Abstract
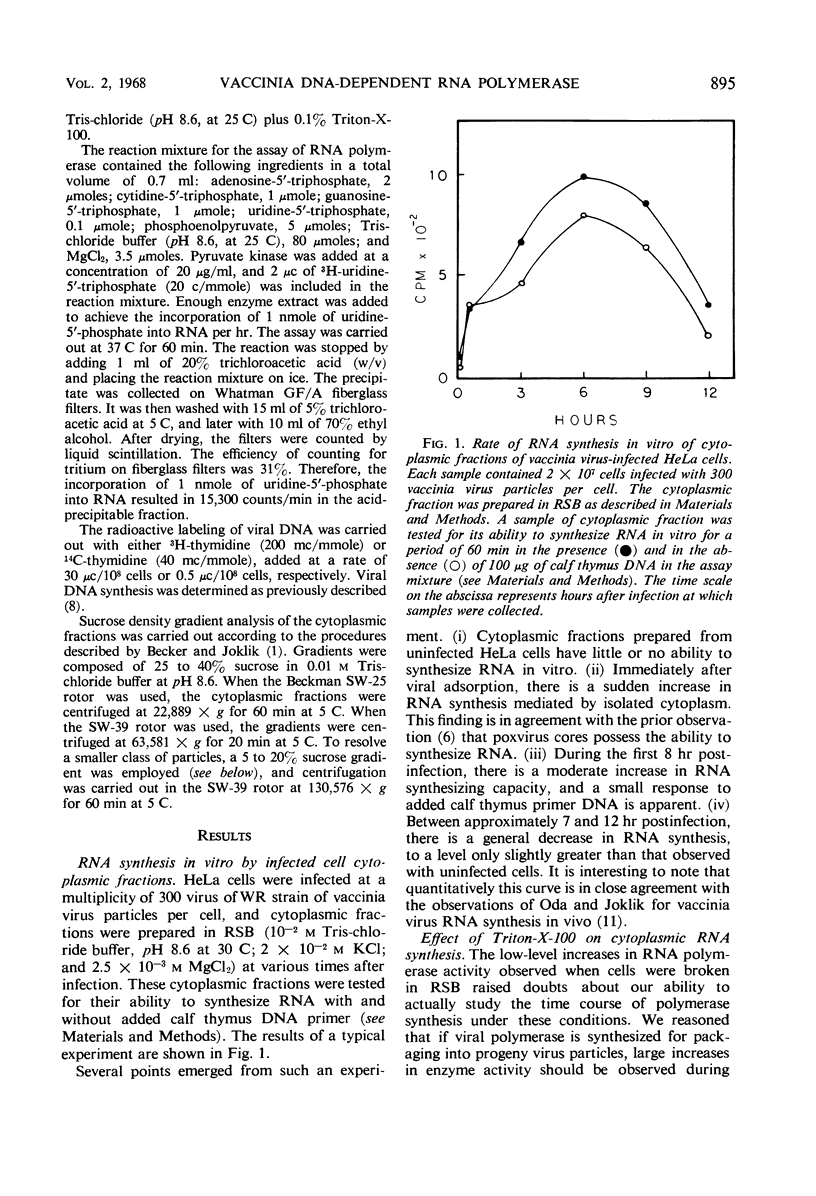
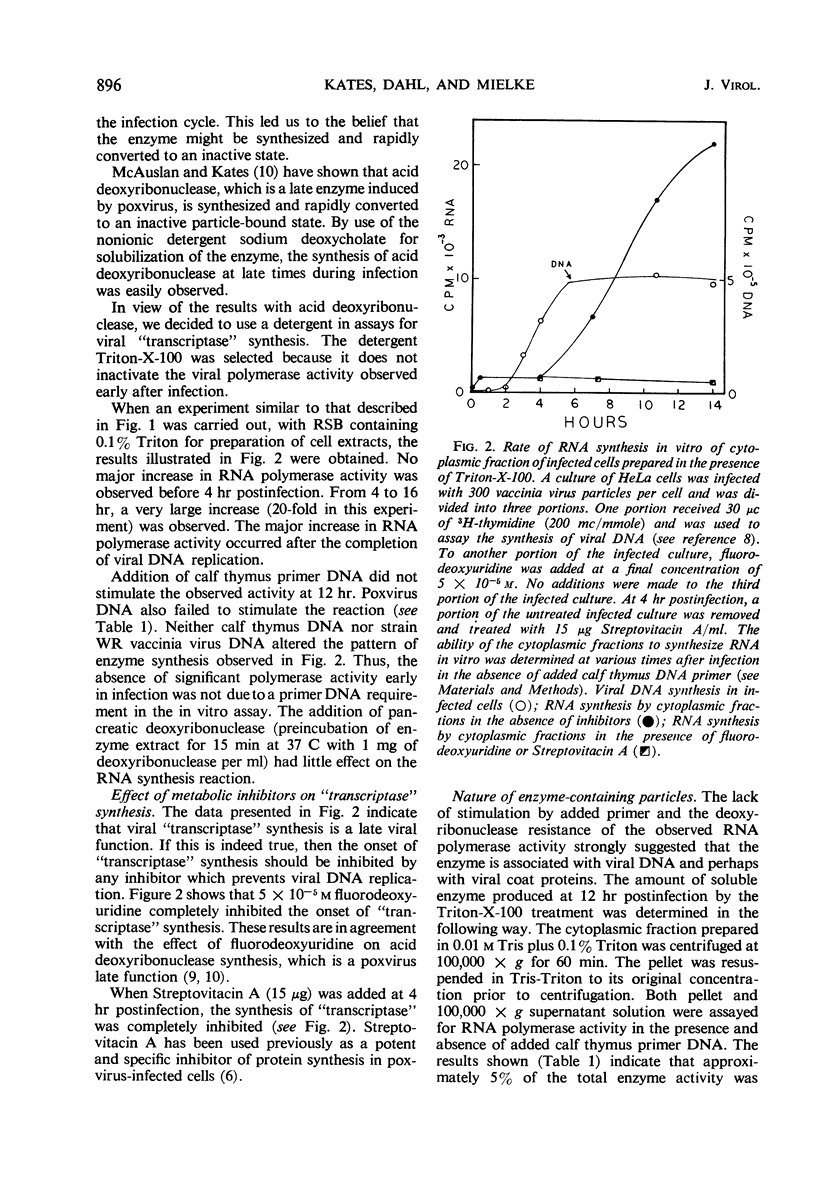
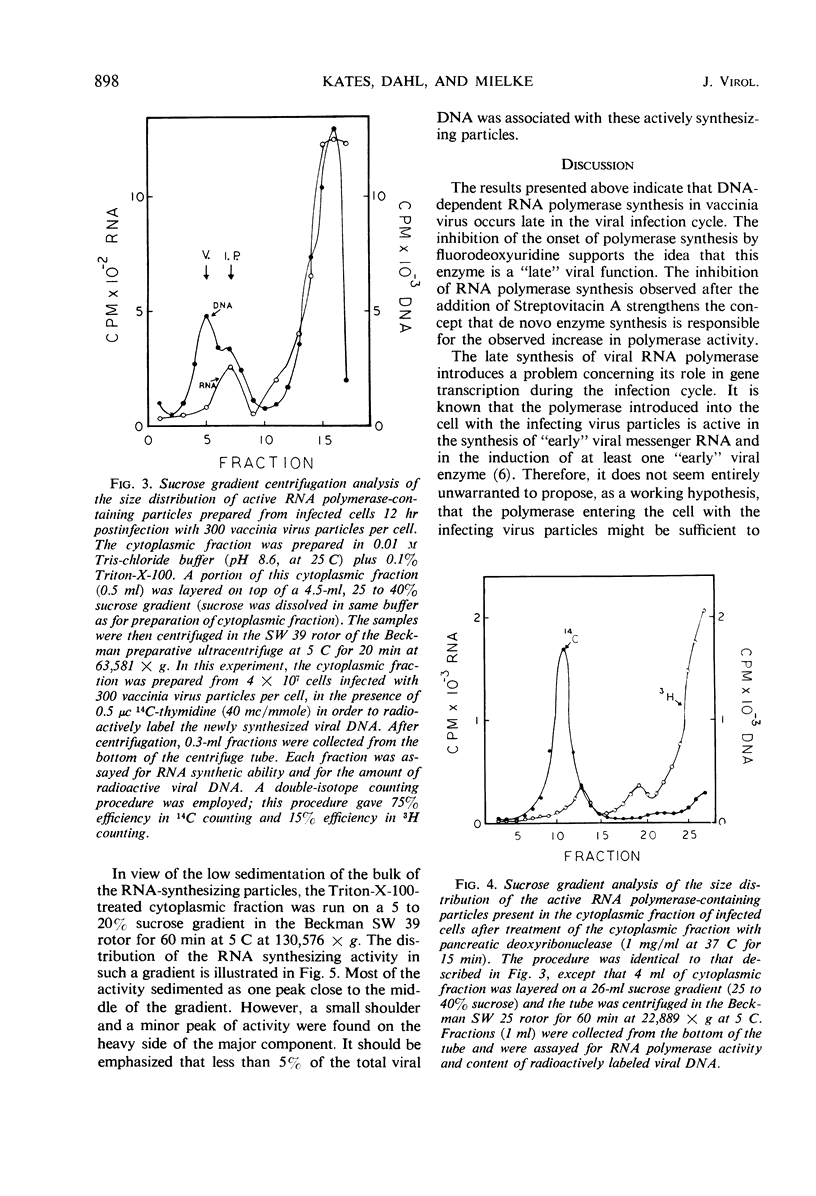
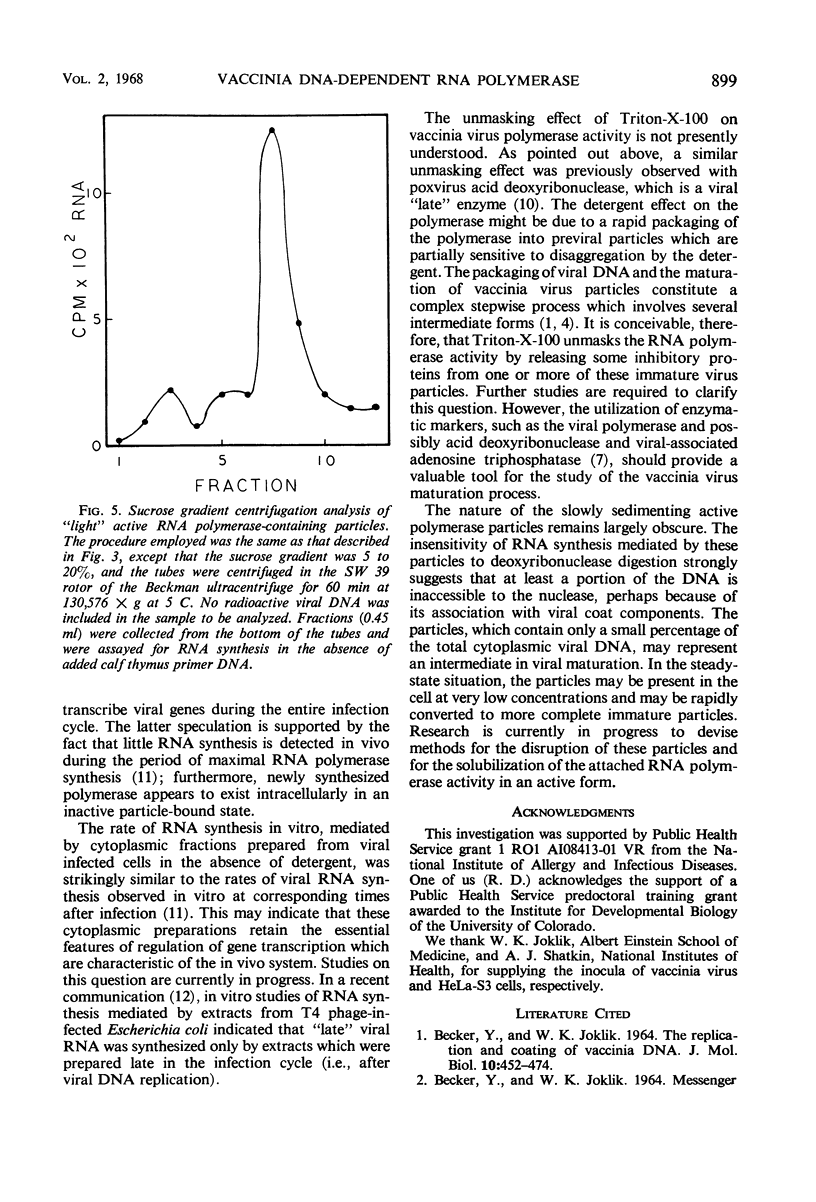
The time course of vaccinia deoxyribonucleic acid (DNA)-dependent ribonucleic acid (RNA) polymerase synthesis and its intracellular localization were studied with virus-infected HeLa cells. Viral RNA polymerase activity could be meassured shortly after viral infection in the cytoplasmic fraction of infected cells in vitro. However, unless the cells were broken in the presence of the nonionic detergent Triton-X-100, no significant synthesis of new RNA polymerase was detected during the viral growth cycle. When cells were broken in the presence of this detergent, extensive increases in viral RNA polymerase activity were observed late in the infection cycle. The onset of new RNA polymerase synthesis was dependent on prior viral DNA replication. Fluorodeoxyuridine (5 × 10−5m) prevented the onset of viral polymerase synthesis. Streptovitacin A, a specific and complete inhibitor of protein synthesis in HeLa cells, prevented the synthesis of RNA polymerase. Thus, the synthesis of RNA polymerase is a “late” function of the virus. The newly synthesized RNA polymerase activity was primarily bound to particles which sedimented during high-speed centrifugation. These particles have been characterized by sucrose gradient centrifugation. A major class of active RNA polymerase particles were considerably “lighter” than whole virus in sucrose gradients. These particles were entirely resistant to the action of added pancreatic deoxyribonuclease, and they were not stimulated by added calf thymus primer DNA. It is concluded that these particles are not active in RNA synthesis in vivo, and that activation occurs as a result of detergent treatment in vitro.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAIRNS J. The initiation of vaccinia infection. Virology. 1960 Jul;11:603–623. doi: 10.1016/0042-6822(60)90103-3. [DOI] [PubMed] [Google Scholar]
- DALES S. The uptake and development of vaccinia virus in strain L cells followed with labeled viral deoxyribonucleic acid. J Cell Biol. 1963 Jul;18:51–72. doi: 10.1083/jcb.18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOKLIK W. K., BECKER Y. THE REPLICATION AND COATING OF VACCINIA DNA. J Mol Biol. 1964 Dec;10:452–474. doi: 10.1016/s0022-2836(64)80066-8. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The preparation and characteristics of highly purified radioactively labelled poxvirus. Biochim Biophys Acta. 1962 Aug 20;61:290–301. doi: 10.1016/0926-6550(62)90091-9. [DOI] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Messenger RNA synthesis by a "coated" viral genome. Proc Natl Acad Sci U S A. 1967 Feb;57(2):314–320. doi: 10.1073/pnas.57.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Poxvirus DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Jul;58(1):134–141. doi: 10.1073/pnas.58.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Relationship between protein synthesis and viral deoxyribonucleic acid synthesis. J Virol. 1967 Feb;1(1):110–114. doi: 10.1128/jvi.1.1.110-114.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuslan B. R., Kates J. R. Regulation of virus-induced deoxyribonucleases. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1581–1587. doi: 10.1073/pnas.55.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K. I., Joklik W. K. Hybridization and sedimentation studies on "early" and "late" vaccinia messenger RNA. J Mol Biol. 1967 Aug 14;27(3):395–419. doi: 10.1016/0022-2836(67)90047-2. [DOI] [PubMed] [Google Scholar]
- Snyder L., Geiduschek E. P. In vitro synthesis of T4 late messenger RNA. Proc Natl Acad Sci U S A. 1968 Feb;59(2):459–466. doi: 10.1073/pnas.59.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]



