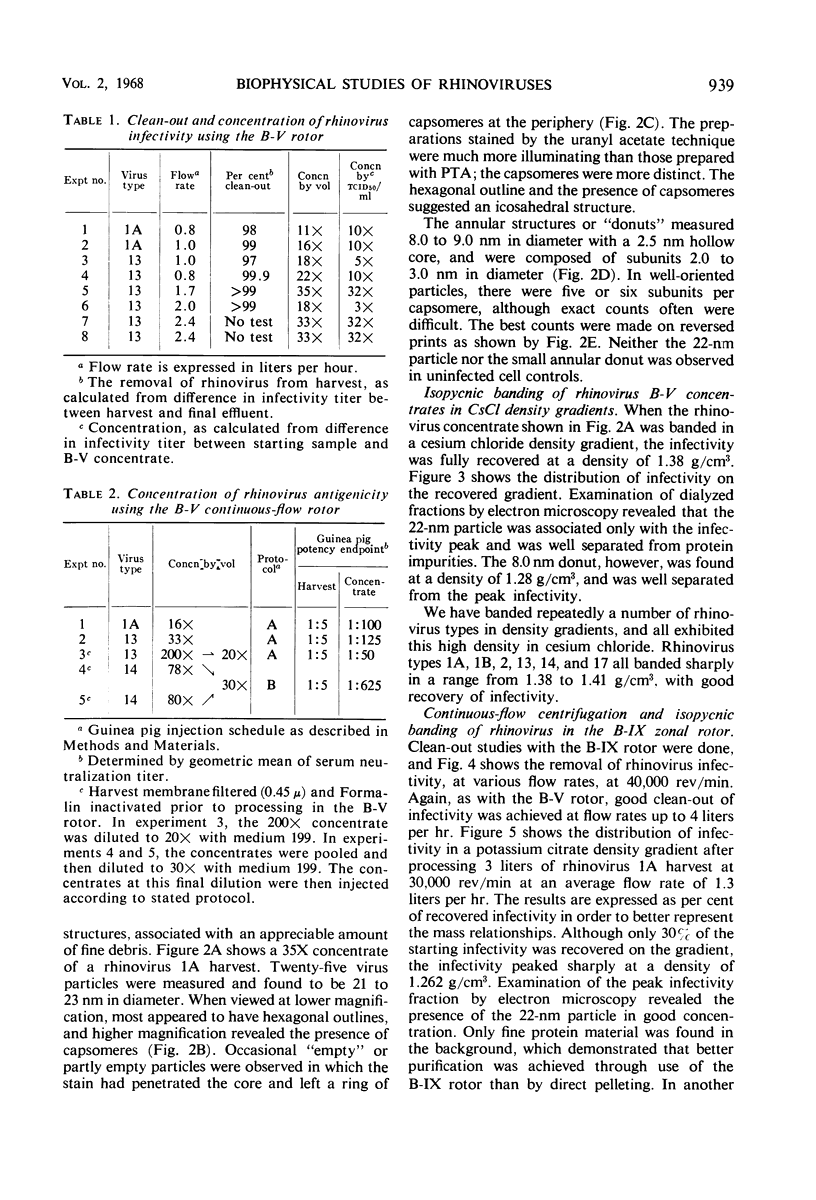
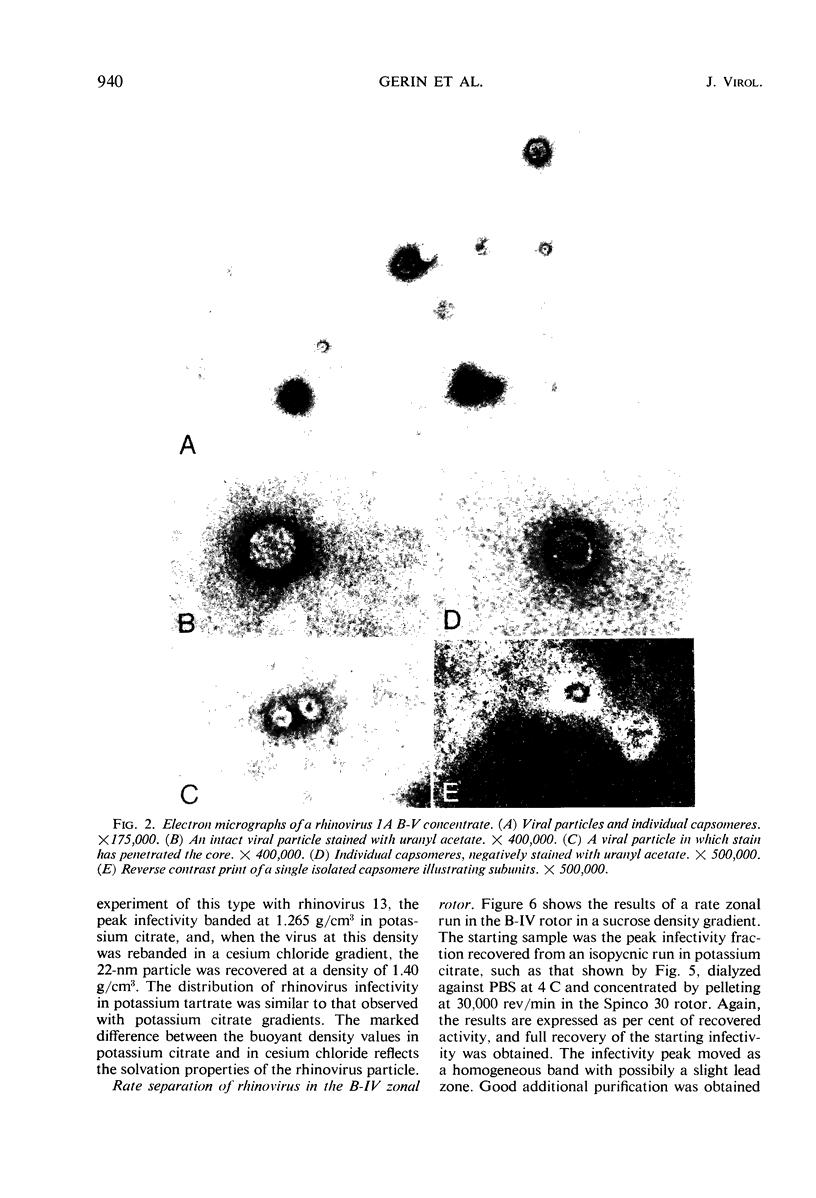
Abstract
This paper reports the use of zonal ultracentrifuge techniques to conduct biophysical studies of rhinoviruses grown with WI-38 cells. Good clean-out of infectivity from rhinovirus harvests was obtained with the continuous-flow B-V and B-IX rotors. Use of the B-V rotor resulted in the successful concentration of rhinovirus infectivity and antigenicity. Additional purification was achieved by the combined use of continuous-flow centrifugation and isopycnic banding procedures. Two particle sizes were found to be associated with the virus-infected cell harvests. The infectious 22-nm particle banded in density ranges of 1.38 to 1.40 g/cm3 in CsCl and 1.26 to 1.27 g/cm3 in potassium citrate. The 8.0 nm capsomere was composed of 2.0 nm subunits and banded with a density of protein at 1.28 g/cm3 in CsCl. Equivalent sedimentation coefficients of 155 or 185, depending on particle density in sucrose, were calculated from rate zonal experiments by use of the B-IV zonal rotor.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. G., Barringer H. P., Amburgey J. W., Jr, Cline G. B., Nunley C. E., Berman A. S. Continuous-flow centrifugation combined with isopycnic banding: rotors B-8 and B-IX. Natl Cancer Inst Monogr. 1966 Jun;21:199–216. [PubMed] [Google Scholar]
- Anderson N. G., Barringer H. P., Babelay E. F., Nunley C. E., Bartkus M. J., Fisher W. D., Rankin C. T., Jr The design and operation of the B-IV zonal centrifuge system. Natl Cancer Inst Monogr. 1966 Jun;21:137–164. [PubMed] [Google Scholar]
- BREESE S. S., Jr, TRAUTMAN R., BACHRACH H. L. Analysis by electron microscopy and infectivity of foot-and mouth disease virus in moving-boundary and zone ultracentrifugation. Arch Biochem Biophys. 1960 Mar;87:1–8. doi: 10.1016/0003-9861(60)90115-6. [DOI] [PubMed] [Google Scholar]
- Barringer H. P., Anderson N. G., Nunley C. E. Design of the B-V continuous-flow centrifuge system. Natl Cancer Inst Monogr. 1966 Jun;21:191–198. [PubMed] [Google Scholar]
- Bishop B. S. Digital computation of sedimentation coefficients in zonal centrifuges. Natl Cancer Inst Monogr. 1966 Jun;21:175–188. [PubMed] [Google Scholar]
- Chapple P. J., Harris W. J. Biophysical studies of a rhinovirus. Ultracentrifugation and electron microscopy. Nature. 1966 Feb 19;209(5025):790–792. doi: 10.1038/209790a0. [DOI] [PubMed] [Google Scholar]
- DIMMOCK N. J., TYRRELL D. A. Physicochemical properties of some viruses isolated from common colds (rhinoviruses). Lancet. 1962 Sep 15;2(7255):536–537. doi: 10.1016/s0140-6736(62)90404-x. [DOI] [PubMed] [Google Scholar]
- Dans P. E., Forsyth B. R., Chanock R. M. Density of infectious virus and complement-fixing antigens of two rhinovirus strains. J Bacteriol. 1966 Apr;91(4):1605–1611. doi: 10.1128/jb.91.4.1605-1611.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenters J. D., Fordyce P. A., Gerin J. L., Holper J. C. Propagation of Rhinovirus on WI-38 Cell Monolayers in Rolling Bottles. Appl Microbiol. 1967 Nov;15(6):1460–1464. doi: 10.1128/am.15.6.1460-1464.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenters J. D., Gillum S. S., Holper J. C., Marquis G. S. Serotypic relationships among rhinoviruses. Am J Epidemiol. 1966 Jul;84(1):10–20. doi: 10.1093/oxfordjournals.aje.a120614. [DOI] [PubMed] [Google Scholar]
- HAMPARIAN V. V., LEAGUS M. B., HILLEMAN M. R. ADDITIONAL RHINOVIRUS SEROTYPES. Proc Soc Exp Biol Med. 1964 Aug-Sep;116:976–984. doi: 10.3181/00379727-116-29426. [DOI] [PubMed] [Google Scholar]
- HOWATSON A. F., CRAWFORD L. V. DIRECT COUNTING OF THE CAPSOMERES IN POLYOMA AND PAPILLOMA VIRUSES. Virology. 1963 Sep;21:1–6. doi: 10.1016/0042-6822(63)90296-4. [DOI] [PubMed] [Google Scholar]
- KETLER A., HAMPARIAN V. V., HILLEMAN M. R. Characterization and classification of ECHO 28-rhinovirus-coryzavirus agents. Proc Soc Exp Biol Med. 1962 Aug-Sep;110:821–831. doi: 10.3181/00379727-110-27662. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McGregor S., Mayer H. D. Biophysical studies on rhinovirus and poliovirus. I. Morphology of viral ribonucleoprotein. J Virol. 1968 Feb;2(2):149–154. doi: 10.1128/jvi.2.2.149-154.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor S., Phillips C. A., Mayor H. D. Purification and biophysical properties of rhinoviruses. Proc Soc Exp Biol Med. 1966 May;122(1):118–121. doi: 10.3181/00379727-122-31068. [DOI] [PubMed] [Google Scholar]
- Reimer C. B., Newlin T. E., Havens M. L., Baker R. S., Anderson N. G., Cline G. B., Barringer H. P., Nunley C. E. An evaluation of the B-V (continuous-flow) and B-IV (density gradient) rotors by use of live polio virus. Natl Cancer Inst Monogr. 1966 Jun;21:375–388. [PubMed] [Google Scholar]
- Rhinoviruses: a numbering system. Nature. 1967 Feb 25;213(5078):761–762. doi: 10.1038/213761a0. [DOI] [PubMed] [Google Scholar]
- SCHULZE P., GRATHEER H. VISUALIZATION OF CAPSOMERES OF FOOT-AND MOUTH DISEASE VIRUS. Acta Virol. 1964 Sep;8:473–473. [PubMed] [Google Scholar]
- SCHWERDT C. E., SCHAFFER F. L. Some physical and chemical properties of purified poliomyelitis virus preparations. Ann N Y Acad Sci. 1955 Sep 27;61(4):740-50; discussion, 750-3. doi: 10.1111/j.1749-6632.1955.tb42530.x. [DOI] [PubMed] [Google Scholar]




