid disorders, such as ADHD or obsessive–compulsive disorders (OCD). The presence of ADHD or OCD symptoms in children often leads to poorer performance on executive tasks (Bornstein, 1990; Harris et al., 1995). Despite this, there have been consistent reports of deficits in fine motor dexterity and visuo-motor integration in both children and adults with persistent GTS.
Recent etiological studies have all implicated fronto-striato-thalamo-cortical circuits in the cognitive and motor functioning of GTS patients, but assess indirectly cerebral motor functions and the underlying brain structures involved in response processing. A dopaminergic imbalance (Singer and Minzer, 2005; Leckman et al., 2006) has been proposed, as well as a loss of basal ganglia control, a thalamo-cortical neuronal dysrhythmia and a frontal compensation, which impacts on the dysregulation of striatal and thalamo-cortical electrical oscillations (see Leckman et al., 2006). These hypotheses are supported by brain imaging studies reporting volumetric and metabolic reductions in lentiform (Braun et al., 1995; Eidelberg et al., 1997) and caudate nuclei (Hyde et al., 1995; Stoetter et al., 1992; Bloch et al., 2005), while observing larger prefrontal volume (Peterson et al., 2001). Other investigators have shown a metabolic increase reflecting heightened activation in pre-motor cortex and supplementary motor area (SMA) through anatomical (Braun et al., 1993; Eidelberg et al., 1997; Stoetter et al., 1992) or functional magnetic resonance imaging (fMRI) during a finger tapping task (Biswal et al., 1998). More recently, another study showed an increase in alpha EEG coherence in the pre-motor cortex during execution of a go–no-go task in GTS patients (Serrien et al., 2002). In brief, these observations suggest that anomalies in cerebral regions, associated with motor processing and tic generation, are likely to interfere with accurate planning and execution of voluntary movements in GTS.
Despite recent advances in the understanding of GTS etiology, neurobiological and cognitive factors have mostly been addressed independently. For that purpose, the brain event-related potentials (ERPs) offer a useful tool for monitoring cerebral activity, recorded in synchrony with cognitive events. Earlier investigations found anomalies in motor ERPs with patients suffering from GTS and chronic tics. For instance, the Bereitschaftpotentials (BP), or readiness potential, reflecting motor preparation, was consistently larger over frontal and smaller over central areas in the GTS group (Rothenberger and Kemmerling 1982; Rothenberger et al., 1986). In a more recent ERP study, chronic tic disorder patients failed to demonstrate a relationship between motor output and preparation of cortical activation (i.e. BP) during a foreperiod reaction time task (O’Connor et al., 2005), supporting the idea that people with tic disorders may not be able to modulate cortical activation optimally when planning and executing motor responses. The BP was nonetheless highly variable in these cohorts, and it might well have reflected overlapping non-motor as well as motor activity. Also, its early onset may have implicated general anticipatory processes rather than the specific cortical preparation preceding movement (Trevena and Miller, 2002). To circumvent this problem, the lateralized-readiness potential (LRP) component, which has its generator sources in the primary motor cortex (Requin and Riehle, 1995), the SMA (Rektor, 2002) and the basal ganglia (Rektor et al., 2003), represents a good candidate measure of motor processing anomalies in GTS. Specifically, the LRP has been shown to be a marker of selective motor activation, representing the differential engagement of the left and right motor cortices in the preparation and initiation of motor responses (Coles, 1989; Kutas and Donchin, 1980). Only one study has investigated this component in a group of patients with GTS, and failed to show any group difference in LRP (Johannes et al., 2001b). However, the LRP was pooled across conditions and analyzed as a non-specific measure of motor processing, which may have reduced its sensitivity to detect any subtle motor processing differences in GTS. To remedy this limitation, it would be advisable to compare LRPs across diverse conditions of stimulus–response compatibility for instance.
Overall, evidence from brain imaging and electro-physiology suggests that (1) participants with GTS present problems in executing complex motor actions, which is consistent with a probable medial frontal, SMA and striatal dysfunction; and (2) difficulties experienced by participants with GTS might not be limited to motor processing and could extend to stimulus evaluation and response inhibition stages. The aim of the current study was to look at electrophysiological measures related to stimulus evaluation and categorization processes (P300), inhibition (no-go anteriorization; NGA), and motor processing, within the same stimulus–response compatibility (SRC) paradigm. We also administered the Stroop Color–Word Test (SCWT) condition as a verbal measure of cognitive interference (Stroop, 1935), complementary to the SRC paradigm’s non-verbal incompatible condition. Specific hypotheses were as follows: GTS participants would show problems in attentional resource allocation and response selection and thus would be more susceptible to the conflict generated in incompatible or incongruent conditions. Group effects would be particularly pronounced in the SCWT interference condition.
2. Method
2.1. Participants
Fifteen adult participants suffering from GTS were matched to 20 control participants on age (range=21–54 years old) and gender. All participants were right-handed (Edinburgh Handedness Inventory; Oldfield, 1971), had normal visual acuity (Snellen notation system) and color perception (Ishihara test for color blindness). The study was approved by the local ethics committee and all participants gave their written informed consent. Control participants were recruited among Lafontaine hospital employees or via local newspapers. None of the control participants were diagnosed with a psychiatric or a neurological disease or were taking psycho-active medication. All participants with GTS fulfilled diagnostic criteria according to the DSM-IV-TR (307.23), including the presence of multiple single motor tics and at least one phonic tic. Simple motor tics are sudden, brief, meaningless movements. Complex motor tics are more purposive stereotyped movements of longer duration, such as facial gestures and grooming-like movements. Simple phonic tics are fast, meaningless sounds or noises, while complex phonic tics may include syllables, words or phrases, as well as odd patterns of speech. Diagnosis was based on a consensus between a certified psychiatrist (E.S.) and a clinical psychologist (supervised by K.O.). Symptom severity was assessed by an independent rater using the Tourette Syndrome Global Scale (TSGS: Harcherik et al., 1984) and participants obtained global scores distributed across mild (56%), moderate (31%) and severe (13%) symptom intensity (Table 1). The mean age at tic onset was 8 years old ranging between 4 and 16 years old (Table 2). Exclusion criteria for all participants included the presence of a diagnosis, other than GTS, on Axis I or any other diagnosed problem on Axes II, III or IV of the DSM-IV-TR. Participants currently receiving any form of treatment (behavioral and pharmacological) for their tic symptoms were also excluded.
Table 1.
Means and standard deviations for descriptive variables, including demographic and clinical variables.
| GTS group
|
Control group
|
t-test | |||
|---|---|---|---|---|---|
| (n=15)
|
(n=20)
|
||||
| Mean | σ | Mean | σ | ||
| Demographic and characteristics | |||||
| Age (years) | 37 | 8 | 40 | 12 | n.s. |
| Education (years) | 15 | 3 | 15 | 2 | n.s. |
| Laterality (right) | 100 | – | 100 | – | n.s. |
| Gender (M/F%) | 50/50 | – | 50/50 | – | n.s. |
| Visual acuity (Snellen) | 1.2 | 0.27 | 1.4 | 0.28 | n.s. |
| Color perception (Ishihara) | 11 | 1 | 11 | 1 | n.s. |
| Clinical variables | |||||
| Intelligence (Raven matrices) | 76 | 25 | 75 | 21 | n.s. |
| Depression (BDI) | 8 | 7 | 3 | 3 | ** |
| Anxiety (BAI) | 11 | 9 | 3 | 3 | ** |
| OCD (Padua Inventory) | 37 | 22 | 12 | 8 | ** |
| Style of planning (global) a | 8 | 27 | 39 | 17 | ** |
| Over-preparation | 2 | 9 | 12 | 7 | ** |
| Over-activity | 1 | 5 | 17 | 7 | ** |
| Inflexibility | 5 | 9 | 10 | 7 | * |
| TSGS (global=severity+disruption) | 24 | 12 | – | – | n.a. |
| Tic severity | 13 | 7 | – | – | n.a. |
| Behavioral disruption | 11 | 7 | – | – | n.a. |
| Simple motor | 25 | 39 | – | – | n.a. |
| Complex motor | 7 | 5 | – | – | n.a. |
| Simple vocal | 13 | 20 | – | – | n.a. |
| Complex vocal | 2 | 5 | – | – | n.a. |
Note. n.s = non significant; BAI = Beck Anxiety Inventory; BDI = Beck Depression Inventory; SM = simple motor; CM = complex motor; SP = simple phonic; CP = complex phonic.
P<0.05;
P<0.01;
n.a.: non-applicable.
For the style of planning (STOP) questionnaire, a lower score means greater symptom severity.
Table 2.
Description of past medication and the history of tic severity for each year.
| Years | Threshold | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||||||||||||||
| Tourette subjectsa | Past medication | Current age | Age at tic onset | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25+ | 31+ | 36+ | 41+ | 46+ | 51+ |
| 1 | 39 | 10 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | |||||||||||||||||||||
| 2 | 29 | 7 | 5 | 5 | 5 | 4 | 4 | 4 | 4 | 4 | 4 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | |||||||||
| 3 | Zoloft | 32 | 11 | 4 | 4 | 5 | 5 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 2 | 5 | 4 | |||||||||||
| 4 | 46 | 5 | ¥ | |||||||||||||||||||||||||||
| 5 | Rivotril | 35 | 9 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | 4 | 4 | 5 | 5 | 5 | |||||||||
| 6 | 43 | 10 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 4 | |||||||||
| 7 | Nitoman | 54 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | 4 |
| 8 | 21 | 6 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | ||||||||||||
| 9 | 35 | 16 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | 5 | 5 | |||||||||||||||||
| 10 | 38 | 6 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | ||||||
| 11 | 32 | 4 | 4 | 4 | 4 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 4 | 4 | 4 | 4 | 4 | 3 | 3 | 3 | 3 | 3 | 4 | 4 | |||||
| 12 | Haldoperidol/Nitoman | 36 | 4 | 12 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 4 | 4 | 4 | |||
| 13 | Not specified | 30 | 9 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 4 | 4 | 4 | 4 | 4 | ||||||||||
| 14 | Not specified | 36 | 5 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | ||||
| 15 | 44 | 12 | 2 | 2 | 2 | 2 | 2 | 3 | 4 | |||||||||||||||||||||
Note. The numerical values indicate subjective tic severity for each year: 1 = milder; 2 = mild; 3 = moderate; 4 = severe; 5 = more severe.
The grey shadings indicate the years where the tics were present.
The severity scores were not available for subject 4.
In order to control for comorbidity, all participants completed questionnaires assessing depression (Beck Depression Inventory; BDI; Beck et al., 1961), anxiety (Beck Anxiety Inventory; BAI; Beck et al., 1988), over-active style of planning (STOP questionnaire; O’Connor, 2005) and obsessive–compulsive symptoms (Padua Inventory; Sanavio, 1988). The Anxiety Disorders Interview Schedule for DSM-IV (ADIS-IV; Brown et al., 1994) was also administered to the GTS group to screen the presence of anxiety disorders. The clinical evaluation also included an estimation of the non-verbal intelligence (Raven’s matrices—short version?; Raven, 1938, 1996).
2.2. Procedure and experimental tasks
2.2.1. Stroop Color–Word Test
The SCWT included three conditions. In the word and color conditions, stimuli were color words (RED, BLUE, GREEN) printed in black ink or color spots (red, blue or green spots). In the interference condition, stimuli consisted of the same color words, printed in an incongruent color ink (e.g. BLUE written with red ink). Participants read the words, named the colors or named the ink color, as fast as possible. Total execution time for each condition was recorded and entered for further analysis.
2.2.2. Stimulus–response compatibility paradigm
Stimuli display consisted of blue, black and red arrows subtending 2°×2° of visual angle, pointing to the left or to the right. Each stimulus was presented for 350 milliseconds (ms) on a white background at the center of a monitor screen (Viewsonic SVGA 17-in. flat screen monitor). Stimulus sequence was pseudo-random, with less than four identical trials in a row and inter-stimulus interval (ISI) randomly varied between 2200 and 2800 ms. Depending on the color of the arrow, participants had to press a button in the same direction as the arrow (compatible trials), in the opposite direction (incompatible trials) or give no response (no-go trials). A total of 250 trials were administered: 100 blue arrows (50 pointing right and 50 pointing left), 100 black arrows (50 pointing right and 50 pointing left) and 50 red arrows (25 pointing right and 25 pointing left). Participants were instructed to respond as fast as possible and responses were classified as hits when generated with the correct hand or when no response was correctly made (no-go trials). Median reaction times were recorded for further analysis.
2.3. Electrophysiological recordings
Participants were comfortably seated in a dimly lit room, their head at a distance of 90 cm from the monitor and a response box fixed on an adjustable tablet placed over their knees. The response box comprised three horizontally placed buttons, easily activated with the index fingers of each hand. All electrophysiological signals were acquired through an analog amplifier (SA Instrumentation Inc., San Diego, CA). The electroencephalogram (EEG) was recorded from 26 tin electrodes mounted in a nylon cap (ElectroCap International, Eaton, OH), referenced to linked mastoids with impedance kept below 5 kΩ. EEG recordings were continuously sampled at 250 Hz and amplified with a calibrated gain of 10,000 and high–low pass filter settings at 0.01 and 30 Hz, respectively. The electro-oculogram (EOG) was recorded from four tin bi-polar electrodes placed horizontally at the outer canthus of each eye and vertically at an infra- and a supra-orbital position on the right eye, aligned with the pupil when looking straight ahead. The EOG was amplified with a gain of 3000. Stimuli presentation and data acquisition were controlled automatically by an acquisition program (InstEP Systems, Montréal, QC).
2.4. EEG signal extraction
EOG artifacts contaminating the EEG signal were corrected offline using dynamic multiple regression in the frequency domain, by the Woestenburg method (InstEP-TALO). Remaining epochs exceeding 100 μV and clippings due to amplifier saturation or blockage were eliminated automatically during the averaging procedure. Signals were averaged offline, time-locked to the stimulus onset (from 100 ms before to 1900 ms after stimulus onset) and to the response onset (from 1000 ms before to 500 ms after reaction time). The P300 component was scored baseline-to-peak across frontal, central and parietal regions from the stimulus-locked EEG data, in a 250- to 550-ms post-stimulus window. The no-go anteriority (NGA) was scored in the same time window, except that compatible and incompatible trials were pooled in the go condition while the inhibition trials were considered in the no-go condition. The NGA was identified after subtraction of the average response related to the no-go from the response related to the go response. Twelve electrodes were included for the P300 analysis: F3, F4, FC3, FC4 (frontal region), C1, C2, C3, C4 (central region), P3, P4, CP3 and CP4 (parietal region). The LRP was obtained after elimination, through a double subtraction, of lateralized potentials of non-motor origin common to the left- and right-hand responses with the following equation: LRP = (Mean(C2−C1)left hand + Mean(C1−C2)right hand)/2 (see Coles, 1989). Its onset was scored using the proportional method (Kornblum et al., 1990), where onset corresponds to 20% of the peak. The LRP was average stimulus-locked (S-LRP) or response-locked (R-LRP) and compared in order to specify the stimulus–response (S–R) incompatibility interference effect on either pre-motor or motor processes, respectively (Mordkoff and Gianaros, 2000). Motor inhibition was operationalized as LRP mean amplitude to the no-go condition. However, the onset to the no-go was not scored because, as expected, no peak could be reliably identified. The time window for onset detection corresponded to 150- to 900-ms post-stimulus onset for the S-LRP and to −500 ms prior to the response onset for the R-LRP.
2.5. Statistical analysis
Age, education, depression (BDI) and anxiety (BAI) scores, over-active style of planning (STOP), non-verbal intelligence and OCD symptoms (Padua Inventory) were analyzed using t-tests comparing the two groups. Gender effect was analyzed with a non-parametric chi-square test. SCWT raw execution times were analyzed using a multivariate repeated-measures analysis of variance (MANOVA) with a between-group factor, Group (GTS, controls), and a within-group factor, Condition, with three levels (word, color and interference). For the SRC task, median reaction times, number of hits, peak amplitude and onset latency for S-LRP and R-LRP were analyzed using a MANOVA with a between-group factor (Group with two levels: GTS/controls), and within-group factors with two levels each (Hand: right/left; and Condition: compatible/incompatible). P300 component peak latency and amplitude were also analyzed using a repeated-measures MANOVA with a between-group factor (Group with two levels: GTS/controls) and several within-groups factors (Hand: right/left; Condition: compatible/incompatible/inhibition; Region: frontal/central/parietal; Hemisphere: left/right). Subsidiary ANOVAs and independent groups’ t-tests were also performed to explore further some of the significant interaction effects. The NGA peak latency and amplitude were analyzed using a repeated-measures MANOVA with the same between-group factor and the within-groups factors of Conditions, with two levels (go, no-go) and Regions, with three levels (frontal, central, parietal).
In order to control for significant comorbidity, separate multivariate analyses of covariance (MAN-COVA) including BDI, BAI, STOP and Padua Inventory scores as covariates were performed on all electrophysiological data. Finally, Pearson correlation analyses were calculated between the following: incompatible SRC paradigm and SCWT interference condition reaction times, compatible–incompatible S-LRP onset latency discrepancy values and TSGS scores, compatible–incompatible S-LRP onset latency discrepancy values and SWCT interference execution time.
3. Results
3.1. Demographic and clinical evaluation
No group difference was reported regarding age, gender, education and non-verbal intelligence (Table 1). However, participants with GTS had significantly higher scores than the control group on the BDI (t[33]=−2.81, P<0.01), on the BAI (t[33]=−3.83, P<0.01), on the STOP questionnaire (t[33]=−3.92, P<0.001) and on the Padua Inventory (t[33]=−4.74, P<0.01). In the control group (n=20), participants obtained scores corresponding to the sub-clinical level of symptoms on these questionnaires. In the GTS group, participants obtained scores corresponding to sub-clinical (n=13) and borderline (n=2) levels of depressive symptoms (Beck et al., 1961) and very low (n=12) to moderate (n=3) levels of anxious symptoms (Beck et al., 1988). However, administration of the ADIS-IV in this group revealed that none of the participants met the diagnostic criteria for any anxiety disorder. Concerning obsessive–compulsive symptoms, four participants with GTS obtained scores corresponding to “very much disturbing” symptoms according to their age and gender (Sanavio, 1988). However, none of the participants fulfilled the DSM-IV-TR diagnostic criteria for obsessive–compulsive disorder (300.3).
3.2. Behavior and performance
3.2.1. Stroop Color–Word Test
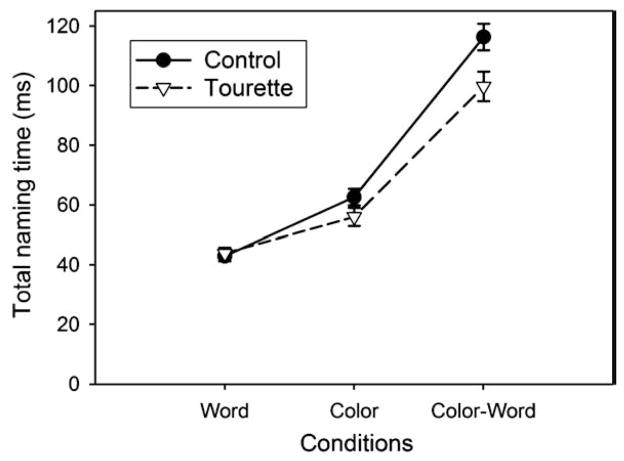
Analysis of the SCWT data revealed significant Condition (F[2,32]= 169.41; P <0.001) and Group (F[1,33]=8.33; P<0.01) main effects, along with a Group by Condition interaction (F[2,32] = 4.34; P<0.05). This interaction remained significant after covariance with the BDI (P<0.01), the BAI (P<0.005) the Padua Inventory (P<0.05) and the STOP questionnaire (P<0.005). Post hoc (Bonferroni) tests contrasting execution times in the three conditions revealed that execution times were longer in the interference condition than in the color (P<0.01) and word (P<0.01) conditions and in the color compared with the word condition (P<0.01). An independent groups t-test comparing groups within each condition separately revealed that participants with GTS were significantly faster than control participants in the interference condition (t[33]= 2.50; P<0.05) but not in the color (P=0.11) and word (P=0.74) conditions (see Fig. 1). Correlations between naming time in the interference condition and symptom severity failed to reach significance.
Fig. 1.
Performance in the three conditions of the Stroop Color–Word Test. Participants with GTS were significantly faster to name the color compared to the control participants in the interference condition.
3.2.2. SRC paradigm
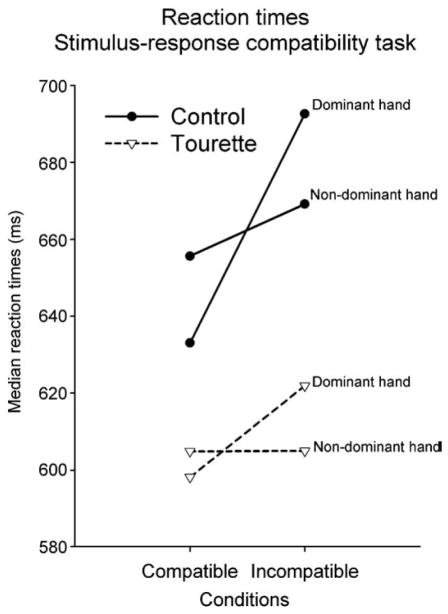
Analysis of median reaction times (RTs) revealed a significant Condition main effect (F[1,33] = 17.34; P<0.001) reflecting delayed reaction times in the incompatible compared with the compatible condition (Table 3). There is a significant Condition by Hand interaction (F[1,33] = 9.39; P <0.001) reflecting a reduced incompatibility effect for the non-dominant hand which corresponds to the classical SRC effect (see Fig. 2). Despite the fact that participants with GTS were generally faster to respond than the control participants, the analysis revealed only a tendency toward a significant group main effect (P=0.09) and toward a Group by Condition (P=0.06) interaction. No other interaction was significant and correlations between reaction times and symptom intensity were not significant. Finally, a two-tailed Pearson correlational analysis revealed a significant positive correlation (r=0.48; P<0.05) between reaction times in the SRC incompatible trials and execution time in the SCWT interference condition. Longer incompatible S-LRP onset latency corresponded to longer execution times in the SCWT interference condition.
Table 3.
Data for behavioral and performance variables on the stimulus–response compatibility task.
| GTS group
|
Control group
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| (n=15)
|
(n=20)
|
||||||||
| Conditions | Hands of response | RT | (SE) | Commission error (%) | Intra-subject (S.D.) | RT | (SE) | Commission error (%) | Intra-subject (S.D.) |
| Incompatible | Left | 605 | (23) | 4 | 128 | 669 | (20) | 8 | 154 |
| Right | 622 | (27) | 4 | 133 | 693 | (23) | 9 | 163 | |
| Compatible | Left | 604 | (26) | 2 | 143 | 656 | (23) | 5 | 163 |
| Right | 598 | (25) | 3 | 128 | 633 | (22) | 4 | 146 | |
| Inhibition | No response | – | – | 0.7 | – | – | – | 0.6 | – |
Note: GTS = Gilles de la Tourette Syndrome; RT = median reaction times; SE = standard error of the mean; S.D. = intra-subject standard deviation.
Fig. 2.
Illustration depicting the reaction times as a function of the condition in the stimulus–response compatibility task. Reaction times were delayed in the incompatible condition in both groups. This effect was larger for the non-dominant hand.
Analysis of commission errors (Table 3) revealed significantly more errors for the incompatible than for the compatible and inhibition conditions, respectively (F[1,33]=6.70; P<0.05). However, no significant differences were demonstrated across groups (P=0.59) or hand of response (P=0.45). Similarly, intra-subject variability of the reaction times revealed no significant difference across groups (P=0.19), conditions (P=0.84) or hand of response (P=0.10).
3.3. Electrophysiological results
3.3.1. The P300 component
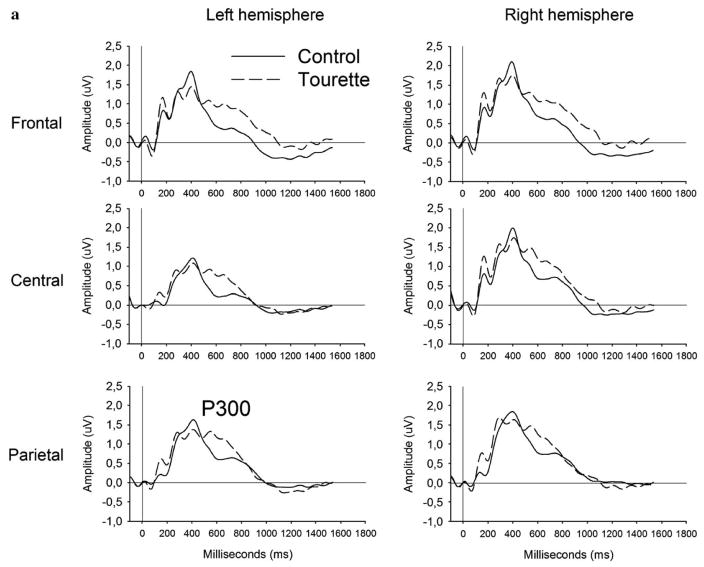
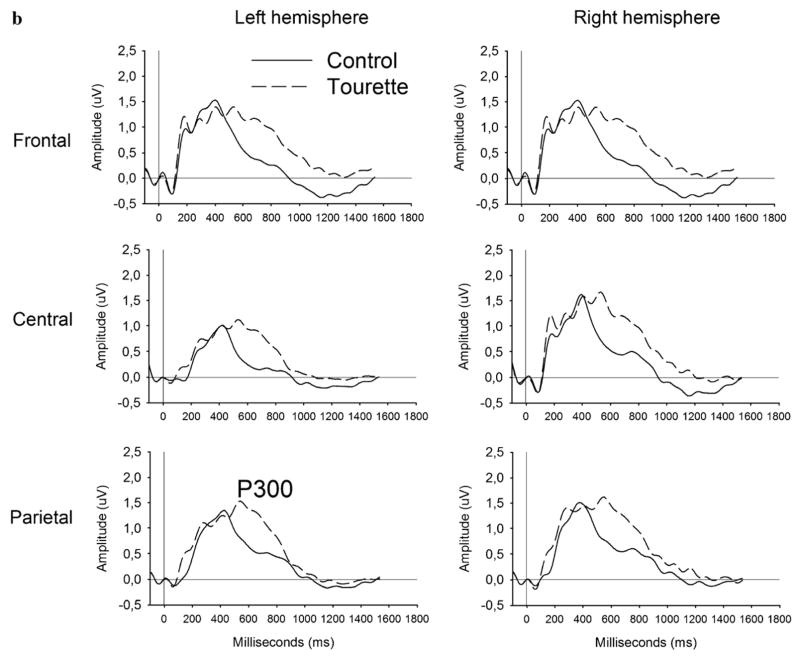
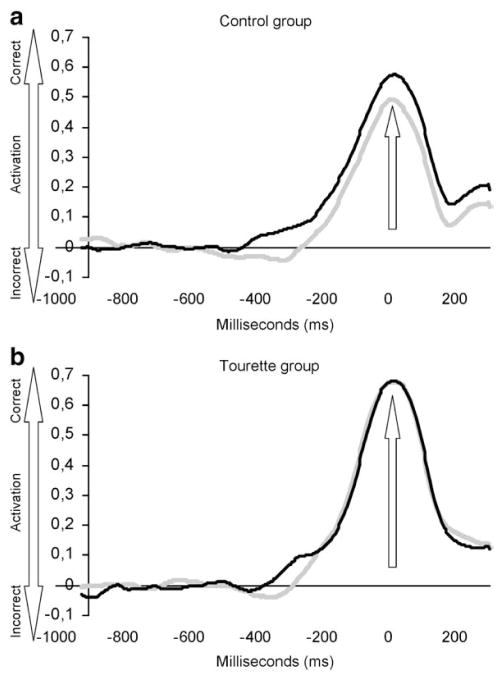
Analysis of the P300 peak amplitude applied to compatible and incompatible conditions revealed a significant Region (F[1,33]=15.28; P<0.001) main effect represented by a more prominent amplitude over frontal and central regions. Analysis of the P300 peak latency revealed significant Condition (F[1,33]=8.90; P<0.01) and Group (F[1,33]=4.81; P<0.05) main effects, along with a Group by Region (F[2,32]=5.89; P<0.01) and a Group by Condition interaction (F[1,33]=4.22; P<0.05) as shown in Fig. 3. These two interactions remain significant after covariance with the BDI (P<0.05), the BAI (P<0.01), the Padua Inventory (P<0.05) and the STOP questionnaire (P<0.01). Independent group t-tests comparing the two groups, within each condition separately, revealed that the GTS group had a significantly delayed P300 latency in the incompatible (t[33]=2.71; P <0.05), but not in the compatible (t[33] = 1.33; P=0.19) condition (see Fig. 3a and b). Correlations between the P300 latency and the tic symptom intensity reached significance over central areas, more precisely the Cz (r=0.40; P<0.01), C2 (r=0.41; P<0.01) and C4 (r=0.46; P<0.01) electrodes only in the incompatible condition.
Fig. 3.
Comparison of the stimulus-locked ERP between the GTS (dotted curve) and the control (solid curve) group in response to the compatible (panel a), incompatible (panel b) in the stimulus–response compatibility task. The P300 peak latency in the compatible and no-go trials was equivalent across groups, while the incompatible trials elicited delayed peak latency in the GTS (dotted curve) compared to the control (solid curve) group.
3.3.2. The no-go anteriorization (NGA)
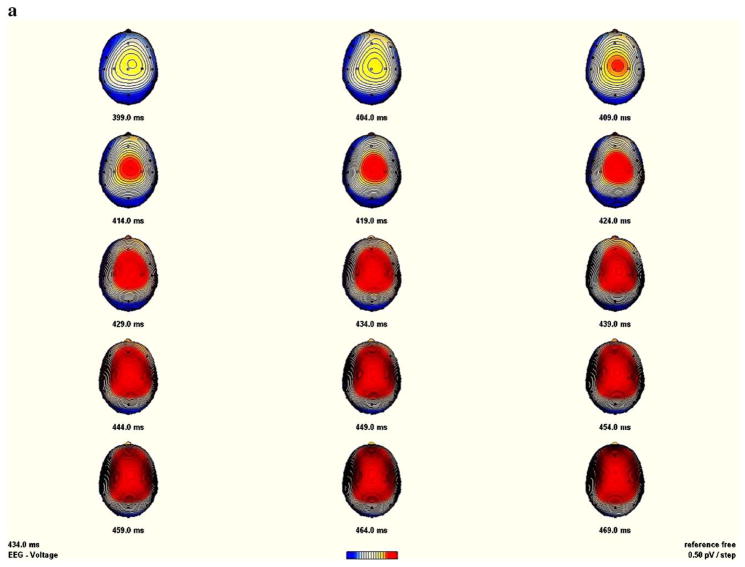
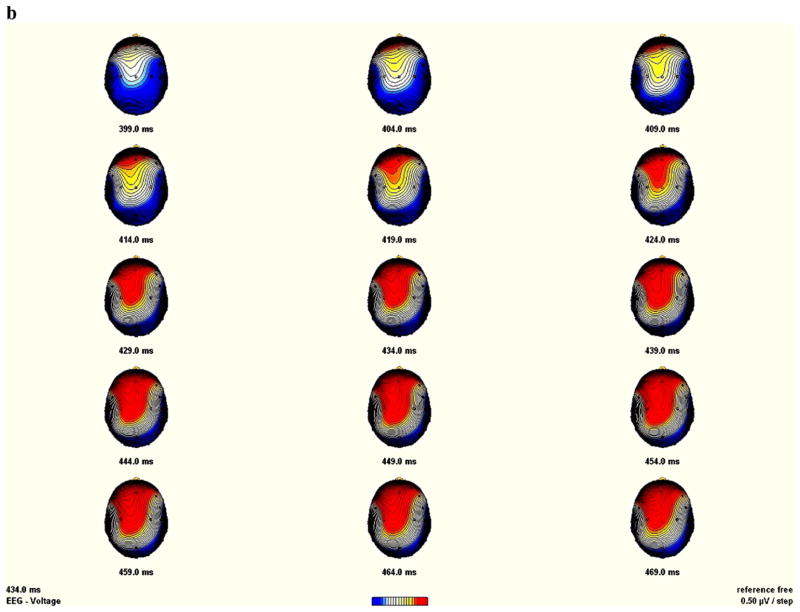
Analysis of the NGA amplitude revealed a significant Condition main effect (F[1,33]=13.46; P<0.001), along with a Condition by Region interaction (F[2,32]= 18.89; P<0.001), reflecting larger amplitude for the no-go than for the go condition over frontal and central electrode sites, which reflect typical NGA topography. The topographical mapping of the NGA showed more prominent amplitude over central than over anterior regions in the control group (see Fig. 4a), while it was more frontally distributed in the GTS group (Fig. 4b). However, and despite this frontal shift associated with the GTS patients, the ANOVA revealed no significant main effect of Group (P=0.52), Group by Region (P=0.97) or Group by Condition (P=0.47) interactions.
Fig. 4.
Sequential brain maps (5 ms steps) between 399 ms until 469 ms post-stimulus, of the no-go anteriorization (NGA) in the control (panel A) and the GTS (panel b) group (nasion upward). The NGA was obtained by subtracting the no-go from the go condition.
Analysis of the NGA peak latency also revealed a significant main effect of Condition (F[1,33]=13.74; P<0.001), indicating a delayed latency elicited by the no-go condition, along with a Condition by Region interaction (F[2,32]=8.21; P<0.001) of a delayed go–no-go peak latency, more prominent over frontal (96 ms), central (49 ms) and parietal (25 ms) regions, respectively. There was no further interaction involving group or condition.
3.3.3. Stimulus-locked LRP peak amplitude and onset latency
Analysis of the S-LRP peak amplitude revealed a significant main Condition effect (F[2,32] = 72.20; P<0.001). Post hoc (Bonferroni) tests, contrasting the three task conditions, revealed that peak amplitudes in the compatible and incompatible conditions were equivalent (P=0.81), but that amplitude in the no-go condition was significantly smaller than in the two other conditions (P<0.001), which is a typical LRP effect in the no-go condition. However, no main effects of Group or interactions were observed with the LRP peak amplitude.
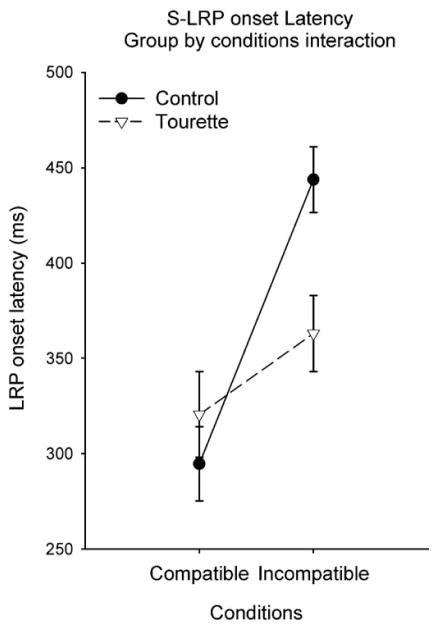
Analysis of the stimulus-locked LRP onset latency showed a significant main Condition effect (F[1,33]= 31.78; P<0.001) and a significant Group by Condition interaction (F[1,33]=9.48; P<0.05). The Group by Condition interaction (Fig. 5) remained significant after covariance with the BDI (F[1,33]=14.80; P<0.001), the BAI (F[1,33]=7.21; P<0.05), the global score of the STOP questionnaire (F[1,33]=9.96; P<0.005) and the Padua Inventory (F[1,33]=7.99; P<0.005). Subsequent analysis contrasting the three conditions within each group revealed that, in the controls, LRP onset was significantly shorter (Fig. 6a) in the compatible (294 ms) than in the incompatible (443 ms) condition (F[1,19] = 51.57; P<0.001). In the GTS group (Fig. 6b), the discrepancy between compatible (321 ms) and incompatible (363 ms) S-LRP onset was not significant (P=0.98). An independent group t-test, applied separately to compatible and incompatible conditions, confirmed that the S-LRP onset latency differed significantly between groups for the incompatible condition (t[33]=2.23, P<0.05), but not for the compatible condition (t[33]=−0.44, P=0.66). In comparison with the control group, the GTS group had a significantly shorter S-LRP onset latency to the incompatible trials, but similar S-LRP onset latency to the compatible trials (Fig. 5). Pearson correlational analysis revealed a significant negative correlation between compatible–incompatible S-LRP onset discrepancy and the TSGS tic score (r=−0.50; P<0.01). A larger amount of tic symptoms corresponded to a smaller compatible–incompatible S-LRP onset discrepancy.
Fig. 5.
Illustration depicting the stimulus-locked LRP onset latency in function of the condition of the stimulus–response compatibility task. Stimulus-locked LRP onset latency was significantly delayed in the control (black circle) compared to the GTS (white triangle) group for the incompatible condition. The compatible condition was not affected.
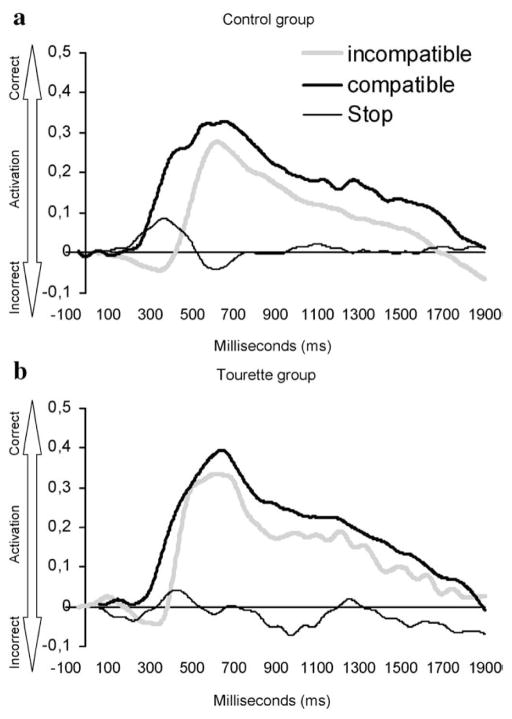
Fig. 6.
Grand average stimulus-locked LRP waveforms (derived from C1 and C2 electrodes) for incompatible (grey line), compatible (black line) and no-go (thin line) conditions in the control group (panel a) and the GTS group (panel b). Time point zero indicates the stimulus presentation onset.
3.3.4. Response-locked LRP peak amplitude and onset latency
Analysis of the R-LRP peak amplitude revealed no significant Condition (P=0.25) or Group (P=0.26) main effects, and no significant Condition (P=0.18) or Group (P=0.79) main effects in relation to R-LRP onset latency (Fig. 7a and b).
Fig. 7.
Grand average response-locked LRP waveforms (derived from C1 and C2 electrodes) for incompatible (grey line) and compatible (black line) conditions in the control group (panel a) and the GTS group (panel b). Time point zero represents the moment of the button press indicated by the arrow.
4. Discussion
4.1. Behavioral performances
At the behavioral level, a reaction time (RT) delay was detected in response to incompatible trials for all participants. This result is congruent with studies using similar SRC paradigms and is generally interpreted to be a consequence of S–R incompatibility interference (e.g. Masaki et al., 2000). Even if participants with GTS showed this pattern in their reaction times, they demonstrated a tendency to be generally faster than control participants in both compatible and incompatible conditions. Similarly, the GTS group was also faster than the control group in the SCWT interference condition, and a strong positive relationship was observed between naming times at the SCWT and the RTs on the SRC tasks. Even if these two tasks involve generally different modalities of conflict (i.e. verbal and non-verbal), neuroimaging results support the view that they both involve an attentional modulation generated by a common activation pattern in the dorsolateral prefrontal, middle occipital and inferior temporal cortices (Liu et al., 2004; Peterson et al., 2002).
Altogether, this performance profile suggests that participants with GTS are less sensitive to stimulus–response incompatibility both in the verbal (SCWT) and the non-verbal (SRC) modality. Other studies also confirmed that patients with GTS show a tendency toward faster RTs for both congruent and incongruent stimuli on the SCWT (Johannes et al., 2003; Lavoie et al., 2007). A similar pattern emerged in a recent study using a flankers task (Crawford et al., 2005), which found that the mean RTs of the GTS group were slightly faster than those of the control group. However, their correlations suggested a speed–accuracy trade-off for the incompatible trials, only in the GTS group. In that particular study, this may indicate impulsive responding in some individuals with GTS, since less accurate performance tended to be associated with faster RTs. With our GTS group, these patterns of response were not associated with more errors or response variability, which means that the response accuracy of the GTS group remained comparable to that of the control group despite their faster RTs.
4.2. Stimulus evaluation and the parietal P300 component
Current results revealed that the parietal P300 peak latency was not affected by S–R incompatibility in the control group. These data are in line with previous studies, which found the P300 latency to be relatively independent of response selection and execution processes (Magliero et al., 1984; McCarthy and Donchin, 1981).
Following our hypothesis, we expected that S–R incompatibility would affect the P300 component in the GTS group, by lengthening its peak latency. Our results confirmed this hypothesis and showed that this group difference remained significant, even after controlling for clinical variables such as depression, over-activity, anxiety and obsessive–compulsive symptoms. Moreover, correlations revealed that P300 latency was positively correlated to tic symptom severity, suggesting that delayed peak latency to incompatibility interference was related to more frequent tic symptoms. This finding strongly suggests that the presence of GTS symptoms does constitute an important factor contributing to this significant incompatibility effect at the P300 level. In sum, the P300 latency delay showed that, in GTS, stimulus evaluation and categorization processes (Duncan-Johnson and Donchin, 1982; Verleger, 1997) are more sensitive to the context of incongruent S–R mapping, compared with the controls. This P300 sensitivity to the SRC interference could be explained, in part, by limited attentional resource allocation in participants with GTS, since delayed P300 latencies have been observed in tasks requiring divided attention (Johannes et al., 1997; Johannes et al., 2001a).
Nonetheless, patients with GTS also showed an intact P300 in an auditory oddball (van Woerkom et al., 1988; Van de Wetering et al., 1985; Oades et al., 1996) and a Stroop task (Johannes et al., 2003), while a larger P300 amplitude was elicited during a counting oddball task, where no motor responses are required (Thibault et al., 2008). In the current paradigm, the incompatibility condition required that inhibition of the automatically activated response occur concurrently with comparison, abortion and retrieval of the correct motor program, which likely required dividing attentional resources, and which in GTS, delayed stimulus evaluation and categorization processes.
4.3. Motor inhibition and the NGA
Our results showed an enhanced and delayed NGA component over anterior regions, which corresponds to the frequently replicated classical topography of this component in a go–no-go task (Fallgatter et al., 2002, 1997; Strik et al., 1998). Our results with the GTS group generally showed a tendency toward a more frontally distributed NGA while, in the control group, the NGA was more centrally distributed. This seems in accord with one earlier study, which found evidence of a frontal shift of the NGA in a comparable adult GTS cohort (Johannes et al., 2001a). This effect has been interpreted as resulting from overactive frontal inhibitory functions during no-go responses, which is consistent with previous studies reporting cortical motor inhibitory anomalies in GTS (Braun et al., 1995; Ziemann et al., 1997). However, our NGA results must be interpreted cautiously, as we failed to find clear group differences on the no-go S-LRP amplitude, the NGA peak amplitude or the inhibition accuracy suggesting that, even in the presence of a NGA frontal shift, the GTS participants showed appropriate capacity of motor inhibition.
4.4. Motor activation and the lateralized-readiness potentials
As expected, S-LRPs obtained in the context of incongruent stimulus–response mapping showed an “incorrect” negative activation, followed by an opposite “correct” positive activation. These deflections correspond to the differential engagement of the motor cortices in the preparation of a uni-manual response (Eimer, 1998). They represent, in a temporally precise manner, the automatic compatible response activation and its gradual replacement by activation of the incompatible correct activation after parallel comparison and abortion stages have taken place (Coles et al., 1992). In the control group, these temporal fluctuations led to an onset latency delay in the incompatible compared with the compatible condition for the S-LRP only, reflecting a specific interference effect on pre-motor processes (Mordkoff and Gianaros, 2000). As opposed to the control group, the GTS group showed no significant difference in LRP onset latency between compatible and incompatible conditions, neither on the S-LRP or the R-LRP, indicating that they did not demonstrate a compatibility effect at the pre-motor (S-LRP) or the motor level (R-LRP). In other words, participants with GTS showed faster S-LRP onset latency than control participants in the context of incongruent S–R mapping. This group difference was even more robust after the contributions of depression, anxiety or over-activity scores were partialled out, once more confirming the primary contribution of GTS symptomatology to this significant group effect. This finding is congruent with our behavioral results and confirms that participants with GTS are characterized by a reduced sensitivity to S–R incompatibility interference at the pre-motor level. Moreover, correlational analysis revealed that S-LRP onset latency was negatively correlated to GTS symptom severity, suggesting that reduced sensitivity to S–R incompatibility interference was related to more frequent tic symptoms. This reduced sensitivity is also supported by SCWT results, demonstrating that participants with GTS, who were less affected by S–CR incompatibility interference at the pre-motor level, were also less affected by the interference generated by the SCWT.
According to what is known about LRP functional significance, a shorter S-LRP onset latency in the context of incongruent S–R mapping suggests that the switch, from compatible to incompatible cortical activation, is faster and, to some extent, more efficiently activated in participants with GTS. This switch requires either effective abortion of the automatic response, a faster retrieval of the required motor program, or both (Kornblum et al., 1990). The current results provide some clarification by disentangling these processes. The hypothesis of a faster retrieval of motor programs seems plausible and congruent with the etiological hypothesis of a motor cortical over-activation in GTS (Biswal et al., 1998; Eidelberg et al., 1997). In the case where an automatic congruent response activation has to be aborted and replaced, over-activation of motor cortical regions like the SMA and the pre-motor cortex (Eidelberg et al., 1997) could create higher baseline activation in these structures, which might lower the threshold for retrieval of the motor program and lead to a more rapid activation of the required response. In this case, the smaller S–R incompatibility interference effect characterizing participants with GTS could be most likely related to faster retrieval of the required motor program, which is consistent with the faster RTs.
Previous studies, using transcranial magnetic stimulation (TMS), showed that GTS patients are characterized by disinhibition of the motor cortex (Gilbert et al., 2004; Ziemann et al., 1997). Neuronal motor inhibitory difficulties of the motor system as measured with TMS in these tasks are not cognitively influenced (Daskalakis et al., 2002). So, this disinhibition could be interpreted as an indication of either an enhanced voluntary motor drive or a facilitated accessibility of motor commands to the motor cortex (Gilbert et al., 2004). However, we cannot fully transpose these TMS results to our own since the SRC task also reflects cognitive inhibition. But in other domains, some authors consistently reported that participants with GTS performed better in fast, goal-directed movements such as aiming at targets (Georgiou et al., 1997). Finally, O’Connor (2002) proposed that people with GTS would be characterized by a specific style of action, expressed by a motor over-preparedness. This over-preparation includes making too much effort over-investing in response and sometimes implicating too many muscles when executing an action, which would be congruent with the higher level of baseline sensorimotor activation, hypothesized in the current study.
4.5. Limitations and future directions
The presence of significantly more anxious, depressed, obsessive–compulsive and hyperactive traits in our GTS group could have contributed to the pattern of results. One could argue that this comorbidity could have contributed to the difference observed in the patient group. It has been repeatedly shown that major depression is related to reduced P300 amplitude and delayed peak latency (Anderer et al., 2002; Blackwood et al., 1987; Gangadhar et al., 1993; Röschke and Wagner, 2003), while other research has shown that hyperactivity fails to significantly affect the P300 component in an adult population (Ohlmeier et al., 2007; Prox et al., 2007). Additional investigations, with OCD patients, also found diminished amplitude and faster P300 peak latency (Towey et al., 1990, 1993, 1994). With our sample, however, group differences remained statistically significant after comorbid symptoms had been controlled as covariables. These results suggest that comorbidity did not affect significantly our measures, possibly because these symptoms were present at a sub-clinical level. One way to clarify this issue would be to compare a GTS group with other comorbid GTS groups suffering from clinically significant depression, OCD or hyperactive deficit.
Finally, another limitation was our small group sample size, which could have reduced the power and the generalizability of our conclusions. Nonetheless, our sample size compares favorably to earlier samples in the ERP field, which had samples ranging between 6 and 24 GTS patients (Johannes et al., 1997; Johannes et al., 2001a,b, 2003; van Woerkom et al., 1988; van Woerkom et al., 1988, 1994).
5. Conclusion
In sum, our results suggest that participants with GTS are characterized by a specific style of motor processing. The stimulus evaluation and categorization processes were delayed, possibly due to limited allocation of attentional resources, and they consistently failed to demonstrate the stimulus–response incompatibility effect, both at the pre-motor and at the motor level. Another interesting finding is the absence of group differences in both the ability to inhibit motor responses, along with the frontal shift related to this successful inhibition in GTS. These findings could be parsimoniously explained by faster retrieval of required motor programs, related to over-activation of motor cortical areas. The current study strongly suggests that previous assumptions about motor execution and inhibition deficits in GTS may need to be reconsidered.
Acknowledgments
This work was supported by a Canadian Institutes of Health Research (CIHR) operating grant (MOP57936), a Fonds pour la Recherche en Santé du Québec (FRSQ), clinical research grant (5271) and the laboratory infrastructure grant from the Fernand-Seguin research center. Geneviève Thibault was supported by a Ph.D. fellowship from the CIHR (139370). We wish to express our gratitude to Marie-Claude Pélissier, Frederic Aardema, Anick Laverdure, Ariane Fontaine and Valérie Poulin for research coordination and clinical screening, to Martine Germain and Nerly Jeudin for electrophysiological recordings, to Tine Timmermans, Véronique Labelle and Tina Imbriglio, for technical assistance and Sophie Lecours, Maria-Teresa Hernandez, Cathy Léveillé and Anne-Marie Daoust for the neuropsychometric testing. And last but not the least, we thank all the participants for their participation in this study.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington DC: American Psychiatric Association; 2000. Text revision ed. [Google Scholar]
- Anderer P, Saletu B, Semlitsch HV, Pascual-Marqui RD. Structural and energetic processes related to P300: LORETA findings in depression and effects of antidepressant drugs. Methods and Findings in Experimental and Clinical Pharmacology. 2002;24:85–91. Suppl D. [PubMed] [Google Scholar]
- Baron-Cohen S, Cross P, Crowson M, Robertson M. Can children with Gilles de la Tourette syndrome edit their intentions? Psychological Medicine. 1994;24 (1):29–40. doi: 10.1017/s0033291700026805. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56 (6):893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Biswal B, Ulmer JL, Krippendorf RL, Harsch HH, Daniels DL, Hyde JS, Haughton VM. Abnormal cerebral activation associated with a motor task in Tourette syndrome. American Journal of Neuroradiology. 1998;19 (8):1509–1512. [PMC free article] [PubMed] [Google Scholar]
- Blackwood DH, Whalley LJ, Christie JE, Blackburn IM, St Clair DM, McInnes A. Changes in auditory P3 event-related potential in schizophrenia and depression. British Journal of Psychiatry. 1987;150:154–160. doi: 10.1192/bjp.150.2.154. [DOI] [PubMed] [Google Scholar]
- Bloch MH, Leckman JF, Zhu H, Peterson BS. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology. 2005;65 (8):1253–1258. doi: 10.1212/01.wnl.0000180957.98702.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein RA. Neuropsychological performance in children with Tourette’s syndrome. Psychiatry Research. 1990;33 (1):73–81. doi: 10.1016/0165-1781(90)90150-4. [DOI] [PubMed] [Google Scholar]
- Bornstein RA. Neuropsychological performance in adults with Tourette’s syndrome. Psychiatry Research. 1991;37:229–236. doi: 10.1016/0165-1781(91)90059-x. [DOI] [PubMed] [Google Scholar]
- Bornstein RA, King G, Carroll A. Neuropsychological abnormalities in Gilles de la Tourette’s syndrome. J Nerv Ment Dis. 1983;171(8):497–502. doi: 10.1097/00005053-198308000-00007. [DOI] [PubMed] [Google Scholar]
- Bornstein RA, Baker GB, Bazylewich T, Douglass AB. Tourette syndrome and neuropsychological performance. Acta Psychiatr Scand. 1991;84(3):212–216. doi: 10.1111/j.1600-0447.1991.tb03131.x. [DOI] [PubMed] [Google Scholar]
- Braun AR, Stoetter B, Randolph C, Hsiao JK, Vladar K, Gernert J, Carson RE, Herscovitch P, Chase TN. The functional neuroanatomy of Tourette’s syndrome: an FDG-PET study. I. Regional changes in cerebral glucose metabolism differentiating patients and controls. Neuropsychopharmacology. 1993;9 (4):277–291. doi: 10.1038/npp.1993.64. [DOI] [PubMed] [Google Scholar]
- Braun AR, Randolph C, Stoetter B, Mohr E, Cox C, Vladar K, Sexton R, Carson RE, Herscovitch P, Chase TN. The functional neuroanatomy of Tourette’s syndrome: an FDG–PET Study. II: relationships between regional cerebral metabolism and associated behavioral and cognitive features of the illness. Neuropsychopharmacology. 1995;13 (2):151–168. doi: 10.1016/0893-133X(95)00052-F. [DOI] [PubMed] [Google Scholar]
- Brookshire BL, Butler IJ, Ewing-Cobbs L, Fletcher JM. Neuropsychological characteristics of children with Tourette syndrome: Evidence for a nonverbal learning disability? Journal of Clinical and Experimental Neuropsychology. 1994;16:289–302. doi: 10.1080/01688639408402639. [DOI] [PubMed] [Google Scholar]
- Brown TA, DiNardo PA, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV. Graywind Publications; Boulder, CO: 1994. [Google Scholar]
- Coles MG. Modern mind-brain reading: psychophysiology, physiology, and cognition. Psychophysiology. 1989;26 (3):251–269. doi: 10.1111/j.1469-8986.1989.tb01916.x. [DOI] [PubMed] [Google Scholar]
- Coles GH, Gehring WJ, Gratton G, Donchin E. Response activation and verification: A psychophysiological analysis. In: Requin GESJ, editor. Tutorials in Motor Behavior II. Elsevier; Amsterdam: 1992. pp. 779–792. [Google Scholar]
- Como PG. Neuropsychological function in Tourette syndrome. 2001. pp. 103–111. [PubMed] [Google Scholar]
- Crawford S, Channon S, Robertson MM. Tourette’s syndrome: performance on tests of behavioural inhibition, working memory and gambling. Journal of Child Psycholology and Psychiatry. 2005;46 (12):1327–1336. doi: 10.1111/j.1469-7610.2005.01419.x. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Chen R. Transcranial magnetic stimulation: a new investigational and treatment tool in psychiatry. Journal of Neuropsychiatry and Clinical Neuroscience. 2002;14 (4):406–415. doi: 10.1176/jnp.14.4.406. [DOI] [PubMed] [Google Scholar]
- Duncan-Johnson CC, Donchin E. The P300 component of the event-related brain potential as an index of information processing. Biological Psychology. 1982;14 (1–2):1–52. doi: 10.1016/0301-0511(82)90016-3. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Antonini A, Kazumata K, Dhawan V, Budman C, Feigin A. The metabolic anatomy of Tourette’s syndrome. Neurology. 1997;48 (4):927–934. doi: 10.1212/wnl.48.4.927. [DOI] [PubMed] [Google Scholar]
- Eimer M. The lateralized readiness potential as an on-line measure of central response processes. Behavior Research Methods, Instruments, & Computers. 1998;30 (1):146–156. [Google Scholar]
- Fallgatter AJ, Bartsch AJ, Herrmann MJ. Electrophysiological measurements of anterior cingulate function. Journal of Neural Transmission. 2002;109 (5–6):977–988. doi: 10.1007/s007020200080. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Brandeis D, Strik WK. A robust assessment of the NoGo-anteriorisation of P300 microstates in a cued Continuous Performance Test. Brain Topography. 1997;9 (4):295–302. doi: 10.1007/BF01464484. [DOI] [PubMed] [Google Scholar]
- Freeman RD, Fast DK, Burd L, Kerbeshian J, Robertson MM, Sandor P. An international perspective on Tourette syndrome: selected findings from 3,500 individuals in 22 countries. Developmental Medicine and Child Neurology. 2000;42 (7):436–447. doi: 10.1017/s0012162200000839. [DOI] [PubMed] [Google Scholar]
- Gangadhar BN, Ancy J, Janakiramaiah N, Umapathy C. P300 amplitude in non-bipolar, melancholic depression. Journal of Affective Disorders. 1993;28 (1):57–60. doi: 10.1016/0165-0327(93)90077-w. [DOI] [PubMed] [Google Scholar]
- Georgiou N, Bradshaw JL, Phillips JG, Cunnington R, Rogers M. Functional asymmetries in the movement kinematics of patients with Tourette’s syndrome. Journal of Neurology, Neurosurgery, and Psychiatry. 1997;63 (2):188–195. doi: 10.1136/jnnp.63.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DL, Bansal AS, Sethuraman G, Sallee FR, Zhang J, Lipps T, Wassermann EM. Association of cortical disinhibition with tic, ADHD, and OCD severity in Tourette syndrome. Movement Disorders. 2004;19 (4):416–425. doi: 10.1002/mds.20044. [DOI] [PubMed] [Google Scholar]
- Hallett M. Neurophysiology of tics. Advances in Neurology. 2001;85:237–244. [PubMed] [Google Scholar]
- Harcherik DF, Leckman JF, Detlor J, Cohen DJ. A new instrument for clinical studies of Tourette’s syndrome. Journal of the American Academy of Child Psychiatry. 1984;23 (2):153–160. doi: 10.1097/00004583-198403000-00006. [DOI] [PubMed] [Google Scholar]
- Harris EL, Schuerholz LJ, Singer HS, Reader MJ, Brown JE, Cox C, Mohr J, Chase GA, Denckla MB. Executive function in children with Tourette syndrome and/or attention deficit hyperactivity disorder. Journal of the International Neuropsychological Society. 1995;1 (6):511–516. doi: 10.1017/s1355617700000631. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Stacey ME, Coppola R, Handel SF, Rickler KC, Weinberger DR. Cerebral morphometric abnormalities in Tourette’s syndrome: a quantitative MRI study of monozygotic twins. Neurology. 1995;45 (6):1176–1182. doi: 10.1212/wnl.45.6.1176. [DOI] [PubMed] [Google Scholar]
- Johannes S, Weber A, Muller-Vahl KR, Kolbe H, Dengler R, Munte TF. Event-related brain potentials show changed attentional mechanisms in Gilles de la Tourette syndrome. European Journal of Neurology. 1997;4:152–161. doi: 10.1111/j.1468-1331.1997.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Mantey M, Nager W, Rada D, Muller-Vahl KR, Emrich HM, Dengler R, Münte TF, Dietrich D. Altered inhibition of motor responses in Tourette syndrome and obsessive–compulsive disorder. Acta Neurologica Scandinavica. 2001a;104:36–43. doi: 10.1034/j.1600-0404.2001.00308.x. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Nager W, Muller-Vahl KR, Dengler R, Munte TF. Electrophysiological measures and dual-task performance in Tourette syndrome indicate deficient divided attention mechanisms. European Journal of Neurology. 2001b;8 (3):253–260. doi: 10.1046/j.1468-1331.2001.00199.x. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Nager W, Rada D, Muller-Vahl KR, Emrich HM, Dengler R, Münte TF, Dietrich D. Tourette syndrome and obsessive–compulsive disorder: event-related brain potentials show similar mechanisms of frontal inhibition but dissimilar target evaluation processes. Behavioural Neurology. 2003;14:9–17. doi: 10.1155/2003/326468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblum S, Hasbroucq T, Osman A. Dimensional overlap: cognitive basis for stimulus–response compatibility—a model and taxonomy. Psychological Review. 1990;97 (2):253–270. doi: 10.1037/0033-295x.97.2.253. [DOI] [PubMed] [Google Scholar]
- Kutas M, Donchin E. Preparation to respond as manifested by movement-related brain potentials. Brain Research. 1980;202 (1):95–115. [PubMed] [Google Scholar]
- Lavoie ME, Thibault G, Stip E, O’Connor KP. Memory and executive functions in adults with Gilles de la Tourette syndrome and chronic tic disorder. Cognitive Neuropsychiatry. 2007;12 (2):165–181. doi: 10.1080/13546800600826371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Zhang H, Vitale A, Lahnin F, Lynch K, Bondi C, Kim YS, Peterson BS. Course of tic severity in Tourette syndrome: the first two decades. Pediatrics. 1998;102 (1 Pt 1):14–19. doi: 10.1542/peds.102.1.14. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Vaccarino FM, Kalanithi PS, Rothenberger A. Annotation: Tourette syndrome: a relentless drumbeat–driven by misguided brain oscillations. J Child Psychol Psychiatry. 2006;47 (6):537–550. doi: 10.1111/j.1469-7610.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Banich MT, Jacobson BL, Tanabe JL. Common and distinct neural substrates of attentional control in an integrated Simon and spatial Stroop task as assessed by event-related fMRI. Neuroimage. 2004;22 (3):1097–1106. doi: 10.1016/j.neuroimage.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Magliero A, Bashore TR, Coles MG, Donchin E. On the dependence of P300 latency on stimulus evaluation processes. Psychophysiology. 1984;21 (2):171–186. doi: 10.1111/j.1469-8986.1984.tb00201.x. [DOI] [PubMed] [Google Scholar]
- Mahone EM, Koth CW, Cutting L, Singer HS, Denckla MB. Executive function in fluency and recall measures among children with Tourette syndrome or ADHD. J Int Neuropsychol Soc. 2001;7(1):102–111. doi: 10.1017/s1355617701711101. [DOI] [PubMed] [Google Scholar]
- Masaki H, Takasawa N, Yamazaki K. An electrophysiological study of the locus of the interference effect in a stimulus–response compatibility paradigm. Psychophysiology. 2000;37:464–472. [PubMed] [Google Scholar]
- McCarthy G, Donchin E. A metric for thought: a comparison of P300 latency and reaction time. Science. 1981;211 (4477):77–80. doi: 10.1126/science.7444452. [DOI] [PubMed] [Google Scholar]
- Mordkoff JT, Gianaros PJ. Detecting the onset of the lateralized readiness potential: a comparison of available methods and procedures. Psychophysiology. 2000;37 (3):347–360. [PMC free article] [PubMed] [Google Scholar]
- Oades RD, Dittmann-Balcar A, Schepker R, Eggers C, Zerbin D. Auditory event-related potentials (ERPs) and mismatch negativity (MMN) in healthy children and those with attention-deficit or tourette/tic symptoms. Biological Psychology. 1996;43 (2):163–185. doi: 10.1016/0301-0511(96)05189-7. [DOI] [PubMed] [Google Scholar]
- O’Connor K. A cognitive–behavioral/psychophysiological model of tic disorders. Behaviour Research and Therapy. 2002;40:1113–1142. doi: 10.1016/s0005-7967(02)00048-7. [DOI] [PubMed] [Google Scholar]
- O’Connor KP. Cognitive–Behavioral Management of Tic Disorders. John-Wiley and Sons; Chichester: 2005. Testing the cognitive–psychophysiological model: validation of a style of planning action (STOP) as a discriminator between tic disorder, obsessive–compulsive disorder and generalized anxiety; pp. 65–73. [Google Scholar]
- O’Connor KP, Lavoie ME, Stip E, Borgeat F, Laverdure A. Cognitive-behaviour therapy and skilled motor performance in adults with chronic tic disorder. Neuropsychological Rehabilitation. 2008;18 (1):45–64. doi: 10.1080/09602010701390835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlmeier MD, Prox V, Zhang Y, Zedler M, Ziegenbein M, Emrich HM, Dietrich DE. Effects of methylphenidate in ADHD adults on target evaluation processing reflected by event-related potentials. Neuroscience Letters. 2007;424 (3):149–154. doi: 10.1016/j.neulet.2007.06.055. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9 (1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ozonoff S, Jensen J. Brief report: specific executive function profiles in three neurodevelopmental disorders. Journal of autism and developmental disorders. 1999;29 (2):171–177. doi: 10.1023/a:1023052913110. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Staib L, Scahill L, Zhang H, Anderson C, Leckman JF, Cohen DJ, Gore JC, Albert J, Webster R. Regional brain and ventricular volumes in Tourette syndrome. Archives of General Psychiatry. 2001;58 (5):427–440. doi: 10.1001/archpsyc.58.5.427. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Kane MJ, Alexander GM, Lacadie C, Skudlarski P, Leung HC, May J, Gore JC. An event-related functional MRI study comparing interference effects in the Simon and Stroop tasks. Brain Research. Cognitive Brain Research. 2002;13 (3):427–440. doi: 10.1016/s0926-6410(02)00054-x. [DOI] [PubMed] [Google Scholar]
- Prox V, Dietrich DE, Zhang Y, Emrich HM, Ohlmeier MD. Attentional processing in adults with ADHD as reflected by event-related potentials. Neuroscience Letters. 2007;419 (3):236–241. doi: 10.1016/j.neulet.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Raven JC. Individual Form. 1938, 1996. Progressive Matrices: A Perceptual Test of Intelligence. [Google Scholar]
- Rektor I. Scalp-recorded Bereitschaftspotential is the result of the activity of cortical and subcortical generators–a hypothesis. Clinical Neurophysiology. 2002;113 (12):1998–2005. doi: 10.1016/s1388-2457(02)00286-9. [DOI] [PubMed] [Google Scholar]
- Rektor I, Kaiiovsky P, Bares M, Brazdil M, Streitova H, Klajblova H, Kuba R, Daniel P. A SEEG study of ERP in motor and premotor cortices and in the basal ganglia. Clinical Neurophysiology. 2003;114 (3):463–471. doi: 10.1016/s1388-2457(02)00388-7. [DOI] [PubMed] [Google Scholar]
- Requin J, Riehle A. Neural correlates of partial transmission of sensorimotor information in the cerebral cortex. Acta Psychologica. 1995;90 (1–3):81–95. doi: 10.1016/0001-6918(95)00039-w. [DOI] [PubMed] [Google Scholar]
- Röschke J, Wagner P. A confirmatory study on the mechanisms behind reduced P300 waves in depression. Neuropsychopharmacology. 2003;28 (Suppl 1):S9–S12. doi: 10.1038/sj.npp.1300139. [DOI] [PubMed] [Google Scholar]
- Rothenberger A, Kemerling S. Bereitschaftpotential in children with multiple tics and Gilles de la Tourette syndrome. In: Rothenberger A, editor. Event-Related Potentials in children. Amsterdam: Elsevier Biomedical Press; 1982. pp. 257–270. [Google Scholar]
- Rothenberger AK, Schenk GK, Zerbin D, Voss M. Movement-Related potentials in children with hypermotoric behaviour. In: McCallum RZWC, Denoth F, editors. Cerebral Psychophysiology: studies in event-related potentials. suppl 38. Amsterdam: Elsevier Science publishers, B.V. (Biomedical division); 1986. pp. 496–499. Vol. EEG. [Google Scholar]
- Sanavio E. Obsessions and compulsions: the Padua inventory. Behaviour Research and Therapy. 1988;26 (2):169–177. doi: 10.1016/0005-7967(88)90116-7. [DOI] [PubMed] [Google Scholar]
- Schuerholz LJ, Baumgardner TL, Singer HS, Reiss AL, Denckla MB. Neuropsychological status of children with Tourette’s syndrome with and without attention deficit hyper-activity disorder. Neurology. 1996;46 (4):958–965. doi: 10.1212/wnl.46.4.958. [DOI] [PubMed] [Google Scholar]
- Serrien DJ, Nirkko AC, Loher TJ, Lovblad KO, Burgunder JM, Wiesendanger M. Movement control of manipulative tasks in patients with Gilles de la Tourette syndrome. Brain. 2002;125 (Pt 2):290–300. doi: 10.1093/brain/awf024. [DOI] [PubMed] [Google Scholar]
- Singer HS, Minzer K. Neurobiological issues in Tourette’s syndrome. In: Kurlan R, editor. Handbook of Tourette’s syndrome and related tic and behavioral disorders. 2. New York: Marcel Dekker; 2005. [Google Scholar]
- Stoetter B, Braun AR, Randolph C, Gernert J, Carson RE, Herscovitch P, Chase TN. Functional neuroanatomy of Tourette syndrome. Limbic-motor interactions studied with FDG PET. Advances in Neurology. 1992;58:213–226. [PubMed] [Google Scholar]
- Strik WK, Fallgatter AJ, Brandeis D, Pascual-Marqui RD. Three-dimensional tomography of event-related potentials during response inhibition: evidence for phasic frontal lobe activation. Electroencephalography and Clinical Neurophysiology. 1998;108 (4):406–413. doi: 10.1016/s0168-5597(98)00021-5. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Sutherland RJ, Kolb B, Schoel WM, Whishaw IQ, Davies D. Neuropsychological assessment of children and adults with Tourette syndrome: a comparison with learning disabilities and schizophrenia. Advances in Neurology. 1982;35:311–322. [PubMed] [Google Scholar]
- Thibault G, Felezeu M, O’Connor KP, Todorov C, Stip E, Lavoie ME. Influence of comorbid obsessive-compulsive symptoms on brain event-related potentials in Gilles de la Tourette syndrome. Progress in Neuropsychopharmacology & Biological Psychiatry. 2008;32 (3):803–815. doi: 10.1016/j.pnpbp.2007.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towey J, Bruder G, Hollander E, Friedman D, Erhan H, Liebowitz M, Sutton S. Endogenous event-related potentials in obsessive–compulsive disorder. Biological Psychiatry. 1990;28 (2):92–98. doi: 10.1016/0006-3223(90)90626-d. [DOI] [PubMed] [Google Scholar]
- Towey J, Bruder G, Tenke C, Leite P, DeCaria C, Friedman D, Hollander E. Event-related potential and clinical correlates of neurodysfunction in obsessive–compulsive disorder. Psychiatry Research. 1993;49 (2):167–181. doi: 10.1016/0165-1781(93)90103-n. [DOI] [PubMed] [Google Scholar]
- Towey JP, Tenke CE, Bruder GE, Leite P, Friedman D, Liebowitz M, Hollander E. Brain event-related potential correlates of overfocused attention in obsessive–compulsive disorder. Psychophysiology. 1994;31 (6):535–543. doi: 10.1111/j.1469-8986.1994.tb02346.x. [DOI] [PubMed] [Google Scholar]
- Trevena JA, Miller J. Cortical movement preparation before and after a conscious decision to move. Consciousness and Cognition. 2002;11 (2):162–190. doi: 10.1006/ccog.2002.0548. discussion 314–125. [DOI] [PubMed] [Google Scholar]
- Van de Wetering BJ, Martens CM, Fortgens C, Slaets JP, van Woerkom TC. Late components of the auditory evoked potentials in Gilles de la Tourette syndrome. Clinical Neurology and Neurosurgery. 1985;87 (3):181–186. doi: 10.1016/0303-8467(85)90004-6. [DOI] [PubMed] [Google Scholar]
- van Woerkom TC, Fortgens C, Rompel-Martens CM, Van de Wetering BJ. Auditory event-related potentials in adult patients with Gilles de la Tourette’s syndrome in the oddball paradigm. Electroencephalography and Clinical Neurophysiology. 1988;71 (6):443–449. doi: 10.1016/0168-5597(88)90048-2. [DOI] [PubMed] [Google Scholar]
- van Woerkom TC, Fortgens C, Van de Wetering BJ, Martens CM. Contingent negative variation in adults with Gilles de la Tourette syndrome. Journal of Neurology, Neurosurgery and Psychiatry. 1988;51 (5):630–634. doi: 10.1136/jnnp.51.5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Woerkom TC, Roos RA, van Dijk JG. Altered attentional processing of background stimuli in Gilles de la Tourette syndrome: a study in auditory event-related potentials evoked in an oddball paradigm. Acta Neurologica Scandinavica. 1994;90 (2):116–123. doi: 10.1111/j.1600-0404.1994.tb02690.x. [DOI] [PubMed] [Google Scholar]
- Verleger R. On the utility of P3 latency as an index of mental chronometry. Psychophysiology. 1997;34 (2):131–156. doi: 10.1111/j.1469-8986.1997.tb02125.x. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Paulus W, Rothenberger A. Decreased motor inhibition in Tourette’s disorder: evidence from transcranial magnetic stimulation. American Journal of Psychiatry. 1997;154 (9):1277–1284. doi: 10.1176/ajp.154.9.1277. [DOI] [PubMed] [Google Scholar]