Background: Synaptic and extrasynaptic GABAA receptors mediate phasic and tonic inhibition, respectively.
Results: Overexpression of extrasynaptic GABAA receptors decreases synaptic GABAergic transmission.
Conclusion: Synaptic and extrasynaptic GABAA receptors may have intrinsic competition in single individual neurons.
Significance: Phasic and tonic inhibition interacts to maintain homeostasis of total inhibition.
Keywords: GABA Receptors, Neurobiology, Neurons, Neurotransmitter Release, Synapses
Abstract
The GABAA receptors are the major inhibitory receptors in the brain and are localized at both synaptic and extrasynaptic membranes. Synaptic GABAA receptors mediate phasic inhibition, whereas extrasynaptic GABAA receptors mediate tonic inhibition. Both phasic and tonic inhibitions regulate neuronal activity, but whether they regulate each other is not very clear. Here, we investigated the functional interaction between synaptic and extrasynaptic GABAA receptors through various molecular manipulations. Overexpression of extrasynaptic α6β3δ-GABAA receptors in mouse hippocampal pyramidal neurons significantly increased tonic currents. Surprisingly, the increase of tonic inhibition was accompanied by a dramatic reduction of the phasic inhibition, suggesting a possible homeostatic regulation of the total inhibition. Overexpressing the α6 subunit alone induced an up-regulation of δ subunit expression and suppressed phasic inhibition similar to overexpressing the α6β3δ subunits. Interestingly, blocking all GABAA receptors after overexpressing α6β3δ receptors could not restore the synaptic GABAergic transmission, suggesting that receptor activation is not required for the homeostatic interplay. Furthermore, insertion of a gephyrin-binding-site (GBS) into the α6 and δ subunits recruited α6GBSβ3δGBS receptors to postsynaptic sites but failed to rescue synaptic GABAergic transmission. Thus, it is not the positional effect of extrasynaptic α6β3δ receptors that causes the down-regulation of phasic inhibition. Overexpressing α5β3γ2 subunits similarly reduced synaptic GABAergic transmission. We propose a working model that both synaptic and extrasynaptic GABAA receptors may compete for limited receptor slots on the plasma membrane to maintain a homeostatic range of the total inhibition.
Introduction
GABAA receptor-mediated inhibition is critical to maintain normal brain functions. GABAA receptors are localized at both synaptic and extrasynaptic membranes and mediate phasic and tonic inhibition, respectively (1). Synaptic GABAA receptors typically contain the γ2 subunit, whereas δ subunit-containing GABAA receptors often localize at extrasynaptic sites. Our recent work revealed that, in addition to γ2 and δ subunits, α subunits also play a direct role in targeting GABAA receptors to synaptic and extrasynaptic sites (2). Dysfunction of both synaptic and extrasynaptic GABAA receptors has been associated with neurological disorders such as anxiety, depression, and epilepsy (1, 3–5). Because synaptic and extrasynaptic GABAA receptors have distinct spatial localization and mediate distinct temporal inhibition, it is unclear how synaptic and extrasynaptic GABAA receptors may interact with each other to regulate the total inhibition.
Synaptic GABAA receptors are mainly composed of α1–3β2–3γ2 subunits. The γ2 and α1–3 subunits together target the GABAA receptors to postsynaptic sites, juxtaposing to presynaptic GABAergic terminals to respond rapidly to vesicular GABA release (2, 6, 7). Gene deletion of either the γ2 or α1/α2/α3 subunit will result in a significant decrease of GABAA receptor puncta in neuronal subcellular regions (8–12). Loss of postsynaptic GABAA receptor clusters often leads to a reduction of presynaptic GABAergic terminals, possibly because of a retrograde feedback (13–16). The extrasynaptic GABAA receptors are mainly composed of α4βδ, α6βδ, and α5βγ2 subunits (17, 18). The α5-GABAA receptors are mainly localized at extrasynaptic membranes but are also found in synaptic sites (19, 20). The δ-containing GABAA receptors are mostly found in the dentate and cerebellar granule cells, as well as in the thalamic neurons (21–24). In developing cerebellum, the level of tonic inhibition increases significantly in the first postnatal month, but synaptic GABAergic transmission decreases substantially in the same time (25, 26). It has also been reported that in transgenic mice with ectopic expression of the α6 subunit, tonic conductance increases, but synaptic GABA responses decrease simultaneously (27). These studies suggest a possible interplay between synaptic and extrasynaptic GABAA receptors, but the molecular mechanism underlying such interaction is currently unknown.
In this study, we employed a series of molecular manipulations of extrasynaptic GABAA receptors to investigate their functional effects on fast GABAergic synaptic transmission. Overexpression of α6β3δ receptors in cultured hippocampal pyrimidal neurons resulted in a dramatic decrease of synaptic GABAergic events as well as a significant reduction of presynaptic GABAergic terminals. Surprisingly, such a functional outcome did not require the activation of extrasynaptic GABAA receptors. We further demonstrated that the down-regulation of phasic inhibition is not due to the positional effect of α6β3δ receptors because inserting a gephyrin-binding site (GBS)3 into the α6 and δ subunits targeted α6GBSβ3δGBS receptors to postsynaptic sites but could not rescue phasic inhibition. Interestingly, overexpressing α5β3γ2-receptors also decreased synaptic GABAergic transmission. On the basis of these results, we propose that synaptic and extrasynaptic GABAA receptors will compete for limited receptor slots on the cell surface so that the total GABA inhibition can be regulated in a homeostatic manner.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection
Hippocampal and cortical neurons were prepared form newborn C57BL/6 mouse pups (of either sex) as described previously (2). Briefly, the hippocampi or cerebral cortex tissue was cut into 1-mm3 cubes and digested with 0.05% trypsin-EDTA containing 50 units/ml DNase I. Dissociated single neurons were then plated onto a monolayer of astrocytes on poly-D-lysine-coated coverglass at a density of 1 × 104 cells/cm2. The neuronal culture medium contained 500 ml minimum Eagle's medium (Invitrogen), 5% fetal bovine serum (HyClone, Logan, UT), 10 ml B-27 supplement (Invitrogen), 100 mg NaHCO3, 0.5 mm l-glutamine, 25 unit/ml penicillin/streptomycin, and 4 μm AraC to suppress the excessive proliferation of astrocytes. Cells were maintained in a 5% CO2 incubator at 37 °C for 2–3 weeks.
Neurons were transfected at 2–4 days in vitro using our modified high-efficiency calcium phosphate transfection protocol (28). About 1 μg of each plasmid was used for the α6, α6β3δ, or α2β3γ2 transfection. The α6GBS, β3, and δGBS subunits were cotransfected at a ratio of 2:1:0.5 μg. About 0.4 μg each of GFP or mCherry was coexpressed to identify the transfected neurons. Neurons at 10–14 days in vitro were used for electrophysiological and immunocytochemical analyses.
HEK 293T cells were maintained in high-glucose DMEM (Invitrogen) and supplemented with 10% fetal bovine serum and 25 units/ml penicillin/streptomycin. HEK cells were transfected with PEI (molecular weight 25,000, Polysciences, Inc.). HEK cells were split 1 day ahead and allowed to reach 90% confluence by the time of transfection. For each well in a 24-well plate, 1 μg of total DNA was diluted in 50 μl of Opti-MEM (Invitrogen), mixed with 4 μl of PEI (1 μg/μl), sat for 5 min, and added drop-by-drop to the culture well containing 500 μl of media. After 5 h of incubation, the cells were rinsed with fresh medium to remove the excess reagents. The transfected HEK cells were used for electrophysiological study 1–3 days after transfection.
Constructs
The CMV-based expression vectors for the GABAA receptor α2, β3, and mycγ2 subunits were described previously (6). The α6 and δ constructs in the pCMVneo backbone were gifts from Dr. R. L. MacDonald (Vanderbilt University Medical Center). The α5 subunit was a gift from Dr. Matthias Kneussel (University of Hamburg Medical School). The α6GBS and δGBS were constructed in our laboratory as described recently (2). The GBS originated from the glycine receptor β subunit (29) was inserted into the intracellular loop of the δ and α6 subunits after amino acid 341 and amino acid 340, respectively.
Drugs and Treatment
GABA and tetrodotoxin (TTX) were purchased from Sigma. Bicuculline methobromide (BIC), 6,7-dinitroquinoxaline-2,3-dione (DNQX), and 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol hydrochloride (THIP) were purchased from Tocris (Ellisville, MO). DNQX and THIP were dissolved in dimethyl sulfoxide to make concentrated stock solutions. Other drugs were dissolved in deionized water. All drugs were freshly diluted to working concentrations in the bath solution on the day of the experiment. The final dimethyl sulfoxide concentration was lower than 0.1%. For chronic BIC treatment, BIC (20 μm) was added to the neuronal culture medium 2–4 days before electrophysiological recordings.
Immunocytochemistry
Neurons were fixed with 4% paraformaldehyde diluted in PBS for 12 min and permeabilized with 0.01% Triton X-100 in the blocking solution (PBS with 3% normal goat serum and 2% normal donkey serum) for 30 min. All steps were conducted at room temperature unless specified otherwise. Antibodies were diluted in the same blocking solution. The δ/δGBS subunit on the neuronal surface was labeled before permeabilization with rabbit-anti-δ-Nterm antibody (1:500, PhosphoSolutions, Aurora, CO). Other primary antibodies were applied after Triton X-100 and incubated overnight at 4 °C. The following primary antibodies were used: mouse-anti-GAD65 (1:100, Developmental Studies Hybridoma Bank), mouse-anti-gephyrin mAb7a (1:500, Synaptic Systems, Göttingen, Germany), and chicken-anti-GFP (1:2000, Abcam, Cambridge, MA). The following secondary antibodies were used as appropriate to detect primary antibodies: Alexa Fluor 488 goat-anti-chicken (1:500), Alexa Fluor 488 donkey-anti-rabbit (1:300), and Alexa Fluor 647 goat-anti-mouse (1:300, Molecular Probes, Eugene, OR).
Images for GAD immunoreactivity were captured on an inverted epifluorescent microscope (Nikon Eclipse TE2000S) equipped with a digital charge-coupled device (CCD) camera (Hamamatsu C4742-95). To quantify GABAergic synaptic terminals, images of 60 neurons were taken from each group. For each transfected neuron, two 50-μm segments of major dendrites were randomly chosen on the basis of the GFP image, and GAD-positive puncta were counted in each segment. The total number of GAD puncta along the 100-μm dendrite was compared among neurons transfected with GFP, α6β3δ, or α6 subunit alone.
To investigate the surface expression of δ or δGBS, confocal images were acquired from an Olympus FV1000 microscope. Quantification of the δ immunosignal was conducted using ImageJ software. The neuronal soma together with two major dendrites of each transfected neuron was traced manually under the mCherry image. The mean fluorescence intensity of δ immunostaining in the region of interest was analyzed. The background signal in the nearby area without any neurons was subtracted during analysis.
Electrophysiology
Whole cell recordings were performed in voltage clamp mode using a Multiclamp 700A amplifier (Molecular Devices) as described before (30). A coverglass with cultured cells was placed in the recording chamber with continuous perfusion of the bath Tyrode's solution (128 mm NaCl, 30 mm glucose, 25 mm HEPES, 5 mm KCl, 2 mm CaCl2, and 1 mm MgCl2 (pH 7.3), osmolarity ∼320 mm). Fire-polished borosilicate glass pipettes with a resistance of 3–6 MΩ were used for recording. The internal pipette solution contained 135 mm KCl, 10 mm HEPES, 2 mm EGTA, 10 mm Tris-phosphocreatine, 4 mm MgATP, and 0.5 mm Na2GTP (pH 7.3), osmolarity ∼300 mm). Recordings were conducted at room temperature. The membrane potential was held at −70 mV. Data were acquired using pClamp 9 software (Molecular Devices), sampled at 5 kHz, and filtered at 1 kHz.
Drugs were applied through a six-channel manifold connected to a valve control system (VC-6, Warner Instruments, Hamden, CT) to achieve a fast switch between different solutions. Whole cell GABA/THIP-induced currents were recorded in the presence of TTX (0.5 μm) and DNQX (10 μm) to block voltage-gated sodium channels and AMPA receptors. TTX and DNQX were also added to the bath solution to record miniature inhibitory postsynaptic currents (mIPSCs), whereas mEPSCs were recorded in the presence of TTX and bicuculline (GABAA receptor antagonist, 20 μm). To examine tonic currents in neurons, GABA (2 μm) was bath-applied, and, after the GABA-induced current reached a plateau (normally after 1 min), 40 μm bicuculline was added to block all GABAA receptors. The shift in holding current after bicuculline application was quantified as the tonic current. DNQX (10 μm) was applied throughout the entire recording period to block AMPA receptor activation.
To analyze the kinetics of mIPSCs, single events recorded from each cell were identified using MiniAnalysis software (Synaptosoft). The events were aligned at the 50% rise point, and the average traces were used for curve fitting. The decay was fitted using two-exponential equations, and the weighted time constant (τweighted) was calculated using the following equation: τweighted = (τ1 × A1 + τ2 × A2)/(A1 + A2). Pooled data were presented as mean ± S.E., and unpaired Student's t test was used for two-group comparison, whereas one-way ANOVA was used for multiple group comparison.
RESULTS
Overexpression of Extrasynaptic GABAA Receptors Down-regulates Synaptic GABAergic Transmission
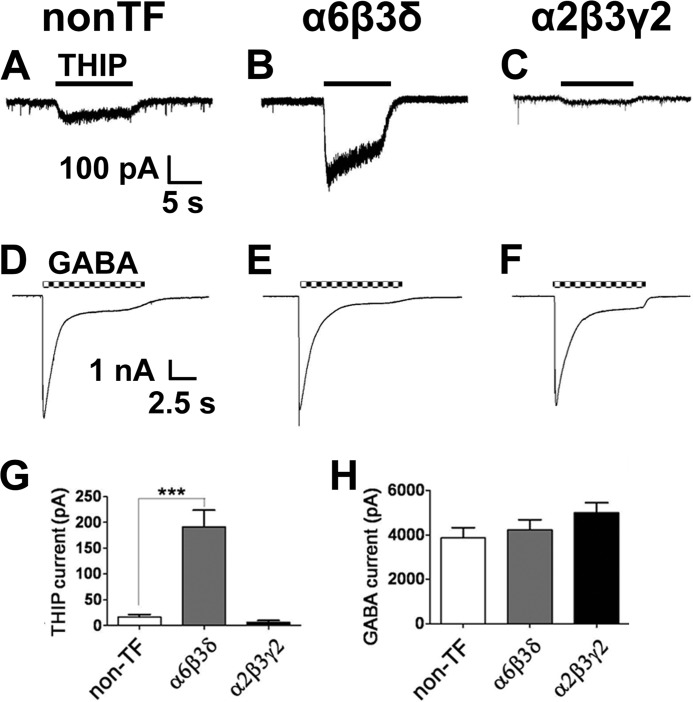
To investigate the interrelationship between synaptic and extrasynaptic GABAA receptors, we overexpressed the α6, β3, and δ subunits in cultured hippocampal neurons to increase tonic inhibition and examined the effect on synaptic GABAergic transmission. Pyramidal neurons with a large apical dendrite and multiple basal dendrites were selected for recordings. THIP (also called Gaboxadol) is a relatively selective agonist for δ-containing GABAA receptors at low concentrations (31–33). As expected, neurons transfected with α6β3δ subunits (together with GFP) showed significantly larger THIP (5 μΜ) currents, whereas α2β3γ2 overexpression did not increase THIP currents compared with non-transfected neurons (Fig. 1, A–C and G; non-TF, 17 ± 5 pA, n = 22; α6β3δ, 192 ± 32 pA, n = 11; α2β3γ2, 6 ± 3 pA, n = 10; p < 0.001, one-way ANOVA). Interestingly, overexpression of either α6β3δ or α2β3γ2 receptors did not change the whole-cell GABA (100 μΜ) currents (Fig. 1, D–F and H; non-TF, 3861 ± 461 pA, n = 20; α6β3δ, 4228 ± 453 pA, n = 12; α2β3γ2, 4997 ± 445 pA, n = 8; p > 0.3, one-way ANOVA). The γ2 subunit was myc-tagged, and immunostaining confirmed that it was expressed after transfection and clustered at postsynaptic sites (2). Thus, overexpression of α6β3δ subunits in hippocampal neurons results in a large tonic current, but the total GABA current remains unchanged. On the other hand, overexpression of α2β3γ2 subunits has no effect on the tonic current nor on the total GABA current, as if the total number of GABAA receptors on the cell surface had reached the plateau before overexpression.
FIGURE 1.
Overexpression of the α6β3δ receptors increased tonic current in cultured hippocampal neurons. A–C, representative traces showing the whole-cell currents induced by 5 μΜ THIP in non-transfected (A), α6β3δ-transfected (Β), and α2β3γ2-transfected neurons (C). D–F, representative traces showing the whole-cell currents induced by 100 μΜ GABA in non-transfected (D), α6β3δ-transfected (E), and α2β3γ2-transfected neurons (F). G, neurons expressing α6β3δ receptors showed significantly larger THIP-induced currents. Non-TF, non-transfected. ***, p < 0.001. H, overexpression of α6β3δ or α2β3γ2 receptors did not change the GABA-induced whole-cell currents.
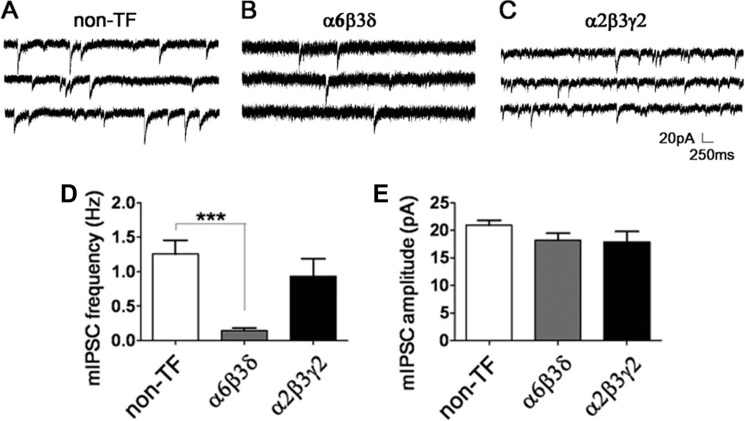
We next examined whether the increased expression of extrasynaptic GABAA receptors might affect synaptic GABAergic transmission. mIPSCs were recorded in the presence of TTX (0.5 μm) and DNQX (10 μm) to block action potentials and glutamatergic responses, respectively. The average mIPSC frequency recorded in non-transfected control neurons was 1.25 ± 0.20 Hz (n = 30, Fig. 2, A and D). Surprisingly, in neurons overexpressing α6β3δ-receptors, the mIPSC frequency was reduced significantly to 0.14 ± 0.04 Hz (Fig. 2B, n = 9, p < 0.001), a dramatic 9-fold decrease from the control level. We wondered whether such a drastic reduction was caused by transfecting multiple plasmids in neurons. Therefore, we overexpressed α2β3γ2 subunits as a control but found no significant change in the frequency of mIPSCs (Fig. 2, C and D, 0.94 ± 0.25 Hz, n = 9, p > 0.5), suggesting that transfection of multiple plasmids did not cause mIPSC reduction. Furthermore, the average mIPSC amplitude was found to be not different among the three groups (Fig. 2E, non-TF, 20.9 ± 0.9 pA, n = 30; α6β3δ, 18.2 ± 1.3 pA, n = 9; α2β3γ2, 17.9 ± 1.9 pA, n = 9; p > 0.15, one-way ANOVA). These experiments revealed that increasing extrasynaptic α6β3δ receptor expression in hippocampal neurons significantly down-regulates synaptic GABAergic transmission.
FIGURE 2.
Overexpression of the α6β3δ receptors decreased synaptic GABAergic transmission in cultured hippocampal neurons. A–C, representative traces showing mIPSCs in non-transfected (A), α6β3δ-expressing (B), and α2β3γ2-expressing neurons (C). D, the frequency of mIPSCs was reduced significantly after overexpression of α6β3δ receptors, whereas α2β3γ2 overexpression did not change the mIPSC frequency. Non-TF, non-transfected. ***, p < 0.001. E, overexpression of GABAA receptors did not change the amplitude of mIPSCs.
Overexpression of the α6 Subunit Alone Decreases Synaptic GABAergic Transmission
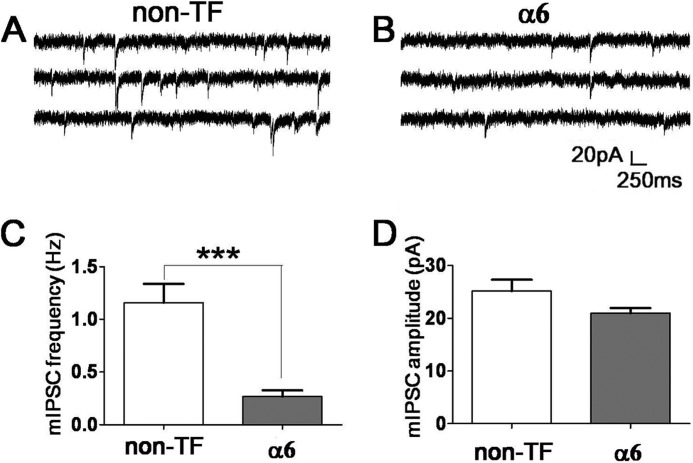
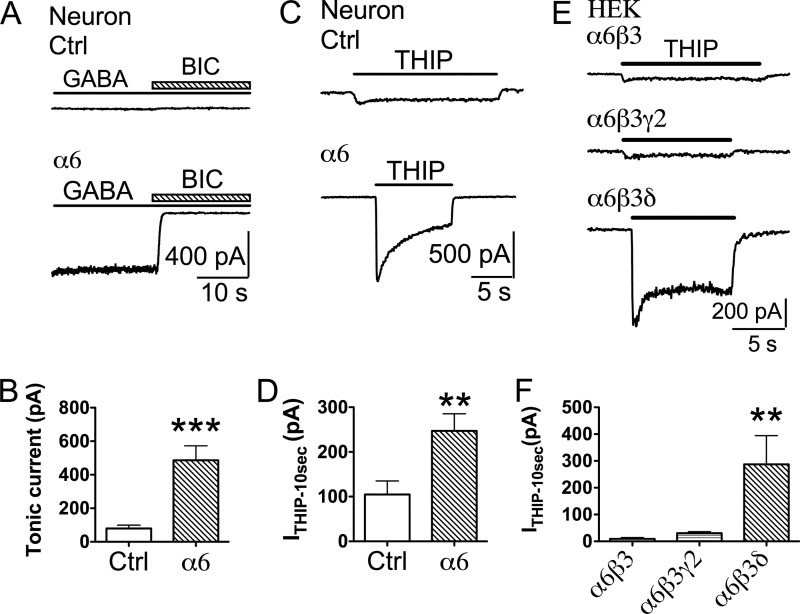
Our recent studies demonstrated that, in addition to the γ2 and δ subunits, the α subunits also play a direct role in GABAA receptor targeting (2). Therefore, we tested whether expressing the α6 subunit alone in cultured hippocampal neurons can affect synaptic GABAergic transmission. Similar to α6β3δ cotransfection, we found that transfection with the α6 subunit alone (together with mCherry for identification purposes) significantly decreased the mIPSC frequency (Fig. 3, A–C; control, 1.2 ± 0.2 Hz, n = 19; α6, 0.27 ± 0.06 Hz, n = 21; p < 0.001, unpaired Student's t test). Despite the remarkable 77% reduction in mIPSC frequency, α6 expression did not change the amplitude of mIPSCs in a significant way (Fig. 3D; control, 25.2 ± 2.2 pA, n = 19; α6, 21.0 ± 1.0 pA, n = 19; p = 0.09, unpaired Student's t test). We next investigated whether overexpressing the α6 subunit alone resulted in any changes of tonic current. In control neurons (non-transfected or mCherry-transfected), a small tonic GABA current was revealed by the application of the GABAA receptor antagonist BIC (40 μm) in the continuous presence of GABA (2 μm) and DNQX (10 μm) (Fig. 4, A and B, 79 ± 20 pA, n = 13). In contrast, the α6-transfected neurons displayed much larger tonic GABA currents (Fig. 4, A and B, 486 ± 86 pA, n = 8, p < 0.001), a 6-fold increase over the control level. Furthermore, application of THIP (5 μΜ), a relatively selective agonist for δ-containing GABAA receptors, also revealed a significantly larger tonic current in α6-transfected neurons than the control (Fig. 4, C and D; control, 104.8 ± 30.3 pA, n = 12; α6, 247.1 ± 38.3 pA, n = 8; p < 0.01; unpaired Student's t test). Therefore, overexpression of the α6 subunit alone increased tonic inhibition and down-regulated synaptic GABAergic transmission at the same time, consistent with previous findings in α6 transgenic mice (27).
FIGURE 3.
Overexpression of the α6 subunit alone decreased the mIPSC frequency. A and B, representative traces showing mIPSCs in non-transfected (non-TF) (A) and α6-transfected neurons (B). C, quantitative analysis showed a significant reduction of the mIPSC frequency in α6-transfected neurons. ***, p < 0.001. D, ectopic expression of the α6 subunit did not change the amplitude of mIPSCs significantly.
FIGURE 4.
Overexpression of the α6 subunit increased the tonic current in hippocampal pyramidal neurons. A, application of BIC (40 μΜ) revealed a significantly larger tonic current activated by 2 μm GABA (in the presence of TTX and DNQX) in α6-transfected neurons. Ctrl, control. B, quantified data illustrating larger tonic currents in α6-transfected neurons. ***, p < 0.001, unpaired Student's t test. C and D, representative traces (C) and quantified data (D) illustrating significantly larger THIP-induced (5 μΜ) currents in α6-transfected neurons. TTX (0.5 μm) and DNQX (10 μm) were used to block action potentials and glutamate receptors. **, p < 0.01. E, THIP (5 μΜ) induced small whole-cell currents in HEK cells expressing α6β3 or α6β3γ2 receptors but activated a large current in HEK cells transfected with α6β3δ receptors. F, quantified data illustrating that HEK cells expressing α6β3δ-GABAA receptors showed a significantly larger whole-cell current induced by 5 μm THIP. **, p < 0.01.
Because the α6 subunit can form multiple subtypes of functional receptors, such as α6β, α6βγ2, and α6βδ receptors (22, 34), we wondered what kind of GABAA receptor subtype was responsible for such a large tonic current in α6-transfected neurons. To answer this question, we overexpressed the α6β3, α6β3γ2, or α6β3δ subunits in HEK293T cells and tested their responses to THIP (5 μΜ). As shown in Fig. 4E, THIP-induced currents in HEK cells transfected with α6β3 or α6β3γ2 subunits were very small, but HEK cells transfected with α6β3δ subunits showed a significantly larger tonic current. Quantitatively, the average sustained current induced by THIP (5 μm) was 9.2 ± 4.8 pA (n = 6) for α6β3 receptors and 30.7 ± 5.4 pA (n = 7) for α6β3γ2 receptors, respectively (Fig. 4F). In comparison, the THIP-induced current in α6β3δ-expressing cells was 287 ± 107 pA (Fig. 4F, n = 5, p < 0.01, one-way ANOVA), a 9-fold increase over that mediated by α6β3γ2 receptors. Because overexpression of α6β3γ2 receptors in HEK cells can only generate a very small THIP current, the large tonic current observed in α6-overexpressing neurons is likely not mediated by α6βγ2 receptors but possibly by THIP-sensitive α6βδ receptors (33). However, most of our recordings were performed in hippocampal pyramidal neurons, which usually have a low level of δ receptor expression. We therefore hypothesized that the δ subunit expression level may be up-regulated in α6-transfected neurons to form α6β3δ receptors.
The α6 Subunit Induces the Expression of the δ Subunit in Pyrimidal Neurons
To test our hypothesis that the δ subunit expression can be induced by overexpressing the α6 subunit in hippocampal pyramidal neurons, we directly examined the expression level of the δ subunit on the cell surface of neurons with or without α6 transfection. Cultured neurons were transfected with mCherry alone or α6 plus mCherry, and the antibodies specific for the δ subunit (PhosphoSolutions) were used to label the neuronal surface expression in the non-permeabilized condition. As expected, the endogenous δ subunit expression level was very low in hippocampal and cortical pyrimidal neurons (Fig. 5, A and B). Intriguingly, neurons transfected with the α6 subunit alone showed a significantly elevated expression level of the δ subunit on the cell surface of both soma and dendrites (Fig. 5, C and D). It is noteworthy that the δ immunosignal showed a diffused distribution pattern in α6-transfected neurons, consistent with the pattern of α6β3δ receptors (Fig. 8B). Quantitative analysis of the fluorescent intensity showed that the expression level of the δ subunit on the plasma membrane was significantly higher after α6 transfection (Fig. 5E, mCherry, 12.1 ± 1.0 arbitrary units, n = 24; α6, 40.3 ± 3.7 arbitrary units, n = 24; p < 0.001, unpaired Student's t test). Therefore, overexpression of the α6 subunit alone in pyramidal neurons can enhance tonic inhibition through the induction of the δ subunit expression, suggesting that tonic inhibition in pyramidal neurons is much more plastic than we thought before.
FIGURE 5.
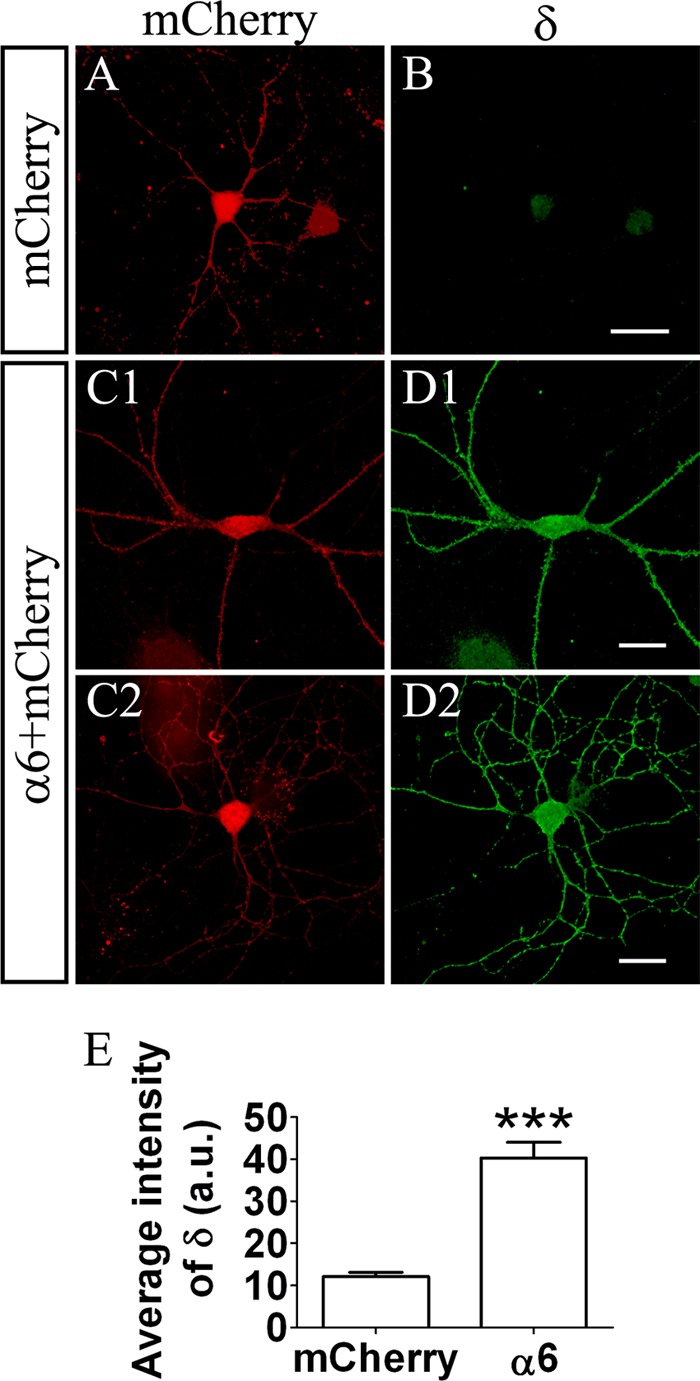
Ectopic expression of α6 subunit-induced δ subunit expression in pyramidal neurons. A and B, a control neuron transfected with mCherry (red) showed a very low level of δ immunofluorescence signal (green). C and D, two representative neurons transfected with the α6 subunit and mCherry showed increased an δ immunofluorescence signal on the soma and dendrites. Scale bars = 20 μm. E, quantified fluorescence intensity showing elevated δ expression in α6-transfected neurons (n = 24 in both groups). ***, p < 0.001, unpaired Student's t test. a.u., arbitrary units.
FIGURE 8.
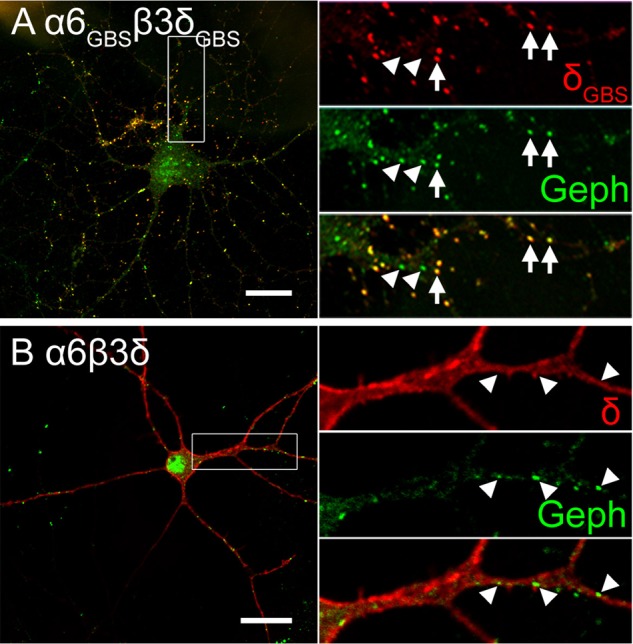
Clustering of α6GBSβ3δGBS receptors at postsynaptic sites. A, the α6GBSβ3δGBS receptors formed clusters and colocalized with gephyrin (Geph) at postsynaptic membranes. Arrows indicate δGBS puncta that colocalized with gephyrin puncta. Arrowheads indicate some gephyrin puncta devoid of δGBS receptors. B, ectopically expressed α6β3δ receptors were distributed diffusely along the plasma membrane without colocalization with gephyrin puncta. Arrowheads indicate gephyrin clusters without corresponding δ-containing receptors. Scale bar = 20 μm.
Overexpressing Extrasynaptic GABAA Receptors Reduces GABAergic Synapses
The substantial reduction of the mIPSC frequency after transfection of the α6 or α6β3δ subunits in pyramidal cells prompted us to further examine the changes in GABAergic synapses using an immunostaining method. As shown in Fig. 6A, control neurons transfected with GFP alone received a normal level of GABAergic innervation, as indicated by the GAD-positive presynaptic puncta (red) apposing the dendrites (green). However, significantly less GAD puncta were detected on the dendrites of neurons transfected with α6β3δ or α6 subunits (Fig. 6, B and C). On average, the GABAergic synaptic density on control neurons was 8.8 ± 0.5 puncta/100 μm dendrites (randomly chosen), whereas on α6β3δ- and α6-transfected neurons, the GABAergic density reduced substantially to 2.9 ± 0.3 and 4.3 ± 0.4 puncta/100 μm dendrites, respectively (Fig. 6D, p < 0.001, one-way ANOVA followed by Bonferroni's correction). No significant difference was found in the GAD puncta density between α6β3δ- and α6-transfected neurons (p > 0.05). Therefore, overexpression of extrasynaptic GABAA receptors resulted in a decrease of presynaptic GABAergic innervation, consistent with the substantial decrease of synaptic GABAergic transmission.
FIGURE 6.
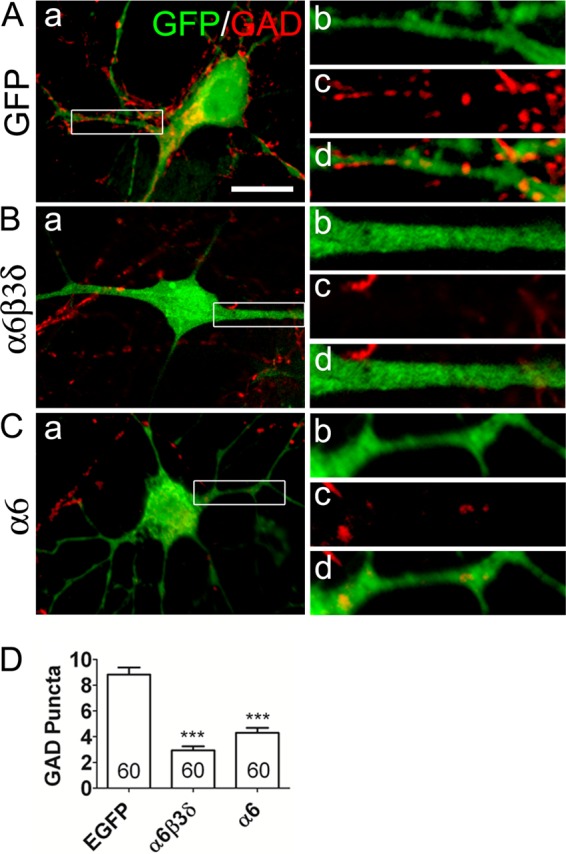
Overexpression of extrasynaptic α6β3δ-GABAA receptors decreased GABAergic innervation. A, GAD-positive presynaptic puncta (red) along the dendrites of a control neuron expressing GFP (green). B, α6β3δ-transfected neurons showed fewer GAD-positive puncta along the dendrites. C, neurons transfected with the α6 subunit also showed fewer GAD puncta. b, c, and d show enlarged views of the areas marked in a. Scale bar = 10 μm. D, quantification of GAD puncta/100 μm dendrites. ***, p < 0.001, one-way ANOVA followed by Bonferroni's correction.
Interaction between Synaptic and Extrasynaptic GABAA Receptors Is Independent of Receptor Activation
So far we have demonstrated that enhancing tonic inhibition through the overexpression of extrasynaptic GABAA receptors resulted in a significant decrease of fast synaptic GABAergic transmission. This led to our working hypothesis that, in any given neuron, there is a constant interplay between synaptic and extrasynaptic GABAA receptors to maintain a normal range of total inhibition. If tonic inhibition is substantially up-regulated, the phasic inhibition will be down-regulated and vice versa. Maintaining the total inhibition in each individual neuron may be critical for homeostatic modulation of neural network activity (35, 36). To test our hypothesis, we first investigated whether the activation of synaptic and extrasynaptic GABAA receptors is required for their interplay. For this purpose, after α6β3δ transfection, neurons were treated with bicuculline (20 μΜ) for 2–4 days to completely block all GABAA receptors, including both synaptic and extrasynaptic GABAA receptors. Global suppression of GABAergic inhibition in non-transfected neurons did result in a significant increase in mIPSC frequency (Fig. 7, A, B, and E; control, 1.04 ± 0.17 Hz, n = 19; BIC, 3.24 ± 0.61 Hz, n = 20; p < 0.01). Similarly, in α6β3δ-transfected neurons, BIC treatment also resulted in an increase in mIPSC frequency (Fig. 7, C–E; α6β3δ, 0.08 ± 0.03 Hz, n = 8; α6β3δ + BIC, 0.33 ± 0.11 Hz, n = 16; p < 0.05). The average amplitude of mIPSCs was also increased significantly by BIC treatment in non-transfected neurons (Fig. 7F; control, 24.5 ± 1.1 pA, n = 19; BIC, 29.9 ± 2.1 pA, n = 20; p < 0.05). The mIPSC amplitude in α6β3δ-transfected neurons was not changed by chronic BIC treatment (Fig. 7F; α6β3δ, 28.1 ± 4.1, n = 8; α6β3δ + BIC, 27.6 ± 1.4, n = 15; p > 0.9). Importantly, after BIC treatment, the mIPSC frequency in α6β3δ-transfected neurons remained very low compared with non-transfected neurons (Fig. 7, B, D, and E; p < 0.001). If increased tonic inhibition is a prerequisite for the down-regulation of phasic inhibition, blocking all GABAA receptor activity with bicuculline should have abolished the decrease of mIPSC frequency in the α6β3δ-transfected neurons. In contrast, our results indicate that overexpression of extrasynaptic GABAA receptors can down-regulate synaptic GABAergic transmission regardless of whether the overexpressed receptors are activated or not.
FIGURE 7.
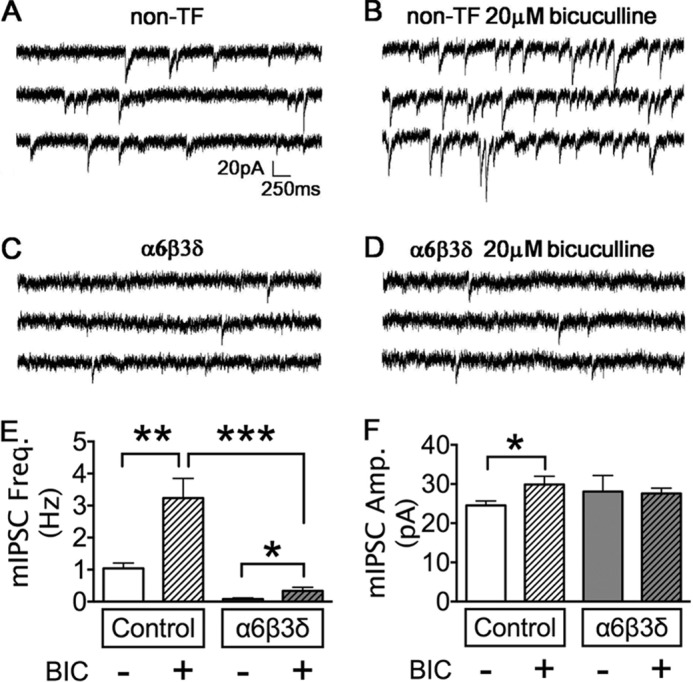
Chronic block of GABAA receptors increased GABAergic synaptic transmission but did not rescue the α6β3δ-induced mIPSC deficit. A and B, representative traces showing that chronic BIC treatment increased the mIPSC frequency in non-transfected (non-TF) neurons. C and D, neurons overexpressing α6β3δ receptors showed a low mIPSC frequency without (C) or with bicuculline treatment (D). E, quantified data showed that chronic BIC treatment significantly increased the mIPSC frequency in control neurons. The mIPSC frequency in α6β3δ-transfected neurons remained very low after BIC treatment. *, p < 0.05; **, p < 0.01; ***, p < 0.001. F, chronic BIC treatment increased the mIPSC amplitude in control but not α6β3δ-transfected neurons. *, p < 0.05.
Down-regulation of Phasic Inhibition Is Not Caused by the Positional Effect of Extrasynaptic Localization of α6β3δ Receptors
Because α6β3δ receptors are mainly localized on the extrasynaptic membranes, we wondered whether their positional effect could be causing the down-regulation of synaptic GABAergic transmission. We reasoned that if it is due to the positional effect, the defect in synaptic GABAergic transmission may be rescued if the α6β3δ receptors could be targeted to GABAergic postsynaptic sites. Indeed, we have recently engineered chimeric subunits by inserting GBSs into the intracellular loop of the α6 (α6GBS) and δ (δGBS) subunits so that they can interact with the scaffold protein gephyrin and localize to synaptic sites (2). Neurons were transfected with α6β3δ or α6GBSβ3δGBS subunits and double-immunostained for gephyrin and surface δ expression. We confirmed that α6GBSβ3δGBS receptors formed clusters in 90% of the transfected neurons (18 of 20 cells). Of the 18 cells, 14 cells showed a high level of colocalization between δGBS and gephyrin clusters (Fig. 8A, arrows). Some gephyrin-positive puncta did not colocalize with δGBS clusters (Fig. 8A, arrowheads), indicating that the α6GBSβ3δGBS receptors were not recruited to all GABAergic synapses. In contrast, in α6β3δ-transfected neurons (n = 9), δ immunoreactivity (red) was distributed diffusely throughout the soma and dendrites and not colocalized with the gephyrin clusters (Fig. 8B, green). Thus, α6GBSβ3δGBS receptors are efficiently recruited to GABAergic postsynaptic sites, distinct from the extrasynaptic localization of α6β3δ receptors.
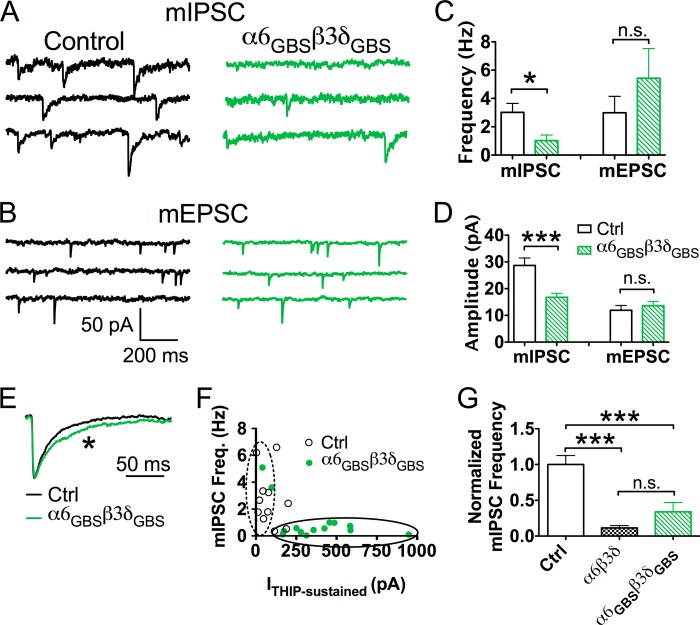
After changing their localization, we next examined the effect of α6GBSβ3δGBS receptors on the fast GABAergic synaptic transmission in pyramidal neurons. Surprisingly, the mIPSC frequency recorded in α6GBSβ3δGBS-transfected neurons still showed a significant reduction compared with the control neurons (Fig. 9, A and C; control, 3.0 ± 0.6 Hz, n = 12; α6GBSβ3δGBS, 1.0 ± 0.4 Hz, n = 14; p < 0.05). Unlike the overexpression of α6β3δ receptors, which only decreased mIPSC frequency, the amplitude of mIPSCs in the α6GBSβ3δGBS-transfected neurons was also reduced significantly compared with the control cells (Fig. 9, A and D; control, 28.7 ± 2.8 pA, n = 12; α6GBSβ3δGBS, 16.8 ± 1.5 pA, n = 14; p < 0.001), likely because of lower conductance or opening probability of α6β3δ-receptors (37, 38). No changes were found in mEPSC frequency (Fig. 9, B and C; control, 3.0 ± 1.2 Hz, n = 9; α6GBSβ3δGBS, 5.4 ± 2.1 Hz, n = 13; p > 0.3) or mEPSC amplitude (B and D; control, 11.9 ± 1.8 pA, n = 9; α6GBSβ3δGBS, 13.6 ± 1.6 pA, n = 13; p > 0.4). These results suggest that although α6GBSβ3δGBS receptors are now localized at synaptic sites, they still down-regulate GABAergic synaptic transmission.
FIGURE 9.
Overexpression of α6GBSβ3δGBS receptors decreased both the frequency and amplitude of mIPSCs. A, representative mIPSC traces in control (black) and α6GBSβ3δGBS-transfected neurons (green). B, mEPSC traces in control (black) and α6GBSβ3δGBS-transfected neurons (green). C, neurons expressing α6GBSβ3δGBS receptors had a significantly lower frequency of mIPSCs but no change in mEPSCs. *, p < 0.05; n.s., not significant. D, expression of α6GBSβ3δGBS receptors significantly reduced mIPSC amplitude, but mEPSCs remained unaffected. Ctrl, control. ***, p < 0.001. E, scaled overlay showing a slower decay phase of mIPSCs in α6GBSβ3δGBS-expressing neurons. *, p < 0.05. F, inverse relationship between the mIPSC frequency and whole-cell THIP current. The α6GBSβ3δGBS-transfected neurons showed a large THIP (5 μm) current but low mIPSC frequency, whereas control neurons showed a small THIP current but a large mIPSC frequency. G, normalized mIPSC frequency showing a similar reduction of mIPSC frequency in neurons transfected with α6GBSβ3δGBS or α6β3δ receptors. ***, p < 0.001.
We further analyzed the mIPSCs in α6GBSβ3δGBS-transfected neurons. The δ-containing GABAA receptors are known to have slower desensitization compared with many other receptor subtypes, such as γ2-containing receptors (39, 40). When examining the kinetics of the mIPSC events in the α6GBSβ3δGBS-expressing neurons, we found that these events decayed much slower than those in control neurons (Fig. 9E; control, τweighted = 37.3 ± 4.1 ms, n = 12; α6GBSβ3δGBS, τweighted = 52.3 ± 5.3 ms, n = 14; p < 0.05; unpaired Student's t test). The 10–90% rise time did not differ significantly between control and α6GBSβ3δGBS-expressing neurons (control, 2.2 ± 0.2 ms, n = 12; α6GBSβ3δGBS, 2.8 ± 0.3 ms, n = 14; p > 0.1, unpaired Student's t test). The slower decay in α6GBSβ3δGBS-expressing neurons indicated that the α6GBSβ3δGBS receptors were indeed incorporated into postsynaptic receptor clusters and contributed to the mIPSC events. Interestingly, we also observed a negative correlation between the mIPSC frequency and THIP-induced current in α6GBSβ3δGBS-transfected neurons (Fig. 9F, Pearson's test, r = −0.6, p < 0.01). 10 of 14 neurons transfected with α6GBSβ3δGBS receptors showed a THIP-induced current greater than 200 pA, and the mIPSC frequency in these 10 neurons were all lower than 1 Hz (Fig. 9F, green dots circled by the solid line). On the other hand, THIP currents in 7 of 11 control neurons were smaller than 100 pA, and the mIPSC frequency in these neurons was much higher, up to 6.2 Hz (Fig. 9F, white dots circled by the dashed line). Lastly, we compared the relative reduction in mIPSC frequency caused by α6β3δ or α6GBSβ3δGBS receptors. As shown in Fig. 9G, normalized mIPSC frequency showed that overexpressing α6β3δ receptors reduced the mIPSC frequency to 11.4 ± 3.2% of the control level (n = 10), whereas the α6GBSβ3δGBS receptors down-regulated the mIPSC frequency to 33.4 ± 13.0% of the control level (n = 14). Nevertheless, there was no statistical difference between the relative mIPSC frequency in α6β3δ- and α6GBSβ3δGBS-expressing neurons (Fig. 9G, α6β3δ versus α6GBSβ3δGBS, p > 0.2, one-way ANOVA followed by Bonferroni's correction). In conclusion, the clustering of α6GBSβ3δGBS receptors at postsynaptic sites cannot rescue the defect in fast GABAergic synaptic transmission. Therefore, the interplay between synaptic and extrasynaptic GABAA receptors cannot be attributed to the positional effect of α6β3δ receptors.
Overexpressing α5β3γ2 Receptors Also Decreased Synaptic GABAergic Transmission
Besides δ subunit-containing extrasynaptic GABAA receptors, α5 subunit-containing receptors are also largely localized at extrasynaptic membranes, although some synaptic puncta were also reported (19, 20). We therefore investigated whether overexpressing a distinctly different subtype of extrasynaptic GABAA receptors will affect synaptic GABA transmission. Immunocytochemistry experiments confirmed that the HA-α5 subunit was significantly expressed in cultured hippocampal neurons (Fig. 10, A–F). Our recent study also demonstrated that overexpressing HA-α5β3γ2 subunits significantly increased the tonic current in cultured hippocampal neurons (41). Interestingly, we found that after overexpressing α5β3γ2 receptors, the frequency of mIPSCs was reduced significantly compared with non-transfected controls (Fig. 10, G and H). Quantified data showed that the frequency, but not the amplitude, of mIPSCs was decreased in α5β3γ2-transfected neurons (Fig. 10, I and J). Therefore, similar to the overexpression of α6β3δ receptors, increasing the expression level of α5β3γ2 receptors also leads to a reduction of synaptic GABA transmission.
FIGURE 10.
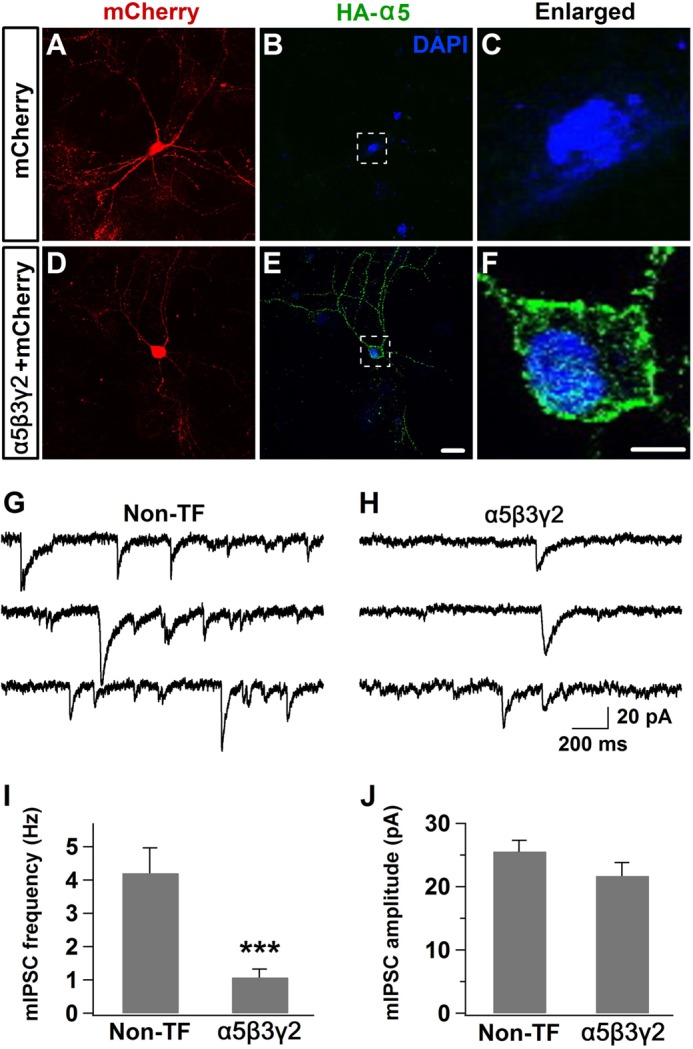
Overexpression of α5-GABAA receptors also decreased synaptic GABAergic transmission. A–C, control hippocampal neurons overexpressing mCherry showed no HA staining. D–F, hippocampal neurons overexpressing the HA-α5β3γ2 subunits showed a substantial HA staining signal throughout the soma and dendrites. G and H, representative traces showing reduced mIPSC events in hippocampal neurons overexpressing α5-GABAA receptors. Non-TF, non-transfected. I and J, quantitative data showing the reduction of mIPSC frequency but not the amplitude after overexpressing α5-GABAA receptors (n = 10–11). ***, p < 0.001, Student's t test. Scale bars = 20 μm for A, B, D, and E and 5 μm for C and F.
DISCUSSION
About half of the GABAA receptors are at synaptic sites, and the other half are on extrasynaptic membranes. How synaptic and extrasynaptic GABAA receptors interact with each other to maintain the homeostasis of GABAergic inhibition has not been well studied. In this work, we demonstrated that overexpression of extrasynaptic α6β3δ receptors in cultured pyramidal neurons resulted in a substantial decrease of the mIPSC frequency and presynaptic GABAergic terminals. Thus, there appears to be a homeostatic interplay between tonic and phasic inhibition. Interestingly, such an interaction between synaptic and extrasynaptic GABAA receptors does not require the receptor activation, suggesting a novel form of homeostatic plasticity. The extrasynaptic localization of the α6β3δ-receptors is also not a precondition for the suppression of synaptic GABAergic transmission because molecularly engineered α6GBSβ3δGBS receptors can cluster at synaptic sites but fail to rescue the mIPSC defects. These results suggest that synaptic and extrasynaptic GABAA receptors may interact with each other in an unconventional way to maintain a delicate balance between tonic and phasic inhibition.
Homeostatic Regulation of GABAergic Inhibition
Homeostatic plasticity in the central nervous system refers to the capability of neural networks to maintain their activity level within a normal range in response to a variety of stimulations. One type of homeostatic plasticity, synaptic scaling, was first demonstrated in excitatory synapses, and alteration of network activity by TTX or BIC may uniformly change the synaptic strength in all synapses (35). The mechanism of such homeostatic scaling has been studied extensively in excitatory synapses (42–44). In contrast, homeostatic plasticity in inhibitory synapses is much less studied (45). Global inhibition of network activity by TTX decreased the mIPSC amplitude as well as the number of GAD-positive puncta (46). Interestingly, homeostatic synaptic scaling at inhibitory synapses only occurred after inhibiting global activity with TTX, whereas hyperpolarizing individual neurons by overexpressing potassium channels did not change the GABAergic synaptic efficacy (47). Conversely, increasing network activity resulted in an increase of mIPSC frequency and amplitude (36, 48). Our bicuculline experiments confirmed that perturbing global activity can induce homeostatic plasticity at inhibitory synapses. However, in individual neurons overexpressing α6β3δ-GABAA receptors, the mIPSC frequency was still decreased drastically in the presence of BIC (Fig. 7). Our results have two significant implications. 1) The interplay between synaptic and extrasynaptic GABAA receptors does not require the activation of these receptors. 2) The homeostatic down-regulation of synaptic GABAergic transmission after overexpressing extrasynaptic GABAA receptors is cell-autonomous and not because of global activity changes. Therefore, the interaction between tonic and phasic inhibition may represent a novel form of homeostatic plasticity.
In fact, GABAergic inhibition may have several different types of homeostatic plasticity. One well studied area is the compensatory changes of GABAA receptors following targeted gene deletion of specific subunits. For example, targeted deletion of the GABAA receptor α1 subunit resulted in a significant increase in α2 and α3 subunit expression as well as an elevated number of morphological synapses. However, electrophysiological study showed that the amplitude and frequency of mIPSCs were reduced in α1−/− mice, suggesting that the compensation by α2 and α3 subunits cannot completely rescue the loss of GABAergic transmission because of the deletion of the α1 subunit (49). Another type of plasticity is the change of specific GABAA receptor subunits induced by pathological conditions such as epileptic seizures (50–56). Moreover, GABAA receptor-mediated inhibition may be interacting with other channel-mediated inhibition to maintain homeostatic neuronal activity. For example, in α6−/− neurons, α6βδ-GABAA receptor-mediated tonic conductance was reduced greatly, but a continuously active form of potassium conductance emerged to maintain normal neuronal excitability (23). On the other hand, in HCN1 (hyperpolarization-activated cyclic nucleotide-gated channel 1) knockout neurons, the inhibitory function of HCN1 was partially compensated by increased tonic inhibition mediated by α5-containing extrasynaptic GABAA receptors (57). These various forms of homeostatic plasticity suggest that it is pivotal to maintaining neural inhibition in a normal range for proper neural network function.
Interplay between Synaptic and Extrasynaptic GABAA Receptors
A potential interplay between tonic and phasic inhibition came from observations during normal brain development, particularly in cerebellar granule cells. At postnatal day 7 (P7), spontaneous IPSCs contribute to most of the total charge transfer mediated by GABAA receptors (25). As the animals grow older (P21), both the frequency and amplitude of IPSCs decrease substantially, whereas a tonic form of inhibition takes over to control the granule cell firing rate as well as the information flow through the cerebellar cortex (25, 26, 58, 59). Such a developmental shift from phasic to tonic inhibition correlates very well with the up-regulation of α6 and δ subunit expression in the cerebellum, with the tonic current contributing to the majority of the total charge transfer by P21 (25, 59–61). The more direct evidence suggesting a possible homeostatic interplay between synaptic and extrasynaptic GABAA receptors came from transgenic mice overexpressing the GABAA receptor α6 subunit (27). Ectopic expression of the α6 subunit in hippocampal pyramidal neurons increased the tonic current but decreased the spontaneous IPSC frequency. An immunoelectron microscopic assay revealed an extrasynaptic localization of the α6-containing receptors (27). However, the authors suggested that α6 was assembled into receptors composed of the α6βγ2, α1α6βγ2, and α3α6βγ2 subunits. In this study, overexpressing the α6 subunit alone is mimicking the expression of α6β3δ subunits in increasing tonic inhibition and decreasing phasic inhibition, which is also reminiscent of the finding in α6 transgenic mice. However, we demonstrated that α6βγ2 receptors cannot generate a large tonic current (Fig. 4). More importantly, our immunostaining experiments directly demonstrated that α6 overexpression can induce an up-regulation of δ subunit expression (Fig. 5). Therefore, the overexpressed α6 subunit is more likely assembled with the up-regulated δ subunit to form α6βδ receptors, which will mediate the enhanced tonic inhibition.
Such an intimate association between the α6 and δ subunits is not surprising. A previous study has demonstrated that the inactivation of α6 subunit expression suppresses δ subunit expression (62). Similarly, in α6−/− mice, δ subunit expression is almost abolished in cerebellar granule cells (23). Our study suggests that the opposite may also be true. In hippocampal pyramidal neurons where the δ subunit expression level is typically very low, overexpression of the α6 subunit can strongly up-regulate δ subunit expression. Thus, tonic inhibition mediated by δ-containing receptors may be quite plastic, particularly in brain regions with a low expression level of the δ subunit under normal physiological conditions. In support of this notion, it has been reported that the mRNA encoding the δ subunit was present (at a low level) in the CA1-CA3 region of the adult hippocampus (63) and that the δ subunit gene promoter may be active in a subpopulation of pyramidal cells (64). It is unclear at this time whether the δ subunit in pyramidal neurons is constantly translated but fails to assemble into functional receptors because of the lack of endogenous α6/α4 partners or whether the translation of the δ subunit itself requires the α6/α4 partners. In any case, the low level of δ subunit expression at basal conditions may leave ample room for the up-regulation of tonic inhibition in pyramidal cells.
The α5 subunit is initially expressed widely in the developing brain but gradually restricted to the hippocampus and olfactory bulb (60). The decrease of the α5 subunit is accompanied with an increase of the α1 subunit during brain development (60). Thus, it is easier to understand a potential competition between the α5 and α1 subunits. The overexpression of the α5 subunit may displace some α1 subunit and, hence, decrease synaptic GABAergic inhibition. Our recent work demonstrated that overexpression of both the α6β3δ and α5β3γ2 receptors significantly inhibited the formation of epileptiform activity in hippocampal neurons (41). This work suggests that synaptic GABA inhibition is actually reduced after enhancing tonic inhibition. Therefore, it is pivotal to look at both synaptic and extrasynaptic GABAA receptors when assessing the net effect of GABA inhibition on neural network activity. In conclusion, tonic inhibition mediated by extrasynaptic GABAA receptors is highly dynamic, and the interaction between synaptic and extrasynaptic GABAA receptors plays an important role in controlling the homeostatic level of the total inhibition.
A Working Model. Synaptic and Extrasynaptic GABAA Receptors Compete for Limited Receptor Slots on the Plasma Membrane
The easiest explanation for the decreased phasic inhibition after an increased tonic inhibition is that neurons have to maintain an optimal level of total inhibition to control overall neuronal excitability and global network activity. The difficult question is how neurons sense the increase of tonic inhibition and how they send the signal to decrease phasic inhibition. We thought initially that, after overexpressing extrasynaptic GABAA receptors, the enhanced tonic inhibition reduced neuronal firing in the transfected neurons and that the reduced neuronal activity down-regulates synaptic IPSCs through the homeostatic scaling mechanism. However, we discovered that chronic blockage of GABAA receptor activity by bicuculline did not restore the GABAergic synaptic transmission in α6β3δ-overexpressed neurons. Under bicuculline treatment, neurons cannot sense the enhanced tonic inhibition, suggesting that the down-regulation of phasic inhibition cannot be attributed to the elevated tonic inhibition. Because both synaptic and extrasynaptic GABAA receptors are blocked by bicuculline, we suspected that there might be a “physical competition” between these receptors.
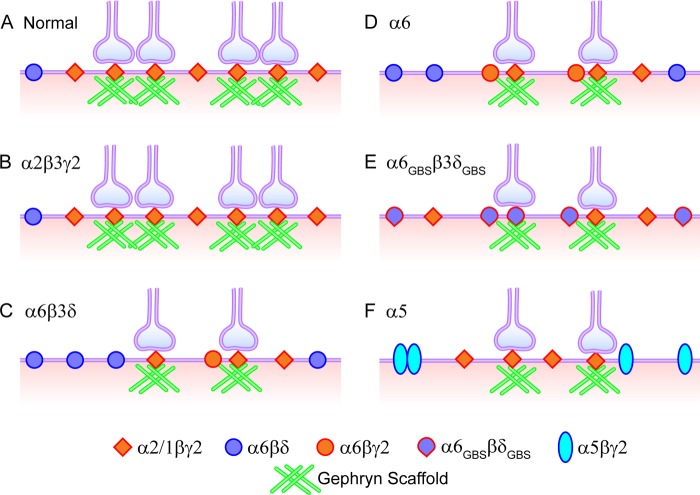
Although there may be several possible models to explain all of the data observed here, we propose a working model that both synaptic and extrasynaptic GABAA receptors have to compete for the limited receptor slots on the plasma membrane. It is the total number of GABAA receptors on the cell surface that controls the total inhibition (Fig. 11). Under normal conditions, about half of the GABAA receptors are at synaptic sites, and half are on extrasynaptic membranes. Some of the γ2-containing receptors on the extrasynaptic membranes will diffuse laterally and be stabilized eventually at synaptic sites (65). When additional extrasynaptic GABAA receptors are overexpressed, they competitively occupy more receptor slots on the cell surface so that the number of slots available for γ2-receptors is reduced greatly. As a result, the fast synaptic GABAergic transmission is reduced. Such a competition model does not require the activation of the synaptic or extrasynaptic GABAA receptors. This model not only explains the results of overexpressing the α6 and α6β3δ subunits but also explains the results of overexpressing the α2β3γ2, α5β3γ2, and α6GBSβ3δGBS subunits. Overexpressing the α2β3γ2 subunits did not increase the whole-cell GABA current nor change the mIPSC frequency or amplitude, suggesting that α2β3γ2 receptors usually occupy the synaptic receptor slots in neurons and that α2β3γ2 overexpression will not further increase their occupancy. In the case of overexpressing α6GBSβ3δGBS subunits, although α6GBSβ3δGBS receptors can be recruited to synaptic sites, the total number of γ2-receptor clusters at synaptic sites is still reduced because of some receptor slots occupied by α6GBSβ3δGBS receptors. Because α6GBSβ3δGBS receptors cannot generate large GABA responses to compensate for the loss of γ2-receptors (2), the phasic inhibition is reduced significantly. It is worth to point out that besides receptor slot competition, the α6 subunit may compete with the α1–3 subunits for the γ2 subunit and that the resulting α6βγ2 receptors may likely reside at perisynaptic sites, between synaptic and extrasynaptic sites, as demonstrated by our recent work (2). It is not clear how many α6 subunits will assemble into α6β3γ2 and how many will assemble into α6β3δ receptors when overexpressing the α6 or α6β3δ subunits in pyramidal neurons. The overexpression of the α5 subunit may result in direct competition with the α1–3 subunits and increase extrasynaptic receptors but, simultaneously, reduce synaptically localized receptors.
FIGURE 11.
A working model illustrating the homeostatic competition between synaptic and extrasynaptic GABAA receptors. There are limited slots for GABAA receptors on the plasma membrane, with half being synaptic and half extrasynaptic. Overexpression of α2β3γ2 receptors does not further increase the total receptor number or the number of GABAergic synapses. In contrast, overexpression of α6β3δ receptors takes some slots that are usually occupied by α1–3βγ2 receptors, causing the reduction of postsynaptic GABAA receptor clusters and presynaptic GABAergic terminals. Overexpression of the α6 subunit induces surface expression of α6β3δ and α6β3γ2 receptors, also resulting in the down-regulation of synaptic GABAergic transmission. The α6GBSβ3δGBS receptors can bind with gephyrin and cluster at synaptic sites, still occupying the receptor slots typically occupied by α1–3βγ2 receptors, causing a reduction of synaptic GABAergic transmission. Finally, overexpression of α5β3γ2 receptors also reduces the number of α1–3βγ2 receptors on the plasma membrane, resulting in decreased synaptic GABAergic transmission.
Our working model predicts that the total number of receptor slots for GABAA receptors may be limited on the plasma membrane and that both synaptic and extrasynaptic GABAA receptors will compete to occupy the same pool of receptor slots on the cell surface. Alternatively, it is also possible that receptor assembly or receptor trafficking may be the rate-limiting step for different receptors to compete. For example, the exocytotic machinery for GABAA receptors may be limited, so that when more α6β3δ receptors are exocytosed to the cell surface, fewer γ2-receptors will be exocytosed (7, 17). The limited slot hypothesis has been proposed in the study of calcium channels at nerve terminals, where N-type and P/Q-type Ca2+ channels may compete for channel slots to trigger vesicular release (66). Considering the large number of membrane proteins and a limited cell surface, it is possible that only limited membrane slots are allocated for each specific type of receptor or channel and that different subtypes within the same family will have to compete with each other to achieve functional homeostasis.
This work was supported, in whole or in part, by National Institute of Health Grants MH083911 and MH092740 (to G. C.). This work was also supported by Natural Science Foundation of China Grant 31129003 (to G. C. and Y. W.).
- GBS
- gephyrin-binding site
- TTX
- tetrodotoxin
- BIC
- bicuculline methobromide
- DNQX
- 6,7-dinitroquinoxaline-2,3-dione
- THIP
- 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol hydrochloride
- mIPSC
- miniature inhibitory postsynaptic current
- mEPSC
- miniature excitatory postsynaptic current
- ANOVA
- analysis of variance
- GAD
- glutamic acid decarboxylase
- P7
- postnatal day 7.
REFERENCES
- 1. Brickley S. G., Mody I. (2012) Extrasynaptic GABAa receptors. Their function in the CNS and implications for disease. Neuron 73, 23–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu X., Wu Z., Ning G., Guo Y., Ali R., Macdonald R. L., De Blas A. L., Luscher B., Chen G. (2012) γ-Aminobutyric acid type A (GABAa) receptor α subunits play a direct role in synaptic versus extrasynaptic targeting. J. Biol. Chem. 287, 27417–27430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Macdonald R. L., Kang J. Q., Gallagher M. J. (2010) Mutations in GABAa receptor subunits associated with genetic epilepsies. J. Physiol. 588, 1861–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fritschy J. M. (2008) Epilepsy, E/I balance and GABAa receptor plasticity. Front. Mol. Neurosci. 1, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hines R. M., Davies P. A., Moss S. J., Maguire J. (2012) Functional regulation of GABAa receptors in nervous system pathologies. Curr. Opin. Neurobiol. 22, 552–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alldred M. J., Mulder-Rosi J., Lingenfelter S. E., Chen G., Lüscher B. (2005) Distinct γ2 subunit domains mediate clustering and synaptic function of postsynaptic GABAa receptors and gephyrin. J. Neurosci. 25, 594–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luscher B., Fuchs T., Kilpatrick C. L. (2011) GABAa receptor trafficking-mediated plasticity of inhibitory synapses. Neuron 70, 385–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Essrich C., Lorez M., Benson J. A., Fritschy J. M., Lüscher B. (1998) Postsynaptic clustering of major GABAa receptor subtypes requires the γ 2 subunit and gephyrin. Nat. Neurosci. 1, 563–571 [DOI] [PubMed] [Google Scholar]
- 9. Fritschy J. M., Panzanelli P., Kralic J. E., Vogt K. E., Sassoè-Pognetto M. (2006) Differential dependence of axo-dendritic and axo-somatic GABAergic synapses on GABAa receptors containing the α1 subunit in Purkinje cells. J. Neurosci. 26, 3245–3255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kralic J. E., Sidler C., Parpan F., Homanics G. E., Morrow A. L., Fritschy J. M. (2006) Compensatory alteration of inhibitory synaptic circuits in cerebellum and thalamus of γ-aminobutyric acid type A receptor α1 subunit knockout mice. J. Comp. Neurol. 495, 408–421 [DOI] [PubMed] [Google Scholar]
- 11. Studer R., von Boehmer L., Haenggi T., Schweizer C., Benke D., Rudolph U., Fritschy J. M. (2006) Alteration of GABAergic synapses and gephyrin clusters in the thalamic reticular nucleus of GABAa receptor α3 subunit-null mice. Eur. J. Neurosci. 24, 1307–1315 [DOI] [PubMed] [Google Scholar]
- 12. Panzanelli P., Gunn B. G., Schlatter M. C., Benke D., Tyagarajan S. K., Scheiffele P., Belelli D., Lambert J. J., Rudolph U., Fritschy J. M. (2011) Distinct mechanisms regulate GABAa receptor and gephyrin clustering at perisomatic and axo-axonic synapses on CA1 pyramidal cells. J. Physiol. 589, 4959–4980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li R. W., Yu W., Christie S., Miralles C. P., Bai J., Loturco J. J., De Blas A. L. (2005) Disruption of postsynaptic GABA receptor clusters leads to decreased GABAergic innervation of pyramidal neurons. J. Neurochem. 95, 756–770 [DOI] [PubMed] [Google Scholar]
- 14. Fang C., Deng L., Keller C. A., Fukata M., Fukata Y., Chen G., Lüscher B. (2006) GODZ-mediated palmitoylation of GABAa receptors is required for normal assembly and function of GABAergic inhibitory synapses. J. Neurosci. 26, 12758–12768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yuan X., Yao J., Norris D., Tran D. D., Bram R. J., Chen G., Luscher B. (2008) Calcium-modulating cyclophilin ligand regulates membrane trafficking of postsynaptic GABAa receptors. Mol. Cell Neurosci. 38, 277–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yu W., Jiang M., Miralles C. P., Li R. W., Chen G., de Blas A. L. (2007) Gephyrin clustering is required for the stability of GABAergic synapses. Mol. Cell Neurosci. 36, 484–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jacob T. C., Moss S. J., Jurd R. (2008) GABAa receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat. Rev. Neurosci. 9, 331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Farrant M., Nusser Z. (2005) Variations on an inhibitory theme. Phasic and tonic activation of GABAa receptors. Nat. Rev. Neurosci. 6, 215–229 [DOI] [PubMed] [Google Scholar]
- 19. Brünig I., Scotti E., Sidler C., Fritschy J. M. (2002) Intact sorting, targeting, and clustering of γ-aminobutyric acid A receptor subtypes in hippocampal neurons in vitro. J. Comp. Neurol. 443, 43–55 [DOI] [PubMed] [Google Scholar]
- 20. Christie S. B., de Blas A. L. (2002) α5 Subunit-containing GABAa receptors form clusters at GABAergic synapses in hippocampal cultures. Neuroreport 13, 2355–2358 [DOI] [PubMed] [Google Scholar]
- 21. Nusser Z., Sieghart W., Somogyi P. (1998) Segregation of different GABAa receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J. Neurosci. 18, 1693–1703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nusser Z., Ahmad Z., Tretter V., Fuchs K., Wisden W., Sieghart W., Somogyi P. (1999) Alterations in the expression of GABAa receptor subunits in cerebellar granule cells after the disruption of the α6 subunit gene. Eur. J. Neurosci. 11, 1685–1697 [DOI] [PubMed] [Google Scholar]
- 23. Brickley S. G., Revilla V., Cull-Candy S. G., Wisden W., Farrant M. (2001) Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature 409, 88–92 [DOI] [PubMed] [Google Scholar]
- 24. Chandra D., Jia F., Liang J., Peng Z., Suryanarayanan A., Werner D. F., Spigelman I., Houser C. R., Olsen R. W., Harrison N. L., Homanics G. E. (2006) GABAa receptor α 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc. Natl. Acad. Sci. U.S.A. 103, 15230–15235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brickley S. G., Cull-Candy S. G., Farrant M. (1996) Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAa receptors. J. Physiol. 497, 753–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wall M. J., Usowicz M. M. (1997) Development of action potential-dependent and independent spontaneous GABAa receptor-mediated currents in granule cells of postnatal rat cerebellum. Eur. J. Neurosci. 9, 533–548 [DOI] [PubMed] [Google Scholar]
- 27. Wisden W., Cope D., Klausberger T., Hauer B., Sinkkonen S. T., Tretter V., Lujan R., Jones A., Korpi E. R., Mody I., Sieghart W., Somogyi P. (2002) Ectopic expression of the GABAa receptor α6 subunit in hippocampal pyramidal neurons produces extrasynaptic receptors and an increased tonic inhibition. Neuropharmacology 43, 530–549 [DOI] [PubMed] [Google Scholar]
- 28. Jiang M., Chen G. (2006) High Ca2+-phosphate transfection efficiency in low-density neuronal cultures. Nat. Protoc. 1, 695–700 [DOI] [PubMed] [Google Scholar]
- 29. Meyer G., Kirsch J., Betz H., Langosch D. (1995) Identification of a gephyrin binding motif on the glycine receptor beta subunit. Neuron 15, 563–572 [DOI] [PubMed] [Google Scholar]
- 30. Deng L., Yao J., Fang C., Dong N., Luscher B., Chen G. (2007) Sequential postsynaptic maturation governs the temporal order of GABAergic and glutamatergic synaptogenesis in rat embryonic cultures. J. Neurosci. 27, 10860–10869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krogsgaard-Larsen P., Frølund B., Liljefors T., Ebert B. (2004) GABAa agonists and partial agonists. THIP (Gaboxadol) as a non-opioid analgesic and a novel type of hypnotic. Biochem. Pharmacol. 68, 1573–1580 [DOI] [PubMed] [Google Scholar]
- 32. Brown N., Kerby J., Bonnert T. P., Whiting P. J., Wafford K. A. (2002) Pharmacological characterization of a novel cell line expressing human α(4)β(3)δ GABAa receptors. Br. J. Pharmacol. 136, 965–974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meera P., Wallner M., Otis T. S. (2011) Molecular basis for the high THIP/gaboxadol sensitivity of extrasynaptic GABAa receptors. J. Neurophysiol. 106, 2057–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jechlinger M., Pelz R., Tretter V., Klausberger T., Sieghart W. (1998) Subunit composition and quantitative importance of hetero-oligomeric receptors. GABAa receptors containing α6 subunits. J. Neurosci. 18, 2449–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Turrigiano G. G., Leslie K. R., Desai N. S., Rutherford L. C., Nelson S. B. (1998) Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391, 892–896 [DOI] [PubMed] [Google Scholar]
- 36. Peng Y. R., Zeng S. Y., Song H. L., Li M. Y., Yamada M. K., Yu X. (2010) Postsynaptic spiking homeostatically induces cell-autonomous regulation of inhibitory inputs via retrograde signaling. J. Neurosci. 30, 16220–16231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fisher J. L., Macdonald R. L. (1997) Single channel properties of recombinant GABAa receptors containing γ 2 or δ subtypes expressed with α 1 and β 3 subtypes in mouse L929 cells. J. Physiol. 505, 283–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Keramidas A., Harrison N. L. (2008) Agonist-dependent single channel current and gating in α4β2δ and α1β2γ2S GABAa receptors. J. Gen. Physiol. 131, 163–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mody I., Pearce R. A. (2004) Diversity of inhibitory neurotransmission through GABAa receptors. Trends Neurosci. 27, 569–575 [DOI] [PubMed] [Google Scholar]
- 40. Bianchi M. T., Haas K. F., Macdonald R. L. (2001) Structural determinants of fast desensitization and desensitization-deactivation coupling in GABAa receptors. J. Neurosci. 21, 1127–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sun Y., Wu Z., Kong S., Jiang D., Pitre A., Wang Y., Chen G. (2013) Regulation of epileptiform activity by two distinct subtypes of extrasynaptic GABAa receptors. Mol. Brain 6, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Turrigiano G. G., Nelson S. B. (2004) Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 5, 97–107 [DOI] [PubMed] [Google Scholar]
- 43. Turrigiano G. G. (2008) The self-tuning neuron. Synaptic scaling of excitatory synapses. Cell 135, 422–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yu L. M., Goda Y. (2009) Dendritic signalling and homeostatic adaptation. Curr. Opin. Neurobiol. 19, 327–335 [DOI] [PubMed] [Google Scholar]
- 45. Mody I. (2005) Aspects of the homeostatic plasticity of GABAa receptor-mediated inhibition. J. Physiol. 562, 37–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kilman V., van Rossum M. C., Turrigiano G. G. (2002) Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABAa receptors clustered at neocortical synapses. J. Neurosci. 22, 1328–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hartman K. N., Pal S. K., Burrone J., Murthy V. N. (2006) Activity-dependent regulation of inhibitory synaptic transmission in hippocampal neurons. Nat. Neurosci. 9, 642–649 [DOI] [PubMed] [Google Scholar]
- 48. Rannals M. D., Kapur J. (2011) Homeostatic strengthening of inhibitory synapses is mediated by the accumulation of GABAa receptors. J. Neurosci. 31, 17701–17712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schneider Gasser E. M., Duveau V., Prenosil G. A., Fritschy J. M. (2007) Reorganization of GABAergic circuits maintains GABAa receptor-mediated transmission onto CA1 interneurons in α1-subunit-null mice. Eur. J. Neurosci. 25, 3287–3304 [DOI] [PubMed] [Google Scholar]
- 50. Brooks-Kayal A. R., Shumate M. D., Jin H., Rikhter T. Y., Coulter D. A. (1998) Selective changes in single cell GABAa receptor subunit expression and function in temporal lobe epilepsy. Nat. Med. 4, 1166–1172 [DOI] [PubMed] [Google Scholar]
- 51. Goodkin H. P., Joshi S., Mtchedlishvili Z., Brar J., Kapur J. (2008) Subunit-specific trafficking of GABAa receptors during status epilepticus. J. Neurosci. 28, 2527–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Scimemi A., Semyanov A., Sperk G., Kullmann D. M., Walker M. C. (2005) Multiple and plastic receptors mediate tonic GABAa receptor currents in the hippocampus. J. Neurosci. 25, 10016–10024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Naylor D. E., Liu H., Wasterlain C. G. (2005) Trafficking of GABAa receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J. Neurosci. 25, 7724–7733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Peng Z., Huang C. S., Stell B. M., Mody I., Houser C. R. (2004) Altered expression of the δ subunit of the GABAa receptor in a mouse model of temporal lobe epilepsy. J. Neurosci. 24, 8629–8639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. González M. I., Brooks-Kayal A. (2011) Altered GABAa receptor expression during epileptogenesis. Neurosci. Lett. 497, 218–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sun C., Mtchedlishvili Z., Erisir A., Kapur J. (2007) Diminished neurosteroid sensitivity of synaptic inhibition and altered location of the α4 subunit of GABAa receptors in an animal model of epilepsy. J. Neurosci. 27, 12641–12650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen X., Shu S., Schwartz L. C., Sun C., Kapur J., Bayliss D. A. (2010) Homeostatic regulation of synaptic excitability: tonic GABAa receptor currents replace I (h) in cortical pyramidal neurons of HCN1 knock-out mice. J. Neurosci. 30, 2611–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hamann M., Rossi D. J., Attwell D. (2002) Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron 33, 625–633 [DOI] [PubMed] [Google Scholar]
- 59. Tia S., Wang J. F., Kotchabhakdi N., Vicini S. (1996) Developmental changes of inhibitory synaptic currents in cerebellar granule neurons. Role of GABAa receptor α 6 subunit. J. Neurosci. 16, 3630–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Laurie D. J., Wisden W., Seeburg P. H. (1992) The distribution of thirteen GABAa receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J. Neurosci. 12, 4151–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zheng T., Santi M. R., Bovolin P., Marlier L. N., Grayson D. R. (1993) Developmental expression of the α 6 GABAa receptor subunit mRNA occurs only after cerebellar granule cell migration. Brain Res. Dev. Brain Res. 75, 91–103 [DOI] [PubMed] [Google Scholar]
- 62. Jones A., Korpi E. R., McKernan R. M., Pelz R., Nusser Z., Mäkelä R., Mellor J. R., Pollard S., Bahn S., Stephenson F. A., Randall A. D., Sieghart W., Somogyi P., Smith A. J., Wisden W. (1997) Ligand-gated ion channel subunit partnerships. GABAa receptor α6 subunit gene inactivation inhibits δ subunit expression. J. Neurosci. 17, 1350–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wisden W., Laurie D. J., Monyer H., Seeburg P. H. (1992) The distribution of 13 GABAa receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J. Neurosci. 12, 1040–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lüscher B., Häuselmann R., Leitgeb S., Rülicke T., Fritschy J. M. (1997) Neuronal subtype-specific expression directed by the GABAa receptor δ subunit gene promoter/upstream region in transgenic mice and in cultured cells. Brain Res. Mol. Brain Res. 51, 197–211 [DOI] [PubMed] [Google Scholar]
- 65. Bogdanov Y., Michels G., Armstrong-Gold C., Haydon P. G., Lindstrom J., Pangalos M., Moss S. J. (2006) Synaptic GABAa receptors are directly recruited from their extrasynaptic counterparts. EMBO J. 25, 4381–4389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cao Y. Q., Piedras-Rentería E. S., Smith G. B., Chen G., Harata N. C., Tsien R. W. (2004) Presynaptic Ca2+ channels compete for channel type-preferring slots in altered neurotransmission arising from Ca2+ channelopathy. Neuron 43, 387–400 [DOI] [PubMed] [Google Scholar]