Abstract
Unveiling the mechanisms participating in the damage and repair of traumatic brain injury (TBI) is fundamental to develop new therapies. The P2Y-like GPR17 receptor has recently emerged as a sensor of damage and a key actor in lesion remodeling/repair in the rodent brain, but its role in humans is totally unknown. Here, we characterized GPR17 expression in brain specimens from seven intensive care unit TBI patients undergoing neurosurgery for contusion removal and from 28 autoptic TBI cases (and 10 control subjects of matched age and gender) of two university hospitals. In both neurosurgery and autoptic samples, GPR17 expression was strong inside the contused core and progressively declined distally according to a spatio-temporal gradient. Inside and around the core, GPR17 labeled dying neurons, reactive astrocytes, and activated microglia/macrophages. In peri-contused parenchyma, GPR17 decorated oligodendrocyte precursor cells (OPCs) some of which had proliferated, indicating re-myelination attempts. In autoptic cases, GPR17 expression positively correlated with death for intracranial complications and negatively correlated with patients’ post-traumatic survival. Data indicate lesion-specific sequential involvement of GPR17 in the (a) death of irreversibly damaged neurons, (b) activation of microglia/macrophages remodeling the lesion, and (c) activation/proliferation of multipotent parenchymal progenitors (both reactive astrocytes and OPCs) starting repair processes. Data validate GPR17 as a target for neurorepair and are particularly relevant to setting up new therapies for TBI patients.
Electronic supplementary material
The online version of this article (doi:10.1007/s11302-013-9366-3) contains supplementary material, which is available to authorized users.
Keywords: Activated microglia, Adult neural precursors, Human brain injury, Lesion repair, Reactive astrocytes
Introduction
After injury, the brain undergoes profound remodeling in an attempt to restore the most important functions. This process requires a complex cross-talk between damaged neurons releasing alerting signals and responding glial cells [1]. Microglia, the brain’s immunocytes, release chemokines and cytokines and phagocytize irreversibly damaged neurons to clear space and resources for recovering cells. Astrocytes become “reactive” by increased expression of glial fibrillary acidic protein (GFAP) and release trophic molecules. A third type of normally quiescent glia, the so-called NG2 cells [2], primarily known as oligodendrocyte precursor cells (OPCs), start proliferating and differentiating to mature myelin-producing oligodendrocytes sustaining re-myelination, reconstruction of neuronal circuitries, and re-establishment of cell-to-cell communication [3, 4]. Interestingly, after injury, both reactive astrocytes [5–8] and NG2 cells [9, 10] reacquire features typical of multipotent precursors and could represent a source for new neurons or glia replacing damaged cells. Unfortunately, the neurogenic/gliogenic program of these cells is inhibited by inflammatory signals at the lesion site [6, 11].
Obviously, exploitment of the beneficial actions of glial cells to foster repair in human neurodegenerative diseases would greatly benefit from (a) identification of the molecular players regulating their activation after injury and (b) demonstration of their presence and behavior in the human brain. In this respect, the P2Y-like membrane receptor GPR17 has emerged as a key player in brain repair after injury. Both rodent and human GPR17 dually respond to uracil nucleotides (UDP and UDP-glucose) and arachidonic acid-derived cysteinyl-leukotrienes (LTD4 and LTE4), two families of alerting “danger signals” accumulating at injury sites [12–16]. The purinergic component of GPR17 has been confirmed in two additional independent studies [17, 18], whereas response to cysteinyl-leukotrienes has been proposed to be related to the formation of heteromers [17, 19–21]. In this respect, in a previous in silico and in vitro study on GPR17 ligands performed by our group [22], we proposed a possible allosteric effect of the CysLT1R antagonist montelukast on GPR17 uracil ligands, suggesting that montelukast may act on a yet uncharacterized binding site differing from the principal orthosteric one.
In rodent brain, GPR17 is normally present on some neurons and on quiescent parenchymal OPCs (very early bi-/tripolar NG2 cells and more advanced ramified immature pre-oligodendrocytes expressing early myelin markers like O4, [23]). After brain ischemic, traumatic, demyelinating [12, 24], or spinal cord [25] lesions, GPR17 was transiently and sequentially upregulated/expressed first in neurons and then in microglia, astrocytes, and OPCs participating in lesion remodeling. In 2012, based on these and other data, FAST FORWARD, a nonprofit organization of the National Multiple Sclerosis Society USA aimed at speeding up basic discoveries to the clinics, has independently recognized GPR17 as a main target and a “model receptor” for innovative therapies for multiple sclerosis and neurodegenerative diseases (see FAST FORWARD 2012 Request for proposals, http://www.nationalmssociety.org/fast-forward/index.aspx).
However, the relevance of these data to human brain injury is totally unknown. Here, we present, for the first time, a detailed analysis of GPR17 expression in neurosurgical and autoptic samples from patients with traumatic brain injury (TBI). Data are particularly relevant to setting up new therapies for TBI, especially in view of the fact that GPR17 represents a target for already marketed drugs like the anti-asthmatic agent montelukast [26].
Materials and methods
Neurosurgical TBI samples
Samples were obtained from seven TBI patients undergoing neurosurgery for contusion removal [27] at the Neuroscience Intensive Care Unit of Milan Policlinico Hospital (patients’ characteristics in Table 1). According to the admission Glasgow Coma Scale (GCS), four severe (GCS < 8), two moderate (GCS = 9–12), and one mild (GCS = 13–15) cases were included. In five patients, contusions were evacuated within day 1, and two patients showing a slow evolution of intracranial lesion leading to increase of intracranial pressure underwent surgery 4 and 5 days after TBI, respectively. The study was approved by the Local Research Ethics Committee of Milan Policlinico Hospital.
Table 1.
Summary of patients’ characteristics for neurosurgical samples
| Patient | Age (years) | Sex | Mechanism of TBI | GCS at admission | Main lesion | Post-traumatic survival time | GOS | Presence of GPR17+ cells |
|---|---|---|---|---|---|---|---|---|
| P1 | 21 | M | MVA | 4 | Traumatic SDH, ICH | 96 | NA | Y |
| P2 | 32 | F | Fall | 9 | Traumatic EDH, ICH | 4 | 3 | Y |
| P3 | 67 | M | MVA | 11 | Traumatic ICH | 4.2 | 3 | N |
| P4 | 48 | F | MVA | 5 | Traumatic SDH, ICH | 3.15 | 5 | Y |
| P5 | 58 | F | Fall | 14 | Traumatic ICH | >120 | 3 | N |
| P6 | 54 | F | MVA | 8 | Traumatic ICH | 8.5 | 2 | N |
| P7 | 63 | F | MVA | 8 | Traumatic ICH | 16 | NA | Y |
GCS Glasgow Coma Scale, GOS Glasgow Outcome Score, SDH subdural hematoma, EDH epidural hematoma, MVA motor vehicle accident, Y yes, N no, ICH intracerebral hemorrhage, NA not available
Autoptic TBI specimens
Peri-traumatic cortical samples from 28 TBI and 10 control subjects of matched age and gender were removed at autopsy. The 28 specimens were from 10 females and 18 males (ages 18–87 years, means 49.57 ± 3.77 vs 48.40 ± 4.6 years in TBI and control groups, respectively); postmortem interval was between 17.5 h and 4.5 days. In TBI cases, post-traumatic survival time (PTI) ranged from 0 to 2,208 h. In control subjects, the cause of death was other than TBI (cardiac infarction, multiple stroke, strangulation, aortic rupture). Subjects’ characteristics are reported in Table 2. All procedures were approved by the ethical committee and authorities at University of Leipzig.
Table 2.
Summary of patients’ characteristics for autoptic samples
| Patient | Age (years) | Sex | Trauma region | Cause of death | Post-traumatic survival time (day/h/min) | Presence of GPR17+ cells |
|---|---|---|---|---|---|---|
| 1 | 85 | F | PFC r, TC r, l | Traumatic ICH | 0.4 day (10.8 h) | Y |
| 2 | 44 | M | PFC r, l | Traumatic ICH, SDH | 14 days (336 h) | Y |
| 3 | 44 | F | PFC r | Traumatic ICH, SDH | 11 days (264 h) | Y |
| 4 | 38 | M | PFC r | Traumatic SAH | 0 | Y |
| 5 | 87 | M | PFC l | Traumatic SAH, ICH | 16 days | Y |
| 6 | 47 | F | PFC l | Traumatic ICH | 0 h | Y |
| 7 | 18 | M | OC r, l | Traumatic SAH, ICH, EDH | 0 day (10 min) | Y |
| 8 | 50 | F | PFC r | Traumatic SAH, ICH | 2 days (48 h) | Y |
| 9 | 68 | M | PFC r | Traumatic SAH, ICH, lung contusion, skull base fracture, fat embolism of the lung | 0 day (1 h) | Y |
| 10 | 26 | M | PFC l, HC | Traumatic SAH, SDH, ICH | 0 day (15 min) | Y |
| 11 | 53 | M | PFC l | Traumatic ICH | 0.2 day (5 h) | Y |
| 12 | 55 | M | PFC | Traumatic SAH, SDH | 7.4 days (178 h) | Y |
| 13 | 70 | F | HC l | Traumatic SDH, SAH, ICH | 2.5 days (60 h) | Y |
| 14 | 45 | M | PFC l | Hypothermia, traumatic SAH | 0 h | Y |
| 15 | 35 | M | PFC | Lung and heart contusions, skull base fracture, traumatic SAH | 0 h | N |
| 16 | 29 | M | PFC | Lung contusion, traumatic aortic rupture, traumatic EDH | 0 h | N |
| 17 | 18 | F | HC l, OC r | Traumatic SAH, ICH | 0 h | N |
| 18 | 19 | F | OC l | Hemorrhagic shock, traumatic SAH, ICH | 0 h | N |
| 19 | 26 | F | PFC | Hemorrhagic shock, traumatic EDH, skull brain fracture | 0.1 day (2 h) | N |
| 20 | 48 | M | PFC r, PC r | Traumatic ICH | 3–5 days | N |
| 21 | 75 | M | PC r | Traumatic ICH | 1.2 days (28.8 h) | N |
| 22 | 59 | M | PFC l, TC l | Traumatic EDH, SDH, SAH, ICH, skull base fracture | 3.1 days (75 h) | N |
| 23 | 70 | F | HC l | Traumatic SDH, SAH, ICH | 2.5 days (60 h) | N |
| 24 | 51 | F | PFC l | Traumatic SDH, SAH, ICH | 2.3 days (55 h) | N |
| 25 | 58 | M | PFC | Pulmonary embolism, traumatic SDH | 33 days | N |
| 26 | 57 | M | PFC | Septicemia, traumatic SAH, SDH, ICH | 92 days | N |
| 27 | 34 | M | PFC | Septicemia, traumatic SDH | 50 days | N |
| 28 | 79 | M | PFC | Pneumonia, traumatic SDH | 37 days | N |
| 29 | 56 | M | PFC | Septicemia, overrun by car | 41 days | N |
| 30 | 30 | M | PFC | Aortic rupture | 1.7 day (40 h) | N |
| 31 | 75 | F | PFC | Multiple stroke | 13 days | N |
| 32 | 61 | M | PFC r | Hemorrhagic shock, thoracic trauma | 0 h | Y |
| 33 | 47 | M | PFC l | Traumatic thoracic dissection | 0.4 h (25 min) | Y |
| 34 | 43 | M | PFC l | Cardiac infarction | 0 h | Y |
| 35 | 57 | M | PFC l | Cardiac infarction | 0 h | Y |
| 36 | 27 | M | PFC r | Drowning | 0 h | Y |
| 37 | 44 | F | PFC r | Strangulation | 0 h | Y |
| 38 | 44 | M | PFC l | Acute coronary insufficiency | 0 h | Y |
ICH intracerebral hemorrhage, SDH subdural hematoma, SAH subarachnoid hemorrhage, EDH epidural hematoma, l left, r right, PFC prefrontal cortex, OC occipital cortex, PC parietal cortex, TC temporal cortex, HC hippocampus, Y yes, N no
Histology, immunohistochemistry, and confocal laser scanning/light microscopy
Antibodies are described in Supplementary Tables 3 and 4. For standard histology, immunohistochemistry, and confocal laser scanning/light microscopy for detection of GPR17 and other markers, see Supplementary material.
Statistical analysis
Differences in GPR17 expression between patients who died from extra- or intracranial complications were estimated by Pearson’s chi-square test. Spearman’s correlation test was applied to evaluate correlations between GPR17 expression and PTI. For comparison of time courses of GPR17 expression in TBI and control cases, a Mann–Whitney U test was applied. Level of significance was set at 0.05. Descriptive statistical data are given as mean ± standard error. Statistical analyses were accomplished using IBM SPSS statistics software (Version 19, IBM, Ehningen, Germany).
Results
In neurosurgical TBI samples, GPR17 labels dying neurons, reactive astrocytes, and activated microglia/macrophages infiltrating the lesion
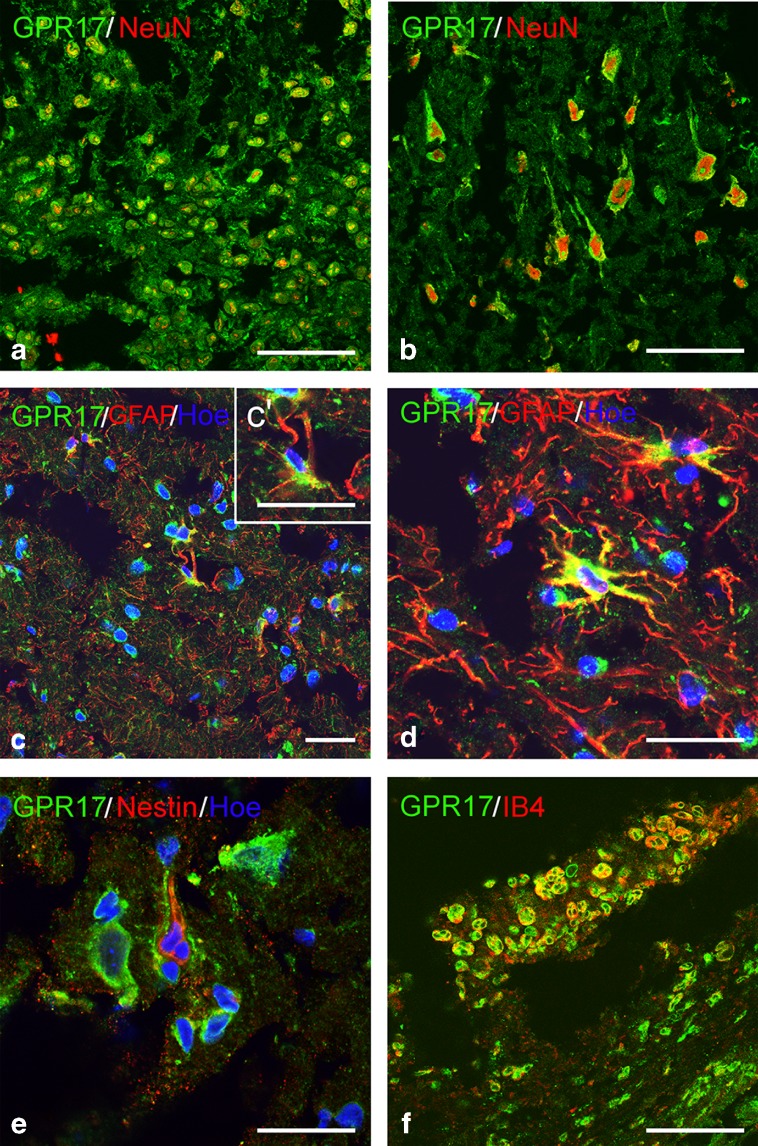
As a first step of our analysis, to confirm the specificity of our results, we tested three different antibodies against GPR17: two were from the same host (rabbit) and based on the same epitope (an “in-house” produced antibody and a commercial one); only for the Iba1 co-staining (where an antibody from a different host was needed), a murine monoclonal antibody was used (see “Materials and methods”). After a careful setting of the staining conditions, the first two antibodies gave similar and comparable results (data not shown). We then analyzed samples from patients undergoing surgery for contusion removal. In two neurosurgical samples, GPR17 was found in shrunk neurons (green cells in Fig. 1a) also staining for the neuronal marker NeuN (red fluorescence) and in triangular cells (Fig. 1b) reminiscent of pyramidal cortical neurons overexpressing GPR17 in rats after ischemia [14].
Fig. 1.
GPR17 expression in human brain neurosurgical samples after contusion. In neurosurgical samples from some patients, GPR17 was found to be expressed in a wide population of shrunk neurons (green fluorescence in a), most of which were also positive for the neuronal marker NeuN (in red). GPR17 was also expressed in NeuN-positive neurons with a triangular shape (b). In specimens from three patients, many GFAP-expressing reactive astrocytes (red cells in c and d) were found; a subset of these cells also positively stained for GPR17 (green fluorescence), as shown in c′; an example of such cells showing immunoreactivity for both GFAP and GPR17 (yellow cell) is reported in d. Reactive astrocytes expressing the stem cell marker nestin (red fluorescence) were also found throughout the cortical parenchyma; no such cells expressed GPR17 (green fluorescence) e. Only in one patient, in which infiltrating cells were visible, numerous cells expressing both GPR17 (green fluorescence) and the microglial marker IB4 (red fluorescence) were found (yellow cells in f). Nuclei are labeled with Hoechst 33258 dye (Hoe). Chromogens in immunohistochemistry micrographs were digitally pseudocolored. Scale bars = 20 μm
Many hypertrophic GFAP-positive astrocytes were found, some of which coexpressed GPR17 (yellow cells in Fig. 1c, c′ inset, and d), suggesting a role in reactive astrocytes. This is at variance from rodent central nervous system (CNS) tissues, where GPR17 was never found in GFAP-positive astrocytes under normal or injury conditions [12, 14, 24]. Only after spinal cord trauma, GPR17 was induced in GFAP-positive ependymal cells lining the spinal cord’s central canal, the true adult “stem-like” progenitors in this part of the CNS, suggesting a role for GPR17 in reacquisition of multipotency and stem cellness after this kind of injury [25]. In the injured rodent CNS, reactive astrocytes are heterogeneous, as demonstrated by the fact that only a subpopulation of these cells re-express the multipotency marker nestin [25]. Nestin-positive cells were also found in human neurosurgical samples (Fig. 1e), but they did not express GPR17, indicating that, as in rodents, human reacting astrocytes are heterogeneous and GPR17 specifically labels a nestin-negative subset of these cells.
In one patient where cells infiltrating the lesion were visible, rounded cells coexpressing GPR17 and IB4, a marker of activated microglia/macrophages, were detected (Fig. 1f). These cells that were very similar to GPR17/IB4-expressing cells found in rodent injured brain and spinal cord [12, 14, 24, 25] likely represent local reacting microglia and/or blood-borne macrophages infiltrating the lesion to phagocytize dead cells and initiate remodeling.
Spatio-temporal changes of GPR17 in various cell types in autoptic TBI samples
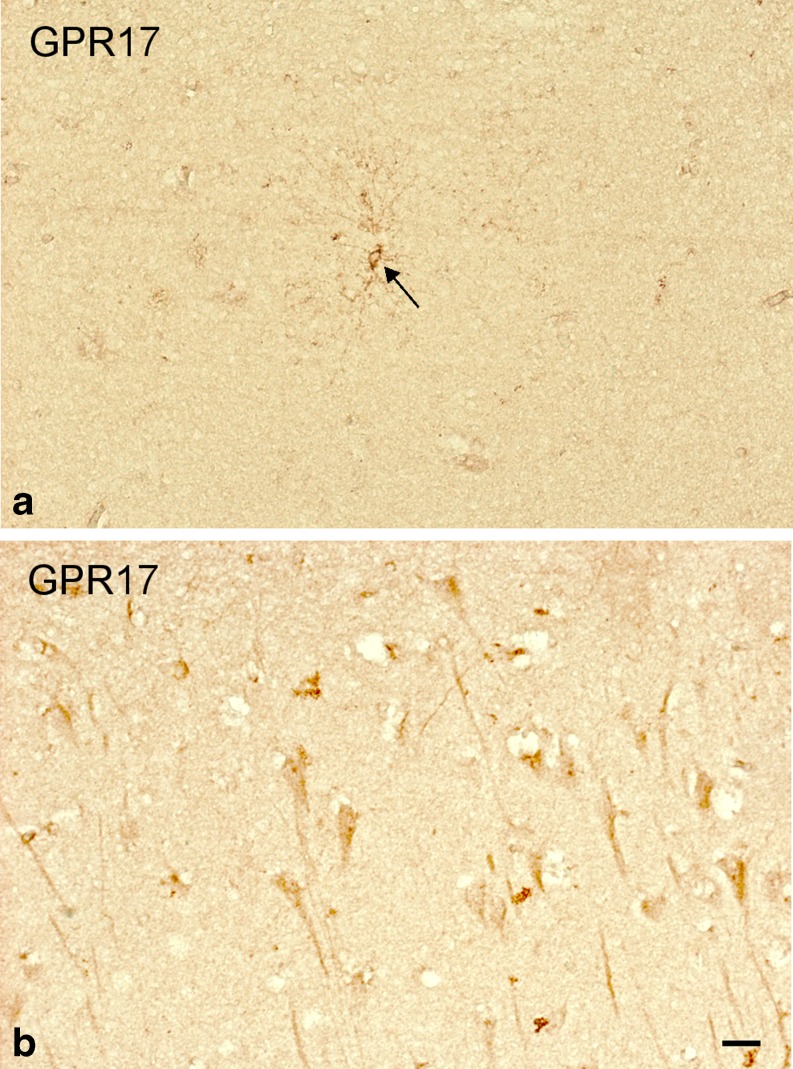
We then performed a more detailed study in autoptic specimens from a larger number of TBI patients. In cortical autoptic samples from control subjects, GPR17 was found in a low number of cells with two distinct morphologies: (a) small, variously ramified cells reminiscent of precursor cells [28] and (b) triangular cells with a long single process (a and b of Fig. 2, respectively). Based on these features and subsequent stainings (see also below), we confirmed these cells to be OPCs and pyramidal neurons, respectively.
Fig. 2.
GPR17 expression in the human brain of control subjects. Immunohistochemical analysis of autoptic samples collected from patients deceased from causes other than TBI (controls) showed that, in these subjects, GPR17 (brown staining) is expressed in a small number of cells typically resembling OPCs (a) and in cells whose morphology is reminiscent of pyramidal neurons (b). Scale bars = 20 μm
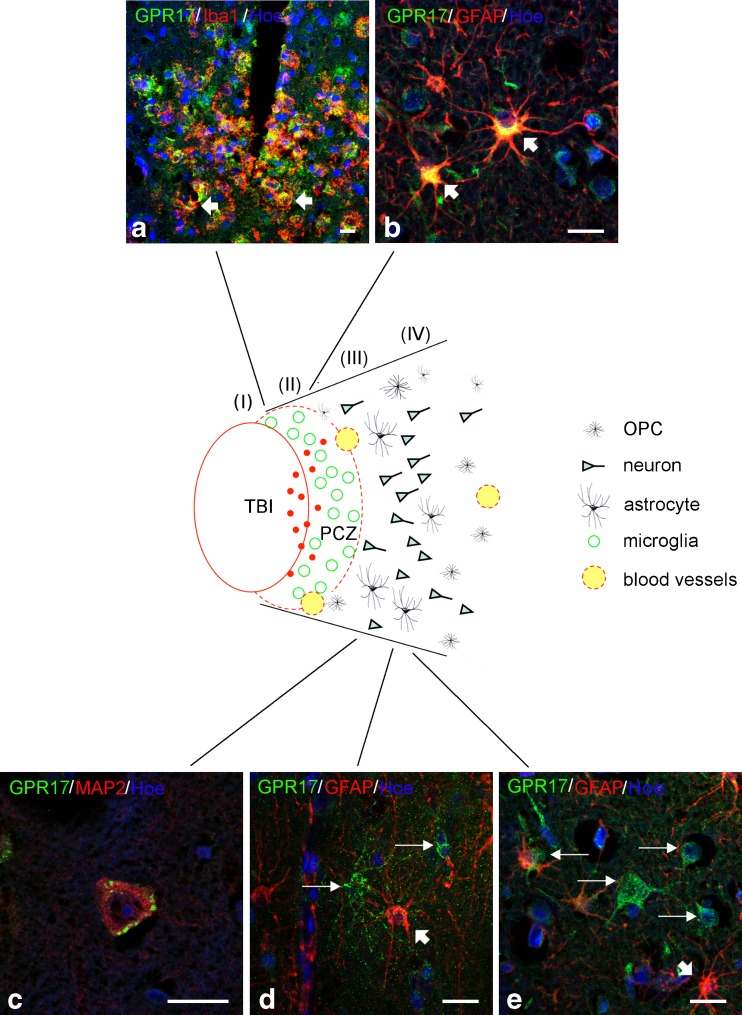
At variance from neurosurgical samples, where a clear spatio-temporal analysis of GPR17 was not possible, the larger number of autoptic TBI cases allowed us to establish a gradient of GPR17 induction in different cells at distinct distances from the traumatic core (TBI) that were arbitrarily named zones (I), (II) (peri-contused zone, PCZ), (III), and (IV) (Fig. 3). Depending on TBI severity and bleeding extent, the full area illustrated in Fig. 3 central drawing spanned from 1 to 10 (or more) mm; cells in zone (I) were found at approximately 50–500 μm from TBI.
Fig. 3.
Spatial gradient of GPR17 expression in various types of neural cells in autoptic specimens from TBI patients. GPR17 expression was very strong in cells inside and at the borders of the necrotic core (zones I and II, also indicated as PCZ in the central drawing) and then gradually declined in zones (III) and (IV). Dense immunoreactive red spots in zones (I) and (II) of the central drawing likely represent shrunk dying or already dead cells, or fragments of necrotized cells. Green circles in zone (II) represent cells costaining for both GPR17 and the microglial marker Iba1 (a), thus representing activated globose microglia. In zone (III), stellate GPR17/GFAP double-positive reactive astrocytes were found (yellow cells indicated by arrows in b). GFAP-positive, GPR17-negative astrocytes (red cells in b; see also cells indicated by thick short arrows in d) were also present. Starting from zone (III) and throughout zone (IV), many pyramidal cells costaining for GPR17 and the neuronal marker MAP2 were present (c). In these zones, several GPR17-expressing cells that, however, did not stain for anyone of the previously mentioned markers (including GFAP) were also detected (green fluorescent cells indicated by long thin arrows in d and e). The morphology of these cells was reminiscent of that of OPCs (see also text and Fig. 6). For further details, please see “Results.” Nuclei are labeled with Hoechst 33258 dye (Hoe, blue fluorescence). Scale bars = 10 μm (a, b, d, e) and 20 μm (c)
In contused zone (I) and PCZ zone (II), many GPR17-positive dots (in red in Fig. 3 drawing) representing fragments of shrunk dying/dead cells expressing high receptor levels were observed (see also below). In zone (II), many rounded GPR17-expressing cells were detected at the borders or in the immediate vicinity of TBI (green circles in Fig. 3 drawing and green fluorescent cells in Fig. 3a). These cells were very similar to the globose-activated microglia infiltrating the lesion in one neurosurgical sample (Fig. 1f) and also stained for the microglial/macrophage marker Iba1 (yellow cells indicated by arrows, Fig. 3a) which confirmed that they are indeed GPR17-expressing microglia/macrophages infiltrating the core.
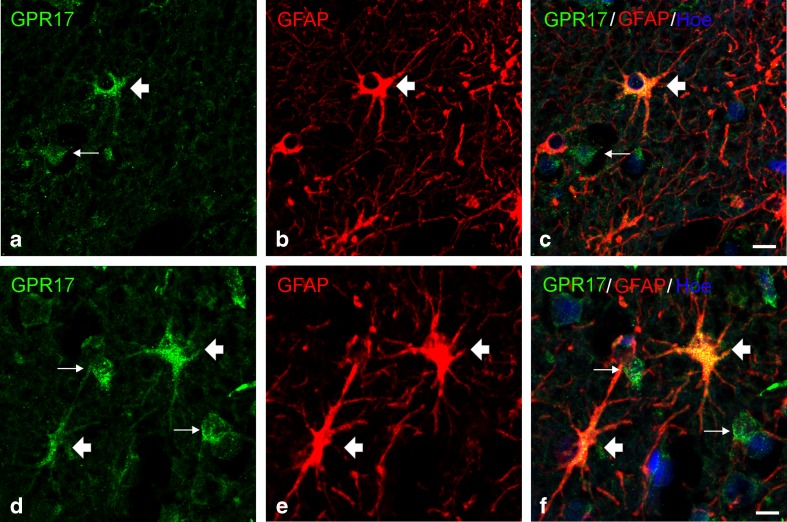
In zones (II) and (III), two other GPR17-expressing types of cells were found: the morphology of one cell type was reminiscent of type 2 astrocytes [29] as confirmed by GFAP staining (yellow cells indicated by arrows, Fig. 3b); additional examples of GPR17-positive type 2 or fibrous astrocytes in prefrontal cortical samples are shown in Fig. 4. As already observed in neurosurgical samples, there were also many GPR17-negative activated astrocytes (Fig. 3d, e).
Fig. 4.
GPR17 expression in activated astrocytes after TBI. After TBI, in tissues collected from human autoptic samples, GPR17 expression was also found in activated astrocytes (thick arrows). Examples of such cells are shown in a–c (cells with a morphology resembling type 2 astrocytes) and in d–f (fibrous astrocytes). Green immunofluorescence for GPR17 (a, d); red fluorescence for GFAP (b, e); merging of images showing coexpression of both GPR17 and GFAP in the same cells (yellow double-labeled cells, thick arrows) (c, f). GFAP-negative/GPR17-positive cells were also observed (examples are indicated with thin arrows). Nuclei are labeled with Hoechst 33258 dye (Hoe, blue fluorescence). Scale bars = 10 μm
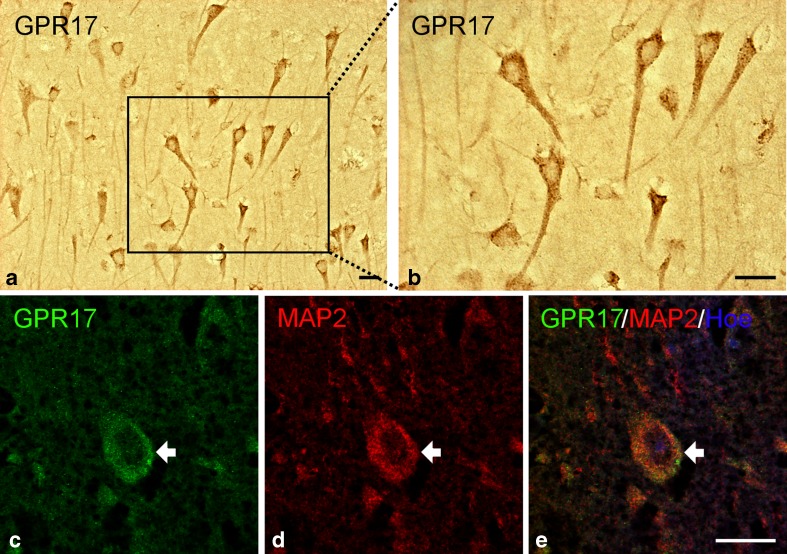
The second type of cells found in zones (II) and (III) and throughout zone (IV) were the triangular cells already observed in the cortex of control subjects (see also DAB staining in Fig. 5a, b); we confirmed these cells to be pyramidal neurons by MAP2 staining (Figs. 3c and 5c–e). A clear gradient of GPR17 expression was observed in these neurons, with very strong immunoreactivity in zone (II) and progressively fainter staining in more distal zones (III) and (IV) (data not shown), suggesting that GPR17 is markedly induced in damaged neurons and that its expression is proportional to the extent of damage extent.
Fig. 5.
GPR17 expression in neurons after TBI. Immunohistochemical analysis of tissues collected from human autoptic TBI samples revealed expression of GPR17 in cells resembling pyramidal neurons (a) and higher magnification detail (b). Immunofluorescence staining confirmed the neuronal nature of these cells: green fluorescence for GPR17 (c); red fluorescence for the neuronal marker MAP2 (d); merge of the two images showing coexpression of GPR17 and MAP2 in the same cell (e). Nuclei are labeled with Hoechst 33258 dye (Hoe, blue fluorescence). Scale bars = 20 μm (a), 50 μm (b), and 20 μm (c–e)
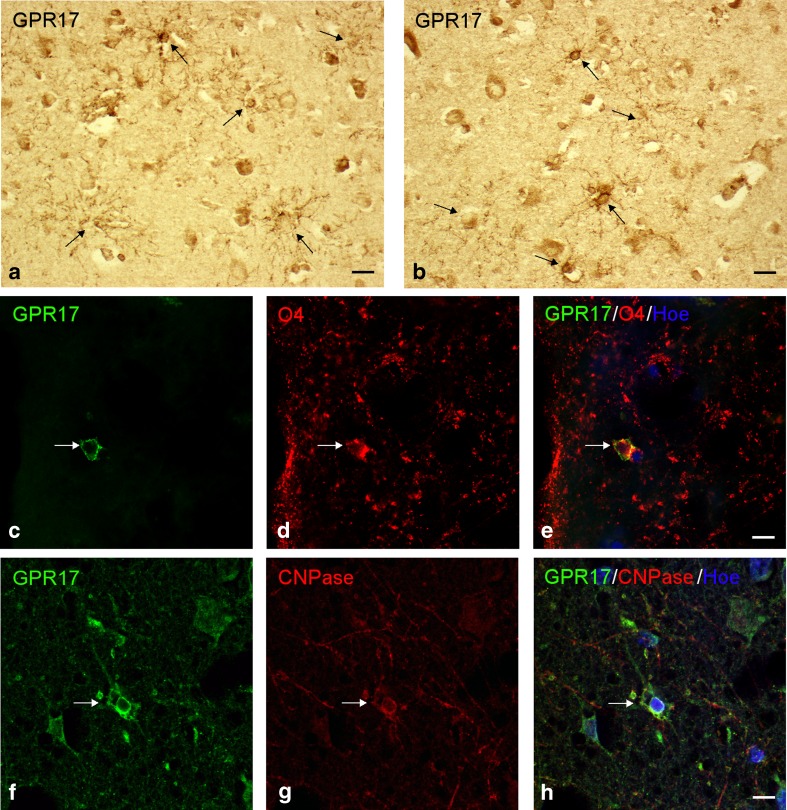
A fourth GPR17-positive cell type was found in zones (II)–(IV): smaller, variously ramified cells reminiscent of some cells found in control samples (green fluorescent cells indicated by thin arrows in Fig. 3d, e; see also DAB stainings in Fig. 6a, b). These cells were clearly different from astrocytes (the red cells indicated by short arrows in Fig. 3d, e) and never co-stained for GFAP. Based on their mean diameter (~10 μm, considerably smaller compared to neurons, astrocytes, and microglia) and coexpression of O4 and CNPase that typically stain immature pre-oligodendrocytes [23] (Fig. 6c–e and f–h, respectively), we confirmed these cells to be OPCs.
Fig. 6.
Expression of GPR17 in oligodendroglial cells after TBI. Immunohistochemical analysis of autoptic samples from TBI patients showed GPR17 expression in cells with a variously ramified morphology that was typically reminiscent of OPCs (brown stained cells indicated by arrows in a and b). The oligodendroglial phenotype of these cells was confirmed by immunofluorescence double staining analysis showing that GPR17-positive cells (c and f, in green fluorescence) co-localized with two typical oligodendroglial markers like O4 (red fluorescence in d and merge image of same cells in e) and CNPase (red fluorescence in g and merge image of same cells in h). Nuclei were labeled with Hoechst 33258 dye (Hoe, blue fluorescence). Scale bars = 10 μm
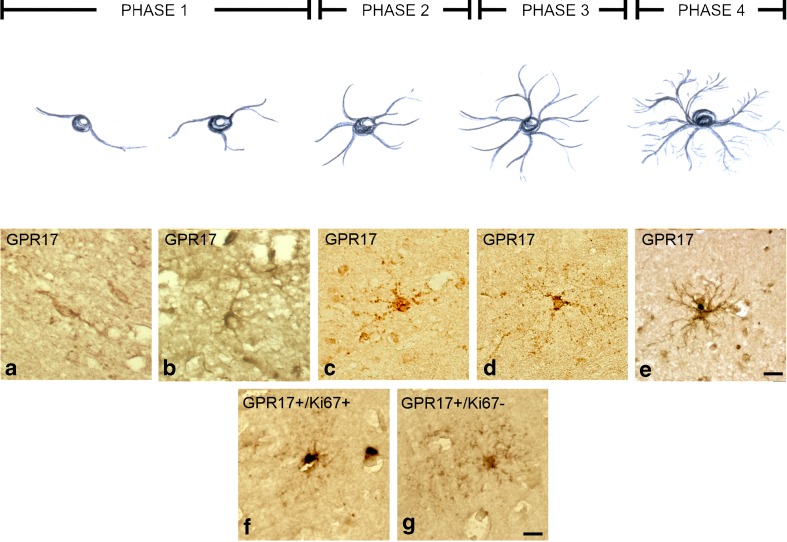
In rodents, GPR17 transiently labels OPCs from early bi-/tripolar NG2 stages (phase 1, Fig. 7 drawing) to more advanced phase 2/phase 3 ramified stages. GPR17 is maximally expressed at the transition between phase 2 and phase 3, when cells exit the cell cycle and become committed to differentiation; thereafter, GPR17 is gradually downregulated along with appearance of more ramified morphologies and markers of myelination [23, 24]. Moreover, the number of GPR17-expressing cells is increased in rodent brain after lesions [24] when proliferation and differentiation of normally quiescent OPCs is stimulated [4] to face increased demand for re-myelination and repair.
Fig. 7.
Differentiation phases of GPR17-positive OPCs in human TBI autoptic specimens. Immunohistochemical analysis of cortical samples collected from TBI patients showed that GPR17 is indeed present in OPCs belonging to distinct differentiation phases, each identified by a typical morphology (i.e., bi- and tripolar phase 1 OPCs, more ramified phase 2 OPCs, and highly ramified phase 3 and phase 4 OPCs, upper drawing; see text for details). GPR17 was found in early bipolar (a) or tripolar (b) cells and in cells of more advanced stages (c–e). In samples from some patients, early phase 1 and phase 2 GPR17-expressing OPCs (f) markedly co-stained for the nuclear proliferative marker dye Ki67, suggesting a proliferative behavior that was not observed in more ramified, later stages GPR17-positive OPCs whose nuclei were negative for Ki67 (g). Scale bars = 10 μm
In various TBI cases, bi-/tripolar or more ramified GPR17-positive OPCs were found (Fig. 7a–e). In some samples, GPR17-positive, phase 2 OPCs intensively stained for the nuclear proliferation marker Ki67, suggesting that, also in humans, early OPCs respond to injury by resuming proliferation (Fig. 7f). We also found several phase 4 highly ramified Ki67-negative OPCs showing fainter GPR17 expression (Fig. 7g), confirming that, also in the human brain, GPR17 expression is downregulated at more advanced stages when cells assume more mature morphologies typical of myelinating cells.
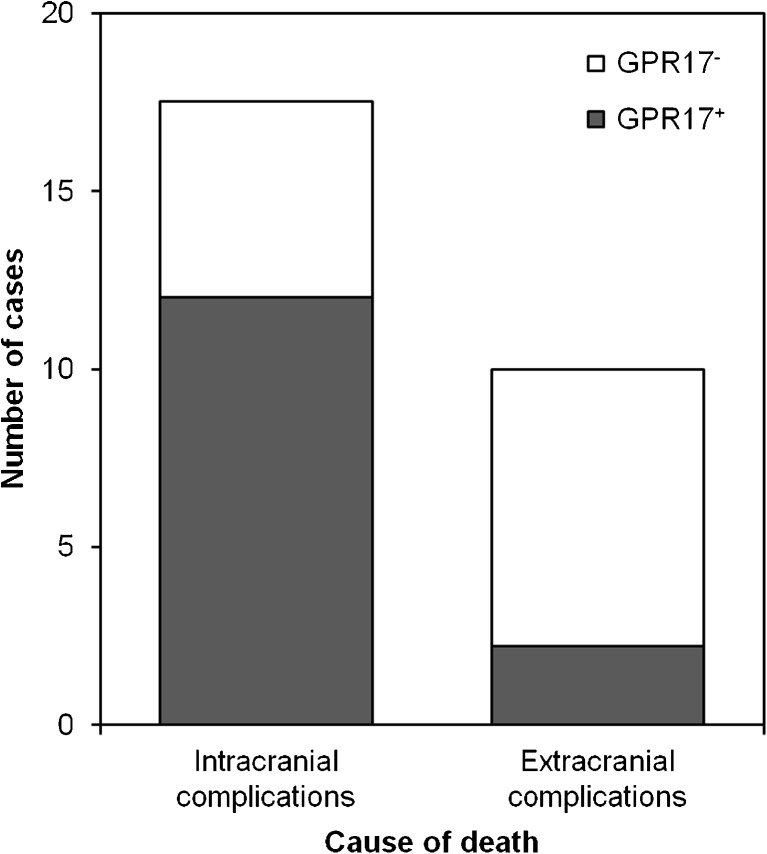
GPR17 is selectively induced in patients deceased from intracranial complications and negatively correlates with patients’ PTI
TBI autoptic cases (n = 28) belong to two groups depending on the cause of death, extracranial vs intracranial complications (Fig. 8). Subgroup analysis revealed that the majority (66.7 %, 12 out of 18) of patients deceased from intracranial complications displayed GPR17 expression in OPCs, whereas only 2 out of 10 patients deceased from extracranial complications (20 %) displayed detectable GPR17 expression, a statistically significant difference (p = 0.018). Thus, receptor induction results from reactions occurring as a consequence of local injury and likely involves release of diffusible factors from damaged cells. Furthermore, there was a clear negative correlation between GPR17 expression and patient’s PTI after TBI (p = 0.027). GPR17 immunoreactivity was already detected within 2 h after TBI, and very numerous GPR17-expressing OPCs were found in patients deceased within 16 days (384 h). Patients showed GPR17 expression until a mean survival time of 92 ± 37 vs 0.06 ± 0.06 h in controls (p = 0.07). At PTI longer than 16 days, GPR17 expression was markedly decreased, suggesting a clear time window for receptor induction after injury.
Fig. 8.
Correlation analysis: selective expression of GPR17 in patients deceased from intracranial complications. Traumatic brain-injured patients from the autopsy study (n = 28) could be divided in two categories based on the cause of death: namely extracranial complications (left column) or intracranial complications (right column). The majority of those who died upon their focal damage had GPR17 expression (in gray). In contrast, the vast majority of patients that deceased from extracranial complications showed no GPR17 expression (in white; chi-square test, linear-by-linear association, two sided, p = 0.02)
Discussion
Here we show that, in normal human brain, GPR17 labels OPCs and neurons and that, after TBI, GPR17 undergoes profound changes in cells participating in damage and repair according to a precise spatio-temporal pattern. Our findings are obtained on contusions neurosurgically dissected from TBI patients and on autoptic specimens. Both approaches complementarily revealed the strong expression of GPR17 in the injured brain tissue. The larger number of autoptic samples allowed a detailed analysis of GPR17 expression in different brain cells and its spatio-temporal gradient in relation to the injury core, with very strong expression within the lesion and gradually fainter stainings in more distal areas. The existence of a spatio-temporal gradient for GPR17 induction is also confirmed by statistical analysis showing that (a) GPR17 is selectively induced in patients deceased from intracranial but not extracranial complications and that (b) receptor expression negatively correlates with patients’ PTI, being very high at short survival periods and markedly decreased at PTI times longer than 16 days.
Importantly, the neurosurgical samples demonstrated that GPR17 is actually expressed in the living tissue, thus underlying the relevance of this sensor in TBI patients and ruling out the possibility of postmortem artefacts.
Strong GPR17 immunoreactivity was found in shrunk dying pyramidal neurons inside or in the immediate vicinity of the traumatic core. This is consistent with rodent data showing early and transient GPR17 upregulation in neurons inside and at the borders of ischemic lesions [12, 14] and in necrotic core neurons after spinal cord trauma [25]. In both types of injury in rodents, early in vivo GPR17 knockdown by antisense oligonucleotide strategy reduced damage, suggesting that pathological GPR17 overexpression at very early injury phases may contribute to the damage [12, 14, 25].
The detected gradient of GPR17 expression also suggests that receptor induction is proportional to the damage and likely induced by diffusible factor(s) released by injured cells. In this respect, GPR17 endogenous ligands (UDP-glucose and LTD4) are massively released at lesion sites [30] and can themselves induce GPR17 in both OPCs [12] and microglia (Wypych, Lecca, and Abbracchio, unpublished); these ligands are therefore likely candidates for GPR17 induction (and activation) in humans as well.
In rodents, the initial GPR17 deregulation inside the lesion is followed by sequential induction/activation of GPR17 in microglia/macrophages infiltrating the lesion, reactive astrocytes, and parenchymal OPCs. Data from TBI patients show that GPR17 is specifically induced in activated IB4-positive microglia/macrophages infiltrating the lesion, confirming GPR17 participation in the lesion remodeling that precedes reconstruction of neuronal circuitries. At variance from rodents where GFAP/GPR17 coexpression was detected in spinal cord injury-activated ependymal cells [25] but not in brain astrocytes [12, 14, 24], in the cortex of TBI patients, GPR17 decorated a subset of reactive astrocytes, suggesting that, in humans, GPR17 may also regulate astrocytic post-traumatic activation. Thus, the role of GPR17 in the injured human brain may be broader than that observed in rodents. Moreover, in several human samples, GPR17 was found in bi-/tripolar or more ramified OPCs, suggesting a role in post-injury-induced OPC differentiation. Some GPR17-expressing OPCs were clearly resuming proliferation, as already shown in rodents [28]. Thus, in both humans and rodents, GPR17 participates to post-injury attempts to generate new myelinating oligodendrocytes.
Based on these findings, we suggest that GPR17 plays a role in sensing damage and in orchestrating lesion remodeling and repair in the humans brain and, thus, represents a novel target for new therapies for TBI. Highly relevant, GPR17 is indeed the target of already marketed drugs, such as the anti-asthmatic agent montelukast [12, 14], whose efficacy against cerebral ischemia in rodents has been recently demonstrated [26].
Electronic supplementary material
(DOC 37 kb)
(DOC 40 kb)
(DOC 47 kb)
Acknowledgments
The authors thank Prof. Enzo Wanke, University of Bicocca-Milan and Dr. Annalisa Buffo, University of Turin for useful discussion. The authors are also grateful to Prof. J. Dreßler and Benjamin Ondruschka (Institute for Legal Medicine, Medical Faculty University of Leipzig) for providing autoptic samples, to Dr. Mauro Pluderi, MD, and Dr. Marco Locatelli, MD, from the Department of Neurosurgery of the Ospedale Maggiore Policlinico di Milano for assistance in resection of contusions and collection of neurosurgical brain samples, and to Mrs. Katrin Becker (RBI of Pharmacology and Toxicology, Leipzig) for expert technical assistance. This work was partially supported by the Italian PRIN-COFIN (Project Prot. 2006059022 and 2008XFMEA3 to M.P.A) and by Deutsche Forschungsgemeinschaft (FR 1253/3-2); C.P. was supported by a Fondazione Cariplo fellowship (project number 2008.2907); M.F. is supported by a fellowship from Fondazione Italiana Sclerosi Multipla (grant N. 2010/R/2); C.H. was supported by a funding from the German Federal Ministry of Education and Research (BMBF, PtJ-Bio, 0313909), the European Fund for Regional Development (EFRE), and the Free State of Saxony (grant no. SAB12525).
Conflict of interest
None declared.
References
- 1.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nature medicine. 2008;14(5):497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- 2.De Biase LM, Nishiyama A, Bergles DE. Excitability and synaptic communication within the oligodendrocyte lineage. J Neurosci Off J Soc Neurosci. 2010;30(10):3600–3611. doi: 10.1523/JNEUROSCI.6000-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fumagalli M, Lecca D, Abbracchio MP. Role of purinergic signalling in neuro-immune cells and adult neural progenitors. Front Biosci. 2011;16:2326–2341. doi: 10.2741/3856. [DOI] [PubMed] [Google Scholar]
- 4.Simon C, Gotz M, Dimou L. Progenitors in the adult cerebral cortex: cell cycle properties and regulation by physiological stimuli and injury. Glia. 2011;59(6):869–881. doi: 10.1002/glia.21156. [DOI] [PubMed] [Google Scholar]
- 5.Buffo A, Vosko MR, Erturk D, Hamann GF, Jucker M, Rowitch D, Gotz M. Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair. Proc Natl Acad Sci U S A. 2005;102(50):18183–18188. doi: 10.1073/pnas.0506535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buffo A, Rite I, Tripathi P, Lepier A, Colak D, Horn AP, Mori T, Gotz M. Origin and progeny of reactive gliosis: a source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A. 2008;105(9):3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berninger B, Costa MR, Koch U, Schroeder T, Sutor B, Grothe B, Gotz M. Functional properties of neurons derived from in vitro reprogrammed postnatal astroglia. J Neurosci Off J Soc Neurosci. 2007;27(32):8654–8664. doi: 10.1523/JNEUROSCI.1615-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heinrich C, Gascon S, Masserdotti G, Lepier A, Sanchez R, Simon-Ebert T, Schroeder T, Gotz M, Berninger B. Generation of subtype-specific neurons from postnatal astroglia of the mouse cerebral cortex. Nat Protoc. 2011;6(2):214–228. doi: 10.1038/nprot.2010.188. [DOI] [PubMed] [Google Scholar]
- 9.Richardson WD, Young KM, Tripathi RB, McKenzie I. NG2-glia as multipotent neural stem cells: fact or fantasy? Neuron. 2011;70(4):661–673. doi: 10.1016/j.neuron.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kondo T, Raff M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science. 2000;289(5485):1754–1757. doi: 10.1126/science.289.5485.1754. [DOI] [PubMed] [Google Scholar]
- 11.Marchetti B, Abbracchio MP. To be or not to be (inflamed)—is that the question in anti-inflammatory drug therapy of neurodegenerative disorders? Trends Pharmacol Sci. 2005;26(10):517–525. doi: 10.1016/j.tips.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Lecca D, Trincavelli ML, Gelosa P, Sironi L, Ciana P, Fumagalli M, Villa G, Verderio C, Grumelli C, Guerrini U, Tremoli E, Rosa P, Cuboni S, Martini C, Buffo A, Cimino M, Abbracchio MP. The recently identified P2Y-like receptor GPR17 is a sensor of brain damage and a new target for brain repair. PLoS One. 2008;3(10):e3579. doi: 10.1371/journal.pone.0003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pugliese AM, Trincavelli ML, Lecca D, Coppi E, Fumagalli M, Ferrario S, Failli P, Daniele S, Martini C, Pedata F, Abbracchio MP. Functional characterization of two isoforms of the P2Y-like receptor GPR17: [35S]GTPgammaS binding and electrophysiological studies in 1321N1 cells. Am J Physiol Cell Physiol. 2009;297(4):C1028–1040. doi: 10.1152/ajpcell.00658.2008. [DOI] [PubMed] [Google Scholar]
- 14.Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, Ferrario S, Parravicini C, Capra V, Gelosa P, Guerrini U, Belcredito S, Cimino M, Sironi L, Tremoli E, Rovati GE, Martini C, Abbracchio MP. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. EMBO J. 2006;25(19):4615–4627. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daniele S, Lecca D, Trincavelli ML, Ciampi O, Abbracchio MP, Martini C. Regulation of PC12 cell survival and differentiation by the new P2Y-like receptor GPR17. Cell Signal. 2010;22(4):697–706. doi: 10.1016/j.cellsig.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Daniele S, Trincavelli ML, Gabelloni P, Lecca D, Rosa P, Abbracchio MP, Martini C. Agonist-induced desensitization/resensitization of human G protein-coupled receptor 17: a functional cross-talk between purinergic and cysteinyl-leukotriene ligands. J Pharmacol Exp Ther. 2011;338(2):559–567. doi: 10.1124/jpet.110.178715. [DOI] [PubMed] [Google Scholar]
- 17.Benned-Jensen T, Rosenkilde MM. Distinct expression and ligand-binding profiles of two constitutively active GPR17 splice variants. Br J Pharmacol. 2010;159(5):1092–1105. doi: 10.1111/j.1476-5381.2009.00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buccioni M, Marucci G, Dal Ben D, Giacobbe D, Lambertucci C, Soverchia L, Thomas A, Volpini R, Cristalli G. Innovative functional cAMP assay for studying G protein-coupled receptors: application to the pharmacological characterization of GPR17. Purinergic Signal. 2011;7(4):463–468. doi: 10.1007/s11302-011-9245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maekawa A, Balestrieri B, Austen KF, Kanaoka Y. GPR17 is a negative regulator of the cysteinyl leukotriene 1 receptor response to leukotriene D4. Proc Natl Acad Sci U S A. 2009;106(28):11685–11690. doi: 10.1073/pnas.0905364106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wunder F, Tinel H, Kast R, Geerts A, Becker EM, Kolkhof P, Hutter J, Erguden J, Harter M. Pharmacological characterization of the first potent and selective antagonist at the cysteinyl leukotriene 2 (CysLT(2)) receptor. Br J Pharmacol. 2010;160(2):399–409. doi: 10.1111/j.1476-5381.2010.00730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Back M, Dahlen SE, Drazen JM, Evans JF, Serhan CN, Shimizu T, Yokomizo T, Rovati GE. International Union of Basic and Clinical Pharmacology. LXXXIV: leukotriene receptor nomenclature, distribution, and pathophysiological functions. Pharmacol Rev. 2011;63(3):539–584. doi: 10.1124/pr.110.004184. [DOI] [PubMed] [Google Scholar]
- 22.Eberini I, Daniele S, Parravicini C, Sensi C, Trincavelli ML, Martini C, Abbracchio MP. In silico identification of new ligands for GPR17: a promising therapeutic target for neurodegenerative diseases. J Comput Aided Mol Des. 2011;25(8):743–752. doi: 10.1007/s10822-011-9455-8. [DOI] [PubMed] [Google Scholar]
- 23.Fumagalli M, Daniele S, Lecca D, Lee PR, Parravicini C, Fields RD, Rosa P, Antonucci F, Verderio C, Trincavelli ML, Bramanti P, Martini C, Abbracchio MP. Phenotypic changes, signaling pathway, and functional correlates of GPR17-expressing neural precursor cells during oligodendrocyte differentiation. J Biol Chem. 2011;286(12):10593–10604. doi: 10.1074/jbc.M110.162867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boda E, Vigano F, Rosa P, Fumagalli M, Labat-Gest V, Tempia F, Abbracchio MP, Dimou L, Buffo A. The GPR17 receptor in NG2 expressing cells: focus on in vivo cell maturation and participation in acute trauma and chronic damage. Glia. 2011;59(12):1958–1973. doi: 10.1002/glia.21237. [DOI] [PubMed] [Google Scholar]
- 25.Ceruti S, Villa G, Genovese T, Mazzon E, Longhi R, Rosa P, Bramanti P, Cuzzocrea S, Abbracchio MP. The P2Y-like receptor GPR17 as a sensor of damage and a new potential target in spinal cord injury. Brain. 2009;132(Pt 8):2206–2218. doi: 10.1093/brain/awp147. [DOI] [PubMed] [Google Scholar]
- 26.Yu GL, Wei EQ, Zhang SH, Xu HM, Chu LS, Zhang WP, Zhang Q, Chen Z, Mei RH, Zhao MH. Montelukast, a cysteinyl leukotriene receptor-1 antagonist, dose- and time-dependently protects against focal cerebral ischemia in mice. Pharmacology. 2005;73(1):31–40. doi: 10.1159/000081072. [DOI] [PubMed] [Google Scholar]
- 27.Zanier ER, Ortolano F, Ghisoni L, Colombo A, Losappio S, Stocchetti N. Intracranial pressure monitoring in intensive care: clinical advantages of a computerized system over manual recording. Crit Care. 2007;11(1):R7. doi: 10.1186/cc5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boda E, Buffo A. Glial cells in non-germinal territories: insights into their stem/progenitor properties in the intact and injured nervous tissue. Arch Ital Biol. 2010;148(2):119–136. [PubMed] [Google Scholar]
- 29.Raff MC. Glial cell diversification in the rat optic nerve. Science. 1989;243(4897):1450–1455. doi: 10.1126/science.2648568. [DOI] [PubMed] [Google Scholar]
- 30.Lecca D, Ceruti S. Uracil nucleotides: from metabolic intermediates to neuroprotection and neuroinflammation. Biochem Pharmacol. 2008;75(10):1869–1881. doi: 10.1016/j.bcp.2007.12.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 37 kb)
(DOC 40 kb)
(DOC 47 kb)