Abstract
Previous studies indicate that carbohydrate intake influences prostate cancer biology, as mice fed a no-carbohydrate ketogenic diet (NCKD) had significantly smaller xenograft tumors and longer survival than mice fed a Western diet. As it is nearly impossible for humans to consume and maintain NCKD, we determined whether diets containing 10% or 20% carbohydrate kcal showed similar tumor growth as NCKD. A total of 150 male severe combined immunodeficient mice were fed a Western diet ad libitum, injected with the human prostate cancer cell line LAPC-4, and then randomized 2 weeks later to one of three arms: NCKD, 10% carbohydrate, or 20% carbohydrate diets. Ten mice not injected were fed an ad libitum low-fat diet (12% fat kcal) serving as the reference in a modified-paired feeding protocol. Mice were sacrificed when tumors reached 1,000 mm3. Despite consuming extra calories, all mice receiving low-carbohydrate diets were significantly lighter than those receiving a low-fat diet (P < 0.04). Among the low-carbohydrate arms, NCKD-fed mice were significantly lighter than the 10% or 20% carbohydrate groups (P < 0.05). Tumors were significantly larger in the 10% carbohydrate group on days 52 and 59 (P < 0.05), but at no other point during the study. Diet did not affect survival (P = 0.34). There were no differences in serum insulin-like growth factor-I or insulin-like growth factor binding protein-3 at sacrifice among the low-carbohydrate arms (P = 0.07 and P = 0.55, respectively). Insulin was significantly lower in the 20% carbohydrate arm (P = 0.03). LAPC-4 xenograft mice fed a low-carbohydrate diet (10–20% carbohydrate kcal) had similar survival as mice consuming NCKD (0% carbohydrate kcal).
Introduction
Prostate cancer is the leading cancer diagnosis for men in the United States and the second leading cause of cancer-related deaths (1). Overall, men in Western countries have an ~6-fold increase in prostate cancer incidence relative to other countries such as China and Japan (2). One hypothesis for this discrepancy is differences in dietary intake, although much of the research has resulted in no clear-cut conclusion (3–7). Contrary to the mixed epidemiologic data, reducing fat intake in animal studies has repeatedly been shown to slow tumor growth (8–11). The question was recently posed of whether reducing dietary carbohydrates is as effective as fat reduction in slowing prostate cancer growth (12). Such diets are safe in humans (13–15), useful in the management of epilepsy (16), and shown to help control brain tumors in animals (17, 18). Thus, it is plausible to believe that carbohydrate-restricted diets may slow tumor growth (12). To test this hypothesis, we performed a xenograft study and found that mice consuming a no-carbohydrate ketogenic diet (NCKD; 84% fat–0% carbohydrate–16% protein kcal) had slowed tumor growth and an increase in overall survival compared with mice on a Western diet, whereas such a survival benefit was not seen for mice consuming a low-fat diet (19). Mice consuming NCKD were also found to have an increase in urinary ketones and decrease in signaling of the insulin-like growth factor (IGF) axis, a pathway known not only to be integral in the progression of prostate cancer but also well known to be highly controlled through dietary intake (10, 20–26). We confirmed these results in a second independent xenograft study using the LNCaP cell line (27).
Although these results provoke ideas of dietary carbohydrate restriction as a treatment for cancer, implementing a no-carbohydrate diet into clinical trials would be difficult, as asking a patient to maintain such a diet in the long term would be near impossible. However, it is reasonable for patients to maintain a long-term diet that is low in carbohydrates, similar to that of Atkins diet (28). Indeed, a reduced carbohydrate diet (10% carbohydrate as opposed to NCKD) delays LNCaP xenograft growth (29). However, in that study, mice on the low-carbohydrate diet lost weight, suggesting that caloric restriction may have played a role in the reported growth delay. That study, moreover, did not test a diet containing 0% carbohydrate and offers no insight as to whether the results would have been better with NCKD. Alternatively, whether a diet allowing a small amount of carbohydrates would compromise the benefit observed in our earlier studies is unknown. Therefore, we sought to determine if diets low in carbohydrates (10–20% kcal, respectively) resulted in similar tumor growth as NCKD in the same xenograft model in which we had previously shown that NCKD slowed tumor growth relative to a Western diet (19).
Materials and Methods
Cell culture
LAPC-4 human prostate cancer cells were a generous gift from William J. Aronson (University of California at Los Angeles School of Medicine, Los Angeles, CA). Cells were maintained in Iscove’s modified medium with 10% fetal bovine serum and supplemented with the synthetic androgen R1881 at 1 nmol/L. Cells were grown in 5% CO2 at 37°C and harvested by trypsinization at ~80% confluence in log-phase growth.
Animal studies
After approval from the Duke University Institutional Animal Care and Use Committee, 160 male severe combined immunodeficient (CB.17 scid/scid) mice, ages 6 weeks, were purchased from Taconic Farms, Inc. Animals were fed an ad libitum Western diet (35% fat–49% carbohydrate–16% protein kcal) for a 5-day acclimation period, after which they were injected subcutaneously with 1 × 105 LAPC-4 tumor cells in 0.1 mL of Matrigel (Becton Dickinson). All mice were housed one mouse per cage 10 days postinjection to permit precise measurements of caloric intake (30). Two weeks postinjection, all mice were randomized to one of three diets: NCKD (84% fat–0% carbohydrate–16% protein kcal), 10% carbohydrate diet (74% fat–10% carbohydrate–16% protein kcal), or 20% carbohydrate diet (64% fat–20% carbohydrate–16% protein kcal). The diets were prepared by TestDiet (Table 1). Another 10 mice not injected were fed a low-fat diet (12% fat–72% carbohydrate–16% protein kcal) ad libitum and served as the reference group in a modified paired-feeding protocol. We previously observed that mice on NCKD tended to overeat and gained weight when fed ad libitum relative to mice fed a low-fat diet. On the contrary, isocaloric feeding for this diet group led to substantial weight loss (19). Based on these observations, we performed an initial 4-week pilot feeding study (without tumor injection) to determine the precise amount of excess calories all low-carbohydrate-fed mice must consume to maintain body weights similar to those ad libitum fed with the low-fat diet. We observed that NCKD-fed mice consumed ~10% extra calories relative to those ad libitum fed with the low-fat diet, whereas mice fed with 10% and 20% carbohydrates required ~7.5% extra calories to maintain similar body weights (data not shown). Such an excess in calorie intake is less than the natural proclivity of these mice to overeat, and thus forced feeding was not necessary. Mice were weighed three times per week, and tumor dimensions were measured twice a week with calipers once palpable. Tumor volumes were then calculated using the formula width × height × length × 0.5236 (10).
Table 1.
Ingredients of experimental diets
| Low-fat % Energy | NCKD % Energy | 10% Carbohydrate % Energy | 20% Carbohydrate % Energy | |
|---|---|---|---|---|
| Fat—total | 12.0 | 83.0 | 73.6 | 64 |
| Corn oil | 0.6 | 2.8 | 2.2 | 1.7 |
| Milk fat | 5.7 | 27.8 | 21.6 | 17.5 |
| Lard | 5.7 | 27.8 | 21.6 | 17.5 |
| Protein | 15.7 | 15.8 | 15.8 | 15.8 |
| Casein | 15.5 | 26.8 | 23.9 | 22.1 |
| DL-Methionine | 0.2 | 0.4 | 0.4 | 0.4 |
| Carbohydrate | 72.3 | 1.2 | 10.5 | 20.2 |
| Dextrin | 7.2 | 0.0 | 0.0 | 0 |
| Maltodextrin 10 | 14.5 | 0.0 | 0.0 | 0 |
| Sucrose | 50.6 | 0.0 | 0.0 | 0 |
| Corn starch | 0.0 | 0.0 | 12.5 | 23.7 |
| Cholesterol | 0.0 | 0.2 | 0.2 | 0.2 |
| AIN-76 mineral mix | 0.0 | 4.8 | 4.4 | 4.4 |
| AIN-76 vitamin mix | 0.0 | 1.4 | 1.3 | 1.3 |
| Cellulose | 0.0 | 6.9 | 10.9 | 10.3 |
| Calcium carbonate | 0.0 | 0.0 | 0.0 | 0.6 |
| Choline bitartrate | 0.0 | 0.3 | 0.3 | 0.3 |
| Total grams | 100.0 | 100.0 | 100.0 | 100 |
Two days before randomization and then again 4 weeks postrandomization (both posttumor injection), mice were bled through the facial vein to measure blood glucose, using a handheld Accu-Chek Active glucometer (Roche Diagnostics). At this time, urine was also expressed by gentle suprapubic pressure to measure urinary ketones using Ketostix semiquantitative urine strips (Bayer Corporation). Animals were euthanized using a lethal dose of nembutol when tumors reached 1,000 mm3 or when the health of the animal seemed compromised per Duke institutional criteria (ruffled fur, hunched posture, lethargy, severe weight loss, etc.). Serum was obtained through cardiac puncture. Tumor samples were cut in half and either snap-frozen at −80°C or preserved in a 10% formalin solution for subsequent analysis. Serum from the median surviving 10 mice from each experimental group (total 30 mice) were assayed for murine levels of insulin, IGF-I, and IGF binding protein-3 (IGFBP-3) using a mouse-specific enzyme-linked immunoassay (Millipore Corp.; R&D Systems, Inc.). Serum levels of human prostate-specific antigen (PSA) were determined using human-specific enzyme-linked immunoassay (Abazyme LLC).
Statistical analysis
The primary end-point was survival, defined as time from randomization to sacrifice, which we examined using a log-rank test. Graphically, survival was represented using Kaplan-Meier curves. Comparisons in calories consumed and body weights, tumor volumes, IGF-hormone levels, and serum glucose and ketone levels were determined using the Kruskal-Wallis test. All statistical analyses were done using STATA 10.0 (Stata Corp.), with P ≤ 0.05 considered statistically significant.
Results
Caloric intake and body weights
Despite the overall extra consumption of calories compared with the low-fat group (+9.8% kcal NCKD, +6.0% kcal 10% carbohydrate, +4.8% kcal 20% carbohydrate, respectively; Fig. 1A), all mice in the low-carbohydrate groups weighed less than those in the low-fat group (P < 0.04; data not shown). Among the low-carbohydrate groups, mice consuming NCKD weighed significantly less than the other two groups (P < 0.05), although they consumed the most calories (Fig. 1B). Consistent with the increase in caloric intake, NCKD-fed mice had the highest glucose levels at the time of sacrifice (P < 0.001; Fig. 2A, bottom), although all mice had similar serum glucose levels before randomization (P = 0.62; Fig. 2A, top). Moreover, although not significant, all groups had reduced glucose levels at sacrifice relative to prerandomization (P > 0.07). At the time of sacrifice, all low-carbohydrate-fed mice had increased their ketone production (Fig. 2B, bottom) compared with their levels before randomization (Fig. 2B, top). There were no significant differences in ketone production among the groups consuming NCKD, 10% carbohydrate, and 20% carbohydrate diets either before randomization or at the time of sacrifice (prerandomization, P = 0.79; sacrifice, P = 0.37).
Fig. 1.
Mouse energy intake and body weights. A total of 150 severe combined immunodeficient mice (6-week-old males) were fed NCKD, 10% carbohydrate (Carb) diet, or 20% carbohydrate diet 2 weeks after injection with LAPC-4 tumor cells. An extra 10 mice not injected with tumor were fed a low-fat diet and used as the reference group in a modified paired-feeding protocol. A, energy intake was measured for each mouse three times per week by subtracting the weight of uneaten food from the weight of the food placed into the cages at the beginning of each feeding period. B, body weights of mice were measured three times per week. Each value represents the mean of each group (*, P < 0.05). The SEM is represented by error bars.
Fig. 2.
Analysis of glucose and ketones. Box-plot representation of serum glucose (A) and urinary ketone (B) levels before randomization (day -3; top) and at sacrifice (bottom). Closed circles represent outliers.
Tumor growth and survival
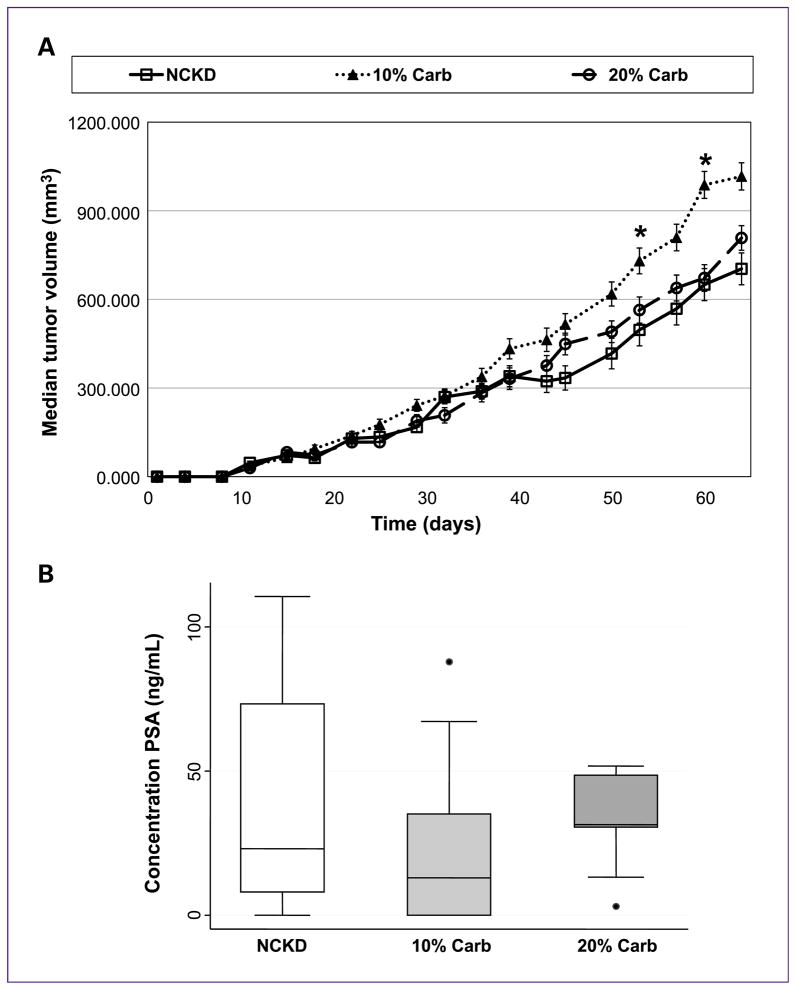
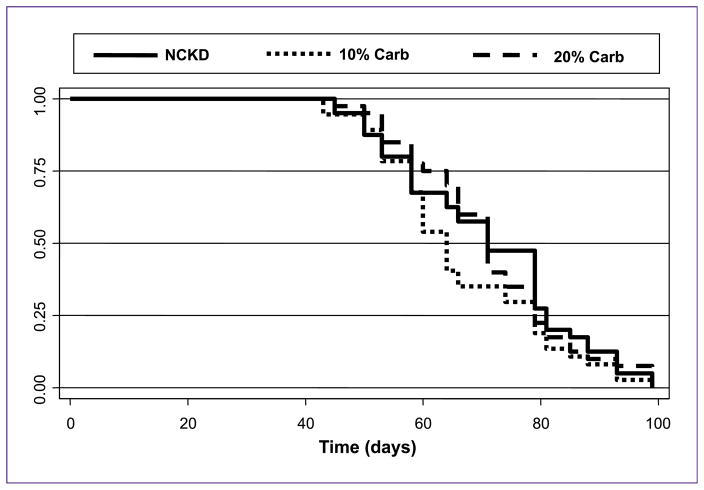
Although NCKD-fed mice were significantly lighter than those fed with 10% and 20% carbohydrates at multiple time points throughout the study, there were no significant differences in tumor volumes among groups at any time except at days 52 and 59, where mice fed with 10% carbohydrate had larger tumors (P < 0.05; Fig. 3A). Moreover, analysis of serum PSA levels at sacrifice confirmed no significant differences in tumor size (P = 0.43; Fig. 3B). There was also no significant difference in the overall survival between the three low-carbohydrate groups (P = 0.32; Fig. 4).
Fig. 3.
LAPC-4 xenograft tumor growth in severe combined immunodeficient mice. Mice were injected subcutaneously in the flank with 1 × 105 LAPC-4 tumor cells in 0.1 mL of Matrigel. Once the tumors became palpable, tumor volume was measured two times per week. A, values are expressed as the median of each group. Curves only extend to 65 days because after this time, >50% of the mice fed with the 10% carbohydrate diet had been sacrificed, and therefore the median tumor volume is not meaningful (*, P < 0.04, Kruskal-Wallis). The SEM is represented by error bars. B, box-plot representation of serum PSA levels at the time of sacrifice. Closed circles represent outliers.
Fig. 4.
Kaplan-Meier survival plot of overall mouse survival by dietary group (P = 0.32).
Analysis of the IGF axis
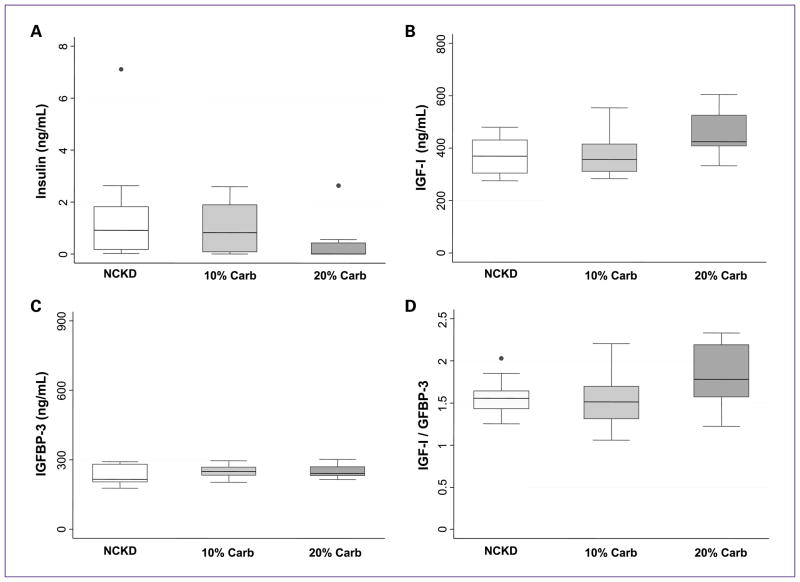
To investigate the effect of the various low-carbohydrate diets on the IGF axis, we quantified levels of insulin, IGF-I, and IGFBP-3 at the time of sacrifice (Fig. 5). Insulin levels were significantly lower in the 20% carbohydrate group compared with all other groups (P = 0.03; Fig. 5A). Differences in expression trended toward significance for IGF-I (P = 0.07; Fig. 5B) and the IGF-I/IGFBP-3 ratio, an indication of the level of active IGF-I (P = 0.12; Fig. 5D). Although not statistically significant, the levels of both IGF-I and the IGF-I:IGFBP-3 ratio trended to be the highest in mice fed with 20% carbohydrate. Expression of IGFBP-3 was not significantly different among all three groups (P = 0.55; Fig. 5C).
Fig. 5.
Analysis of the IGF axis. Box-plot representation of serum hormone levels within the IGF axis at the time of sacrifice. A, insulin. B, IGF-I. C, IGFBP-3. D, ratio between IGF-I and IGFBP-3. Closed circles represent outliers.
Discussion
Diet as a treatment for cancer is an exciting and novel therapy option. Although much evidence indicates that a low-fat diet is successful in slowing tumor growth in animal studies (8–11), recent data suggest that carbohydrate-restricted diets may also act in a similar fashion, although the extent of carbohydrate restriction required to maintain these effects is unknown (19, 27). Carbohydrate-restricted diets also do not affect androgen levels (31), which in turn leads to better quality of life for the patient. However, several of the animal studies used NCKD, which is nearly impossible to translate into human clinical trials. Therefore, in this study, we investigated the effect of consuming one of several low-carbohydrate diets on prostate tumor growth in a xenograft model. Our results showed that mice consuming a low-carbohydrate diet (10–20% kcal carbohydrates) had similar tumor growth and overall survival to mice consuming NCKD (Figs. 3 and 4), which is further supported by similar expression levels of PSA in all three groups (Fig. 3). Taken together, these results led us to conclude that there is no difference between low-carbohydrate and no-carbohydrate diets in terms of prostate cancer growth and progression.
Consistent with previous xenograft studies, we found that despite the NCKD-consuming mice eating ~10% more calories than the low-fat reference group, they weighed less. Moreover, the percentage of extra calories needed to maintain similar body weights across the low-carbohydrate arms decreased as the carbohydrate content in the diets increased (Fig. 1). This is consistent with many clinical trials that show that low-carbohydrate diets are effective tools for weight loss (5, 26, 32, 33). All three diets also lowered serum glucose levels and increased urinary ketone production by the time of sacrifice compared with levels before randomization (Fig. 2). Given the hypothesized importance of these factors in cancer control, these findings suggest that these diets may slow cancer growth. Indeed, we have previously shown in two independent xenograft studies that mice consuming NCKD have slower tumor growth and an increase in overall survival compared with mice consuming a Western diet (19, 27).
We also quantified levels of IGF-I, IGFBP-3, and insulin to determine the effect of low-carbohydrate diets on the regulation of the IGF axis. Although not statistically significant, we found that levels of IGF-I and the IGF-I:IGFBP-3 ratio trended to be higher in mice fed with the 20% carbohydrate diet (Fig. 5B–D). Several studies indicate that lower expression levels of IGF-I are linked with slower prostate tumor growth in vitro and in vivo (10, 20, 23–25). High levels of IGFBP-3 are also thought to be anticancer, as binding of this protein to IGF-I sequesters the growth factor, rendering it inactive (reviewed in ref. 34). Interestingly, IGFBP-3 may have proapoptotic activity independent of IGF-I (35). Despite the higher levels of IGF-I, which would presumably promote growth in the 20% carbohydrate arm, survival times were similar. This is perhaps explained by the significantly lower levels of insulin in the 20% carbohydrate arm (Fig. 5A). Indeed, multiple pre-clinical studies suggest that insulin is a growth factor for prostate cancer (36). In fact, there are data to suggest that patients with early-onset diabetes mellitus whose pancreas is no longer able to produce insulin are significantly less likely to develop prostate cancer, independent of diet changes, oral medications, and insulin use (37). It was unexpected that the lowest levels of insulin were observed in mice fed with 20% carbohydrate, but there are possible explanations for this phenomenon. First, there is always the possibility for a type I error in the analysis. Second, it is known that low-carbohydrate diets promote insulin sensitivity in animals (38) and humans (39, 40). Thus, it is possible that a diet containing a small amount of carbohydrates may actually improve insulin sensitivity compared with a diet completely lacking of carbohydrates.
Initially, we hypothesized that our low-carbohydrate diets would cause an increase in tumor growth and a decrease in overall survival compared with the no-carbohydrate diet, as diets rich in carbohydrates have been shown to promote tumor growth (41) and lead to an overall increase in the incidence of prostate cancer (2, 42). However, results from the study showed no difference in either of these outcomes between the three dietary interventions, suggesting that severe carbohydrate restriction is not necessary to achieve optimal benefit. It is plausible that a carbohydrate intake threshold exists in which lowering the carbohydrate content below this threshold no longer affects baseline insulin levels and therefore overall tumor growth inhibition, and potentially makes sense from an evolutionary standpoint. It is believed that early humans consumed diets that were rich in fat and protein, and low, but not completely void, of carbohydrates (43, 44). These carbohydrates tended to be unrefined and high in fiber. As societies became more industrialized, Western diets steadily became more carbohydrate based, correlating with an increase in obesity, cardiovascular disease, and cancer incidence. In fact, one study by Frassetto et al. examined the effect of a paleolithic “caveman”-type diet—high in meat, vegetables, and nuts—in nonobese, healthy patients compared with their consumption of a typical Western diet (45). They observed a significant reduction in blood pressure, plasma insulin, glucose, total cholesterol, low-density lipoproteins, and triglycerides during the period in which these patients consumed the paleolithic-type diet. Although this study examined diet intervention in healthy patients, it is of interest that lowered insulin levels were observed in their study, similar to our results in a tumor xenograft model. Therefore, it is possible that reduction of carbohydrates aids in well-being, but a small quantity is still necessary for optimal benefit. For example, although no-carbohydrate diets are effective inhibitors of tumor growth, they also restrict the brain of proper nutrition, which primarily metabolizes glucose. In a starvation state, the brain metabolizes ketone bodies instead of glucose (46). Although ketosis seems to be beneficial for brain function (16, 47), some studies suggest that ketogenic diets increase fatigue and mood changes (16, 47–49). Therefore, it is plausible that a low-carbohydrate diet provides proper nutrients to the brain while still restricting the tumor of dietary needs. Further studies would need to be done to determine where the precise threshold of carbohydrate content for optimal health yet maintenance of tumor control lies.
Although the current study does not conclusively indicate that low-carbohydrate diets slow tumor growth, prior xenograft studies by our group show that NCKD is successful in slowing tumor growth relative to a Western diet across multiple prostate cancer models (19, 27). NCKD-fed mice also had higher urinary ketone levels and expression of serum IGFBP-3 and lower expression of serum IGF-I and insulin levels compared with Western diet–fed mice at the time of sacrifice in these prior studies. In this study, we found all of these levels except for insulin to be similar between mice consuming NCKD, 10% carbohydrate diet, and 20% carbohydrate diet, making it plausible that a low-carbohydrate diet may indeed slow tumor growth relative to a Western diet. However, further analysis would need to be done to test this hypothesis directly.
Conclusion
The results from this study show that mice consuming diets containing 10% to 20% kcal carbohydrates have similar tumor growth, overall survival, and IGF axis signaling as mice consuming NCKD. Given our previous results that NCKD slowed tumor growth versus a Western diet, this implies that survival benefits may be possible through the use of less-restrictive low-carbohydrate dietary interventions.
Acknowledgments
Grant Support
Department of Veterans Affairs; Division of Urology, Department of Surgery, Duke University; and NIH grant R01 CA131235.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.American Cancer Society. Cancer Statistics 2009. 2009 [cited; Available from: http://www.cancer.org/docroot/CRI/content/CRI_2_4_1X_What_are_the_key_statistics_for_prostate_cancer_36.asp.
- 2.Baade PD, Youlden DR, Krnjacki LJ. International epidemiology of prostate cancer: geographical distribution and secular trends. Mol Nutr Food Res. 2009;53:171–84. doi: 10.1002/mnfr.200700511. [DOI] [PubMed] [Google Scholar]
- 3.Gann PH, Hennekens CH, Sacks FM, Grodstein F, Giovannucci E, Stampfer MJ. Prospective study of plasma fatty acids and risk of prostate cancer. J Natl Cancer Inst. 1994;86:281–6. doi: 10.1093/jnci/86.4.281. [DOI] [PubMed] [Google Scholar]
- 4.Kolonel LN, Nomura AM, Cooney RV. Dietary fat and prostate cancer: current status. J Natl Cancer Inst. 1999;91:414–28. doi: 10.1093/jnci/91.5.414. [DOI] [PubMed] [Google Scholar]
- 5.Lopez Fontana CM, Recalde Rincon GM, Messina Lombino D, Uvilla Recupero AL, Perez Elizalde RF, Lopez Laur JD. Body mass index and diet affect prostate cancer development. Actas Urol Esp. 2009;33:741–6. doi: 10.1016/s0210-4806(09)74225-1. [DOI] [PubMed] [Google Scholar]
- 6.Walker M, Aronson KJ, King W, et al. Dietary patterns and risk of prostate cancer in Ontario, Canada. Int J Cancer. 2005;116:592–8. doi: 10.1002/ijc.21112. [DOI] [PubMed] [Google Scholar]
- 7.Wu K, Hu FB, Willett WC, Giovannucci E. Dietary patterns and risk of prostate cancer in U.S. men. Cancer Epidemiol Biomarkers Prev. 2006;15:167–71. doi: 10.1158/1055-9965.EPI-05-0100. [DOI] [PubMed] [Google Scholar]
- 8.Kobayashi N, Barnard RJ, Said J, et al. Effect of low-fat diet on development of prostate cancer and Akt phosphorylation in the Hi-Myc transgenic mouse model. Cancer Res. 2008;68:3066–73. doi: 10.1158/0008-5472.CAN-07-5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ngo TH, Barnard RJ, Anton T, et al. Effect of isocaloric low-fat diet on prostate cancer xenograft progression to androgren independence. Cancer Res. 2004;64:1252–4. doi: 10.1158/0008-5472.can-03-3830. [DOI] [PubMed] [Google Scholar]
- 10.Ngo TH, Barnard RJ, Cohen P, et al. Effect of isocaloric low-fat diet on human LAPC-4 prostate cancer xenografts in sever combined immunodeficient mice and the insulin-like growth factor axis. Clin Cancer Res. 2003;9:2734–43. [PubMed] [Google Scholar]
- 11.Wang Y, Corr JG, Thaler HT, Tao Y, Fair WR, Heston WD. Decreased growth of established human prostate LNCaP tumors in nude mice fed a low-fat diet. J Natl Cancer Inst. 1995;87:1456–62. doi: 10.1093/jnci/87.19.1456. [DOI] [PubMed] [Google Scholar]
- 12.Mavropoulos JC, Isaacs WB, Pizzo SV, Freedland SJ. Is there a role for a low-carbohydrate ketogenic diet in the management of prostate cancer? Urology. 2006;68:15–8. doi: 10.1016/j.urology.2006.03.073. [DOI] [PubMed] [Google Scholar]
- 13.Dyson PA. A review of low and reduced carbohydrate diets and weight loss in type 2 diabetes. J Hum Nutr Diet. 2008;21:530–8. doi: 10.1111/j.1365-277X.2008.00896.x. [DOI] [PubMed] [Google Scholar]
- 14.Last AR, Wilson SA. Low-carbohydrate diets. Am Fam Physician. 2006;73:1942–8. [PubMed] [Google Scholar]
- 15.Mann J, McAuley K. Carbohydrates: is the advice to eat less justified for diabetes and cardiovascular health? Curr Opin Lipidol. 2007;18:9–12. doi: 10.1097/MOL.0b013e328012b63c. [DOI] [PubMed] [Google Scholar]
- 16.Baranano KW, Hartman AL. The ketogenic diet: uses in epilepsy and other neurologic illnesses. Curr Treat Options Neurol. 2008;10:410–9. doi: 10.1007/s11940-008-0043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seyfried TN, Kiebish M, Mukherjee P, Marsh J. Targeting energy metabolism in brain cancer with calorically restricted ketogenic diets. Epilepsia. 2008;(Suppl 8):114–6. doi: 10.1111/j.1528-1167.2008.01853.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhou W, Mukherjee P, Kiebish MA, Markis WT, Mantis JG, Seyfried TN. The calorically restricted ketogenic diet, and effective alternative therapy for malignant brain cancer. Nutr Metab. 2007;4:1–15. doi: 10.1186/1743-7075-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedland SJ, Mavropoulos J, Wang A, et al. Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate. 2008;68:11–9. doi: 10.1002/pros.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barnard RJ, Ngo TH, Leung PS, Aronson WJ, Golding LA. A low-fat diet and/or strenuous exercise alters the IGF axis in vivo and reduces prostate tumor cell growth in vitro. Prostate. 2003;56:201–6. doi: 10.1002/pros.10251. [DOI] [PubMed] [Google Scholar]
- 21.Dewell A, Weidner G, Sumner MD, et al. Relationship of dietary protein and soy isoflavones to serum IGF-1 and IGF binding proteins in the Prostate Cancer Lifestyle Trial. Nutr Cancer. 2007;58:35–42. doi: 10.1080/01635580701308034. [DOI] [PubMed] [Google Scholar]
- 22.Giovannucci E, Pollak M, Liu Y, et al. Nutritional predictors of insulin-like growth factor I and their relationships to cancer in men. Cancer Epidemiol Biomarkers Prev. 2003;12:84–9. [PubMed] [Google Scholar]
- 23.Gunnell D, Oliver SE, Peters TJ, et al. Are diet-prostate cancer associations mediated by the IGF axis? A cross-sectional analysis of diet, IGF-1 and IGFBP-3 in healthy middle-aged men. Br J Cancer. 2003;88:1682–6. doi: 10.1038/sj.bjc.6600946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ngo TH, Barnard RJ, Tymchuk CN, Cohen P, Aronson WJ. Effect of diet and exercise on serum insulin, IGF-1, and IGFBP-1 levels and growth of LNCaP cells in vitro. Cancer Causes Control. 2002;13:929–35. doi: 10.1023/a:1021911517010. [DOI] [PubMed] [Google Scholar]
- 25.Powolny AA, Wang S, Carlton PS, Hoot DR, Clinton SK. Interrelationships between dietary restriction, the IGF-1 axis, and expression of vascular endothelial growth factor by prostate adenocarcinoma in rats. Mol Carcinog. 2008;47:458–65. doi: 10.1002/mc.20403. [DOI] [PubMed] [Google Scholar]
- 26.Saxton JM. Diet, physical activity and energy balance and their impact on breast and prostate cancers. Nutr Res Rev. 2006;19:197–215. doi: 10.1017/S095442240720294X. [DOI] [PubMed] [Google Scholar]
- 27.Mavropoulos JC, Buschmeyer WC, III, Tewari AK, et al. The effects of varying dietary carbohydrate and fat content on survival in a murine LNCaP prostate cancer xenograft model. Cancer Prev Res. 2009;2:557–65. doi: 10.1158/1940-6207.CAPR-08-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low- carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359:229–41. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 29.Vekateswaran V, Haddad AQ, Fleshner NE, et al. Association of diet-induced hyperinsulinemia with accelerated growth of prostate cancer (LNCaP) xenografts. J Natl Cancer Inst. 2007;99:1793–800. doi: 10.1093/jnci/djm231. [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee P, Sotnikov AV, Mangian HJ, Zhou J, Visek WJ, Clinton SK. Energy intake and prostate tumor growth, angiogenesis, and vascular endothelial growth factor expression. J Natl Cancer Inst. 1999;91:512–23. doi: 10.1093/jnci/91.6.512. [DOI] [PubMed] [Google Scholar]
- 31.Volek JS, Sharman MJ, Love DM, et al. Body composition and hormonal responses to a carbohydrate-restricted diet. Metabolism. 2002;51:864–70. doi: 10.1053/meta.2002.32037. [DOI] [PubMed] [Google Scholar]
- 32.Brinkworth GD, Buckley JD, Noakes M, Clifton PM, Wilson CJ. Long-term effects of a very low-carbohydrate diet and a low-fat diet on mood and cognitive function. Arch Inter Med. 2009;169:1873–80. doi: 10.1001/archinternmed.2009.329. [DOI] [PubMed] [Google Scholar]
- 33.Lim SS, Noakes M, Keogh JB, Clifton PM. Long-term effects of a low-carbohydrate, low-fat, or high unsaturated fat diet compared to no-intervention control. Nutr Metab Cardiovasc Dis. doi: 10.1016/j.numecd.2009.05.003. Epub 2009 Aug 19. [DOI] [PubMed] [Google Scholar]
- 34.Ali O, Cohen P, Lee KW. Epidemiology and biology of insulin-like growth factor binding protein-3 (IGFBP-3) as an anti-cancer molecule. Horm Metab Res. 2003;35:726–33. doi: 10.1055/s-2004-814146. [DOI] [PubMed] [Google Scholar]
- 35.Liu B, Lee KW, Anzo M, et al. Insulin-like growth factor-binding protein-3 inhibition of prostate cancer growth involves suppression of angiogenesis. Oncogene. 2007;26:1811–9. doi: 10.1038/sj.onc.1209977. [DOI] [PubMed] [Google Scholar]
- 36.Lima GA, Correa LL, Gabrich R, Miranda LC, Gadelha MR. IGF-1, insulin, and prostate cancer. Arq Bras Endocrinol Metabol. 2009;53:969–75. doi: 10.1590/s0004-27302009000800010. [DOI] [PubMed] [Google Scholar]
- 37.Pierce BL, Plymate S, Ostrander EA, Stanford JL. Diabetes mellitus and prostate cancer risk. Prostate. 2008;68:1126–32. doi: 10.1002/pros.20777. [DOI] [PubMed] [Google Scholar]
- 38.Leite JO, DeOgburn R, Ratliff JC, et al. Low-carbohydrate diet disrupts the association between insulin resistance and weight gain. Metabolism. 2009;58:1116–22. doi: 10.1016/j.metabol.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 39.McKeown NM, Meigs JB, Liu S, et al. Dietary carbohydrates and cardiovascular disease risk factors in the Framingham offspring cohort. J Am Coll Nutr. 2009;28:150–8. doi: 10.1080/07315724.2009.10719766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volek JS, Phinney SD, Forsythe CE, et al. Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet. Lipids. 2009;44:297–309. doi: 10.1007/s11745-008-3274-2. [DOI] [PubMed] [Google Scholar]
- 41.Aronson WJ, Barnard RJ, Freedland SJ, et al. Growth inhibitory effect of low fat diet in prostate cancer cells: results of a prospective, randomized dietary intervention trial in men with prostate cancer. J Urol. 2010;183:345–50. doi: 10.1016/j.juro.2009.08.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ambrosini GL, Fritschi L, de Klerk NH, Mackerras D, Leavy J. Dietary patterns identified using factor analysis and prostate cancer risk: a case control study in Western Australia. Ann Epidemiol. 2008;18:364–70. doi: 10.1016/j.annepidem.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 43.Samaras T, Bartke A, Rollo CD. Human body size and the laws of scaling. Hauppauge (NY): Nova Science Publishers; 2007. [Google Scholar]
- 44.Ungar PS, Teaford MF. Human diet: its origin and evolution. Santa Barbara (CA): Praeger; 2002. [Google Scholar]
- 45.Frassetto LA, Schloetter M, Mietus-Snyder M, Morris RC, Jr, Sebastian A. Metabolic and physiologic improvements from consuming a paleolithic, hunter-gatherer type diet. Eur J Clin Nutr. 2009;63:947–55. doi: 10.1038/ejcn.2009.4. [DOI] [PubMed] [Google Scholar]
- 46.Cahill GF, Jr, Veech RL. Ketoacids? Good medicine? Trans Am Clin Climatol Assoc. 2003;114:149–63. [PMC free article] [PubMed] [Google Scholar]
- 47.Noh HS, Kim YS, Choi WS. Neuroprotective effects of the ketogenic diet. Epilepsia. 2008;49:120–3. doi: 10.1111/j.1528-1167.2008.01855.x. [DOI] [PubMed] [Google Scholar]
- 48.Brown AJ. Lowcarb diets, fasting and euphoria: is there a link between ketosis and γ-hydroxybutyrate (GHB)? Med Hypotheses. 2007;68:268–71. doi: 10.1016/j.mehy.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 49.White AM, Johnston CS, Swan PD, Tjonn SL, Sears B. Blood ketones are directly related to fatigue and perceived effort during exercise in overweight adults adhering to low-carbohydrate diets for weight loss: a pilot study. J Am Diet Assoc. 2007;107:1792–6. doi: 10.1016/j.jada.2007.07.009. [DOI] [PubMed] [Google Scholar]