Abstract
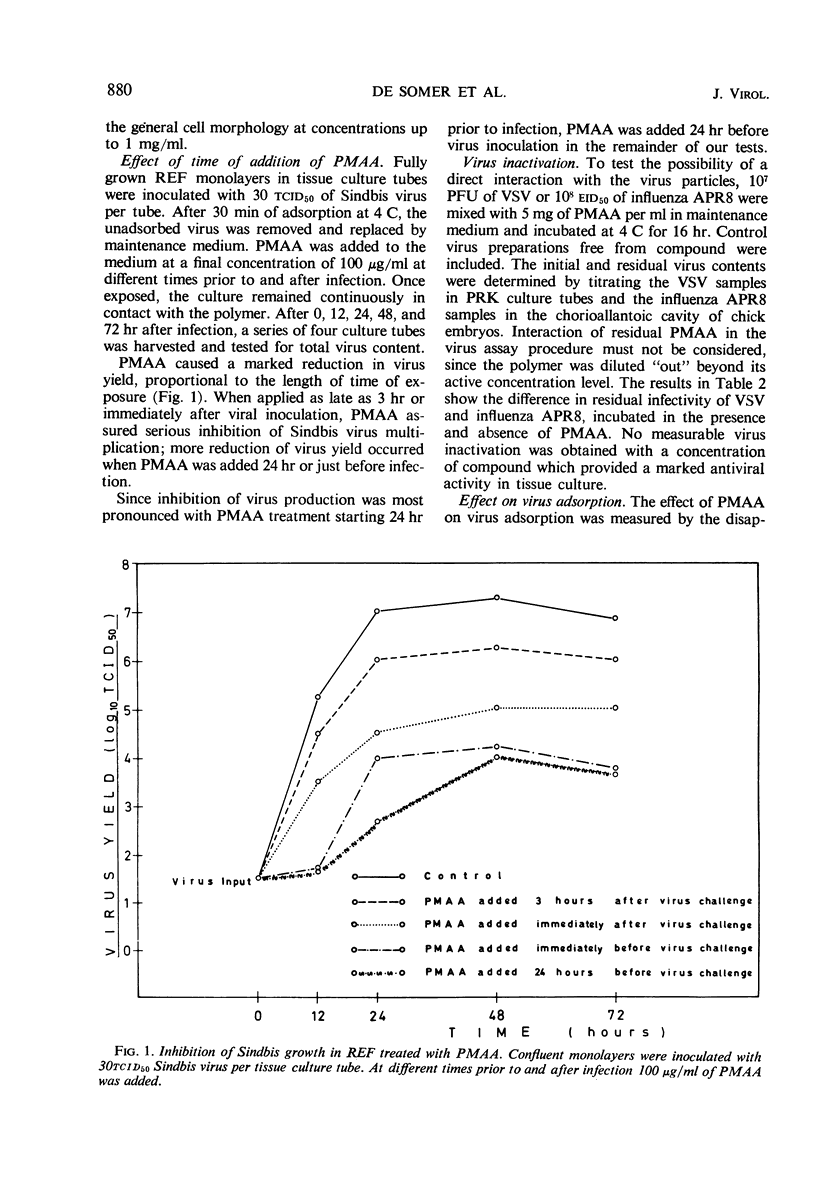
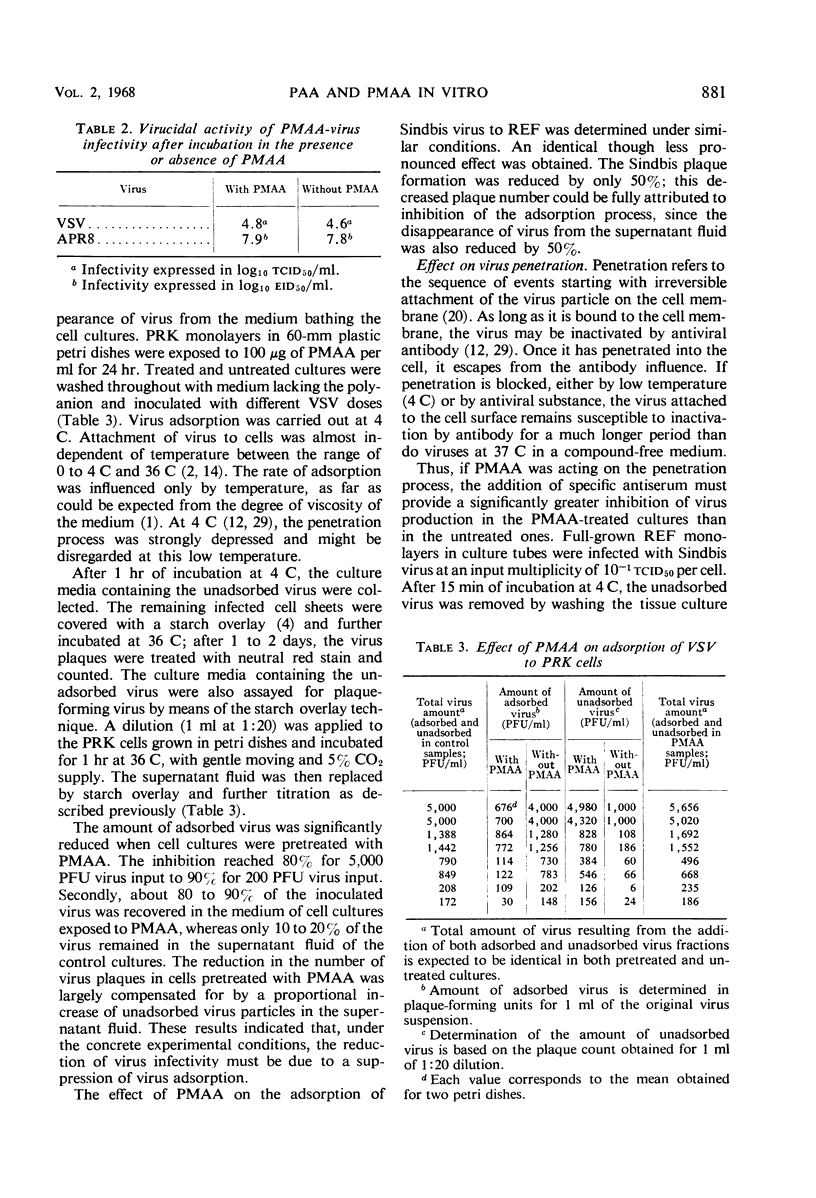
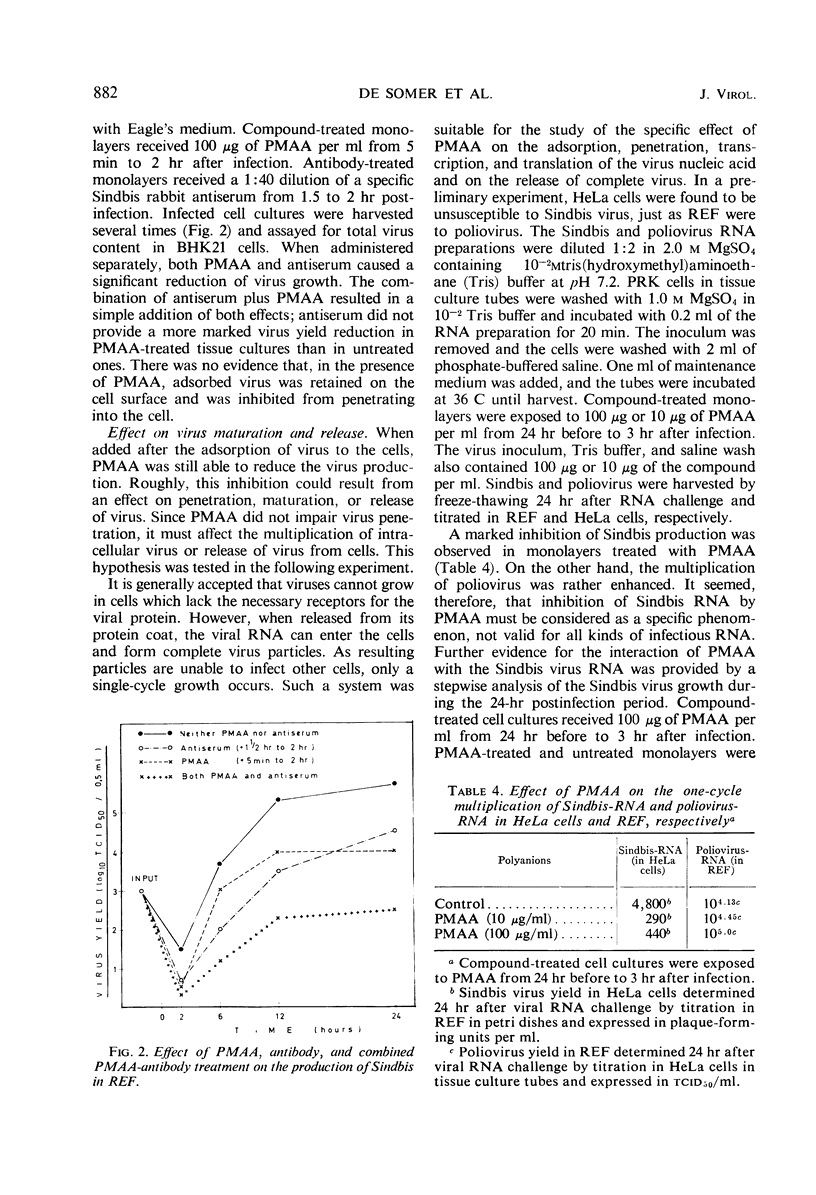
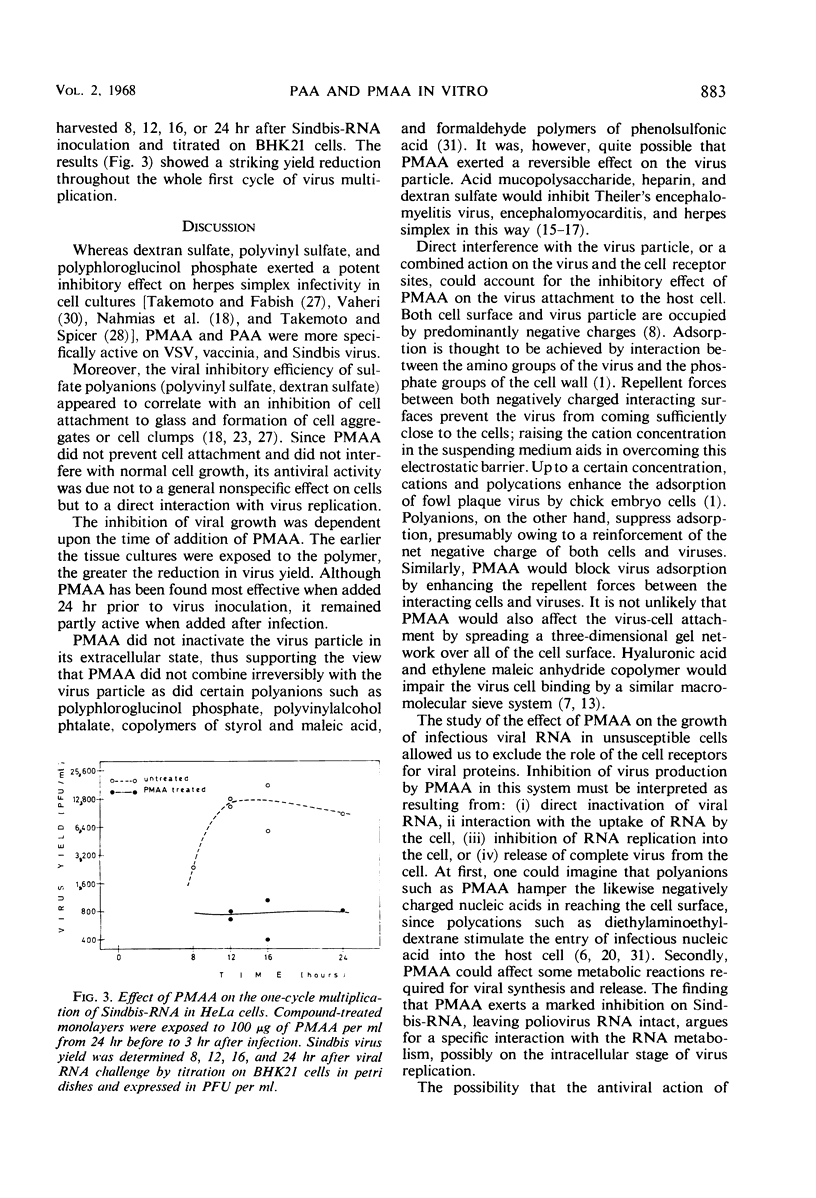
Polyacrylic acid (PAA) and polymethacrylic acid (PMAA) were investigated for their antiviral properties in tissue culture. Compared to other related polyanions, as dextran sulfate, polystyrene sulfonate, polyvinyl sulfate, and polyphloroglucinol phosphate, PAA and PMAA were found to be significantly more antivirally active and less cytotoxic. PMAA added 24 hr prior to virus inoculation inhibited viral growth most efficiently but it was still effective when added 3 hr after infection. Neither a direct irreversible action on the virus nor inhibition of virus penetration into the cell could explain the antiviral activity of PMAA. PMAA inhibited the adsorption of the virus to the host cell and suppressed the one-cycle viral synthesis in tissue cultures inoculated with infectious RNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON A. C., VALENTINE R. C. Virus particle adsorption, III. Adsorption of viruses by cell monolayers and effects of some variables on adsorption. Biochim Biophys Acta. 1960 Jun 3;40:400–410. doi: 10.1016/0006-3002(60)91380-9. [DOI] [PubMed] [Google Scholar]
- BACHTOLD J. G., BUBEL H. C., GEBHARDT L. P. The primary interaction of poliomyelitis virus with host cells of tissue culture origin. Virology. 1957 Dec;4(3):582–589. doi: 10.1016/0042-6822(57)90087-9. [DOI] [PubMed] [Google Scholar]
- BURGER W. C., STAHMANN M. A. The combination of lysine polypeptides with tobacco mosaic virus. J Biol Chem. 1951 Nov;193(1):13–22. [PubMed] [Google Scholar]
- DEMAEYER E., SCHONNE E. STARCH GEL AS AN OVERLAY FOR THE PLAQUE ASSAY OF ANIMAL VIRUSES. Virology. 1964 Sep;24:13–18. doi: 10.1016/0042-6822(64)90142-4. [DOI] [PubMed] [Google Scholar]
- De Somer P., De Clercq E., Billiau A., Schonne E., Claesen M. Antiviral activity of polyacrylic and polymethacrylic acids. II. Mode of action in vivo. J Virol. 1968 Sep;2(9):886–893. doi: 10.1128/jvi.2.9.886-893.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELTZ E. T., REGELSON W. Ethylene maleic anhydride copolymers as viral inhibitors. Nature. 1962 Nov 17;196:642–645. doi: 10.1038/196642a0. [DOI] [PubMed] [Google Scholar]
- Fayet M. T. Augmentation du pouvoir infectant de l'acide ribonucléique du virus de la fièvre aphteuse par addition de diéthylaminoéthyl-dextran. C R Acad Sci Hebd Seances Acad Sci D. 1967 Jan 30;264(5):783–784. [PubMed] [Google Scholar]
- GREEN M., STAHMANN M. A. Inhibition of mumps virus multiplication by a synthetic polypeptide. Proc Soc Exp Biol Med. 1953 Aug-Sep;83(4):852–858. doi: 10.3181/00379727-83-20514. [DOI] [PubMed] [Google Scholar]
- GREEN M., STAHMANN M. A., RASMUSSEN A. F., Jr Protection of embryonated eggs infected with infectious bronchitis or Newcastle disease virus by polypeptides. Proc Soc Exp Biol Med. 1953 Jul;83(3):641–643. doi: 10.3181/00379727-83-20444. [DOI] [PubMed] [Google Scholar]
- HERMODSSON S., PHILIPSON L. A SENSITIVE METHOD FOR INTERFERON ASSAY. Proc Soc Exp Biol Med. 1963 Dec;114:574–579. doi: 10.3181/00379727-114-28735. [DOI] [PubMed] [Google Scholar]
- HUANG A. S., WAGNER R. R. PENETRATION OF HERPES SIMPLEX VIRUS INTO HUMAN EPIDERMOID CELLS. Proc Soc Exp Biol Med. 1964 Aug-Sep;116:863–869. doi: 10.3181/00379727-116-29392. [DOI] [PubMed] [Google Scholar]
- LAURENT T. C., PIETRUSZKIEWICZ A. The effect of hyaluronic acid on the sedimentation rate of other substances. Biochim Biophys Acta. 1961 May 13;49:258–264. doi: 10.1016/0006-3002(61)90125-1. [DOI] [PubMed] [Google Scholar]
- LEVINE S., SAGIK B. P. The interactions of Newcastle disease virus (NDV) with chick embryo tissue culture cells: attachment and growth. Virology. 1956 Feb;2(1):57–68. doi: 10.1016/0042-6822(56)90076-9. [DOI] [PubMed] [Google Scholar]
- LIEBHABER H., TAKEMOTO K. K. THE BASIS FOR THE SIZE DIFFERENCES IN PLAQUES PRODUCED BY VARIANTS OF ENCEPHALOMYOCARDITIS (EMC) VIRUS. Virology. 1963 Aug;20:559–566. doi: 10.1016/0042-6822(63)90280-0. [DOI] [PubMed] [Google Scholar]
- MANDEL B. Inhibition of Theiler's encephalomyelitis virus (GDVII strain) of mice by an intestinal mucopolysaccharide. III. Studies on factors that influence the virus-inhibitor reaction. Virology. 1957 Jun;3(3):444–463. doi: 10.1016/0042-6822(57)90002-8. [DOI] [PubMed] [Google Scholar]
- NAHMIAS A. J., KIBRICK S., BERNFELD P. EFFECT OF SYNTHETIC AND BIOLOGICAL POLYANIONS ON HERPES SIMPLEX VIRUS. Proc Soc Exp Biol Med. 1964 Apr;115:993–996. doi: 10.3181/00379727-115-29098. [DOI] [PubMed] [Google Scholar]
- Nahmias A. J., Kibrick S. Inhibitory effect of heparin on herpes simplex virus. J Bacteriol. 1964 May;87(5):1060–1066. doi: 10.1128/jb.87.5.1060-1066.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILIPSON L. THE EARLY INTERACTION OF ANIMAL VIRUSES AND CELLS. Prog Med Virol. 1963;5:43–78. [PubMed] [Google Scholar]
- Pagano J. S., Vaheri A. Enhancement of infectivity of poliovirus RNA with diethylaminoethyl-dextran (DEAE-D). Arch Gesamte Virusforsch. 1965;17(3):456–464. doi: 10.1007/BF01241201. [DOI] [PubMed] [Google Scholar]
- RUBINI J. R., RASMUSSEN A. F., Jr, STAHMANN M. A. Inhibitory effect of synthetic lysine polypeptides on growth of influenza virus in embryonated eggs. Proc Soc Exp Biol Med. 1951 Apr;76(4):662–665. doi: 10.3181/00379727-76-18588. [DOI] [PubMed] [Google Scholar]
- SCHULZE I. T., SCHLESINGER R. W. Inhibition of infectious and hemagglutinating properties of type 2 dengue virus by aqueous Agar extracts. Virology. 1963 Jan;19:49–57. doi: 10.1016/0042-6822(63)90023-0. [DOI] [PubMed] [Google Scholar]
- Solomon J. J., Glatt K. A., Okazaki W. Inhibitory effect of heparin on Rous sarcoma virus. J Bacteriol. 1966 Dec;92(6):1855–1856. doi: 10.1128/jb.92.6.1855-1856.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEMOTO K. K., FABISCH P. INFLUENCE OF ACID POLYSACCHARIDES ON PLAQUE FORMATION BY INFLUENZA A2 AND B VIRUSES. Proc Soc Exp Biol Med. 1963 Dec;114:811–814. doi: 10.3181/00379727-114-28806. [DOI] [PubMed] [Google Scholar]
- THORNE H. V. Kinetics of cell infection and penetration by the virus of foot-and-mouth disease. J Bacteriol. 1962 Nov;84:929–942. doi: 10.1128/jb.84.5.929-942.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAHERI A., PENTINEN K. Effect of polyphloroglucinol-phosphate, an acid polymer, on herpes simplex virus. Ann Med Exp Biol Fenn. 1962;40:334–341. [PubMed] [Google Scholar]
- Vaheri A., Pagano J. S. Infectious poliovirus RNA: a sensitive method of assay. Virology. 1965 Nov;27(3):434–436. doi: 10.1016/0042-6822(65)90126-1. [DOI] [PubMed] [Google Scholar]



