Abstract
Inflammation is responsible for secondary organ failure after trauma and hemorrhagic shock (T/HS). Adenosine, acting through four G protein-coupled cell surface receptors, A1, A2A, A2B, and A3, exerts a number of tissue protective and anti-inflammatory effects. The goal of the present study was to test the effect of A2B adenosine receptor stimulation on T/HS-induced organ injury and inflammation in rats. Rats after T/HS were resuscitated with Ringer’s lactate containing the A2B receptor agonist BAY 60–6583 or its vehicle. We found that BAY 60–6583 decreased T/HS-induced lung permeability and plasma creatine kinase levels but failed to affect T/HS-induced lung neutrophil infiltration and IκBα expression and plasma alanine aminotransferase levels. Thus, we conclude that stimulation of A2B receptors protects against T/HS-induced lung and muscle injury.
Keywords: Trauma, Hemorrhagic shock, A2B adenosine receptor, BAY 60–6583
Background
Organ failure following trauma and hemorrhage is a consequence of immune dysfunction and cellular damage. Trauma patients who survive the early post-injury period can develop a wide range of conditions, such as acute lung injury, acute respiratory distress syndrome, systemic inflammatory response syndrome, and multiple organ dysfunction syndrome [1, 2].
The endogenous purine nucleoside adenosine has been implicated in the regulation of the development of multiple organ dysfunction following trauma. Adenosine has long been known as a cytoprotective and anti-inflammatory agent that accumulates in response to shock, trauma, ischemia, and inflammation [3, 4]. Cells release adenine nucleotides such as adenosine triphosphate (ATP) or adenosine diphosphate (ADP) in response to stress by a number of mechanisms, including membrane damage, connexin/pannexin channels, and hormone transporting vesicles [3]. Extracellular ATP and ADP are then degraded to adenosine by ecto-nucleoside triphosphate diphosphohydrolase 1 (CD39) and ecto-5′-nucleotidase (CD73) enzymes that are located on the surface of various cell types [5]. Adenosine is also released directly from cells such as neutrophils or endothelial cells to the extracellular space via nucleoside transporters [5, 6]. Extracellular adenosine signals through four G protein-coupled cell surface receptors, A1, A2A, A2B, and A3 adenosine receptors (ARs) [7]. ARs are expressed on virtually all cell types that are involved in orchestrating an inflammatory/immune response, and these cell types include polymorphonuclear neutrophil (PMN)s, monocytes/macrophages, endothelial cells, lymphocytes, and dendritic cells [8–10]. While all adenosine receptors have immunomodulatory functions, stimulation of A2AARs and A2BARs is primarily anti-inflammatory, whereas A1ARs and A3ARs have both pro- and anti-inflammatory effects [8].
We have previously shown the protective effects of pre-treatment of rats with an A2AAR agonist against organ injury in trauma/hemorrhagic shock (T/HS) and resuscitation, a clinically relevant model comprising global ischemia and reperfusion [11]. The goal of present study was to evaluate the effect of A2BAR stimulation on T/HS-induced organ injury in rats.
Materials and methods
Drugs and reagents
The selective A2BAR agonist 2-[6-amino-3,5-dicyano-4-[4-(cyclopropylmethoxy) phenyl]pyridin-2-ylsulfanyl]acetamide (BAY 60–6583) was provided by Bayer Healthcare [12]. Stock solutions of BAY606583 were prepared using dimethylsulfoxide (Sigma Aldrich, St. Louis, MO, USA). Ringer’s lactate (RL) was obtained from Fisher Scientific (Pittsburgh, PA, USA).
Experimental animals and T/HS
Male Sprague Dawley rats weighing 300–400 g obtained from the Jackson Laboratory (Bar Harbor, ME, USA) were used for the experiments. Rats were anesthetized with intraperitoneal sodium pentobarbital (50 mg/kg), and their right femoral arteries and jugular veins were isolated using aseptic techniques and were cannulated with polyethylene (PE-50) tubing or 50-gauge silicone catheter containing 0.1 ml of heparinized saline (10 U/ml), respectively. A laparotomy was then performed on each rat, and the abdominal cavity was closed with a running 3–0 silk surgical suture. The arterial catheter was attached to a continuous blood-pressure monitoring system (Powerlab 8/30, ADinstruments, Colorado Springs, CO, USA) during the experiment. The jugular vein catheter was used for blood withdrawal, volume resuscitation, and Evan's blue dye (Sigma Aldrich, St. Louis, MO, USA) administration. Rectal temperature was monitored throughout the shock period and was maintained at 37 °C by a heat lamp positioned over the animal. T/HS rats were exsanguinated to a mean arterial pressure of 30–35 mmHg through the internal jugular vein (at a rate of 1 ml/min) and were maintained at that level for 90 min by withdrawing or reinfusing shed blood (kept at 37 °C) as needed. Trauma/sham-shock (T/SS) rats underwent a laparotomy and vascular cannulation, but no blood was withdrawn. At the end of the 90-min shock or sham-shock period, the animals were resuscitated with RL containing either 0.5 mg/kg BAY 60–6583 or its vehicle [13, 14]. All rats were maintained in accordance with the recommendations of the “Guide for the Care and Use of Laboratory Animals,” and the experiments were approved by the Institutional Animal Care and Use Committee of the New Jersey Medical School.
Measurement of lung permeability
Three hours after the end of the 90-min shock period, the rats were injected with 10 mg of Evan’s blue dye through the internal jugular catheter. After 5 min, to allow for complete circulation of the dye, a blood sample (1.5 ml) was withdrawn from the femoral artery catheter, and the plasma was used to determine the plasma Evan’s blue dye concentration. Twenty minutes after injection of the dye, the rats were killed and the lungs harvested. Bronchoalveolar lavage was performed on the excised lungs by lavaging the lungs three times with 5 ml aliquots of physiological saline. The recovered bronchoalveolar lavage fluid (BALF) was then centrifuged at 1,500×g at 4 °C for 20 min to remove any cells. The supernatant fluid was assayed spectrophotometrically at 620 nm to measure the concentration of the Evan’s blue dye in the BALF. The concentration of Evan’s blue dye in the BALF was expressed as the percentage of that present in the plasma.
Assessment of myeloperoxidase activity
For measuring myeloperoxidase (MPO) activity in lung, tissue samples were homogenized in extraction buffer (20 mM acetate buffer (pH 4.7) containing 0.2 M NaCl, 0.5 % cetyltrimethylammonium bromide, 10 μg/ml of phenylmethylsulfonyl fluoride, and 1 mM EDTA). MPO activity was measured using the NWLSS Myeloperoxidase Activity Assay (Northwest Life Science Specialties, Vancouver, WA, USA) and according to the instructions provided with the kit.
Protein isolation and Western blot
For Western blot analysis, lungs were homogenized in a Dounce homogenizer in modified radioimmunoprecipitation assay buffer (50 mM Tris HCl, 150 mM NaCl, 1 mM EDTA, 0.25 % sodium deoxycholate, 1 % Nonidet P-40, 100× diluted proteinase inhibitor cocktail mix). Then the lysates were centrifuged at 15,000×g for 15 min, and the supernatant was recovered. The Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA) was used to determine the protein concentrations. Protein samples (40 μg from each organ) were separated on 8–12 % Tris-glycine gel (Invitrogen Life Technologies, Carlsbad, CA, USA) and transferred to nitrocellulose membrane. The membranes were probed with a monoclonal antibody against IκBα (Cell Signaling Technology, Danvers, MA, USA), which was followed by incubating with a secondary HRP conjugated anti-rabbit antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA). To assess equal protein loading, HRP-conjugated polyclonal goat anti-β actin antibody was used from Santa Cruz Biotechnology. Bands were detected using Chemiluminescent HRP Detection Reagent (Denville Scientific, South Plainfield, NJ, USA).
Determination of alanine aminotransferase and creatine kinase levels in blood of T/HS rats
Plasma concentrations of alanine aminotransferase (ALT) and creatine kinase (CK) were analyzed using a clinical chemistry analyzer system (VetTest8008, IDEXX Laboratories).
Statistical analysis
Values in the figures are expressed as mean plus or minus of the standard error of mean (SEM) of the indicated number of observations. Statistical analyses of the data were performed using Student t test.
Results
A2BAR stimulation protects against T/HS-induced lung injury
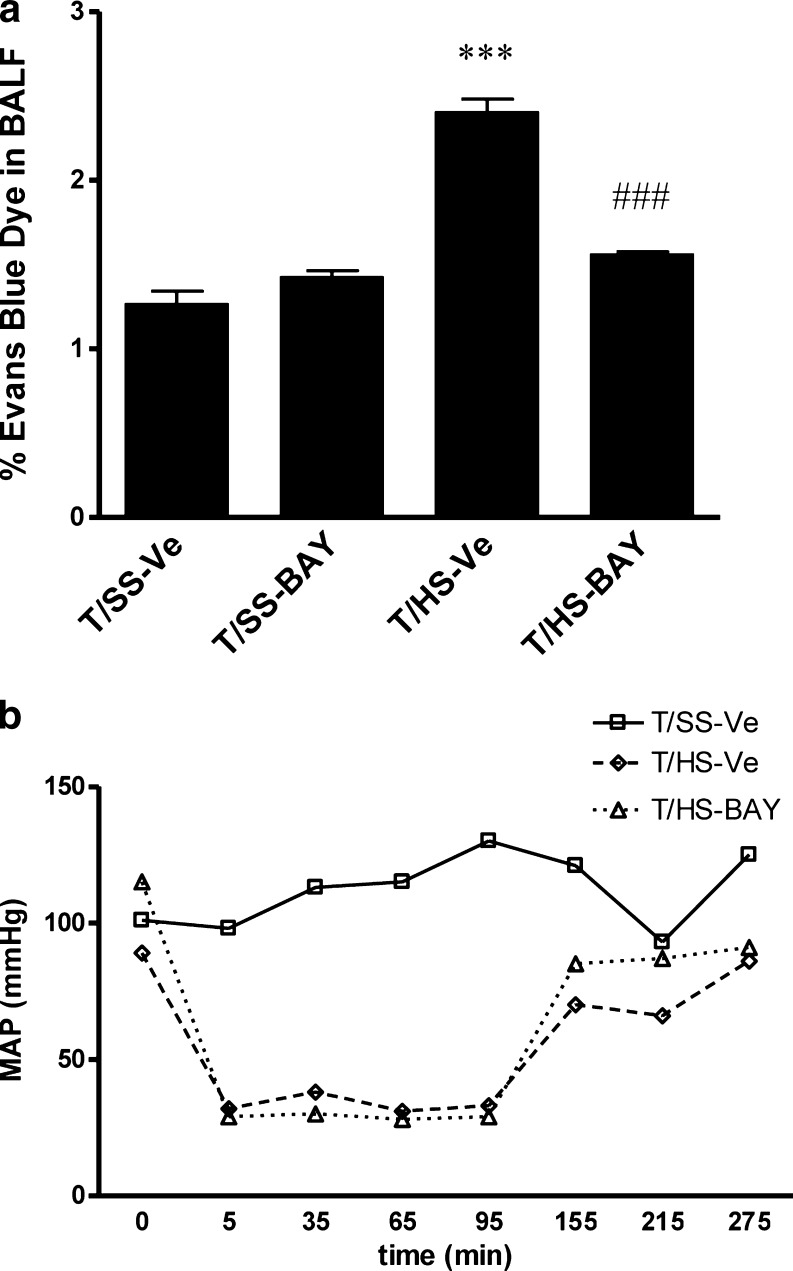
We began our experiments by testing the efficacy of BAY 60–6583 in preventing T/HS-induced lung injury. We found that lung permeability was increased in rats subjected to T/HS in comparison to T/SS. Resuscitation with RL containing BAY 60–6583 provided an almost complete protection against the T/HS-induced increase in lung permeability (Fig. 1a). We also monitored blood pressure and found that BAY 60–6583 did not have any adverse effect on blood pressure (Fig. 1b).
Fig. 1.
Effect of BAY 60–6583 on lung permeability and mean arterial pressure (MAP) in rats subjected to trauma/hemorrhagic shock (T/HS). a Trauma/sham shock (T/SS) or T/HS rats were resuscitated with Ringer’s lactate (RL) containing 0.5 mg/kg BAY 60–6583 or its vehicle. Lung permeability was evaluated using Evan’s blue dye. ***p < 0.001 vs T/SS-vehicle, ###p < 0.001 vs. T/HS-vehicle. Data shown are mean ± SEM (n = 7 to 14). b MAP of T/SS rats resuscitated with RL containing vehicle and T/HS rats resuscitated with RL containing vehicle or 0.5 mg/kg BAY 60–6583. Data shown are representative of seven experiments in each group
A2BAR stimulation fails to inhibit T/HS-induced lung inflammation
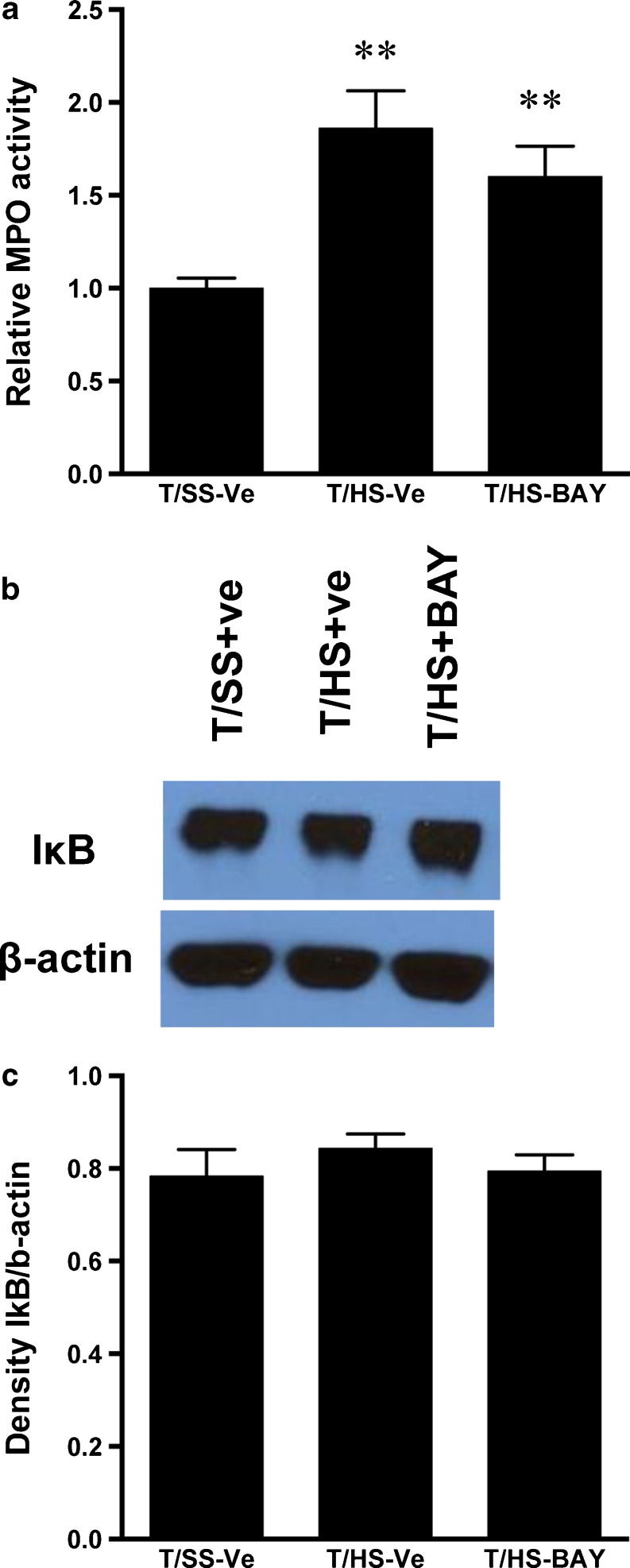
We next studied the effect of A2BAR stimulation on neutrophil infiltration into the lung. Our results showed that BAY 60–6583 failed to decrease T/HS-induced neutrophil infiltration as indicated by MPO activity (Fig. 2a). We then examined the effect of BAY 60–6583 on inflammation in the lung by determining IκBα levels. We found that both T/HS and BAY 60–6583 failed to affect IκBα levels (Fig. 2b, c).
Fig. 2.
BAY 60–6583 fails to protect against T/HS-induced neutrophil infiltration and inflammation in the lung. a Myeloperoxidase (MPO) level in lungs of T/SS rats resuscitated with RL containing vehicle or T/HS rats resuscitated with RL containing vehicle or 0.5 mg/kg BAY 60–6583. Data shown are mean ± SEM of fold increase relative to T/SS-vehicle. **p < 0.01 vs. T/SS-vehicle (n = 8–10). b Expression of IκBα protein in lungs of T/SS rats resuscitated with RL containing vehicle, T/HS rats resuscitated with RL containing vehicle or 0.5 mg/kg BAY 60–6583. Data shown are representative of seven experiments in each group. c Densitometric analysis of IκBα protein expression. Data shown are mean ± SEM (n = 4)
A2BAR stimulation protects against T/HS-induced muscle injury
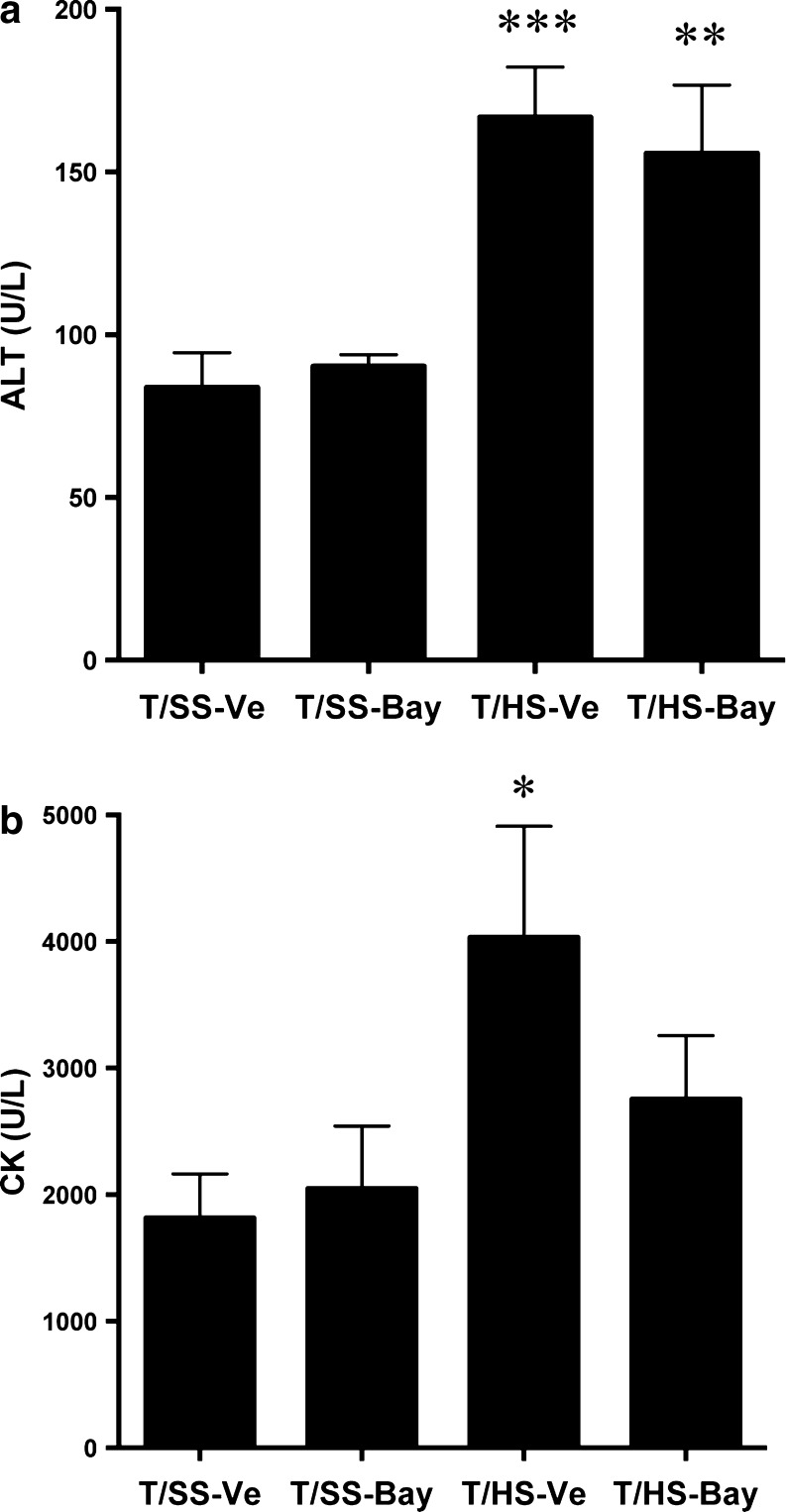
Finally, we tested the effect of BAY 60–6583 on markers of liver and muscle injury. We found that T/HS induced significant increases in the plasma levels of ALT and CK. While plasma ALT concentration was not affected by BAY 60–6583 (Fig. 3a), BAY 60–6583 reversed the increase in plasma CK levels caused by T/HS (Fig. 3b). Thus, A2BAR stimulation protects against T/HS-induced muscle, but not liver injury.
Fig. 3.
BAY 60–6583 protects against T/HS-induced muscle injury. Alanine aminotransferase (ALT), an indicator of liver injury (a), or creatine kinase (CK), an indicator of muscle injury (b) of T/SS or T/HS rats resuscitated with RL containing vehicle or 0.5 mg/kg BAY 60–6583. *p < 0.05, **p < 0.01, ***p < 0.001 vs. T/SS-vehicle. Mean ± SEM (n = 9–13)
Discussion
Here we have provided evidence that A2BAR stimulation with BAY 60–6583 during resuscitation protects against T/HS-induced lung injury. As we have recently demonstrated, A2AAR activation also protects against acute lung injury following T/HS [11], but there are differences between the mechanisms of action of stimulating A2A vs. A2B receptors. The protection afforded by A2AAR stimulation was accompanied by a decrease in PMN sequestration in the lung [11]. In contrast, our results suggest that A2BAR stimulation does not provide protection by inhibiting the PMN infiltration to the lung because BAY 60–6583 did not reverse the T/HS-induced increase in lung MPO activity. This finding is surprising because PMNs reportedly express A2BARs and activation of these receptors suppresses PMN transmigration through both endothelium and epithelium [15, 16]. On the other hand, it has also been shown that A2BARs are important in moderating vascular injury in systemic endotoxin challenge [17] and have a key role in dampening hypoxia-induced vascular leak in vivo [15, 18]. A2BAR KO mice exposed to ambient hypoxia had increased vascular permeability in the majority of organs tested and A2BAR KO mice suffering from ventilator-induced lung injury demonstrate increased pulmonary vascular leak and edema, which is due to lack of A2BARs on pulmonary and not hematopoietic cells [18]. These findings together with our results raise the possibility that BAY 60–6583 protects against T/HS-induced lung injury by stimulating the A2BARs of pulmonary parenchyma cells.
Muscle injury, a marker of which is elevated CK level in plasma, is a well-known consequence of T/HS [19–21]. The role of adenosine signaling in protecting against ischemia–reperfusion induced myocardial injury has been described, and A1ARs [22], A2BARs [12, 23], or A3ARs [24, 25] have all been shown to be protective. Our data showing that resuscitation with BAY 60–6583 reverses T/HS-induced increases in plasma CK levels suggest that A2BARs also have protective roles against muscle injury following T/HS.
We have previously shown that A2BAR stimulation augments IL-10 expression [26–28]. In addition, recent studies have shown the protective role of IL-10 in hemorrhagic shock induced lung inflammation and injury [29, 30]. Our results together with these data raise the possibility that one of the mechanisms of action of adenosine in protecting against lung injury is through augmenting IL-10 expression. This will be an interesting aspect to explore in the future.
In conclusion, we found that stimulation of A2BARs during resuscitation following T/HS protects against lung and muscle injury without any adverse effect on blood pressure, suggesting the therapeutic potential of A2BAR agonists in the treatment of hemorrhage.
Acknowledgments
This work was supported by National Institutes of Health grants R01GM066189; USAMRMC grant log# 09065004 (contract W81XWH-10-1-1015); Hungarian Scientific Research Fund (OTKA) grant CK 78275 to GH; National Heart Institute Grants R01-HL0921, R01-DK083385, and R01-HL098294; and a grant by the Crohn’s and Colitis Foundation of America to HKE.
Footnotes
Balázs Koscsó and Alexey Trepakov contributed equally to this work.
References
- 1.Hierholzer C, Billiar TR. Molecular mechanisms in the early phase of hemorrhagic shock. Langenbecks Arch Surg. 2001;386(4):302–308. doi: 10.1007/s004230100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992;216(2):117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bours MJL, Swennen ELR, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112(2):358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 4.Hasko G, Deitch EA, Szabo C, Nemeth ZH, Vizi ES. Adenosine: a potential mediator of immunosuppression in multiple organ failure. Curr Opin Pharmacol. 2002;2(4):440–444. doi: 10.1016/S1471-4892(02)00172-8. [DOI] [PubMed] [Google Scholar]
- 5.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004;25(1):33–39. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Cronstein BN, Kramer SB, Weissmann G, Hirschhorn R. Adenosine: a physiological modulator of superoxide anion generation by human neutrophils. J Exp Med. 1983;158(4):1160–1177. doi: 10.1084/jem.158.4.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fredholm BB, IJzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53(4):527–552. [PMC free article] [PubMed] [Google Scholar]
- 8.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7(9):759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hasko G, Pacher P, Deitch EA, Vizi ES. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol Ther. 2007;113(2):264–275. doi: 10.1016/j.pharmthera.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hasko G, Pacher P. Regulation of macrophage function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32(4):865–869. doi: 10.1161/ATVBAHA.111.226852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasko G, Xu DZ, Lu Q, Nemeth ZH, Jabush J, Berezina TL, Zaets SB, Csoka B, Deitch EA. Adenosine A2A receptor activation reduces lung injury in trauma/hemorrhagic shock. Crit Care Med. 2006;34(4):1119–1125. doi: 10.1097/01.CCM.0000206467.19509.C6. [DOI] [PubMed] [Google Scholar]
- 12.Eckle T, Krahn T, Grenz A, Kohler D, Mittelbronn M, Ledent C, Jacobson MA, Osswald H, Thompson LF, Unertl K, Eltzschig HK. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115(12):1581–1590. doi: 10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- 13.Hart ML, Jacobi B, Schittenhelm J, Henn M, Eltzschig HK. Cutting edge: A2B adenosine receptor signaling provides potent protection during intestinal ischemia/reperfusion injury. J Immunol. 2009;182(7):3965–3968. doi: 10.4049/jimmunol.0802193. [DOI] [PubMed] [Google Scholar]
- 14.Schingnitz U, Hartmann K, Macmanus CF, Eckle T, Zug S, Colgan SP, Eltzschig HK. Signaling through the A2B adenosine receptor dampens endotoxin-induced acute lung injury. J Immunol. 2010;184(9):5271–5279. doi: 10.4049/jimmunol.0903035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eckle T, Faigle M, Grenz A, Laucher S, Thompson LF, Eltzschig HK. A2B adenosine receptor dampens hypoxia-induced vascular leak. Blood. 2008;111(4):2024–2035. doi: 10.1182/blood-2007-10-117044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberger P, Schwab JM, Mirakaj V, Masekowsky E, Mager A, Morote-Garcia JC, Unertl K, Eltzschig HK. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10(2):195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 17.Yang D, Zhang Y, Nguyen HG, Koupenova M, Chauhan AK, Makitalo M, Jones MR, St Hilaire C, Seldin DC, Toselli P, Lamperti E, Schreiber BM, Gavras H, Wagner DD, Ravid K. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J Clin Invest. 2006;116(7):1913–1923. doi: 10.1172/JCI27933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckle T, Grenz A, Laucher S, Eltzschig HK. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest. 2008;118(10):3301–3315. doi: 10.1172/JCI34203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehnert M, Relja B, Sun-Young Lee V, Schwestka B, Henrich D, Czerny C, Froh M, Borsello T, Marzi I. A peptide inhibitor of C-jun N-terminal kinase modulates hepatic damage and the inflammatory response after hemorrhagic shock and resuscitation. Shock. 2008;30(2):159–165. doi: 10.1097/SHK.0b013e31815dd623. [DOI] [PubMed] [Google Scholar]
- 20.Sato H, Tanaka T, Kasai K, Kita T, Tanaka N. Role of p38 mitogen-activated protein kinase on cardiac dysfunction after hemorrhagic shock in rats. Shock. 2007;28(3):291–299. doi: 10.1097/SHK.0b013e3180326e3d. [DOI] [PubMed] [Google Scholar]
- 21.Korff S, Falsafi R, Czerny C, Jobin C, Nau C, Jakob H, Marzi I, Lehnert M. Time dependency and topography of hepatic nuclear factor kappaB activation after hemorrhagic shock and resuscitation in mice. Shock. 2012;38(5):486–492. doi: 10.1097/SHK.0b013e3182699072. [DOI] [PubMed] [Google Scholar]
- 22.Kalk P, Eggert B, Relle K, Godes M, Heiden S, Sharkovska Y, Fischer Y, Ziegler D, Bielenberg GW, Hocher B. The adenosine A1 receptor antagonist SLV320 reduces myocardial fibrosis in rats with 5/6 nephrectomy without affecting blood pressure. Br J Pharmacol. 2007;151(7):1025–1032. doi: 10.1038/sj.bjp.0707319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, Kominsky DJ, Colgan SP, Eltzschig HK. Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia. Nat Med. 2012;18(5):774–782. doi: 10.1038/nm.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thourani VH, Ronson RS, Jordan JE, Guyton RA, Vinten-Johansen J. Adenosine A3 pretreatment before cardioplegic arrest attenuates postischemic cardiac dysfunction. Ann Thorac Surg. 1999;67(6):1732–1737. doi: 10.1016/S0003-4975(99)00316-1. [DOI] [PubMed] [Google Scholar]
- 25.Thourani VH, Nakamura M, Ronson RS, Jordan JE, Zhao ZQ, Levy JH, Szlam F, Guyton RA, Vinten-Johansen J. Adenosine A(3)-receptor stimulation attenuates postischemic dysfunction through K(ATP) channels. Am J Physiol. 1999;277(1 Pt 2):H228–H235. doi: 10.1152/ajpheart.1999.277.1.H228. [DOI] [PubMed] [Google Scholar]
- 26.Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol. 1996;157(10):4634–4640. [PubMed] [Google Scholar]
- 27.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, Marton A, Szabo C. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000;14(13):2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 28.Csoka B, Nemeth ZH, Virag L, Gergely P, Leibovich SJ, Pacher P, Sun CX, Blackburn MR, Vizi ES, Deitch EA, Hasko G. A2A adenosine receptors and C/EBPbeta are crucially required for IL-10 production by macrophages exposed to Escherichia coli. Blood. 2007;110(7):2685–2695. doi: 10.1182/blood-2007-01-065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobbe P, Schmidt J, Stoffels B, Chanthaphavong RS, Bauer AJ, Pape HC. IL-10 administration attenuates pulmonary neutrophil infiltration and alters pulmonary iNOS activation following hemorrhagic shock. Inflamm Res Off J Eur Hist Res Soc. 2009;58(3):170–174. doi: 10.1007/s00011-009-8116-z. [DOI] [PubMed] [Google Scholar]
- 30.Kobbe P, Stoffels B, Schmidt J, Tsukamoto T, Gutkin DW, Bauer AJ, Pape HC. IL-10 deficiency augments acute lung but not liver injury in hemorrhagic shock. Cytokine. 2009;45(1):26–31. doi: 10.1016/j.cyto.2008.10.004. [DOI] [PubMed] [Google Scholar]