Abstract
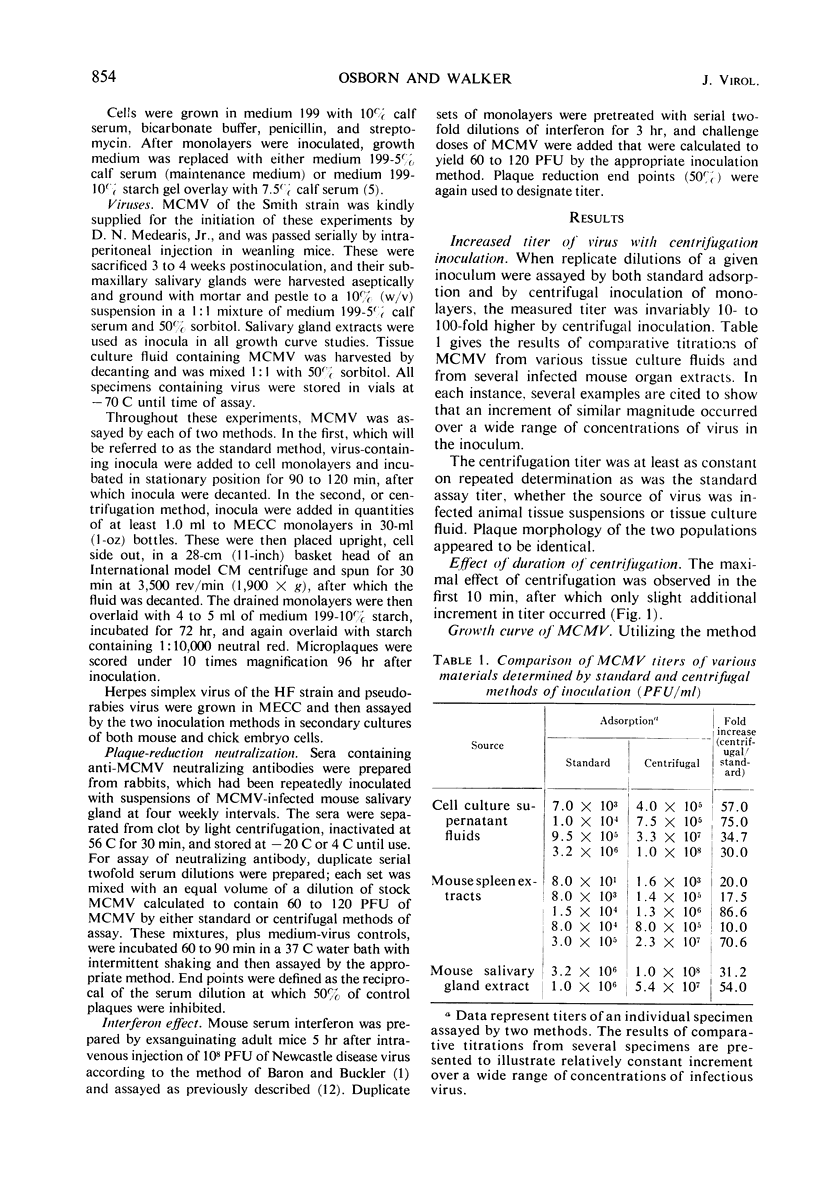
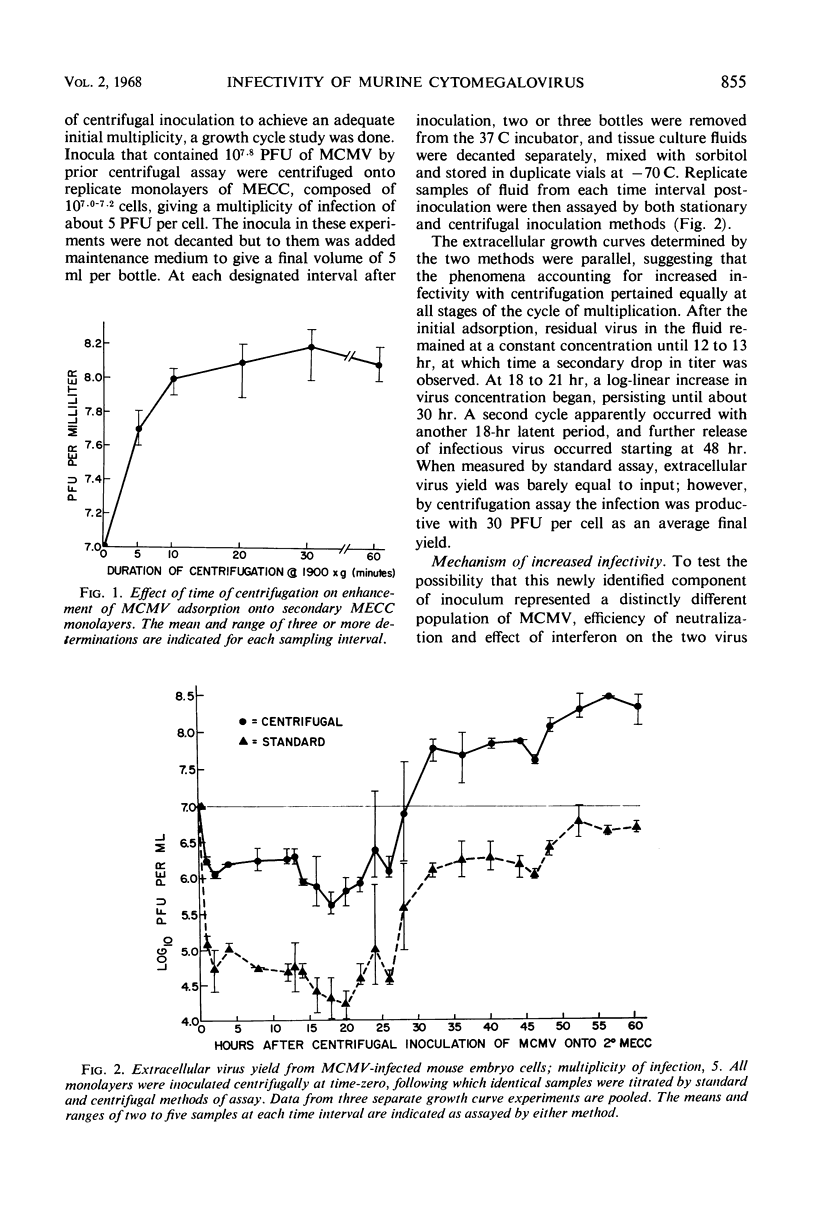
Centrifugation of murine cytomegalovirus inocula from a variety of sources onto secondary mouse embryo cell monolayers at 1,900 × g for 30 min regularly revealed 10- to 100-fold more infectious virus than could be found in the same materials using standard inoculation methods. Virus demonstrable only by centrifugation was present throughout the entire growth cycle in a constant proportion to virus measured without centrifugation. Extracellular growth curves of both populations revealed an 18- to 21-hr latent period, followed by a long-linear increase over the next 12 hr; final yield was 30 plaque-forming units (PFU) per cell. Centrifugation of cells prior to inoculation or after standard adsorption and removal of inoculum failed to result in any significant change in measured virus titer. However, even after 4-hr adsorption, the supernatant inoculum could be transferred and centrifuged onto a fresh monolayer resulting in the same increment of measurable virus. Neutralizing antibody and interferon were equally efficacious against 100 PFU of virus as defined by either method. Thus, this newly identified population of cytomegalo-virus represents the vast majority of potentially infectious units and appears to differ solely in ease of adsorption onto cell monolayers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARON S., BUCKLER C. E. CIRCULATING INTERFERON IN MICE AFTER INTRAVENOUS INJECTION OF VIRUS. Science. 1963 Sep 13;141(3585):1061–1063. doi: 10.1126/science.141.3585.1061. [DOI] [PubMed] [Google Scholar]
- Brunell P. A. Separation of infectious Varicella-Zoster virus from human embryonic lung fibroblasts. Virology. 1967 Apr;31(4):732–734. doi: 10.1016/0042-6822(67)90207-3. [DOI] [PubMed] [Google Scholar]
- CAUNT A. E. GROWTH OF VARICELLA-ZOSTER VIRUS IN HUMAN THYROID TISSUE CULTURES. Lancet. 1963 Nov 9;2(7315):982–983. doi: 10.1016/s0140-6736(63)90680-9. [DOI] [PubMed] [Google Scholar]
- CHAPARAS S. D., SCHLESINGER R. W. Plaque assay of Toxoplasma on monolayers of chick embryo fibroblasts. Proc Soc Exp Biol Med. 1959 Nov;102:431–437. doi: 10.3181/00379727-102-25275. [DOI] [PubMed] [Google Scholar]
- DEMAEYER E., SCHONNE E. STARCH GEL AS AN OVERLAY FOR THE PLAQUE ASSAY OF ANIMAL VIRUSES. Virology. 1964 Sep;24:13–18. doi: 10.1016/0042-6822(64)90142-4. [DOI] [PubMed] [Google Scholar]
- GEY G. O., BANG F. B., GEY M. K. Responses of a variety of normal and malignant cells to continuous cultivation, and some practical applications of these responses to problems in the biology of disease. Ann N Y Acad Sci. 1954 Nov 17;58(7):976–999. doi: 10.1111/j.1749-6632.1954.tb45886.x. [DOI] [PubMed] [Google Scholar]
- GORDON F. B., QUAN A. L., TRIMMER R. W. Morphologic observations on trachoma virus in cell cultures. Science. 1960 Mar 11;131(3402):733–734. doi: 10.1126/science.131.3402.733. [DOI] [PubMed] [Google Scholar]
- Henle G., Henle W., Diehl V. Relation of Burkitt's tumor-associated herpes-ytpe virus to infectious mononucleosis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):94–101. doi: 10.1073/pnas.59.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan M. M., Wiktor T. J., Maes R. F., Campbell J. B., Koprowski H. Effect of polyions on the infectivity of rabies virus in tissue culture: construction of a single-cycle growth curve. J Virol. 1967 Feb;1(1):145–151. doi: 10.1128/jvi.1.1.145-151.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn J. E., Medearis D. N., Jr Studies of relationship between mouse cytomegalovirus and interferon. Proc Soc Exp Biol Med. 1966 Mar;121(3):819–824. doi: 10.3181/00379727-121-30897. [DOI] [PubMed] [Google Scholar]
- PADGETT B. L., WALKER D. L. Use of centrifugal force to promote adsorption of myxoma virus to cell monolayers. Proc Soc Exp Biol Med. 1962 Nov;111:364–367. doi: 10.3181/00379727-111-27793. [DOI] [PubMed] [Google Scholar]
- SMITH K. O. PHYSICAL AND BIOLOGICAL OBSERVATIONS ON HERPESVIRUS. J Bacteriol. 1963 Nov;86:999–1009. doi: 10.1128/jb.86.5.999-1009.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH K. O., RASMUSSEN L. MORPHOLOGY OF CYTOMEGALOVIRUS (SALIVARY GLAND VIRUS). J Bacteriol. 1963 Jun;85:1319–1325. doi: 10.1128/jb.85.6.1319-1325.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH K. O. RELATIONSHIP BETWEEN THE ENVELOPE AND THE INFECTIVITY OF HERPES SIMPLEX VIRUS. Proc Soc Exp Biol Med. 1964 Mar;115:814–816. doi: 10.3181/00379727-115-29045. [DOI] [PubMed] [Google Scholar]
- Vonka V., Benyeshmelnick M. Thermoinactivation of human cytomegalovirus. J Bacteriol. 1966 Jan;91(1):221–226. doi: 10.1128/jb.91.1.221-226.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISS E., DRESSLER H. R. Centrifugation and Rickettsiae and viruses onto cells and its effect on infection. Proc Soc Exp Biol Med. 1960 Apr;103:691–695. doi: 10.3181/00379727-103-25637. [DOI] [PubMed] [Google Scholar]
- WELLER T. H., WITTON H. M., BELL E. J. The etiologic agents of varicella and herpes zoster; isolation, propagation, and cultural characteristics in vitro. J Exp Med. 1958 Dec 1;108(6):843–868. doi: 10.1084/jem.108.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]



