Abstract
How does long-term training and the development of motor skill modify the activity of the primary motor cortex (M1)? To address this issue we trained monkeys for ~1–6 years to perform visually-guided and internally-generated sequences of reaching movements. Then, we used 14C-2-deoxyglucose (2DG) uptake and single neuron recording to measure metabolic and neuron activity in M1. After extended practice, we observed a profound reduction of metabolic activity in M1 for the performance of internally-generated compared to visually-guided tasks. In contrast, measures of neuron firing displayed little difference during the two tasks. These findings suggest that the development of skill through extended practice results in a reduction in the synaptic activity required to produce internally-generated, but not visually-guided sequences of movements. Thus, practice leading to skilled performance results in more efficient generation of neuronal activity in M1.
INTRODUCTION
The development of motor skill involves a gradual transition from sensory-driven responses to highly integrated patterns of behavior that rely on anticipatory planning1,2. Large amounts of practice are commonly required to achieve this transition to the internal generation of movements, and to maintain a high level of performance. In humans, expert performance on a motor task is acquired through years of training and is associated with changes in the structural and functional organization of the cortical motor areas3. For example, the volume of the primary motor cortex (M1) and the premotor areas is increased in professional musicians compared to amateurs or non-musicians4,5. Similarly, the sensory and motor representations of the body parts used for skilled performance are enlarged in professional musicians6,7.
Paradoxically, some studies using functional magnetic resonance imaging (fMRI) show that cortical activation (BOLD) is decreased after long-term training. Specifically, the functional activation observed in premotor areas or M1 during the performance of various sequential tasks is reduced or becomes more focused in professional musicians compared to amateurs or non-musicians8–12. The reduced activation in highly skilled performers is often taken as evidence for “increased efficiency of the motor system,” and the need for a smaller number of active neurons to perform a given set of movements9–11. Others have argued that lower levels of activation are the product of reduced attention or task difficulty13,14. In addition, elevated activation at rest seen in skilled performers may mask task-related activation in M1 in traditional “task” versus “rest” contrasts15.
A fundamental difficulty with the interpretation of functional activation associated with skilled performance is that the level of neuron discharge during the task is unknown. It is unclear how the decreases in functional activation observed in humans relate to changes in neuron spiking. The firing rate and other response properties of neurons in M1 and the premotor areas are altered by motor learning16–20. However, to our knowledge, functional activation and neuron firing rate have not been previously measured during the same motor task. Thus, it is not possible to evaluate whether motor learning and the development of skill modify the efficiency of neural processing.
Here, we examine the consequence of practice-dependent motor learning on the metabolic and neuron activity in M1 of monkeys who had extensive training (~1–6 years) on sequential movement tasks. We assessed both measures of activity during the performance of visually-guided and internally-generated sequences of movements. This comparison captures the extreme stages in the continuum of motor skill learning from sensory-driven responses to highly skilled performance. We observed a profound reduction of metabolic activity in M1 for performance of internally-generated compared to visually-guided tasks. In contrast, measures of neuron firing were similar during the tasks. Thus, our results provide direct evidence for a widespread alteration in the relationship between metabolic activity and neuron activity associated with practice on a skilled sequence of movements. This decoupling of metabolic and neuron activity implies that practice leading to skilled performance results in more efficient generation of neuronal activity in M1.
RESULTS
We trained ten monkeys to perform two of four sequential reaching tasks (Track, Rem, Random, and Repeating) (see Online Methods). In all cases, we rewarded the monkeys for contacting targets displayed at arm’s length in the frontal plane. In the Track and Random tasks, each reaching movement was instructed by a visual cue. We refer to these tasks as the “visually-guided” tasks. In the Rem and Repeating tasks, the monkeys produced sequences of movements that were learned through practice and internally-generated from memory. We refer to these tasks as the “internally-generated” tasks. During the Track (visually-guided) and Rem (internally-generated) tasks, 3 correct movements were followed by an inter-trial interval. During the Random (visually-guided) and Repeating (internally-generated) tasks, movements were performed continuously. We trained two additional monkeys on a Lick task in which they received rewards without performing arm movements.
We used the 14C-2-deoxyglucose (2DG) technique21,22 to examine the pattern of metabolic activity in the arm representation of M1 (Fig. 1a) during each task (Track, n = 2; Rem, n = 2; Random, n = 3; Repeating, n = 3 and Lick, n = 2). We also recorded single neuron activity in arm M1 during the Random and Repeating Tasks20 (n = 2). One of these animals performed the Random task during the subsequent 2DG experiment (2DG-Random animal, N15). In this animal, neuron recording occurred over 6.7 months and the 2DG experiment was carried out 26 months after the end of recording. The other animal performed the Repeating task during the 2DG experiment (2DG-Repeating animal, N14). In this animal, neuron recording occurred over 7.9 months and the 2DG experiment followed 1 month later. Results were highly consistent within groups. In particular, the pattern of 2DG uptake in the animals with neuron recording was comparable to that observed in the other animals for the same task. Thus, prior neuron recording did not influence the 2DG results.
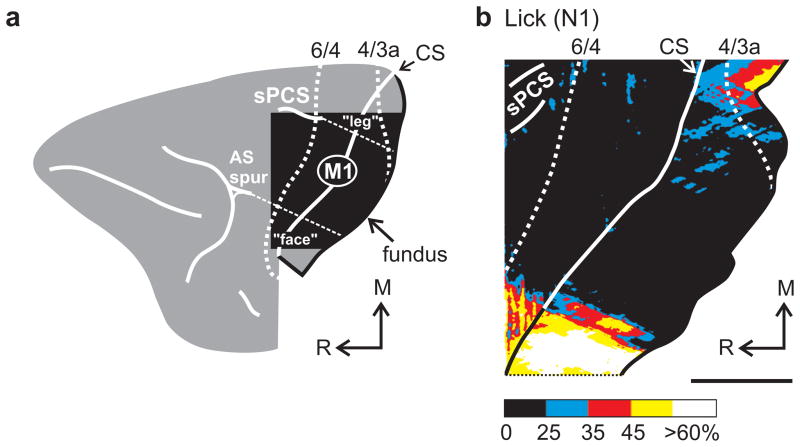
Figure 1.
Activation during the Lick task. (a) The square cutout outlines the region of the frontal lobe studied and shown in the 2DG maps. We defined the hindlimb, forelimb, and face representations based on data from prior anatomical and physiological studies. The arm representation is approximately located within the area defined by a 45° line from the caudal tip of the superior precentral sulcus (sPCS) and a 45° line from the caudal tip of the spur of the arcuate sulcus (AS spur) (dashed lines). CS marks the edge of the central sulcus. The anterior bank of the central sulcus is shown on the right. M, medial; R, rostral. (b) 2DG uptake observed in one animal during the Lick task. 2DG uptake within the banks of the sPCS is not illustrated. The color scale represents normalized activation (% 2DG uptake, see Online Methods). Scale bars = 5 mm.
Patterns of activation in M1
In the Lick animals, we observed two main sites of 2DG uptake in M1 (Fig. 1b). One was located laterally in the face representation, and was related to the consumption of liquid rewards. The second site was located medially in the lower body representation, and was related to the maintenance of posture in the primate chair. Similar activations were present in the animals that performed the Track, Rem, Random and Repeating tasks because they regularly licked for fluid rewards while seated in a primate chair. On the other hand, 2DG uptake in the arm area of M1 was low in the Lick animals (Fig. 1b, Table 1). In fact, the average 2DG uptake in the arm area was not significantly different (< 3 standard deviations) from background uptake in the Lick animals.
Table 1.
Global activation (% 2DG uptake) in arm M1
| Central Sulcus | Precentral Gyrus | |
|---|---|---|
| Lick (n = 2) | 12.2 ± 6.9 ns | 5.0 ± 5.6 ns |
| Track (n = 2) | 47.8 ± 4.1** | 39.6 ± 1.1** |
| Random (n = 3) | 36.7 ± 5.8** | 37.1 ± 4.7** |
| Rem (n = 2) | 17.2 ± 3.3 ns | 14.0 ± 0.7 ns |
| Repeating (n = 3) | 20.8 ± 4.7* | 11.4 ± 6.5 ns |
Values are mean ± 1 s.d. of the entire arm area.
ns, not significantly different than background in any animal
significantly greater than background in 1 monkey
significantly greater than background in all monkeys
We compared the average 2DG uptake in arm M1 on the precentral gyrus and in the anterior bank of the central sulcus during the tasks (2-way ANOVA with post-hoc comparison of means and Bonferroni correction). Task was a significant factor for 2DG uptake in arm M1 (d.f. = 4, 14, p < 0.01 × 10−4), as was area (gyrus, sulcus) (d.f. = 1, 14, p = 0.02). There was no interaction between task and area (d.f. = 4, 14, p = 0.48). Compared to the Lick task, there was marked 2DG uptake in arm M1 during visually-guided reaching movements (Fig. 2a–b, Fig. 3a–b, Table 1 (ANOVA, d.f. = 4, 14; Track vs Lick, p = 0.01 × 10−4; Random vs Lick, p = 0.05 × 10−4).
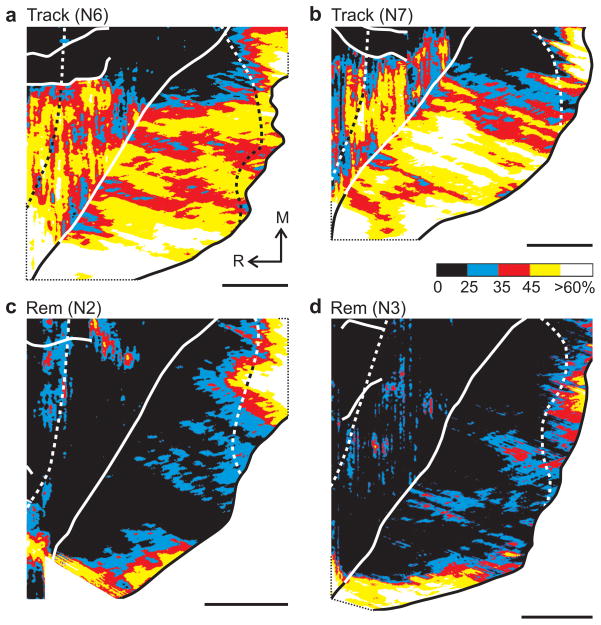
Figure 2.
Activation during the Track and Rem tasks. Each panel shows the 2DG uptake in M1 of one animal who performed the Track (a,b) or Rem (c,d) task for the 2DG experiment. See Fig. 1 for conventions.
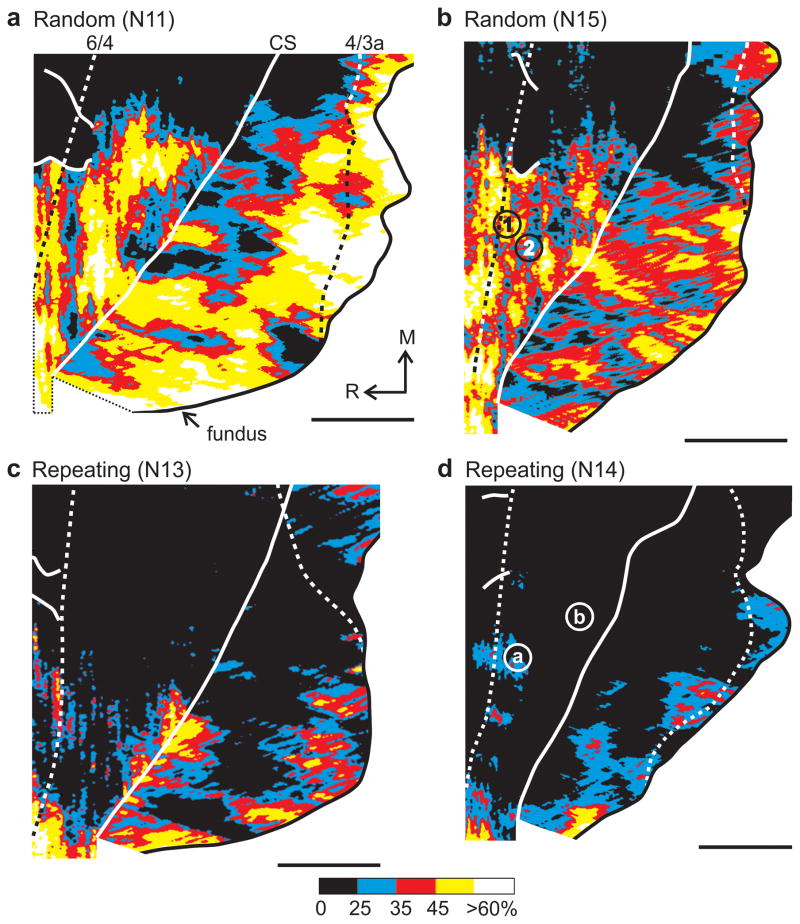
Figure 3.
Activation during the Random and Repeating tasks. Each panel shows the 2DG uptake in M1 of one animal who performed the Random (a,b) or Repeating (c,d) task for the 2DG experiment. See Fig. 1 for conventions. Monkeys N15 (b) and N14 (d) were involved in single neuron recording as well as 2DG experiments. For monkey N15, numbers mark penetrations in regions of high (1) and lower (2) 2DG uptake. The activity of some neurons recorded at these penetration sites is illustrated in Supplementary Figs. 2–3. For monkey N14, letters mark penetrations in regions of high (a) and lower (b) 2DG uptake. The activity of some neurons recorded at these penetration sites is illustrated in Supplementary Figs. 4–5.
In contrast, during internally-generated reaching movements, limited 2DG uptake was present in arm M1 (Fig. 2c–d, Fig. 3c–d, Table 1). Indeed, the average 2DG uptake in arm M1 during internally-generated movements was not significantly different from the uptake during the Lick task (ANOVA, d.f. = 4, 14; Rem vs Lick, p = 0.67; Repeating vs Lick, p = 0.35). This observation is surprising given the absence of arm movements during the Lick task and the prolonged use of the arm during the Rem and Repeating tasks. These results indicate that the manner of movement generation rather than movement per se determines the extent of 2DG uptake in M1during the performance of sequential arm movements.
Later we will argue that the striking differences in 2DG uptake associated with the visually-guided and internally-generated tasks are due to the practice required to develop skill on the internally-generated sequences. This argument depends, in part, on the demonstration that the differences in 2DG uptake are not a function of simple variations in task performance or other task-related variables. Measures of performance and task parameters are listed in Supplementary Tables 1 and 2. None of the variables measured, including number of movements performed, movement rate, number of rewards, reward rate, and average movement speed could individually or jointly account for the differences of 2DG uptake between animals or task categories (Supplementary Fig. 1). For example, there was no significant difference in the average movement rate during the Track and Rem tasks (ANOVA, d.f. = 3, 6; p = 0.77, Bonferroni corrected) and yet, the average 2DG uptake in arm M1 was much greater in the Track than in the Rem task (ANOVA, d.f. = 4, 14; p = 0.015 × 10−3, Bonferroni corrected). On the other hand, the average movement rate during the Random task was significantly less (by 49%) than that during the Repeating task (ANOVA, d.f. = 3, 6; p = 0.022, Bonferroni corrected). In contrast, 2DG uptake in arm M1 during the Random task was significantly greater than during the Repeating task (ANOVA, d.f. = 4, 14; p = 0.046 × 10−3, Bonferroni corrected). Thus, there was no simple correlation across tasks between 2DG uptake and movement rate or other performance parameters.
The high spatial resolution of 2DG uptake (~50–90 μm) allowed us to compare both the local peak intensity and the spatial extent of activation during visually-guided and internally-generated reaching movements. We measured local peak activation in 2 mm2 areas of interest. We defined the spatial extent of activation as the percentage of significantly activated pixels in arm M1. Peak activation during internally-generated movements was nearly half of that observed during visually-guided movements (Fig. 4a). Similarly, the area significantly activated during internally-generated movements amounted to only 22–35% of that activated during visually-guided movements (Fig. 4b). In essence, large portions of arm M1 (up to 86% in individual animals) were devoid of significant activation during the two internally-generated tasks.
Figure 4.
Comparison of activation measures in arm M1. (a) Peak activation was measured in 2 mm2 areas of interest over the most intense activation in arm M1. (b) Area measures were normalized by calculating the percentage of significantly activated pixels within each region of M1. We assessed group differences (Lick, n = 2; Rem and Repeating [internally-generated], n = 5; Track and Random [visually-guided], n = 5) with a 2-way (measure, group) ANOVA (d.f. = 2, 9) and post-hoc comparison of means. The probabilities shown are after Bonferroni correction. Error bars are s.d..
We examined whether the length of practice on a task had an effect on the peak of activation in arm M1. Animals practiced visually-guided reaching movements for 12 to 74 months (Track, n = 2, 16–18 months; Random, n = 3, 12–74 months). For these tasks we saw no evidence that the duration of practice had an effect on the peak amplitude of activation in M1 either on the precentral gyrus or in the central sulcus (linear regression, r = −0.17 and −0.33; ANOVA, d.f. = 1, 3, p = 0.79 and 0.59).
On the other hand, the length of practice did appear to have an effect on the peak activation (in the central sulcus) for animals that performed internally-generated movements. Our animals practiced internally-generated reaching movements for 7.4 to 39 months. The peak activation in M1 for animals with less practice averaged 39% (7.4–8 months practice, Repeating, n = 2) (e.g., Fig. 3c). In contrast, the peak activation for animals with more practice averaged 21% (31–39 months practice, Repeating, n = 1, Rem, n = 2) (Fig. 3d). Altogether, peak activation in M1 was inversely related to the duration of practice on internally-generated movements (linear regression, r = −0.91; ANOVA, d.f. = 1, 3, p = 0.01). Although the number of observations is limited, this strong association suggests that the peak activation in arm M1 declined in relation to the amount of practice on an internally-generated sequence.
Comparison of activation patterns and neuron activity
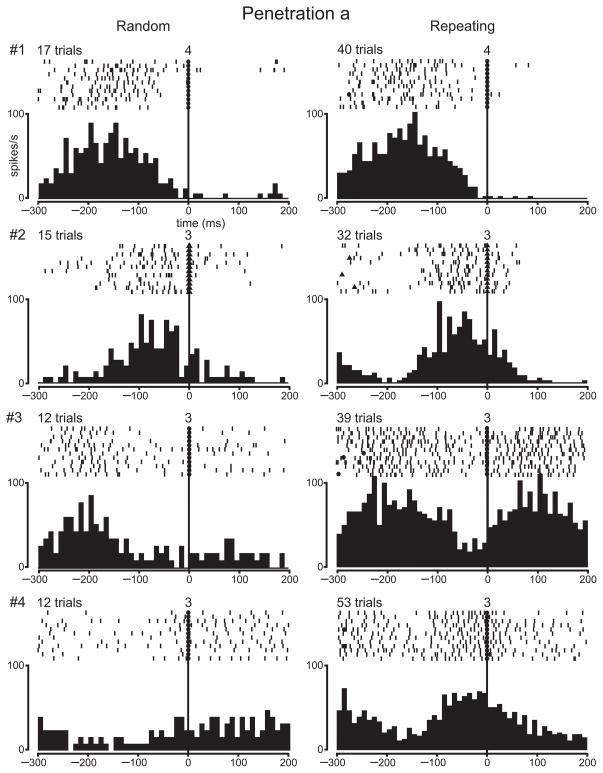
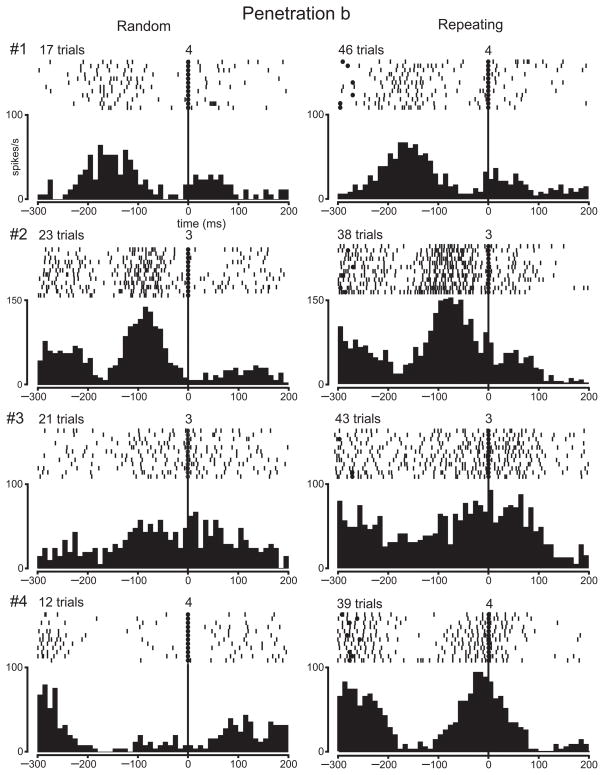
Our observation of a relative decrease in 2DG uptake in arm M1 for highly practiced sequences of movements raises a fundamental question — Is there a similar decrease in neuron activity in M1 for highly practiced sequences of movements? To answer this question, we recorded the activity of M1 neurons in two monkeys as they performed the Random and Repeating tasks. Then, we performed a 2DG experiment in the same monkeys to compare directly neuron activity and 2DG uptake. We have previously reported the results from recording single neuron activity in these animals during the Random and Repeating tasks20. Briefly, we recorded 234 task-related neurons in the proximal arm representation of M1. Many task-related neurons displayed changes in activity that were comparable during the two tasks. However, 40% of the task-related neurons were differentially active during the two tasks: 27% displayed enhanced activity during the Repeating task (e.g., Fig., 5, neuron #4; Fig. 6, neuron #4), and 12% displayed enhanced activity during the Random task. The discovery of such a substantial number of differential responses was part of the motivation for exploring the patterns of metabolic activity in the same animals during the Random and Repeating tasks.
Figure 5.
Single neuron activity in a penetration through an area of high 2DG uptake (N14). The rasters and histograms illustrate the activity of four neurons recorded in penetration “a” (see Fig. 3d). 2DG uptake at this site was relatively high (20%). Each dash in the raster is a spike. The rasters and histograms show the activity for the same specific movements during the Random and Repeating tasks. Rasters and histograms are aligned at the time of target contact (filled circles) or release (filled triangles). The numbers above the rasters at time 0 indicate which target was contacted (or released). The number of trials included in the histograms is indicated above the rasters. The rasters are limited to 12 trials. Histogram bin width, 10 ms.
Figure 6.
Single neuron activity in a penetration through an area of low 2DG uptake (N14). The rasters and histograms illustrate the activity of four neurons recorded in penetration “b” (see Fig. 3d). 2DG uptake at this site was relatively low (−3%). See Fig. 5 for conventions.
The remainder of this section will focus on the comparison between the topographic distributions of neuron activity and 2DG uptake. For this analysis, we included electrode penetrations placed not only in the proximal arm representation of M1, but also in adjacent regions of M1 (e.g., finger and wrist representations). To achieve a comprehensive sample, we included every well-isolated neuron, task-related or not, in this analysis. In the 2DG-Random animal, our analysis is based on 214 neurons recorded in 52 penetrations (Fig. 7a). In the 2DG-Repeating animal, our analysis is based on 504 neurons recorded in 95 penetrations (Fig. 7b). In both animals neurons recorded in electrode penetrations throughout arm M1 displayed activity that was well-modulated during the Random and Repeating tasks. These observations are illustrated in Figs. 5 and 6 for the 2DG-Repeating animal (see also Supplementary Figs. 2–5).
Figure 7.
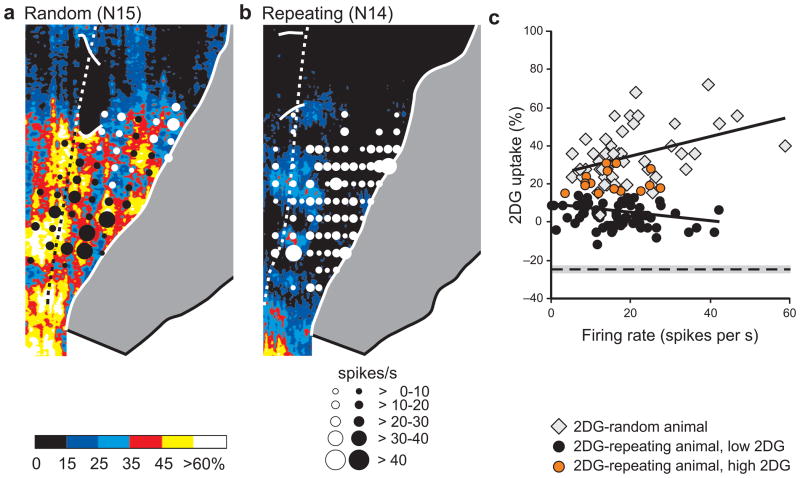
Relation between local average 2DG uptake and neuron activity. (a) The 2DG data for the 2DG-Random animal (Fig. 3b) and (b) the 2DG-Repeating animal (Fig. 3d) are shown on an expanded color scale to reveal lower levels of 2DG uptake. Black or white circles are centered at penetration sites and scaled to the average firing rate (spikes/s) in each microelectrode penetration. Only penetrations in cortex exposed on the surface are shown. See Fig. 1 for conventions. (c) The local average firing rate is plotted against the average 2DG uptake for each penetration site. Gray diamonds indicate results from the 2DG-Random animal (N15, n = 50). Circles indicate results from the 2DG-Repeating animal (N14, n = 81). Orange circles mark penetrations through areas of ≥ 15% 2DG uptake, whereas black circles mark penetrations through areas of < 15% 2DG uptake. The regression lines are based on the activity averages for all penetrations on the precentral gyrus of each animal. For comparison, the average level of 2DG uptake in a control area (anterior cingulate cortex) is shown by the dashed line ± 1 s.d. (shaded area).
2DG-Random animal
We defined the average firing rate for each neuron sampled during a task and then generated a group average firing rate for all the neurons sampled. For the 2DG-Random animal the group average firing rate was 18.5 ± 13.6 (s.d.) spikes/s during the Random task and 20.1 ± 14.4 spikes/s during the Repeating task. We also examined the relationship between local neuron activity and metabolic activity during the Random task. We did this by regressing the average 2DG uptake within a 50 μm radius centered on an electrode penetration against the average firing rate for the neurons recorded in the penetration during the Random task. Our analysis revealed a modest, but significant positive correlation between local 2DG uptake and local average firing rate (linear regression, n = 50, r = 0.44; ANOVA, d.f. = 1, 48, p = 0.014 after Bonferroni correction, see Online Methods) (Fig. 7c, diamonds). Thus, as expected23–26, we found a relationship between local 2DG uptake and local neuron activity in the 2DG-Random animal.
2DG-Repeating animal
In this animal, the group average firing rate was 17.6 ± 14.9 spikes/s during the Random task. The group average during the Repeating task was 16.6 ± 14.7 spikes/s. Although this value is slightly less than the group average for the 2DG-Random animal during the Random task (18.5 spikes/s), the total neuron activity generated during the two 2DG experiments was not statistically significant (Mann-Whitney U = 5.5, p = 0.81). Thus, the global neuron activity in M1 of the 2DG-Repeating animal was similar to that of the 2DG-Random animal.
However, in the 2DG-Repeating animal we found a striking mismatch between local 2DG uptake and local neuron activity. Neurons recorded in regions of low and high 2DG uptake could have comparable average firing rates and modulation in activity (e.g., Figs. 5 and 6). Unlike the 2DG-Random animal, we found no significant correlation in the 2DG-Repeating animal between local 2DG uptake and local neuron activity (linear regression, n = 81, r = −0.21; ANOVA, d.f. = 1, 79, p = 1 after Bonferroni correction, see Online Methods) (Fig. 7c, circles).
To examine further the relationship between the local average firing rate and local 2DG uptake, we defined electrode penetrations as falling in regions of low or high 2DG uptake in the 2DG-Repeating animal. Low regions were defined as uptake ≤ 10% and at least 0.5 mm away from a site of high uptake (≥ 15%). Overall, the average firing rate of neurons recorded in penetrations through regions of low 2DG uptake (n = 52) was 16.7 ± 8.3 (s.d.) spikes/s (Fig. 7c, black circles). This rate was not significantly different from that of neurons recorded in penetrations through regions of high 2DG uptake (n = 15) in the same animal (15.4 ± 7.1 spikes/s) (t-test, d.f. = 65, p = 0.57) (Fig. 7c, orange circles). It is noteworthy that the range of average firing rates in the 2DG-Repeating animal was similar to the range in the 2DG-Random animal (Fig. 7c, compare circles with diamonds). The recordings in the 2DG-Repeating animal clearly demonstrate that there was substantial neuron activity in arm M1 even at sites where there was low 2DG uptake. Thus, local 2DG uptake was not a reliable indicator of local neuron activity in M1 in an animal that performed highly skilled, internally generated sequences of movement.
DISCUSSION
There are two surprising results from the present study. First, we found that 2DG uptake in arm M1 is unexpectedly low in animals that performed highly practiced, internally-generated sequences of movements. Second, the low 2DG uptake was not matched by low neuron activity in the same area. Indeed, we found that the level of neuron activity in arm M1 during internally-generated movements was comparable to that observed during visually-guided movements. Thus, we found a marked dissociation between metabolic and neuron activity in M1. Each of these observations raises distinct issues which we discuss below.
What factors contribute to low 2DG uptake in arm M1?
It is noteworthy that we observed low 2DG uptake in arm M1 in all of the animals that performed internally-generated movements whether they were performed during the Rem or Repeating tasks. In contrast, we observed marked 2DG uptake in the arm area in all animals that performed visually-guided movements whether they were performed during the Track or Random tasks. The reproducibility of these patterns of activation across animals and task categories (Rem/Repeating tasks versus Track/Random tasks) indicates that the phenomenon is robust and dependent on the mode of the task rather than performance specifics. The animals that displayed low 2DG uptake for internally-generated movements also were trained to perform visually-guided movements. Similarly, the animals that displayed high 2DG uptake for visually-guided movements also were trained to perform internally-generated movements. Thus, both sets of data came from animals with the same training experience. We argue below that the low 2DG uptake during internally-generated movements reflects plastic mechanisms associated with motor learning and long-term practice of a motor skill. Before doing so, we argue against five alternative explanations.
The low 2DG uptake is not an artefact of 2DG analysis
Our analysis relies on semi-quantitative measures of 2DG uptake relative to background (leg M1) and peak activation (face S1). Relative measures (e.g., z scores) are widely used in neuroimaging, but must be applied with caution for comparisons between subjects. For example, wide fluctuations in the metabolic rate of the areas referenced for normalization could affect between subject comparisons. In our case, all animals shared common patterns of activation in multiple cortical areas (Supplementary Fig. 6), including the two areas used for normalization. Indeed, the basic pattern of activation we observed in areas outside of arm M1 is comparable to that seen by others with alternative analysis methods in animals performing similar behavioral tasks27–29. In addition, using alternative normalization procedures, such as normalizing based on background 2DG uptake only, gave essentially the same results. Thus, the low 2DG uptake in arm M1 of animals who performed internally-generated movements is not a consequence of our analysis methods.
The low 2DG uptake is not a reflection of movement parameters
It would not be surprising to see altered 2DG uptake if movement parameters during two tasks differed markedly. However, we did not see consistent differences in kinematics or muscle activity between the visually-guided and internally-generated tasks (Supplementary Fig. 1 and Supplementary Tables 1 and 2; see also refs. 20, 30). Thus, the striking difference between 2DG uptake in M1 for the two task categories cannot be explained by the minor variations in motor output that occurred during the tasks.
The low 2DG uptake is not a consequence of reduced visual input
The two task categories differed notably in the utilization of visual signals. However, M1 neurons do not respond to simple visual stimuli and are poorly responsive to visual cues used as instruction signals for movement31–33. In our tasks, the visual cues that specify where to move were replaced by internal instruction signals that direct movement. Thus, any reduction in synaptic input to guide movement based on vision must be compensated by input from another source to internally generate movement.
The low 2DG uptake is not a “repetition suppression” effect
Because decreased 2DG uptake was observed during the performance of highly practiced, repeating or familiar sequences of movement, one could argue that it is linked to repetition suppression. Repetition suppression is the attenuation of stimulus-evoked neural activity observed upon stimulus repetition34,35. A similar reduction in BOLD responses has been observed in M1 with movement repetition36. However, neuron recordings in M1 during the Rem37 and Repeating tasks20 provide no evidence of repetition suppression of neural activity during the performance of these tasks. For example, we compared the magnitude of neural activity in M1 for the same movement during the Random and Repeating tasks20. If repetition suppression were a major factor then the majority of neurons would show reduced activity during the Repeating task. On the contrary, 68% of the differentially active neurons were more active for the Repeating movements than for the Random movements. Extending this analysis to all neurons sampled confirms that the average discharge rate was quite similar during the Repeating (22.5 spikes/s) and Random (23.2 spikes/s) tasks (234 neurons × 6 moves; Wilkoxon signed rank test, p = 0.003). Thus, the marked differences in 2DG uptake during the tasks cannot be explained by repetition suppression.
The low 2DG uptake is not a general use effect
Our animals trained on the various reaching tasks for ~10–74 months. Extended practice in and of itself could result in alterations in 2DG uptake. For example, motor exercise in rats results in structural changes in M1 such as angiogenesis38. However, our animals displayed patterns of 2DG uptake that were task- and not use-dependent. For example, animals N11 and N13 were trained for 10–12 months on the Random and Repeating tasks. N11 displayed pronounced 2DG uptake in arm M1 during the Random task (Fig. 3a), whereas N13 did not during the Repeating task (Fig. 3c). Animal N14 was trained 37 months on the Random and Repeating tasks. During the 2DG infusion, N14 performed the internally-generated task (Repeating) and displayed low 2DG uptake in arm M1 (Fig. 3d). In contrast, animal N15 was trained for 74 months on the Random and Repeating tasks. During the 2DG infusion, this animal performed the visually-guided task (Random) and displayed high 2DG uptake in arm M1 (Fig. 3b). Clearly, low 2DG uptake was a consequence of performing the internally-generated tasks at a high level of motor skill.
Low 2DG Uptake is Associated with Motor Skill
Extended practice is a critical requirement for the skilled performance of internally-guided movements. For the Repeating task, continued practice was accompanied by incremental improvements in performance even after one year of training20. In addition, the amount of practice on the internally-generated tasks was inversely correlated with 2DG uptake in arm M1. These observations imply that the reduction in 2DG uptake is related to an animal’s skill in performing the movements of an internally-generated task.
2DG uptake is thought to be most closely associated with presynaptic activity at both excitatory and inhibitory synapses26,39. Therefore, the most likely explanation for the low 2DG uptake in M1 during internally-generated movements is that synaptic activity during these movements is reduced relative to the synaptic activity for visually-guided movements. In other words, our results suggest that the development of skill through extended practice on an internally-generated sequence of movements results in a reduction in the synaptic activity required to produce the neural activity necessary to generate the movements.
In general, metabolic processes and hemodynamic measures of cerebral activity are tightly coupled40. For this reason, 2DG and other measures of “functional activation”, such as the BOLD signal of fMRI, should be similarly modulated. Our results predict that the magnitude and/or the extent of the BOLD signal for a task requiring internally-generated movements should decrease with the development of skilled performance as a consequence of extended practice (months to years). This prediction is supported by the relative reduction in M1 activation seen in professional musicians during the performance of a practiced task9,10. We want to emphasize that our results are relevant only for studies of activation after long-term practice. In fact, increased activation in M1 and other motor structures is frequently seen in unskilled subjects after short periods of training (a single session to a few weeks)3,41–43. For example, increased activation in hand M1 is associated with practice on an internally-generated finger opposition sequence41. M1 activation reached an asymptote after 3 weeks of training. By comparison, the shortest training duration on an internally-generated sequence in our experiments was 30 weeks. The negative correlation between peak 2DG uptake and training duration that we found suggests that extensive training is a critical element for our results. It is also noteworthy that the subjects in ref. 41 performed the finger opposition sequence as fast as possible during practice sessions, but performed at a fixed movement rate (2 Hz) during scanning. The additional timing requirement during scanning may have influenced the activation observed in M1.
Dissociation of neuron activity and 2DG uptake in arm M1
Perhaps the most surprising observation of our study is the dissociation between metabolic activity and neuron activity in M1. Prior studies show 2DG uptake to be spatially coincident with cortical sites where neurons are active24,44,45. It is possible for an increase in 2DG uptake to occur without a concomitant increase in neuron activity26. For example, inhibitory synaptic activity can result in increased 2DG uptake46. However, we are not aware of prior examples in which 2DG uptake decreased, but neuron activity remained unaltered.
As noted in the Introduction, BOLD signals in M1 during the performance of sequential movements are lower in professional musicians than in unskilled subjects. Our results suggest that the lower signal may occur without a concomitant reduction in neuron activity. We believe that the development of expertise through extended practice is a special circumstance that leads to a reduction in overall synaptic activity which is not accompanied by a reduction in spiking activity.
At this point we can only speculate about the origin of the dissociation between synaptic activity and cellular activity. Training on a motor skill engages multiple plastic mechanisms in M1 such as long-term potentiation, synaptogenesis, circuit reorganization, and modifications of cortical dynamics47–50. As a consequence, processing in M1 may become more efficient. At the neural level, efficiency can be expressed through any number of processes such as changes in synaptic efficacy, relocation of active synapses to more effective sites, and synchronization of synaptic activity. The end result would be what we observed — less synaptic activity is required to generate a given amount of neuronal activity. Whatever the mechanism, our observations prompt a new caution for the interpretation of functional imaging results, particularly in the context of skilled performance acquired through extended practice. Low activation is not always a sign of low neuronal activity. Instead, it may be a reflection of plastic mechanisms involved in the development of expertise.
ONLINE METHODS
We trained twelve monkeys (Macaca nemestrina or mulatta, 3.1–10.4 kg, 6 females) to perform sequential reaching tasks or a control task. The care of the animals and the experimental protocols adhered to the NIH Guide for the Care and Use of Laboratory Animals and the US Public Health Service Policy on Humane Care and Use of Laboratory Animals. All procedures used followed institutional guidelines and were approved by the Institutional Animal Care and Use Committee. The monkeys were singly housed with a 12 h light/dark cycle. We performed all experiments during the light cycle. Data collection and analysis were not performed blind to the conditions of the experiments, nor processed randomly. For all statistical comparisons, we tested for data normality using the Shapiro-Wilk test and equality of variances using Levene’s test. Based on this, we selected parametric or non-parametric tests for specific comparisons. In cases where data normality could not be evaluated (i.e., samples of n = 2 for individual task comparisons), we assumed a normal distribution of the data. We report two-sided probabilities.
Behavioral tasks
Ten monkeys were trained to perform two of four sequential reaching tasks with the right arm20,31. In two of the tasks, the sequences of movement were visually-guided (Track, n = 2; Random, n = 3). In the other two tasks, the sequences were internally generated (Rem, n = 2; Repeating, n = 3). No statistical methods were used to pre-determine group sizes. Our group sizes are similar to those reported in previous publications27–29,37,44–45. Monkeys were assigned to their experimental group based on need and availability.
Track task
Two monkeys faced a panel with five touch-sensitive targets positioned in a horizontal row. A red light-emitting diodes (LEDs) located above each target served as instruction cue. The monkey placed its hand on a hold key to initiate a trial. An auditory tone signaled contact with the hold key. The monkey had to maintain contact with the hold key for a variable time (0.6–1.5 s). At the end of the hold period, one target cue was lit and a brief auditory “Go” signal was presented. The monkey had to release the hold key and quickly (< 1 s) contact the cued target. A correct response immediately triggered the illumination of a second target cue over another target. The monkey had to quickly move from the first to the second target. A third cycle of cue-target contact followed. A brief tone signaled the correct completion of the movement sequence and the monkey received fruit juice. If the monkey made an error, the trial was aborted and the same set of target cues was repeated on the next trial. Otherwise, a new trial was initiated with a different sequence selected pseudo-randomly from a set of eight. Trials were separated by 1.1–1.5 s intervals. The monkeys were also trained on the Rem task and alternated between the two within training sessions. For the 2DG experiments, they only performed the Track task. During the 2DG experiment, the two monkeys performed correctly on 97% and 98% of the trials.
Rem task
The two monkeys trained on the Rem task were also trained on the Track task and alternated between the two within training sessions. For the 2DG experiment, they performed the Rem task exclusively. The monkey placed its hand on a hold key to initiate a trial. At the end of the hold period, three LEDs, each above a target, were illuminated in sequence. The LEDs served as instruction cues for the upcoming reaching movements. The LEDs remained illuminated until the end of the trial. The monkey had to keep his hand on the hold key until the Go signal was given, 1.25–2.15 s after presentation of the instruction cues. After the Go signal, the monkeys had to release the hold key and quickly contact each of the three targets in the instructed order. Thus, the series of movements that the monkeys made on each trial were based on memorized sequence information. Correct completion of the sequence was signaled and rewarded as above. The sequences of movements performed in the Rem task were the same as those performed in the Track task. At the time of the 2DG experiment, the monkeys performed correctly on ~90% of the trials.
Random task
Three monkeys performed continuous reaching movements to visual targets. The monkeys were also trained on the Repeating task and alternated between the two tasks within training sessions. For the 2DG experiment, they performed the Random task exclusively. The monkey faced a touch-sensitive monitor that displayed the outlines of five targets arranged in a horizontal row. On each trial, one of the targets was filled with yellow. The targets were randomly selected without repetition. The monkeys had to quickly (< 800 ms) touch the filled target. Contact outside of the target area was signaled with an error tone. Trials were repeated at the occurrence of an incorrect response. Correct contact within the filled target was indicated by a brief tone. A new target was filled 100 ms after a correct response and the monkey again had to quickly touch the indicated target. This way the monkey performed continuously, moving from one target directly to the next without pause. The monkey received water after every fourth correct response. During the 2DG experiments, the monkeys performed correctly on 93%, 96% and 98% of the trials.
Repeating task
Three monkeys performed repeating sequences of reaching movements. The monkeys were also trained on the Random task and alternated between the two within training sessions. For the 2DG experiment, they performed the Repeating task exclusively. In the Repeating task, the targets followed a repeating sequence 3 elements long. A new target was colored yellow 400 ms after a correct response. The task allowed the monkeys to contact the next target in the sequence during the 400 ms delay before it was shown. When they made these predictive responses, the task was incremented to the next element of the sequence without display of the touched target. As a result, the monkeys could perform continuously without visual cues. The monkeys received water after every fifth correct response. During the 2DG experiments, the monkeys performed correctly on 90%, 96% and 97% of the trials. They made predictive responses without visual cues on 92%, 96% and 94% of correct trials.
Lick task
To control for activations that were not directly related to the performance of the reaching movements, we trained another two monkeys to perform a licking task. In the Lick task, the monkey faced the panel of targets used for the Track and Rem tasks. No arm movement was required. The animal received fruit juice at variable intervals. Visual and auditory signals comparable to those of the Track and Rem tasks were generated. However, these signals were meaningless for the Lick task and the monkeys did not respond to them.
2DG experiments
For the 2DG experiments, 5 monkeys performed a visually-guided task, 5 monkeys performed an internally-generated task and 2 monkeys performed the Lick task. We followed conventional procedures for semi-quantitative analysis of 2-deoxyglucose (2DG) uptake as previously described22. On the day of the 2DG experiment, the monkeys performed the trained task after receiving an i.v. injection 14C-2DG in sterile saline (60–100 μCi/kg, 55 mCi/mmol, American Radiolabeled Chemicals: St. Louis, MO). After 35–45 min the animal was quickly perfused, the brain extracted, frozen, and kept at −70° C for later processing. Autoradiographs of 30 μm thick brain sections (25 μm for monkey N1) were digitized at a pixel resolution of 55–77 μm by 45–62 μm and transformed to 14C concentration values based on calibrated standards applied to each film. Average 14C values in the middle cortical layers were used as the local measurements of activation in our maps of M1. These maps were constructed using sections spaced 90 μm apart (100 μm for N1) and were smoothed slightly for illustration only.
To compare patterns of activation between animals, we transformed 14C concentration values to a normalized activation scale (% 2DG uptake). To accomplish this, the range of activation was defined as the difference between peak 14C tissue concentration and background concentration. Peak concentration was measured in the face representation of the primary somatosensory cortex on the precentral gyrus where 2DG uptake was always high. We measured background cortical concentration in a portion of the leg representation of M1 on the precentral gyrus where 2DG uptake was consistently low. The values assigned to peak and background were the median 14C concentration in a 2 mm2 area in each region. 14C concentration value of each pixel was expressed as a percentage of the range determined for each animal. We validated the normalization for the group of 12 animals included here as described previously22 (Supplementary Fig. 6). Normalizing the 2DG data relative to background uptake did not influence the conclusions (not shown). Indeed, there was a high correlation between data normalized relative to the full range of 2DG uptake and that normalized relative to background uptake (r = 0.97, measured across subjects for 2 areas of interest in arm M1 and 3 in control areas).
We outlined the location of arm M1 based on data from prior anatomical and physiological studies (Fig. 1a). Only activations in the left hemisphere (contralateral to the moving arm) were examined. We measured activation separately for the portion of M1 on the precentral gyrus and in the central sulcus because of the growing evidence for their anatomical and functional distinction51. We obtained global measures of activation by calculating the median 2DG uptake across all pixels in the rostral (gyrus) and caudal (sulcus) portions of arm M1. We obtained peak activation measures from the median value in 2 mm2 areas of interest centered on the most intensely activated region of rostral and caudal M1. We considered 2DG uptake significant if it was ≥ 3 standard deviations above background (corresponding to 22–24%). We quantified the extent of activation as the percentage of significantly activated pixels in arm M1.
Comparison of neuron activity and 2DG uptake
For each of two monkeys, we recorded the activity of neurons in M1 during the Random and Repeating tasks prior to the 2DG experiments. We used conventional recording techniques described in detail in ref. 20. In one of these monkeys (N14), we subsequently measured 2DG uptake in M1 during the Repeating task. In the other animal (N15), we measured 2DG uptake during the Random task.
The sample of neurons considered in the current analysis includes, but is not limited to, the neurons described in ref. 20. We recorded from a wide area in M1 including axial, proximal and distal representations. Most penetrations were made in the precentral gyrus (Fig. 7a,b). The database includes a total of 714 task-related and unrelated neurons (502 from monkey N14, 212 from N15) sampled during 95 (N14) and 52 (N15) penetrations. Neurons were sampled across the cortical thickness with a similar distribution in the 2 animals (Supplementary Fig. 7). The median number of neurons recorded in electrode penetrations of both monkeys was 4. For each neuron, we calculated the average discharge frequency as the average spike count per trial divided by the average trial time, scaled to spikes/second. We calculated separately the spikes/s in correct trials and in 3 types of error trials (no response, wrong target touched, and corrective responses). We then calculated the estimated activity for the 2DG session as the weighted average of spikes/s in the four trial types based on their proportion in the session of the 2DG experiment. This measure of neuron activity parallels the cumulative uptake of 2DG during the experimental session. To correct for slight differences in the duration of the 2DG experiments, and thus the total activity associated with it, we calculated the total amount of spikes for each neuron as the neuron’s average firing rate multiplied by the experiment duration.
For illustration, we generated histograms of activity representing the average spike counts in 10 ms time bins (scaled to spikes/s) for correct trials aligned on the time of a target hit or release. Histograms were derived for single neurons and single moves (e.g., Figs. 5–6), or collapsed across moves to show cumulative activity on a longer time scale (e.g., Supplementary Figs. 2–5).
We examined the relationship between local neuron activity and local 2DG uptake with a linear regression analysis. For this purpose, we calculated several measures of neuron activity: 1) the average firing rate (spikes/s as described above), 2) the average of peak activity measured in any 3 consecutive 20 ms bins on each trial, weighted by trial type as described above for spikes/s, and 3) the average modulation of activity (peak value minus the lowest value in any 3 consecutive 20 ms bins on each trial), weighted by trial type as described above. However, peak frequency and modulation of activity were strongly correlated with the average firing rate in both animals (2DG-Random animal, r = 0.88 and 0.92; ANOVA, d.f. = 1, 212, p < 0.001; 2DG-Repeating animal, r = 0.90 and 0.95; ANOVA, d.f. = 1,502, p < 0.001). Consequently, using peak or modulation measures for the regression with 2DG uptake gave results similar to the analysis using the average firing rate. Thus, for the sake of brevity, we report only the relation between average firing rate and 2DG uptake. This choice is also congruent with: 1) the fact that 2DG uptake represents the integrated rate of metabolism over a long period of time21 and 2) the association between total neuronal activity and neuroenergetics52. Thus, average neuron activity is the most appropriate measure for comparison with 2DG uptake.
Because of the greater uncertainty in the location of recordings along the anterior bank of the central sulcus, we included only penetration sites on the precentral gyrus in the regression analysis. We averaged the firing rate of all neurons at each recording site. For each monkey, the 2-dimensional grid of neuron activity was registered with the 2-dimensional grid of 2DG uptake in M1. We averaged the 2DG uptake in a 100 μm by 100 μm area centered at each penetration site. In monkey N14 (2DG-Repeating animal), the initial registration of 2DG and neuron data was based on the location of 3 small electrolytic lesions made in the white matter of M1 5 days prior to the 2DG experiment. In monkey N15 (2DG-Random animal), the initial registration was performed by: 1) matching the location and course of the central sulcus in the reconstructed 2DG data to photographs of the animal’s brain (through the exposed dura) and 2) fitting the map of M1 obtained with intracortical microstimulation (see ref. 20 for details) to anatomical landmarks. Although these procedures provided a good fit, we could not rule out the possibility of a small registration error between the two data sets. With this concern in mind, we re-computed the regression analysis following multiple combinations of rotation and translation of one data set relative to the other. Rotations of up to ± 6° were performed in 2° increments, and translations of up to ± 1 mm were performed in 0.1 mm increments in the anterior-posterior and medio-lateral directions. The probabilities in these calculations were adjusted using Bonferroni correction. Alterations in alignment as large as ± 0.5 mm in all directions, and rotations as large as ± 6° did not significantly alter the results. In fact, neighboring pixels in maps of 2DG activity (i.e., within 0.5 mm of each other) are highly correlated (spatial correlogram, r ~0.7). This makes the analysis of the relationship between 2DG and neural activity robust to small differences in registration.
Relation between 2DG uptake and performance parameters
We examined the relation between 2DG uptake in arm M1 and each performance parameter (Supplementary Tables 1 and 2) using a regression analysis. Previous studies in monkeys and humans have shown positive linear relations between neuron activity or metabolic signals in M1 and movement parameters such as velocity or amplitude within the range of the present study53–59. Based on this, we used linear models (least squares) for our analyses. In each case, the significance of the association was assessed with an ANOVA. The regressions were calculated for the average of 2DG uptake on the precentral gyrus and in the anterior bank of the central sulcus for each animal (Supplementary Fig. 1). In addition, we performed a multiple regression analysis to assess the joint influence of performance parameters on 2DG uptake. Several parameters could not be included in the regression model due to strong multicollinearity. The most complete model that could be tested without multicollinearity confounds included: constant, number of rewards, movement rate, reward rate, movement speed, and movement amplitude. We found no significant relation between 2DG uptake and any performance parameter (Supplementary Fig. 1).
Supplementary Material
Acknowledgments
This material is based upon work supported in part by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, NIH Grants R01 NS24328 (PLS) and P01 NS044393 (PLS). The contents do not represent the views of the Department of Veterans Affairs or the United States Government. We are grateful to Mike Page for the development of computer programs and to Michele O’Malley and Kathleen McDonald for their expert technical assistance.
Footnotes
AUTHOR CONTRIBUTIONS
N.P. conducted the 2DG experiments, Y.M. recorded single neuron activity, N.P. analyzed data, N.P. and P.L.S. wrote the manuscript.mailto: strickp@hscsyr.edu
References
- 1.Adams JA. Historical review and appraisal of research on the learning, retention, and transfer of human motor skills. Psychol Bull. 1987;101:41–74. [Google Scholar]
- 2.Proctor RW, Dutta A. Skill acquisition and human performance. Sage Publications; Thousand Oaks, CA: 1995. [Google Scholar]
- 3.Dayan E, Cohen LG. Neuroplasticity subserving motor skill learning. Neuron. 2011;72:443–454. doi: 10.1016/j.neuron.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amunts K, et al. Motor cortex and hand motor skills: Structural compliance in the human brain. Hum Brain Mapp. 1997;5:206–215. doi: 10.1002/(SICI)1097-0193(1997)5:3<206::AID-HBM5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. J Neurosci. 2003;23:9240–9245. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E. Increased cortical representation of the fingers of the left hand in string players. Science. 1995;270:305–307. doi: 10.1126/science.270.5234.305. [DOI] [PubMed] [Google Scholar]
- 7.Schwenkreis P, et al. Assessment of sensorimotor cortical representation asymmetries and motor skills in violin players. Eur J Neurosci. 2007;26:3291–3302. doi: 10.1111/j.1460-9568.2007.05894.x. [DOI] [PubMed] [Google Scholar]
- 8.Hund-Georgiadis M, von Cramon DY. Motor-learning-related changes in piano players and non-musicians revealed by functional magnetic-resonance signals. Exp Brain Res. 1999;125:417–425. doi: 10.1007/s002210050698. [DOI] [PubMed] [Google Scholar]
- 9.Krings T, et al. Cortical activation patterns during complex motor tasks in piano players and control subjects. A functional magnetic resonance imaging study. Neurosci Lett. 2000;278:189–193. doi: 10.1016/s0304-3940(99)00930-1. [DOI] [PubMed] [Google Scholar]
- 10.Jäncke L, Shah NJ, Peters M. Cortical activations in primary and secondary motor areas for complex bimanual movements in professional pianists. Cogn Brain Res. 2000;10:177–183. doi: 10.1016/s0926-6410(00)00028-8. [DOI] [PubMed] [Google Scholar]
- 11.Haslinger B, et al. Reduced recruitment of motor association areas during bimanual coordination in concert pianists. Hum Brain Mapp. 2004;22:206–215. doi: 10.1002/hbm.20028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meister I, et al. Effects of long-term practice and task complexity in musicians and nonmusicians performing simple and complex motor tasks: Implications for cortical motor organization. Hum Brain Mapp. 2005;25:345–352. doi: 10.1002/hbm.20112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Floyer-Lea A, Matthews PM. Changing brain networks for visuomotor control with increased movement automaticity. J Neurophysiol. 2004;92:2405–2412. doi: 10.1152/jn.01092.2003. [DOI] [PubMed] [Google Scholar]
- 14.Landau SM, D’Esposito M. Sequence learning in pianists and nonpianists: An fMRI study of motor expertise. Cogn Affect Behav Neurosci. 2006;6:246–259. doi: 10.3758/cabn.6.3.246. [DOI] [PubMed] [Google Scholar]
- 15.Xiong J, et al. Long-term motor training induced changes in regional cerebral blood flow in both task and resting states. Neuroimage. 2009;45:75–82. doi: 10.1016/j.neuroimage.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li CS, Padoa-Schioppa C, Bizzi E. Neuronal correlates of motor performance and motor learning in the primary motor cortex of monkeys adapting to an external force field. Neuron. 2001;30:593–607. doi: 10.1016/s0896-6273(01)00301-4. [DOI] [PubMed] [Google Scholar]
- 17.Paz R, Boraud T, Natan C, Bergman H, Vaadia E. Preparatory activity in motor cortex reflects learning of local visuomotor skills. Nature Neurosci. 2003;6:882–890. doi: 10.1038/nn1097. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Shaul Y, et al. Neuronal activity in motor cortical areas reflects the sequential context of movement. J Neurophysiol. 2004;91:1748–1762. doi: 10.1152/jn.00957.2003. [DOI] [PubMed] [Google Scholar]
- 19.Lu X, Ashe J. Anticipatory activity in primary motor cortex codes memorized movement sequences. Neuron. 2005;45:967–973. doi: 10.1016/j.neuron.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 20.Matsuzaka YM, Picard N, Strick PL. Skill representation in the primary motor cortex after long-term practice. J Neurophysiol. 2007;97:1819–1832. doi: 10.1152/jn.00784.2006. [DOI] [PubMed] [Google Scholar]
- 21.Sokoloff L, et al. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 22.Picard N, Strick PL. Activation of the supplementary motor area (SMA) during performance of visually guided movements. Cereb Cortex. 2003;13:977–986. doi: 10.1093/cercor/13.9.977. [DOI] [PubMed] [Google Scholar]
- 23.Sokoloff L. Sites and mechanisms of function-related changes in energy metabolism in the nervous system. Dev Neurosci. 1993;15:194–206. doi: 10.1159/000111335. [DOI] [PubMed] [Google Scholar]
- 24.Smith AJ, et al. Cerebral energetics and spiking frequency: The neurophysiological basis of fMRI. Proc Natl Acad Sci USA. 2002;99:10765–10770. doi: 10.1073/pnas.132272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D-S, et al. Spatial relationship between neuronal activity and BOLD functional MRI. Neuroimage. 2004;21:876–885. doi: 10.1016/j.neuroimage.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 26.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453:869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 27.Matsunami K, Kawashima T. Radioactive 2-DG incorporation patterns in the mesial frontal cortex of task-performing monkeys. Neurosci Lett. 1995;23:365–375. doi: 10.1016/0168-0102(95)00964-U. [DOI] [PubMed] [Google Scholar]
- 28.Savaki HE, Dalezios Y. 14C-deoxyglucose mapping of the monkey brain during reaching to visual targets. Prog Neurobiol. 1999;58:473–540. doi: 10.1016/s0301-0082(98)00080-x. [DOI] [PubMed] [Google Scholar]
- 29.Gregoriou GG, Luppino G, Matelli M, Savaki HE. Frontal cortical areas of the monkey brain engaged in reaching behavior: A 14C-deoxyglucose imaging study. Neuroimage. 2005;27:442–464. doi: 10.1016/j.neuroimage.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 30.Mushiake H, Strick PL. Preferential activity of dentate neurons during limb movements guided by vision. J Neurophysiol. 1993;70:2660–2664. doi: 10.1152/jn.1993.70.6.2660. [DOI] [PubMed] [Google Scholar]
- 31.Johnson PB, Ferraina S, Bianchi L, Caminiti R. Cortical networks for visual reaching: Physiological and anatomical organization of frontal and parietal lobe arm regions. Cereb Cortex. 1996;6:102–119. doi: 10.1093/cercor/6.2.102. [DOI] [PubMed] [Google Scholar]
- 32.Crammond DJ, Kalaska JF. Prior information in motor and premotor cortex: Activity during the delay period and effect on pre-movement activity. J Neurophysiol. 2000;84:986–1005. doi: 10.1152/jn.2000.84.2.986. [DOI] [PubMed] [Google Scholar]
- 33.Hanakawa T, Dimyan MA, Hallett M. Motor planning, imagery, and execution in the distributed motor network: A time-course study with functional MRI. Cereb Cortex. 2008;18:2775–2788. doi: 10.1093/cercor/bhn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poldrack RA. Imaging brain plasticity: Conceptual and methodological issues - A theoretical perspective. Neuroimage. 2000;12:1–13. doi: 10.1006/nimg.2000.0596. [DOI] [PubMed] [Google Scholar]
- 35.Grill-Spector K, Henson R, Martin A. Repetition and the brain: neural models of stimulus-specific effects. Trends Cogn Sci. 2006;10:14–23. doi: 10.1016/j.tics.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton AFC, Grafton ST. Repetition suppression for performed hand gestures revealed by fMRI. Hum Brain Mapp. 2009;30:2898–2906. doi: 10.1002/hbm.20717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mushiake H, Inase M, Tanji J. Neuronal activity in primate premotor, supplementary motor and precentral motor cortex during visually guided and internally determined sequential movements. J Neurophysiol. 1991;66:705–718. doi: 10.1152/jn.1991.66.3.705. [DOI] [PubMed] [Google Scholar]
- 38.Swain RA, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neurosci. 2003;117:1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- 39.Jueptner M, Weiller C. Does measurement of regional cerebral blood flow reflect synaptic activity?—Implications for PET and fMRI. Neuroimage. 1995;2:148–156. doi: 10.1006/nimg.1995.1017. [DOI] [PubMed] [Google Scholar]
- 40.Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- 41.Karni A, et al. Functional evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–158. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- 42.Hluštik P, Solodkin A, Noll DC, Small SL. Cortical plasticity during three-week motor skill learning. J Clin Neurophysiol. 2004;21:180–190. doi: 10.1097/00004691-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Kelly AMC, Garavan H. Human functional neuroimaging of brain changes associated with practice. Cereb Cortex. 2005;15:1089–1102. doi: 10.1093/cercor/bhi005. [DOI] [PubMed] [Google Scholar]
- 44.Juliano SA, Whitsel BL. A combined 2-deoxyglucose and neurophysiological study of primate somatosensory cortex. J Comp Neurol. 1987;263:514–525. doi: 10.1002/cne.902630405. [DOI] [PubMed] [Google Scholar]
- 45.Devor A, et al. Stimulus-induced changes in blood flow and 2-deoxyglucose uptake dissociate in ipsilateral somatosensory cortex. J Neurosci. 2008;28:14347–14357. doi: 10.1523/JNEUROSCI.4307-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nudo RJ, Masterton RB. Stimulation induced [14C]2-deoxyglucose labeling of synaptic activity in the central nervous system. J Comp Neurol. 1986;245:553–565. doi: 10.1002/cne.902450410. [DOI] [PubMed] [Google Scholar]
- 47.Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–536. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 48.Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol. 2006;101:1776–1782. doi: 10.1152/japplphysiol.00515.2006. [DOI] [PubMed] [Google Scholar]
- 49.Kilavik BE, et al. Long-term modifications in motor cortical dynamics induced by intensive practice. J Neurosci. 2009;29:12653–12663. doi: 10.1523/JNEUROSCI.1554-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu M, Yu X, Lu J, Zuo Y. Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature. 2012;483:92–95. doi: 10.1038/nature10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rathelot JA, Strick PLS. Subdivisions of primary motor cortex based on cortico-motoneuronal cells. Proc Natl Acad Sci USA. 2009;106:918–923. doi: 10.1073/pnas.0808362106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shulman RG, Rothman DL, Hyder F. Stimulated changes in localized cerebral energy consumption under anesthesia. Proc Natl Acad Sci USA. 1999;96:3245–3250. doi: 10.1073/pnas.96.6.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fu QG, Suarez JI, Ebner TJ. Neuronal specification of direction and distance during reaching movements in the superior precentral premotor area and primary motor cortex of monkeys. J Neurophysiol. 1993;70:2097–2116. doi: 10.1152/jn.1993.70.5.2097. [DOI] [PubMed] [Google Scholar]
- 54.Ashe J, Georgopoulos AP. Movement parameters and neural activity in motor cortex and area 5. Cereb Cortex. 1994;6:1047–3211. doi: 10.1093/cercor/4.6.590. [DOI] [PubMed] [Google Scholar]
- 55.Turner RS, Desmurget M, Grethe J, Crutcher MD, Grafton ST. Motor subcircuits mediating the control of movement extent and speed. J Neurophysiol. 1998;90:3958–3966. doi: 10.1152/jn.00323.2003. [DOI] [PubMed] [Google Scholar]
- 56.Moran DW, Schwartz AB. Motor cortical representation of speed and direction during reaching. J Neurophysiol. 1999;82:2676–2692. doi: 10.1152/jn.1999.82.5.2676. [DOI] [PubMed] [Google Scholar]
- 57.Stark E, Drori R, Asher I, Ben-Shaul Y, Abeles M. Distinct movement parameters are represented by different neurons in the motor cortex. Eur J Neurosci. 2007;26:1055–1066. doi: 10.1111/j.1460-9568.2007.05711.x. [DOI] [PubMed] [Google Scholar]
- 58.Wand W, Chan SS, Heldman DA, Moran DW. Motor cortical representation of position and velocity during reaching. J Neurophysiol. 2007;97:4258–4270. doi: 10.1152/jn.01180.2006. [DOI] [PubMed] [Google Scholar]
- 59.Orban P, et al. Functional neuroanatomy associated with the expression of distinct movement kinematics in motor sequence learning. Neurosci. 2011;179:94–103. doi: 10.1016/j.neuroscience.2011.01.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.