Abstract
Extracellular ATP and related nucleotides promote a wide range of pathophysiological responses via activation of cell surface purinergic P2 receptors. Almost every cell type expresses P2 receptors and/or exhibit regulated release of ATP. In this review, we focus on the purinergic receptor distribution in inflammatory cells and their implication in diverse immune responses by providing an overview of the current knowledge in the literature related to purinergic signaling in neutrophils, macrophages, dendritic cells, lymphocytes, eosinophils, and mast cells. The pathophysiological role of purinergic signaling in these cells include among others calcium mobilization, actin polymerization, chemotaxis, release of mediators, cell maturation, cytotoxicity, and cell death. We finally discuss the therapeutic potential of P2 receptor subtype selective drugs in inflammatory conditions.
Keywords: ATP, Extracellular nucleotides, P2 receptors, Inflammatory cells, Regulation of immune responses
Introduction
Inflammation encompasses a complex homeostatic mechanism that enables the body to detect and fight foreign nonself antigens and restore tissue integrity. While mediators of inflammation originating from outside are often responsible for initiating the immune response, some constitutive intracellular molecules from the host—that are implicated in housekeeping functions during normal physiology—can be released into the extracellular space and regulate the function of immune cells, and consequently equally participate and/or modulate inflammatory responses under pathological conditions [1, 2]. Evidence has accumulated showing that ATP and related purine and pyrimidine nucleotides fulfill these requirements [3–5] further evidenced by recent studies using bioluminescence imaging techniques that visualized ATP release in vivo in response to contact allergen [6], irradiation, allograft rejection [7], and intraperitoneal LPS administration [8].
Indeed, while ATP is found inside almost every living cell—predominantly known for its central role in the cellular energy metabolism-, it is also widely distributed outside the cell where it appears to influence a diverse scale of biological processes such as the generation of chemotactic signals and/or the activation of different immune cells, causing inflammatory cells to migrate, proliferate, differentiate, or release diverse inflammatory mediators [9, 10]. Under inflammatory conditions, ATP is often regarded to be released passively following cellular stress or cell death. However, nucleotides can also be released into the extracellular space via nonlytic mechanisms through regulated transport as a neurotransmitter from neurons or from immune cells during inflammation and even under normal physiological conditions; this process is regulated via an autocrine feedback mechanism [11, 12].
Extracellular ATP and the related purine and pyrimidine nucleotides exert their functions via signaling through membrane-bound purinergic P2 receptors. These receptors are widely expressed throughout the body on various immune and nonimmune cells. The P2 receptors are subdivided into two families: the G protein-coupled P2Y receptors and the ligand-gated P2X ion channels. The International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification recognizes eight distinct P2Y receptor subtypes: P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14 [13] and seven P2X subunits that may form six homomeric (P2X1–P2X5 and P2X7), and at least six heteromeric P2X1/2, P2X1/4, P2X1/5, P2X2/3, P2X2/6, and P2X4/6, receptors [14].
Either constitutively present or induced under pathological conditions, P2 receptors are differentially expressed on a large scale of cells including immune cells (Table 1) [13, 15–17]. Although P2 receptors are clearly implicated in diverse inflammatory reactions [18], the mechanisms involved are complex and not fully elucidated. Their expression profiles and biological activities are diverse as evidenced by results in the literature regarding eosinophils [10, 19], neutrophils [20, 21], mast cells [22, 23], monocytes/macrophages [24], lymphocytes [25, 26], and others [4]. Identifying the precise receptors and pathways involved is a first step in the challenge which could lead to the discovery of a new therapeutic class of drugs that suppress inflammation.
Table 1.
Expression profiles and functional responses of the purinergic receptor subtypes in different immune cells
| Inflammatory cell type | Functional response to purines | P2 receptor subtype (expression profile and/or involvement in functional response) | References |
|---|---|---|---|
| Neutrophils | Undefined roles | P2Y1, P2Y4, P2Y11, P2Y14, and P2X7a | [34, 36, 38–40] |
| Calcium mobilization | P2Y2 | [40, 50, 51] | |
| Actin polymerization | P2Y2 | [52] | |
| Primary granule release | P2Y2 | [40] | |
| Chemotaxis | P2Y2, P2Y6, and P2X1 | [43, 55, 57, 58] | |
| Reduced cAMP accumulation | P2Y14 | [41] | |
| Delay in constitutive neutrophil apoptosis | P2Y11 | [35] | |
| Macrophages | Undefined roles | P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, P2Y14, and P2X1–P2X6 | [75–78] |
| Intracellular calcium increase | P2Y1, P2Y2, P2Y4, P2Y11, P2X4, and P2X7 | [73–78] | |
| IL-1β/IL-18 maturation/release via the NLRP3 inflammasome, caspase-1 and cytosolic K+ depletion | P2X7 | [24, 78, 80, 82–88, 90–96, 108] | |
| Release of cathepins, PGE2, MMP-9 phosphatidilserine (caspase independent) | P2X7 | [8, 111–114] | |
| Promoting chemotaxis/phagocytosisb | P2Y2, P2Y12, P2X1, and P2X3 | [115, 117–119] | |
| Regulation of autophagy | P2X4 and P2X7 | [98, 100, 108, 109] | |
| Multinucleated giant cells formation | P2X7 | [73, 83, 120, 121] | |
| Dendritic cells | Undefined roles | P2X1, P2X4, P2X5, P2X7, P2Y1, P2Y4, P2Y6, and P2Y11 | [123–125, 127] |
| Regulation in cytokine release | P2Y11 | [128–131] | |
| DC maturation | P2Y11, P2Y12, and P2Y14 | [123, 126, 128, 129, 131, 134] | |
| Apoptosis | P2X7 | [136, 137] | |
| DC migration | P2Y2 and P2Y11 | [125, 141] | |
| Lymphocytes | |||
| B and T cells | Undefined roles | P2X2, P2X3, P2X5, P2X6, and all P2Y | [21, 150–153] |
| T cell activation (p38 MAPK activation and IL-2 gene transcription ) | P2X1, P2X4, and P2X7 | [166, 168] | |
| T cell activation (CD62L shedding) | P2X7 | [25, 178, 179] | |
| cAMP accumulation | P2Y14 | [154] | |
| Inhibition of immunosuppressive potential of Tregs | P2X7 | [169] | |
| Natural killer cells | Regulation of NK cytoxicity and chemotaxis | P2Y11 | [183] |
| Eosinophils | Undefined roles | P2Y1, P2Y4, P2Y6, P2Y11, P2Y14, P2X1, P2X4, and P2X7 | [10, 19, 191, 192] |
| Chemotaxis | P2Y2 | [141, 193] | |
| Release of chemokines and cytokines | P2Y2, P2X1, P2X7, and P2Y6 | [196, 197] | |
| Mast cells | Undefined roles | P2X1, P2X4, P2X6, P2X7, P2Y1, P2Y2, P2Y11, P2Y12, and P2Y13 | [23, 200, 213, 214] |
| Degranulation | P2Y13 and P2Y14 | [215, 216, 221] | |
aAmbiguous data reporting the presence [34, 38] or absence [36] of P2X7 on neutrophils
bAmbiguous data reporting the ATP to be a long-range chemoattractant without phagocytic activity [115] vs. ATP as an autocrine/paracrine signal limited to influence chemotaxis but accompanied with phagocytosis by locally inducing lamellipodial membrane extensions [117, 118]
This review summarizes the history and the current knowledge regarding the role of purinergic signaling in different inflammatory cells and focuses on the purinergic receptor distribution in immune cells and their implication in diverse immune responses.
Neutrophils
Neutrophils (Fig. 1) are essential effector cells of the innate immune system, forming the first line of defense responsible for the initial clearance of invading microorganisms. They are highly motile phagocytic cells that rapidly migrate from the blood circulation [27]. Their recruitment and activation into peripheral tissues is crucial for an efficient host defense but needs to be tightly regulated in order to avoid host damage. The latter is under control of a diverse set of neutrophil chemoattractants such as chemokines, eicosanoids, cytokines and other peptides [28], but also extracellular ATP and its derivates can play a role herein. In this chapter, the current knowledge regarding the effects of extracellular nucleotides in neutrophil function and its influence on the immune response will be presented.
Fig. 1.
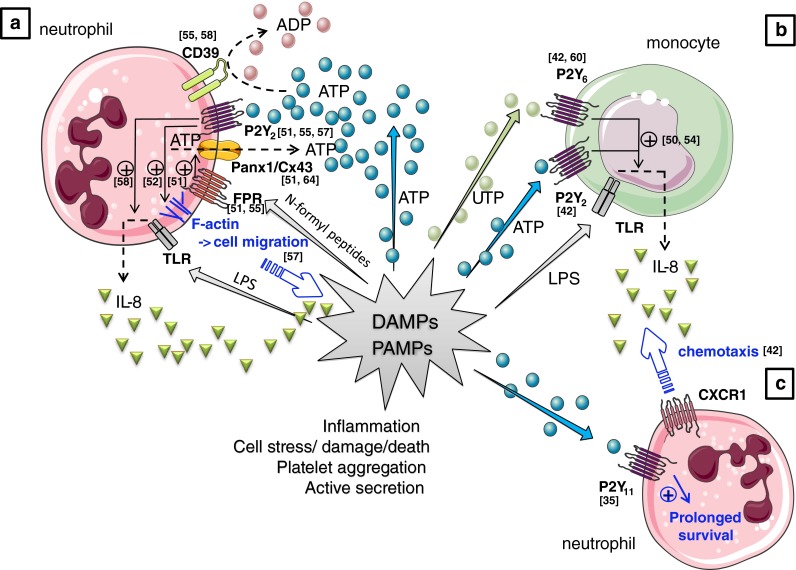
Purinergic mechanisms in neutrophil biology. a Activation of the formyl-peptide receptor (FPR) by N-formyl peptides produced by the degradation of either bacterial or host cells (which constitutes together with many other molecules, including ATP, the group of PAMPs and DAMPs) stimulates localized ATP release from neutrophils [51, 55] which occurs through pannexin-1 (panx1) and/or connexin 43 (Cx43) hemichannels [51, 64] resulting in the activation of nearby P2Y2 receptors on the neutrophils [51, 55, 57] This autocrine P2Y2 receptor activation will subsequently amplify gradient sensing of chemotactic signals (e.g., N-formyl peptides [51, 55] and interleukin (IL)-8 [57] by stimulating F-actin to the leading edge [51, 52, 55]. P2Y2 receptor ligation is also implicated in the potentiation of IL-8 production by human neutrophils in response to the bacterial endotoxin LPS [58], a mechanism under tight control by the ectonucleotidase “Ecto-nucleoside triphosphate diphosphohydrolase 1” (E-NTPDase1 or CD39) expressed on the surface of neutrophils [55, 58] by breaking down ATP to ADP. b Next to neutrophilic P2Y2 receptors, extracellular nucleotides (UTP and ATP) were also reported to regulate neutrophil migration by controlling TLR-induced IL-8 release from monocytes via P2Y2 and/or P2Y6 receptors expressed on the latter cell types [42, 60]. c ATP, via P2Y11 might also cause a long-lasting delay in constitutive neutrophil apoptosis [35]. DAMP damage-associated molecular patterns, LPS lipopolysaccharide, PAMP pathogen-associated molecular patterns, TLR toll-like receptor. Values between brackets in the figure represent reference numbers
Ward et al. were the first to describe the role of purines in neutrophil functionality, showing the release of ATP/ADP from platelets to induce an enhanced superoxide anion production in stimulated rat and human neutrophils [29–31]. The involvement of a purinergic pathway in the platelet–neutrophil interaction was corroborated by others who reported its implication in the respiratory burst in human neutrophils isolated from peripheral blood of healthy donors [32, 33]. Suh et al. characterized the P2X7 receptor subtype to be involved in the purinergic mechanism of enhanced respiratory burst, consequently suggesting its presence on human neutrophils [34]. In contrast, others refuted the presence of P2X7 receptors in human neutrophils by means of a large scale of techniques [35, 36]. While on mRNA level, human neutrophils were previously reported to express P2Y2, P2Y4, P2Y6, P2Y11, P2Y14 (at that time called GPR105) [37], and P2X7 [38, 39], only the P2Y2 and P2Y14 but not P2Y1, P2Y4 P2Y6, and P2Y11 receptors were initially reported to be functionally active [40, 41]. However, later on also the P2Y6 and P2Y11 were assigned to a specific task in neutrophil functionality [35, 42] (see below). Besides the ambiguous data on P2X7 and a single paper claiming the involvement of P2X1 in neutrophil chemotaxis [43], more information on the expression of other P2X receptor subtypes in neutrophils is lacking. Hence further investigation regarding the functional expression of the different P2X receptor subunits in this cell type is required.
Functionally, Ward et al. [44] observed an increase in intracellular calcium levels in rat neutrophils during stimulation by ATP, which was furthermore corroborated by others [29, 45–48]. The purinergic pathway that induces the elevation of calcium levels in neutrophils involves the activation of phospholipase C (PLC) which generates inositol 1,4,5-triphosphate (IP3) that subsequently induces calcium mobilization [45, 46, 49, 50]. The P2Y2 receptor was identified to be the receptor subtype implicated in these responses [40, 50, 51]. Although not mediated via an increased Fcγ receptor-triggered Ca2+ response but via tyrosine phosphorylation, ATP released from platelets and exogenously applied ATP showed to potentiate actin polymerization with associated increased IgG-mediated phagocytosis and to enhance respiratory burst in neutrophils [33]. P2U—now being characterized to functionally correspond to the P2Y2 receptor based on the agonist profile reported in the referred study—was again suggested as the P2 receptor responsible for this actin polymerization process in neutrophils [52]. As actin polymerization is also involved in cell motility such as chemotaxis and phagocytosis, exogenous ATP, and adenosine have indeed been shown to induce chemotaxis of neutrophils [52–54]. Chen et al. reported a specific role for purinergic P2Y2 receptor and the A3 adenosine receptor subtype respectively in gradient sensing and controlling migration speed of neutrophils in an autocrine response to ATP release during stimulation of human neutrophils with the chemoattractant N-formyl-Met-Leu-Phe (FMLP) [55]. The same researchers translated their findings into an in vivo model showing A3 and P2Y2 receptors to control the recruitment of neutrophils to the lung in a mouse model of sepsis [35, 56]. The involvement of the neutrophilic P2Y2 receptor in neutrophil migration was corroborated by several other research papers by the group of Sévigny who described that P2Y2 receptor activation on neutrophils is required for IL-8 (released from monocytes/macrophages and/or epithelial cells at sites of inflammation) to induce most efficient neutrophil chemotaxis [57], and that P2Y2 receptors on neutrophils are implicated in the potentiation of IL-8 production by human neutrophils themselves, a mechanism under tight control by the ectonucleotidase “Ecto-nucleoside triphosphate diphosphohydrolase 1” (E-NTPDase1 or CD39) expressed on the surface of neutrophils [58]. E-NTPDase1 also regulates the migration speed of neutrophils but not their ability to detect the orientation of the gradient field generated by fMLP that induces an autocrine ATP release via stimulation of the formyl peptide receptor [59]. Besides neutrophilic P2Y2 receptors, extracellular nucleotides were also reported to regulate neutrophil migration by controlling TLR-induced IL-8 release from monocytes via P2Y2 and/or P2Y6 receptors expressed on the latter cell types [42, 60] or potentiate LPS-induced transendothelial migration of neutrophils via P2Y2 receptors on endothelial cells via an IL-8 independent way [61]. Similarly, macrophages stimulated with extracellular ATP produce macrophage inflammatory protein-2 (MIP-2) which is an important factor for neutrophil migration [62]. Vaughan et al. reported that exposure of neutrophils to physiologically relevant concentrations of ATP causes a long-lasting delay in constitutive neutrophil apoptosis mediated via P2Y11 receptors [35].
While purinergic activation of neutrophils thus seems to be clearly a regulatory component implicated in the immune response mediated by neutrophils [63], the activation of neutrophils can conversely also lead to the release of ATP, which was reported to occur via connexin (Cx43) and pannexin-1 (panx-1) hemichannels [51, 64]. Stimulation of the formyl peptide receptor, which colocalizes with P2Y2 nucleotide receptors on the cell surface, forms a purinergic signaling system that facilitates neutrophil activation and leads to ATP release through panx1 hemichannels [51]. While UDP-glucose, an agonist at P2Y14 receptors, is also reported to be functionally active in neutrophils—as evidenced by inducing an inhibition of forskolin-stimulated cAMP accumulation and an increase in ERK1/2 phosphorylation-, it does not enhance neutrophil degranulation triggered by fMLP [41].
In conclusion, with the exception of a single study suggesting P2X1 to be implicated in chemotaxis of neutrophils [43], a fast majority of publications describes the P2Y2 receptor to be the main P2 receptor subtype implicated the functional regulation of neutrophils regarding calcium mobilization, actin polymerization, cell motility, and an autocrine feedback mechanism releasing ATP through hemichannels. Neutrophil apoptosis on the other hand rather seems to be mediated via P2Y11 receptors [35] (Table 1).
Macrophages
Macrophages (Fig. 2) are phagocytes [65] that are strategically located throughout the body tissues where they ingest and process foreign material and dead cells. Macrophages are implicated in antigen presentation and immunomodulation by production of various cytokines, playing an important role in the initiation, maintenance, and resolution of inflammation. Macrophages are known to be able to rapidly change their function in response to local microenvironmental signals [66]; such a signal derived from dying, necrotic, and apoptotic cells is ATP.
Fig. 2.
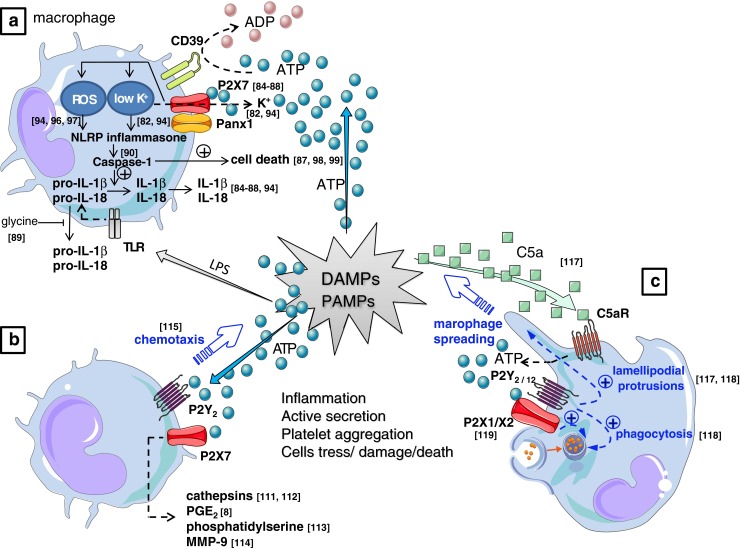
a Challenging macrophages with PAMPs such as LPS stimulates synthesis of pro-IL-1β and pro-IL-18. Secretion of the active cytokines requires a secondary signal that is provided by ATP through P2X7 ligation [84–88], yielding the conversion of pro-IL-1β into active IL-1β by NLRP3 inflammasome activated caspase-1 enzymatic cleavage [90]. The activation process of caspase-1 is initiated via P2X7-mediated cytosolic K+ outflow [82, 94], or alternatively via the activation of ROS [94, 96, 97]. Also the pro-IL-1β and pro-IL-18 can be released via a distinct caspase-1 independent pathway which is blocked by glycine [89]. Prolonged stimulation with ATP also leads to caspase-1 dependent, but IL-1β and IL-18 cytokine processing independent cell death in macrophages [87, 98, 99]. b P2X7 receptor activation has also been shown to signal caspase-1 and IL-1β/IL-18 independent release of cathepsins [111, 112], PGE2 [8], phosphatidylserine [113], and MMP-9 [114], all of which are implicated in cellular processes that play a defined role in inflammation. ATP is also reported to act as a long-range “find me” signal (chemoattractant) to recruit monocytes and macrophages [115]. c The role of ATP as “real” direct chemoattractant for macrophages is however controversial; the purinergic system can also act as an indirect chemoattractant that navigates macrophages in a gradient of the “real” chemoattractant C5a via autocrine release of ATP induced by the C5a receptor (C5aR), yielding an amplification in the gradient sensing via an orientated generation of lamellipodial membrane protrusions involving P2Y2 and P2Y12 receptors to furthermore induce an indirect effect of chemotaxis and the promotion of phagocytosis [117, 118]. Enhanced phagocytic effect in macrophages by purinergic stimuli can also involve the ligation of P2X1 or P2X3 receptors [119]. DAMP damage-associated molecular patterns, LPS lipopolysaccharide, MMP-9 matrix metalloproteinase-9, PAMP pathogen-associated molecular patterns, TLR toll-like receptor, PGE2 prostaglandin E2. Values between brackets in the figurerepresent reference numbers
The influence of exogenous nucleotides on macrophages was identified by Cohn and Parks which paved the way for further research into the involvement of extracellular purine and pyrimidine nucleotides during inflammation [67]. The authors showed adenine nucleotides to be implicated in the pinocytic vesicle formation in mouse macrophage cultures. Later Silverstein and colleagues revealed ATP to induce a mechanism which facilitates calcium fluxes in mouse macrophages [68–71]. Since calcium is mobilized during stimulation with ATP, assessment of calcium levels is used in the functional characterization of the receptors involved in purinergic responses. The P2Z receptor—later on being characterized as the P2X7 receptor [72]—was the first P2 receptor subtype identified to augment intracellular calcium levels in murine and human macrophages [73, 74].
The expression and functional characterization of other P2 receptor subtypes in macrophages was assessed in different cell lines: all murine and rat macrophage cell lines were shown to express multiple P2Y and P2X subtypes. However, only P2Y1, P2Y2, P2Y4, P2X4, and P2X7 receptors were demonstrated to be functionally implicated in evoking ATP-mediated increases of cytosolic calcium levels [75–77]. In humans, alveolar macrophages were reported to express all P2 receptor subtypes except P2Y12, P2X2, P2X3, and P2X6 [78], whereas only, P2Y1, P2Y2, P2Y11, and P2X7 exhibit functional responses in inducing an intracellular calcium elevation.
Besides phagocytosis and antigen presentation, macrophages are implicated in inflammatory reactions via the secretion of a diverse set of mediators [79]. A role for extracellular ATP in modulating the mediators’ expression and/or release was first reported by Tonneti et al. who observed increased production of nitric oxide, IL1-β, and TNF-α by ATP in lipopolysaccharide-treated mouse macrophages [80, 81]. IL1-β maturation and release in response to ATP in murine macrophages was confirmed by Perregaux and Gabel who reported a concomitant decrease in intracellular K+ as a requisite [82]. In contrast to murine macrophages, in human macrophages the expression of TNF-α was reported to be downregulated, whereas IL-1β secretion was also here upregulated by extracellular nucleotides [78]. In rat alveolar macrophages, IL-1α, IL-1β, and IL-6 cytokine release was also reported to be facilitated by P2X7 receptor activation, while TNF-α release was not affected [83]. The P2Z/P2X7 was indeed earlier identified to be involved in the increase in IL1-β production in human macrophages [24, 84–87]. Later on, secretion of IL-1β and IL-18 in macrophages was found to be mediated by caspase-1, in association with the involvement of the NLRP3 inflammasome [88]. Priming of macrophages with PAMPs such as LPS is sufficient to stimulate synthesis of pro-IL-1β, but secretion of the active IL-1β cytokine requires a secondary signal that is provided by ATP through P2X7 ligation, yielding the cleavage of pro-IL-1β into active IL-1β by the cysteine proteases caspase-1. In macrophages, both pro-IL-1β and processed IL-1β are reported to be released via two distinct pathways respectively; the former pathway being caspase-1 and panx-1 independent but blocked by glycine [89] while the latter does depend on these structures, with both pathways being independent of cell death processes [88]. Caspase-1 itself is expressed as an inactive pro-caspase-1 that needs cleavage and activation in association with protein complexes called inflammasomes; the specific inflammasome that is activated by P2X7 activation contains the adaptor molecule NLRP3 [90] The NLRP3 inflammasome can be activated via P2X7-ligation by DAMPs such as ATP [91], but also biglycan [92] and serum amyloid A [93] are reported to elicit signaling through P2X7. P2X7-ligation can lead to pore formation and cytosolic K+ outflow which results in activation of caspase-1 and the subsequent secretion of active IL-1β and IL-18 in macrophages that had been primed with LPS [94] confirming the early observations by Perregaux and Gabel as mentioned above [82]. However, unlike caspase-1 activated by TLR agonists (extracellular pathogenic products) and ATP, the activation of the macrophage inflammasome by intracellular bacteria is reported to proceed normally in the absence of P2X7 receptor-mediated cytoplasmic K+ perturbations [95] ATP also shows to process and secrete IL-1β and IL-18 via the activation of reactive oxygen species (ROS) in rat alveolar macrophages through P2X7 [96]. ATP-mediated ROS production can activate ERK1/2 and subsequently caspase-1, which is required for IL-1β and IL-18 processing [94, 96, 97]
While prolonged stimulation with ATP also leads to caspase-1-dependent cell death in macrophages [98], this process seems to be independent from the concomitant occurring IL-1β and IL-18 cytokine processing and release [87, 99]. Biswas et al. reported that ATP-treated human monocytes/macrophages undergo rapid cell autophagy which involves the activation of P2X7 receptors and is associated with augmented mycobactericidal activity of macrophages[100]. Some intracellular pathogens modulate the function of the macrophage phagosome in order to escape the host defense responses [101]. However the host macrophages have developed a purinergic-mediated mechanism to counteract the escape efforts of these pathogens such as Mycobacterium [102], Vibrio cholerae [103], Pseudomonas aeruginosa [104], and Chlamydia trachomatis [105]; P2X7 receptor ligation can stimulate the inflammasome associated with secretion of cytokines but it can also lead to the direct killing of intracellular pathogens in infected macrophages, involving macrophage cell death and potentially related to macrophage autophagy [106, 107]. The co-expression of P2X4 receptors with P2X7 receptors was subsequently found to suppress P2X7-mediated autophagy and to facilitate the release of pro-inflammatory mediators in mouse macrophage RAW264.7 cells, consequently enhancing inflammation [108]. This association of P2X4 with P2X7 was also described in relation to macrophage cell death [109] but for which the underlying molecular mechanism is not yet unveiled. The effects by P2X7 receptor activation can also be tempered by E-NTPDase1 which degrades ATP at the cell surface of marcophages, potentially contributing to the fact that P2X7 is activated by higher concentrations of ATP compared with other P2 receptors [110].
Besides the caspase-1 dependent processes described above, P2X7 receptor activation has also been shown to signal caspase-1 and IL-1β/IL-18 independent release of cathepsins [111, 112], prostaglandin (PG)E2 [8], phosphatidylserine [113], and matrix metalloproteinase 9 [114], all of which are implicated in cellular processes that play a defined role in inflammation.
Extracellular purines and pyrimidines might also be implicated in controlling the movement of macrophages; Elliott et al. reported that ATP released from apoptotic cells acts as a long-range “find me” signal (chemoattractant) to recruit motile monocytes and macrophages. The authors showed that the increased recruitment of monocytes/macrophages to apoptotic cell supernatants in a transwell migration assay and in an in vivo murine subcutaneous air-pouche model was reduced by apyrase and under P2Y2−/− conditions [115]. The identification of the P2Y2 receptor in purinergic-mediated chemotaxis of macrophages is however not in agreement with the initial observation by McCloskey et al. [116] who observed that ADP was a chemoattractant for the murine J774 macrophage cell line because this agonist is not active on the P2Y2 receptor. However, Elliot et al. could not exclude the possibility that other chemotactic factors work alone or together with nucleotides in mediating the observed chemoattractant effect. Moreover, the role of nucleotides in chemotaxis remains equivocal as evidenced by several recent papers that do not consider ATP any longer as a “real” direct chemoattractant for macrophages. One report describes ATP as an indirect chemoattractant that navigates macrophages in a gradient of the chemoattractant C5a via autocrine release of ATP, generating amplification in gradient sensing via a “purinergic feedback loop” [117]. The same paper also reports the stimulation of macrophages with ATP to generate lamellipodial membrane protrusions that induce an indirect effect of chemotaxis [117]. The latter two mechanisms were found to involve P2Y2 and P2Y12 receptors [117]. The same authors confirmed in another recent paper that ATP does not recruit macrophages but locally induces lamellipodial membrane extensions and that ATP can promote chemotaxis and phagocytosis via autocrine/paracrine signaling involving P2Y2 and P2Y12 receptors but that it is itself not a chemoattractant as was evidenced from a microscope-based real-time chemotaxis assay that allows quantification of migration velocity and chemotaxis [118]. The increase in phagocytotic effect of marcophages by P2Y2 and/or P2Y12 ligation also contrasts to the findings of Elliott et al. who characterized ATP as a long-range chemoattractant -as discussed above- but without any effect on phagocytic activity [115]. Marques-da-Silva et al. on the other hand confirmed an enhanced phagocytic effect in macrophages by purinergic stimuli but proposed the engagement of P2X1 or P2X3 receptors based on the agonists profile [119]
Macrophages can furthermore undergo fusion with other macrophages to form multinucleated giant cells (MGC), a common feature of granulomas that develop during various inflammatory reactions. The involvement of purinergic receptors in MGC formation was first reported by the group of Di Virgilio who showed that high levels of P2X7 expression leads to spontaneous macrophage fusion in vitro [73, 120], being confirmed by Lemaire and Leduc [83]. Both groups later on attributed this effect to the C terminal part of the P2X7 receptor [121].
In conclusion, the implication of purinergic P2 receptors in inflammatory responses is evident in macrophages, being dominated by the P2X7 receptor subtype. Recent evidence suggests the possible regulatory function for P2X4 in P2X7-mediated responses. Further research is needed in order to assess whether other purinergic P2 receptors might contribute to the regulation of macrophage function (Table 1).
Dendritic cells
Dendritic cells (DCs) (Fig. 3) are the most potent antigen presenting cells of the immune system. In the absence of infection, DCs participate in central and peripheral T cell tolerance. During an infection, a dramatic increase in the movement of DCs from the tissue via afferent lymphatics into lymph nodes occurs where they trigger a specific T cell response [122].
Fig. 3.
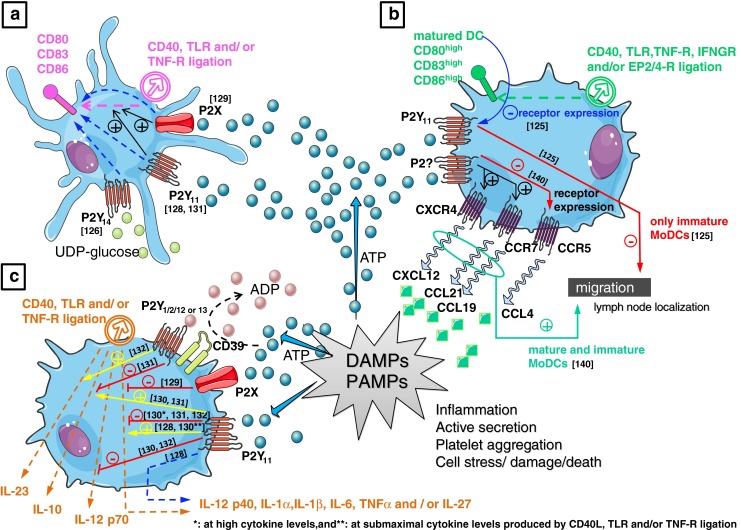
Purinergic responses in monocyte-derived dendritic cells (MoDCs). a MoDC maturation: chronic stimulation of DCs with noncytotoxic doses of ATP increases membrane expression of CD80, CD83, and CD86, Moreover, ATP also enhanced soluble CD40 ligand (sCD40L), LPS, and/or TNF-α-induced maturation of moDCs via P2X receptor activation [129] These processes might also involve P2Y11 [128] and/or P2Y14 [126] receptor activation. b MoDC migration: ATP provides a signal for enhanced lymph node localization of DCs; ATP increased migration of immature and mature DCs to CXCL12, CCL19, and CCL21, whereas responses to CCL4 were reduced [140]. In contrast, exposure of MoDCs to gradients of ATP inhibited their migratory capacity via P2Y11 receptors [125]. During maturation (by sCD40L, LPS, TNF-α, INFγ, and/or PGE2), MoDCs downregulated P2Y11 receptor expression which yield mature DCs that are less sensitive to ATP-mediated inhibition of migration [125]. c MoDC cytokine release—equivocal data in the literature report either a potentiating or inhibitory effect on sCD40L LPS and/or TNF-α-induced expression of inflammatory cytokines. CCL19 chemokine (C–C motif) ligand 19, CCR7 C–C chemokine receptor type 7, CXCL12 chemokine (C–X–C motif) ligand 12, CXCR4 C–X–C chemokine receptor type 4, DAMP damage-associated molecular patterns, INFγ interferon gamma, INFGR INFγ receptor, LPS lipopolysaccharide, PAMP pathogen-associated molecular patterns, PGE2 prostaglandin E2, EP2/4-R PGE2 receptor 2 and/or 4, TLR toll-like receptor, TNF-α tumor necrosis factor alpha, TNFR TNF receptor. Values between brackets in figurerepresent reference numbers
Human dendritic cells were found to express mRNA for P2X1, P2X4, P2X5, and P2X7 as well as for P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, and P2Y14 receptors [123–126]. The expression of P2X7 on protein level in DCs during immune responses was suggested by Buell et al. who observed immunopositive staining within human tonsils [127].
Functional responses induced by purinergic stimuli in DCs involve the release of IL-1β and TNF-α in LPS-matured DCs [124]. While Wilkin et al.[128] also reported ATP to synergize with TNF-α and LPS in increasing IL-12 secretion, la Sala et al. [129] reported ATP to inhibit LPS—and soluble CD40 ligand (sCD40L)—induced expression of IL12, but also of IL-1α, IL-1β, TNF-α, and IL-6. When discriminating between IL-12p40 and IL-12p70, the stable ATP analogue ATPγS potentiated the IL-12p40 release from human monocyte-derived DCs (MoDCs) induced by TNF-α, lipopolysaccharides (LPS), or by sCD40L [130]. The combinations ATPγS-TNF-α and ATPγS-sCD40L were however unable to induce detectable bioactive IL-12p70 production and at concentrations of LPS that induced a significant stimulation of IL-12p40 andIL-12p70, the effect of ATPγS was inhibitory. The authors also showed that that ATPγS synergized with LPS and sCD40L, but not TNF- α, to stimulate IL-10 production [130]. Except for the increase in IL-12p40 levels by ATPγS, above data were corroborated by another study reporting that ATPγS inhibited the levels of IL-12p40, IL-12p70, and TNFα produced during concomitant LPS challenge, whereas the IL-10 secretion was observed to be increased [131]. Conversely, IL10 production in these DCs could be reduced by ADPβS stimulation, which equally reduced IL-12 levels [131]. The inhibition by ATP of IL-12p40 and IL-12p70-production by DCs was also observed by Schnur et al. who moreover found that ATP also inhibited IL-27 expression but enhanced IL-23 expression in E. Coli induced MoDCs [132].
The involvement of the purinergic system in DC functionality was equally confirmed by assessing the maturation status based on the levels of DC surface markers. The implication of P2 receptors in DC maturation was already suggested by Schnurr et al. in showing that activation and maturation of DCs was blocked by suramin [133]. Later studies showed that in combination with TNF-α, ATP increased the expression of the DC surface markers CD80, CD83, and CD86 confirming a maturation-promoting effect [123]. Similarly, la Sala et al. reported that chronic stimulation of DCs with low, noncytotoxic doses of ATP increased membrane expression of CD54, CD80, CD83, and CD86, slightly reduced the endocytic activity of DCs, and augmented their capacity to promote proliferation of allogeneic naive T lymphocytes. Moreover, ATP also enhanced LPS- and sCD40L-induced CD54, CD83, and CD86 expression [129]. While Wilkin et al. assigned these processes to cAMP accumulation via P2Y11 receptor activation in human DCs based on the rank order of potency of various ATP analogues [128], a study on mice DCs reported that also ADP—via P2Y12—can promote antigen endocytosis by DCs and enhance T cell response [134]. Moreover, also the P2Y14 receptor was proposed to be implicated in DC activation/maturation as evidenced by the induction of Ca2+ fluxes and an increase in expression of the costimulatory molecule CD86 during stimulation with UDP glucose [126].
Other functional responses induced by purinergic stimuli in human DCs involve the reorientation of dendrites and changes in cell shape via functionally coupled P2Y receptors but not P2X receptors, being deduced from the pharmacological selectivity of the applied agonists [135]. However in this study, prolonged exposure to ATP was found to be toxic to dendritic cells that swelled, lost typical dendrites, and eventually died, which was highly suggestive for the expression of the cytotoxic receptor P2X7, as confirmed by ability of dendritic cells to become permeate to dyes such as Lucifer yellow or ethidium bromide [124]. ATP via P2X7 indeed also mediates apoptosis of dendritic cells as was confirmed in murine DCs [136, 137]. Epidermal Langerhans cells have previously been shown to express membrane ATPase which appears to protect them against the cytotoxic effects of extracellular ATP [138]. Similarly, human peripheral blood-derived DCs express the ecto-apyrase CD39 and the ecto-5P-nucleotidase CD73 as demonstrated by RT-PCR which rapidly hydrolyze extracellular ATP [123].
The reorientation of dendrites and changes of cell shape by P2Y receptor activation as observed by Liu et al. [135] suggests a possible role for ATP in mediating DC chemoatxis. The chemotactic effect of ATP was confirmed by Idzko et al. in immature but not mature DCs via P2Y receptors while P2X receptor activation only showed minor responses [139]. ATP indeed provides a signal for enhanced lymph node localization of DCs but it may, at the same time, diminish the capacity of DCs to amplify type 1 immune responses as was reported by la Sala et al. [140]. In contrast, exposure of MoDCs and CD1a+ dermal DCs to gradients of ATP inhibited their migratory capacity in a dose-dependent manner. However, during maturation, MoDCs downregulated P2Y11 receptor expression which yielded mature DCs that are less sensitive to ATP-mediated inhibition of migration [125]. These data indicate that the physiologic consequences of exposure to ATP on DC migration depend on ATP concentration, DC type, and DC maturational stage. The authors speculated that the formation of ATP gradients at sites of inflammation may transiently inhibit the migration of local DCs, thus prolonging the time of antigen encounter [125]. Also, the P2Y2 receptor appears to be involved in ATP-triggered migration of DCs as was reported for myeloid DCs in an experimental model of asthmatic airway inflammation [141]. The importance of ATP in dendritic cell on airway inflammation was earlier reported to be implicated in the pathogenesis of human asthma and experimental asthmatic airway inflammation in mice [142]. The authors showed that all the cardinal features of asthma, including eosinophilic airway inflammation, Th2 cytokine production and bronchial hyper-reactivity, were abrogated when lung ATP levels were locally neutralized using apyrase or when mice were treated with broad-spectrum P2-receptor antagonists. In addition to these effects of ATP in established inflammation, Th2 sensitization to inhaled antigen was enhanced by endogenous or exogenous ATP. The adjuvant effects of ATP were due to the recruitment and activation of lung myeloid dendritic cells that induced Th2 responses in mediastinal lymph nodes [142].
In conclusion, nucleotides can significantly modify DC functions such as cytokine release, maturation status and migration characteristics. Although more in vivo studies are welcome to confirm the observations made on the models of asthmatic inflammation, targeting P2 receptor function might indeed represent a promising approach to develop new therapeutics for asthma as evidenced by a recent study in a birth cohort at high risk for development of asthma that reported an attenuated P2X7 function to be associated with lower asthma rates [143] (Table 1).
Lymphocytes
The three major types of lymphocyte (Fig. 4 and Table 1) are T cells, B cells and natural killer (NK) cells. T cells are characterized by the presence of a T cell receptor (TCR) on the cell surface. There are several subsets of T cells (T helper cells (Th cells), cytotoxic T cells (CTLs), memory T cells, regulatory T cells (Tregs), and natural killer T cells (NKT cells)), each with distinct functions; Th cells, CTLs and Treg cells are reported to possess a purinergic component. Th cells (CD4+ T cells) assist other leukocytes in immunologic processes by means of secreting different sets of cytokine subtypes (Th1, Th2, Th17, etc.) Th cells themselves become activated when they are presented with peptide antigens by MHC class II molecules on the surface of antigen presenting cells (APCs), such as macrophages, dendritic cells, and B cells. CTLs (CD8+ T cells) destroy virally infected cells and tumor cells. These cells recognize their targets by binding to antigen associated with MHC class I, which is present on the surface of nearly every cell of the body. Treg cells (CD4+CD25+ T cells) are crucial for the maintenance of immunological tolerance by dampening the T cell-mediated immunity, terminating the immune reaction and suppressing auto-reactive T cells that escaped the process of negative selection in the thymus [144]. B cells are characterized by the presence of a B cell receptor on the cell surface which allows B cells to bind to a specific antigen. The principal functions of B cells are to make antibodies against antigens, to perform the role of APCs, and to develop into memory B cells after activation by antigen interaction [145]. Similar to CTL’s, NK cells provide responses to virally infected cells and tumor formations but can act much faster as they have the ability to recognize stressed cells in the absence of antibodies or MHC molecules [146].
Fig. 4.
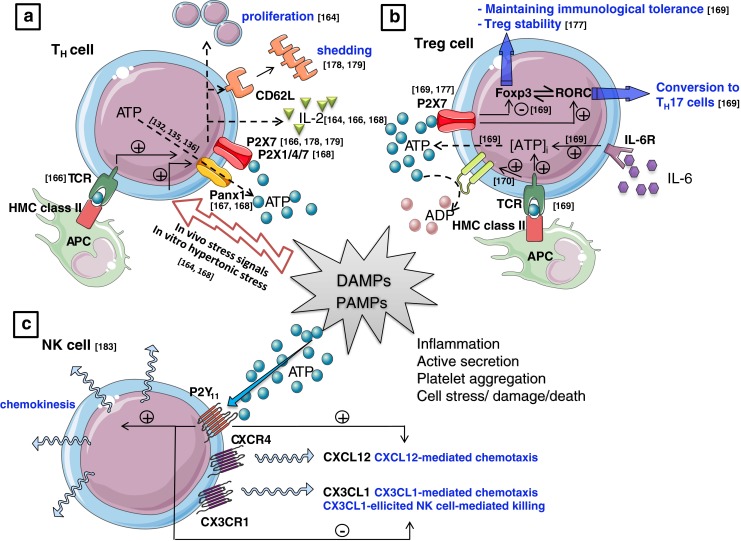
a T helper (TH) cells: hypertonic saline promotes T cell proliferation and enhances IL-2 production of stimulated T cells by inducing the release of cellular ATP and by the autocrine activation of stimulatory P2X receptors [164]. Also, T cell receptor (TCR) stimulation itself triggers the release of ATP from the T cells which - via an autocrine feedback mechanism through P2X7 receptors [166] and/or P2X1 and P2X4 [168]—is required for the effective activation of T cells. This ATP release occurs through pannexin-1 hemichannels [167, 168]. ATP also triggers P2X7 receptor-dependent CD62L shedding in lymphocytes [178, 179] which is associated with T cell activation. b T regulatory (Treg) cells: CD4+ helper T cells upon stimulation of the TCR and potentiated by the inflammatory cytokine IL-6, increases the synthesis and release of ATP [169] which subsequently induces an autocrine P2X7-mediated signaling in Tregs [169] that inhibits the suppressive potential and stability of Tregs [177], and promotes their conversion to IL-17-secreting T Th17 effector cells [169]. However, TCR stimulation also enhances the expression of CD39 [170] which degrades ATP to ADP and AMP. Consequently, activated Treg cells are able to abrogate the P2X7-mediated suppression of Treg function and stability in an autologous way [169]. c Natural killer (NK) cells: NK cells stimulated with ATP increases chemokinesis and chemotaxis to CXCL12 via P2Y11 receptor activation, while chemotaxis and NK cell-mediated killing in response to CX3CL1 chemokine is inhibited [183]. APC antigen presenting cell, CX3CL1 chemokine (C–X3–C motif) ligand 1, CX3CR1 CX3C chemokine receptor 1, CXCL12 chemokine (C–X–C motif) ligand 12, CXCR4 C–X–C chemokine receptor type 4, DAMP damage-associated molecular patterns, Foxp3 forkhead box P3, HMC class II major histocompatibility complex class II, IL-6R interleukin-6 receptor, PAMP pathogen-associated molecular patterns, RORC retinoic acid receptor-related orphan receptor C. Values between brackets in figurerepresent reference numbers
B and T cells
Based on results generated in the late 1980s and begin 1990s, lymphocytes were initially regarded to exclusively express ionotropic P2X receptors as they react on exogenous ATP via enhanced Ca2+ influx across the plasma membrane while mobilization of Ca2+ from intracellular stores (P2Y) could not be observed [147, 148]. Others reported that P2 receptors are expressed only on B lymphocytes and not on T lymphocytes [149]. However, later studies revealed the presence of both P2X and P2Y receptor subtypes in both T and B lymphocytes [21, 150–153]. By means of confocal immunofluorescence microscopy imaging using a panel of polyclonal antibodies raised against extracellular epitopes of the seven P2X subtypes, Sluyter et al. confirmed the presence of P2X1, P2X2, P2X4, and P2X7 subtypes but not P2X3, P2X5, and P2X6 subtypes on human B lymphocytes [152]. In contrast to the selective expression of P2X receptors in the latter study, Lee et al. [151] demonstrated human B lymphocytes to express mRNA of all P2X and P2Y receptors. All P2Y receptors showed similar rates of expression within 1- or 2-fold of each other. The same is true within the P2X receptor family with the exception of the P2X3 and P2X7 receptors, which were expressed in lower quantities.
The expression of most P2 receptors was suppressed during Epstein–Barr virus-induced B cell transformation into lymphoblastoid cell lines (LCLs); however, the suppression of P2X1, P2X4, P2X5, and P2Y11 receptors was not as pronounced as for other subtypes, leaving them to be the most abundantly expressed subtypes in LCLs. Only P2X7 receptor was significantly upregulated, suggesting some plasticity in P2-receptor expression in B cells. Western blot analysis confirmed the mRNA data for all P2 receptors [151]. Similarly, Wang et al. [153] showed mRNA from all P2Y receptor subtypes to be expressed in lymphocytes. Among the P2Y receptor subtypes, P2Y2 and P2Y12 had highest expression, but did not exceed a 2.5-fold change. The lowest expressed P2Y receptor subtype was P2Y4. The P2X4 receptor was found to be the most abundant P2X receptor subtype expressed in lymphocytes compared with the P2X1, P2X4, and P2X7 subtypes that were assessed in that study. In contrast to the study by Lee et al., Wang et al. found similar expression levels between P2X1 and P2X7 [153]. Moore et al. furthermore showed expression of P2Y14 in peripheral immune cells including lymphocytes [39], which was in a further study reported to be predominantly expressed in T lymphocytes rather than in B lymphocytes [154].
Functionally, the effects of ATP on lymphocytes were investigated extensively. Early observations, from the late 1970s on, already indicated nucleotides to equivocally affect thymocytes/lymphocyte mitogenesis/blastogenesis [147, 155–161]. Barankiewicz et al. suggested that extracellular ATP catabolism may serve as a means of communication between B and T cells in lymphoid organs, with B lymphocytes but not T lymphocytes being the producers of adenosine via ATP breakdown, and T lymphocytes being the recipients of this signal [162]. However, it is now more commonly appreciated that also T lymphocytes can release and hydrolyze extracellular ATP to adenosine, and that ATP is involved in promoting T cell activation while adenosine inhibits T cell functions [163–165]. Indeed, Loomis et al. showed that hypertonic saline promotes T cell proliferation and enhances IL-2 production of stimulated T cells by inducing the release of cellular ATP and by the autocrine activation of stimulatory P2X receptors [164]. The same group also showed that T cells express enzymes that convert ATP to its inhibitory metabolite adenosine which inhibits IL-2 production by activating A2 receptors expressed on the surface of T cells [165]. Later studies demonstrated TCR stimulation itself to trigger the release of ATP from the T cells which—via an autocrine feedback mechanism through P2X7 receptors—is required for the effective activation of T cells as was shown in Jurkat cells and human purified CD4+ T cells [166]. This ATP release from naive CD4+ T cells occurs through pannexin-1 hemichannels [167, 168] and the released ATP promotes T cell function as evidenced by p38 MAPK activation and increased IL-2 gene transcription through stimulation of P2X1, P2X4, and P2X7 receptors [168]. Another recent paper describes that pharmacological antagonism of P2X7 receptors promotes cell-autonomous conversion of naïve CD4+ T cells into Tregs after TCR stimulation, while the activation of P2X7 by autocrine ATP inhibits the suppressive potential and stability of Tregs [169]. However, TCR stimulation also enhances the expression of CD39 via the induction of the Treg-specific transcription factor Foxp3 [170]. As CD39 is an ectoenzyme that degrades ATP to AMP, activated Treg cells are consequently not only able to abrogate ATP-driven maturation of dendritic cells [170], but also P2X7-mediated suppression of Treg function and stability in an autologous way [169].
The breakdown product of ATP was initially also reported as an inhibitor of lymphocyte mediated cytolysis which was associated with an elevation of cyclic AMP within the cytotoxic T cells [171, 172]. Later on, others also revealed extracellular ATP to be a potent modulator in the cytolytic activity of cytotoxic T cells [173, 174]. Filippini et al. described the ability of both cytotoxic T cells and T helper cells to accumulate ATP in response to TCR crosslinking, suggesting a general role of ATP in the effector functions of T lymphocytes. When secreted by cytotoxic T cells towards the target cell, ATP may contribute to the death of the target cell, while secretion of ATP by T helper cells toward the antigen-presenting cell may modulate the action of lymphokines by involving purinergic receptors or ectoprotein kinases [174]. Similarly, Blanchard et al. provided evidence that ATP is released from cytotoxic T cells during antigen presentation, and that IFN-γ-activated macrophages underwent necrosis [173].Besides the induction of cell death in the cytotoxic T lympocytes’ target cells, also the T cells themselves are reported to undergo ATP-mediated cell death [175]. Unlike cytotoxic T cells and T helper cells, it is the T regulatory cells that are reported to be particularly sensitive to undergo cell death accompanied by CD62L shedding in response to ATP [176, 177]. As it is well documented that ATP triggers P2X7 receptor-dependent CD62L shedding in lymphocytes [25, 178, 179] and because it is also known that the release of CD62L form T cells is associated with T cell activation [180], the involvement of this P2 receptor subtype in T cell responses could be suggested. Indeed, in murine T cells, also Aswad et al. reported that ATP induces the release of CD62L but then associated to the initiation of T cell death [176]. The extent to one or another of both actions depends on the level of P2X7 receptor cell surface expression in the T cell subtype; CD4+ CD25+ T regulatory cells are particularly sensitive to ATP-induced cell death and this high sensitivity correlates with a high expression level of the P2X7 receptor. T helper cells showed intermediate sensitivity to ATP, whereas cytotoxic T cells are the least sensitive and showed the lowest expression of the P2X7 receptor [177].
Although P2X7 receptors have indeed a strong pro-apoptotic and/or necrotic activity, it can also support cell growth. Baricordi et al. proposed the operation of an ATP-based autocrine/paracrine loop that supports lymphoid cell growth in the absence of serum-derived growth factors [181]. Adinolfi et al. described a mechanism that helps to understand the dual effect of P2X7 receptor stimulation on cell growth and cell death; the authors showed that expression and basal activation of the P2X7 receptor modulates mitochondrial ion homeostasis and increases cellular energy stores which correlates with a better survival of P2X7 receptor transfectants in the absence of growth factors. Contrariwise, a strong stimulation of the P2X7 receptor causes a dramatic overflow of basal mitochondrial Ca2+ levels, a resting mitochondrial potential collapse, and extensive fragmentation of the mitochondrial network, resulting in cell death [182].
The only P2Y receptor for which functional data are available in lymphocytes is P2Y14; in T cells UDP, glucose induces significant inhibition of forskolin-stimulated cAMP accumulation via P2Y14 receptors [154].
NK cells
NK cells are reported to express P2X1, P2X4-7, and all P2Y receptor subtypes on m RNA level [183]. Schmidt et al. were the first to study the functional effect of ATP on NK cells showing that the treatment of human NK cells with ATP resulted in a strong inhibition of their cytotoxic activity on K562 target cells [184], a result that was corroborated by others [185, 186]. Miller et al. equally confirmed that ATP and related adenine nucleotides play a role in decreased NK cell activity [187] but in contrast to the previous studies, the authors reported that this was in fact due to an inhibition of proliferation because despite a reduced thymidine incorporation induced by extracellular adenine nucleotides, NK cells maintained their capacity to lyse K562 cells in their experiments [187]. Extracellular ATP furthermore regulates NK cell cytotoxicity via P2Y11 receptor activation; NK cells stimulated with ATP increased chemokinesis and inhibited NK chemotaxis in response to CX3CL1 chemokine [183].
In contrast to the reduced cytotoxic activity of NK cells by ATP in the reports above, Beldi et al. described limited tissue damage mediated by NK cells during an experimental model of hepatic ischemia/reperfusion injury in CD39-null mice as a consequence of increased peri-cellular ATP levels on NK cells [188]. As these peri-cellular ATP levels are required for regulated IFN-γ secretion by NK cells, alterations in the levels of ATP by CD39 deletions therefore modulate tissue injury. Indeed, IFN-γ secretion by NK cells in response to interleukin-12 and interleukin-18 was inhibited by ATPγS in vitro [188].
Eosinophils
Eosinophils (Fig. 5) are granulocytic leukocytes implicated in the pathogenesis of diverse inflammatory processes such as parasitic, bacterial and viral infections, tissue injury, tumor immunity, and allergic diseases. In response to a set of diverse stimuli, eosinophils are recruited from the circulation into inflammatory foci where they modulate immune responses [189].
Fig. 5.
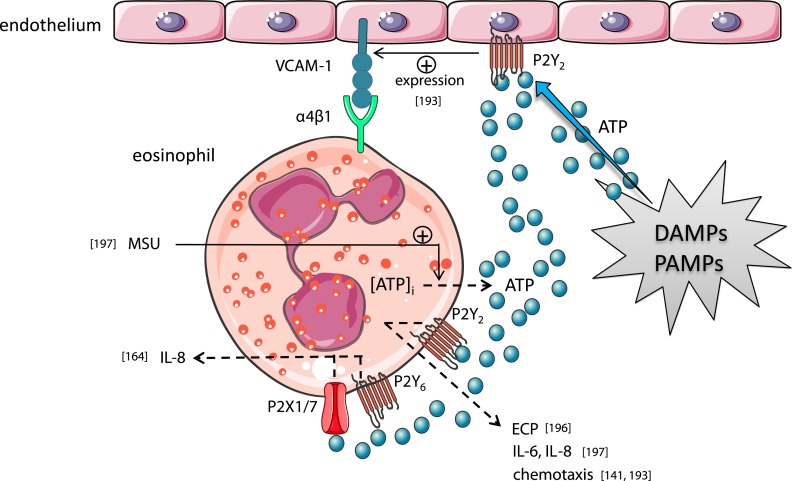
Human eosinophils release eosinophil cationic protein (ECP) [196] IL-6 and IL-8 [197] by P2Y2 receptor stimulation, while P2X1, P2X7, and P2Y6 receptors might also be implicated in the release of IL-8 [196]. The P2Y2 receptor subtype expressed on eosinophils is also suggested to be implicated in eosinophil chemotaxis [141, 193]. Also endothelial P2Y2 receptors can affect eosinophil migration by regulating the expression of endothelial VCAM-1 which mediates migration and adhesion of eosinophils via the α4β1 integrin [193]. Monosodium urate (MSU) crystals induce the release of autocrine ATP from eosinophils which provides a pivotal positive feedback signal via P2 receptors on eosinophils [197]. DAMP damage-associated molecular patterns, PAMP pathogen-associated molecular patterns, VCAM-1 vascular cell adhesion molecule 1. Values between brackets in the figurerepresent reference numbers
The first notion for a functional effect of purines on eosinophils comes from experiments showing that the ATP released from the granules of platelets can activate eosinophilic granulocytes and induces chemotaxis, a mechanism mimicked by exogenously added ATP [190].
To date human eosinophils are reported to express mRNA for P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y14, P2X1, P2X4, and P2X7 purinergic receptor subtypes [10, 19, 191, 192] (Table 1).
Both the P2X and P2Y receptor family members are implicated in eosinophil chemotaxis, but P2Y receptors are reported to be more potent than P2X receptors in doing so [192]. Especially, the purinergic P2Y2 receptor subtype stood recently in the spotlights as it was reported to be a potential therapeutic target for the treatment of asthma because of its implication in the chemotaxis of dendritic cells and eosinophils in allergic lung inflammation [141, 193]. The functional expression of P2Y2 receptors in human and murine eosinophils revealed that the migratory response to ATP was absent in eosinophils derived from P2Y2-deficient animals. The hypothesis that P2Y2 receptor signaling might contribute to the pathogenesis of asthma was further supported by the observation that eosinophils from asthmatic subjects showed a higher expression of P2Y2 receptor compared with healthy individuals [141]. At the same time, also Anderstocken et al. demonstrated that allergic eosinophilic lung inflammation is decreased in animals lacking the P2Y2 receptor [193]. The authors observed that eosinophil accumulation was defective in OVA-challenged P2Y2-deficient mice compared with OVA-treated wild type animals. In contrast, comparable infiltration of macrophages and neutrophils in the BALF of LPS-aerosolized P2Y2+/+ and P2Y2−/− mice were observed. In the same study, the P2Y2 receptor was defined as a regulator of endothelial membrane and soluble forms of VCAM-1 mediating the adhesion and migration of eosinophils that express the α4β1 integrin which binds to VCAM-1 [193].
Besides its implication in chemotoxis, ATP has been shown to be involved in the induction of ROS production, CD11b upregulation, and cytokine production in eosinophils [19, 141, 192, 194, 195]. These effects occur through Ca2+ mobilization [19, 194, 195]. ATP can either interact directly with P2X ion channels or indirectly activate transmembranous Ca2+ fluxes following G protein activation. ATP is reported to induce actin polymerization and production of ROS via pertussis toxin–sensitive Gi proteins (P2Y) in eosinophils, while the upregulation of integrin CD11b by ATP occurs directly via Ca2+ transients (P2X) [194] although the latter has also been reported to occur equipotently by both P2Y and P2X receptor agonists [192].
With regard to the release of pro-inflammatory mediators, human eosinophils release eosinophil cationic protein (ECP) and IL-8 by P2 receptor-stimulation; the release of ECP was triggered by ATP, UTP, and UDP via a member of the P2Y receptor family, possibly P2Y2 while IL-8 release is triggered by UDP, ATP, α,β-meATP, and BzATP mediated by P2X1, P2X7, and P2Y6 receptor subtypes [196]. Eosinophils cultured with monosodium urate (MSU) crystals also produce cytokines and chemokines; Kobayashi et al. found that MSU crystals induce the release of autocrine ATP from eosinophils which provides a pivotal positive feedback signal in eosinophils to release cytokines and chemokines (including IL-8, IL-6, CCL2-4, IL-1β, IL-10, IL-17, IFN-γ, and TGF-β) via P2Y2 receptors [197]. Others reported P2Y6 receptors to mediate MSU-induced inflammation in both keratinocyte and monocytes and P2Y6 antagonists to suppress neutrophil infiltration in both mouse air pouch and peritonitis models [198]. They also demonstrated MSU-induced inflammatory cytokine and chemokine production (including IL-1α, IL-8, IL-6, IL-18, and to a lesser extend TNF-α and IL-1β) by keratinocytes and monocytes. In contrast to the study of Kobayashi et al. [197], the authors could not confirm a MSU-induced release of ATP to be the mechanism of action explaining the involvement of the P2Y6 receptors in the responses described in their study [198].
Mast cells
Mast cells (Fig. 6) are key components of allergic and anaphylactic reactions. When mast cells are activated by allergens binding to IgE antibodies attached to FcεRI receptors, they produce cytokines, chemokines, leukotriens, and eicosanoids and release secretory granules. Mast cells are also increasingly regarded to be involved in chronic inflammatory diseases during which they can be activated or modulated by diverse nonallergic triggers such as neuropeptides and cytokines [199, 200].
Fig. 6.
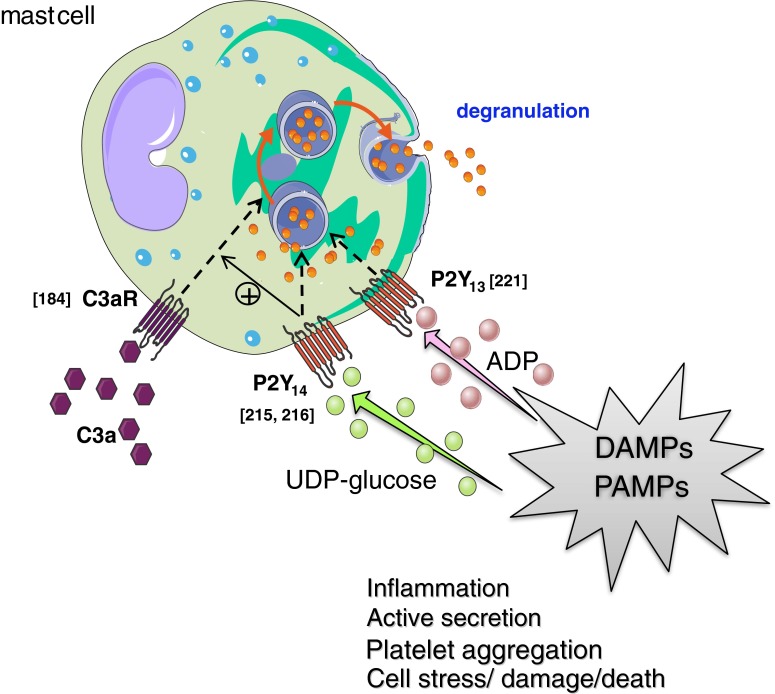
P2Y13 and P2Y14 receptor-activation directly induces mast cell degranulation [215, 221] while P2Y14 receptors also potentiate C3a-induced degranulation [216]. DAMP damage-associated molecular patterns, PAMP pathogen-associated molecular patterns, C3a complement 3a, C3aR complement 3a receptor. Values between brackets in the figurerepresent reference numbers
In the presence of Ca2+, ATP is known to directly induce responses [199, 201–207] and/or modulate responses induced by other mediators such as compound 48/80 [208], anti-IgE [200], and ionophore A23187 [209–211] in mast cells, resulting in the release of histamine [199–204, 208, 209, 211, 212], PGD2 [199] or phosphorylated metabolites and nucleotides [206, 207]. However, for most of above observations, no P2 receptor-mediated mechanisms were reported; the events were described to be associated to either Ca2+-dependent exocytotic degranulation [206, 210], permeability changes [199, 205–207], the metabolic state of the cells [202, 209–212], or morphological changes such as cell swelling [204].
The number of studies that investigated P2 receptor expression on mRNA and protein levels in human mast cells is scarce. Human lung mast cells (HLMC) were reported to express mRNA transcripts for P2X1 and P2X4 but not for P2X2, P2X3, and P2X5-P2X7 [213] in a study that did not investigate the P2Y receptor family, while others found that of the 3 P2 receptor transcripts assessed in the study, P2Y1 and P2Y2 but not P2X7 were expressed [200]. In contrast to the absence of the P2X7 receptor in above mentioned studies, Wareham et al. reported its presence in HLMC next to P2X1 and P2X4 [214] (Table 1). Also, the human mast cell line HMC-1 was shown to highly express the P2X7 receptor being further upregulated upon PMA (phorbol 12-myristate 13-acetate) or ionomycin stimulation [200], while Wareham et al. also confirmed the presence of P2X7 receptors next to P2X1, P2X4, and P2X6 in the LAD2 human cell line [214]. Among the P2Y receptors, the presence of P2Y1, P2Y2, P2Y11, P2Y12, and P2Y13 subtypes were shown by RT-PCR on cord blood-derived human mast cells (hMCs) [23]. Recently, the presence of all P2Y receptors in mast cells was reported including P2Y4 and P2Y14 receptors as was shown in both murine RBL-2H3 and human LAD2 cell lines, the former being most highly expressed together with the P2Y11 receptor[215, 216].
The application of ATP is known to evoke inward currents in the mast cells [214], yielding an increase in intracellular Ca2+ which is necessary for IgE-mediated and/or ATP-mediated/potentiated mediator release from mast cells [23, 195, 200, 202, 213, 217–220]. The first paper functionally linking P2 receptors to Ca2+-dependent mediator release by mast cells was the work from Schulman et al. who reported that adenine nucleotides consistently enhanced anti-IgE-induced histamine release with a rank order of potency of ATP > 2mSATP > αβmATP > βγmATP [200]. Schulman et al. confirmed the presence of P2Y1 and P2Y2, but not P2X7 transcript in HLMC’s as discussed above. Recent studies indicate that P2Y13 and P2Y14 might also be involved in mast cell degranulation, since these receptors induce β-hexosaminidase release in RBL-2H3 rat mast cells [215, 221] while P2Y14 was also reported to potentiate C3a-induced β-hexosaminidase release in human LAD2 cells [216] (Table 1). Triggering of intracellular Ca2+ signaling in mast cells is also linked to cytokine expression and release. ADP and ATP inhibited the generation of TNF-α in response to the TLR2 ligand peptidoglycan, and blocked the production of TNF-γ, IL-8, and MIP-1β in response to leukotriene D4 via P2Y receptors in hMCs for which the presence of P2Y1, P2Y2, P2Y11, P2Y12, and P2Y13 subtypes was confirmed on mRNA level [23]. Finally, ATP has also been reported to be liberated from mast cells itself which could diffuse to elicit responses in distant cells; this ATP release from mast cells is triggered either by FcεR cross-linking, by extracellular nucleotide application, or by mechanic stimulation and is independent on gap-junctional coupling [222, 223] .
Adenine nucleotides have also been reported to participate in the recruitment of mast cells, an event where also a role for Ca2+ influx has been suggested in the process; ADP, ATP, and UTP are indeed reported to be effective chemoattractants for rat-bone-marrow-cultured mast cells [116].
Therapeutic potential of P2 receptor targeting
While this review provides an overview of purinergic receptors implicated in various functions of different immune cells, based on the currently explored therapeutic interventions we may suggest that especially the P2X7 receptor may have an important mechanistic role in inflammation. Indeed, several P2X7 antagonists are found to be promising tools in the fight against chronic inflammatory conditions such as Crohn’s disease, rheumatoid arthritis, psoriasis, allergic dermatitis, chronic pulmonary disease, and asthma [224–226]. Albeit not directly affecting inflammatory cells, also the P2Y2 receptor agonists Denufsol is currently under investigation in the treatment of cystic fibrosis (CF), improving lung function in CF patients with mildly impaired lung function by enhancing airway hydration and mucociliary clearance, and thus indirectly reducing the susceptibility to infection, inflammation and the resultant progressive airway damage [227].
While a possible therapeutic potential of other P2 receptor subtype selective drugs in inflammatory conditions cannot be excluded, more studies are required to confirm the importance of these receptors. Here, we discuss a few ideas regarding potential therapeutic possibilities on which was speculated in the literature: as Vaughan et al. [35] reported that ATP causes a substantial delay in neutrophil apoptosis which is mediated via P2Y11 receptors, specific antagonizing P2Y11 receptors could reduce the injurious effects of increased neutrophil longevity induced by ATP during neutrophilic inflammation while retaining their key immune functions such as chemotaxis and phagocytosis which are primary mediated via P2Y2 receptors (see paragraph on neutrophils). The P2Y11 receptor could thus be a novel potential therapeutic target for the amelioration of neutrophilic inflammation in a wide range of inflammatory diseases [35]. Another possible mechanism of action that involves to the inhibition of P2Y11 receptors relates to DC migration; the observation that extracellular ATP at sites of inflammation inhibits the migration of local DCs via P2Y11 [125] makes P2Y11 receptor-inhibition a strategy to improve the migration of antigen-loaded DCs from the vaccination site to lymph nodes as a result of a prolonged time of antigen encounter [125]. As also the P2Y2 receptor appears to be involved in ATP-triggered migration of DCs and eosinophils, the latter showing increased expression of P2Y2 in allergic asthmatics [141], P2Y2 receptor targeting could also represent another potential therapeutic option for the treatment of allergic asthma and other allergic inflammatory conditions.
Besides inflammatory conditions, there is also an interest in the therapeutic potential of purinergic compounds in a wide range of other pathologies [228] including atherosclerotic vascular diseases [229], cancer [230, 231], ocular problems [232], lower urinary tract dysfunction [233], and neurodegenerative disorders including amyotropic lateral sclerosis, multiple sclerosis and Alzheimer’s disease [225]. To date only two P2 receptor antagonist are marketed: the irreversible P2Y12 receptor antagonist clopidogrel (Plavix®), which is used as an antithrombotic agent in patients with atherosclerotic vascular disease [234] and another orally active but reversible antagonist of P2Y12, Ticagrelor (Brilinta®), which showed to even have a greater efficacy in reducing cardiovascular death compared with clopidogrel [235].
The diversification and the introduction of new clinical targets will intensify the ongoing effort to design more selective agonists and antagonists for the P2 receptors, both as pharmacological tools and as potential therapeutic agents [236, 237].
Conclusions
Extracellular nucleotides are important players in regulating inflammatory responses through binding to purinergic P2 receptors present on various inflammatory cell types. All immune cells express a wide range of P2 receptors which modulate a diverse set of responses. Among the different P2 receptors expressed, it can be generally concluded that particularly the P2X4, P2X7, P2Y2, P2Y4, P2Y6, P2Y11, and P2Y12 receptor subtypes come into the picture when analyzing the effects of purines in immune responses. However, the picture of P2 receptors is still not final and further research is needed in order to gain a more complete overview of their role in inflammation. The recent development of P2 receptor-targeting drugs has provided potential candidates as therapeutics in inflammatory diseases. While currently several selective P2 receptor ligands are available, much more work is needed to fully cover the wide range of P2X and P2Y receptor subtypes with selective agonists and antagonists.
Acknowledgments
This work was supported by funding from The Research Foundation Flanders (FWO); research project Nr. G.0641.10 to KVC, and a Concerted Research Action project (01G01009) from the Special Research Fund of Ghent University. The figures were generated using Servier Medical Art’s Powerpoint image banks available at www.servier.com.
Abbreviations
- cAMP
Cyclic adenosine monophosphate
- DCs
Dendritic cells
- ECP
Eosinophil cationic protein
- ERK
Extracellular signal-regulated kinases
- fMLP
fMet-Leu-Phe receptor
- HLMC
Human lung mast cell
- HMC
Human mast cell
- IP3
Inositol 1,4,5-triphosphate
- LAD 2
Leukocyte adhesion deficiency
- LCL
Lymphoblastoid cell lines
- MIP-2
Macrophage inflammatory protein-2
- MoDCs
Monocyte-derived dendritic cells
- MSU
Monosodium urate
- E-NTPDase1
Ecto-nucleoside triphosphate diphosphohydrolase 1
- PLC
Phospholipase C
Footnotes
Fenila Jacob and Koen Van Crombruggen contributed equally to this work
References
- 1.Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, et al. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunol Rev. 2007;220:60–81. doi: 10.1111/j.1600-065X.2007.00579.x. [DOI] [PubMed] [Google Scholar]
- 2.Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28(10):429–436. doi: 10.1016/j.it.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442(7102):527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 4.Myrtek D, Idzko M. Chemotactic activity of extracellular nucleotideson human immune cells. Purinergic Signal. 2007;3(1–2):5–11. doi: 10.1007/s11302-006-9032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vitiello L, Gorini S, Rosano G, la Sala A (2012) Immunoregulation through extracellular nucleotides. Blood 120(3):511–518 [DOI] [PubMed]
- 6.Weber FC, Esser PR, Muller T, Ganesan J, Pellegatti P, et al. Lack of the purinergic receptor P2X(7) results in resistance to contact hypersensitivity. J Exp Med. 2010;207(12):2609–2619. doi: 10.1084/jem.20092489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilhelm K, Ganesan J, Muller T, Durr C, Grimm M, et al. Graft-versus-host disease is enhanced by extracellular ATP activating P2X7R. Nat Med. 2010;16(12):1434–1438. doi: 10.1038/nm.2242. [DOI] [PubMed] [Google Scholar]
- 8.Barbera-Cremades M, Baroja-Mazo A, Gomez AI, Machado F, Di Virgilio F, et al. P2X7 receptor-stimulation causes fever via PGE2 and IL-1beta release. FASEB J. 2012;26(7):2951–2962. doi: 10.1096/fj.12-205765. [DOI] [PubMed] [Google Scholar]
- 9.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, et al. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97(3):587–600. doi: 10.1182/blood.V97.3.587. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari D, la Sala A, Panther E, Norgauer J, Di Virgilio F et al (2006) Activation of human eosinophils via P2 receptors: novel findings and future perspectives. J Leukoc Biol 79(1):7–15 [DOI] [PubMed]
- 11.Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26(8–9):959–969. doi: 10.1023/A:1012388618693. [DOI] [PubMed] [Google Scholar]
- 12.Junger WG. Immune cell regulation by autocrine purinergic signalling. Nat Rev Immunol. 2011;11(3):201–212. doi: 10.1038/nri2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, et al. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58(3):281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarvis MF, Khakh BS. ATP-gated P2X cation-channels. Neuropharmacology. 2009;56(1):208–215. doi: 10.1016/j.neuropharm.2008.06.067. [DOI] [PubMed] [Google Scholar]
- 15.Burnstock G , Knight GE (2004) Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol 240:31–304 [DOI] [PubMed]
- 16.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64(12):1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volonte C, Amadio S, D'Ambrosi N, Colpi M, Burnstock G. P2 receptor web: complexity and fine-tuning. Pharmacol Ther. 2006;112(1):264–280. doi: 10.1016/j.pharmthera.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Rayah A, Kanellopoulos JM, Di Virgilio F. P2 receptors and immunity. Microbes Infect. 2012;14(14):1254–1262. doi: 10.1016/j.micinf.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohanty JG, Raible DG, McDermott LJ, Pelleg A, Schulman ES. Effects of purine and pyrimidine nucleotides on intracellular Ca2+ in human eosinophils: activation of purinergic P2Y receptors. J Allergy Clin Immunol. 2001;107(5):849–855. doi: 10.1067/mai.2001.114658. [DOI] [PubMed] [Google Scholar]
- 20.Sak K, Boeynaems JM, Everaus H. Involvement of P2Y receptors in the differentiation of haematopoietic cells. J Leukoc Biol. 2003;73(4):442–447. doi: 10.1189/jlb.1102561. [DOI] [PubMed] [Google Scholar]
- 21.Jin J, Dasari VR, Sistare FD, Kunapuli SP. Distribution of P2Y receptor subtypes on haematopoietic cells. Br J Pharmacol. 1998;123(5):789–794. doi: 10.1038/sj.bjp.0701665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bulanova E, Budagian V, Orinska Z, Hein M, Petersen F, et al. Extracellular ATP induces cytokine expression and apoptosis through P2X7 receptor in murine mast cells. J Immunol. 2005;174(7):3880–3890. doi: 10.4049/jimmunol.174.7.3880. [DOI] [PubMed] [Google Scholar]
- 23.Feng C, Mery AG, Beller EM, Favot C, Boyce JA. Adenine nucleotides inhibit cytokine generation by human mast cells through a Gs-coupled receptor. J Immunol. 2004;173(12):7539–7547. doi: 10.4049/jimmunol.173.12.7539. [DOI] [PubMed] [Google Scholar]
- 24.Humphreys BD, Dubyak GR. Modulation of P2X7 nucleotide receptor expression by pro- and anti-inflammatory stimuli in THP-1 monocytes. J Leukoc Biol. 1998;64(2):265–273. doi: 10.1002/jlb.64.2.265. [DOI] [PubMed] [Google Scholar]
- 25.Gu B, Bendall LJ, Wiley JS. Adenosine triphosphate-induced shedding of CD23 and l-selectin (CD62L) from lymphocytes is mediated by the same receptor but different metalloproteases. Blood. 1998;92(3):946–951. [PubMed] [Google Scholar]
- 26.Chen JR, Gu BJ, Dao LP, Bradley CJ, Mulligan SP, et al. Transendothelial migration of lymphocytes in chronic lymphocytic leukaemia is impaired and involved down-regulation of both l-selectin and CD23. Br J Haematol. 1999;105(1):181–189. doi: 10.1111/j.1365-2141.1999.01278.x. [DOI] [PubMed] [Google Scholar]
- 27.Amulic B, Cazalet C, Hayes GL, Metzler KD, Zychlinsky A (2012) Neutrophil function: from mechanisms to disease. Annu Rev Immunol 30:459–489 [DOI] [PubMed]
- 28.Sadik CD, Kim ND, Luster AD. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32(10):452–460. doi: 10.1016/j.it.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Axtell RA, Sandborg RR, Smolen JE, Ward PA, Boxer LA. Exposure of human neutrophils to exogenous nucleotides causes elevation in intracellular calcium, transmembrane calcium fluxes, and an alteration of a cytosolic factor resulting in enhanced superoxide production in response to FMLP and arachidonic acid. Blood. 1990;75(6):1324–1332. [PubMed] [Google Scholar]
- 30.Ward PA, Cunningham TW, McCulloch KK, Johnson KJ. Regulatory effects of adenosine and adenine nucleotides on oxygen radical responses of neutrophils. Lab Investig. 1988;58(4):438–447. [PubMed] [Google Scholar]
- 31.Ward PA, Cunningham TW, McCulloch KK, Phan SH, Powell J, et al. Platelet enhancement of O2−. responses in stimulated human neutrophils. Identification of platelet factor as adenine nucleotide. Lab Investig. 1988;58(1):37–47. [PubMed] [Google Scholar]
- 32.Bengtsson T, Zalavary S, Stendahl O, Grenegard M. Release of oxygen metabolites from chemoattractant-stimulated neutrophils is inhibited by resting platelets: role of extracellular adenosine and actin polymerization. Blood. 1996;87(10):4411–4423. [PubMed] [Google Scholar]
- 33.Zalavary S, Grenegard M, Stendahl O, Bengtsson T. Platelets enhance Fc(gamma) receptor-mediated phagocytosis and respiratory burst in neutrophils: the role of purinergic modulation and actin polymerization. J Leukoc Biol. 1996;60(1):58–68. doi: 10.1002/jlb.60.1.58. [DOI] [PubMed] [Google Scholar]
- 34.Suh BC, Kim JS, Namgung U, Ha H, Kim KT. P2X7 nucleotide receptor mediation of membrane pore formation and superoxide generation in human promyelocytes and neutrophils. J Immunol. 2001;166(11):6754–6763. doi: 10.4049/jimmunol.166.11.6754. [DOI] [PubMed] [Google Scholar]
- 35.Vaughan KR, Stokes L, Prince LR, Marriott HM, Meis S, et al. Inhibition of neutrophil apoptosis by ATP is mediated by the P2Y11 receptor. J Immunol. 2007;179(12):8544–8553. doi: 10.4049/jimmunol.179.12.8544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martel-Gallegos G, Rosales-Saavedra MT, Reyes JP, Casas-Pruneda G, Toro-Castillo C, et al. Human neutrophils do not express purinergic P2X7 receptors. Purinergic Signal. 2010;6(3):297–306. doi: 10.1007/s11302-010-9178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, et al. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci. 2003;24(2):52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Shukla A, Namiki S, Insel PA, Junger WG. A putative osmoreceptor system that controls neutrophil function through the release of ATP, its conversion to adenosine, and activation of A2 adenosine and P2 receptors. J Leukoc Biol. 2004;76(1):245–253. doi: 10.1189/jlb.0204066. [DOI] [PubMed] [Google Scholar]
- 39.Moore DJ, Murdock PR, Watson JM, Faull RL, Waldvogel HJ, et al. GPR105, a novel Gi/o-coupled UDP-glucose receptor expressed on brain glia and peripheral immune cells, is regulated by immunologic challenge: possible role in neuroimmune function. Brain Res Mol Brain Res. 2003;118(1–2):10–23. doi: 10.1016/S0169-328X(03)00330-9. [DOI] [PubMed] [Google Scholar]
- 40.Meshki J, Tuluc F, Bredetean O, Ding Z, Kunapuli SP. Molecular mechanism of nucleotide-induced primary granule release in human neutrophils: role for the P2Y2 receptor. Am J Physiol Cell Physiol. 2004;286(2):C264–C271. doi: 10.1152/ajpcell.00287.2003. [DOI] [PubMed] [Google Scholar]
- 41.Scrivens M, Dickenson JM. Functional expression of the P2Y14 receptor in human neutrophils. Eur J Pharmacol. 2006;543(1–3):166–173. doi: 10.1016/j.ejphar.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 42.Ben YF, Kukulski F, Tremblay A, Sevigny J. Concomitant activation of P2Y(2) and P2Y(6) receptors on monocytes is required for TLR1/2-induced neutrophil migration by regulating IL-8 secretion. Eur J Immunol. 2009;39(10):2885–2894. doi: 10.1002/eji.200939347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lecut C, Frederix K, Johnson DM, Deroanne C, Thiry M, et al. P2X1 ion channels promote neutrophil chemotaxis through rho kinase activation. J Immunol. 2009;183(4):2801–2809. doi: 10.4049/jimmunol.0804007. [DOI] [PubMed] [Google Scholar]
- 44.Ward PA, Cunningham TW, Johnson KJ. Signal transduction events in stimulated rat neutrophils: effects of adenine nucleotides. Clin Immunol Immunopathol. 1989;50(1 Pt 1):30–41. doi: 10.1016/0090-1229(89)90219-5. [DOI] [PubMed] [Google Scholar]
- 45.Cowen DS, Lazarus HM, Shurin SB, Stoll SE, Dubyak GR. Extracellular adenosine triphosphate activates calcium mobilization in human phagocytic leukocytes and neutrophil/monocyte progenitor cells. J Clin Invest. 1989;83(5):1651–1660. doi: 10.1172/JCI114064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dubyak GR, Cowen DS, Lazarus HM (1988) Activation of the inositol phospholipid signaling system by receptors for extracellular ATP in human neutrophils, monocytes, and neutrophil/monocyte progenitor cells. Ann N Y Acad Sci 551:218–237 [DOI] [PubMed]
- 47.Kuhns DB, Wright DG, Nath J, Kaplan SS, Basford RE. ATP induces transient elevations of [Ca2+]i in human neutrophils and primes these cells for enhanced O2− generation. Lab Investig. 1988;58(4):448–453. [PubMed] [Google Scholar]
- 48.Merritt JE, Moores KE. Human neutrophils have a novel purinergic P2-type receptor linked to calcium mobilization. Cell Signal. 1991;3(3):243–249. doi: 10.1016/0898-6568(91)90050-5. [DOI] [PubMed] [Google Scholar]
- 49.Krause KH, Campbell KP, Welsh MJ, Lew DP. The calcium signal and neutrophil activation. Clin Biochem. 1990;23(2):159–166. doi: 10.1016/0009-9120(90)80030-M. [DOI] [PubMed] [Google Scholar]
- 50.Meshki J, Tuluc F, Bredetean O, Garcia A, Kunapuli SP. Signaling pathways downstream of P2 receptors in human neutrophils. Purinergic Signal. 2006;2(3):537–544. doi: 10.1007/s11302-006-9007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Y, Yao Y, Sumi Y, Li A, To UK, et al. Purinergic signaling: a fundamental mechanism in neutrophil activation. Sci Signal. 2010;3(125):ra45. doi: 10.1126/scisignal.2000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verghese MW, Kneisler TB, Boucheron JA. P2U agonists induce chemotaxis and actin polymerization in human neutrophils and differentiated HL60 cells. J Biol Chem. 1996;271(26):15597–15601. doi: 10.1074/jbc.271.26.15597. [DOI] [PubMed] [Google Scholar]
- 53.Becker EL, Kermode JC, Naccache PH, Yassin R, Marsh ML, et al. The inhibition of neutrophil granule enzyme secretion and chemotaxis by pertussis toxin. J Cell Biol. 1985;100(5):1641–1646. doi: 10.1083/jcb.100.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Junger WG. Purinergic regulation of neutrophil chemotaxis. Cell Mol Life Sci. 2008;65(16):2528–2540. doi: 10.1007/s00018-008-8095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006;314(5806):1792–1795. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 56.Inoue Y, Chen Y, Hirsh MI, Yip L, Junger WG. A3 and P2Y2 receptors control the recruitment of neutrophils to the lungs in a mouse model of sepsis. Shock. 2008;30(2):173–177. doi: 10.1097/shk.0b013e318160dad4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kukulski F, Ben YF, Lecka J, Kauffenstein G, Levesque SA, et al. Extracellular ATP and P2 receptors are required for IL-8 to induce neutrophil migration. Cytokine. 2009;46(2):166–170. doi: 10.1016/j.cyto.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kukulski F, Bahrami F, Ben YF, Lecka J, Martin-Satue M, et al. NTPDase1 controls IL-8 production by human neutrophils. J Immunol. 2011;187(2):644–653. doi: 10.4049/jimmunol.1002680. [DOI] [PubMed] [Google Scholar]
- 59.Corriden R, Chen Y, Inoue Y, Beldi G, Robson SC, et al. Ecto-nucleoside triphosphate diphosphohydrolase 1 (E-NTPDase1/CD39) regulates neutrophil chemotaxis by hydrolyzing released ATP to adenosine. J Biol Chem. 2008;283(42):28480–28486. doi: 10.1074/jbc.M800039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kukulski F, Ben YF, Lefebvre J, Warny M, Tessier PA, et al. Extracellular nucleotides mediate LPS-induced neutrophil migration in vitro and in vivo. J Leukoc Biol. 2007;81(5):1269–1275. doi: 10.1189/jlb.1206758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kukulski F, Ben YF, Bahrami F, Fausther M, Tremblay A, et al. Endothelial P2Y2 receptor regulates LPS-induced neutrophil transendothelial migration in vitro. Mol Immunol. 2010;47(5):991–999. doi: 10.1016/j.molimm.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kawamura H, Kawamura T, Kanda Y, Kobayashi T, Abo T. Extracellular ATP-stimulated macrophages produce macrophage inflammatory protein-2 which is important for neutrophil migration. Immunology. 2012;136(4):448–458. doi: 10.1111/j.1365-2567.2012.03601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu DJ, Grinstein S. ATP and guanine nucleotide dependence of neutrophil activation. Evidence for the involvement of two distinct GTP-binding proteins. J Biol Chem. 1990;265(23):13721–13729. [PubMed] [Google Scholar]
- 64.Eltzschig HK, Eckle T, Mager A, Kuper N, Karcher C, et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99(10):1100–1108. doi: 10.1161/01.RES.0000250174.31269.70. [DOI] [PubMed] [Google Scholar]
- 65.Underhill DM, Goodridge HS. Information processing during phagocytosis. Nat Rev Immunol. 2012;12(7):492–502. doi: 10.1038/nri3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cohn ZA, Parks E. The regulation of pinocytosis in mouse macrophages. 3. The induction of vesicle formation by nucleosides and nucleotides. J Exp Med. 1967;125(3):457–466. doi: 10.1084/jem.125.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buisman HP, Steinberg TH, Fischbarg J, Silverstein SC, Vogelzang SA, et al. Extracellular ATP induces a large nonselective conductance in macrophage plasma membranes. Proc Natl Acad Sci U S A. 1988;85(21):7988–7992. doi: 10.1073/pnas.85.21.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Greenberg S, Di Virgilio F, Steinberg TH, Silverstein SC. Extracellular nucleotides mediate Ca2+ fluxes in J774 macrophages by two distinct mechanisms. J Biol Chem. 1988;263(21):10337–10343. [PubMed] [Google Scholar]
- 70.Steinberg TH, Silverstein SC. Extracellular ATP4− promotes cation fluxes in the J774 mouse macrophage cell line. J Biol Chem. 1987;262(7):3118–3122. [PubMed] [Google Scholar]
- 71.Sung SS, Young JD, Origlio AM, Heiple JM, Kaback HR, et al. Extracellular ATP perturbs transmembrane ion fluxes, elevates cytosolic [Ca2+], and inhibits phagocytosis in mouse macrophages. J Biol Chem. 1985;260(25):13442–13449. [PubMed] [Google Scholar]
- 72.Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272(5262):735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 73.Falzoni S, Munerati M, Ferrari D, Spisani S, Moretti S, et al. The purinergic P2Z receptor of human macrophage cells. Characterization and possible physiological role. J Clin Invest. 1995;95(3):1207–1216. doi: 10.1172/JCI117770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hickman SE, el KJ, Greenberg S, Schieren I, Silverstein SC. P2Z adenosine triphosphate receptor activity in cultured human monocyte-derived macrophages. Blood. 1994;84(8):2452–2456. [PubMed] [Google Scholar]
- 75.Bowler JW, Bailey RJ, North RA, Surprenant A. P2X4, P2Y1 and P2Y2 receptors on rat alveolar macrophages. Br J Pharmacol. 2003;140(3):567–575. doi: 10.1038/sj.bjp.0705459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Coutinho-Silva R, Ojcius DM, Gorecki DC, Persechini PM, Bisaggio RC, et al. Multiple P2X and P2Y receptor subtypes in mouse J774, spleen and peritoneal macrophages. Biochem Pharmacol. 2005;69(4):641–655. doi: 10.1016/j.bcp.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 77.Bar I, Guns PJ, Metallo J, Cammarata D, Wilkin F, et al. Knockout mice reveal a role for P2Y6 receptor in macrophages, endothelial cells, and vascular smooth muscle cells. Mol Pharmacol. 2008;74(3):777–784. doi: 10.1124/mol.108.046904. [DOI] [PubMed] [Google Scholar]
- 78.Myrtek D, Muller T, Geyer V, Derr N, Ferrari D, et al. Activation of human alveolar macrophages via P2 receptors: coupling to intracellular Ca2+ increases and cytokine secretion. J Immunol. 2008;181(3):2181–2188. doi: 10.4049/jimmunol.181.3.2181. [DOI] [PubMed] [Google Scholar]
- 79.Fujiwara N, Kobayashi K. Macrophages in inflammation. Curr Drug Targets Inflamm Allergy. 2005;4(3):281–286. doi: 10.2174/1568010054022024. [DOI] [PubMed] [Google Scholar]
- 80.Tonetti M, Sturla L, Bistolfi T, Benatti U, De Flora A. Extracellular ATP potentiates nitric oxide synthase expression induced by lipopolysaccharide in RAW 264.7 murine macrophages. Biochem Biophys Res Commun. 1994;203(1):430–435. doi: 10.1006/bbrc.1994.2200. [DOI] [PubMed] [Google Scholar]
- 81.Tonetti M, Sturla L, Giovine M, Benatti U, De Flora A. Extracellular ATP enhances mRNA levels of nitric oxide synthase and TNF-alpha in lipopolysaccharide-treated RAW 264.7 murine macrophages. Biochem Biophys Res Commun. 1995;214(1):125–130. doi: 10.1006/bbrc.1995.2265. [DOI] [PubMed] [Google Scholar]
- 82.Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269(21):15195–15203. [PubMed] [Google Scholar]
- 83.Lemaire I, Leduc N. Purinergic P2X7 receptor function in lung alveolar macrophages: pharmacologic characterization and bidirectional regulation by Th1 and Th2 cytokines. Drug Dev Res. 2003;59(1):118–127. doi: 10.1002/ddr.10209. [DOI] [Google Scholar]
- 84.el-Moatassim C, Dubyak GR. Dissociation of the pore-forming and phospholipase D activities stimulated via P2z purinergic receptors in BAC1.2F5 macrophages. Product inhibition of phospholipase D enzyme activity. J Biol Chem. 1993;268(21):15571–15578. [PubMed] [Google Scholar]
- 85.Gudipaty L, Munetz J, Verhoef PA, Dubyak GR. Essential role for Ca2+ in regulation of IL-1beta secretion by P2X7 nucleotide receptor in monocytes, macrophages, and HEK-293 cells. Am J Physiol Cell Physiol. 2003;285(2):C286–C299. doi: 10.1152/ajpcell.00070.2003. [DOI] [PubMed] [Google Scholar]
- 86.Perregaux DG, Laliberte RE, Gabel CA. Human monocyte interleukin-1beta posttranslational processing. Evidence of a volume-regulated response. J Biol Chem. 1996;271(47):29830–29838. doi: 10.1074/jbc.271.47.29830. [DOI] [PubMed] [Google Scholar]
- 87.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, et al. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor of human macrophages. J Immunol. 1997;159(3):1451–1458. [PubMed] [Google Scholar]
- 88.Pelegrin P, Barroso-Gutierrez C, Surprenant A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol. 2008;180(11):7147–7157. doi: 10.4049/jimmunol.180.11.7147. [DOI] [PubMed] [Google Scholar]
- 89.Verhoef PA, Kertesy SB, Lundberg K, Kahlenberg JM, Dubyak GR. Inhibitory effects of chloride on the activation of caspase-1, IL-1beta secretion, and cytolysis by the P2X7 receptor. J Immunol. 2005;175(11):7623–7634. doi: 10.4049/jimmunol.175.11.7623. [DOI] [PubMed] [Google Scholar]
- 90.Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440(7081):228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 91.Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28(9):465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 92.Babelova A, Moreth K, Tsalastra-Greul W, Zeng-Brouwers J, Eickelberg O, et al. Biglycan, a danger signal that activates the NLRP3 inflammasome via toll-like and P2X receptors. J Biol Chem. 2009;284(36):24035–24048. doi: 10.1074/jbc.M109.014266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Niemi K, Teirila L, Lappalainen J, Rajamaki K, Baumann MH, et al. Serum amyloid A activates the NLRP3 inflammasome via P2X7 receptor and a cathepsin B-sensitive pathway. J Immunol. 2011;186(11):6119–6128. doi: 10.4049/jimmunol.1002843. [DOI] [PubMed] [Google Scholar]
- 94.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, et al. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14(9):1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 95.Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282(26):18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- 96.Cruz CM, Rinna A, Forman HJ, Ventura AL, Persechini PM, et al. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J Biol Chem. 2007;282(5):2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sekiyama A, Ueda H, Kashiwamura S, Sekiyama R, Takeda M, et al. A stress-induced, superoxide-mediated caspase-1 activation pathway causes plasma IL-18 upregulation. Immunity. 2005;22(6):669–677. doi: 10.1016/j.immuni.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 98.Chiozzi P, Murgia M, Falzoni S, Ferrari D, Di Virgilio F. Role of the purinergic P2Z receptor in spontaneous cell death in J774 macrophage cultures. Biochem Biophys Res Commun. 1996;218(1):176–181. doi: 10.1006/bbrc.1996.0031. [DOI] [PubMed] [Google Scholar]
- 99.Le Feuvre RA, Brough D, Iwakura Y, Takeda K, Rothwell NJ. Priming of macrophages with lipopolysaccharide potentiates P2X7-mediated cell death via a caspase-1-dependent mechanism, independently of cytokine production. J Biol Chem. 2002;277(5):3210–3218. doi: 10.1074/jbc.M104388200. [DOI] [PubMed] [Google Scholar]
- 100.Biswas D, Qureshi OS, Lee WY, Croudace JE, Mura M et al. (2008) ATP-induced autophagy is associated with rapid killing of intracellular mycobacteria within human monocytes/macrophages. BMC Immunol 9:35 [DOI] [PMC free article] [PubMed]
- 101.Ernst RK, Guina T, Miller SI. How intracellular bacteria survive: surface modifications that promote resistance to host innate immune responses. J Infect Dis. 1999;179(Suppl 2):S326–S330. doi: 10.1086/513850. [DOI] [PubMed] [Google Scholar]
- 102.Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, et al. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z(P2X7) receptors. Immunity. 1997;7(3):433–444. doi: 10.1016/S1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- 103.Punj V, Zaborina O, Dhiman N, Falzari K, Bagdasarian M, et al. Phagocytic cell killing mediated by secreted cytotoxic factors of Vibrio cholerae. Infect Immun. 2000;68(9):4930–4937. doi: 10.1128/IAI.68.9.4930-4937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zaborina O, Misra N, Kostal J, Kamath S, Kapatral V, et al. P2Z-independent and P2Z receptor-mediated macrophage killing by Pseudomonas aeruginosa isolated from cystic fibrosis patients. Infect Immun. 1999;67(10):5231–5242. doi: 10.1128/iai.67.10.5231-5242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Coutinho-Silva R, Stahl L, Raymond MN, Jungas T, Verbeke P, et al. Inhibition of chlamydial infectious activity due to P2X7R-dependent phospholipase D activation. Immunity. 2003;19(3):403–412. doi: 10.1016/S1074-7613(03)00235-8. [DOI] [PubMed] [Google Scholar]
- 106.Coutinho-Silva R, da Monteiro CC, Persechini PM, Ojcius DM. The role of P2 receptors in controlling infections by intracellular pathogens. Purinergic Signal. 2007;3(1–2):83–90. doi: 10.1007/s11302-006-9039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Coutinho-Silva R, Correa G, Sater AA, Ojcius DM. The P2X(7) receptor and intracellular pathogens: a continuing struggle. Purinergic Signal. 2009;5(2):197–204. doi: 10.1007/s11302-009-9130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kawano A, Tsukimoto M, Mori D, Noguchi T, Harada H, et al. Regulation of P2X7-dependent inflammatory functions by P2X4 receptor in mouse macrophages. Biochem Biophys Res Commun. 2012;420(1):102–107. doi: 10.1016/j.bbrc.2012.02.122. [DOI] [PubMed] [Google Scholar]
- 109.Kawano A, Tsukimoto M, Noguchi T, Hotta N, Harada H, et al. Involvement of P2X4 receptor in P2X7 receptor-dependent cell death of mouse macrophages. Biochem Biophys Res Commun. 2012;419(2):374–380. doi: 10.1016/j.bbrc.2012.01.156. [DOI] [PubMed] [Google Scholar]
- 110.Levesque SA, Kukulski F, Enjyoji K, Robson SC, Sevigny J. NTPDase1 governs P2X7-dependent functions in murine macrophages. Eur J Immunol. 2010;40(5):1473–1485. doi: 10.1002/eji.200939741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol. 2007;179(3):1913–1925. doi: 10.4049/jimmunol.179.3.1913. [DOI] [PubMed] [Google Scholar]
- 112.Lopez-Castejon G, Theaker J, Pelegrin P, Clifton AD, Braddock M, et al. P2X(7) receptor-mediated release of cathepsins from macrophages is a cytokine-independent mechanism potentially involved in joint diseases. J Immunol. 2010;185(4):2611–2619. doi: 10.4049/jimmunol.1000436. [DOI] [PubMed] [Google Scholar]
- 113.Moore SF, MacKenzie AB. Murine macrophage P2X7 receptors support rapid prothrombotic responses. Cell Signal. 2007;19(4):855–866. doi: 10.1016/j.cellsig.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 114.Gu BJ, Wiley JS. Rapid ATP-induced release of matrix metalloproteinase 9 is mediated by the P2X7 receptor. Blood. 2006;107(12):4946–4953. doi: 10.1182/blood-2005-07-2994. [DOI] [PubMed] [Google Scholar]
- 115.Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature. 2009;461(7261):282–286. doi: 10.1038/nature08296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McCloskey MA, Fan Y, Luther S. Chemotaxis of rat mast cells toward adenine nucleotides. J Immunol. 1999;163(2):970–977. [PubMed] [Google Scholar]
- 117.Kronlage M, Song J, Sorokin L, Isfort K, Schwerdtle T, et al. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci Signal. 2010;3(132):ra55. doi: 10.1126/scisignal.2000588. [DOI] [PubMed] [Google Scholar]
- 118.Isfort K, Ebert F, Bornhorst J, Sargin S, Kardakaris R, et al. Real-time imaging reveals that P2Y2 and P2Y12 receptor agonists are not chemoattractants and macrophage chemotaxis to complement C5a is phosphatidylinositol 3-kinase (PI3K)- and p38 mitogen-activated protein kinase (MAPK)-independent. J Biol Chem. 2011;286(52):44776–44787. doi: 10.1074/jbc.M111.289793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marques-da-Silva C, Burnstock G, Ojcius DM, Coutinho-Silva R. Purinergic receptor agonists modulate phagocytosis and clearance of apoptotic cells in macrophages. Immunobiology. 2011;216(1–2):1–11. doi: 10.1016/j.imbio.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 120.Chiozzi P, Sanz JM, Ferrari D, Falzoni S, Aleotti A, et al. Spontaneous cell fusion in macrophage cultures expressing high levels of the P2Z/P2X7 receptor. J Cell Biol. 1997;138(3):697–706. doi: 10.1083/jcb.138.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lemaire I, Falzoni S, Leduc N, Zhang B, Pellegatti P, et al. Involvement of the purinergic P2X7 receptor in the formation of multinucleated giant cells. J Immunol. 2006;177(10):7257–7265. doi: 10.4049/jimmunol.177.10.7257. [DOI] [PubMed] [Google Scholar]
- 122.Summers dL, Gommerman JL. Fine-tuning of dendritic cell biology by the TNF superfamily. Nat Rev Immunol. 2012;12(5):339–351. doi: 10.1038/nri3193. [DOI] [PubMed] [Google Scholar]
- 123.Berchtold S, Ogilvie AL, Bogdan C, Muhl-Zurbes P, Ogilvie A, et al. Human monocyte derived dendritic cells express functional P2X and P2Y receptors as well as ecto-nucleotidases. FEBS Lett. 1999;458(3):424–428. doi: 10.1016/S0014-5793(99)01197-7. [DOI] [PubMed] [Google Scholar]
- 124.Ferrari D, la Sala A, Chiozzi P, Morelli A, Falzoni S, et al. The P2 purinergic receptors of human dendritic cells: identification and coupling to cytokine release. FASEB J. 2000;14(15):2466–2476. doi: 10.1096/fj.00-0031com. [DOI] [PubMed] [Google Scholar]
- 125.Schnurr M, Toy T, Stoitzner P, Cameron P, Shin A, et al. ATP gradients inhibit the migratory capacity of specific human dendritic cell types: implications for P2Y11 receptor signaling. Blood. 2003;102(2):613–620. doi: 10.1182/blood-2002-12-3745. [DOI] [PubMed] [Google Scholar]
- 126.Skelton L, Cooper M, Murphy M, Platt A. Human immature monocyte-derived dendritic cells express the G protein-coupled receptor GPR105 (KIAA0001, P2Y14) and increase intracellular calcium in response to its agonist, uridine diphosphoglucose. J Immunol. 2003;171(4):1941–1949. doi: 10.4049/jimmunol.171.4.1941. [DOI] [PubMed] [Google Scholar]
- 127.Buell G, Chessell IP, Michel AD, Collo G, Salazzo M, et al. Blockade of human P2X7 receptor function with a monoclonal antibody. Blood. 1998;92(10):3521–3528. [PubMed] [Google Scholar]
- 128.Wilkin F, Duhant X, Bruyns C, Suarez-Huerta N, Boeynaems JM, et al. The P2Y11 receptor mediates the ATP-induced maturation of human monocyte-derived dendritic cells. J Immunol. 2001;166(12):7172–7177. doi: 10.4049/jimmunol.166.12.7172. [DOI] [PubMed] [Google Scholar]
- 129.la Sala A, Ferrari D, Corinti S, Cavani A, Di Virgilio F, et al. Extracellular ATP induces a distorted maturation of dendritic cells and inhibits their capacity to initiate Th1 responses. J Immunol. 2001;166(3):1611–1617. doi: 10.4049/jimmunol.166.3.1611. [DOI] [PubMed] [Google Scholar]
- 130.Wilkin F, Stordeur P, Goldman M, Boeynaems JM, Robaye B. Extracellular adenine nucleotides modulate cytokine production by human monocyte-derived dendritic cells: dual effect on IL-12 and stimulation of IL-10. Eur J Immunol. 2002;32(9):2409–2417. doi: 10.1002/1521-4141(200209)32:9<2409::AID-IMMU2409>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 131.Marteau F, Communi D, Boeynaems JM, Suarez GN. Involvement of multiple P2Y receptors and signaling pathways in the action of adenine nucleotides diphosphates on human monocyte-derived dendritic cells. J Leukoc Biol. 2004;76(4):796–803. doi: 10.1189/jlb.0104032. [DOI] [PubMed] [Google Scholar]
- 132.Schnurr M, Toy T, Shin A, Wagner M, Cebon J, et al. Extracellular nucleotide signaling by P2 receptors inhibits IL-12 and enhances IL-23 expression in human dendritic cells: a novel role for the cAMP pathway. Blood. 2005;105(4):1582–1589. doi: 10.1182/blood-2004-05-1718. [DOI] [PubMed] [Google Scholar]
- 133.Schnurr M, Then F, Galambos P, Scholz C, Siegmund B, et al. Extracellular ATP and TNF-alpha synergize in the activation and maturation of human dendritic cells. J Immunol. 2000;165(8):4704–4709. doi: 10.4049/jimmunol.165.8.4704. [DOI] [PubMed] [Google Scholar]
- 134.Ben AA, Cammarata D, Conley PB, Boeynaems JM, Robaye B. Role of the P2Y12 receptor in the modulation of murine dendritic cell function by ADP. J Immunol. 2010;185(10):5900–5906. doi: 10.4049/jimmunol.0901799. [DOI] [PubMed] [Google Scholar]
- 135.Liu QH, Bohlen H, Titzer S, Christensen O, Diehl V, et al. Expression and a role of functionally coupled P2Y receptors in human dendritic cells. FEBS Lett. 1999;445(2–3):402–408. doi: 10.1016/S0014-5793(99)00161-1. [DOI] [PubMed] [Google Scholar]
- 136.Coutinho-Silva R, Persechini PM, Bisaggio RD, Perfettini JL, Neto AC, et al. P2Z/P2X7 receptor-dependent apoptosis of dendritic cells. Am J Physiol. 1999;276(5 Pt 1):C1139–C1147. doi: 10.1152/ajpcell.1999.276.5.C1139. [DOI] [PubMed] [Google Scholar]
- 137.Nihei OK, de Carvalho AC, Savino W, Alves LA. Pharmacologic properties of P(2Z)/P2X(7)receptor characterized in murine dendritic cells: role on the induction of apoptosis. Blood. 2000;96(3):996–1005. [PubMed] [Google Scholar]
- 138.Girolomoni G, Santantonio ML, Pastore S, Bergstresser PR, Giannetti A, et al. Epidermal Langerhans cells are resistant to the permeabilizing effects of extracellular ATP: in vitro evidence supporting a protective role of membrane ATPase. J Investig Dermatol. 1993;100(3):282–287. doi: 10.1111/1523-1747.ep12469769. [DOI] [PubMed] [Google Scholar]
- 139.Idzko M, Dichmann S, Ferrari D, Di Virgilio F, la Sala A, et al. Nucleotides induce chemotaxis and actin polymerization in immature but not mature human dendritic cells via activation of pertussis toxin-sensitive P2y receptors. Blood. 2002;100(3):925–932. doi: 10.1182/blood.V100.3.925. [DOI] [PubMed] [Google Scholar]
- 140.la Sala A, Sebastiani S, Ferrari D, Di Virgilio F, Idzko M, et al. Dendritic cells exposed to extracellular adenosine triphosphate acquire the migratory properties of mature cells and show a reduced capacity to attract type 1T lymphocytes. Blood. 2002;99(5):1715–1722. doi: 10.1182/blood.V99.5.1715. [DOI] [PubMed] [Google Scholar]
- 141.Muller T, Robaye B, Vieira RP, Ferrari D, Grimm M, et al. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy. 2010;65(12):1545–1553. doi: 10.1111/j.1398-9995.2010.02426.x. [DOI] [PubMed] [Google Scholar]
- 142.Idzko M, Hammad H, van NM, Kool M, Willart MA, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13(8):913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 143.Manthei DM, Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, et al. Protection from asthma in a high-risk birth cohort by attenuated P2X(7) function. J Allergy Clin Immunol. 2012;130(2):496–502. doi: 10.1016/j.jaci.2012.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Islam SA, Luster AD. T cell homing to epithelial barriers in allergic disease. Nat Med. 2012;18(5):705–715. doi: 10.1038/nm.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9(1):15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- 146.Vivier E, Ugolini S, Blaise D, Chabannon C, Brossay L. Targeting natural killer cells and natural killer T cells in cancer. Nat Rev Immunol. 2012;12(4):239–252. doi: 10.1038/nri3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Baricordi OR, Ferrari D, Melchiorri L, Chiozzi P, Hanau S, et al. An ATP-activated channel is involved in mitogenic stimulation of human T lymphocytes. Blood. 1996;87(2):682–690. [PubMed] [Google Scholar]
- 148.Wiley JS, Dubyak GR. Extracellular adenosine triphosphate increases cation permeability of chronic lymphocytic leukemic lymphocytes. Blood. 1989;73(5):1316–1323. [PubMed] [Google Scholar]
- 149.Padeh S, Cohen A, Roifman CM. ATP-induced activation of human B lymphocytes via P2-purinoceptors. J Immunol. 1991;146(5):1626–1632. [PubMed] [Google Scholar]
- 150.Conigrave AD, Fernando KC, Gu B, Tasevski V, Zhang W, et al. P2Y(11) receptor expression by human lymphocytes: evidence for two cAMP-linked purinoceptors. Eur J Pharmacol. 2001;426(3):157–163. doi: 10.1016/S0014-2999(01)01222-5. [DOI] [PubMed] [Google Scholar]
- 151.Lee DH, Park KS, Kong ID, Kim JW, Han BG (2006) Expression of P2 receptors in human B cells and Epstein-Barr virus-transformed lymphoblastoid cell lines. BMC Immunol 7:22 [DOI] [PMC free article] [PubMed]
- 152.Sluyter R, Barden JA, Wiley JS. Detection of P2X purinergic receptors on human B lymphocytes. Cell Tissue Res. 2001;304(2):231–236. doi: 10.1007/s004410100372. [DOI] [PubMed] [Google Scholar]
- 153.Wang L, Jacobsen SE, Bengtsson A, Erlinge D (2004) P2 receptor mRNA expression profiles in human lymphocytes, monocytes and CD34+ stem and progenitor cells. BMC Immunol 5:16 [DOI] [PMC free article] [PubMed]
- 154.Scrivens M, Dickenson JM. Functional expression of the P2Y14 receptor in murine T-lymphocytes. Br J Pharmacol. 2005;146(3):435–444. doi: 10.1038/sj.bjp.0706322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Duhant X, Schandene L, Bruyns C, Gonzalez NS, Goldman M, et al. Extracellular adenine nucleotides inhibit the activation of human CD4+ T lymphocytes. J Immunol. 2002;169(1):15–21. doi: 10.4049/jimmunol.169.1.15. [DOI] [PubMed] [Google Scholar]
- 156.Duhant X, Suarez GN, Schandene L, Goldman M, Communi D, et al. Molecular mechanisms of extracellular adenine nucleotides-mediated inhibition of human Cd4(+) T lymphocytes activation. Purinergic Signal. 2005;1(4):377–381. doi: 10.1007/s11302-005-8077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.el-Moatassim C, Dornand J, Mani JC. Extracellular ATP increases cytosolic free calcium in thymocytes and initiates the blastogenesis of the phorbol 12-myristate 13-acetate-treated medullary population. Biochim Biophys Acta. 1987;927(3):437–444. doi: 10.1016/0167-4889(87)90110-8. [DOI] [PubMed] [Google Scholar]
- 158.Fishman RF, Rubin AL, Novogrodsky A, Stenzel KH. Selective suppression of blastogenesis induced by different mitogens: effect of noncyclic adenosine-containing compounds. Cell Immunol. 1980;54(1):129–139. doi: 10.1016/0008-8749(80)90196-3. [DOI] [PubMed] [Google Scholar]
- 159.Gregory S, Kern M. Adenosine and adenine nucleotides are mitogenic for mouse thymocytes. Biochem Biophys Res Commun. 1978;83(3):1111–1116. doi: 10.1016/0006-291X(78)91510-3. [DOI] [PubMed] [Google Scholar]
- 160.Huang N, Wang DJ, Heppel LA. Extracellular ATP is a mitogen for 3T3, 3T6, and A431 cells and acts synergistically with other growth factors. Proc Natl Acad Sci U S A. 1989;86(20):7904–7908. doi: 10.1073/pnas.86.20.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ikehara S, Pahwa RN, Lunzer DG, Good RA, Modak MJ. Adenosine-5′-triphosphate-(ATP) mediated stimulation and suppression of DNA synthesis in lymphoid cells. I. Characterization of ATP responsive cells in mouse lymphoid organs. J Immunol. 1981;127(5):1834–1838. [PubMed] [Google Scholar]
- 162.Barankiewicz J, Dosch HM, Cohen A. Extracellular nucleotide catabolism in human B and T lymphocytes. The source of adenosine production. J Biol Chem. 1988;263(15):7094–7098. [PubMed] [Google Scholar]
- 163.Corriden R, Insel PA, Junger WG. A novel method using fluorescence microscopy for real-time assessment of ATP release from individual cells. Am J Physiol Cell Physiol. 2007;293(4):C1420–C1425. doi: 10.1152/ajpcell.00271.2007. [DOI] [PubMed] [Google Scholar]
- 164.Loomis WH, Namiki S, Ostrom RS, Insel PA, Junger WG. Hypertonic stress increases T cell interleukin-2 expression through a mechanism that involves ATP release, P2 receptor, and p38 MAPK activation. J Biol Chem. 2003;278(7):4590–4596. doi: 10.1074/jbc.M207868200. [DOI] [PubMed] [Google Scholar]
- 165.Yip L, Cheung CW, Corriden R, Chen Y, Insel PA, et al. Hypertonic stress regulates T-cell function by the opposing actions of extracellular adenosine triphosphate and adenosine. Shock. 2007;27(3):242–250. doi: 10.1097/01.shk.0000245014.96419.3a. [DOI] [PubMed] [Google Scholar]
- 166.Yip L, Woehrle T, Corriden R, Hirsh M, Chen Y, et al. Autocrine regulation of T-cell activation by ATP release and P2X7 receptors. FASEB J. 2009;23(6):1685–1693. doi: 10.1096/fj.08-126458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schenk U, Westendorf AM, Radaelli E, Casati A, Ferro M, et al. Purinergic control of T cell activation by ATP released through pannexin-1 hemichannels. Sci Signal. 2008;1(39):ra6. doi: 10.1126/scisignal.1160583. [DOI] [PubMed] [Google Scholar]
- 168.Woehrle T, Yip L, Manohar M, Sumi Y, Yao Y, et al. Hypertonic stress regulates T cell function via pannexin-1 hemichannels and P2X receptors. J Leukoc Biol. 2010;88(6):1181–1189. doi: 10.1189/jlb.0410211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schenk U, Frascoli M, Proietti M, Geffers R, Traggiai E, et al. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci Signal. 2011;4(162):ra12. doi: 10.1126/scisignal.2001270. [DOI] [PubMed] [Google Scholar]
- 170.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110(4):1225–1232. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 171.Wolberg G, Zimmerman TP, Hiemstra K, Winston M, Chu LC. Adenosine inhibition of lymphocyte-mediated cytolysis: possible role of cyclic adenosine monophosphate. Science. 1975;187(4180):957–959. doi: 10.1126/science.167434. [DOI] [PubMed] [Google Scholar]
- 172.Wolberg G, Zimmerman TP, Duncan GS, Singer KH, Elion GB. Inhibition of lymphocyte-mediated cytolysis by adenosine analogs. Biochemical studies concerning mechanism of action. Biochem Pharmacol. 1978;27(10):1487–1495. doi: 10.1016/0006-2952(78)90105-3. [DOI] [PubMed] [Google Scholar]
- 173.Blanchard DK, Wei S, Duan C, Pericle F, Diaz JI, et al. Role of extracellular adenosine triphosphate in the cytotoxic T-lymphocyte-mediated lysis of antigen presenting cells. Blood. 1995;85(11):3173–3182. [PubMed] [Google Scholar]
- 174.Filippini A, Taffs RE, Sitkovsky MV. Extracellular ATP in T-lymphocyte activation: possible role in effector functions. Proc Natl Acad Sci U S A. 1990;87(21):8267–8271. doi: 10.1073/pnas.87.21.8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tsukimoto M, Maehata M, Harada H, Ikari A, Takagi K, et al. P2X7 receptor-dependent cell death is modulated during murine T cell maturation and mediated by dual signaling pathways. J Immunol. 2006;177(5):2842–2850. doi: 10.4049/jimmunol.177.5.2842. [DOI] [PubMed] [Google Scholar]
- 176.Aswad F, Dennert G. P2X7 receptor expression levels determine lethal effects of a purine based danger signal in T lymphocytes. Cell Immunol. 2006;243(1):58–65. doi: 10.1016/j.cellimm.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Aswad F, Kawamura H, Dennert G. High sensitivity of CD4+ CD25+ regulatory T cells to extracellular metabolites nicotinamide adenine dinucleotide and ATP: a role for P2X7 receptors. J Immunol. 2005;175(5):3075–3083. doi: 10.4049/jimmunol.175.5.3075. [DOI] [PubMed] [Google Scholar]
- 178.Jamieson GP, Snook MB, Thurlow PJ, Wiley JS. Extracellular ATP causes of loss of l-selectin from human lymphocytes via occupancy of P2Z purinocepters. J Cell Physiol. 1996;166(3):637–642. doi: 10.1002/(SICI)1097-4652(199603)166:3<637::AID-JCP19>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 179.Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, et al. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168(12):6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 180.Smalley DM, Ley K. l-selectin: mechanisms and physiological significance of ectodomain cleavage. J Cell Mol Med. 2005;9(2):255–266. doi: 10.1111/j.1582-4934.2005.tb00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Baricordi OR, Melchiorri L, Adinolfi E, Falzoni S, Chiozzi P, et al. Increased proliferation rate of lymphoid cells transfected with the P2X(7) ATP receptor. J Biol Chem. 1999;274(47):33206–33208. doi: 10.1074/jbc.274.47.33206. [DOI] [PubMed] [Google Scholar]
- 182.Adinolfi E, Callegari MG, Ferrari D, Bolognesi C, Minelli M, et al. Basal activation of the P2X7 ATP receptor elevates mitochondrial calcium and potential, increases cellular ATP levels, and promotes serum-independent growth. Mol Biol Cell. 2005;16(7):3260–3272. doi: 10.1091/mbc.E04-11-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gorini S, Callegari G, Romagnoli G, Mammi C, Mavilio D, et al. ATP secreted by endothelial cells blocks CX(3)CL 1-elicited natural killer cell chemotaxis and cytotoxicity via P2Y(1)(1) receptor activation. Blood. 2010;116(22):4492–4500. doi: 10.1182/blood-2009-12-260828. [DOI] [PubMed] [Google Scholar]
- 184.Schmidt A, Ortaldo JR, Herberman RB. Inhibition of human natural killer cell reactivity by exogenous adenosine 5′-triphosphate. J Immunol. 1984;132(1):146–150. [PubMed] [Google Scholar]
- 185.Dombrowski KE, Cone JC, Bjorndahl JM, Phillips CA. Irreversible inhibition of human natural killer cell natural cytotoxicity by modification of the extracellular membrane by the adenine nucleotide analog 5′-p-(fluorosulfonyl)benzoyl adenosine. Cell Immunol. 1995;160(2):199–204. doi: 10.1016/0008-8749(95)80028-H. [DOI] [PubMed] [Google Scholar]
- 186.Krishnaraj R. Negative modulation of human NK cell activity by purinoceptors. 1. Effect of exogenous adenosine triphosphate. Cell Immunol. 1992;141(2):306–322. doi: 10.1016/0008-8749(92)90150-N. [DOI] [PubMed] [Google Scholar]
- 187.Miller JS, Cervenka T, Lund J, Okazaki IJ, Moss J. Purine metabolites suppress proliferation of human NK cells through a lineage-specific purine receptor. J Immunol. 1999;162(12):7376–7382. [PubMed] [Google Scholar]
- 188.Beldi G, Banz Y, Kroemer A, Sun X, Wu Y, et al. Deletion of CD39 on natural killer cells attenuates hepatic ischemia/reperfusion injury in mice. Hepatology. 2010;51(5):1702–1711. doi: 10.1002/hep.23510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, et al. Eosinophils: biological properties and role in health and disease. Clin Exp Allergy. 2008;38(5):709–750. doi: 10.1111/j.1365-2222.2008.02958.x. [DOI] [PubMed] [Google Scholar]
- 190.Burgers JA, Schweizer RC, Koenderman L, Bruijnzeel PL, Akkerman JW. Human platelets secrete chemotactic activity for eosinophils. Blood. 1993;81(1):49–55. [PubMed] [Google Scholar]
- 191.Ferrari D, Idzko M, Dichmann S, Purlis D, Virchow C, et al. P2 purinergic receptors of human eosinophils: characterization and coupling to oxygen radical production. FEBS Lett. 2000;486(3):217–224. doi: 10.1016/S0014-5793(00)02306-1. [DOI] [PubMed] [Google Scholar]
- 192.Idzko M, Dichmann S, Panther E, Ferrari D, Herouy Y, et al. Functional characterization of P2Y and P2X receptors in human eosinophils. J Cell Physiol. 2001;188(3):329–336. doi: 10.1002/jcp.1129. [DOI] [PubMed] [Google Scholar]
- 193.Vanderstocken G, Bondue B, Horckmans M, Di Pietrantonio L, Robaye B, et al. P2Y2 receptor regulates VCAM-1 membrane and soluble forms and eosinophil accumulation during lung inflammation. J Immunol. 2010;185(6):3702–3707. doi: 10.4049/jimmunol.0903908. [DOI] [PubMed] [Google Scholar]
- 194.Dichmann S, Idzko M, Zimpfer U, Hofmann C, Ferrari D, et al. Adenosine triphosphate-induced oxygen radical production and CD11b up-regulation: Ca(++) mobilization and actin reorganization in human eosinophils. Blood. 2000;95(3):973–978. [PubMed] [Google Scholar]
- 195.Saito H, Ebisawa M, Reason DC, Ohno K, Kurihara K, et al. Extracellular ATP stimulates interleukin-dependent cultured mast cells and eosinophils through calcium mobilization. Int Arch Allergy Appl Immunol. 1991;94(1–4):68–70. doi: 10.1159/000235327. [DOI] [PubMed] [Google Scholar]
- 196.Idzko M, Panther E, Bremer HC, Sorichter S, Luttmann W, et al. Stimulation of P2 purinergic receptors induces the release of eosinophil cationic protein and interleukin-8 from human eosinophils. Br J Pharmacol. 2003;138(7):1244–1250. doi: 10.1038/sj.bjp.0705145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kobayashi T, Kouzaki H, Kita H. Human eosinophils recognize endogenous danger signal crystalline uric acid and produce proinflammatory cytokines mediated by autocrine ATP. J Immunol. 2010;184(11):6350–6358. doi: 10.4049/jimmunol.0902673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Uratsuji H, Tada Y, Kawashima T, Kamata M, Hau CS, et al. P2Y6 receptor signaling pathway mediates inflammatory responses induced by monosodium urate crystals. J Immunol. 2012;188(1):436–444. doi: 10.4049/jimmunol.1003746. [DOI] [PubMed] [Google Scholar]
- 199.Jaffar ZH, Pearce FL. Some characteristics of the ATP-induced histamine release from and permeabilization of rat mast cells. Agents Actions. 1993;40(1–2):18–27. doi: 10.1007/BF01976747. [DOI] [PubMed] [Google Scholar]
- 200.Schulman ES, Glaum MC, Post T, Wang Y, Raible DG, et al. ATP modulates anti-IgE-induced release of histamine from human lung mast cells. Am J Respir Cell Mol Biol. 1999;20(3):530–537. doi: 10.1165/ajrcmb.20.3.3387. [DOI] [PubMed] [Google Scholar]
- 201.Dahlquist R, Diamant B. Further observations on ATP-induced histamine release from rat mast cells. Acta Pharmacol Toxicol (Copenh) 1970;28(1):43. [PubMed] [Google Scholar]
- 202.Dahlquist R. Relationship of uptake of sodium and 45calcium to ATP-induced histamine release from rat mast cells. Acta Pharmacol Toxicol (Copenh) 1974;35(1):11–22. doi: 10.1111/j.1600-0773.1974.tb00720.x. [DOI] [PubMed] [Google Scholar]
- 203.Diamant B (1967) The mechanism of histamine release from rat mast cells induced by adenosine triphosphate. Acta Pharmacol Toxicol (Copenh) 25(S4):33 [DOI] [PubMed]
- 204.Kruger PG, Diamant B, Dahlquist R. Morphological changes induced by ATP on rat mast cells and their relationship to histamine release. Int Arch Allergy Appl Immunol. 1974;46(5):676–688. doi: 10.1159/000231168. [DOI] [PubMed] [Google Scholar]
- 205.Bennett JP, Cockcroft S, Gomperts BD. Rat mast cells permeabilized with ATP secrete histamine in response to calcium ions buffered in the micromolar range. J Physiol. 1981;317:335–345. doi: 10.1113/jphysiol.1981.sp013828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Cockcroft S, Gomperts BD. ATP induces nucleotide permeability in rat mast cells. Nature. 1979;279(5713):541–542. doi: 10.1038/279541a0. [DOI] [PubMed] [Google Scholar]
- 207.Cockcroft S, Gomperts BD. Activation and inhibition of calcium-dependent histamine secretion by ATP ions applied to rat mast cells. J Physiol. 1979;296:229–243. doi: 10.1113/jphysiol.1979.sp013002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Peterson C. Histamine release induced by compound 48–80 from isolated rat cells: dependence on endogenous ATP. Acta Pharmacol Toxicol (Copenh) 1974;34(5):356–367. doi: 10.1111/j.1600-0773.1974.tb03532.x. [DOI] [PubMed] [Google Scholar]
- 209.Johansen T, Chakravarty N. Dependence of histamine release from rat mast cells on adenosine triphosphate. Naunyn Schmiedebergs Arch Pharmacol. 1972;275(4):457–463. doi: 10.1007/BF00501133. [DOI] [PubMed] [Google Scholar]
- 210.Johansen T. Further observations on the utilization of adenosine triphosphate in rat mast cells during histamine release induced by the ionophore A23187. Br J Pharmacol. 1980;69(4):657–662. doi: 10.1111/j.1476-5381.1980.tb07918.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Peterson C, Diamant B. Utilization of endogenous ATP during histamine release from isolated rat mast cells. Agents Actions. 1973;3(3):189–190. doi: 10.1007/BF01965746. [DOI] [PubMed] [Google Scholar]
- 212.Diamant B, Kruger PG. Histamine release from isolated rat peritoneal mast cells induced by adenosine-5′-triphosphate. Acta Physiol Scand. 1967;71(4):291–302. doi: 10.1111/j.1748-1716.1967.tb03736.x. [DOI] [PubMed] [Google Scholar]
- 213.Bradding P, Okayama Y, Kambe N, Saito H. Ion channel gene expression in human lung, skin, and cord blood-derived mast cells. J Leukoc Biol. 2003;73(5):614–620. doi: 10.1189/jlb.1202602. [DOI] [PubMed] [Google Scholar]
- 214.Wareham K, Vial C, Wykes RC, Bradding P, Seward EP. Functional evidence for the expression of P2X1, P2X4 and P2X7 receptors in human lung mast cells. Br J Pharmacol. 2009;157(7):1215–1224. doi: 10.1111/j.1476-5381.2009.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gao ZG, Ding Y, Jacobson KA. UDP-glucose acting at P2Y14 receptors is a mediator of mast cell degranulation. Biochem Pharmacol. 2010;79(6):873–879. doi: 10.1016/j.bcp.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gao ZG, Wei Q, Jayasekara MP, Jacobson KA (2012) The role of P2Y(14) and other P2Y receptors in degranulation of human LAD2 mast cells. Purinergic Signal [DOI] [PMC free article] [PubMed]
- 217.Dahlquist R, Diamant B. Interaction of ATP and calcium on the rat mast cell: effect on histamine release. Acta Pharmacol Toxicol (Copenh) 1974;34(5):368–384. doi: 10.1111/j.1600-0773.1974.tb03533.x. [DOI] [PubMed] [Google Scholar]
- 218.Qian YX, McCloskey MA. Activation of mast cell K+ channels through multiple G protein-linked receptors. Proc Natl Acad Sci U S A. 1993;90(16):7844–7848. doi: 10.1073/pnas.90.16.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Tatham PE, Lindau M. ATP-induced pore formation in the plasma membrane of rat peritoneal mast cells. J Gen Physiol. 1990;95(3):459–476. doi: 10.1085/jgp.95.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sudo N, Tanaka K, Koga Y, Okumura Y, Kubo C, et al. Extracellular ATP activates mast cells via a mechanism that is different from the activation induced by the cross-linking of Fc receptors. J Immunol. 1996;156(10):3970–3979. [PubMed] [Google Scholar]
- 221.Gao ZG, Ding Y, Jacobson KA. P2Y(13) receptor is responsible for ADP-mediated degranulation in RBL-2H3 rat mast cells. Pharmacol Res. 2010;62(6):500–505. doi: 10.1016/j.phrs.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bulanova E, Bulfone-Paus S. P2 receptor-mediated signaling in mast cell biology. Purinergic Signal. 2010;6(1):3–17. doi: 10.1007/s11302-009-9173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Osipchuk Y, Cahalan M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature. 1992;359(6392):241–244. doi: 10.1038/359241a0. [DOI] [PubMed] [Google Scholar]
- 224.Carroll WA, Donnelly-Roberts D, Jarvis MF. Selective P2X(7) receptor antagonists for chronic inflammation and pain. Purinergic Signal. 2009;5(1):63–73. doi: 10.1007/s11302-008-9110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Romagnoli R, Baraldi PG, Cruz-Lopez O, Lopez-Cara C, Preti D, et al. The P2X7 receptor as a therapeutic target. Expert Opin Ther Targets. 2008;12(5):647–661. doi: 10.1517/14728222.12.5.647. [DOI] [PubMed] [Google Scholar]
- 226.Arulkumaran N, Unwin RJ, Tam FW. A potential therapeutic role for P2X7 receptor (P2X7R) antagonists in the treatment of inflammatory diseases. Expert Opin Investig Drugs. 2011;20(7):897–915. doi: 10.1517/13543784.2011.578068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Accurso FJ, Moss RB, Wilmott RW, Anbar RD, Schaberg AE, et al. Denufosol tetrasodium in patients with cystic fibrosis and normal to mildly impaired lung function. Am J Respir Crit Care Med. 2011;183(5):627–634. doi: 10.1164/rccm.201008-1267OC. [DOI] [PubMed] [Google Scholar]
- 228.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58(1):58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- 229.Cattaneo M. Platelet P2 receptors: old and new targets for antithrombotic drugs. Expert Rev Cardiovasc Ther. 2007;5(1):45–55. doi: 10.1586/14779072.5.1.45. [DOI] [PubMed] [Google Scholar]
- 230.White N, Burnstock G. P2 receptors and cancer. Trends Pharmacol Sci. 2006;27(4):211–217. doi: 10.1016/j.tips.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 231.Di Virgilio F. Purines, purinergic receptors, and cancer. Cancer Res. 2012;72(21):5441–5447. doi: 10.1158/0008-5472.CAN-12-1600. [DOI] [PubMed] [Google Scholar]
- 232.Nakamura M, Imanaka T, Sakamoto A. Diquafosol ophthalmic solution for dry eye treatment. Adv Ther. 2012;29(7):579–589. doi: 10.1007/s12325-012-0033-9. [DOI] [PubMed] [Google Scholar]
- 233.Ford AP, Gever JR, Nunn PA, Zhong Y, Cefalu JS, et al. Purinoceptors as therapeutic targets for lower urinary tract dysfunction. Br J Pharmacol. 2006;147(Suppl 2):S132–S143. doi: 10.1038/sj.bjp.0706637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Cattaneo M. New P2Y(12) inhibitors. Circulation. 2010;121(1):171–179. doi: 10.1161/CIRCULATIONAHA.109.853069. [DOI] [PubMed] [Google Scholar]
- 235.Wartak SA, Lotfi A, Rothberg M. Ticagrelor versus clopidogrel in acute coronary syndromes. N Engl J Med. 2009;361(24):2386–2387. [PubMed] [Google Scholar]
- 236.Gum RJ, Wakefield B, Jarvis MF. P2X receptor antagonists for pain management: examination of binding and physicochemical properties. Purinergic Signal. 2012;8(Suppl 1):41–56. doi: 10.1007/s11302-011-9272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Jacobson KA, Boeynaems JM. P2Y nucleotide receptors: promise of therapeutic applications. Drug Discov Today. 2010;15(13–14):570–578. doi: 10.1016/j.drudis.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]