Abstract
P2X receptors participate in cardiovascular regulation and disease. After myocardial ischemic injury, sensory–sympathetic coupling between rat cervical DRG nerves and superior cervical ganglia (SCG) facilitated sympathoexcitatory action via P2X7 receptor. The results showed that after myocardial ischemic injury, the systolic blood pressure, heart rate, serum cardiac enzymes, IL-6, and TNF-α were increased, while the levels of P2X7 mRNA and protein in SCG were also upregulated. However, these alterations diminished after treatment of myocardial ischemic (MI) rats with the P2X7 antagonist oxATP. After siRNA P2X7 in MI rats, the systolic blood pressure, heart rate, serum cardiac enzymes, the expression levels of the satellite glial cell (SGC) or P2X7 were significantly lower than those in MI group. The phosphorylation of ERK 1/2 in SCG participated in the molecular mechanism of the sympathoexcitatory action induced by the myocardial ischemic injury. Retrograde tracing test revealed the sprouting of CGRP or SP sensory nerves (the markers of sensory afferent fibers) from DRG to SCG neurons. The upregulated P2X7 receptor promoted the activation of SGCs in SCG, resulting in the formation of sensory–sympathetic coupling which facilitated the sympathoexcitatory action. P2X7 antagonist oxATP could inhibit the activation of SGCs and interrupt the formation of sensory–sympathetic coupling in SCG after the myocardial ischemic injury. Our findings may benefit the treatment of coronary heart disease and other cardiovascular diseases.
Keywords: P2X7 receptor, Superior cervical ganglia, Dorsal root ganglia, Myocardial ischemic injury, Sensory–sympathetic coupling
Introduction
Sympathoexcitatory action in cervical sympathetic ganglia is induced by cardiac afferent activation [4, 25, 43]. Sympathoexcitatory action can exaggerate myocardial ischemia further by increasing myocardial oxygen consumption while maintaining cardiac function transiently by enhancing contractility [25, 44]. Surgical interventions of sympathetic afferent pathways abolish or relieve angina pectoris [43]. Myocardial ischemia can produce a variety of chemical substances, which act on the cardiac afferent nerves. Adenine triphosphate (ATP) is released from the sympathetic ganglia [10, 11, 48] and is involved in signal transmission via acting on P2X receptors [10, 11]. The P2X7 receptor is a member of P2X gene family, which encodes membrane glycoproteins that function as ATP-gated cation channels [11, 12, 56]. P2X receptors participate in cardiovascular regulation and disease [15, 30, 47].
Expression of P2X receptors is found in the cervical sympathetic ganglia [1, 20, 27, 28, 30, 38, 54, 58, 59]. Previous works in our laboratory showed that acute myocardial ischemic (MI) injury induced up-expressions of P2X3 and P2X2/3 receptors in superior cervical ganglia (SCG) and increased blood pressure and heart rate via sympathoexcitatory reflex [27, 28, 30, 38, 58, 59]. We also found that P2X3 and P2X2/3 receptors might be involved in the signal transmission of MI injury since A-317491, an antagonist of these receptors, could inhibit these events [27, 28, 30, 38, 50, 51, 58, 59]. P2X7 receptor plays an important role in functions and diseases of the nervous system [13, 40, 41]. ATP and P2X7 receptor are involved in the responses of injury [3, 8, 9, 11, 18, 41]. P2X7 blockade reduced brain damage after ischemia [3].This study investigated the effects of P2X7 receptor on the increased sympathoexcitatory action in SCG caused by MI injury and examined if there is a specific connection between SCG and the cervical dorsal root ganglion (DRG) afferents which would exaggerate the sympathoexcitatory reflex due to activation of P2X7 receptor during MI injury.
Materials and methods
Animals and myocardial ischemic rat model
Sprague–Dawley rats weighing 180–230 g were used in all experiments. Use of the animals was approved by the Animal Care and Use Committee of Medical College of Nanchang University. Myocardial ischemic rat model was used in our studies [27, 28, 50, 51]. Rats were randomly divided (with six to eight rats in each group) into sham operation group (sham group), control rats treated with the P2X7 receptor antagonist oxATP group (con+oxATP group), myocardial ischemic group (MI group), and oxATP-treated myocardial ischemic rats group (MI+oxATP group).
MI injury was established by ligating the left anterior descending coronary artery [22]. The rats were anesthetized with 10 % chloral hydrate (0.3 ml/100 g). Mechanical ventilation and thoracotomy were undergone in MI rat model. In rats with left coronary artery (LCA) occlusion, a 5–0 suture on a small, curved needle was passed through the myocardium beneath the LCA. Subsequently, both suture ends were passed through a small vinyl tube to make a snare. The suture was pulled tightly against the vinyl tube and ligated to occlude the LCA. After the surgery, the chest was closed and electrocardiogram (ECG) changes were continuously monitored. The relative voltage of negative peak of the S-wave against the QQ line was defined as the ST segment. The abnormal Q wave or ST-segment displacement in a lead II ECG was clearly observed in MI rats. The rats in the sham group were not ligated via LCA, but only probed up the cardiac pericardium 24 h after surgery. Rats in MI+oxATP group and con+oxATP group were treated with oxATP (1 mg/kg day) by intragastric administration (i.g.) for 20 days. Rats in sham group and MI group were given the same volume of normal saline (i.g.) for 20 days. OxATP was dissolved in saline and the final concentration was 0.2 mg/ml.
Measurement of blood pressure, cardiac enzymes, and cytokines
Blood pressure and heart rate were measured with an indirect tail-cuff method (Softron BP-98A, Softron Co, Tokyo, Japan) [28]. Twenty days after myocardial ischemia,the rats were anesthetized with 10 % chloral hydrate. Blood samples were obtained from carotid arterial cannulation. After statically left for 30 min, blood samples were centrifuged at 1,000 rpm for 15 min, and the serum was collected. Serum enzyme levels of lactate dehydrogenase (LDH), creatine kinase (CK), CK isoform MB (CK-MB), and cardiac troponin I (cTn-I) were measured with automatic electrochemiluminescence immunoassay analyzer (Roche COBAS E601, USA) [50].
Serum tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) levels were quantified with enzyme-linked immunosorbent assay (ELISA) by utilizing commercially available antibodies, according to the protocol provided by the supplier (Senxiong company, Shanghai, China). The reactions were recorded with an ELISA reader (Rayto, RT-6000, USA) at 450 nm. The concentrations of TNF-α and IL-6 were determined based on a standard curve [28].
Immunohistochemistry and double-label immunofluorescence
The expression levels of receptors or makers were measured by immunohistochemistry [28, 50, 51]. Co-expression was observed by double-label immunofluorescence [27, 49]. Twenty days after myocardial ischemia,the rats in four groups were anesthetized. Superior cervical ganglia (SCG) and cervical dorsal root ganglia (DRG) were dissected immediately and washed in phosphate-buffered saline (PBS), fixed in 4 % PFA for 24 h at 4 °C, and transferred to 20 % sucrose in 4 % PFA overnight. Tissues were sectioned at 12 μm thick and stored at −20 °C until immunohistochemical processing. The expression levels of P2X7 receptor, P2X3 receptor, glutamine synthetase (GS), neuronal nuclei (NeuN), calcitonin gene-related peptide (CGRP), substance P (SP) or tyrosine hydroxylase (TH) were measured by immunohistochemistry. After washed by PBS for three times, the sections were incubated in 3 % H2O2 for 10 min to block the endogenous peroxidase. Following rinses in PBS, sections were incubated with 5 % BSA for 60 min, then the sections were incubated with rabbit anti-P2X7 (1:100; Alomone Labs, Jerusalem, Israel) [8], rabbit anti-P2X3 (1:2,500, Millipore International, Inc, USA), mouse anti- GS (1:100 dilution; Abcam Inc., USA), mouse anti-NeuN (1:200 dilution, Millipore International, Inc, USA), rabbit monoclonal anti-CGRP (1:500 dilution, bioss, CO), rabbit monoclonal anti-SP (1:500 dilution, bioss, CO) or mouse anti-TH (1:1,000 dilution; Abcam International, Inc. USA) diluted in PBS for overnight at 4 °C. Sections were rinsed and incubated with biotinylated goat anti-rabbit secondary antibody or goat anti-mouse secondary antibody (1:100 dilution; Beijing Zhongshan Biotech. CO.) for 1 h at room temperature. The sections were washed in PBS and added streptavidin-horseradish peroxidase (HRP; Beijing Zhongshan Biotech. CO.) for 30 min. After development of the diaminobenzidine chromogen for 2 min, the slides were washed with distilled water and cover-slipped. Image scanning analysis system (Image-Pro Plus) was used to analyze the changes in integrated optical density (IOD) of P2X7, P2X3, GS, NeuN, CGRP, SP and TH. In the double-label immunofluorescence, the differences are those we used 3 % Triton-X100 (Beijing Zhongshan Biotech. CO.) instead of 3 % H2O2 and the sections were incubated with mixed primary antibodies. Fluorescein- or rhodamine-conjugated goat anti-rabbit and goat anti-mouse secondary antibodies (1:100 dilution; Beijing Zhongshan Biotech. CO.) were also used for 1 h at room temperature. Other sections for “ABC staining” were incubated with biotinylated secondary antibodies (Beijing Zhongshan Biotech. CO.) for 45 min at room temperature, processed with avidin-coupled horseradish peroxidase, and developed with diamino-benzidine. The sections of double-label immunofluorescence were incubated with fluorescent goat anti-rabbit FITC and goat anti-mouse TRITC secondary antibody (1:100 dilution; Beijing Zhongshan Biotech. CO.). Stained slides were mounted, coverslipped, and examined under Olympus microscope (Olympus TH4-200, Japan). The changes of IOD for ganglia were analyzed by Image Pro-Plus software. To verify the specificity of immunoreactivity of primary antibodies, 10 % normal goat serum in PBS was substituted for the primary antibody as a negative control.
Retrograde tracing labeling of horseradish peroxidase
Horseradish peroxidase (HRP) is a retrograde tracer. Retrograde tracing of HRP [31] in the cardiac afferent endings was used to observe the retrograde neuronal labeling from the cardiac afferent endings to SCG. The rats were anesthetized with 10 % chloral hydrate (0.3 ml/100 g). Mechanical ventilation and thoracotomy were undergone in the experimental rats. HRP (15 μl, diluted in 0.1 M PBS, 10 mg/ml) was injected into cardiac apex and conus arteriosus at five to seven points by a microinjector. Penicillin (2 × 105 U) was injected in the muscle of experimental rats. After 7 days, the SCG was isolated to carry out the test of labelled immunofluorescence.
HRP in SCG was used to observe the retrograde neuronal labeling from the cervical DRG (C1-T2) to SCG. The rats were anesthetized and cervical skin was cut open. Left SCG was softly fixed. HRP (15 μl, diluted in 0.1 M PBS, 10 mg/ml) was injected into SCG by a microinjector. Penicillin (2 × 105 U) was injected in the muscle of experimental rats. After 7 days, the cervical DRG (C1-T2) were isolated for the examination of labelled immunofluorescence.
SCG and DRG were isolated from the experimental rats. Mouse anti-HRP antibody (1:100, dilute with 0.1 M PBS) was mixed with the secondary antibody (1:200 Rhodamine (or TRITC)-conjugated AffiniPure Goat anti- mouse lgG) to carry out the double-label immunofluorescence test.
In situ hybridization
The mRNA expression was assessed by in situ hybridization (ISH) [28, 50]. The rats of four groups were anaesthetized. The ganglia were dissected immediately and fixed in 4 % paraformaldehyde (PFA) for 24 h at 4 °C, and then transferred to 20 % sucrose in 4 % PFA overnight. Tissues were sectioned at 12 μm thick and stored in refrigerator at −20 °C.
Diethyl pyrocarbonate water was used for all solutions and appliances necessary for ISH. Sections were treated with 0.05 % H2O2, followed by digestion with pepsin at 37 °C for 1–2 min, terminated with 0.5 mol/L phosphate-buffered saline (PBS), and washed for 15 min. The sections were incubated in prehybridization for 4–6 h at 37 °C and then in hybridization overnight at 37 °C. The in situ hybridization kit for P2X7 receptors (Wuhan Boster Co.) was used. P2X7 mRNA probe sequences are (1)5′-AATTA CGGCA CCATC AAGTG GATCT TGCAC ATGAC-3′; (2)5′-TACTG GGACT GCAAC CTGGA CAGCT GGTCC CATCG-3′; (3)5′-TTTGT GGACG AGCCC CACAT TTGGA TGGTG GACCA-3′. The sections were washed with gradient SSC (2× SSC 17.6 g sodium chloride, 8.8 g sodium citrate in 1,000 ml distilled water) thoroughly, 2× SSC for 10 min, 0.5× SSC for 15 min, and 0.2× SSC for 15 min to remove the background signals and followed by treatment of biotinylated digoxim antibody at 37 °C for 2 h. After strongly washed with PBS, the sections were incubated with SABC-POD for 30 min and with biotinylated peroxidase (Beijing Zhongshan Biotech. CO.) for 30 min at 37 °C. The sections were developed in DAB substrate (Beijing Zhongshan Biotech. CO.) then dehydrated and mounted with neutral gum.
Western blotting
The protein expression was determined by Western blotting [28, 51]. The rats were anesthetized with 10 % chloral hydrate (0.3 ml/100 g) and sacrificed. The ganglia were isolated and flushed with ice-cold phosphate-buffered saline (PBS). Ganglia were homogenized by mechanical disruption in lysis buffer (50 mmol/L TrisCl, pH 8.0, 150 mmol/L NaCl, 0.1 % dodecyl sodium sulfate, 1 % Nonidet P-40, 0.5 % sodium deoxycholate, 100 μg/mL phenylmethylsulfonyl fluoride, and 1 μg/mL Aprotinin) and incubated on ice for 40 min. Homogenate was then centrifuged at 12,000 rpm for 10 min, and supernatant was collected. Using Lowry method, the quantity of total protein was determined in the supernatant. After diluted with sample buffer (100 mmol/L TrisCl, 200 mmol/L dithiothreitol, 4 % sodium deodecylsulfate (SDS), 0.2 % Bromophenol Blue, 20 % glycerol) and heated to 95 °C for 5 min, samples containing equal amounts of protein (20 μg) were separated by SDS-polyacrylamide gel electrophoresis by using Bio-Rad system and 10 % gel. In the wake of electrophoretic transfer onto a PVDF membrane using the same system, the membrane was blocked with 5 % non-fat dry milk in 25 mmol/L Tris-buffered saline, pH 7.2, plus 0.1 % Tween 20 (TBST) for 3 h at room temperature, followed by incubation with primary antisera (rabbit anti-P2X7 1:200; Alomone Labs, Jerusalem, Israel) in 5 % non-fat dry milk for overnight at 4 °C. Recovering to room temperature on the second day, the membrane was washed in TBST, and incubated with the secondary antibody, horseradish peroxidase (HRP)-conjugated goat anti- rabbit IgG (1:1,000, Beijing Zhongshan Biotech. CO.) in 5 % non-fat dry milk for 1 h at room temperature. After a final wash in TBST and then using the enhanced chemiluminescence kit (Shanghai Pufei Biotech. CO.), chemiluminescent signals were collected on autoradiography film. The quantity of band intensity was determined using AlphaImager 2200 software. The primary antibodies and dilutions used were as follows: rabbit polyclonal anti-P2X7 (1:200; Alomone Labs, Jerusalem, Israel) and monoclonal β-actin (1:1,000; Advanced Immunochemicals, VB Long Beach, CA). Band densities were normalized to each β-actin internal control.
The expression of ERK1/2 and p-ERK1/2 protein was examined by the same protocol used for P2X7 protein. The primary antibodies and dilutions used were as follows: rabbit polyclonal anti-phospho-p44/42 MAPK (ERK1/2, 1:1,000, Cell Signal CO.) and rabbit polyclonal anti-p44/42 MAPK (ERK1/2, 1:1000, Cell Signal CO.)
P2X7 receptor small interference RNA treatment
The small interference RNA (siRNA) specific for rat P2X7 was purchased from Invitrogen (Carlsbad, CA). siRNA oligonucleotides targeted specifically to rat P2X7 were used in this experiment [35]. P2X7-receptor knockdown was achieved by RNA interference (RNAi) using an Entranster™ in vivo Transfection Reagent [57]. The siRNA was diluted with 80 μL RNA-free water, then added 80 μL 10 % glucose, we got the mixture A. Mixture B was obtained by mixing 80 μL Transfection Reagent and 80 μL 10 % glucose. B was added into A immediately, and 15 min later, the 320-μL siRNA solution was injected into the sublingual vein. The siRNA target sequence 5′-GUGCAGUGAAUGAGUACUATT-3′ (Invitrogen) was selected for the P2X7 receptor. Rats were assigned in a random blind fashion to one of four groups, as follows: sham operation group (sham group), myocardial ischemic group (MI group), P2X7 siRNA vector-treated myocardial ischemic rats group (MI+P2X7 siRNA group), and MI rats treated with scramble siRNA vector group (MI+scramble siRNA group).
Data analysis
Statistical analyses of the data were performed using SPSS 11.5. All results were expressed as mean ± SE. Statistical significance was determined by one-way analysis of variance followed by the Fisher post hoc test for multiple comparisons. p < 0.05 was considered as significant difference.
Results
Effects of oxATP or knockdown P2X7 on blood pressure, heart rate, and electrocardiogram
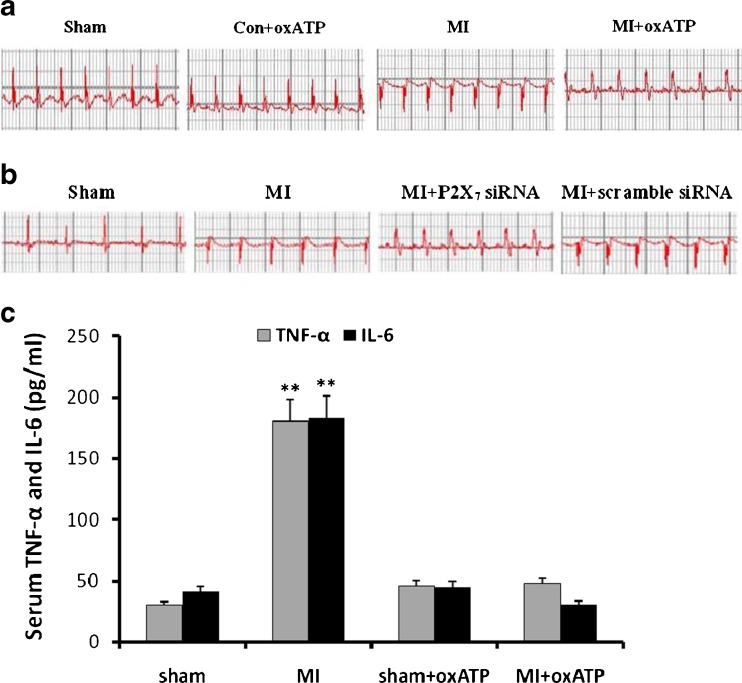
After 20 days of myocardial ischemia, systolic blood pressure and heart rate in the MI group (MI; n = 8) were increased in comparison with those in sham group and control rats treated with oxATP (con+oxATP; n = 8, respectively; p < 0.05). After the treatment with P2X7 antagonist oxATP in the MI rats (MI+oxATP) (n = 8), systolic blood pressure and heart rate were lower than those in MI rats (p < 0.05; A of Table 1). Heart rate in MI+oxATP group was still higher than con+oxATP group and sham group (n = 8; p < 0.05). There was no significant difference between con+oxATP group and sham group (p > 0.05; A of Table 1). After ligating the left anterior descending coronary artery, ST segment in the ECG was highly upward. After 20 days of myocardial ischemia, the abnormal Q wave appeared obviously in MI rats compared with that in sham or oxATP-treated control rats. After MI rats treated with oxATP, abnormal Q wave induced by myocardial ischemia was improved in comparison with that in MI group (Fig. 1a).
Table 1.
Effects of oxATP or knockdown P2X7 on systolic blood pressure, diastolic blood pressure, heart rate
| Group | Systolic blood pressure (mmHg) | Diastolic blood pressure (mmHg) | Heart rate (/min) |
|---|---|---|---|
| A | |||
| Sham (n = 8) | 110.04 ± 9.53 | 72.35 ± 7.35 | 341.88 ± 35.43 |
| con+oxATP (n = 8) | 108. 65 ± 10.72 | 73.43 ± 8.56 | 334.70 ± 36.90 |
| MI (n = 8) | 128.00 ± 13.54* | 91.06 ± 9.14* | 426.72 ± 44.21* |
| MI+oxATP (n = 8) | 111.16 ± 10.87 | 73.65 ± 9.87 | 398.72 ± 39.81** |
| B | |||
| Sham (n = 8) | 110.17 ± 10.56 | 76.66 ± 7.14 | 352.24 ± 21.38 |
| MI (n = 8) | 133.40 ± 8.37*** | 97.89 ± 8.23*** | 438.42 ± 30.53*** |
| MI+P2X7 siRNA (n = 8) | 111.16 ± 7.62 | 77.27 ± 6.98 | 349.78 ± 25.74 |
| MI+Scramble siRNA (n = 8) | 131.93 ± 10.65*** | 100.81 ± 7.35*** | 428.20 ± 28.89*** |
Data are presented as means ± SE
*p < 0.05, compared with sham group, oxATP control group, and myocardial ischemic rats treated with oxATP group; **p < 0.05, compared with sham and oxATP control group; ***p < 0.05, compared with sham group and myocardial ischemic rats treated with P2X7 siRNA group.
Fig. 1.
Representative traces of ECG in the different groups of rats. Representative traces of ECG were measured 20 days after myocardial ischemia. a The abnormal Q wave appeared obviously in MI rats (n = 8) compared with sham (n = 8) and con+oxATP rats (n = 8). In MI+oxATP group (n = 8), abnormal Q wave induced by MI injury was improved. b After MI rats treated with siRNA P2X7, abnormal Q wave induced by MI injury was improved in comparison with that in MI and MI+scramble siRNA rats. c The serum concentration of TNF-α and IL-6 was measured by ELISA. The concentrations of TNF-α and IL-6 in MI group were higher than those in sham group, con+oxATP group, MI+oxATP group (p < 0.01). No significant difference was found in the concentration of TNF-α or IL-6 among sham group, con+oxATP group, and MI+oxATP group (p > 0.05). The n value is 8 in all groups. Results are mean ± SE. **p < 0.05 vs sham group, con+oxATP group, and MI+oxATP group
In order to validate P2X7 inhibition in SCG after the myocardial ischemic injury, siRNA P2X7 receptor in vivo was used. After the treatment with siRNA P2X7 in myocardial ischemic rats (MI+siRNA P2X7), systolic blood pressure and heart rate were lower than those in MI rats and MI+scramble siRNA group (n = 8, respectively; p < 0.05). There was no significant difference between sham group and MI+P2X7 siRNA group (n = 8, respectively; p > 0.05; B of Table 1). After MI rats treated with siRNA P2X7, abnormal Q wave induced by myocardial ischemia was improved in comparison with that in MI group and MI+scramble siRNA group (Fig. 1b).
Effects of oxATP on serum TNF-α and IL-6
The serum levels of TNF-α and IL-6 were measured by ELISA kit. Both TNF-α and IL-6 were higher in MI group than in sham group, con+oxATP group, or MI+oxATP group (n = 6; p < 0.01). There were no significant differences among sham group, con+oxATP group, and MI+oxATP group (n = 6; p > 0.05; Fig. 1c).
Effects of oxATP or knockdown P2X7 on serum myocardial enzymes
MI injury induced elevations in serum cardiac enzymes. CK-MB, CK, LDH, and cTn-I in the blood serum were markers for the ischemic injury of myocardial tissue. After 20 days of MI, serum concentrations of CK-MB, CK, LDH, and cTn-I in the MI rats were significantly increased compared with those in the sham or con+oxATP rats (p < 0.05). P2X7 receptor antagonist oxATP significantly attenuated the elevated serum concentrations of these enzymes due to myocardial ischemia, indicative of reduced myocardial injury. Serum concentrations of CK-MB, CK, LDH, and cTn-I in MI+oxATP group were higher than those in con+oxATP group or sham group (p < 0.05; A of Table 2). After MI rats treated with siRNA P2X7, serum concentrations of CK-MB, CK, LDH, and cTn-I were decreased in comparison with that in MI group and MI+scramble siRNA group (p < 0.05; B of Table 2).
Table 2.
Effects of oxATP or knockdown P2X7 on the serum concentration of CK-MB, CK, LDH, and cTn-I
| Group | CK-MB (u/l) | CK (u/l) | LDH (u/l) | cTn-I (ng/ml) |
|---|---|---|---|---|
| A | ||||
| Sham (n = 8) | 57.34 ± 5.843 | 533.65 ± 52.12 | 321.85 ± 31.24 | 0.058 ± 0.005 |
| con+oxATP (n = 8) | 31.48 ± 3.98 | 400.35 ± 40.65 | 234.90 ± 20.96 | 0.048 ± 0.005 |
| MI (n = 8) | 82.20 ± 8.76* | 2231.95 ± 135.04* | 936.85 ± 91.49* | 0.186 ± 0.009* |
| MI+oxATP (n = 8) | 68.10 ± 7.66** | 874.25 ± 91.60** | 487.80 ± 50.76** | 0.084 ± 0.007** |
| B | ||||
| Sham (n = 8) | 32.80 ± 2.53 | 559.57 ± 55.36 | 311.43 ± 37.50 | 0.051 ± 0. 005 |
| MI (n = 8) | 87.50 ± 8.45*** | 2252.44 ± 220.50*** | 889.16 ± 88.34*** | 0.185 ± 0.02*** |
| MI+P2X7 siRNA (n = 8) | 35.11 ± 4.21 | 547.43 ± 55.89 | 340.24 ± 35.54 | 0.049 ± 0.005 |
| MI+Scramble siRNA (n = 8) | 85.45 ± 6.35*** | 2103.47 ± 217.48*** | 823.89 ± 82.58*** | 0.163 ± 0.01*** |
Data are presented as means ± SE
*p < 0.05, compared with sham group, oxATP control group and myocardial ischemic rats treated with oxATP group; **p < 0.05, compared with sham and oxATP control group; ***p < 0.05, compared with sham group and myocardial ischemic rats treated with P2X7 siRNA group
Effects of oxATP on the expressions of P2X7 mRNA and protein in SCG
The expressions of P2X7 mRNA and protein in SCG were assessed by ISH (Fig. 2a), immunohistochemistry (Fig. 2b), and Western blotting (Fig. 2d). Higher expression of P2X7 mRNA and protein occurred in MI group in comparison with sham group, con+oxATP group, and MI+oxATP group (n = 8; p < 0.05; Fig. 2a–e). The bar graphs (Fig. 2c, e) showed the result of ISH, IHC, and Western blotting. The relative expression was the IOD ratio of P2X7 to β-actin. The results showed that the expression of P2X7 receptor in MI group was significantly higher than that in sham group, con+oxATP, and MI+oxATP (p < 0.05). No significant difference was found among sham group, con+oxATP group, and MI+oxATP group (n = 8; p > 0.05; Fig. 2).
Fig. 2.
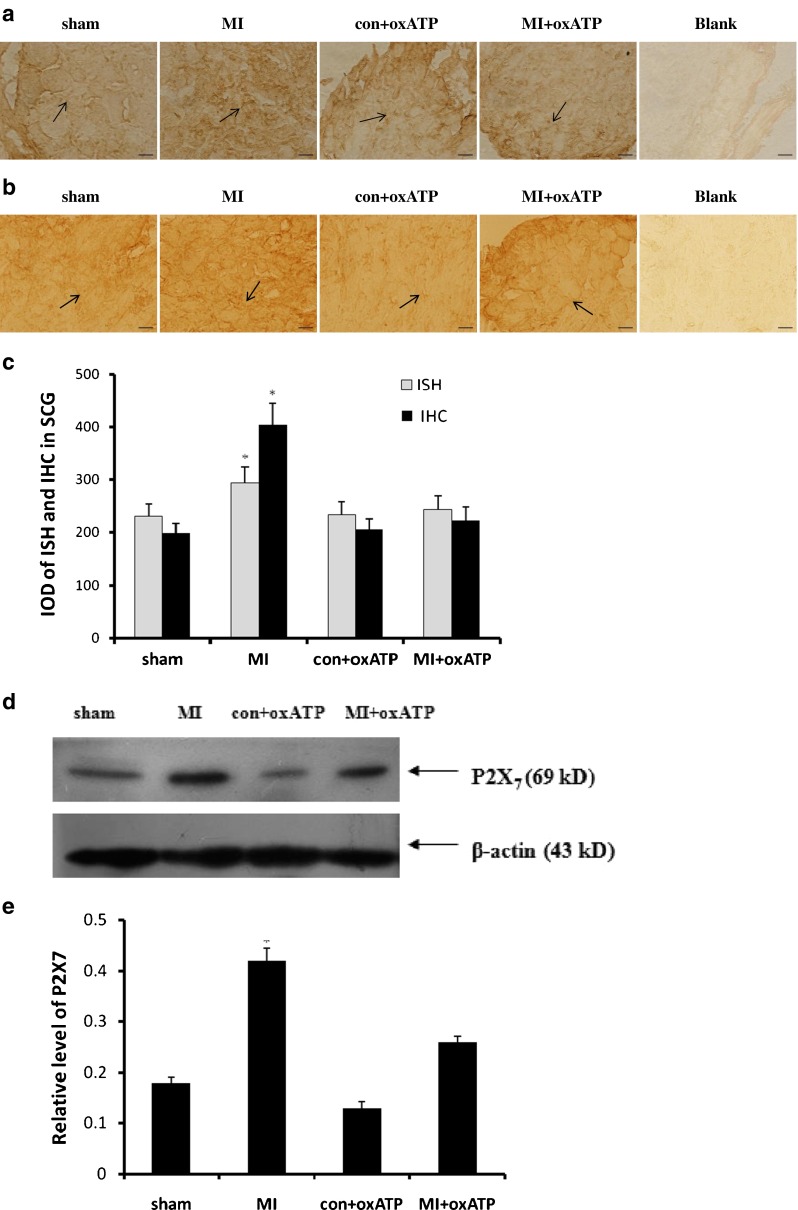
Effects of oxATP on the increased P2X7 expression induced by ischemic injury. The expressions of P2X7 mRNA and protein were measured by in situ hybridization (a), immunohistochemistry (b), and Western blotting (d). The bar graphs (c, e) showed the statistical results for expression of P2X7 mRNA or protein. The relative expression in Western blotting was expressed by the IOD ratio of P2X7 protein to β-actin. The results showed that the expression levels of P2X7 mRNA or protein in MI group (n = 8) were significantly higher than those in sham group (n = 8), con+oxATP group (n = 8), and MI+oxATP group (n = 8; p < 0.05). No difference was found among sham group, con+oxATP group, and MI+oxATP group (p > 0.05). Arrows indicate the immunostaining neurons. Scale bars, 20 μm. Results are mean ± SE. *p < 0.05 vs sham group, con+oxATP group, and MI+oxATP group
Effects of oxATP on the levels of ERK1/2 and p-ERK1/2 in SCG
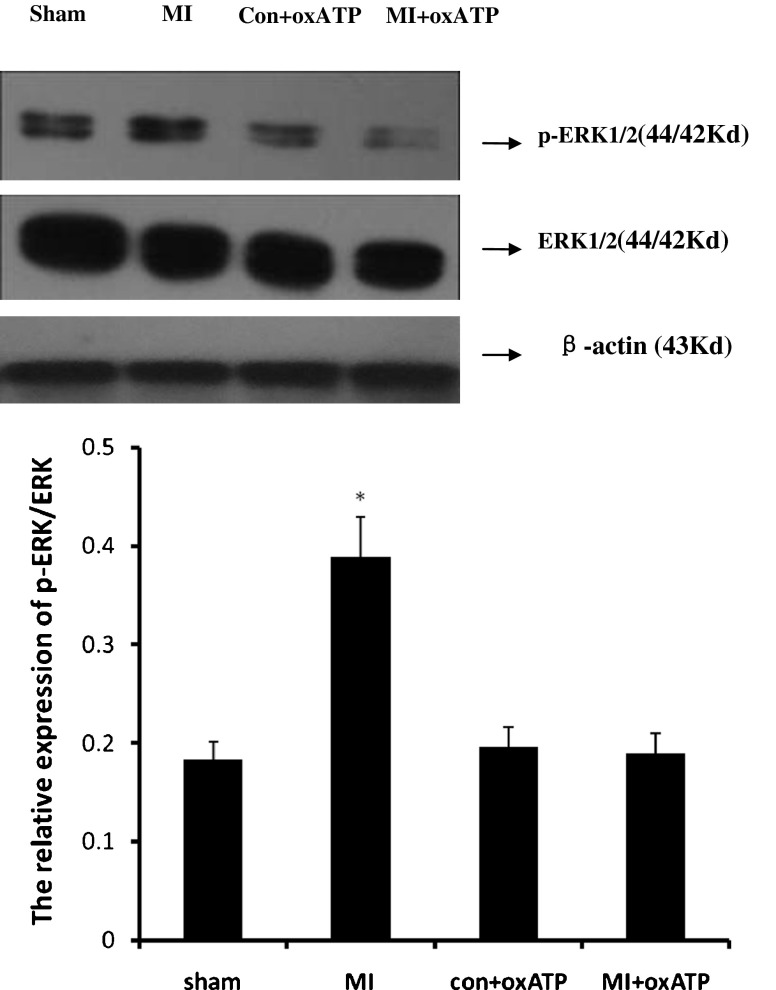
Phosphorylation and activation of ERK1/2 is involved in the activation of inflammatory mediators. The expressions of ERK1/2 and p-ERK1/2 in SCG were analyzed by Western blotting. The IOD ratio of ERK1/2 to β-actin was not significantly different among four groups (p > 0.05). But the IOD ratio of p-ERK1/2 to total ERK1/2 in MI group was higher than that in sham group (n = 8). In addition, oxATP attenuated nearly 50 % of the upregulated expression due to MI (Fig. 3).
Fig. 3.
The expression of ERK1/2 and p-ERK1/2 protein in SCG. The IOD ratio of p-ERK1/2 to total ERK1/2 in SCG in MI group (n = 8) was higher than that in sham group (n = 8), con+oxATP group (n = 8), and MI+oxATP group (n = 8; p < 0.05). No difference was found among sham group, con+oxATP group, and MI+oxATP group (p > 0.05). There was no difference in ERK1/2 protein among the four groups (p > 0.05). Results are mean ± SE. *p < 0.05 vs sham group, con+oxATP group, and MI+oxATP group
Double-label immunofluorescence of P2X7 and TH, GS, or HRP in SCG
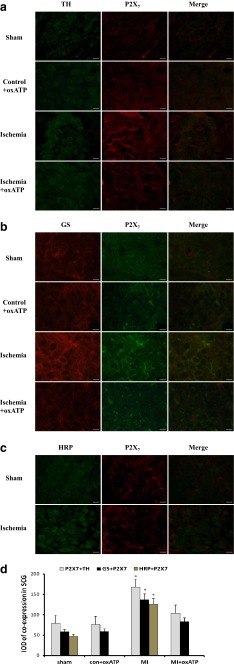
The double-label immunofluorescence of P2X7 and TH in SCG was detected with fluorescence immunohistochemistry in four groups after 20 days of myocardial ischemia (Fig. 4a). P2X7 and TH were co-expressed in SCG. The double-label immunofluorescence of P2X7 and TH in MI group exhibited higher density than that in sham group, con+oxATP group, and MI+oxATP group (n = 6, respectively; p < 0.05; Fig. 4a, d). There was no significant difference among con+oxATP group, sham group, and MI+oxATP group (n = 6, respectively; p > 0.05; Fig. 4a, d).
Fig. 4.
The double-label immunofluorescence of P2X7 and TH, GS or HRP in SCG. a There was the coexpression of P2X7 and TH in SCG. Red signal represents P2X7 staining with TRITC, and green signal indicates TH staining with FITC. Merge represents P2X7 and TH double staining image. Scale bar, 20 μm. b GS is the typical feature of satellite glial cells (SGCs) in sympathetic ganglia. P2X7 receptor was co-expressed with GS, so it was mainly expressed in SGCs. Red signal represents P2X7 staining with TRITC, and green signal indicates GS staining with FITC. Merge represents P2X7 and GS double staining image. Scale bar, 20 μm. c There was double-label immunofluorescence of P2X7 and HRP from the cardiac afferent endings to the SCG, as assessed by the retrograde tracing neuronal labeling using HRP as a retrograde tracing marker. Red signal represents P2X7 staining with TRITC, and green signal indicates HRP staining with FITC. Merge represents P2X7 and HRP double staining image. Scale bar, 20 μm. d The bar graphs showed the statistical results for co-expression of P2X7 and TH, GS or HRP in SCG (n = 6 in all groups)
The main type of glial cells in most sympathetic ganglia is the satellite glial cells (SGCs). SGCs usually form envelopes around individual ganglionic neurons. The presence of glutamine synthetase (GS) is the typical feature of glial cells. The coexpression of GS and P2X7 receptor in SCG was measured by double-label immunofluorescence (Fig. 4b). After 20 days of myocardial ischemia, the staining of GS and P2X7 by double-label immunofluorescence in MI group was more intense than that in con+oxATP group, sham group, and MI+oxATP group (n = 6, respectively; p < 0.05; Fig. 4b, d). There was no significant difference among sham group, con+oxATP group, and MI+oxATP group (n = 6, respectively; p > 0.05; Fig. 4b, d). Horseradish peroxidase (HRP) was used as a retrograde tracing marker to observe the retrograde neuronal labeling from the cardiac afferent endings to SCG. Two groups of rats, sham control and myocardial ischemia, were studied in these experiments. In MI group, HRP was injected into cardiac apex and conus arteriosus at 13 days after MI operation. The double immunofluorescence labelling of HRP and P2X7 in the SCG of sham group was observed 7 days later, which displayed barely staining. By contrast, the staining of P2X7 and HRP by double immunofluorescence labelling in MI group was more intense (n = 6, respectively; p < 0.05; Fig. 4c, d), suggesting the neuronal labeling of P2X7 receptor from the cardiac afferent endings to SCG.
Effects of knockdown P2X7 on the expression or co-expression of P2X7 or/and GS in SCG
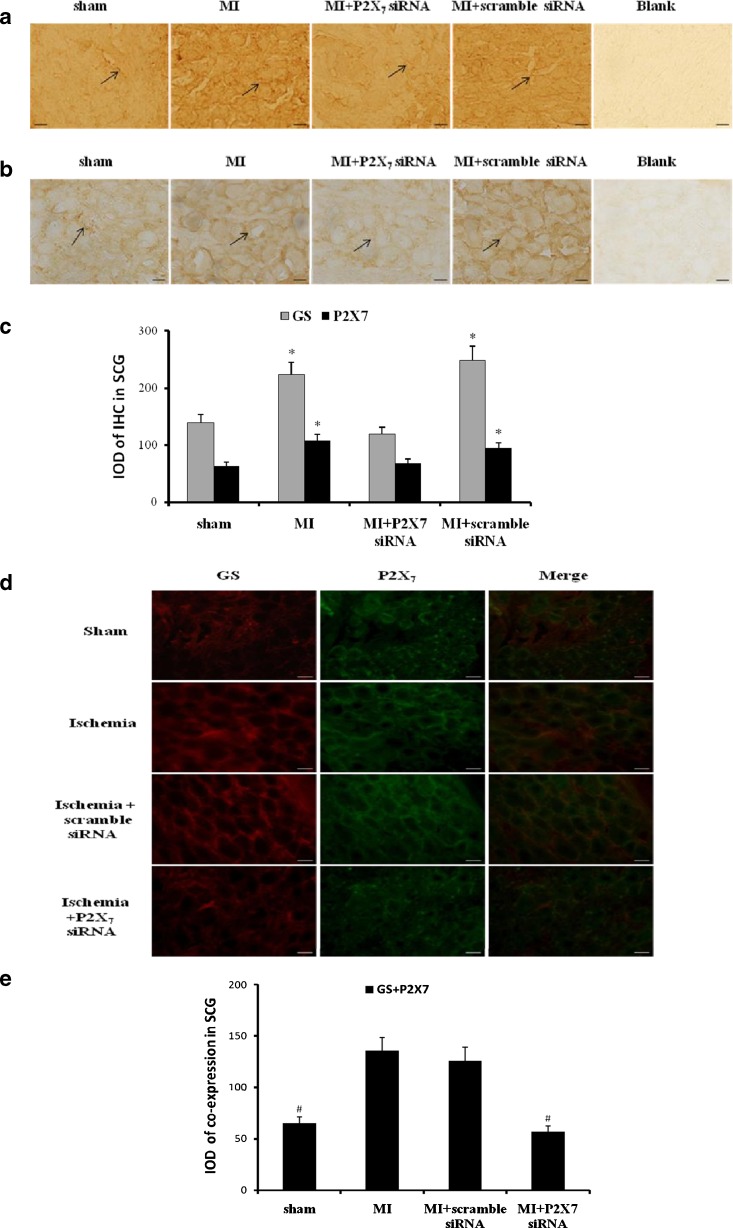
In order to validate P2X7 inhibition interrupting the formation of sensory–sympathetic coupling in SCG after the myocardial ischemic injury, we adopted a second approach based on the expression of siRNA P2X7 receptor. The expressions of P2X7 or GS were assessed by immunohistochemistry (Fig. 5a, b). After siRNA P2X7 in myocardial ischemic rats, the expression levels of GS or P2X7 were significantly lower than those in MI group (n = 6, respectively; p < 0.05). There was no significant difference among sham group, MI+P2X7 siRNA group, and MI+scramble siRNA group (n = 6, respectively; p > 0.05; Fig. 5c).
Fig. 5.
Effects of siRNA P2X7 on the expression or co-expression of P2X7 or/and GS in SCG. The expressions of P2X7 (a) or GS (b) were assessed by immunohistochemistry. After siRNA P2X7 in myocardial ischemic rats, the expression levels of GS or P2X7 were significantly lower than those in MI group and MI+scramble siRNA group (n = 6, respectively; p < 0.05). There was no significant difference among sham group and MI+P2X7 siRNA group (n = 6, respectively; p > 0.05). Arrows indicate the immunostaining neurons. Scale bars, 20 μm. The bar graphs (c) showed the statistical results for expression of GS or P2X7 immunoactivity. Results are mean ± SE. *p < 0.05 vs sham group, con+oxATP group, and MI+oxATP group. The co-expression of GS and P2X7 receptor in SCG was measured by double-label immunofluorescence after siRNA P2X7 receptor (d). After siRNA P2X7 in myocardial ischemic rats, the co-expression staining of GS and P2X7 was significantly lower than that in MI group and MI+scramble siRNA group (n = 6, respectively; p < 0.05). There was no significant difference among sham group and MI+P2X7 siRNA group (n = 6, respectively; p > 0.05). The results further revealed that P2X7 receptor was involved in myocardial ischemic injury. Red signal represents GS staining with TRITC, and green signal indicates P2X7 staining with FITC. Merge represents GS and P2X7 double staining image. Scale bar, 20 μm. The bar graphs showed the statistical results for co-expression of GS and P2X7 in SCG (e)
The coexpression of GS and P2X7 receptor in SCG was measured by double-label immunofluorescence (Fig. 5d). After siRNA P2X7 in myocardial ischemic rats, the coexpression staining of GS and P2X7 was significantly lower than that in MI group (n = 6, respectively; p < 0.05). There was no significant difference among sham group, con+scramble siRNA group, and MI+P2X7 siRNA group (n = 6, respectively; p > 0.05; Fig. 5e). The results further revealed that P2X7 receptor was involved in myocardial ischemic injurious response.
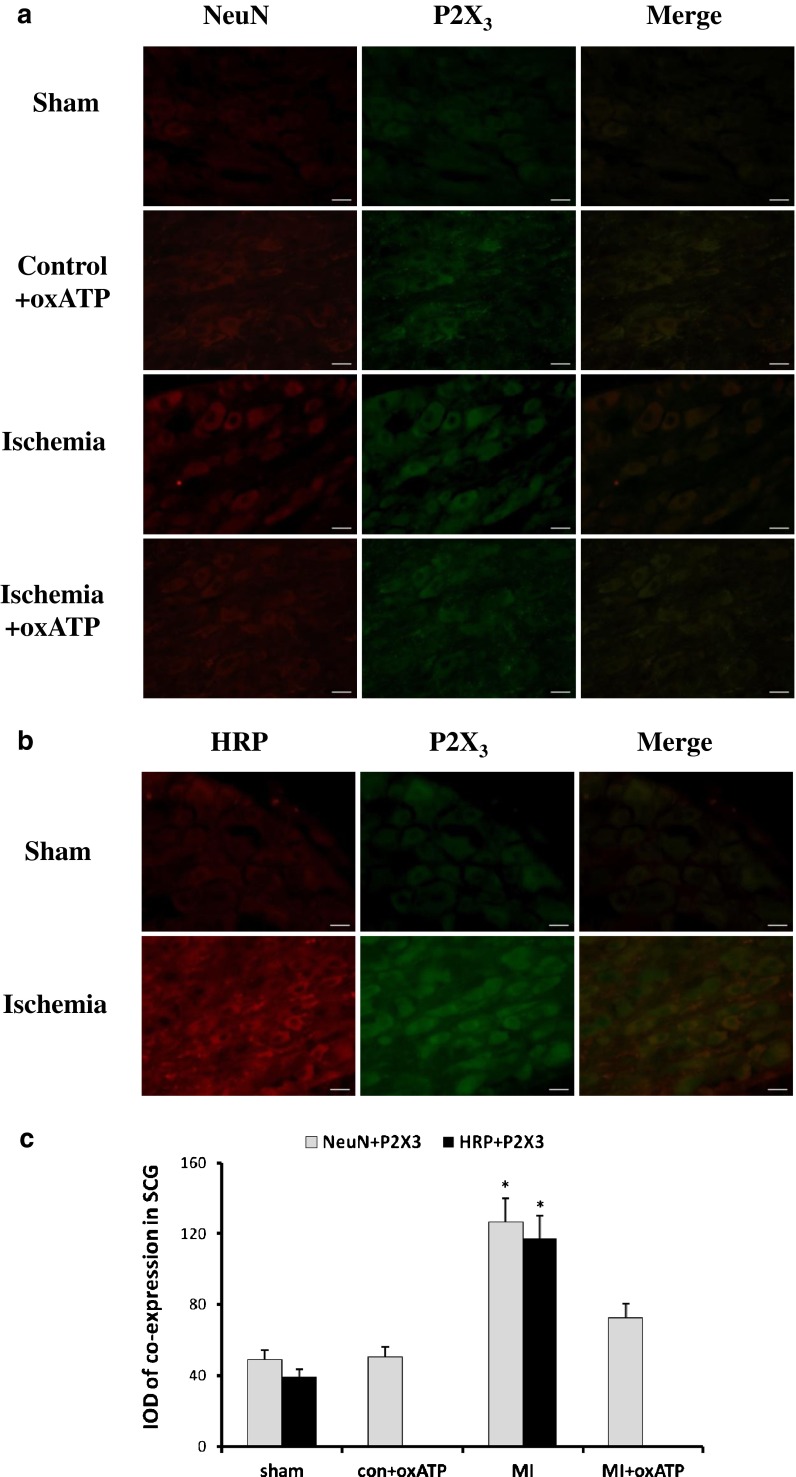
Double immunofluorescence labelling of P2X3 and NeuN or HRP in SCG
NeuN is the marker of neurons. The presence of NeuN immunoreactivity is the typical feature of sympathetic neurons. After 20 days of myocardial ischemia, the staining of NeuN and P2X3 by double-label immunofluorescence in SCG of MI group was more intense than that in sham group, con+oxATP group, and MI+oxATP group(n = 6, respectively; p < 0.05; Fig. 6a, c). There was no significant difference among the latter three groups (n = 6, respectively; p > 0.05; Fig. 6a, c). The results indicated that P2X3 receptor was mainly expressed in SCG neurons.
Fig. 6.
The double-label immunofluorescence of P2X3 and NeuN or HRP in SCG. a NeuN is the marker of neurons. The image shows that P2X3 receptor was mainly expressed in SCG neurons. Red signal represents P2X3 staining with TRITC, and green signal indicates NeuN staining with FITC. Merge represents P2X3 and NeuN double staining image. Scale bar, 20 μm. b There was double immunofluorescence of P2X3 and HRP from the cardiac afferent endings to SCG, as tested by the retrograde neuronal labeling. Red signal represents P2X3 staining with TRITC, and green signal indicates HRP staining with FITC. Merge represents P2X3 and HRP double staining image. Scale bar, 20 μm. c The bar graphs showed the statistical results for co-expression of P2X3 and NeuN or HRP in SCG (n = 6 in all groups)
The retrograde neuronal labeling from the cardiac afferent endings to SCG was also performed after injection of HRP as above. A little staining of P2X3 and HRP was observed in the SCG of sham group by double-label immunofluorescence. The staining of P2X3 and HRP in MI group was more intense than that in sham group (n = 6, respectively; p < 0.05; Fig. 6b, c), indicating the presence of P2X3 from the cardiac afferent endings to SCG.
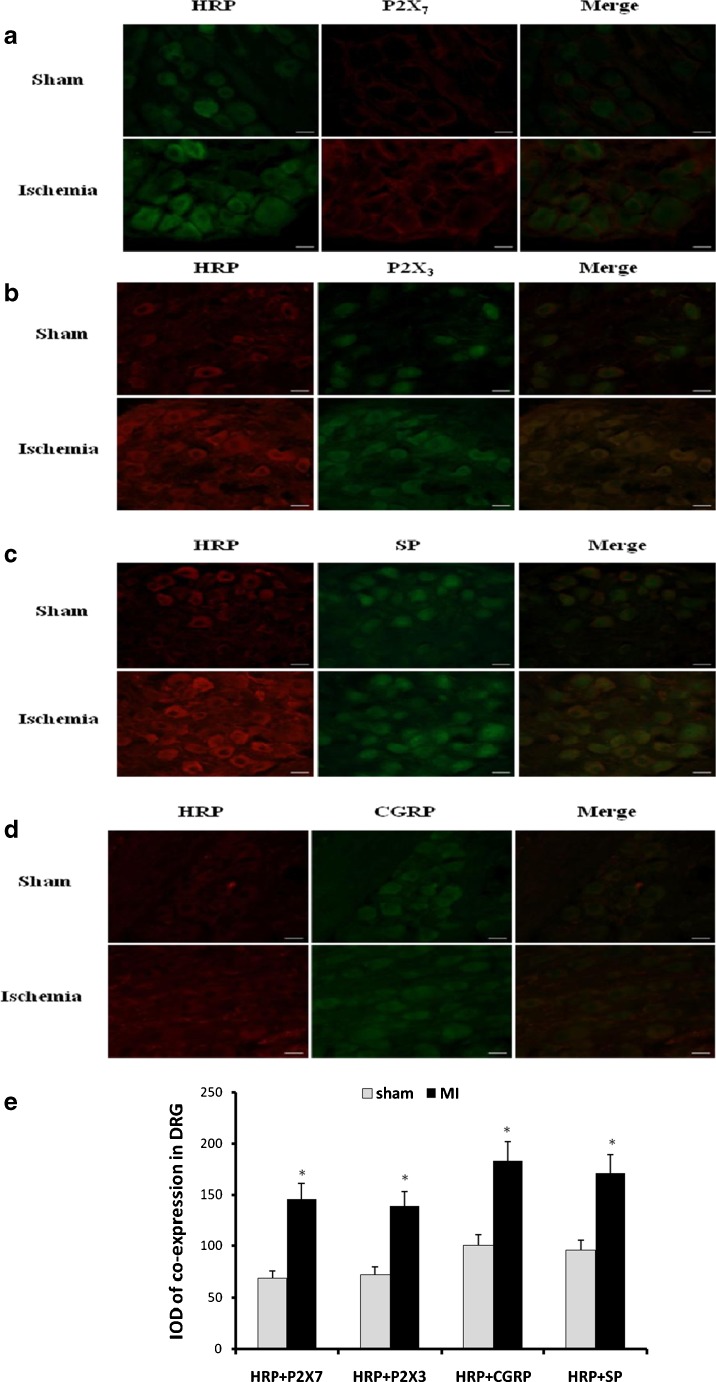
Double-label immunofluorescence of HRP and P2X7, P2X3, CGRP, or SP in DRG
HRP was used to observe the retrograde neuronal labeling from the superior cervical ganglia (SCG) to the cervical dorsal root ganglia (DRG). In MI group, HRP was injected into SCG at 13 days after MI operation. HRP was injected into SCG for 7 days in sham group.
The double-label immunofluorescence of HRP and P2X7 in the DRG was observed. The staining of HRP and P2X7 in the DRG of sham group was lower than that in MI group (n = 6, respectively; p < 0.05; Fig. 7a, e). In addition, double-label immunofluorescence of HRP and P2X3 in the DRG of sham group was also lower than that in MI group (n = 6, respectively; p < 0.05; Fig. 7b, e). Likewise, the staining of double-label immunofluorescence of HRP and CGRP (Fig. 7c, e) or HRP and SP (Fig. 7d, e) in the DRG of sham group was lower than that in MI group (n = 6, respectively; p < 0.05). These double-label immunofluorescence results indicated that there was the retrograde neuronal labeling from DRG to SCG.
Fig. 7.
The double-label immunofluorescence of P2X7, P2X3, CGRP, or SP with HRP in DRG. HRP was used as retrograde tracing marker. a There was double immunofluorescence of P2X7 and HRP from the cervical DRG to SCG, tested by the retrograde tracing neuronal labeling. Red signal represents P2X7 staining with TRITC, and green signal indicates HRP staining with FITC. Merge represents P2X7 and HRP double staining image. Scale bar, 20 μm. b Double immunofluorescence of P2X3 and HRP from the cervical DRG to SCG was detected by the retrograde tracing neuronal labeling. Red signal represents P2X3 staining with TRITC, and green signal indicates HRP staining with FITC. Merge represents P2X3 and HRP double staining image. Scale bar, 20 μm. c There was double-label immunofluorescence of CGRP and HRP coexpression from the cervical DRG to SCG, tested by the retrograde tracing neuronal labeling. Red signal represents CGRP staining with TRITC, and green signal indicates HRP staining with FITC. Merge represents CGRP and HRP double staining image. Scale bar, 20 μm. d SP and HRP coexpression from the cervical DRG to SCG was detected by the retrograde tracing neuronal labeling. Red signal represents SP staining with TRITC, and green signal indicates HRP staining with FITC. Merge represents SP and HRP double staining image. Scale bar, 20 μm. e The bar graphs showed the statistical results for co-expression of P2X7, P2X3, CGRP, or SP with HRP in DRG (n = 6 in all groups)
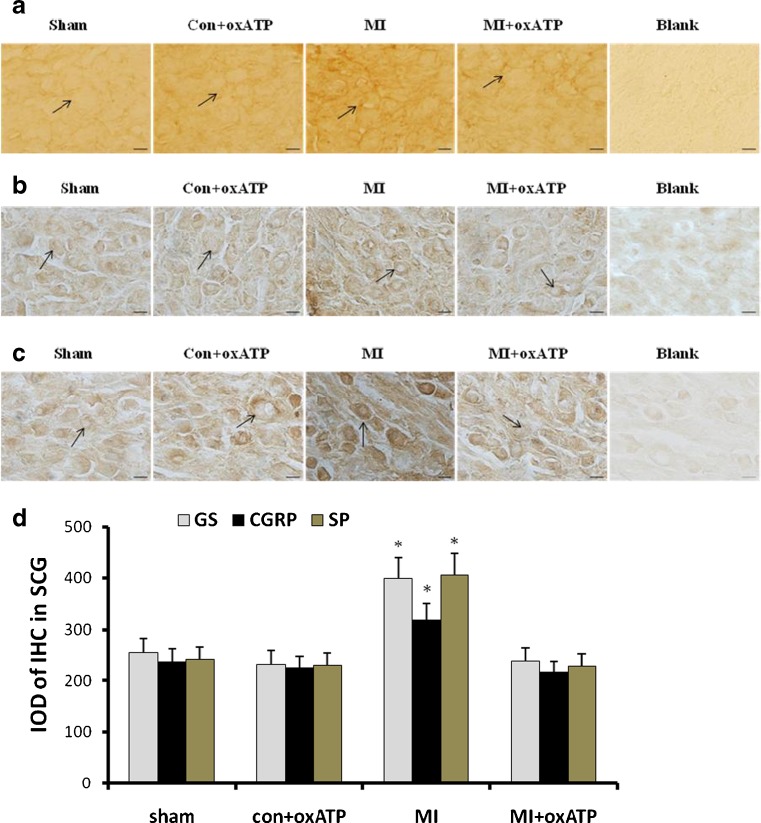
Effects of oxATP on the expression of GS, CGRP, or SP immunoreactivity in SCG
Glutamine synthetase (GS) is the marker of glial cells. Its expression in SCG was examined by immunohistochemistry. The integrated optical density of GS immunoreactivity in sham group, con+oxATP group, MI group and MI+oxATP group was observed (n = 8, respectively) (Fig 8a). The levels of GS immunoreactivity in MI group were higher than those in sham group and con+oxATP group (n = 8) (p < 0.05) (Fig. 8a, d). oxATP treatment could completely reverse the up-regulation of GS due to MI. There was no significant difference among sham group, con+oxATP group and MI+oxATP group (n = 8) (p > 0.05) (Fig. 8a, d).
Fig. 8.
Effects of oxATP on the immunoreactivity of GS, CGRP, or SP in SCG. The results show that the intensity of immunostaining for GS (a), CGRP (b), and SP (c) in SCG was higher in MI group (n = 8) than that in sham group (n = 8), con+oxATP group (n = 8), and MI+oxATP group (n = 8; p < 0.05). No difference was found for these proteins among sham group, con+oxATP group, and MI+oxATP group (p > 0.05). Arrows indicate the immunostained neurons. Scale bars, 20 μm. The bar graphs (d) show the statistical analysis of immunoreactivity for GS, CGRP, or SP
Calcitonin gene-related peptide (CGRP) and substance P (SP) are the neurochemical markers of primary sensory afferent fibers. The integrated optical density CGRP immunoreactive in sham group, oxATP control group, MI group, and MI+oxATP group were observed by immunohistochemistry (n = 8, respectively) (Fig 8b, c). MI group exhibited higher CGRP or SP immunoreactivity than sham group and con+oxATP group (n = 8; p < 0.05; Fig. 8b–d). oxATP treatment could abolish MI-induced upregulation of CGRP and SP. No significant difference was observed among sham group, con+oxATP group, and MI+oxATP group (n = 8; p > 0.05; Fig. 8b–d).
Discussion
Efferent nerves of cervical sympathetic ganglia control the function of heart and blood vessels [34]. The sympathetic ganglia do not simply relay the pre-ganglionic signals but play an integrative role [6, 30, 58, 59]. Acute myocardial ischemia activates cardiac sympathetic afferent nerves, which are often associated with an increase in blood pressure and sympathetic nerve activity [4, 26–29, 50, 51, 58, 59]. The results in our laboratory showed that the systolic blood pressure and heart rate in the MI rats were higher than those in control rats. This means that the activation of sympathetic afferent fibers after myocardial ischemia gives rise to the sympathoexcitatory reflex. After treated with the P2X7 receptor antagonist oxATP, the systolic blood pressure and heart rate were decreased, suggesting the possible involvement of activation of P2X7 receptor in sympathoexcitatory action after MI injury. P2X7 receptor is expressed in SCG [20, 41]. In some tissue P2X7 antibody gives positive reaction in P2X7 KO mice probably interacting with splice variants of the P2X7 receptors [8]. Our results showed that MI rats displayed higher levels of P2X7 immunoreactivity, mRNA, and protein in SCG. After treated with oxATP, the upregulated expression of P2X7 receptor in MI rats was significantly decreased. This further indicated that ATP and P2X7 receptor in SCG participated in the sympathoexcitatory transmission after MI injury.
Acute MI injury elevated the levels of serum CK, CK-MB, LDH, and cTn-I activities [24, 27, 28, 36, 50, 51]. Serum cTn-I seems more sensitive and more specific than CK, CK-MB, and LDH in minor myocardial injury [2, 5, 16]. In this study, P2X7 receptor antagonist oxATP significantly inhibited the elevations in serum CK, CK-MB, LDH, and cTn-I after acute MI injury. Our ECG records showed that after ligating the left anterior descending coronary artery, ST segment was highly upward, which indicated the appearance of acute MI injury. After 20 days of ligating, abnormal Q wave appeared obviously in MI rats. When MI rats were treated with oxATP, such abnormal Q wave was improved. P2X7 antagonist oxATP may inhibit the nociceptive transmission of sympathoexcitatory action in SCG after acute MI injury to produce the cardioprotective action. Inflammatory mediators could augment the nociceptive responses to ATP [7, 9, 11]. The activation of P2X7 receptor enhanced release of inflammatory mediators and cytokines [11, 12, 18]. TNF-α and the pro-inflammatory cytokine IL-6 are implicated in the pathogenesis of heart ischemic injury [17, 32]. Both IL-6 and TNF-α were significantly increased in the rats of our experiments after MI injury. P2X7 antagonist oxATP could mitigate the enhanced IL-6 and TNF-α due to MI injury. Combined with the results in ECG, it appeared that P2X7 antagonist oxATP could alleviate MI injury via modulating the inflammatory response.
Tyrosine hydroxylase (TH) is a noradrenergic marker [7, 27, 34, 58]. Our study showed that there was the coexpression of P2X7 and TH in SCG, indicating that P2X7 receptor was present in the sympathetic nerves. The coexpression values of P2X7 receptor and TH in the SCG of MI rats were higher than those in sham rats. The upregulated P2X7 receptor may participate in the signal transmission of sympathetic nerve activity after MI injury and enhance the sympathoexcitatory action. P2X7 antagonist oxATP significantly decreased the enhanced P2X7 and TH in the SCG of MI rats. Thus, oxATP could inhibit the activation of P2X7 receptor after the MI injury to decrease the transmission of sympathoexcitatory action.
The mitogen-activated protein kinase (MAPK) ERK1 and ERK2 are the critical mediators of signaling pathways linking to the activation of membrane receptors [14, 52]. Our result showed that p-ERK1/2 in SCG of MI rats was significantly higher than that in control rats. ATP leaking out from the damaged cells can facilitate P2X receptors to cause the phosphorylation of ERK [35, 37]. Therefore, the activation of P2X7 receptor in SCG after myocardial ischemia might be involved in the enhancement of intracellular ERK signaling. Treatment of MI rats with oxATP inhibited the increase of p-ERK1/2 in SCG. Thus, these results suggested that the phosphorylation of ERK 1/2 after the activation of P2X7 receptor may participate in the underlying pathological mechanism for the sympathoexcitatory action during MI injury.
SGCs in sympathetic ganglia form envelopes around individual neurons, which create a distinct functional unit consisting of a neuron and its attending SGCs [21, 46]. P2X3 and P2X7 receptors are closely associated with the transmission of nociceptive signals in DRGs [9–11]. P2X3 receptor is expressed mainly in small- and medium-sized DRG neurons [9–11]. P2X7 receptor is expressed in SGCs [9–11]. Endogenously released ATP activates ischemia-sensitive cardiac afferents through the activation of P2X3 receptor located on the cardiac sensory neurites [19, 27, 28, 30, 38, 49–51, 58, 59]. Glutamine synthetase (GS) as the glial marker is also found in the SGCs of the cervical sympathetic ganglia [21]. NeuN is a marker of neuron. The results of double-label immunofluorescence in our study showed that P2X7 receptor is expressed both neurons and SGCs in SCG. However, P2X7 receptor was mainly co-expressed with GS, whereas P2X3 receptor was mainly co-expressed with NeuN in SCG. There is evidence for ATP release from neurons in sympathetic ganglia [48]. SGCs in SCG may be a target for ATP. Purinergic signaling is a major mode of neuron-glial communication [11, 45, 53, 60]. GS expression in SCG after MI injury was increased. This indicated that there was a communication between neurons and glial cells via P2X7 receptor or P2X3 receptor in SCG. GS expression in SCG was decreased when MI rats were treated with oxATP. The communication between neurons and glial cells in the SCG may be involved in the nociceptive transmission of MI injury. P2X7 receptor antagonist oxATP could inhibit the communication. After P2X7 siRNA in myocardial ischemic rats, the expressions of P2X7 or GS assessed by immunohistochemistry and the co-expression staining of GS and P2X7 tested by double-label immunofluorescence were lower than those in MI group. The results further indicated that P2X7 receptor participated in the nociceptive response of MI injury.
HRP was used as a tracing marker to observe the retrograde neuronal labeling [31]. Evidence in our study for the afferent component from the primary sensory ganglia (cervical DRG) to SCG has been provided by combining retrograde tracing with labeling immunofluorescence. Results showed that the retrograde tracer HRP injected into SCG was found to label the cervical DRG. Double-label immunofluorescence results indicated that there was the coexpression of retrograde tracer HRP and P2X7 receptor in the cervical DRG. After myocardial ischemia, the coexpression values of HRP and P2X7 receptor in the cervical DRG were higher than those in control. This indicated that there was a communication between the afferent nerves of primary sensory ganglia and the efferent nerves of SCG. ATP can activate the cardiac sympathetic afferent nerves during myocardial ischemia [19, 27, 28, 30, 38, 49–51, 58, 59]. Signaling mediated by P2X7 receptor in SCG and the cervical DRG may be involved in the communication which can be facilitated after MI injury.
Substance P (SP) and calcitonin gene-related peptide (CGRP) are the markers of primary sensory nerves [23]. The sympathetic–sensory coupling in dorsal root ganglia occurred following peripheral nerve injury [39, 55]. Our results showed that there was the expression of CGRP or SP immunoreactivity in SCG, which might be the primary sensory nerve terminals. Retrograde tracing test showed that there was coexpression of HRP and CGRP or SP when the retrograde tracer HRP injected into SCG to label the cervical DRG. The coexpression values of HRP and CGRP or SP in the cervical DRG were increased after MI injury. The results further indicated that there was a communication between the afferent nerves of DRG and the efferent nerves of SCG. The levels of CGRP and SP immunoreactivity in SCG were enhanced after the myocardial ischemia. P2X7 receptor antagonist oxATP decreased such upregulation of CGRP and SP in SCG. DRG afferent terminals may be involved in the interaction with the sympathetic postganglionic efferent neurons. Therefore, our data suggested that there was a sensory–sympathetic coupling between the cervical DRG sensory afferent nerves and the sympathetic postganglionic efferent neurons.
Inflammatory mediators and cytokines (such as ATP, TNF-α, and IL-6) may facilitate the formation of sensory–sympathetic coupling. After ischemic injury, inflammatory mediators and cytokines upregulated the expression of SP, CGRP, and P2X3 in the sensory afferent fibers surrounded the SCG neurons. ATP activated P2X7 receptor on the surface membrane of SGCs, which resulted in activation of the SGCs. Activation of SGCs may be the sprouting factor of primary sensory nerves. After activation of SGCs, the plasma membrane in contact with the sympathetic postganglionic neurons revealed numerous ruffles, which promoted the interaction between neurons and SGCs [21]. Inflammatory factors and cytokines also sensitized sympathetic postganglionic neurons. Sensory–sympathetic coupling increased the activity of sympathetic postganglionic neurons thus affecting the efferent outputs into the cardiovascular system. DRG sensory sprouting into SCG neurons may increase the abnormal neuronal discharges and aggravate the sympathoexcitatory action. P2X7 antagonist oxATP inhibited the activation of P2X7 receptor on SGCs, which, in turn, decreased primary sensory sprouting. By interrupting the activation of SGCs, P2X7 antagonist inhibited the nociceptive transmission of sympathoexcitatory action after acute MI injury to bring about the cardioprotective action.
It was reported that angina pain symptoms in 50–60 % of patients were completely relieved after sympathectomy [33, 42]. Our previous studies showed that after the treatment with A-317491 in the MI rats, the upregulated systolic blood pressure and heart rate were decreased [27, 28, 38, 50, 51, 58, 59]. The arrhythmia after the myocardial ischemia could be improved by A-317491 [27, 28, 38, 50, 51, 58, 59]. A-317491 might inhibit the nociceptive transmission of sensory–sympathetic coupling. In the current study, P2X7 receptor antagonist oxATP decreased the up-expression of P2X3, CGRP, and SP in SCG after the myocardial ischemia, which inhibited the nociceptive transmission of sensory–sympathetic coupling. After P2X7 siRNA in myocardial ischemic rats, the co-expression staining of GS and P2X7 was decreased in comparison with that in MI group. Therefore, the activation of P2X7 receptor in SCG was associated with the increased sympathoexcitatory action via sensory–sympathetic coupling induced by MI injury.
Conclusions
In summary, there was a sensory–sympathetic coupling between the rat cervical DRG afferent nerves and the SCG neurons after the MI injury. ATP activated P2X7 receptor, which resulted in activation of the SGCs. The activation of SGCs participated in the increased sympathoexcitatory action after MI injury. P2X7 antagonist oxATP could inhibit the activation of SGCs and interrupt the nociceptive transmission of sensory–sympathetic coupling between the cervical DRG nerves and the SCG neurons after the MI injury.
Acknowledgments
This work was supported by grants (№s. 81171184, 31060139, 30860086, 30660048, and 81200853) from National Natural Science Foundation of China, grants (№s. 0640042 and 2008GZY0029) from Natural Science Foundation of Jiangxi Province, grants (№s. 2010BSA09500 and 20111BBG70009-1) from Technology Pedestal and Society Development Project of Jiangxi Province, and grant (№. GJJ13155) from Education Department of Jiangxi Province.
Abbreviations
- ATP
Adenosine triphosphate
- CGRP
Calcitonin gene-related peptide
- CK
Creatine kinase
- CK-MB
Creatine kinase isoform MB
- cTn-I
Cardiac troponin I
- DRG
Dorsal root ganglia
- ECG
Electrocardiogram
- ELISA
Enzyme-linked immunosorbent assay
- ERK1/2
Extracellular signal-regulated protein kinases
- GS
Glutamine synthetase
- HRP
Horseradish peroxidase
- IOD
Integrated optical density
- IL-6
Interleukin-6
- ISH
In situ hybridization
- LCA
Left coronary artery
- LDH
Lactate dehydrogenase
- MI
Myocardial ischemic
- NeuN
Neuronal Nuclei
- oxATP
Oxidized ATP (ATP with the 2′- and 3′-hydroxyl moieties oxidized to aldehydes by periodate treatment)
- PCR
Polymerase chain reaction
- p-ERK1/2
Phosphorylated extracellular signal-regulated protein kinases
- SP
Substance P
- TH
Tyrosine hydroxylase
- TNF-α
Tumor necrosis factor-α
- SGCs
Satellite glial cells
- SCG
Superior cervical ganglia
- siRNA
Small interference RNA
References
- 1.Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H. Purinergic signalling in the nervous system: an overview. Trends Neurosci. 2009;32:19–29. doi: 10.1016/j.tins.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Adams JE, III, Bodor GS, Davila-Roman VG, Delmez JA, Apple FS, Ladenson JH, Jaffe AS. Cardiac troponin I. A marker with high specificity for cardiac injury. Circulation. 1993;88:101–106. doi: 10.1161/01.CIR.88.1.101. [DOI] [PubMed] [Google Scholar]
- 3.Arbeloa J, Pérez-Samartín A, Gottlieb M, Matute C. P2X7 receptor blockade prevents ATP excitotoxicity in neurons and reduces brain damage after ischemia. Neurobiol Dis. 2012;45:954–961. doi: 10.1016/j.nbd.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 4.Armour JA. Myocardial ischaemia and the cardiac nervous system. Cardiovasc Res. 1999;41:41–54. doi: 10.1016/S0008-6363(98)00252-1. [DOI] [PubMed] [Google Scholar]
- 5.Bertinchant JP, Larue C, Pernel I, Ledermann B, Fabbro-Peray P, Beck L, Calzolari C, Trinquier S, Nigond J, Pau B. Release kinetics of serum cardiac troponin I in ischemic myocardial injury. Clin Biochem. 1996;29(6):587–594. doi: 10.1016/S0009-9120(96)00105-1. [DOI] [PubMed] [Google Scholar]
- 6.Boehm S, Kubista H. Fine tuning of sympathetic transmitter release via ionotropic and metabotropic presynaptic receptors. Pharmacol Rev. 2002;54:43–99. doi: 10.1124/pr.54.1.43. [DOI] [PubMed] [Google Scholar]
- 7.Bours MJ, Swennen EL, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacol Ther. 2006;112:358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Brass D, Grably MR, Bronstein-Sitton N, Gohar O, Meir A. Using antibodies against P2Y and P2X receptors in purinergic signaling research. Purinergic Signal. 2012;8(Suppl 1):S61–S79. doi: 10.1007/s11302-011-9278-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnstock G. Purinergic P2 receptors as targets for novel analgesics. Pharmacol Ther. 2006;110:433–454. doi: 10.1016/j.pharmthera.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 10.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 11.Burnstock G, Krügel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol. 2011;95:229–274. doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Colomar A, Marty V, Medina C, Combe C, Parnet P, Amedee T. Maturation and release of interleukin-1beta by lipopolysaccharide-primed mouse Schwann cells require the stimulation of P2X7 receptors. J Biol Chem. 2003;278:30732–30740. doi: 10.1074/jbc.M304534200. [DOI] [PubMed] [Google Scholar]
- 13.Cotrina ML, Nedergaard M. Physiological and pathological functions of P2X7 receptor in the spinal cord. Purinergic Signal. 2009;5:223–232. doi: 10.1007/s11302-009-9138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dai Y, Fukuoka T, Wang H, Yamanaka H, Obata K, Tokunaga A, Noguchi K. Contribution of sensitized P2X receptors in inflamed tissue to the mechanical hypersensitivity revealed by phosphorylated ERK in DRG neurons. Pain. 2004;108(3):258–266. doi: 10.1016/j.pain.2003.12.034. [DOI] [PubMed] [Google Scholar]
- 15.Erlinge D, Burnstock G. P2 receptors in cardiovascular regulation and disease. Purinergic Signal. 2008;4:1–20. doi: 10.1007/s11302-007-9078-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falahati A, Sharkey SW, Christensen D, McCoy M, Miller EA, Murakami MA, Apple FS. Implementation of serum cardiac troponin I as marker for detection of acute myocardial infarction. Am Heart J. 1999;137:332–337. doi: 10.1053/hj.1999.v137.92412. [DOI] [PubMed] [Google Scholar]
- 17.Feldman AM, Combes A, Wagner D, Kadakomi T, Kubota T, Li Y, McTiernan C. The role of tumor necrosis factor in the pathophysiology of heart failure. J Am Coll Cardiol. 2000;35:537–544. doi: 10.1016/S0735-1097(99)00600-2. [DOI] [PubMed] [Google Scholar]
- 18.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, Panther E, Virgilio FD. The P2X7 receptor: a key player in IL-1 processing and release. J mmunol. 2006;176:3877–3883. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 19.Fu LW, Longhurst JC. A new function for ATP. Am J Physiol Heart Circ Physiol. 2010;299:H1762–H1771. doi: 10.1152/ajpheart.00822.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gourine AV, Wood JD, Burnstock G. Purinergic signalling in autonomic control. Trends Neurosci. 2009;32:241–248. doi: 10.1016/j.tins.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Hanani M. Satellite glial cells in sympathetic and parasympathetic ganglia: in search of function. Brain Res Rev. 2010;64:304–327. doi: 10.1016/j.brainresrev.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Higuchi T, Stephan G, Nekolla RS, Poethko T, Yu M, Wester HJ, Casebier DS, Robinson SP, Botnar RM, Schwaiger M. A new 18F-labeled myocardial PET tracer: myocardial uptake after permanent and transient coronary occlusion in rats. Nucl Med. 2008;49:1715–1722. doi: 10.2967/jnumed.108.053967. [DOI] [PubMed] [Google Scholar]
- 23.Hukkanen M, Platts LAM, Corbett SA, Santavirta S, Polak JM, Konttinen YT. Reciprocal age-related changes in GAP-43/B-50, substance P and calcitonin gene-related peptide (CGRP) expression in rat primary sensory neurones and their terminals in the dorsal horn of the spinal cord and subintima of the knee synovium. Neurosci Res. 2002;42:251–260. doi: 10.1016/S0168-0102(02)00003-2. [DOI] [PubMed] [Google Scholar]
- 24.Ioannidis JP, Karvouni E, Katritsis DG. Mortality risk conferred by small elevations of creatine kinase-MB isoenzyme after percutaneous coronary intervention. J Am Coll Cardiol. 2003;42:1406–1411. doi: 10.1016/S0735-1097(03)01044-1. [DOI] [PubMed] [Google Scholar]
- 25.Jardine DL, Charles CJ, Ashton RK, Bennett SI, Whitehead M, Frampton CM, Nicholls MG. Increased cardiac sympathetic nerve activity following acute myocardial in a sheep model. J Physiol. 2004;565:325–333. doi: 10.1113/jphysiol.2004.082198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li DP, Pan HL. Responses of neurons in rostral ventrolateral medulla to activation of cardiac receptors in rats. Am J Physiol Heart Circ Physiol. 2000;279:H2549–H2557. doi: 10.1152/ajpheart.2000.279.5.H2549. [DOI] [PubMed] [Google Scholar]
- 27.Li GL, Liu SM, Zhang J, Xu CS, Lin JR, Li X, Liang SD. Increased sympathoexcitatory reflex induced by myocardial ischemic nociceptive signaling via P2X2/3 receptor in rat superior cervical ganglia. Neurochem Int. 2010;56(8):984–390. doi: 10.1016/j.neuint.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Li GL, Liu SM, Yang Y, Xie JY, Liu J, Kong FJ, Tu GH, Wu RP, Li GD, Liang SD. Effects of oxymatrine on sympathoexcitatory reflex induced by myocardial ischemic signaling mediated by P2X3 receptors in rat SCG and DRG. Brain Res Bull. 2011;84:419–424. doi: 10.1016/j.brainresbull.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 29.Li W, Knowlton D, Van Winkle DM, Habecker BA. Infarction alters both the distribution and noradrenergic properties of cardiac sympathetic neurons. Am J Physiol Heart Circ Physiol. 2004;286:H2229–H2236. doi: 10.1152/ajpheart.00768.2003. [DOI] [PubMed] [Google Scholar]
- 30.Liang SD, Xu CS, Li GL, Gao Y. P2X receptors and modulation of pain transmission: focus on effects of drugs and compounds used in traditional Chinese medicine. Neurochem Int. 2010;57:705–712. doi: 10.1016/j.neuint.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Lieu TM, Kollarik M, Myers AC, Undem BJ. Neurotrophin and GDNF family ligand receptor expression in vagal sensory nerve subtypes innervating the adult guinea pig respiratory tract. Am J Physiol Lung Cell Mol Physiol. 2011;300:L790–L798. doi: 10.1152/ajplung.00449.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsmori A. Roles of cytokines in the pathogenesis of heart failure. Nippon Rinsho. 2003;61:745–750. [PubMed] [Google Scholar]
- 33.Meller ST, Gebhart GF. A critical review of the afferent pathways and the potential chemical mediators involved in cardiac pain. Neuroscience. 1992;48:501–24. doi: 10.1016/0306-4522(92)90398-L. [DOI] [PubMed] [Google Scholar]
- 34.Pather N, Partab P, Singh B, Satyapal KS. The sympathetic contribution to the cardiac plexus. Surg Radiol Anat. 2003;25(3–4):210–215. doi: 10.1007/s00276-003-0113-2. [DOI] [PubMed] [Google Scholar]
- 35.Ponnusamy M, Liu N, Gong R, Yan H, Zhuang S. ERK pathway mediates P2X7 expression and cell death in renal interstitial fibroblasts exposed to necrotic renal epithelial cells. Am J Physiol Renal Physiol. 2011;301:F650–F659. doi: 10.1152/ajprenal.00215.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ricciardi MJ, Davidson CJ, Gubernikoff G, Beohar N, Eckman LJ, Parker MA, Bonow RO. Troponin I elevation and cardiac events after percutaneous coronary intervention. Am Heart J. 2003;145:2–8. doi: 10.1067/mhj.2003.2. [DOI] [PubMed] [Google Scholar]
- 37.Seino D, Tokunaga A, Tachibana T, Yoshiya S, Dai Y, Obata K, Yamanaka H, Kobayashi K, Noguchi K. The role of ERK signaling and the P2X receptor on mechanical pain evoked by movement of inflamed knee joint. Pain. 2006;123:93–203. doi: 10.1016/j.pain.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 38.Shao LJ, Liang SD, Li GL, Xu CS, Zhang CP. Exploration of P2X3 in the rat stellate ganglia after myocardial ischemia. Acta Histochem. 2007;109:330–337. doi: 10.1016/j.acthis.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Shinder V, Govrin-Lippmann R, Cohen S, Belenky M, Ilin P, Fried K, Wilkinson HA, Devor M. Structural basis of sympathetic–sensory coupling in rat and human dorsal root ganglia following peripheral nerve injury. J Neurocytol. 1999;28:743–761. doi: 10.1023/A:1007090105840. [DOI] [PubMed] [Google Scholar]
- 40.Skaper SD, Debetto P, Giusti P. The P2X7 purinergic receptor: from physiology to neurological disorders. FASEB J. 2010;24:337–345. doi: 10.1096/fj.09-138883. [DOI] [PubMed] [Google Scholar]
- 41.Sperlagh B, Vizi ES, Wirkner K, Illes P. P2X7 receptors in the nervous system. Prog Neurobiol. 2006;78:327–346. doi: 10.1016/j.pneurobio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 42.Stritesky M, Dobias M, Demes R, Semrad M, Poliachova E, Cermak T, Charvat J, Malek I. Endoscopic thoracic sympathectomy—its effect in the treatment of refractory angina pectoris. Interact Cardiovasc Thorac Surg. 2006;5:464–468. doi: 10.1510/icvts.2005.118976. [DOI] [PubMed] [Google Scholar]
- 43.Swissa M, Zhou SM, Gonzalez-Gomez I, Chang CM, Lai AC, Cates AW, Fishbein MC, Karagueuzian HS, Chen PS, Chen LS. Long-term subthreshold electrical stimulation of the left stellate ganglion and a canine model of sudden cardiac death. J Am Coll Cardiol. 2004;43:858–864. doi: 10.1016/j.jacc.2003.07.053. [DOI] [PubMed] [Google Scholar]
- 44.Thames MD, Kinugawa T, Dibner-Dunlap ME. Reflex sympathoexcitation by cardiac afferents during myocardial ischemia: role of adenosine. Circulation. 1993;87:1698–704. doi: 10.1161/01.CIR.87.5.1698. [DOI] [PubMed] [Google Scholar]
- 45.Verderio C, Matteoli M. ATP in neuron–glia bidirectional signaling. Brain Res Rev. 2011;66:106–114. doi: 10.1016/j.brainresrev.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Verkhratsky A, Parpura V, Rodríguez JJ. Where the thoughts dwell: the physiology of neuronal–glial “diffuse neural net”. Brain Res Rev. 2011;66:133–151. doi: 10.1016/j.brainresrev.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 47.Vassort G. Adenosine 5′-triphosphate: a P2-purinergic agonist in the myocardium. Physiol Rev. 2001;81:767–806. doi: 10.1152/physrev.2001.81.2.767. [DOI] [PubMed] [Google Scholar]
- 48.Vizi ES, Liang SD, Sperlágh B, Kittel A, Jurányi Z. Studies on the release and extracellular metabolism of endogenous ATP in rat superior cervical ganglion: support for neurotransmitter role of ATP. Neuroscience. 1997;79:893–903. doi: 10.1016/S0306-4522(96)00658-6. [DOI] [PubMed] [Google Scholar]
- 49.Wan F, Li GL, Liu SM, Zhu GC, Xu SH, Lin JR, Zhang J, Li X, Liang SD. P2X2/3 receptor activity of rat nodose ganglion neurons contributing to myocardial ischemic nociceptive signaling. Auton Neurosci: Basic Clin. 2010;158:58–64. doi: 10.1016/j.autneu.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 50.Wang YX, Li GL, Liang SD, Wan F, Xu CS, Gao Y, Liu SM, Lin JR. Expression of P2X2 and P2X3 receptors in rat nodose neurons after myocardial ischemia injury. Auton Neurosci. 2009;145:71–75. doi: 10.1016/j.autneu.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Wang YX, Li GL, Liang SD, Zhang AX, Xu CS, Gao Y, Zhang CP, Wan F. Role of P2X3 receptor in myocardial ischemia injury and nociceptive sensory transmission. Auton Neurosci. 2008;139:30–37. doi: 10.1016/j.autneu.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 52.Watters JJ, Sommer JA, Pfeiffer ZA, Prabhu U, Guerra AN, Bertics PJ. A differential role for the mitogen-activated protein kinases in lipopolysaccharide signaling: the MEK/ERK pathway is not essential for nitric oxide and interleukin 1beta production. J Biol Chem. 2002;277:9077–9087. doi: 10.1074/jbc.M104385200. [DOI] [PubMed] [Google Scholar]
- 53.Weick M, Cherkas PS, Härtig W, Pannicke T, Uckermann O, Bringmann A, Tal M, Reichenbach A, Hanani M. P2 receptors in satellite glial cells in trigeminal ganglia of mice. Neuroscience. 2003;120:969–977. doi: 10.1016/S0306-4522(03)00388-9. [DOI] [PubMed] [Google Scholar]
- 54.Xiang Z, Bo X, Burnstock G. Localization of ATP-gated P2X receptor immunoreactivity in rat sensory and sympathetic ganglia. Neurosci Lett. 1998;256:105–108. doi: 10.1016/S0304-3940(98)00774-5. [DOI] [PubMed] [Google Scholar]
- 55.Xie W, Strong JA, Zhang JM. Increased excitability and spontaneous activity of rat sensory neurons following in vitro stimulation of sympathetic fiber sprouts in the isolated dorsal root ganglion. Pain. 2010;151:447–459. doi: 10.1016/j.pain.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan Z, Li S, Liang Z, Tomic M, Stojilkovic SS. The P2X7 receptor channel pore dilates under physiological ion conditions. J Gen Physiol. 2012;132:563–573. doi: 10.1085/jgp.200810059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yao H, Zhang Y, He F, Wang C, Xiao Z, Zou J, Wang F, Liu Z. Short hairpin RNA targeting 2B gene of coxsackievirus B3 exhibits potential antiviral effects both in vitro and in vivo. BMC Infect Dis. 2012;12:177. doi: 10.1186/1471-2334-12-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang CP, Liang SD, Li GL, Xu CS, Gao Y, Wang YX, Zhang AX, Wan F. The involvement of P2X3 receptors of rat sympathetic ganglia in cardiac nociceptive transmission. J Physiol Biochem. 2007;63(3):249–258. doi: 10.1007/BF03165788. [DOI] [PubMed] [Google Scholar]
- 59.Zhang CP, Li GL, Liang SD, Xu CS, Zhu GC, Wang YX, Zhang AX, Wan F. Myocardial ischemic nociceptive signaling mediated by P2X3 receptor in rat stellate ganglion neurons. Brain Res Bull. 2008;75:77–82. doi: 10.1016/j.brainresbull.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 60.Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci USA. 2007;104:9864–9869. doi: 10.1073/pnas.0611048104. [DOI] [PMC free article] [PubMed] [Google Scholar]