Abstract
Inosine is an endogenous purine nucleoside, which is formed during the breakdown of adenosine. The adenosinergic system was already described as capable of modulating mood in preclinical models; we now explored the effects of inosine in two predictive models of depression: the forced swim test (FST) and tail suspension test (TST). Mice treated with inosine displayed higher anti-immobility in the FST (5 and 50 mg/kg, intraperitoneal route (i.p.)) and in the TST (1 and 10 mg/kg, i.p.) when compared to vehicle-treated groups. These antidepressant-like effects started 30 min and lasted for 2 h after intraperitoneal administration of inosine and were not accompanied by any changes in the ambulatory activity in the open-field test. Both adenosine A1 and A2A receptor antagonists prevented the antidepressant-like effect of inosine in the FST. In addition, the administration of an adenosine deaminase inhibitor (1 and 10 mg/kg, i.p.) also caused an antidepressant-like effect in the FST. These results indicate that inosine possesses an antidepressant-like effect in the FST and TST probably through the activation of adenosine A1 and A2A receptors, further reinforcing the potential of targeting the purinergic system to the management of mood disorders.
Keywords: Inosine, Adenosine receptors, Antidepressant, Forced swimming test
Introduction
Depressive disorders are severe types of psychiatric conditions that affect about 16 % of the population during their lifetime and they are predicted to become the second leading cause of disability by the year 2020 [1–2]. There is still a lack of a clear understanding of the molecular basis associated with these conditions and the efficacy of the therapeutic approaches is still far from optimal [2].
Today, it is generally accepted that the purinergic system, in particular the adenosinergic system, is capable of influencing the development and course of several psychiatric disorders [3]. This assumption is typified by clinical and preclinical studies showing that different therapeutic strategies used to manage mood disorders cause effects related to the adenosinergic system modulation. In addition, evidence from animal models shows that the manipulation of adenosine receptors modifies behavioral responses considered relevant for mood function in humans [3, 4]. The involvement of the adenosinergic system in depression is strongly supported by its neuromodulatory role, especially in cellular targets strongly implicated in the pathogenesis of this disorder. Adenosine receptors, especially the inhibitory A1 and the facilitatory A2A receptors, are able to control several neurotransmission systems, such as dopaminergic, glutamatergic, and serotoninergic systems as well as hormones of the hypothalamic–pituitary–adrenal axis [5, 6]. In addition, these receptors control the release of inflammatory cytokines and also the signaling effects promoted by growth factors [3].
Inosine is an endogenous purine nucleoside, which is formed during the breakdown of adenosine by the enzyme adenosine deaminase [7]. The deamination of adenosine to inosine occurs mainly at high intracellular concentrations of adenosine, which are associated with cellular stress or with the activation of the sympathetic nervous system [8, 9]. When inosine reaches high concentrations inside the cell, it is shunted into the extracellular space by bidirectional, equilibrative, nucleoside transporters and many of the cellular actions of inosine occur through its binding to adenosine receptors, especially A1, A2A, and A3 receptors [10]. Of all the nucleosides and their metabolites, inosine has the highest overall concentration in the brain, especially in the basal ganglia and frontal cortex [11], two regions strongly affected in mood-related disorders [12].
In spite of the originally described lack of biological effects, it is increasingly clear that inosine can act as a potent immunomodulatory and neuroprotective compound [10, 13]. Several works emerged indicating that inosine attenuates the production of pro-inflammatory cytokines [14, 15]; induces antinociceptive, antiallodynic, and antihyperalgesic effects [16, 17]; and is protective in animal models of sepsis, ischemia, and autoimmunity [10]. In addition, inosine preserves the viability of glial and neuronal cells during hypoxia, and stimulates axonal regrowth after injury [7, 9, 18]. Recent evidence also indicates that extracellular inosine has the ability to stimulate various growth factors and restore cell energy [8, 19, 20].
Several studies have highlighted that most of the physiological effects of inosine, mainly the neuroprotective and anti-inflammatory effects, are due to its ability to activate adenosine receptors. Since we have previously demonstrated that the administration of adenosine elicits antidepressant-like effect in the forced swim test (FST) and tail suspension test (TST) in mice, mediated by both A1 and A2A receptors [21], the aim of the present work was to elucidate the role of inosine, the primary by-product of adenosine, in predictive models of depression in mice and the possible involvement of both adenosine A1 and A2A receptors.
Materials and methods
Animals
Male Swiss mice (8 weeks old, weighing 35–40 g) were used throughout this study. Animals were maintained at 21–23 °C with free access to water and food, under a 12:12 h light/dark cycle (lights on at 0700 hours). All manipulations were carried out between 9:00 and 1600 hours, with each animal used only once. All procedures in this study were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals and the Ethics Committee of the Institution and all efforts were made to minimize animal suffering.
Drugs and treatment
The following drugs were used: inosine and N6-cyclohexyladenosine (CHA) (Sigma Chemical Co., USA) which were dissolved in saline, N6-[2-(3,5-dimethoxyphenyl)-2-(methylphenyl)ethyl]adenosine (DPMA) (Sigma Chemical Co., USA), 4-(2-[7-amino-2-{2-furyl}{1,2,4}triazolo-{2,3-a}{1,3,5}triazin-5-yl-amino]ethyl)phenol (ZM241385) and 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) (Tocris Cookson, USA), and erythro-9-(2-hydroxy-3nonyl) adenine (EHNA, Sigma Chemical Co., USA) dissolved in saline with 5 % DMSO. Appropriate vehicle-treated groups were also assessed simultaneously. The compounds were administered by intraperitoneal (i.p.) route in a constant volume of 10 ml/kg.
Inosine or EHNA were injected 30 min before the tests, except in the time course experiments in which inosine was tested from 30 min until 480 min after its i.p. administration. The adenosine A1 and A2A receptor agonists CHA and DPMA, respectively, were also administered 30 min before the FST. The adenosine A1 and A2A receptor antagonists DPCPX and ZM241385, respectively, were administered 60 min before the tests. All the doses and the administration schedule were chosen on the basis of previous results [21, 22].
Forced swimming test
The forced swimming test was described by Porsolt et al. [23]. Briefly, mice were individually forced to swim in an open cylindrical container (diameter, 10 cm; height, 25 cm), with water at 25 °C and the total duration of immobility during a 6-min period was scored [24]. Mice were judged to be immobile when they ceased struggling and remained floating motionless in the water, making only those movements necessary to keep its head above water.
Tail suspension test
The total duration of immobility induced by tail suspension was measured according to the method described by Steru et al. [25]. Briefly, mice both acoustically and visually isolated were suspended 50 cm above the floor by adhesive tape placed approximately 1 cm from the tip of the tail. Immobility time was recorded during a 6-min period [26].
Open-field test
Locomotor and exploratory behaviors were monitored using an open-field apparatus, as previously described. The apparatus consisted of a wooden box measuring 40 × 60 × 50 cm with a frontal glass wall. The floor of the arena was divided into 12 equal squares and placed in a sound-free room. Animals were placed in the rear left square and left to explore it freely for 6 min during which time the number of squares crossed with all paws (crossing) was counted. The apparatus was cleaned up with a 10 % alcohol solution and dried after each individual mouse session [24].
Statistical analysis
The comparisons of data between experimental and control groups were performed by one-way or two-way analysis of variance (ANOVA) followed by Duncan’s post hoc test when appropriate. P < 0.05 was considered to represent a significant difference.
Results
Antidepressant-like effect of inosine in the mouse FST and TST
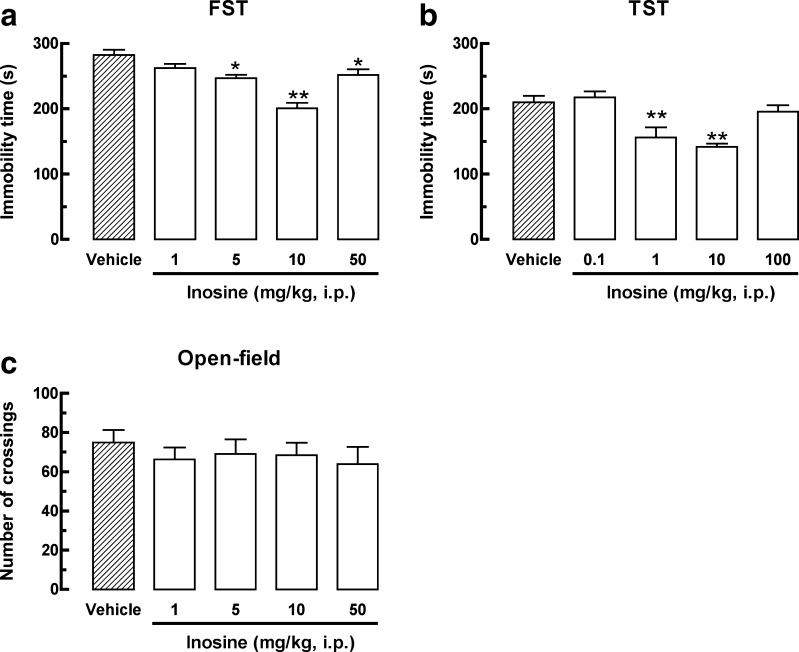
The results depicted in Fig. 1a show that inosine (5 and 50 mg/kg, i.p.) administrated 30 min before the FST significantly decreased the immobility time, as revealed by one-way ANOVA, [F(4, 25) = 15.95, P < 0.01]. Figure 1b shows that inosine (1 and 10 mg/kg, i.p.) 30 min prior to testing significantly decreased mice immobility time in the TST, as revealed by one-way ANOVA [F(4, 25) = 10.11, P < 0.01]. As depicted in Fig. 1c, the specific antidepressant-like effect of inosine was further confirmed in the open-field test since no global change of locomotion was observed after inosine treatment, as revealed by one-way ANOVA [F(4, 23) = 0.35, P = 0.84].
Fig. 1.
Effect of inosine on the immobility time in the FST (1–50 mg/kg, i.p., a) and in the TST (0.1–100 mg/kg, i.p., b) and in the number of crossings in the open-field test (1–50 mg/kg, i.p., c). Values are represented as mean + S.E.M. (n = 5–7). *P < 0.05, **P < 0.01 compared with the control group treated with vehicle
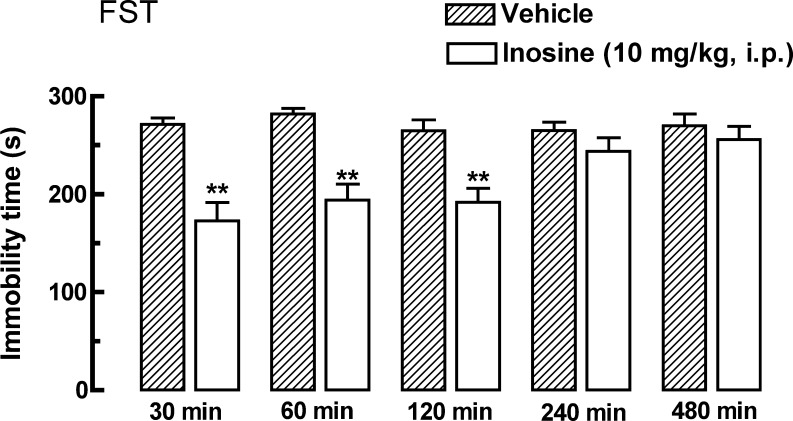
Figure 2 shows the effect of inosine (10 mg/kg, i.p.), administered 30, 60, 180, 240, or 480 min before the FST. The results of two-way ANOVA revealed a significant effect of time [F(1, 44) = 11.87, P < 0.01] and interaction between treatment and time [F(4, 44) = 6.66, P < 0.01], but not of the treatment alone [F(4, 44) = 1.45, P = 0.23]. Thus, the antidepressant-like effect of inosine in the FST starts 30 min after i.p. administration and lasts until 240 min.
Fig. 2.
Time course effect of inosine (10 mg/kg, i.p.) on the immobility time in the FST. Values are represented as mean + S.E.M. (n = 4–6). **P < 0.01 compared with the control group from the same time period treated with vehicle
The antidepressant-like effect of inosine in the FST is prevented by adenosine A1 and A2A receptor antagonists
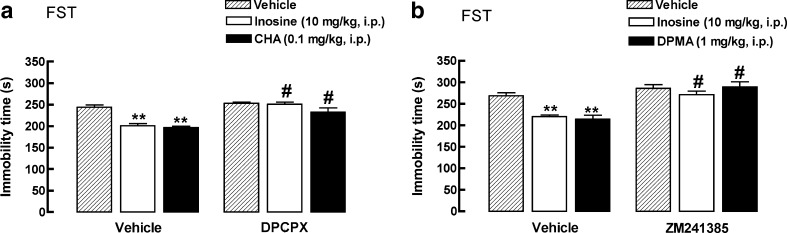
The results depicted in Fig. 3a show that the antidepressant effect of inosine (10 mg/kg, i.p.) and CHA (0.1 mg/kg, i.p., adenosine A1 receptor agonist, positive control) was prevented by the pretreatment of mice with the adenosine A1 receptor antagonist DPCPX (2 mg/kg, i.p.). The two-way ANOVA revealed a main effect of the pretreatment: [F(1, 31) = 39.80, P < 0.01], treatment [F(2, 31) = 15.77, P < 0.01], and of the interaction between pretreatment and treatment [F(2, 31) = 5.35, P < 0.01]. The decrease in the immobility time in the FST elicited by inosine (10 mg/kg, i.p.) and DPMA (1 mg/kg, i.p., adenosine A2A receptor agonist, positive control) was also prevented by the pretreatment with the adenosine A2A receptor antagonist ZM241385 (1 mg/kg, i.p.). The two-way ANOVA revealed a main effect of the pretreatment: [F(1, 31) = 48.36, P < 0.01], treatment [F(2, 31) = 5.97, P < 0.01], and of the interaction between pretreatment and treatment [F(2, 31) = 4.20, P < 0.05]
Fig. 3.
Effect of DPCPX (2 mg/kg, i.p., a) on the antidepressant-like effect of inosine (10 mg/kg, i.p.) or CHA (0.1 mg/kg, i.p., used as a positive control) in the FST. Effect of ZM241385 (1 mg/kg, i.p., b) on the antidepressant-like effect of inosine (10 mg/kg, i.p.) or DPMA (1 mg/kg, i.p., used as a positive control) in the FST. Values are mean + S.E.M. (n = 6–7). **P < 0.01 compared with the control group (vehicle/vehicle). #P < 0.01 when compared with the group vehicle/inosine, vehicle/CHA, or vehicle/DPMA
Antidepressant-like effect of the adenosine deaminase inhibitor in the mouse FST
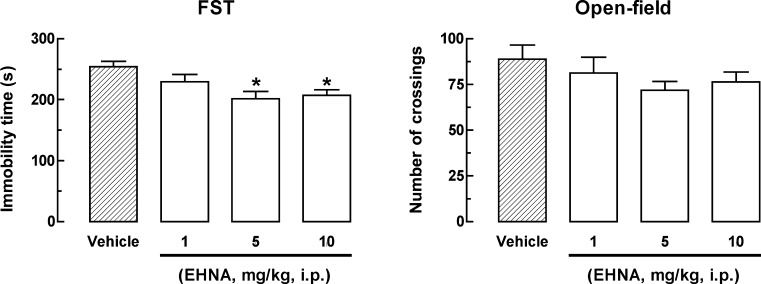
The results depicted in Fig. 4a show that the inhibitor of adenosine deaminase, EHNA (5 and 10 mg/kg, i.p.), administrated 30 min before the FST significantly decreased the immobility time, as revealed by one-way ANOVA [F(3, 20) = 5.10, P < 0.01]. Figure 4b shows that the antidepressant-like effect of EHNA in the FST is specific, since no global change on locomotion was observed in the open-field test, as revealed by one-way ANOVA [F(3, 20) = 1.13, P = 0.35].
Fig. 4.
Effect of EHNA on the immobility time in the FST (1–10 mg/kg, i.p., a) and on the number of crossings in the open-field test (1–10 mg/kg, i.p., b). Values are represented as mean + S.E.M. (n = 6). *P < 0.05 compared with the control group treated with vehicle
Discussion
It is becoming increasingly clear that alterations in the purinergic system are involved in the development and course of psychiatric disorders, including depression [4]. The present study demonstrates for the first time that inosine, a nucleoside formed through the breakdown of adenosine, produced a significant antidepressant-like effect in the FST and TST. Our data also provide evidence for the involvement of adenosine A1 and A2A receptors in the anti-immobility effect of inosine in the FST.
The FST and TST are two of the most widely used predictive animal models of antidepressant activity. They were designed to evaluate antidepressant activity of compounds and to investigate their mechanism of action. In these tests, the immobility time of animals placed in an inescapable and acute stressful situation is measured and an increase of escape-oriented behaviors is considered an antidepressant-like behavior [23, 25]. The validity of the FST and TST is based on the observation that many different classes of antidepressants reduce immobility time of mice in this paradigm [23, 25]. After treatment with inosine, mice displayed a significant reduction of immobility time in both FST and TST. A lower dose of inosine was already able to produce an antidepressant-like behavior in mice when compared to the FST reinforcing the notion that the neurochemical pathways mediating behavior despair in the FST and TST are not identical [27]. One of the major concerns of these models is that psychostimulant compounds may show false-positive effects. However, our data demonstrated that the anti-immobility effect of inosine cannot be attributed to a psychostimulant action, since inosine did not produce alterations in the locomotor activity assessed in the open-field test.
Dysfunction of the purinergic signaling has already been suggested to play an important role in the pathogenesis of depression. There is evidence that different therapeutic strategies used to control mood disorders are associated to the adenosine modulation system. In addition, data from preclinical models show that the manipulation of adenosine receptors modifies behavioral, neuroendocrine, and neurochemical responses that accompany mood disorders [3, 4, 6]. Our group previously demonstrated that adenosine administration elicits an antidepressant-like effect in the FST and TST by a mechanism that involves the activation of adenosine A1 and A2A receptors [21]. In addition, other purine-based nucleosides and nucleotides like guanosine and GMP also present antidepressant properties in these preclinical tests [28, 29].
The antidepressant-like effects of inosine observed in the FST persisted for 2 h after systemic administration. Indeed, literature data have shown that intraperitoneal or even oral administration of inosine may elicit several biological effects, including antinociceptive responses in different models of nociception [16, 17], neuroprotective activity against hypoxic–ischemic brain damage [30], and axonal regrowth [31]. Inosine has a high safety profile and is devoid of any particular side effects. Moreover, literature data show that inosine has been safely given to humans for prolonged periods of time [32, 33].
The cellular and molecular mechanisms underlying the effects of inosine are incompletely understood. Our work corroborates literature data by showing that inosine acts, at least in part, via activation of adenosine A1 and A2A receptors [16]. Indeed, the activation of these receptors produces antidepressant-like effects in the FST [21] and is responsible, at least in part, for the antidepressant-like effects of compounds like adenosine [21] and zinc chloride [34]. However, recent works have indicated that inosine might have other potential targets like the other adenosine receptors, the protein kinase B (Akt), the nuclear enzyme poly ADP-ribose polymerase, K+ channels, and voltage-gated Ca2+ channels [17, 35, 36]. Inosine may also partially act via its breakdown product, uric acid, which has been consistently shown to be a scavenger of oxyradicals and peroxynitrite [37, 38]. Clinical data show that lowered uric acid levels are associated with depression [39]. Ultimately, since extracellular inosine and adenosine access the intracellular space by competing for the same nucleoside transporters [40], it is also possible that extracellular inosine can augment extracellular adenosine levels by preventing adenosine uptake and, thus, generating indirect biological effects secondary to adenosine binding to its receptors. In addition, our work demonstrated that the inhibition of the enzyme adenosine deaminase, which converts adenosine to inosine, also produced an antidepressant-like effect in the FST. This result suggests that the pharmacological inhibition of this enzyme would lead to elevation in local extracellular adenosine concentrations, which results in enhanced nonselective stimulation of adenosine receptors and, subsequently in antidepressant-like effect.
Altogether, our results firstly indicate that inosine exerts an antidepressant-like effect that seems to be mediated through an interaction with both adenosine A1 and A2A receptors. However, it appears that inosine might act through multiple unrelated pathways, and future studies will be necessary to delineate the nature of these various pathways in its antidepressant-like effect.
Acknowledgments
This study was supported by CNPq, CAPES, IBN-Net, Brazil and NENASC project (PRONEX program CNPq/FAPESC).
Conflict of interest
None
References
- 1.Andlin-Sobocki P, Jonsson B, Wittchen HU, Olesen J. Cost of disorders of the brain in Europe. Eur J Neurol. 2005;12(Suppl 1):1–27. doi: 10.1111/j.1468-1331.2005.01202.x. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psych. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 3.Gomes CV, Kaster MP, Tome AR, Agostinho PM, Cunha RA. Adenosine receptors and brain diseases: neuroprotection and neurodegeneration. Biochim Biophys Acta. 2012;1808(5):1380–1399. doi: 10.1016/j.bbamem.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Cunha RA, Ferré S, Vaugeois JM, Chen JF. Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr Pharmacol Des. 2008;14(15):1512–1524. doi: 10.2174/138161208784480090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scaccianoce S, Navarra D, Sciullo AD, Angelucci L, Endröczi E. Adenosine and pituitary–adrenocortical axis activity in the rat. Neuroendocrinology. 1989;50:464–468. doi: 10.1159/000125264. [DOI] [PubMed] [Google Scholar]
- 6.Okada M, Nutt DJ, Murakami T, Zhu G, Kamata A, Kawata Y, Kaneko S. Adenosine receptor subtypes modulate two major functional pathways for hippocampal serotonin release. J Neurosci. 2001;21:628–640. doi: 10.1523/JNEUROSCI.21-02-00628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barankiewicz J, Cohen A. Purine nucleotide metabolism in resident and activated rat macrophages in vitro. Eur J Immunol. 1985;15(6):627–631. doi: 10.1002/eji.1830150618. [DOI] [PubMed] [Google Scholar]
- 8.Fredholm BB, Sollevi A. The release of adenosine and inosine from canine subcutaneous adipose tissue by nerve stimulation and noradrenaline. J Physiol. 1981;313:351–367. doi: 10.1113/jphysiol.1981.sp013670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benowitz LI, Goldberg DE, Irwin N. Inosine stimulates axon growth in vitro and in the adult CNS. Prog Brain Res. 2002;137:389–399. doi: 10.1016/S0079-6123(02)37030-4. [DOI] [PubMed] [Google Scholar]
- 10.Haskó G, Sitkovsky MV, Szabo C. Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacological Sci. 2004;25(3):152–157. doi: 10.1016/j.tips.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Kovacs Z, Dobolyi A, Juhasz G, Kekesi KA. Nucleoside map of the human central nervous system. Neurochem Res. 2009;35(3):452–464. doi: 10.1007/s11064-009-0080-z. [DOI] [PubMed] [Google Scholar]
- 12.Marchand WR, Lee JN, Suchy Y, Johnson S, Thatcher J, Gale P. Aberrant functional connectivity of cortico-basal ganglia circuits in major depression. Neurosci Lett. 2012;514(1):86–90. doi: 10.1016/j.neulet.2012.02.063. [DOI] [PubMed] [Google Scholar]
- 13.Liu F, You SW, Yao LP, Liu HL, Jiao XY, Shi M, Zhao QB, Ju G. Secondary degeneration reduced by inosine after spinal cord injury in rats. Spinal Cord. 2006;44(7):421–426. doi: 10.1038/sj.sc.3101878. [DOI] [PubMed] [Google Scholar]
- 14.Hasko G, Kuhel DG, Nemeth ZH, Mabley JG, Stachlewitz RF, Virag L, Lohinai Z, Southan GJ, Salzman AL, Szabo C. Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J Immunol. 2000;164(2):1013–1019. doi: 10.4049/jimmunol.164.2.1013. [DOI] [PubMed] [Google Scholar]
- 15.Liaudet L, Mabley JG, Soriano FG, Pacher P, Marton A, Hasko G, Szabo C. Inosine reduces systemic inflammation and improves survival in septic shock induced by cecal ligation and puncture. Am J Respir Crit Medicine. 2001;164(7):1213–1220. doi: 10.1164/ajrccm.164.7.2101013. [DOI] [PubMed] [Google Scholar]
- 16.Nascimento FP, Figueredo SM, Marcon R, Martins DF, Macedo SJ, Jr, Lima DA, Almeida RC, Ostroski RM, Rodrigues AL, Santos AR. Inosine reduces pain-related behavior in mice: involvement of adenosine A1 and A2A receptor subtypes and protein kinase C pathways. J Pharmacol Exp Therapeut. 2010;334(2):590–598. doi: 10.1124/jpet.110.166058. [DOI] [PubMed] [Google Scholar]
- 17.Macedo-Júnior SJ, Nascimento FP, Luiz-Cerutti M, Santos AR. Role of pertussis toxin-sensitive G-protein, K+ channels, and voltage-gated Ca2+ channels in the antinociceptive effect of inosine. Purinergic Signal. 2013;9(1):51–58. doi: 10.1007/s11302-012-9327-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irwin N, Li YM, O'Toole JE, Benowitz LI. Mst3b, a purine-sensitive Ste20-like protein kinase, regulates axon outgrowth. Proc Natl Acad Sci U S A. 2006;103(48):18320–18325. doi: 10.1073/pnas.0605135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen P, Goldberg DE, Kolb B, Lanser M, Benowitz LI. Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc Natl Acad Sci USA. 2002;99(13):9031–9036. doi: 10.1073/pnas.132076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannecchini M, Matteucci M, Pesi R, Sgarrella F, Tozzi MG, Camici M. Uptake and utilization of nucleosides for energy repletion. Int J Biochem Cell Biol. 2005;37(4):797–808. doi: 10.1016/j.biocel.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Kaster MP, Rosa AO, Rosso MM, Goulart EC, Santos AR, Rodrigues AL. Adenosine administration produces an antidepressant-like effect in mice: evidence for the involvement of A1 and A2A receptors. Neurosci Lett. 2004;355(1–2):21–24. doi: 10.1016/j.neulet.2003.10.040. [DOI] [PubMed] [Google Scholar]
- 22.Martins DF, Mazzardo-Martins L, Soldi F, Stramosk J, Piovezan AP, Santos AR. High-intensity swimming exercise reduces neuropathic pain in an animal model of complex regional pain syndrome type I: evidence for a role of the adenosinergic system. Neuroscience. 2013;3(234):69–76. doi: 10.1016/j.neuroscience.2012.12.042. [DOI] [PubMed] [Google Scholar]
- 23.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: a primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229(2):327–336. [PubMed] [Google Scholar]
- 24.Kaster MP, Budni J, Binfare RW, Santos AR, Rodrigues AL. The inhibition of different types of potassium channels underlies the antidepressant-like effect of adenosine in the mouse forced swimming test. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(3):690–696. doi: 10.1016/j.pnpbp.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85(3):367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 26.Posser T, Kaster MP, Barauna SC, Rocha JB, Rodrigues AL, Leal RB. Antidepressant-like effect of the organoselenium compound ebselen in mice: evidence for the involvement of the monoaminergic system. Eur J Pharmacol. 2009;602(1):85–91. doi: 10.1016/j.ejphar.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 27.Bai F, Li X, Clay M, Lindstrom T, Skolnick P. Intra- and interstrain differences in models of “behavioral despair”. Pharmacol Biochem Behav. 2001;70:187–192. doi: 10.1016/S0091-3057(01)00599-8. [DOI] [PubMed] [Google Scholar]
- 28.Bettio LE, Cunha MP, Budni J, Pazini FL, Oliveira A, Colla AR, Rodrigues AL. Guanosine produces an antidepressant-like effect through the modulation of NMDA receptors, nitric oxide-cGMP and PI3K/mTOR pathways. Behav Brain Res. 2012;234(2):137–148. doi: 10.1016/j.bbr.2012.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Eckeli AL, Dach F, Rodrigues AL. Acute treatments with GMP produce antidepressant-like effects in mice. Neuroreport. 2000;11(9):1839–1843. doi: 10.1097/00001756-200006260-00008. [DOI] [PubMed] [Google Scholar]
- 30.Deng YH, Kuang SJ, Hei MY, Tian L. Effects of inosine on neuronal apoptosis and the expression of cytochrome C mRNA following hypoxic–ischemic brain damage in neonatal rats. Chinese J Contemp Ped. 2006;8(4):266–271. [PubMed] [Google Scholar]
- 31.Wu MM, You SW, Hou B, Jiao XY, Li YY, Ju G. Effects of inosine on axonal regeneration of axotomized retinal ganglion cells in adult rats. Neurosci Lett. 2003;341(1):84–86. doi: 10.1016/S0304-3940(03)00151-4. [DOI] [PubMed] [Google Scholar]
- 32.Starling RD, Trappe TA, Short KR, Sheffield-Moore M, Jozsi AC, Fink WJ, Costill DL. Effect of inosine supplementation on aerobic and anaerobic cycling performance. Med Science SportsExerc. 1996;28(9):1193–1198. doi: 10.1097/00005768-199609000-00017. [DOI] [PubMed] [Google Scholar]
- 33.McNaughton L, Dalton B, Tarr J. Inosine supplementation has no effect on aerobic or anaerobic cycling performance. Int J Sport Nut. 1999;9(4):333–344. doi: 10.1123/ijsn.9.4.333. [DOI] [PubMed] [Google Scholar]
- 34.Lobato KR, Binfare RW, Budni J, Rosa AO, Santos AR, Rodrigues AL. Involvement of the adenosine A1 and A2A receptors in the antidepressant-like effect of zinc in the forced swimming test. Prog Neuro-Psychopharmacol Biol Psych. 2008;32(4):994–999. doi: 10.1016/j.pnpbp.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 35.Gao Z, Li BS, Day YJ, Linden J. A3 adenosine receptor activation triggers phosphorylation of protein kinase B and protects rat basophilic leukemia 2H3 mast cells from apoptosis. Mol Pharmacol. 2001;59(1):76–82. doi: 10.1124/mol.59.1.76. [DOI] [PubMed] [Google Scholar]
- 36.Szabo C, Dawson VL. Role of poly(ADP-ribose) synthetase in inflammation and ischaemia-reperfusion. Trends Pharmacol Sci. 1998;19(7):287–298. doi: 10.1016/S0165-6147(98)01193-6. [DOI] [PubMed] [Google Scholar]
- 37.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78(11):6858–6862. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Becker BF, Reinholz N, Ozcelik T, Leipert B, Gerlach E. Uric acid as radical scavenger and antioxidant in the heart. Pflugers Arch. 1989;415(2):127–135. doi: 10.1007/BF00370582. [DOI] [PubMed] [Google Scholar]
- 39.Wen S, Cheng M, Wang H, Yue J, Wang H, Li G, Zheng L, Zhong Z, Peng F. Serum uric acid levels and the clinical characteristics of depression. Clinical Biochem. 2012;45(1–2):49–53. doi: 10.1016/j.clinbiochem.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Pastor-Anglada M, Casado FJ, Valdes R, Mata J, Garcia-Manteiga J, Molina M. Complex regulation of nucleoside transporter expression in epithelial and immune system cells. Mol Mem Biol. 2001;18(1):81–85. doi: 10.1080/096876800110033783. [DOI] [PubMed] [Google Scholar]