Abstract
Signaling pathways are often re-used during development in surprisingly different ways. The Hippo tumor suppressor pathway is best understood for its role in the control of growth. The Hippo pathway is also used in a very different context, in the Drosophila eye for the robust specification of R8 photoreceptor neuron subtypes, which complete their terminal differentiation by expressing light-sensing Rhodopsin (Rh) proteins. A double negative feedback loop between the Warts kinase of the Hippo pathway and the PH-domain growth regulator Melted regulates the choice between `pale' R8 (pR8) fate defined by Rh5 expression and `yellow' R8 (yR8) fate characterized by Rh6 expression. Here, we show that the gene encoding the homologue of human Nuclear respiratory factor 1, erect wing (ewg), is autonomously required to repress warts and to promote melted expression to specify pR8 subtype fate and induce Rh5 expression. ewg mutants express Rh6 in most R8s due to ectopic warts expression. Further, ewg is continuously required to maintain repression of Rh6 in pR8s in aging flies. Our work shows that Ewg is a critical factor for the stable down-regulation of Hippo pathway activity to determine neuronal subtype fates. Neural-enriched factors, such as Ewg, may generally contribute to the contextual re-use of signaling pathways in post-mitotic neurons.
Introduction
The Hippo signaling pathway controls growth through the regulation of cell proliferation and apoptosis (Pan, 2010). Warts is the effector kinase of the Hippo tumor suppressor pathway and, along with Hippo, Salvador, and Mats, forms the core of the Hippo pathway that coordinates proliferation and apoptosis in developing tissues (Halder and Johnson, 2011; Pan, 2010; Zhao et al., 2011). Other than its function in growth, the Hippo pathway also regulates non-growth processes, such as follicle cell maturation in the fly oocyte (Polesello and Tapon, 2007) and the establishment of dendritic tiling in larva sensory neurons (Emoto et al., 2006). The core components of the Hippo signaling pathway are also re-used post-mitotically for a dramatically different purpose—to specify terminal photoreceptor fates in the Drosophila retina (Mikeladze-Dvali et al., 2005).
The Drosophila retina contains about 800 repeating unit eyes called ommatidia, each with 8 photoreceptors (Hardie, 1985). There are two main subtypes of ommatidia which are defined by the expression of Rhodopsin proteins in the color-detecting inner photoreceptors, R7 and R8 (Rister et al., 2013). In `pale' (p) ommatidia, pR7 expresses UV-sensitive Rh3 and pR8s expresses blue-sensitive Rh5, whereas in `yellow' (y) ommatidia, yR7 expresses UV-sensitive Rh4 and yR8 expresses green-sensitive Rh6 (Fig. 1A) (Chou et al., 1996; Chou et al., 1999; Papatsenko et al., 1997; Pichaud et al., 1999). The y and p ommatidia are distributed in a stochastic manner in the retina, with roughly 65% y and 35% p ommatidia (Fig. 1C and H) (Fortini and Rubin, 1990; Franceschini et al., 1981).
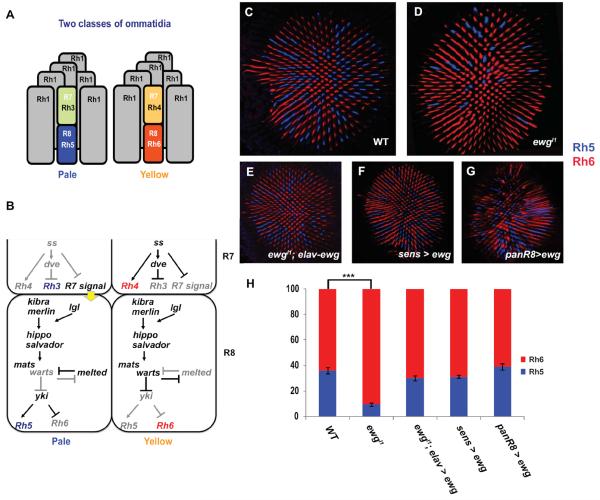
Fig. 1. Rh5 is expressed in fewer R8 cells in ewg mutants.
(A) Two subtypes of ommatidia: pale ommatidia have Rh3 in R7 paired with Rh5 in R8 whereas yellow ommatidia contain Rh4 in R7 and Rh6 in R8. Outer photoreceptors (R1–R6) all express Rh1. (B) Model showing how R7 is specified into pale and yellow R7s, and how the Hippo pathway and Melted regulate R8 subtype specification. Model modified from (Jukam and Desplan, 2011). (C–G) Confocal images of adult retina showing antibody staining of Rh5 (blue) and Rh6 (red) to mark the two R8 subtypes: (C) y1w67 retinas are used as wild-type controls with a ratio of Rh5 to Rh6 of 35:65. (D) ewgl1 mutant retina showing a lower number of Rh5 expressing R8. The ratio of Rh5:Rh6 is 10:90. (E) Using elav-Gal4 to drive expression of the ewg-cDNA in ewgl1 mutant restores the ratio of Rh5:Rh6 to wild type. (F) Overexpression of Ewg using senseless-Gal4 shows no significant changes in the Rh5:Rh6 ratio. (G) panR8>ewg-cDNA retinas have a wild-type Rh5:Rh6 ratio. (H) Quantification of Rh5 and Rh6 in ewg mutants. The graph shows the percentage of Rh5 (blue) and Rh6 (red) in R8. An unpaired t-test was performed to calculate the difference in mean % Rh5. Wild-type: n=10 retinas, N=3675 ommatidia; ewgl1 mutant: n=11 retinas, N=3214 ommatidia; ewgl1, elav>ewg-cDNA: n=11, N=3321 ommatidia; all other genotypes throughout paper: n≥4, N≥ 800 ommatidia. *** p<0.001, error bars are mean ± one standard deviation (s.d.).
The ommatidial subtype decision is made randomly in R7s and then imposed onto R8s. Stochastic expression of Spineless (Ss), a PAS-bHLH transcription factor determines the random mosaic pattern of ommatidial subtypes. In one random subset of R7s, Ss is expressed and induces yR7 fate, including Rh4 expression. yR8 fate, including Rh6 expression is specified by default. In the complementary R7s that lack Ss, pR7 fate is induced including Rh3 expression. (Johnston et al., 2011; Thanawala et al., 2013; Wernet et al., 2006) (Fig 1B). An unknown signal from R7 is then transduced into a stable fate decision in R8s that become yR8 and express Rh6. This decision requires a feedback loop between the Hippo pathway and the Melted growth regulator (Fig.1B). The activity of the Hippo pathway during growth is regulated by multiple inputs to ensure correct proliferation and cell death (Halder and Johnson, 2011). However, only a subset of these upstream regulators of the Hippo pathway are involved in the control of R8 fate. Merlin, Kibra, and Lethal (2) giant larvae (Lgl), appear to constitutively activate the pathway to specify yR8 subtype (Jukam and Desplan, 2011). In pR8s, Warts is repressed while Melted is expressed, yielding expression of Rh5 and repression of Rh6. In yR8s, Warts is expressed and Melted is repressed, promoting expression of Rh6 and repression of Rh5. Mutual repression of Warts and Melted forms a bi-stable double-negative feedback loop for R8 subtype specification (Mikeladze-Dvali et al., 2005) (Fig. 1B).
Although the Hippo pathway has been extensively studied for its role in growth, the factors that determine its various roles in different developmental processes are not well known. Here, we show that erect wing (ewg), which encodes a neuronal transcription factor, is required to antagonize the Hippo pathway in the context of R8 subtype specification. ewg functions autonomously in R8 for terminal differentiation of R8 subtypes. It acts upstream of the Warts-Melted feedback loop to promote pR8 fate and Rh5 expression, and to prevent yR8 fate and Rh6 expression. Moreover, Ewg is required to maintain the repression of the yR8/Rh6 fate in adult R8 photoreceptor neurons. Thus, the input from ewg to down-regulate the Hippo pathway is required to specify and maintain pR8 fate. Such neuron-restricted regulation may help to repurpose the pathway for non-growth functions.
Results
ewg regulates mutually exclusive R8 Rhodopsin expression
We identified a role for ewg in Rhodopsin regulation in an RNAi screen for transcription factors whose knockdown caused changes in Rhodopsin expression. RNAi knockdown of ewg led to ectopic Rh1-GFP expression in inner photoreceptors, a phenotype that will be described elsewhere (H-Y. H. & C.D., in preparation) (Suppl Fig. 1A). However, we also found that the proportion of R8s expressing Rh5 was dramatically lower in eyes expressing ewg-RNAi under the control of two strong eye-specific drivers, the eyeless (ey) and lGMR-Gal4 driver (3% as compared to 17% in RNAi controls; p ≤0.001) (Suppl Fig. S1B–D).
To further investigate the role played by ewg in the regulation of R8 rhodopsin expression, we used the Flp recombinase to excise an elav>ewg-cDNA rescue cassette in ewg11 null flies in order to generate and examine mutant tissue (Haussmann et al., In ewgl1 whole mutant retinas, the proportion of Rh5 was dramatically lower than in the wild-type, with only 10% of R8s expressing Rh5 as compared to 35% in the control (p ≤0.0001) (Fig. 1C, D and H). When the elav>ewg-cDNA cassette was not excised in ewgl1 homozygous mutant flies, we observed a wild-type Rh5:Rh6 ratio, showing that the R8 Rhodopsin phenotype was specifically due to loss of ewg (Fig. 1 E and H). Thus, ewg is required for the normal proportion of Rh5 and Rh6-expressing R8 photoreceptors.
Ewg acts autonomously for the regulation of Rhodopsin expression in R8
We next tested whether over-expression of Ewg was sufficient to affect Rh5 and Rh6 expression. We mis-expressed Ewg using senseless (sens)-Gal4 that drives expression in all R8s from imaginal discs to adulthood (Pepple et al., 2008). The Rh5:Rh6 ratio in sens>ewg flies was not different from wild-type (Fig. 1F and H). We also over-expressed Ewg starting at late pupal stages and continuing through adulthood using panR8-Gal4 (a combination of two drivers, Rh5-Gal4 and Rh6-Gal4); the Rh5:Rh6 ratio in panR8>ewg retinas remained similar to wild type (Fig. 1G and H). Our data suggest that ewg functions permissively for pR8 subtype specification. However, we could not rule out the possibility that the level of overexpressed ewg via the Gal4-UAS system was only marginally higher than endogenous ewg expression levels, in which case overexpressed ewg might not be sufficient to force all R8s to adapt pR8 fate.
To investigate the cellular focus of Ewg function for R8 subtype specification, we examined Ewg expression. Using an anti-Ewg antibody (gift from M. Soller, University of Birmingham), we detected Ewg in all photoreceptor nuclei of the eye disc, starting at the 3rd instar larval stage and continuing throughout pupation and adulthood (Fig. 2A–E). Because Ewg was expressed in all photoreceptors, we tested whether loss of ewg caused defects in photoreceptors other than R8. However, in ewg whole mutant retinas (ewg11, elav<ewg-cDNA>Gal4; ey-flp), R7 Rhodopsins (Rh3 and Rh4) were expressed normally, suggesting that the ewg R8 Rhodopsin defect is not due to mis-specification of R7 subtypes and subsequent mis-regulation of R7 signaling to R8 (Suppl. Fig. S1E-E'). We recombined the ewgl1 allele on an FRT chromosome to generate mutant clones in which Ewg expression was lost, which allowed us to compare wild type and mutant tissue in the same eye (Suppl. Fig. S1F-F') and examine other genes important for photoreceptor specification. In ewgl1 mutant clones, expression of Spalt that specifies inner photoreceptors (Mollereau et al., 2001), and of Prospero that specifies R7 fate (Cook et al., 2003) were both normal (Suppl. Fig. S1G–H'), suggesting that ewg is not required for general inner photoreceptor specification or general R7 fate.
Fig. 2. ewg is expressed in all photoreceptors and functions autonomously to specify R8 subtypes.
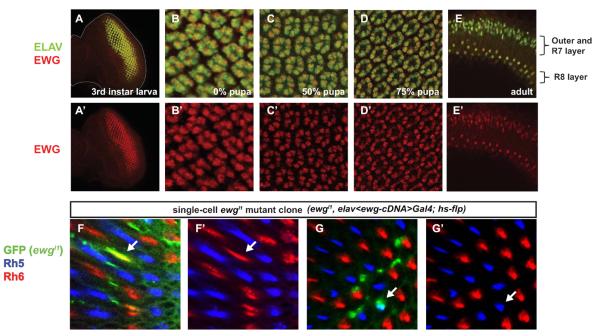
(A–E) Ewg (red) is co-expressed with Elav (green), a neuronal marker, in all photoreceptors starting from 3rd instar larval stage and remains in pupal and adult retinas. (A'–E') Ewg (red) channel only. (A) y1w67eye imaginal disc at the 3rd instar stage. Ewg is co-expressed with Elav only in differentiated photoreceptors. Expression of Ewg remains at 0% (B), 50% (C), 75% pupation (D) and throughout adult stages (E). Ewg is visible both in nuclei of outer and R7 photoreceptors (outer layer) as well as in R8 in the lower nuclear layer. (F) Most ewg R8 mutant cells (arrow) in clones marked by GFP (green) express Rh6 (red). (F') Red (Rh6) and blue (Rh5) channel only. Among 60 mutant R8s, 56 expressed Rh6. (G) One ewg mutant R8 (arrow) marked by GFP (green) expresses Rh5 (blue). (G') Red (Rh6) and blue (Rh5) channel only.
To directly test whether ewg functions autonomously in R8 to control Rh5 and Rh6, we removed ewg function in clones by flipping out the elav>ewg-cDNA rescue construct with hs-flp (see Methods) at 25–50% pupation, i.e. after the last cell division and before R8 subtypes are specified. Of 60 single cell R8 clones, only four expressed Rh5 (arrows in Fig. 2G-G'), whereas 56 expressed Rh6 (arrows in Fig. 2F-F'). The Rh5:Rh6 ratio of 7%:93% in R8 mutant clones is similar to the Rh5:Rh6 ratio of 10%:90% found in whole mutant retinas. This indicates that, although ewg is expressed in all photoreceptors, it functions autonomously in R8 to regulate Rhodopsin expression.
The transcriptional activation domain of Ewg is required for the regulation of R8 Rhodopsins
Different Ewg isoforms have context specific roles in their requirement for viability or synaptic growth (Haussmann and Soller, 2010). Specifically, the `D' exon that encodes a domain missing from the Ewg human homologue Nuclear respiratory factor 1, and the `J' exon that encodes a transcriptional activation domain conserved in humans (Haussmann and Soller, 2010), are included or excluded from different isoforms. To understand which Ewg isoform is responsible for regulating R8 Rhodopsin we tested the ability of four different Ewg isoforms to rescue ewgl1 null mutants (Haussmann and Soller, 2010) (Fig. 3A). The full-length cDNA (SC3 isoform) that rescues viability and synaptic growth in ewg null alleles (Haussmann and Soller, 2010) also fully rescued the ewg R8 defects (Fig. 1E and H). The ΔDJ isoform (Fig. 3A) has the weakest ability to rescue viability and synaptic growth (Haussmann and Soller, 2010) and did not rescue R8 Rhodopsin expression defects (14% Rh5 and 86% Rh6 in R8) (Fig. 3B and E). The ΔD isoform (Fig. 3A) rescued the viability of ewg mutants as well as the Rh5 and Rh6 ratio (Fig. 3C and E). The ΔJ isoform, which can restore viability of ewg mutants, lacks the last exon. However, the ΔJ isoform did not rescue the Rh5:Rh6 phenotype of ewgl1 mutants (p<0.0001) (Fig. 3D and E). Together, these data show that exon J, which contains the activation domain conserved to humans (Haussmann and Soller, 2010), is required for R8 terminal differentiation, whereas exon D is dispensable. As exon J is required for R8 fate regulation while both Exon D and J are required for synaptic growth. Ewg appears to have distinct protein domain requirements for different neuronal-specific functions.
Fig. 3. The Ewg activation domain is required for R8 subtype specification.
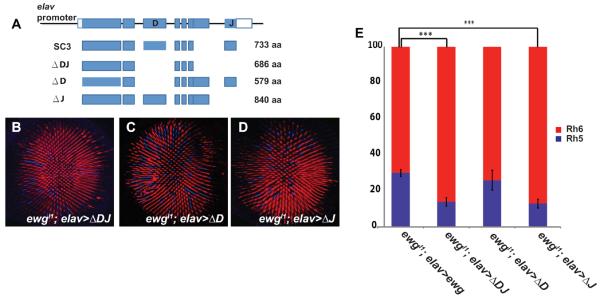
(A) Schematic of ewg genomic organization and four isoforms of Ewg. Exons are indicated in blue. Modified from (Haussmann and Soller, 2010). (B) Expression of the ΔDJ isoform fails to restore the normal ratio of Rh5 (blue) to Rh6 (red). (C) Expression of the ΔD isoform restores a wild type Rh5:Rh6 ratio. (D) Expression of the ΔJ isoform is not able to rescue the ewg mutant phenotype. (E) Quantification of Rh5 and Rh6 rescue with different Ewg isoforms. An unpaired t-test was performed to calculate the difference in mean % Rh5. ewgl1; elav-ewg-cDNA, n=11 retinas, N=3321 ommatidia; ewgl1; elav-ΔDJ, n=5 retinas, N=1656 ommatidia; ewgl1; elav-ΔD, n=6 retinas, N=2096 ommatidia; ewgl1; elav-ΔJ, n=6 retinas, N= 2103 ommatidia. *** p<0.001, error bars are mean ± one standard deviation (s.d.)
ewg is required to maintain pR8 subtype fate
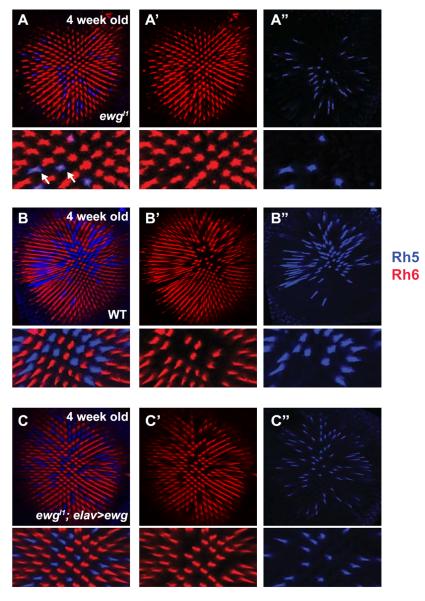
Young adult ewg mutant flies (0–7 days post-eclosion) exhibited a strong reduction in the proportion of R8s that expressed Rh5 (from 35% to 10%), but these remainging pR8s did not contain Rh6. However, in two-week old ewgl1 mutant flies, Rh6 became de-repressed in the remaining pR8s as low levels of Rh6 protein could be observed with In 4 week old ewg mutant flies, Rh6 was expressed in all R8 cells (Fig. 4A-A”), leading to co-expression with Rh5 in all Rh5-expressing cells (10% of R8s), a phenotype never observed in aged wild type flies (Fig. 4B-B”). Restoring ewg function using elav-Gal4 driving ewg-cDNA in ewgl1 mutants rescued the wild-type ratio in old adults (Fig. 4 C-C”). Therefore, ewg appears to have an adult function to specifically maintain repression of Rh6 in pR8s, in addition to its early role in the establishment of pR8 cells. Furthermore, as the Hippo pathway and Rh6 are required late to maintain yR8 fate (Jukam and Desplan, 2011; Vasiliauskas et al., 2011), this late requirement for Ewg in pR8s indicates that all R8s must actively maintain mutually exclusive Rh5 and Rh6 expression.
Fig. 4. ewg functions in R8 subtype specification and is required to maintain repression of Rh6 in pR8 photoreceptors.
(A–C) Confocal images of 4 weeks old adult retina stained with antibodies against Rh5 (blue) and Rh6 (red) with zoomed-in images. (A) ewgl1 mutant retina shows expanded expression of Rh6 in all R8. Arrows point R8 cells co-expressing Rh5 and Rh6. (A') Rh6 channel only. (A”) Rh5 channel only. Note the co-expression of Rh5 and Rh6 in the bottom panels of A and A'. (B) A four week old wild-type retina shows no expansion of Rh6 expression, and maintains mutually exclusive Rh5 and Rh6 expression. (B') Rh6 channel only. (B”) Rh5 channel only. (C) ewgl1 mutant rescued with ewg cDNA shows no expansion of Rh6 in 4 weeks old flies. (C') Rh6 channel only. (C”) Rh5 channel only.
ewg determines pR8 fate by regulating melt and warts expression
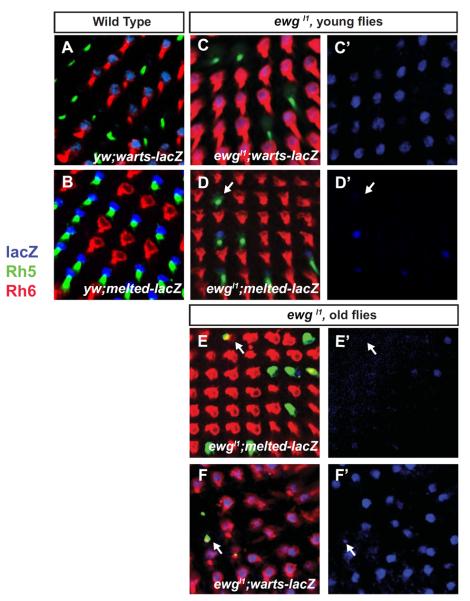
p and y R8 fates are specified by the double-negative transcriptional feedback loop between Warts and Melted (Fig. 1B) (Mikeladze-Dvali et al., 2005). Therefore, ewg might control Rh5 and Rh6 by controlling the R8 subtype fate mechanism. We therefore analyzed expression of warts and melted using transcriptional reporters. In wild-type control retinas, warts-lacZ was perfectly co-expressed with Rh6 to specify yR8 (Fig. 5A). In ewg mutants, the frequency of R8s expressing warts-lacZ increased, but the warts reporter was always co-expressed with Rh6 (Fig. 5C-C'), suggesting that ewg is required to repress warts expression to establish pR8 fate and prevent yR8 fate. melted-lacZ is normally found in pR8s and is always co-expressed with Rh5 (Fig. 5B). The proportion of R8s expressing melted-lacZ was significantly decreased in ewg mutants and paralleled the decrease in the proportion of Rh5-expressing R8s, supporting the notion that ectopic Rh6-expressing R8s had adopted the complete yR8 fate. However, among the Rh5-expressing R8s in ewg mutants, only a subset expressed melted-lacZ (Fig. 5D-D'), suggesting that these Rh5-expressing pR8 cells were in the process of switching fate to becoming yR8. In this case, we would expect melted-lacZ to be completely lost in old flies in which Rh5 and Rh6 were co-expressed in pR8s. Indeed, in aged flies melted-lacZ was lost in R8s that still expressed Rh5, (Fig. 5E-E') whereas warts-lacZ was present in almost all R8s (Fig. 5F-F'). Since the expression of warts and melted is mutually exclusive, the progressive loss of melted and gain of warts is likely responsible for the co-expression of Rh5 and Rh6 in older ewg mutant flies, with Rh5 perduring in R8s that have switched fate late. This indicates that ewg is required to initiate and to maintain expression of melted and repression of warts in adults in order to promote pR8 fate and Rh5 expression.
Fig. 5. ewg is required to specify the pR8 subtype fate.
In controls (A) warts-lacZ (antibody to β–galactosidase, blue) is expressed only in yR8s, marked by Rh6 (red) while pR8s are marked by Rh5 (green) while (B) melted-lacZ expression (blue) in pR8s (labeled with Rh5) is mutually exclusive with warts-lacZ expression in yR8s (marked by Rh6). (C–F) ewgl1 mutant retinas. (C) R8s co-express Rh6 and warts-lacZ. (C') warts-lacZ channel only. (D) Not all R8s expressing Rh5 contain melted-lacZ. The white arrow marks a photoreceptor expressing Rh5 that lacks melted-lacZ expression. (D') melted-lacZ channel only. (E–F) Two weeks old ewgl1 mutant flies. (E) Small amounts of Rh6 are co-expressed in R8s that express Rh5. In those R8s (white arrow), melted-lacZ expression is lost (E') melted-lacZ channel only. (F) warts-lacZ expands in R8s that are co-expressed with Rh5 and Rh6 (arrows). (F') warts-lacZ channel only.
ewg acts genetically upstream of warts and melted
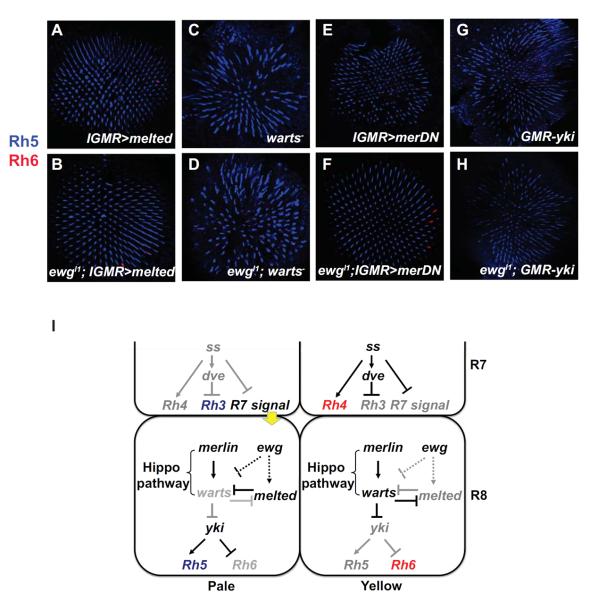
We next performed genetic epistasis tests to determine whether the loss of melted or de-repression of warts caused the R8 Rhodopsin phenotype in ewg mutants. Mis-expression of melted in all photoreceptors with lGMR-Gal4 represses warts transcription and induces Rh5 in all R8s (Mikeladze-Dvali et al., 2005). melted mis-expression (lGMR-Gal4, UAS-melted) suppressed the ewg mutant phenotype, and almost all R8s (>99 %) expressed Rh5 (Fig. 6A–B). Thus, ewg acts genetically upstream of melted to specify the pR8 subtype as melted induces pR8 fate and Rh5 expression in ewg mutants.
Fig. 6. ewg acts genetically upstream of warts and melted.
(A–H) Confocal images of adult retinas stained with antibodies for Rh5 (blue) and Rh6 (red). (A) Over-expression of melted driven by IGMR-Gal4 results in Rh5 expressed in all R8s. (B) In ewgl1; IGMR>melted flies, overexpression of melted suppresses the ewgl1 phenotype, leading to Rh5 expression in all R8s. (C) In a warts mutant, all R8s are converted to pR8 expressing Rh5. (D) ewgl1; warts− double mutants show Rh5 expression in all R8s. (E) Overexpression of a dominant negative form of Merlin (merDN) in all PRs leads to expression of Rh5 in all R8s. (F) A merDN;ewg double mutants show Rh5 expression in all R8s. (G) Over-expression of yki driven by GMR causes Rh5 expression in all R8s. (H) Over-expression of yki in ewgl1mutants suppresses the ewg phenotype and leads to Rh5 expression in all R8s. (I) Model of ewg interaction with the Warts-Melted feedback loop. ewg acts upstream of melted to promote its expression, allowing expression of Rh5 in pR8s. ewg might also be required to repress the Hippo pathway, leading to Rh5 expression.
In warts mutants, all yR8s are converted into pR8s and express Rh5, a phenotype opposite to that of ewg mutants (Fig. 6C). warts mutants also suppressed the ewg mutant phenotype, as ewg;warts double mutants displayed expression of Rh5 in all R8s (Fig. Thus, warts is required to induce yR8 fate in ewg mutants, which suggests that ewg also functions upstream of warts to specify pR8 subtypes.
merlin encodes a FERM-domain containing protein that acts upstream of the Hippo pathway to constitutively promote Warts activity in R8 (Jukam and Desplan, 2011). Loss of merlin resulted in Rh5 expression in most R8s, similar to the warts mutant phenotype (Fig. 6E). When merlin was removed from ewg mutants, almost all R8s expressed Rh5 (Fig. 6F) suggesting that merlin is required to activate the Hippo pathway and Warts to induce yR8 fate in ewg mutants.
Activation of the Hippo pathway leads to the phosphorylation of Warts that negatively regulates the Yorkie (Yki) oncogene (Harvey and Tapon, 2007; Huang et al., 2005). As in growth, Yki is a co-transcriptional regulator that acts with the DNA binding factor Scalloped to activate Rh5 and repress Rh6 (D.J. et al., submitted). We tested whether ewg also acts upstream of yki to regulate Rhodopsin expression. Over-expression of yki caused all R8s to convert to pR8 with Rh5 expression (Fig. 6G). In ewg mutants, overexpression of yki in all PRs with GMR-yki led to Rh5 expression in all R8s (Fig. 6H), indicating that ewg acts upstream of yki to determine the pR8 fate.
These data suggest that ewg is required genetically upstream to activate melted and repress warts to induce pR8 fate (Fig. 6I).
Discussion
The proper specification of photoreceptor subtypes including Rhodopsin expression is critical for proper color-detection and related behavior (Yamaguchi et al., 2010). We found that the neural-specific transcription factor Ewg contributes to R8 subtype specification. In the absence of ewg, most pR8s are mis-specified as yR8s. Our analysis shows that ewg acts autonomously in R8 to regulate the Warts-Melted feedback loop controlling subtype fate. Ewg appears to regulate pR8 fate by promoting melted expression and warts repression, suggesting that ewg is necessary to promote the complete pR8 fate rather than directly regulating Rh5 expression. Furthermore, epistasis experiments with merlin, warts and melted place ewg genetically upstream of the Warts-Melted feedback loop.
In addition to its role in R8 subtype establishment, ewg also functions in subtype maintenance in adult flies, as Rh6 is de-repressed in pR8 and is co-expressed with Rh5 in 4-week old ewg mutant flies. Expression of Rh5 still remains in old pR8s. ewg mutants also progressively lose melted expression and gain warts expression in R8s. The gradual disappearance of melted in old ewg mutant flies likely allowsg expression of warts and reactivation of Rh6. This represents another genetic program required to maintain gene expression in differentiated sensory neuron subtypes of adult animals (Jukam and Desplan, 2011; Vasiliauskas et al., 2011). Previous studies have shown that the Hippo pathway is required both to specify and to maintain yR8 subtypes. Removing merlin after eclosion results in de-repression of Rh5 in all yR8s and co-expression with Rh6 (Jukam and Desplan, 2011), a phenotype opposite to that of old ewg mutant flies. Furthermore, an active Rh6 protein is required to repress Rh5 to maintain its exclusive expression in yR8s, as loss of Rh6 results in the expansion of Rh5 to all R8s in old flies (Vasiliauskas et al., 2011). Our results are consistent with the model that establishment and maintenance programs are coupled by using the same genes, resulting in efficient long-term gene regulation.
How does the Ewg protein function in R8 subtype specification? Ewg has the same consensus DNA binding site as Nuclear respiratory factor 1 (Fazio et al., 2001). However, we could not find motifs matching the Ewg consensus sequence in the regulatory regions of melted and warts. The diverse transcriptional targets of Ewg in various organisms also prevent a clear assignment of a conserved Ewg protein function. For example, Nuclear respiratory factor 1 acts as a transcriptional activator in the regulation of expression of cytochrome C and mitochondrial genes (Efiok et al., 1994; Evans and Scarpulla, 1989). However, the sea urchin Ewg homolog, P3A2, limits expression of the cytoskeletal cyIIIA actin gene (Calzone et al., 1991). As human Nuclear respiratory factor 1 functions as an activator while sea urchin P3A2 negatively regulates cyIIIA (Hough-Evans et al., 1990), Ewg therefore appears to act either as an activator or as a repressor, consistent with the presence of a C-terminal activation domain and an N-terminal repression domain identified in Drosophila (Fazio et al., 2001). Although Ewg functions upstream of the warts/melted loop, neither warts nor melted contain canonical Ewg binding motifs, suggesting that Ewg likely regulates these genes indirectly.
Several other genes are expressed in all photoreceptors and act as permissive factors to regulate specific Rhodopsins and photoreceptor subtypes (Rister et al., 2013). For example, Orthodenticle (Otd), the fly homolog of vertebrate Crx and Otx proteins (Furukawa et al., 1997), is a K50 homeoprotein expressed in all photoreceptors. Loss of otd results in the loss of Rh3 and Rh5 in p ommatidia and de-repression of Rh6 in outer photoreceptors (Tahayato et al., 2003). However, like Ewg, Otd is not sufficient to activate these genes when mis-expressed. Otd is therefore a permissive factor that likely acts with co-factors to specify their activating or repressive functions in particular photoreceptors (Rister and Desplan, 2011; Tahayato et al., 2003). For Rh3, restricted Rh expression is achieved by repression by Dve, Senseless and Prospero (Johnston et al., 2011). The same principle might apply for Ewg: since Ewg is expressed in all photoreceptors, it might recruit co-factors specific to pR8 to promote the expression of melted or to negatively regulate the Hippo pathway. Recently, ewg was shown to be required for the recruitment of the cell specific Armadillo-TCF adaptor, Earthbound 1 (Ebd1), to specific chromatin sites to activate a subset of Wingless target genes (Xin et 2011). Ebd1 shares similar polytene chromatin binding sites with Ewg (Benchabane et 2011; Xin et al., 2011). It is possible that Ewg recruits a specific co-factor such as Ebd1 to function in pR8. However, we did not observe a decrease in Rh5 expression in ebd1 mutant retinas, suggesting that Ewg acts differently in the retina. Nevertheless, it is likely that another subtype specific co-factor functions with Ewg to specify pR8 fate.
In conclusion, ewg is autonomously required to specify the pR8 subtype and induce Rh5 expression. ewg appears to act upstream of the Hippo pathway, of Melted, and the feedback loops to determine pR8 fate. Therefore, a neuronal specific transcription factor, Ewg, contributes to the regulation of the Hippo pathway either directly or indirectly through regulation of melted to specify the fate of R8 photoreceptors.
Material and Methods
Drosophila Stocks and Genetics
Mutant alleles and other fly stocks used in this study include: ewgl1 with elav-FRT-ewg-cDNA-FRT rescue cassette was a generous gift of the Soller lab (Haussmann et al., 2008) as were elav>EwgΔJ, elav>EwgΔJ, elav>EwgΔD flies (Haussmann and Soller, 2010). Other flies stocks include: Rh1-GFP (Pichaud and Desplan, 2001), UAS-merDN (LaJeunesse et al., 1998), wartsP1, warts-lacZ (Xu et al., 1995), UAS-melt, melt-lacZ, panR8-Gal4 (Mikeladze-Dvali et al., 2005), UAS-Dicer2, ey-Gal4+lGMR-Gal4 (Dietzl et al., 2007), GMR-Yki (Huang et al., 2005). y1w67, lGMR-Gal4 (Wernet et al., 2003). ey-FLP, ey3.5-FLP, UAS-CD8:GFP, UAS-GFP, UAS-FLP, hs-flp were obtained from the Bloomington Drosophila Stock Center. The UAS-RNAi stocks used in the RNAi screen were obtained from the Vienna Drosophila RNAi Center (VDRC); UAS-ewg-RNAi was ID# 4559.
Flies were raised on cornmeal-agar-molasses-yeast medium at 25°C. y1w67 flies were used as wild-type controls for Rhodopsin expression. The RNAi screen included ~1700 UAS-RNAi lines which targeted around ~900 transcription factors. The Gal4 driver line contained both eyeless-Gal4 and lGMR-Gal4 drivers recombined on chromosome 2. eyeless-Gal4 is expressed early in the entire eye disc, whereas lGMR-Gal4 is expressed only after the morphogenetic furrow and maintained in adults. Together, these two drivers induce RNAi expression in the whole eye from the time the eye is specified until adulthood. In addition, the RNAi driver stock carries UAS-Dicer2 to enhance the efficiency of generating small interfering RNA. Rh1-GFP, which is expressed only in outer photoreceptors, was used as a readout in the screen. UAS-RNAi lines were crossed to the driver line (eyeless>Gal4, lGMR>Gal4; UAS-Dicer2; Rh1-GFP) at 25°C. The F1 progeny were analyzed under water immersion for a change in rh1-GFP reporter expression (Pichaud and Desplan, 2001).
The ewgl1 mutant allele containing the elav<ewg-cDNA>Gal4 rescue cassette on the same chromosome was used to generate ewg mutant clones (Haussmann et al., 2008). The rescue constructs contains an elav promoter driving the ewg-cDNA flanked by FRTs at each side and followed by Gal4. ey-flp was used to remove ewg specifically in the eye in order to avoid embryonic lethality and generate whole mutant eyes. Mutant clones affecting R8 were generated by using hs-flp in ewgl1 flies containing the ewg-cDNA rescue cassette and UAS-CD8:GFP. These flies were raised at 25°C and were shifted to 37°C for 40 min at 0–25% pupation, when all the photoreceptors have been recruited, but rhodopsins are not yet expressed. After heat shock, the pupae were moved back to 25°C and raised to adulthood. Mutant clones were marked by GFP driven by Gal4 that was activated after removal of the ewg-cDNA. ewgl1 was also recombined with FRT19A to generate mutant clones.
In the ewg isoform rescue experiments, ewgl1/FM7 females were crossed with males carrying elav-EwgΔDJ, elav-EwgΔJ or elav-EwgΔD provided by M. Soller, University of Birmingham.
Immunostaining and statistics
Dissection of adult retina was performed as described (Hsiao et al., 2012). Antibodies and dilutions were as follows: mouse anti-Rh1 (1:10, DSHB) mouse anti-Rh3 (1:100, gift form S. Britt, University of Colorado), rabbit anti-Rh4 (1:100, gift from C. Zuker, Columbia University), mouse anti-Rh5 (1:200, gift from S. Britt), rabbit anti-Rh6 (1:10,000), rabbit anti-Ewg (1:500, gift from M. Soller, University of Birmingham), goat anti-βgal (1:5000, Biogenesis), sheep anti-GFP (1:1000, AbD Serotec), mouse anti-Elav (1:40, DSHB), rabbit anti-Spalt (1:100)(Barrio et al., 1999), mouse anti-Prospero (1:10, DSHB). All secondary antibodies were Alexa Flour (488, 555, or 647)-conjugated made in donkey (1:800, Molecular Probes).
Fluorescent images were taken with a Leica SP5 confocal laser scanning microscope and processed with Leica AF-Lite software. The number of R8 cells that expressed Rh5, Rh6, or both, was counted in a single focal plane of confocal images. The statistical comparison measuring the Rh5% between different genotypes was performed with a two-tailed unpaired t-test.
Supplementary Material
Supplemental Figure 1: ewg is not involved in general photoreceptor or R7 specification. (A) GFP image was taken in live adult flies under water immersion with a Nikon Plan Fluor 40X objective. ewg RNAi mutant retina showed ectopic Rh1-GFP expression (arrows). (B–C) Confocal images of adult retinas stained with antibody against Rh5 and Rh6 (red). (B) Retinas expressing ewg-RNAi exhibit Rh6 in most R8s while a few R8s co-express Rh5. (C) The ey-Gal4+lGMR-Gal4 driver line shows slightly elevated Rh6 expression. (D) Quantification of Rh5 and Rh6 in R8s. An unpaired t-test was performed to calculate the difference in mean % Rh5. Wild-type: n=10 retinas, N=3675 ommatidia; ewg RNAi mutant: n=4 retinas, N=874 ommatidia; ewg RNAi driver (eyeless>Gal4, lGMR>Gal4; UAS-Dicer2; Rh1-GFP): n=4 retinas, N=920 ommatidia *** p<0.001, error bars are mean ± one standard deviation (s.d.). (E) Expression of Rh3 (blue) and Rh4 (red) in R7 cells. The Rh3:Rh4 ratio is roughly 35:65 in wild type flies. (E') ewgl1 mutant retina shows normal Rh3 and Rh4 expression. (F) ewg mutant clones marked by loss of GFP (green). An anti-Ewg antibody (red) was used to show loss of Ewg in the mutant clone (indicated with a white line). (F') Ewg channel only. (G) In an ewg mutant clone, Spalt expression (red) is normal. (G') Spalt channel only. (H) Prospero is only expressed in R7s (arrow) in the wild type and this expression (blue) is unaffected in an ewgl1 mutant clone. (H') Prospero channel only.
Highlights
-
-
erect wing (ewg) controls the fate of photoreceptors in the Drosophila retina.
-
-
ewg is expressed in all photoreceptors: It promotes Rh5 and represses Rh6.
-
-
ewg promotes melted expression and represses warts to specify the pR8 subtype fate.
-
-
ewg is continuously required to maintain repression of Rh6 in pR8s.
-
-
ewg down-regulates Hippo pathway activity to determine neuronal subtype fates.
Acknowledgements
We thank Zhenqing Chen, Xin Li, Jens Rister and Nina Vogt for helpful discussion and comments on the manuscript. We also thank Dr. Matthias Soller for providing us with numerous ewg reagents, including the rescue construct on an ewg mutant chromosome, antibodies, and the UAS-Ewg isoform transgenes. This work was supported by grant from NIH EY13012 to C. D.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barrio R, de Celis JF, Bolshakov S, Kafatos FC. Identification of regulatory regions driving the expression of the Drosophila spalt complex at different developmental stages. Developmental biology. 1999;215:33–47. doi: 10.1006/dbio.1999.9434. [DOI] [PubMed] [Google Scholar]
- Benchabane H, Xin N, Tian A, Hafler BP, Nguyen K, Ahmed A, Ahmed Y. Jerky/Earthbound facilitates cell-specific Wnt/Wingless signalling by modulating beta-catenin-TCF activity. The EMBO journal. 2011;30:1444–1458. doi: 10.1038/emboj.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzone FJ, Hoog C, Teplow DB, Cutting AE, Zeller RW, Britten RJ, Davidson EH. Gene regulatory factors of the sea urchin embryo. I. Purification by affinity chromatography and cloning of P3A2, a novel DNA-binding protein. Development. 1991;112:335–350. doi: 10.1242/dev.112.1.335. [DOI] [PubMed] [Google Scholar]
- Chou WH, Hall KJ, Wilson DB, Wideman CL, Townson SM, Chadwell LV, Britt SG. Identification of a novel Drosophila opsin reveals specific patterning of the R7 and R8 photoreceptor cells. Neuron. 1996;17:1101–1115. doi: 10.1016/s0896-6273(00)80243-3. [DOI] [PubMed] [Google Scholar]
- Chou WH, Huber A, Bentrop J, Schulz S, Schwab K, Chadwell LV, Paulsen R, Britt SG. Patterning of the R7 and R8 photoreceptor cells of Drosophila: evidence for induced and default cell-fate specification. Development. 1999;126:607–616. doi: 10.1242/dev.126.4.607. [DOI] [PubMed] [Google Scholar]
- Cook T, Pichaud F, Sonneville R, Papatsenko D, Desplan C. Distinction between color photoreceptor cell fates is controlled by Prospero in Drosophila. Developmental cell. 2003;4:853–864. doi: 10.1016/s1534-5807(03)00156-4. [DOI] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Efiok BJ, Chiorini JA, Safer B. A key transcription factor for eukaryotic initiation factor-2 alpha is strongly homologous to developmental transcription factors and may link metabolic genes to cellular growth and development. The Journal of biological chemistry. 1994;269:18921–18930. [PubMed] [Google Scholar]
- Emoto K, Parrish JZ, Jan LY, Jan YN. The tumour suppressor Hippo acts with the NDR kinases in dendritic tiling and maintenance. Nature. 2006;443:210–213. doi: 10.1038/nature05090. [DOI] [PubMed] [Google Scholar]
- Evans MJ, Scarpulla RC. Interaction of nuclear factors with multiple sites in the somatic cytochrome c promoter. Characterization of upstream NRF-1, ATF, and intron Sp1 recognition sequences. The Journal of biological chemistry. 1989;264:14361–14368. [PubMed] [Google Scholar]
- Fazio IK, Bolger TA, Gill G. Conserved regions of the Drosophila erect wing protein contribute both positively and negatively to transcriptional activity. The Journal of biological chemistry. 2001;276:18710–18716. doi: 10.1074/jbc.M100080200. [DOI] [PubMed] [Google Scholar]
- Fortini ME, Rubin GM. Analysis of cis-acting requirements of the Rh3 and Rh4 genes reveals a bipartite organization to rhodopsin promoters in Drosophila melanogaster. Genes & development. 1990;4:444–463. doi: 10.1101/gad.4.3.444. [DOI] [PubMed] [Google Scholar]
- Franceschini N, Kirschfeld K, Minke B. Fluorescence of photoreceptor cells observed in vivo. Science. 1981;213:1264–1267. doi: 10.1126/science.7268434. [DOI] [PubMed] [Google Scholar]
- Furukawa T, Morrow EM, Cepko CL. Crx, a novel otx-like homeobox gene, shows photoreceptor-specific expression and regulates photoreceptor differentiation. Cell. 1997;91:531–541. doi: 10.1016/s0092-8674(00)80439-0. [DOI] [PubMed] [Google Scholar]
- Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie R. Functional organization of the fly retina, in Progress in Sensory Physiology. Springer. 1985;5:1–79. [Google Scholar]
- Harvey K, Tapon N. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nature reviews. Cancer. 2007;7:182–191. doi: 10.1038/nrc2070. [DOI] [PubMed] [Google Scholar]
- Haussmann IU, Soller M. Differential activity of EWG transcription factor isoforms identifies a subset of differentially regulated genes important for synaptic growth regulation. Developmental biology. 2010;348:224–230. doi: 10.1016/j.ydbio.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Haussmann IU, White K, Soller M. Erect wing regulates synaptic growth in Drosophila by integration of multiple signaling pathways. Genome biology. 2008;9:R73. doi: 10.1186/gb-2008-9-4-r73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hough-Evans BR, Franks RR, Zeller RW, Britten RJ, Davidson EH. Negative spatial regulation of the lineage specific CyIIIa actin gene in the sea urchin embryo. Development. 1990;110:41–50. doi: 10.1242/dev.110.1.41. [DOI] [PubMed] [Google Scholar]
- Hsiao HY, Johnston RJ, Jr., Jukam D, Vasiliauskas D, Desplan C, Rister J. Dissection and immunohistochemistry of larval, pupal and adult Drosophila retinas. Journal of visualized experiments : JoVE. 2012 doi: 10.3791/4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Johnston RJ, Jr., Otake Y, Sood P, Vogt N, Behnia R, Vasiliauskas D, McDonald E, Xie B, Koenig S, Wolf R, Cook T, Gebelein B, Kussell E, Nakagoshi H, Desplan C. Interlocked feedforward loops control cell-type-specific Rhodopsin expression in the Drosophila eye. Cell. 2011;145:956–968. doi: 10.1016/j.cell.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukam D, Desplan C. Binary regulation of Hippo pathway by Merlin/NF2, Kibra, Lgl, and Melted specifies and maintains postmitotic neuronal fate. Dev Cell. 2011;21:874–887. doi: 10.1016/j.devcel.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaJeunesse DR, McCartney BM, Fehon RG. Structural analysis of Drosophila merlin reveals functional domains important for growth control and subcellular localization. The Journal of cell biology. 1998;141:1589–1599. doi: 10.1083/jcb.141.7.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikeladze-Dvali T, Wernet MF, Pistillo D, Mazzoni EO, Teleman AA, Chen YW, Cohen S, Desplan C. The growth regulators warts/lats and melted interact in a bistable loop to specify opposite fates in Drosophila R8 photoreceptors. Cell. 2005;122:775–787. doi: 10.1016/j.cell.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Mollereau B, Dominguez M, Webel R, Colley NJ, Keung B, de Celis JF, Desplan C. Two-step process for photoreceptor formation in Drosophila. Nature. 2001;412:911–913. doi: 10.1038/35091076. [DOI] [PubMed] [Google Scholar]
- Pan D. The hippo signaling pathway in development and cancer. Developmental cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papatsenko D, Sheng G, Desplan C. A new rhodopsin in R8 photoreceptors of Drosophila: evidence for coordinate expression with Rh3 in R7 cells. Development. 1997;124:1665–1673. doi: 10.1242/dev.124.9.1665. [DOI] [PubMed] [Google Scholar]
- Pepple KL, Atkins M, Venken K, Wellnitz K, Harding M, Frankfort B, Mardon G. Two-step selection of a single R8 photoreceptor: a bistable loop between senseless and rough locks in R8 fate. Development. 2008;135:4071–4079. doi: 10.1242/dev.028951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichaud F, Briscoe A, Desplan C. Evolution of color vision. Current opinion in neurobiology. 1999;9:622–627. doi: 10.1016/S0959-4388(99)00014-8. [DOI] [PubMed] [Google Scholar]
- Pichaud F, Desplan C. A new visualization approach for identifying mutations that affect differentiation and organization of the Drosophila ommatidia. Development. 2001;128:815–826. doi: 10.1242/dev.128.6.815. [DOI] [PubMed] [Google Scholar]
- Polesello C, Tapon N. Salvador-warts-hippo signaling promotes Drosophila posterior follicle cell maturation downstream of notch. Current biology : CB. 2007;17:1864–1870. doi: 10.1016/j.cub.2007.09.049. [DOI] [PubMed] [Google Scholar]
- Rister J, Desplan C. The retinal mosaics of opsin expression in invertebrates and vertebrates. Developmental neurobiology. 2011;71:1212–1226. doi: 10.1002/dneu.20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rister J, Desplan C, Vasiliauskas D. Establishing and maintaining gene expression patterns: insights from sensory receptor patterning. Development. 2013;140:493–503. doi: 10.1242/dev.079095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahayato A, Sonneville R, Pichaud F, Wernet MF, Papatsenko D, Beaufils P, Cook T, Desplan C. Otd/Crx, a dual regulator for the specification of ommatidia subtypes in the Drosophila retina. Developmental cell. 2003;5:391–402. doi: 10.1016/s1534-5807(03)00239-9. [DOI] [PubMed] [Google Scholar]
- Thanawala SU, Rister J, Goldberg GW, Zuskov A, Olesnicky EC, Flowers JM, Jukam D, Purugganan MD, Gavis ER, Desplan C, Johnston RJ., Jr. Regional modulation of a stochastically expressed factor determines photoreceptor subtypes in the Drosophila retina. Developmental cell. 2013;25:93–105. doi: 10.1016/j.devcel.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliauskas D, Mazzoni EO, Sprecher SG, Brodetskiy K, Johnston RJ, Jr., Lidder P, Vogt N, Celik A, Desplan C. Feedback from rhodopsin controls rhodopsin exclusion in Drosophila photoreceptors. Nature. 2011;479:108–112. doi: 10.1038/nature10451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernet MF, Labhart T, Baumann F, Mazzoni EO, Pichaud F, Desplan C. Homothorax switches function of Drosophila photoreceptors from color to polarized light sensors. Cell. 2003;115:267–279. doi: 10.1016/s0092-8674(03)00848-1. [DOI] [PubMed] [Google Scholar]
- Wernet MF, Mazzoni EO, Celik A, Duncan DM, Duncan I, Desplan C. Stochastic spineless expression creates the retinal mosaic for colour vision. Nature. 2006;440:174–180. doi: 10.1038/nature04615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin N, Benchabane H, Tian A, Nguyen K, Klofas L, Ahmed Y. Erect Wing facilitates context-dependent Wnt/Wingless signaling by recruiting the cell-specific Armadillo-TCF adaptor Earthbound to chromatin. Development. 2011;138:4955–4967. doi: 10.1242/dev.068890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Desplan C, Heisenberg M. Contribution of photoreceptor subtypes to spectral wavelength preference in Drosophila. Proc Natl Acad Sci U S A. 2010;107:5634–5639. doi: 10.1073/pnas.0809398107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nature cell biology. 2011;13:877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: ewg is not involved in general photoreceptor or R7 specification. (A) GFP image was taken in live adult flies under water immersion with a Nikon Plan Fluor 40X objective. ewg RNAi mutant retina showed ectopic Rh1-GFP expression (arrows). (B–C) Confocal images of adult retinas stained with antibody against Rh5 and Rh6 (red). (B) Retinas expressing ewg-RNAi exhibit Rh6 in most R8s while a few R8s co-express Rh5. (C) The ey-Gal4+lGMR-Gal4 driver line shows slightly elevated Rh6 expression. (D) Quantification of Rh5 and Rh6 in R8s. An unpaired t-test was performed to calculate the difference in mean % Rh5. Wild-type: n=10 retinas, N=3675 ommatidia; ewg RNAi mutant: n=4 retinas, N=874 ommatidia; ewg RNAi driver (eyeless>Gal4, lGMR>Gal4; UAS-Dicer2; Rh1-GFP): n=4 retinas, N=920 ommatidia *** p<0.001, error bars are mean ± one standard deviation (s.d.). (E) Expression of Rh3 (blue) and Rh4 (red) in R7 cells. The Rh3:Rh4 ratio is roughly 35:65 in wild type flies. (E') ewgl1 mutant retina shows normal Rh3 and Rh4 expression. (F) ewg mutant clones marked by loss of GFP (green). An anti-Ewg antibody (red) was used to show loss of Ewg in the mutant clone (indicated with a white line). (F') Ewg channel only. (G) In an ewg mutant clone, Spalt expression (red) is normal. (G') Spalt channel only. (H) Prospero is only expressed in R7s (arrow) in the wild type and this expression (blue) is unaffected in an ewgl1 mutant clone. (H') Prospero channel only.