Abstract
Objectives
This study assesses the reliability of photographic method with clinical examinations in detecting developmental defects of enamel (DDE) in anterior primary teeth of infants.
Methods
The study sample was a part of an on-going longitudinal study to assess risk factors for early childhood caries, and consisted of 138 and 238 infants who had scheduled follow-up visits at approximately 8 and 18–20 months corrected age, respectively. The modified DDE Index was used to record enamel defects (opacity, hypoplasia, and all types of defects) on anterior primary teeth by trained dentist examiners. Photographs of the teeth were taken using a digital camera. Statistical analysis included Cohen’s Kappa for reliability, and McNemar test and paired t-test for comparison between photographic and clinical examinations.
Results
The level of agreement between clinical and photographic methods was fair to moderate with Kappa values ranging from 0.252 to 0.514. The photographic examination detected significantly more DDE than the clinical examination regardless of age group and type of DDE. The intra- and inter- examiner reliability of the photographic method was excellent with Kappa values ranging from 0.638 to 0.927.
Conclusions
Within the limitation of this study, the photographic method can be a sound approach for verifying the diagnosis of DDE.
Keywords: Photographs, Reproducibility of Results, Diagnosis, Dental Enamel Hypoplasia
Introduction
Developmental defects of enamel (DDE) are visible deviations from the normal appearance of tooth enamel caused by enamel organ dysfunction (1). These defects may be quantitative (i.e. hypoplasia) or qualitative (i.e. opacity) in nature and present with a range of clinical appearances. The prevalence of DDE in the primary teeth has been reported to be in the range of 24% to 45% (2–8). Most studies indicated opacity as the most common type of DDE (4–8), while Li et al. (3) found more children with hypoplasia than those with opacities. The prevalence of DDE among children with very low birth weight (VLBW) has been reported to be higher (ranging between 27% and 96%) compared to those with normal birth weight (NBW) (9–13).
The accurate recording of DDE is important for diagnostic, clinical, and medico-legal purposes, as well as for etiological studies (14). Current in vivo studies of DDE rely on the clinical examination employing visual and/or tactile examination, which has many limitations. Most importantly, the clinical assessment of DDE may be affected by unintentional observer bias, especially when a controversial issue such as water fluoridation is involved (15, 16) or the study population has special medical conditions such as cerebral palsy (17).
Photographic methods have been employed to assist the diagnosis of clinical examinations and increase the accuracy in detecting DDE (18–21). The photographic method may facilitate blinded examinations to avoid observer bias and enable repeated examinations by multiple researchers even remote from the study site (22). Most studies have demonstrated a high reliability of photographic methods for assessing DDE (23–26) with high intra- and inter-examiner reliability (24, 25) although one study reported a relatively lower inter-examiner reliability (22).
The agreement between photographs and clinical examinations in detecting DDE in the permanent teeth has been reported to be substantial to almost perfect with Kappa values ranging from 0.63 to 0.85 (19, 24, 27, 28). However, recent studies by Golkari et al. (23) and Cruz-Orcutt et al. (26) found only moderate agreement (Kappa=0.48 and 0.46, respectively) between two methods suggesting that the photographic method detects significantly more DDE than the clinical examination method.
Previous studies assessing the reliability of photographic methods in detecting DDE were conducted on preschool or school children aged 3–13 years (19, 22–27). There is a dearth of information about the reliability of photographic method in assessing DDE in primary teeth of infants. Because infants are not cooperative during examinations, clinical examiners need to assess DDE as quickly as possible, which may make them fail to detect tiny and obscure DDE. Thus, the advantage of photographic methods over clinical examination may be greater in detecting DDE in the primary teeth of infants.
The aim of this study is to assess the reliability of photographic method with clinical examinations to detect DDE in anterior primary teeth of infants.
Methods
Study Design
A secondary dataset consisting of photographic and clinical examinations was used to calculate the reliability for two age groups of infants who had scheduled follow-up visits at 8 and 18–20 month corrected age.
Study Sample
The study sample was part of an on-going longitudinal study which is investigating DDE and early childhood caries in very low birth weight and normal birth weight infants. Data were collected from infants who returned for their scheduled follow-up visits at approximately 8 months and 18–20 months corrected age. At the time of the present study conducted between June–November 2011, 248 infants had completed 8 month and 285 infants had completed 18–20 month follow-up visits. All infants who had at least one tooth at the examination were eligible for the study with the exception only for infants whose photographs were not clear. Data from 110 infants at 8 month visits were excluded because either they had no erupted teeth (9 infants) or their photographs were not clear enough for DDE diagnoses (101 infants). Similarly, data from 47 infants at 18–10 month visits were excluded because of the clarity of their photographs. Therefore, the comparison between clinical and photographic examination methods in this study was based on 138 infants with a total of 445 anterior primary teeth in the 8 month age group and 238 infants with a total of 1,832 anterior primary teeth in the 18–20 month age group.
The institutional review board of two participating hospitals gave approval and consent was obtained from parents.
Measurement
For the clinical and photographic examinations, DDE of primary teeth were recorded based on the Modified DDE Index (1). The modified DDE Index identifies the type (demarcated opacities, diffuse opacities, and hypoplasia) and severity (number, location, and extent) of enamel defects. For the present study, only type of DDE was assessed as this was crucial for diagnosis. Both demarcated and diffuse opacities are qualitative defects involving an alteration in the translucency of enamel. Demarcated opacity has a distinct and clear boundary with the adjacent normal enamel and can be white, cream, yellow or brown in color. Diffuse opacity has no clear boundary with the adjacent normal enamel. Hypoplasia is a quantitative defect involving the surface of the enamel and associated with pits or missing enamel (1).
In the present study, demarcated opacities and diffuse opacities were combined as opacities, and the study utilized only buccal surfaces of anterior teeth because these surfaces were clearly seen on the photographs. Central and lateral incisors were examined for all infants, and canines were also examined when erupted for the 18–20 month age group. The DDE index type was used to determine the incidence of any type of DDE, opacity, and hypoplasia at both tooth- and subject-level (0=no, 1=yes).
Clinical examinations
The clinical examination for this study was conducted by five trained and calibrated dentist examiners. The reliability was moderate to excellent with inter-examiner Kappa of 0.56–0.90 and intra-examiner Kappa of 0.78–0.93. Each infant was examined in a knee-to-knee position using a lap cushion. Portable LED head lights on lowest light setting and a disposable mirror were used. Prior to the examination, teeth were cleaned with toothbrush and/or sterile gauze to remove any gross plaque or food deposits. Teeth were examined wet, but excess saliva was removed by gauze if necessary. The average time spent on the DDE examination was about 1 minute per infant. The time was recorded on 10 random examinations.
Photographic examinations
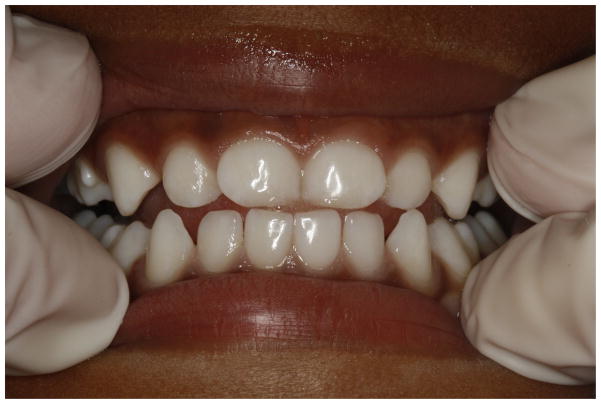
Photographs of the anterior teeth were obtained immediately after the clinical examinations. A digital camera (Canon EOS Rebel T2i 550D) fitted with a 100 mm macro-lens and a ring flash was used. All photographs were taken at 1:3 magnification using f/22 aperture and 1/100 shutter speed based on the best results obtained from pilot testing. The quality of photographs was set on “Large/Fine”, approximately equivalent to 18 megapixels (5,184 × 3,456). The photographs were later saved to personal computer as JPEG files for storage and viewing. An example of a photograph can be seen in Figure 1. Photographs were taken by calibrated research assistants with the infant held in a knee-to-knee position. Cheeks and lips were retracted by examiner’s finger to expose the anterior teeth and part of the upper and lower gums. The teeth were wet when the photographs were taken, but food debris and/or excess saliva was removed with sterile gauze when necessary. At the time of the subject’s visit each photograph was evaluated for clarity, and if not acceptable, the photograph was taken again. Taking photographs added less than 1 minute to the examination time.
Figure 1.
A photograph taken with a digital camera (Canon Eos Rebel T2i 550D) fitted with a 100 mm macro-lens and a ring flash. The anterior primary teeth were assessed by a trained examiner for DDE diagnosis.
Photographs were assessed on a free-angle monitor and scored by a trained examiner who was not involved in the clinical examination. Each photograph was viewed first as actual size, and if necessary for clarity the examiner used the magnifier tool to enlarge the photographs several times.
For the intra-examiner reliability, the selected photographs were scored again by the same study examiner 3 months after the first assessment. For the inter-examiner reliability, the same photographs were scored by another gold standard examiner who was not involved in the clinical examination. The study examiner was told to spend as much time as needed, and approximately 5–7 minutes were spent for the assessment of each photograph.
Statistical Analysis
The level of agreement between the photographic and clinical method, the intra-examiner reliability, and the inter-examiner reliability were assessed using Cohen’s Kappa. Following the suggestion of Landis and Koch (29), Kappa values were interpreted as follows: 0.00–0.20 slight, 0.21–0.40 fair, 0.41–0.60 moderate, 0.61–0.80 substantial, and 0.81–1.00 almost perfect.
The number and percentage of DDE detected by photographic and clinical methods were calculated at subject and tooth level. The same analyses were conducted with the subtypes of DDE (opacity and hypoplasia). Paired t-test and McNemar test were used to assess whether one method detects significantly more DDE than the other method. Statistical significance was determined at the 0.05 alpha-level. IBM SPSS statistics software (version 20) was used for analysis.
Results
The sample consisted of 138 infants with a total of 445 anterior primary teeth (369 central incisors and 76 lateral incisors) at 8 month visit (8m) and 238 infants with a total of 1,832 anterior primary teeth (859 central incisors, 711 lateral incisors, and 262 canines) at 18–20 month visit (18m). The mean age was 9.9 months and 47% were VLBW infants for 8m group, while the mean age was 20.9 months and 57% were VLBW infants for 18m group.
In the 8m group, agreement between two methods in detecting any kind of DDE was moderate at both subject level (Kappa=0.514) and tooth level (Kappa=0.417) (Table 1). The photographic method detected 1.9 times (48/25) more infants and 2.4 times (97/41) more teeth with DDE than the clinical examination (Table 2). Regarding the type of DDE, the photographic method detected more infants and more teeth with both type of defects (opacity and hypoplasia) compared with the clinical examinations (Table 2). In addition to detecting more cases with DDE than clinical examination, McNemar tests indicate that the numbers of subjects and teeth with DDE detected only by the photographic method were significantly (p < 0.05) higher than those detected only by the clinical examination (Table 2).
Table 1.
The level of agreement between clinical and photographic examination methods in detecting all types of, opacity, and hypoplasia DDE in primary teeth of infants
| Type of DDE | Age group | Subject level | Tooth level | ||
|---|---|---|---|---|---|
|
| |||||
| Kappa value | Level of agreement | Kappa value | Level of agreement | ||
| All types of DDE | 8m | 0.514 | Moderate | 0.417 | Moderate |
| 18–20m | 0.252 | Fair | 0.407 | Moderate | |
|
| |||||
| Opacity | 8m | 0.322 | Fair | 0.283 | Fair |
| 18–20m | 0.280 | Fair | 0.338 | Fair | |
|
| |||||
| Hypoplasia | 8m | 0.376 | Fair | 0.346 | Fair |
| 18–20m | 0.269 | Fair | 0.322 | Fair | |
Table 2.
Comparison of clinical and photographic examination methods in detecting all types of, opacity, and hypoplasia DDE in primary teeth of infants
| Type of DDE | Age Group | Method | Number of infants with DDE (%) | Number of teeth with DDE (%) | Mean teeth with DDE per infant |
|---|---|---|---|---|---|
| All types of DDE | 8m | Clinical | 25(18.1%) * | 41 (9.2%) * | 0.30† |
| Photographic | 48 (34.8%) | 97 (21.8%) | 0.70 | ||
|
| |||||
| 18–20m | Clinical | 61 (25.6%) * | 153 (8.4%) * | 0.64† | |
| Photographic | 152 (63.9%) | 367 (20.0%) | 1.54 | ||
|
| |||||
| Opacity | 8m | Clinical | 14 (10.1%) * | 23 (5.2%) * | 0.17† |
| Photographic | 25 (18.1%) | 49 (11.0%) | 0.36 | ||
|
| |||||
| 18–20m | Clinical | 35 (14.7%) * | 90 (4.9%) * | 0.38† | |
| Photographic | 102 (42.9%) | 252 (13.8%) | 1.06 | ||
|
| |||||
| Hypoplasia | 8m | Clinical | 15 (10.9%) * | 20 (4.5%) * | 0.14† |
| Photographic | 32 (23.2%) | 57 (12.8%) | 0.41 | ||
|
| |||||
| 18–20m | Clinical | 38 (16.0%) * | 76 (4.1%) * | 0.32† | |
| Photographic | 95 (39.9%) | 173 (9.4%) | 0.73 | ||
indicates the numbers of infants/teeth with DDE detected only by one method were significantly higher than those detected only by the other method (McNemar test, P < 0.05).
indicates the significant difference in mean numbers between two methods (paired t-test, P < 0.05).
Results were similar in the 18m group. Agreement between the two methods in detecting any type of DDE was fair (Kappa = 0.252) at subject level and moderate (Kappa = 0.407) at tooth level (Table 1). The photographic method detected 2.5 times (152/61) and 2.4 times (367/153) more DDE than the clinical examination at the subject- and tooth-level, respectively. More DDE was detected by the photographic method regardless of the type of DDE (Table 2).
Table 3 shows the intra- and inter-examiner reliability of photo examinations. The level of intra-examiner reliability was almost perfect for both subject level (Kappa = 0.839) and tooth level (Kappa = 0.927). The level of inter-examiner reliability was substantial (Kappa= 0.638) on subject level and almost perfect (Kappa = 0.845) at tooth level.
Table 3.
The level of inter- and intra-photographic examiner reliability
| Number of infants (Number of teeth) | subject level | tooth level | |||
|---|---|---|---|---|---|
|
| |||||
| Kappa value | Level of agreement | Kappa value | Level of agreement | ||
| Intra-examiner reliability | 64 (533) | 0.839 | Almost Perfect | 0.927 | Almost Perfect |
| Inter-examiner reliability | 64 (542) | 0.638 | Substantial | 0.845 | Almost Perfect |
Discussion
The present study investigated the reliability of the photographic method in detecting the type of DDE on the primary teeth of infants by assessing the level of agreement with the clinical examination. Overall, our study indicates fair to moderate agreement between photographs and clinical examination. The photographic method detected significantly more DDE which clinical examinations failed to find. However, the photographic method detected a majority of DDE which were detected by clinical examination (92% at the subject level and 81% at the tooth level). These findings are similar to the Golkari et al. (23) and Cruz-Orcutt et al. (26) studies but differ from the previous studies which reported substantial to almost perfect agreement level (19, 24, 27). Unlike previous studies which compared clinical and photographic methods in detecting DDE on permanent teeth of school children, the present study was conducted among infants, with whom clinical examiners cannot spend as much time as needed due to cooperation issues. With photographs, the examiner was able to spend much more time in detecting DDE. In addition, a highly efficient camera with a macro-lens and a ring flash was very effective to detect changes in color, transparency, or enamel thickness on primary teeth of infants (23). These reasons may have contributed to higher DDE detected through the photographs. Similar to previous studies (23–26), the intra- and inter-examiner reliability of the photographic method was excellent in this study.
Photographic method can be advantageous for epidemiological and public health investigation. First, photographs can be taken quickly compared with clinical examination. Thus, it is advantageous especially when research is focused on young infants. Second, photographic method can be used when clinical examiners are not available all the time. In addition, it allows blinded assessment to avoid examiners’ bias. Thus, photographic methods have been employed in some epidemiological and public health studies (21, 30). For example, Elfrink et al. assessed the relationship between the occurrence of Deciduous Molar Hypomineralization and Molar Incisor Hypomineralization using photographical methods (21). The results of our study indicate that photographic method can be a reliable method for DDE diagnosis in epidemiological and public health investigation.
Despite these advantages over clinical examination the photographic method also has some limitations. First, although taking photograph takes shorter time than the clinical examination, it was still challenging to photograph the teeth of infants. Just like during the clinical examination, many of the infants cried, screamed, or were continuously moving when photographs were taken, which made it hard for the photographer to obtain a clear and focused photograph of the teeth. As a result, although we took photographs of 239 infants at 8 month visits, only 138 (58%) photographs were clear enough for DDE diagnosis. On the other hand, it was relatively easier to take photographs of older infants. Out of 285 photographs taken at 18–20 month visits, 238 (84%) photographs could be used for DDE diagnosis. Second, only labial surfaces of anterior teeth can be photographed easily. Although multiple views can show more teeth and/or more surfaces, some surfaces or parts of a surface may be rotated or overlapped in the photographs because of the arrangement of teeth in the arches (24). Third, photographic examiners cannot check the occlusion of the subjects or ask for the trauma history when it is necessary. It could make examiners mistakenly consider the chip or trauma of the teeth as DDE.
The results of this study need to be interpreted with caution. First, unlike the photographic examination conducted by one examiner, multiple examiners participated in the clinical examinations. However, our dental examiners were all pediatric dental residents or faculty trained and calibrated in the DDE protocol. Since DDE diagnosis is not well taught in the dental school compared with caries diagnosis, there could be some examiner variations that have affected our results. Second, this study was conducted on 8–20 month-old infants. Clinical examinations with such young infants are not common in dentistry probably due to the difficulty of examining infants. Third, we found that the agreement level is notably affected by sample size especially at the tooth-level. The larger samples tended to result in higher Kappa values. This might have contributed to the results of lower and higher Kappa values at the tooth-level for the 8m and 18m groups, respectively compared to the subject-level Kappa values.
In conclusion, the present study demonstrates that agreement between the photographic method and clinical examinations in detecting DDE in infants was moderate because the photographic method detects more DDE compared with clinical examinations. Additionally the intra- and inter-examiner reliability of the photographic methods was excellent. Despite some limitations, this method can be a sound approach for verifying the diagnosis of DDE in anterior primary teeth of infants.
Acknowledgments
This research was supported by a grant from the National Institutes of Health (NIH) R01 DE017947-01. We thank all research staff (Shelley Curtan, Reem Asaad, Samantha Miadich, and Kirk Lang) and dentists (Masahiro Heima, Anchal Malik, Jacqueline Clifford, Margaret Ferretti, Clara Brannan, Amberlee Taylor, and John Gerstenmaier) for their contribution towards data collection. Most importantly, we thank infants and their family that participated in the research project.
References
- 1.Fédération Dentaire Internationale (FDI) A review of the developmental defects of enamel index (DDE Index). Commission on Oral Health, Research & Epidemiology. Report of an FDI Working Group. Int Dent J. 1992 Dec;42(6):411–26. [PubMed] [Google Scholar]
- 2.Needleman HL, Leviton A, Allred E. Macroscopic enamel defects of primary anterior teeth--types, prevalence, and distribution. Pediatr Dent. 1991 Jul-Aug;13(4):208–16. [PubMed] [Google Scholar]
- 3.Li Y, Navia JM, Bian JY. Prevalence and distribution of developmental enamel defects in primary dentition of Chinese children 3–5 years old. Community Dent Oral Epidemiol. 1995 Apr;23(2):72–9. doi: 10.1111/j.1600-0528.1995.tb00204.x. [DOI] [PubMed] [Google Scholar]
- 4.Slayton RL, Warren JJ, Kanellis MJ, Levy SM, Islam M. Prevalence of enamel hypoplasia and isolated opacities in the primary dentition. Pediatr Dent. 2001 Jan-Feb;23(1):32–6. [PubMed] [Google Scholar]
- 5.Lunardelli SE, Peres MA. Prevalence and distribution of developmental enamel defects in the primary dentition of pre-school children. Braz Oral Res. 2005 Apr-Jun;19(2):144–9. doi: 10.1590/s1806-83242005000200013. [DOI] [PubMed] [Google Scholar]
- 6.Farsi N. Developmental enamel defects and their association with dental caries in preschoolers in Jeddah, Saudi Arabia. Oral Health Prev Dent. 2010;8(1):85–92. [PubMed] [Google Scholar]
- 7.Seow WK, Ford D, Kazoullis S, Newman B, Holcombe T. Comparison of enamel defects in the primary and permanent dentitions of children from a low-fluoride District in Australia. Pediatr Dent. 2011 May-Jun;33(3):207–12. [PubMed] [Google Scholar]
- 8.Corrêa-Faria P, Martins-Júnior PA, Vieira-Andrade RG, Oliveira-Ferreira F, Marques LS, Ramos-Jorge ML. Developmental defects of enamel in primary teeth: prevalence and associated factors. Int J Paediatr Dent. 2012 May 1; doi: 10.1111/j.1365-263X.2012.01241.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Johnsen D, Krejci C, Hack M, Fanaroff A. Distribution of enamel defects and the association with respiratory distress in very low birthweight infants. J Dent Res. 1984;63(1):59–64. doi: 10.1177/00220345840630011401. [DOI] [PubMed] [Google Scholar]
- 10.Seow WK, Humphrys C, Tudehope DI. Increased prevalence of developmental dental defects in low-birth-weight children: A controlled study. Pediatr Dent. 1987;9:221–5. [PubMed] [Google Scholar]
- 11.Lai PY, Seow WK, Tudehope DI, Rogers Y. Enamel hypoplasia and dental caries in very-low birthweight children: a case-controlled, longitudinal study. Pediatr Dent. 1997;19(1):42–9. [PubMed] [Google Scholar]
- 12.Aine L, Backström MC, Mäki R, Kuusela AL, Koivisto AM, Ikonen RS, et al. Enamel defects in primary and permanent teeth of children born prematurely. J Oral Pathol Med. 2000 Sep;29(8):403–9. doi: 10.1034/j.1600-0714.2000.290806.x. [DOI] [PubMed] [Google Scholar]
- 13.Takaoka LA, Goulart AL, Kopelman BI, Weiler RM. Enamel defects in the complete primary dentition of children born at term and preterm. Pediatr Dent. 2011 Mar-Apr;33(2):171–6. [PubMed] [Google Scholar]
- 14.Elcock C, Lath DL, Luty JD, Gallagher MG, Abdellatif A, Bäckman B, et al. The new Enamel Defects Index: testing and expansion. Eur J Oral Sci. 2006 May;114(Suppl 1):35–8. doi: 10.1111/j.1600-0722.2006.00294.x. discussion 39–41, 379. [DOI] [PubMed] [Google Scholar]
- 15.Kanagaratnam S, Schluter P, Durward C, Mahood R, Mackay T. Enamel defects and dental caries in 9-year-old children living in fluoridated and nonfluoridated areas of Auckland, New Zealand. Community Dent Oral Epidemiol. 2009 Jun;37(3):250–9. doi: 10.1111/j.1600-0528.2009.00465.x. [DOI] [PubMed] [Google Scholar]
- 16.Ramesh G, Nagarajappa R, Raghunath V, Manohar R. Developmental defects of enamel in children of Davangere District and their relationship to fluoride levels in drinking water. Asia Pac J Public Health. 2011 May;23(3):341–8. doi: 10.1177/1010539509340912. [DOI] [PubMed] [Google Scholar]
- 17.Lin X, Wu W, Zhang C, Lo EC, Chu CH, Dissanayaka WL. Prevalence and distribution of developmental enamel defects in children with cerebral palsy in Beijing, China. Int J Paediatr Dent. 2011 Jan;21(1):23–8. doi: 10.1111/j.1365-263X.2010.01075.x. [DOI] [PubMed] [Google Scholar]
- 18.Nunn JH, Ekanayake L, Rugg-Gunn AJ, Saparamadu KD. Assessment of enamel opacities in children in Sri Lanka and England using a photographic method. Community Dent Health. 1993 Jun;10(2):175–88. [PubMed] [Google Scholar]
- 19.Ellwood RP, Cortea DF, O’Mullane DM. A photographic study of developmental defects of enamel in Brazilian school children. Int Dent J. 1996 Apr;46(2):69–75. [PubMed] [Google Scholar]
- 20.Cochran JA, Ketley CE, Arnadóttir IB, Fernandes B, Koletsi-Kounari H, Oila AM, et al. A comparison of the prevalence of fluorosis in 8-year-old children from seven European study sites using a standardized methodology. Community Dent Oral Epidemiol. 2004 Apr;32( Suppl 1):28–33. doi: 10.1111/j.1600-0528.2004.00136.x. [DOI] [PubMed] [Google Scholar]
- 21.Elfrink ME, ten Cate JM, Jaddoe VW, Hofman A, Moll HA, Veerkamp JS. Deciduous molar hypomineralization and molar incisor hypomineralization. J Dent Res. 2012 Jun;91(6):551–5. doi: 10.1177/0022034512440450. [DOI] [PubMed] [Google Scholar]
- 22.Cochran JA, Ketley CE, Sanches L, Mamai-Homata E, Oila AM, Arnadóttir IB, et al. A standardized photographic method for evaluating enamel opacities including fluorosis. Community Dent Oral Epidemiol. 2004 Apr;32( Suppl 1):19–27. doi: 10.1111/j.1600-0528.2004.00135.x. [DOI] [PubMed] [Google Scholar]
- 23.Golkari A, Sabokseir A, Pakshir HR, Dean MC, Sheiham A, Watt RG. A comparison of photographic, replication and direct clinical examination methods for detecting developmental defects of enamel. BMC Oral Health. 2011 Apr 21;11:16. doi: 10.1186/1472-6831-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong HM, McGrath C, Lo EC, King NM. Photographs as a means of assessing developmental defects of enamel. Community Dent Oral Epidemiol. 2005 Dec;33(6):438–46. doi: 10.1111/j.1600-0528.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 25.Elfrink ME, Veerkamp JS, Aartman IH, Moll HA, Ten Cate JM. Validity of scoring caries and primary molar hypomineralization (DMH) on intraoral photographs. Eur Arch Paediatr Dent. 2009 Nov;10( Suppl 1):5–10. doi: 10.1007/BF03262693. [DOI] [PubMed] [Google Scholar]
- 26.Cruz-Orcutt N, Warren JJ, Broffitt B, Levy SM, Weber-Gasparoni K. Examiner reliability of fluorosis scoring: a comparison of photographic and clinical examination findings. J Public Health Dent. 2012 Spring;72(2):172–5. doi: 10.1111/j.1752-7325.2012.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martins CC, Chalub L, Lima-Arsati YB, Pordeus IA, Paiva SM. Agreement in the diagnosis of dental fluorosis in central incisors performed by a standardized photographic method and clinical examination. Cad Saude Publica. 2009 May;25(5):1017–24. doi: 10.1590/s0102-311x2009000500008. [DOI] [PubMed] [Google Scholar]
- 28.Sabieha AM, Rock WP. A comparison of clinical and photographic scoring using the TF and modified DDE indices. Community Dent Health. 1998 Jun;15(2):82–7. [PubMed] [Google Scholar]
- 29.Landis JR, Koch GG. The Measurement of Observer Agreement for Categorical. Biometrics. 1977 Mar;33(1):159–74. [PubMed] [Google Scholar]
- 30.Iijima Y. Early detection of white spot lesions with digital camera and remineralization therapy. Aust Dent J. 2008 Sep;53(3):274–80. doi: 10.1111/j.1834-7819.2008.00062.x. [DOI] [PubMed] [Google Scholar]