Abstract
P2X receptors are expressed on ventrolateral medulla projecting paraventricular nucleus (PVN) neurons. Here, we investigate the role of adenosine 5′-triphosphate (ATP) in modulating sympathetic nerve activity (SNA) at the level of the PVN. We used an in situ arterially perfused rat preparation to determine the effect of P2 receptor activation and the putative interaction between purinergic and glutamatergic neurotransmitter systems within the PVN on lumbar SNA (LSNA). Unilateral microinjection of ATP into the PVN induced a dose-related increase in the LSNA (1 nmol: 38 ± 6 %, 2.5 nmol: 72 ± 7 %, 5 nmol: 96 ± 13 %). This increase was significantly attenuated by blockade of P2 receptors (pyridoxalphosphate-6-azophenyl-20,40-disulphonic acid, PPADS) and glutamate receptors (kynurenic acid, KYN) or a combination of both. The increase in LSNA elicited by L-glutamate microinjection into the PVN was not affected by a previous injection of PPADS. Selective blockade of non-N-methyl-D-aspartate receptors (6-cyano-7-nitroquinoxaline-2,3-dione disodium salt, CNQX), but not N-methyl-D-aspartate receptors (NMDA) receptors (DL-2-amino-5-phosphonopentanoic acid, AP5), attenuated the ATP-induced sympathoexcitatory effects at the PVN level. Taken together, our data show that purinergic neurotransmission within the PVN is involved in the control of SNA via P2 receptor activation. Moreover, we show an interaction between P2 receptors and non-NMDA glutamate receptors in the PVN suggesting that these functional interactions might be important in the regulation of sympathetic outflow.
Keywords: Sympathetic outflow, ATP, L-Glutamate, Paraventricular nucleus of the hypothalamus
Introduction
The paraventricular nucleus (PVN) of the hypothalamus is considered to be an important integrative center for autonomic and neuroendocrine regulation [1–5]. The PVN is composed of magnocellular and parvocellular neurons. The former synthesize and release vasopressin and oxytocin in the posterior pituitary [1], and the latter project to premotor sympathoexcitatory neurons located either in the rostral ventrolateral medulla (RVLM) [6–8] and/or intermediolateral cell column (IML) of the spinal cord [9–14]. Numerous neurotransmitters and/or neuromodulators act within the PVN to regulate sympathetic nerve activity (SNA). For example, the inhibition of PVN neurons with either a nitric oxide donor [15–17] or γ-aminobutyric acid (GABA) receptor agonist [18, 19] reduces renal SNA and systemic blood pressure (BP) in both normotensive and hypertensive rats. Likewise, activation of PVN neurons with excitatory amino acids [20–22] or GABAA receptor antagonist, biccuculine, [23, 24] increases BP and SNA in both anesthetized and conscious rats. Furthermore, there are several lines of evidence, which highlight the importance of neurotransmitter interactions within the PVN in modulating SNA. For example, Zhang and Patel [25] showed that the inhibitory effect of endogenous NO within the PVN on the renal SNA is mediated by GABA. In relation to the purinergic signaling in hypothalamus, Kapoor and Sladek [26] observed that the application of adenosine 5′-triphosphate (ATP) or phenylephrine in explants of the hypothalamus–neurohypophysial system increases vasopressin release, and this effect was more pronounced when both agonists were applied simultaneously, indicating a synergism between the purinergic and adrenergic systems.
ATP is classically recognized as an intracellular energy source; however, over the last five decades this purine and its metabolites have been shown to be extracellular signaling molecules [30]. The neurotransmitter actions of ATP are mediated by P2 purinoreceptors, which are divided into two main classes: P2Y and P2X [27]. The P2Y receptors are G protein-coupled receptors, while P2X receptors are ligand-gated ion channels, permeable to Na+, K+, and Ca2+. ATP acts as an excitatory neurotransmitter at synapses in the brain, spinal cord, and peripheral nerve terminals. ATP can also be broken down by extracellular ATPases and 5′-nucleotidase to Adenosine diphosphate (ADP), Adenosine monophosphate (AMP), and adenosine, the last of which is taken back up into cells by a specific transporter to resynthesize ATP, which is then incorporated back into secretory vesicles [27, 28, 30]. Adenosine binds to P1 G protein-coupled receptors that have four subtypes (A1, A2A, A2B, and A3); A1 and A3 are inhibitory, while A2A and A2B are excitatory.
Immunohistochemical studies have identified the presence of P2X receptors in the hypothalamus [31–34], especially on PVN neurons that project to the RVLM [33], suggesting that ATP could act as a neurotransmitter within the PVN. Many studies have shown that microinjection of ATP or stable analogs of ATP such as α,β-meATP or purinergic receptor antagonists in the autonomic brain nuclei can affect the arterial BP, heart rate, and respiratory activity [35–40]. However, the physiological actions of ATP on sympathetic outflow at the level of the PVN are still unknown. Furthermore, ATP exerts important neuromodulatory effects on glutamatergic mechanisms [41, 60] In this regard, Pankratov et al. [41] working with CA1 neurons of the rat hippocampus observed that the inhibition of glutamatergic transmission did not blunt all excitatory postsynaptic currents (EPSCs) and that the residual EPSCs were eliminated after the antagonism of P2 receptors with pyridoxalphosphate-6-azophenyl-20,40-disulphonic acid (PPADS), demonstrating an important contribution of purinergic signaling in this brain nucleus in mediating excitatory effects. However, there are no reports about this purinergic–glutamatergic interaction at the PVN modulating sympathetic activity.
Given the evidence cited above, the aim of the current study was to investigate the role of ATP and its functional interactions with the glutamate receptors when microinjected into the PVN on lumbar SNA (LSNA) of rats. Here, we show that purinoceptor activation within the PVN increases LSNA. Additionally, we demonstrate that ATP and glutamate can act as cotransmitters to modulate sympathoexcitatory responses via activation of P2 and non-N-methyl-D-aspartate (non-NMDA) receptors.
Methods
Animals
Juvenile male Wistar rats (3–4 weeks of age) weighing 50–80 g were used in this study (n = 91). The rats were obtained from the colony bred at the Institute of Biomedical Sciences, University of Sao Paulo (ICB/USP) and kept at a constant temperature of 22–24 °C and a relative humidity of 50–60 % under a controlled light/dark cycle (12/12 h) with normal rat chow and drinking water ad libitum. All experimental procedures were performed in accordance with the Ethical Principles in Animal Research of the Brazilian College of Animal Experimentation and were approved by the Ethical Committee for Animal Research of ICB/USP (Protocol # 65/2010).
Decorticate, unanesthetized, arterially perfused in situ preparation of rat
The procedures for the decorticate, unanesthetized, arterially perfused in situ preparation of rat (DAPR) were performed as previously described [43] and are outlined here in brief. Rats were deeply anesthetized with halothane (5 %) until loss of paw withdrawal reflex. The stomach, intestines, and spleen were ligated and removed via midline laparotomy. The sternum was split and the ribcage retracted to allow access to the mediastinum. The pericardium was removed and the left phrenic nerve was isolated. The animal was submerged in cooled artificial cerebrospinal fluid (aCSF, see below) and the cerebral hemispheres exposed by removal of the parietal bones. The cerebral cortices, hippocampus, and thalamic area were removed by gentle aspiration. The removal of these structures abrogates the need for further anesthetic use in this preparation. The preoptic area and its adjacent septal nuclei and hypothalamic areas remained largely intact. The preparation was skinned and transferred to the recording chamber. A double-lumen perfusion cannula was inserted into the ascending aorta via the left ventricle. The preparation was perfused at flow rates of 28 ± 2 ml min−1 using a roller pump (Watson Marlow 505S, UK) with aCSF containing an oncotic agent (Polyethylene Glycol. 20,000, 1.5 %; Sigma-Aldrich, St. Louis, MO, USA), gassed with carbogen (95 % O2 and 5 % CO2), warmed to 32 °C, and filtered using a nylon screen (pore size: 25 μm). After respiratory-related movements commenced, a neuromuscular blocker (vecuronium bromide, 4 mg ml−1, Vecuron, Cristália, SP, Brazil) was added to the perfusate to mechanically stabilize the preparation. The second lumen of the cannula was used to monitor aortic perfusion pressure.
Nerve recordings
Phrenic nerve activity (PNA) was recorded from its distal end using a glass suction bipolar electrode held in a 3-D micromanipulator. Rhythmic ramping PNA gave a continuous physiological index of preparation stability and viability. Preparations that did not show ramping PNA were deemed unviable and not included in the study. The lumbar sympathetic chain (L2–L3) was visualized through a binocular microscope and recordings made from the distal cut end using a bipolar glass suction electrode. Signals were AC-amplified (NL104, Neurolog, UK) and band-pass filtered (100–3 kHz) and displayed on a computer using the Spike 2 software (Cambridge Electronic Design, Cambridge, UK). SNA exhibited marked respiratory modulation and was attenuated by an increase in perfusion pressure (arterial baroreceptor stimulation).
Paraventricular nucleus microinjection
The head of the preparation was fixed by ear bars and a nasal clamp mounted on the perfusion chamber. The head was positioned to a precise horizontal level in every experiment to allow accurate and consistent placement of micropipettes into the PVN was performed based on stereotaxic coordinates relative to the superior colliculus (SC), i.e., 2.5–2.7 mm rostral, 0.3–0.5 mm lateral to midline, and 3.2–3.4 mm below the brain surface [42]. A three-barreled glass micropipette (external tip diameter 10–30 μm) was placed into the PVN using a 3-D micromanipulator. The volume microinjected either unilaterally or bilaterally (100 nL each side) was determined by viewing the movement of the meniscus through a binocular microscope fitted with a precalibrated eyepiece reticule. It should be noted that the unilateral microinjections were performed randomly in either the right or left side of the PVN as we had previously observed that the effects on SNA were similar independent of the side of the microinjection.
Histological analysis
At the end of all experiments, Evans blue dye (2 % w/v), contained in one barrel of the three-way micropipette, was microinjected (100 nL) to mark the drug injection sites. The decorticated brain was removed and fixed in 4 % paraformaldehyde in 0.1 M phosphate-buffered saline and 20 % sucrose. Coronal sections (40 μm of thickness) were cut using a cryostat (CM1900, Leica, Switzerland) and thaw-mounted on gelatin-subbed glass slides. Brain sections were visualized under light microscopy (dark field) and the injection sites mapped according to the rat brain atlas by Paxinos and Watson [43]. Only data in which the microinjections were confirmed to be within the PVN were considered in the statistical analysis.
Data analyses
All data were acquired using biopotential AC amplifiers and filters (Neurolog, Digitimer Ltd., UK) and collected using a CED 1401 A–D interface (CED, Cambridge Electronic Design, Cambridge, UK) and a computer running Spike 2 software (CED) with custom-written scripts for data acquisition and on- and offline analyses. LSNA was displayed as a moving average (100 ms or 2 s time constant, depending on the protocol performed). To standardize the data across preparations, LSNA changes were expressed as a percentage of the basal values. The noise for LSNA was assessed by application of hexamethonium (100 mM–0.5 ml) into the perfusate (final concentration: 5 mM) at the end of each experiment. To analyze the duration of an increase in the LSNA evoked by ATP microinjected into the PVN, a cursor was positioned at the beginning of the response, corresponding to the exact moment of the injection of ATP into the PVN, and a second cursor was positioned at the time when LSNA returned to baseline values. Baseline noise was subtracted from the data prior to analyses. For measurement of the magnitude and duration of response to different doses of ATP and α,β-meATP microinjected into the PVN on LSNA, statistical analysis was also performed on values obtained from measuring area under the curve (AUC) using Prism 5 (GraphPad). One-way ANOVA for repeated measures followed by Bonferroni’s post hoc test was used, and differences were taken as significant at p < 0.05. All values are expressed as the mean ± standard error of mean (SEM) and n is the number of preparations.
Drugs and solutions
Artificial CSF (290 mOsmol/kg) containing in (millimolar): NaCl 120, NaHCO3 24, KCl 5, CaCl2 2.5, MgSO4 1.25, KH2PO4 1.25, dextrose 10; ATP (1, 2.5, and 5 nmol); PPADS (P2 receptor antagonist, 0.5 nmol); α,β-methyleneATP (α,β-meATP, 0.1, 0.5, and 1 nmol); L-glutamate (L-glu, 1 nmol); kynurenic acid (KYN, ionotropic glutamatergic receptors antagonist, 10 nmol); AP5 (NMDA receptor antagonist, 10 nmol); 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt hydrate (CNQX, non-NMDA receptor antagonist, 0.5 nmol); and Evans blue dye (2 % w/v). The drug solutions were freshly dissolved in sterile saline (NaCl 154 mM) and sodium bicarbonate was added to adjust the pH to 7.4. All salts and drugs were purchased from Sigma-Aldrich unless otherwise stated.
Results
Microinjection of adenosine 5′-triphosphate and α,β-methyleneATP into the paraventricular nucleus induces a dose-related increase in the lumbar sympathetic nerve activity
To determine whether the activation of P2 receptors in the PVN would elicit changes to sympathetic outflow, we performed microinjections of random doses of both ATP and α,β-meATP and simultaneously recorded LSNA. We use an L-glu microinjection to functionally identify the PVN as it has been well established that the application of this excitatory amino acid within the PVN increases sympathetic activity. Figure 1a shows traces of LSNA (integrated and raw signals) of four different animals, each representative of groups, which received PVN microinjections of L-glu (1 nmol) and ATP at three different concentrations (1, 2.5, and 5 nmol). Unilateral microinjection of L-glu into the PVN evoked an increase in the LSNA (65 ± 16 %, Fig. 1b) with a rapid onset and a maximum response being reached within 1 s after the injection. Unilateral microinjections of ATP into the PVN elicited a dose-related increase in LSNA with a rapid onset; 1 nmol: 24 ± 6 %; 2.5 nmol: 62 ± 8 %; 5 nmol: 95 ± 12 %, relative to baseline values of LSNA (Fig. 1b and c). The duration of the LSNA responses to ATP from onset until return to baseline values was also dose-dependent. The sympathoexcitation evoked by ATP microinjected into the PVN lasted for approximately 20 to 45 s (Fig. 1b). Moreover, the responses were reproducible as subsequent microinjections at the same site produced similar responses to the initial injection. The magnitude and temporal profile of the response were independent of the order of injection of the various concentrations of ATP. In addition to ATP, the effects of the enzymatically stable ATP analog, α,β-meATP, were also evaluated. Like ATP, unilateral microinjection of α,β-meATP (0.1, 1, and 2 nmol) into the PVN induced a rapid sympathoexcitatory response that was also dose-dependent (Fig. 1d). The LSNA responses to α,β-meATP microinjection into the PVN were also rapid and the following maximal changes were observed: 0.1 nmol: 76 ± 5 %; 1 nmol: 85 ± 20 %; and 2 nmol: 92 ± 32 %, compared to baseline values (Fig. 1d and e). The duration of the sympathoexcitation was also dose-dependent, as observed in the ATP protocol. Microinjection of α,β-meATP at 0.1 nmol produced a sympathoexcitatory response lasting 20 to 30 s before returning to baseline values. At a dose of 1 nmol, the α,β-meATP effect on LSNA lasted 45 to 60 s. When the highest concentration of α,β-meATP (2 nmol) was injected, it evoked a sympathoexcitatory response lasting up to 3 min (Fig. 1d). Additionally, the responses elicited by α,β-meATP microinjected into the PVN were reproducible and did not appear to be subject to desensitization, as repeated microinjections of α,β-meATP with a 5-min interval yielded comparable effects on LSNA both in magnitude and duration.
Fig. 1.
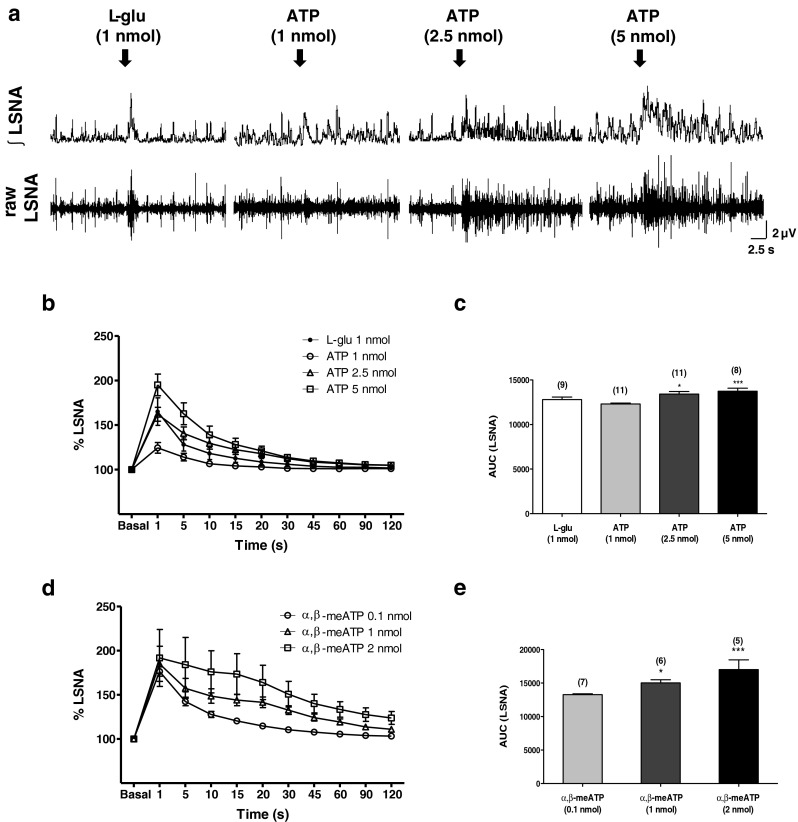
a Representative traces from four animals showing raw and integrated (integral sign) LSNA (μV). Unilateral microinjection (100 nL) of L-glutamate (1 nmol) and ATP (1, 2.5, and 5 nmol) into the PVN increase LSNA in a dose-dependent manner. Arrows show the time of injection into the PVN. b Percentage of changes (basal as 100 %, before injections) in the magnitude of the LSNA (%) and the duration of response(s) elicited by 1 nmol L-glutamate and 1, 2.5, and 5 nmol ATP microinjected into the PVN. L-glutamate induces an increase on LSNA only in the first second, while ATP (2.5 and 5 nmol) maintains this effect for 30 s after the injection with recovery to baseline values 1 min later. c Area under the curve (AUC) of LSNA changes after the injections of 1 nmol L-glutamate and 1, 2.5, and 5 nmol ATP into the PVN. d Percentage of changes (basal as 100 %, before injections) in the magnitude of the LSNA (%) and the duration of response(s) elicited by 0.1, 1, and 2 nmol α,β-metATP microinjected into the PVN on LSNA. The maximum dose of α,β-metATP (2 nmol) increased LSNA for at least 3 min after the injection. Results are shown as mean ± SEM. *p < 0.05 and ***p < 0.001 compared to the lowest dose of ATP or α,β-metATP in the same protocol. One-way repeated measures ANOVA with Bonferroni’s post hoc test. Numbers in parenthesis number of experiments
P2 receptor blockade with pyridoxalphosphate-6-azophenyl-20,40-disulphonic acid attenuates the adenosine 5′-triphosphate-induced sympathoexcitation
The effects of ATP microinjected into the PVN on sympathetic activity were also tested subsequent to treatment with PPADS, a P2 receptor antagonist. Figure 2a shows representative traces of LSNA (integrated and raw signals) from a single experimental animal showing the effects elicited by ATP (2.5 nmol) microinjections into the PVN before and after the administration of PPADS (0.5 nmol) into the same injection site. Unilateral microinjection of PPADS by itself did not elicit any changes to basal LSNA. The amplitude of the sympathoexcitation induced by ATP was not significantly attenuated 5 min after pretreatment with PPADS into the PVN (Fig. 2b, ATP control: 100 % vs. ATP 5 min: 77 ± 13 %, p > 0.05, n = 8). However, 10 and 15 min after the PPADS administration, the amplitude rise in LSNA evoked by ATP was significantly attenuated; 10 min: 59 ± 6 % and 15 min: 59 ± 5 % (p < 0.01, Fig. 2b). A partial recovery of the LSNA response to ATP was observed 30 min after the application of PPADS although the response to ATP remained attenuated (30 min: 70 ± 9 %). The duration of the ATP-induced responses, 5 min after PPADS application into the PVN was not significantly different to saline controls (ATP control: 29 ± 6 s vs. ATP 5 min: 23 ± 5 s, p > 0.05, n = 8, Fig. 2c); however, 10 and 15 min following P2 receptor blockade, the duration of the ATP-induced increase in LSNA was reduced to 17 ± 3 s and 19 ± 3 s, respectively (p < 0.01, Fig. 2c). Unlike the amplitude of the sympathoexcitation, the duration of the effects elicited by ATP on LSNA returned to control values 30 min after PPADS microinjection made into the same site as the ATP microinjections (Fig. 2c, ATP control: 29 ± 6 s vs. ATP 30 min: 28 ± 3 s). Prior microinjection of the vehicle (saline) into the PVN did not affect either the magnitude or the duration of ATP-induced increases in LSNA (Fig. 2b and c). We also evaluated the effects produced by a different concentration of PPADS (0.3 nmol) in ATP-mediated sympathoexcitation at the PVN. Both concentrations of PPADS (0.3 and 0.5 nmol) were able to attenuate the increase of LSNA and the duration of the response elicited by ATP at the same extension (data not shown). Moreover, bilateral microinjection of PPADS (0.5 nmol; n = 7) did not cause significant changes in the basal LSNA (data not shown).
Fig. 2.
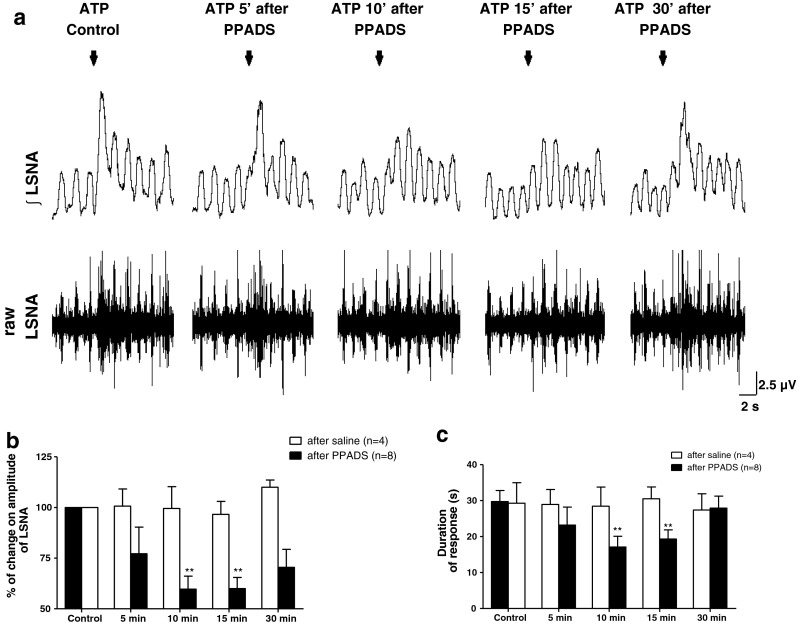
a Representative traces of one animal showing the effects of ATP (2.5 nmol) microinjection into the PVN on raw and integrated (integral sign) LSNA before and after antagonism of P2 receptors (PPADS, 0.5 nmol) at the same site. Arrows show the time of injection. b and c The percentage of change on magnitude of LSNA (%) and duration time of response(s), respectively, induced by ATP before and after PPADS microinjection into the PVN. Note that the ATP-mediated sympathoexcitation is not altered by previous injection of saline. Results are shown as mean ± SEM. **p < 0.01, compared to ATP control vs. ATP in the presence of PPADS, One-way repeated measures ANOVA with Bonferroni’s post hoc test
The sympathoexcitatory responses mediated by adenosine 5′-triphosphate microinjections into the paraventricular nucleus is attenuated after ionotropic glutamate receptor blockade
In order to elucidate a possible interaction between glutamatergic and purinergic systems in the PVN neurons, ATP-evoked increases in LSNA were evaluated following the application of KYN, an ionotropic glutamate receptor antagonist. The increase in sympathetic activity induced by ATP microinjection into the PVN was attenuated at 5 (77 ± 5 %), 10 (76 ± 4 %), and 15 (79 ± 7 %) min after the application of KYN in the same site when compared to ATP control [100 %, (p < 0.05, Fig. 3a)]. Moreover, the rise in LSNA induced by ATP returned to control values 30 min after KYN (104 ± 9 %). With regard to the temporal profile of the sympathoexcitation evoked by ATP, prior administration of KYN significantly reduced the duration of this response at all time periods evaluated (ATP control: 25 ± 4 s; 5 min: 16 ± 2 s; 10 min: 14 ± 2 s; 15 min: 12 ± 2 s; 30 min: 17 ± 2 s, p < 0.001, Fig. 3b). To determine a possible interaction between the glutamatergic and purinergic systems, the sympathoexcitatory effects elicited by L-glu (1 nmol) microinjected into the PVN were tested after P2 purinoceptor blockade with PPADS (0.5 nmol). As demonstrated in Fig. 3c and d, PPADS did not affect the amplitude or temporal profile of the sympathoexcitation elicited by PVN microinjections of L-glu at the same site. In order to verify the effectiveness of the KYN dose (10 nmol), we tested this ionotropic glutamate antagonist on LSNA response to exogenous L-glu (1 nmol) microinjections. As expected, microinjection of L-glu after KYN administration within PVN resulted in a significant reduction in magnitude of sympathoexcitation at 5 min (76 ± 3 %) when compared to L-glu (control: 100 %) or saline (vehicle control: 101 ± 8 %, p < 0.05, n = 4, Fig. 3c) over the same time course.
Fig. 3.
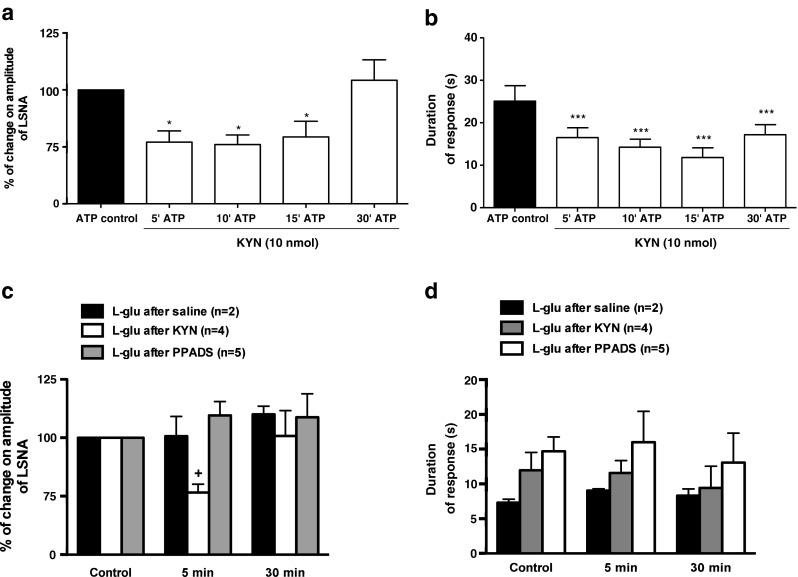
Effects of P2 receptors antagonism (PPADS) or ionotropic glutamatergic receptors antagonism (KYN) on the sympathoexcitation induced by ATP or L-glu microinjected into the PVN. a and b KYN (10 nmol) previously microinjected into the PVN was able to attenuate the percentage of increase on amplitude of LSNA and b the duration of response (onset until recovery to baseline), respectively, induced by ATP (2.5 nmol) microinjection at the same level. c and d Previous antagonism of ionotropic glutamate receptors with KYN (10 nmol), but not P2 receptors (PPADS 0.5 nmol), within the PVN elicits significant attenuation on the amplitude of LSNA and the duration of response (onset until recovery to baseline), respectively, induced by L-glutamate (1 nmol) at the same site. Results are shown as mean ± SEM. *p < 0.05 and ***p < 0.01 compared to control vs. ATP in the presence of KYN; +p < 0.05 compared to control and saline at the same time course. One-way repeated measures ANOVA with Bonferroni’s post hoc test
Kynurenic acid facilitates pyridoxalphosphate-6-azophenyl-20,40-disulphonic acid attenuation of the sympathoexcitatory responses evoked by adenosine 5′-triphosphate in the paraventricular nucleus
Once we had established that both KYN and PPADS alone were able to attenuate the ATP-induced sympathoexcitation within the PVN, we next tested the effects of a double blockade of P2 and ionotropic glutamate receptors (with PPADS and KYN, respectively) on responses elicited by ATP on LSNA. Figure 4a shows representative traces of the increase in the LSNA (raw and integrated) evoked by microinjection of ATP into the PVN before and after the antagonism of P2 and ionotropic glutamatergic receptors in the same site. As observed in Fig. 4b and c, the combination of KYN and PPADS attenuated ATP-induced sympathoexcitation both in amplitude (ATP control: 100 %; 5 min: 70 ± 6 %; 10 min: 61 ± 5 %; 15 min: 75 ± 8 %; 30 min: 82 ± 5 %; n = 6) and duration of the responses (ATP control: 22 ± 2 s; 5 min: 13 ± 1 s; 10 min: 13 ± 2 s; 15 min: 14 ± 3 s; 30 min: 13 ± 2 s; n = 6). The combination of KYN and PPADS treatments appeared to elicit greater attenuation of ATP-induced increases in LSNA than single antagonist treatment alone, although this was not statistically significant. It should be noted that the microinjection of the mixture of the antagonists directly within the PVN did not elicit any visible change to basal LSNA (data not shown).
Fig. 4.
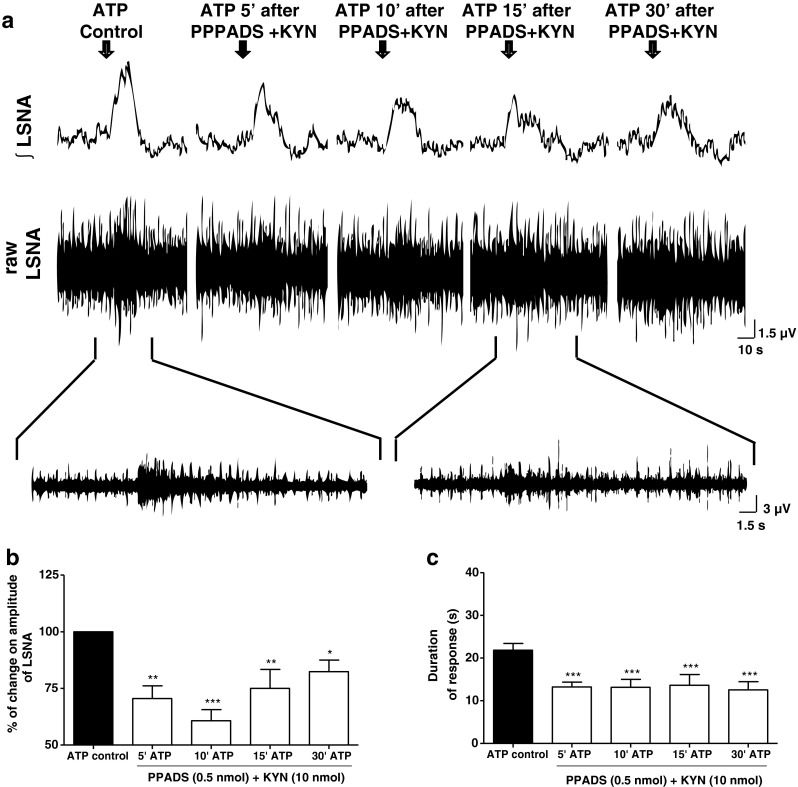
a Representative traces from one animal demonstrating the effect of ATP (2.5 nmol) within the PVN on raw and integrated (integral sign) lumbar sympathetic nerve activity (LSNA) before and after a combination of PPADS (0.5 nmol) and KYN (10 nmol) microinjected at the same site. Arrows show the time of injection into the PVN. b and c The percentage of change on magnitude of the LSNA and duration of response (onset until recovery to baseline), respectively, induced by ATP before and after PPADS plus KYN microinjected into the PVN. Results are shown as mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 compared to ATP control vs. ATP in the presence of PPADS + KYN, One-way repeated measures ANOVA with Bonferroni’s post hoc test; (n = 6)
non-N-methyl-D-aspartate (non-NMDA) receptors contribute to the sympathoexcitation elicited by adenosine 5′-triphosphate into the paraventricular nucleus
Since KYN and the mixture of KYN + PPADS attenuated the ATP-induced sympathoexcitation, we sought to determine which ionotropic glutamatergic receptor subtypes are involved in this response. First, the increase in the LSNA induced by P2 purinoceptor activation into the PVN was evaluated before and after the application of AP-5 (10 nmol), a specific NDMA receptor antagonist. AP5 did not affect LSNA responses, either in amplitude (Fig. 5a) or in duration (Fig. 5b) to microinjections of ATP into the PVN. In a different group of animals, we evaluated the effects of non-NMDA receptor blockade on ATP-induced sympathoexcitation using CNQX (0.5 nmol). Figure 5c shows that the rise in LSNA produced by microinjection of ATP (ATP control: 100 %) into the PVN was significantly attenuated at 10 (80 ± 4 %, p < 0.05) and 15 (74 ± 5 %, p < 0.01) min after the application of CNQX, with recovery of the response observed 30 min later (85 ± 9 %). However, the duration of the increase in the LSNA elicited by ATP was not altered by CNQX administration (Fig. 5d). It is important to mention that the effectiveness of AP-5 and CNQX in blocking NMDA and non-NMDA receptors, respectively, was tested against their agonists (NMDA and L-glu) in the PVN, and the effects elicited by the agonists were blunted 5 min after the application of the antagonists at the same site (L-glu and NMDA control: 100 % vs. CNQX: 54 ± 10 % and AP-5: 53 ± 6 %, p < 0.05, n = 4–6, respectively). It is worth noting that neither AP-5 nor CNQX microinjected into the PVN changed the basal level of SNA (data not shown).
Fig. 5.
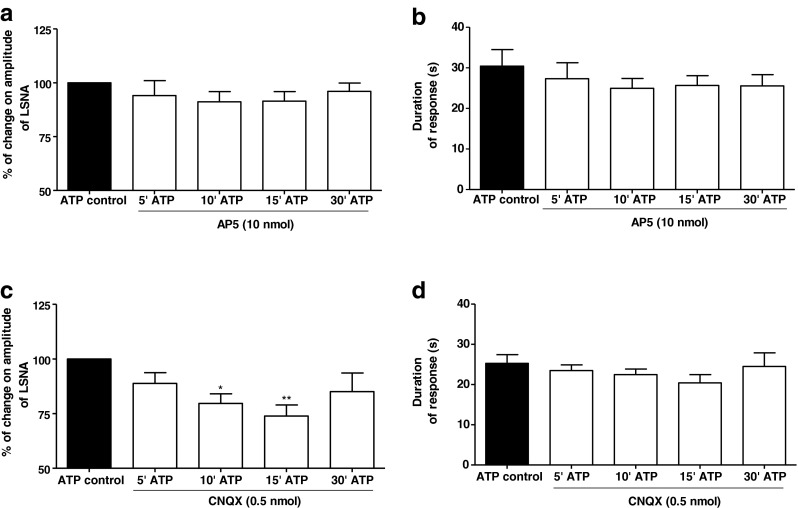
Effects of selective antagonism of the ionotropic glutamatergic receptor subtype on the ATP-induced sympathoexcitation at the PVN level. a and b AP5, a NMDA receptor antagonist with the PVN, did not elicit any significant change on amplitude of LSNA and duration of response (onset until recovery to baseline), respectively, induced by ATP microinjected at the same site (n = 9). c and d Increase in the LSNA elicited by ATP microinjected into the PVN was attenuated in amplitude but not in the duration of that response (onset until recovery to baseline), respectively, by previous injection of CNQX at the same site. Results are shown as mean ± SEM. *p < 0.05, **p < 0.01 compared to ATP control vs. ATP in the presence of CNQX. One-way repeated measures ANOVA with Bonferroni’s post hoc test; (n = 7)
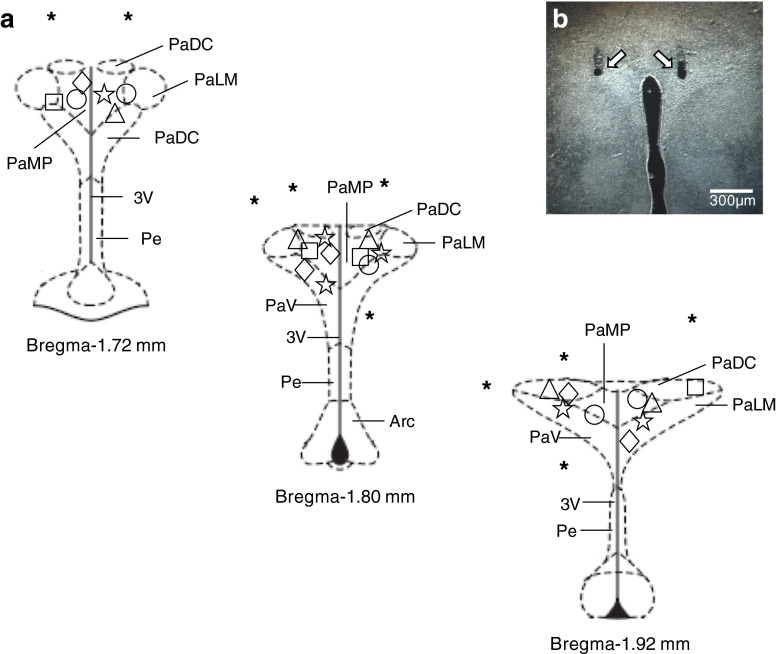
Paraventricular nucleus microinjections
As demonstrated in the schematic diagrams (Fig. 6a) and representative photomicrograph of a typical injection site (Fig. 6b), all of the microinjections reported here were made within the borders of the PVN as defined by the atlas of Paxinos and Watson [43]. Microinjections located outside of the PVN had no significant effects on LSNA, thus demonstrating that the effects of purinergic receptors agonists and/or antagonist were specific to the PVN. Moreover, in our experimental approach, we are able to use both sides of the PVN to develop different protocols without changing the responses on LSNA induced by different agonists and antagonists used in this work.
Fig. 6.
a Coronal sections of the hypothalamus at the level of PVN modified from the brain atlas of Paxinos and Watson (2004), showing the center of the most microinjections of ATP (unfilled star) and α,β-meATP dose–response curve (unfilled diamond), L-glu or ATP vs. PPADS (unfilled circle), ATP vs. KYN or PPADS + KYN (unfilled square), ATP vs. AP5 or CNQX (unfilled triangle) protocols. Asterisks represent microinjection sites of all protocols outside of the PVN. b Representative photomicrograph of a bilateral microinjection into the PVN (dark field microscopy). The arrows show the position of the micropipette tip. PaDC paraventricular hypothalamic nucleus, dorsal cap, PaLM paraventricular hypothalamic nucleus, lateral magnocellular part, PaMP paraventricular hypothalamic nucleus, medial parvicellular part, PaV paraventricular hypothalamic nucleus, ventral part, Pe periventricular hypothalamic nucleus, Arc arcuate hypothalamic nucleus, 3 V third ventricle
Discussion
The present study provides the first functional data to demonstrate that purinergic activation within the PVN increases sympathetic activity in an artificially perfused in situ rat preparation. This preparation is advantageous as it eliminates the need for anesthetic use, which has been shown to have significant depressive effects on the neural control of autonomic function [44]. We have validated and used this preparation extensively in the past to study the central control of SNA [42, 45]. Another advantage of the preparation is the removal of all circulating blood-borne substances such as angiotensin II and other hormones, which can affect sympathetic nerve discharge and obfuscate the data. Our results suggest that ATP and glutamate can act as cotransmitters at this nucleus to modulate sympathoexcitatory responses via the activation of P2 and non-NMDA glutamate receptors.
There is a growing body of evidence suggesting that purinergic signaling within the PVN can modulate autonomic and neuroendocrine functions [26, 40, 46–49]. Furthermore, several immunohistochemical studies have demonstrated that PVN neurons express P2X receptors [31–34]. However, despite the fact that much is known about the anatomical distribution of P2X receptors in the PVN, little is known about the physiological actions of purinergic activation within the PVN on SNA. Our results show that intra-PVN microinjections of ATP and α,β-meATP (a synthetic ATP analog) evoke increases in LSNA in a dose-dependent manner. Moreover, the ATP-mediated sympathoexcitatory responses appear to be specific to the preautonomic neurons located within the PVN, as microinjection of ATP or α,β-meATP in surrounding areas immediately adjacent to the PVN had no effect. As we were primarily interested in the role of the purinergic system in PVN control of sympathetic nerve function, we did not test the effects of ATP in the surrounding hypothalamic areas such as the lateral hypothalamus and dorsomedial hypothalamus. Pharmacological studies showed that α,β-meATP has different affinities for the various P2X receptor subtypes. It is known that P2X1, P2X3 homomeric, and P2X2/3 and P2X1/5 heteromeric receptors have high sensitivity to α,β-meATP [29, 30]. On the other hand, P2X2, P2X4, P2X5, P2X6, and P2X7 homomeric and P2X4/6 heteromeric receptors are fairly insensitive to α,β-meATP [29, 30]. Hence, P2X1, P2X3, P2X2/3, and P2X1/5 are most likely the receptors involved the in PVN-mediated sympathoexcitation evoked by exogenous ATP microinjections. Our results are supported by the findings of Cham et al. [33] who showed that RVLM projecting PVN neurons express P2X1-6 receptors. In addition, ATP induces a rapid, dose-dependent increase in cytosolic Ca2+ in cultured rat hypothalamic neurons [50]. Taken together, we suggest that the activation of ionotropic P2X receptors by exogenous ATP depolarizes preautonomic PVN neurons that, in turn, activate premotor sympathetic neurons projecting to either or both the RVLM and IML to increase sympathetic outflow.
One important difference observed in this study was the more prolonged effect on LSNA induced by the microinjection of α,β-meATP into the PVN compared with ATP. These differences can be attributed to the degradation of ATP to adenosine, which has inhibitory effects when it binds to A1 receptors. α,βme-ATP is a stable ATP analog that is not broken down to adenosine. Recently, a study published by Li et al. [49] showed that adenosine inhibits the excitability of PVN presympathetic neurons through A1 receptor-mediated opening of K(ATP) channels. Taken together, these data suggest that ATP, when applied exogenously, can be degraded to adenosine and thus reduces the duration of ATP-induced sympathoexcitation. This response is different to those mediated by the application of α,β-meATP, a stable synthetic analog of ATP.
An interesting finding was that ATP does not appear to be tonically released in the PVN, since bilateral application of PPADS within this nucleus did not have any significant effects on the LSNA. Our results are concordant with the studies of Kubo et al. [51] who demonstrated that the application of suramin, a nonselective antagonist of P2 receptors, into the PVN did not affect baseline MAP although they did not measure sympathetic nerve responses. In unanesthetized rats, bilateral microinjection of PPADS within the PVN evoked increases in BP and HR, suggesting that ATP is tonically released in the PVN to control autonomic function [40]. The reasons for the differences between their and our current findings are not clear. Perhaps the difference is due to the fact that we studied the lumbar sympathetic branch and cannot exclude the possibility that other sympathetic branches (i.e., thoracic, renal, and cardiac) might exhibit a different activity profile following P2 receptor blockade.
Although PPADS did not alter the basal values of LSNA, it did attenuate the exogenous ATP-evoked sympathoexcitatory effects in the PVN, indicating that this response was partially mediated by purinergic P2 receptors. Interestingly, the ATP-induced sympathoexcitation was not completely antagonized by PPADS. One possible explanation for this might be related to the selectiveness of PPADS for the various P2X receptor subtypes. It is well known that PPADS is a potent antagonist for P2X2, P2X3, and P2X5 homomeric; P2X2/3 and P2X1/5 heteromeric; and P2Y1 receptors [52, 29], but it has little or no effect on P2X1, P2X4, P2X6, and P2X7 homomeric; P2X4/6 heteromeric; and P2Y2, P2Y4, P2Y6, and P2Y11 receptors [29]. Thus, we can postulate that the ATP-evoked sympathoexcitatory responses were not totally blocked by PPADS due to activation of receptors sensitive to α,β-meATP but are PPADS-insensitive.
Our results show that the ATP-mediated sympathoexcitation in the PVN depends, at least in part, on the activation of ionotropic glutamate receptors. In the past, studies have focused on the purinergic–glutamatergic cotransmission within numerous brain nuclei. It is thought that ATP can be released as a transmitter and interact with presynaptic membrane receptors to modulate glutamate release or act on postsynaptic receptors to increase the function or response of glutamate. Moreover, ATP can also play an important role in glial function by stimulating transmitter release or uptake. In the RVLM, Ralevic et al. [53] and Horiuchi et al. [54] have shown that there does not appear to be an interplay between purinergic and glutamatergic systems. However, in other brain nuclei, several studies have demonstrated that ATP and glutamate can function as cotransmitters [39, 41, 55–57]. For example, in nucleus of the solitary tract (NTS) neurons, the fast responses to stimulation of P2X receptors are mediated via glutamatergic ionotropic mechanisms [58]. Furthermore, the ATP-mediated parasympathetic activation within NTS is affected by PPADS and KYN, but the sympathoexcitatory response to microinjection of ATP into the NTS was not affected by the blockade of P2, A1, or excitatory amino acid receptors.
Given the above findings, we investigated the effects of blocking both the P2 and ionotropic glutamate receptors with PPADS and KYN, respectively, on ATP-evoked sympathoexcitation. Interestingly, the combination of KYN and PPADS did not completely attenuate the sympathoexcitatory response induced by ATP when compared to PPADS alone. This might be related to the fact that PPADS did not affect all purinergic receptor subtypes expressed in the PVN neurons. ATP–glutamate interactions have been demonstrated in neural circuits within the NTS, locus coeruleus, hippocampus, cortex, and supraoptic nucleus in both rats and mice in addition to in vitro preparations. Our results demonstrate that the antagonism of NMDA receptors with AP5 did not alter the ATP-induced sympathoexcitation; however, CNQX, an antagonist of non-NMDA receptors, significantly attenuated the increase of sympathetic activity. This finding suggests that the AMPA/kainate receptors may mediate part of the sympathoexcitatory response induced by purinergic activation in the PVN. Our data are in agreement with the observations of Khakh et al. [59], where they used P2X2 knockout mice to show that the activation of presynaptic P2X2 receptors was important for Ca2+ entry and, in turn, increased the frequency of spontaneous and miniature AMPA receptor-mediated glutamatergic currents.
Taken together, we show that purinergic activation of PVN neurons increases LSNA. In addition, there was an interaction between the ATP and glutamate systems at this autonomic brain nucleus. The purinergic–glutamatergic interaction seems to be dependent on activation of postsynaptic P2X receptors located on PVN neurons projecting to sympathetic premotor neurons located either in the RVLM, IML, or both.
Perspectives
Our findings demonstrate, for the first time, that activation of purinergic receptors at the PVN level modulates sympathetic activity. In addition, there is an interaction between purinergic and glutamatergic signaling within PVN neurons that may have important consequences in various physiological and/or pathophysiological conditions. It is well known, for instance, that acute and chronic increases in osmolarity induce alterations in the excitability of PVN neurons, which leads to sympathoexcitation and BP increases. For example, vasopressinergic PVN neurons express P2X4 and P2X6 receptors [34], and as such, the activation of these receptors may contribute to the neuroendocrine and autonomic adjustments induced by hyperosmolarity. Thus, understanding the precise physiological role of this purinergic–glutamatergic interaction as well as the cellular mechanisms involved is important to better understand pathophysiological conditions related to an overactive sympathetic nervous system.
Acknowledgments
This study was supported by Sao Paulo Research Foundation (FAPESP): #07/04085-0 and 10/17997-0. Ferreira-Neto HC is a recipient of a FAPESP fellowship #10/05037-1.
Conflict of interest
None.
Abbreviations
- ATP
Adenosine 5′-triphosphate
- PPADS
Pyridoxalphosphate-6-azophenyl-20,40-disulphonic acid
- α,β-meATP
α,β-MethyleneATP
- L-glu
L-Glutamate
- KYN
Kynurenic acid
- AP5
DL-2-Amino-5-phosphonopentanoic acid
- CNQX
6-Cyano-7-nitroquinoxaline-2,3-dione disodium salt
References
- 1.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 2.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.2466/pr0.1994.74.1.323. [DOI] [PubMed] [Google Scholar]
- 3.Coote JH, Yang Z, Pyner S, Deering J. Control of sympathetic outflows by the hypothalamic paraventricular nucleus. Clin Exp Pharmacol Physiol. 1998;25:461–463. doi: 10.1111/j.1440-1681.1998.tb02235.x. [DOI] [PubMed] [Google Scholar]
- 4.Dampney RA, Horiuchi J, Killinger S, Sheriff MJ, Tan PS, McDowall LM. Long-term regulation of arterial blood pressure by hypothalamic nuclei: some critical questions. Clin Exp Pharmacol Physiol. 2005;32:419–425. doi: 10.1111/j.1440-1681.2005.04205.x. [DOI] [PubMed] [Google Scholar]
- 5.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 6.Dampney RA, Czachurski J, Dembowsky K, Goodchild AK, Seller H. Afferent connections and spinal projections of the pressor region in the rostral ventrolateral medulla of the cat. J Auton Nerv Syst. 1987;20:73–86. doi: 10.1016/0165-1838(87)90083-X. [DOI] [PubMed] [Google Scholar]
- 7.Yang Z, Coote JH. Influence of the hypothalamic paraventricular nucleus on cardiovascular neurones in the rostral ventrolateral medulla of the rat. J Physiol. 1998;513:521–530. doi: 10.1111/j.1469-7793.1998.521bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardy SG. Hypothalamic projections to cardiovascular centers of the medulla. Brain Res. 2001;894:233–240. doi: 10.1016/S0006-8993(01)02053-4. [DOI] [PubMed] [Google Scholar]
- 9.Swanson LW, Kuypers HG. The paraventricular nucleus of the hypothalamus: cytoarchitectonic subdivisions and organization of projections to the pituitary, dorsal vagal complex, and spinal cord as demonstrated by retrograde fluorescence double-labeling methods. J Comp Neurol. 1980;194:555–570. doi: 10.1002/cne.901940306. [DOI] [PubMed] [Google Scholar]
- 10.Sawchenko PE, Swanson LW. Immunohistochemical 546 identification of neurones in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol. 1982;205:260–272. doi: 10.1002/cne.902050306. [DOI] [PubMed] [Google Scholar]
- 11.Lovick TA, Coote JH. Electrophysiological properties of paraventriculo-spinal neurones in the rat. Brain Res. 1988;454:123–130. doi: 10.1016/0006-8993(88)90810-4. [DOI] [PubMed] [Google Scholar]
- 12.Shafton AD, Ryan A, Badoer E. Neurones in the hypothalamic paraventricular nucleus send collaterals to the spinal cord and to the rostral ventrolateral medulla in the rat. Brain Res. 1998;801:239–243. doi: 10.1016/S0006-8993(98)00587-3. [DOI] [PubMed] [Google Scholar]
- 13.Pyner S, Coote JH. Identification of an efferent projection from the paraventricular nucleus of the hypothalamus terminating close to spinally projecting rostral ventrolateral medullary neurones. Neuroscience. 1999;88:949–957. doi: 10.1016/S0306-4522(98)00255-3. [DOI] [PubMed] [Google Scholar]
- 14.Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol. 2001;28:95–99. doi: 10.1046/j.1440-1681.2001.03413.x. [DOI] [PubMed] [Google Scholar]
- 15.Horn T, Smith PM, McLaughlin BE, Bauce L, Marks GS, Pittman QJ, Ferguson AV. Nitric oxide actions in paraventricular nucleus: cardiovascular and neurochemical implications. Am J Physiol. 1994;266:R306–313. doi: 10.1152/ajpregu.1994.266.1.R306. [DOI] [PubMed] [Google Scholar]
- 16.Stern JE, Li Y, Zhang W. Nitric oxide: a local signalling molecule controlling the activity of pre-autonomic neurones in the paraventricular nucleus of the hypothalamus. Acta Physiol Scand. 2003;177:37–42. doi: 10.1046/j.1365-201X.2003.01045.x. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Zhang W, Stern JE. Nitric oxide inhibits the firing activity of hypothalamic paraventricular neurones that innervate the medulla oblongata: role of GABA. Neuroscience. 2003;118:585–601. doi: 10.1016/S0306-4522(03)00042-3. [DOI] [PubMed] [Google Scholar]
- 18.Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39:275–280. doi: 10.1161/hy0202.104272. [DOI] [PubMed] [Google Scholar]
- 19.Silva AQ, Santos RA, Fontes MA. Blockade of endogenous angiotensin-(1–7) in the hypothalamic paraventricular nucleus reduces renal sympathetic tone. Hypertension. 2005;46:341–348. doi: 10.1161/01.HYP.0000179216.04357.49. [DOI] [PubMed] [Google Scholar]
- 20.Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am J Physiol. 1989;256:R1325–1330. doi: 10.1152/ajpregu.1989.256.6.R1325. [DOI] [PubMed] [Google Scholar]
- 21.Busnardo C, Tavares RF, Corrêa FM. Role of N-methyl-D-aspartate and non-N-methyl-D-aspartate receptors in the cardiovascular effects of L-glutamate microinjection into the hypothalamic paraventricular nucleus of unanesthetized rats. J Neurosci Res. 2009;87:2066–2077. doi: 10.1002/jnr.22028. [DOI] [PubMed] [Google Scholar]
- 22.Busnardo C, Crestani CC, Tavares RF, Resstel LB, Correa FM. Cardiovascular responses to L-glutamate microinjection into the hypothalamic paraventricular nucleus are mediated by a local nitric oxide-guanylate cyclase mechanism. Brain Res. 2010;1344:87–95. doi: 10.1016/j.brainres.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 23.Chen QH, Haywood JR, Toney GM. Sympathoexcitation by PVN-injected bicuculline requires activation of excitatory amino acid receptors. Hypertension. 2003;42:725–731. doi: 10.1161/01.HYP.0000085197.20043.44. [DOI] [PubMed] [Google Scholar]
- 24.Chen QH, Toney GM. Responses to GABA-A receptor blockade in the hypothalamic PVN are attenuated by local AT1 receptor antagonism. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1231–1239. doi: 10.1152/ajpregu.00028.2003. [DOI] [PubMed] [Google Scholar]
- 25.Zhang K, Patel KP. Effect of nitric oxide within the paraventricular nucleus on renal sympathetic nerve discharge: role of GABA. Am J Physiol. 1998;275:R728–34. doi: 10.1152/ajpregu.1998.275.3.R728. [DOI] [PubMed] [Google Scholar]
- 26.Kapoor JR, Sladek CD. Purinergic and adrenergic agonists synergize in stimulating vasopressin and oxytocin release. J Neurosci. 2000;20:8868–8875. doi: 10.1523/JNEUROSCI.20-23-08868.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbracchio MP, Burnstock G. Purinoceptors: are there families of P2X and P2Y purinoceptors? Pharmacol Ther. 1994;64:445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin SA, Mackey JR, Cass CE, Young JD. Nucleoside transporters: molecular biology and implications for therapeutic development. Mol Med Today. 1999;5:216–224. doi: 10.1016/S1357-4310(99)01459-8. [DOI] [PubMed] [Google Scholar]
- 29.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 30.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 31.Shibuya I, Tanaka K, Hattori Y, Uezono Y, Harayama N, Noguchi J, Ueta Y, Izumi F, Yamashita H. Evidence that multiple P2X purinoceptors are functionally expressed in rat supraoptic neurones. J Physiol. 1999;514:351–367. doi: 10.1111/j.1469-7793.1999.351ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao ST, Gourine AV, Spyer KM, Barden JA, Lawrence AJ. Localisation of P2X2 receptor subunit immunoreactivity on nitric oxide synthase expressing neurones in the brain stem and hypothalamus of the rat: a fluorescence immunohistochemical study. Neuroscience. 2003;121:411–419. doi: 10.1016/S0306-4522(03)00435-4. [DOI] [PubMed] [Google Scholar]
- 33.Cham JL, Owens NC, Barden JA, Lawrence AJ, Badoer E. P2X purinoceptor subtypes on paraventricular nucleus neurones projecting to the rostral ventrolateral medulla in the rat. Exp Physiol. 2006;91:403–411. doi: 10.1113/expphysiol.2005.032409. [DOI] [PubMed] [Google Scholar]
- 34.Guo W, Sun J, Xu X, Bunstock G, He C, Xiang Z. P2X receptors are differentially expressed on vasopressin- and oxytocin-containing neurones in the supraoptic and paraventricular nuclei of rat hypothalamus. Histochem Cell Biol. 2009;131:29–41. doi: 10.1007/s00418-008-0493-9. [DOI] [PubMed] [Google Scholar]
- 35.de Paula PM, Antunes VR, Bonagamba LG, Machado BH. Cardiovascular responses to microinjection of ATP into the nucleus tractus solitarii of awake rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1164–1171. doi: 10.1152/ajpregu.00722.2003. [DOI] [PubMed] [Google Scholar]
- 36.Antunes VR, Bonagamba LG, Machado BH. Hemodynamic and respiratory responses to microinjection of ATP into the intermediate and caudal NTS of awake rats. Brain Res. 2005;1032:85–93. doi: 10.1016/j.brainres.2004.10.048. [DOI] [PubMed] [Google Scholar]
- 37.Antunes VR, Braga VA, Machado BH. Autonomic and respiratory responses to microinjection of ATP into the intermediate or caudal nucleus tractus solitarius in the working heart–brainstem preparation of the rat. Clin Exp Pharmacol Physiol. 2005;32:467–472. doi: 10.1111/j.1440-1681.2005.04213.x. [DOI] [PubMed] [Google Scholar]
- 38.Yao ST, Lawrence AJ. Purinergic modulation of cardiovascular function in the rat locus coeruleus. Br J Pharmacol. 2005;145:342–352. doi: 10.1038/sj.bjp.0706179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Braga VA, Soriano RN, Braccialli AL, de Paula PM, Bonagamba LG, Paton JF, et al. Involvement of L-glutamate and ATP in the neurotransmission of the sympathoexcitatory component of the chemoreflex in the commissural nucleus tractus solitarii of awake rats and in the working heart-brainstem preparation. J Physiol. 2007;581:1129–1145. doi: 10.1113/jphysiol.2007.129031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cruz JC, Bonagamba LG, Machado BH. Modulation of arterial pressure by P2 purinoceptors in the paraventricular nucleus of the hypothalamus of awake rats. Auton Neurosci. 2010;158:79–85. doi: 10.1016/j.autneu.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 41.Pankratov Y, Castro E, Miras-Portugal MT, Krishtal O. A purinergic component of the excitatory postsynaptic current mediated by P2X receptors in the CA1 neurones of the rat hippocampus. Eur J Neurosci. 1998;10:3898–3902. doi: 10.1046/j.1460-9568.1998.00419.x. [DOI] [PubMed] [Google Scholar]
- 42.Antunes VR, Yao ST, Pickering AE, Murphy D, Paton JF. A spinal vasopressinergic mechanism mediates hyperosmolality-induced sympathoexcitation. J Physiol. 2006;576:569–583. doi: 10.1113/jphysiol.2006.115766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic; 1996. [DOI] [PubMed] [Google Scholar]
- 44.Lash JM, Haase E, Shoukass AA. Systemic responses to carotid 645 occlusion in the anesthetized rat. J Appl Physiol. 1992;72:1247–1254. doi: 10.1152/jappl.1992.72.4.1247. [DOI] [PubMed] [Google Scholar]
- 45.Colombari DS, Colombari E, Freiria-Oliveira AH, Antunes VR, Yao ST, Hindmarch C, Ferguson AV, Fry M, Murphy D, Paton JFR. Switching control of sympathetic activity from forebrain to hindbrain in chronic dehydration. J Physiol. 2011;589:4457–4471. doi: 10.1113/jphysiol.2011.210245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mori M, Tsushima H, Matsuda T. Antidiuretic effects of purinoceptor agonists injected into the hypothalamic paraventricular nucleus of water-loaded, ethanol-anesthetized rats. Neuropharmacology. 1992;31:585–592. doi: 10.1016/0028-3908(92)90191-Q. [DOI] [PubMed] [Google Scholar]
- 47.Song Z, Sladek CD. Site of ATP and phenylephrine synergistic stimulation of vasopressin release from the hypothalamo-neurohypophyseal system. J Neuroendocrinol. 2006;18:266–272. doi: 10.1111/j.1365-2826.2006.01411.x. [DOI] [PubMed] [Google Scholar]
- 48.Knott TK, Marrero HG, Custer EE, Lemos JR. Endogenous ATP potentiates only vasopressin secretion from neurohypophysial terminals. J Cell Physiol. 2008;217:155–161. doi: 10.1002/jcp.21485. [DOI] [PubMed] [Google Scholar]
- 49.Li DP, Chen SR, Pan HL. Adenosine inhibits paraventricular pre-sympathetic neurones through ATP-dependent potassium channels. J Neurochem. 2010;113:530–542. doi: 10.1111/j.1471-4159.2010.06618.x. [DOI] [PubMed] [Google Scholar]
- 50.Chen ZP, Levy A, Lightman SL. Activation of specific ATP receptors induces a rapid increase in intracellular calcium ions in rat hypothalamic neurones. Brain Res. 1994;641:249–256. doi: 10.1016/0006-8993(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 51.Kubo T, Yanagihara Y, Yamaguchi H, Fukumori R. Excitatory amino acid receptors in the paraventricular hypothalamic nucleus mediate pressor response induced by carotid body chemoreceptor stimulation in rats. Clin Exp Hypertens. 1997;19:1117–1134. doi: 10.3109/10641969709083208. [DOI] [PubMed] [Google Scholar]
- 52.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 53.Ralevic V, Thomas T, Burnstock G, Spyer KM. Characterization of P2 receptors modulating neural activity in rat rostral ventrolateral medulla. Neuroscience. 1999;94:867–878. doi: 10.1016/S0306-4522(99)00376-0. [DOI] [PubMed] [Google Scholar]
- 54.Horiuchi J, Potts PD, Tagawa T, Dampney RA. Effects of activation and blockade of P2x receptors in the ventrolateral medulla on arterial pressure and sympathetic activity. J Auton Nerv Syst. 1999;76:118–126. doi: 10.1016/S0165-1838(99)00019-3. [DOI] [PubMed] [Google Scholar]
- 55.Pankratov Y, Lalo UV, Krishtal OA, Verkhratsky A. Ionotropic P2X purinoreceptors mediate synaptic transmission in rat pyramidal neurones of layer II/III of somato-sensory cortex. J Physiol. 2002;542:529–536. doi: 10.1113/jphysiol.2002.021956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pankratov Y, Lalo U, Verkhratsky A, North RA. Quantal release of ATP in mouse cortex. J Gen Physiol. 2007;129:257–265. doi: 10.1085/jgp.200609693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Passamani LM, Pedrosa DF, Mauad H, Schenberg LC, Paton JF, Sampaio KN. Involvement of the purinergic system in central cardiovascular modulation at the level of the nucleus ambiguus of anaesthetized rats. Exp Physiol. 2011;96:262–274. doi: 10.1113/expphysiol.2010.054882. [DOI] [PubMed] [Google Scholar]
- 58.Scislo TJ, O’Leary DS. Differential role of ionotropic glutamatergic mechanisms in responses to NTS P(2x) and A(2a) receptor stimulation. Am J Physiol Heart Circ Physiol. 2000;278:H2057–2068. doi: 10.1152/ajpheart.2000.278.6.H2057. [DOI] [PubMed] [Google Scholar]
- 59.Khakh BS, Gittermann D, Cockayne DA, Jones A. ATP modulation of excitatory synapses onto interneurons. J Neurosci. 2003;23:7426–7437. doi: 10.1523/JNEUROSCI.23-19-07426.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nieber K, Poelchen W, Illes P. Role of ATP in fast excitatory synaptic potentials in locus coeruleus neurones of the rat. Br J Pharmacol. 1997;122:423–430. doi: 10.1038/sj.bjp.0701386. [DOI] [PMC free article] [PubMed] [Google Scholar]