Background: Whether neurogenin3 activation represents beta cell neogenesis in adults is controversial.
Results: Neurogenin3-activated pancreatic duct cells do not become beta cells.
Conclusion: Neurogenin3 activation is not a signature for adult beta cell neogenesis.
Significance: Our data strongly argue against the widely held belief that neurogenin3 is a marker of beta cell neogenesis in the adult pancreas, especially from duct cells.
Keywords: Beta Cell, Flow Cytometry, Pancreas, Reprogramming, Transcription
Abstract
It remains controversial whether adult pancreatic ducts harbor facultative beta cell progenitors. Because neurogenin3 (Ngn3) is a key determinant of pancreatic endocrine cell neogenesis during embryogenesis, many studies have also relied upon Ngn3 expression as evidence of beta cell neogenesis in adults. Recently, however, Ngn3 as a marker of adult beta cell neogenesis has been called into question by reports of Ngn3 expression in fully-developed beta cells. Nevertheless, direct evidence as to whether Ngn3 activation in adult pancreatic duct cells may lead to duct-to-beta cell transdifferentiation is lacking. Here we studied two models of Ngn3 activation in adult pancreatic duct cells (low-dose alloxan treatment and pancreatic duct ligation) and lineage-traced Ngn3-activated duct cells by labeling them through intraductal infusion with a cell-tagging dye, CFDA-SE No dye-labeled beta cells were found during the follow-up in either model, suggesting that activation of Ngn3 in duct cells is not sufficient to direct their transdifferentiation into beta cells. Therefore, Ngn3 activation in duct cells is not a signature for adult beta cell neogenesis.
Introduction
The shortage of donor pancreases is a major obstacle to the widespread application of islet transplantation to treat diabetic patients (1, 2). Therefore, great efforts have been made to identify alternative sources of insulin-producing cells (3). Previous studies have suggested that cell replication is the only mechanism for beta cell expansion in adults (4–9). However, researchers continue to seek evidence of beta cell neogenesis (10–13) since adult beta cells normally show very slow proliferation (14–20), and may quickly undergo senescence or apoptosis if forced into the cell cycle (21).
Adult pancreatic duct cells have been extensively studied for their potential to generate functional beta cells, since embryonic pancreatic ductal structures clearly harbor endocrine precursors (22–24). Recently, lineage tracing studies have been used to evaluate the possible conversion of duct cells into beta cells postnatally, but with conflicting results (25–31). In particular, in the pancreatic duct ligation (PDL)3 model, neurogenin3 (Ngn3), a pancreatic endocrine-determining transcription factor during embryonic development (32–34), was reported to be activated in the duct cells of the ligated pancreas (35). Since Ngn3 has long been regarded as a marker of beta cell neogenesis (36, 37), and since beta cell proliferation after PDL remains at a low level (35), it was logically concluded that the reported doubling of beta cell mass within 1 week of PDL is due to beta cell neogenesis from Ngn3+ duct cells (35). Notably, both this doubling of beta cell mass and the proposed duct-to-beta cell transdifferentiation in the PDL model have been challenged by subsequent studies (8, 25–28, 30, 31, 38). Tissue edema and remodeling after PDL have been sited as potential reasons for over-estimation of the increase in beta cell mass (27, 31, 38). On the other hand, independent reports have shown that Ngn3 is also expressed in adult beta cells, and can be up-regulated after PDL (8, 27, 39–41). However, direct evidence that Ngn3 activation in adult pancreatic duct cells may lead to neogenesis of beta cells is lacking (42).
Here, we examined the fate of Ngn3-activated duct cells in the adult pancreas in two models, low-dose alloxan (ALX) treatment, and PDL. We found that Ngn3 expression was activated in duct cells in both models. To lineage-trace these Ngn3-activated duct cells, we used an intraductal infusion system to efficiently and specifically label pancreatic duct cells with CFDA-SE (carboxyfluorescein diacetate, succinimidyl ester), a dye that passively enters the duct cells, and then is retained within the cells. No dye-labeled beta cells were found in either model, suggesting Ngn3 activation in duct cells is not sufficient to direct their transdifferentiation into beta cells.
EXPERIMENTAL PROCEDURES
Mouse and Cell Manipulation
All mouse experiments were approved by the Animal Research and Care Committee at the Children's Hospital of Pittsburgh and the University of Pittsburgh IACUC. Bacterial-artificial-chromosome (BAC) transgenic Elastase promoter CreERT reporter (Ela-CreERT) mouse was generated and kindly provided by Dr. Craig Logsdon (University of Texas) (43). C57/6, Rosa26CAGTomato (Tomato), BAC transgenic Ngn3 promoter Cre reporter (Ngn3-Cre) (44), BAC transgenic mouse insulin promoter GFP reporter (MIP-GFP) and ROSAmTmG (mTmG) (8) mice were all purchased from Jackson Lab, and all have a C57/6 background. Male Ngn3-Cre and female mTmG mice were crossed to generate the Ngn3-Cre; mTmG mice. Male Ela-CreERT and female Tomato mice were crossed to generate Ela-CreERT; Tomato mice. Tamoxifen (Sigma) solution (20 mg/ml) was freshly prepared the day prior to each injection, by dissolving in filter-sterilized corn oil. To induce Tomato expression in acinar cells, 4 weeks before analysis/operation, Ela-CreERT; Tomato mice were given a single intraperitoneal injection of 1 mg of tamoxifen (50 μl) or corn oil vehicle (control). 99.6 ± 2.1% acinar cells were found labeled without detectable nonspecific labeling of other cell types. Only mice that were heterozygous for both Cre (ERT) and mTmG were used for our experiments. All experiments used 8-week-old males.
The beta-cell toxin alloxan (ALX) was injected into mice via the dorsal tail vein with a dosage of 65 mg/kg or 30 mg/kg. Blood glucose measurements of mice were performed after a 2-h fasting period. PDL was performed and quality controlled (morphology, up-regulation in Ngn3 transcript in ligated tail versus unligated head of pancreas) as described by us previously (8, 45).
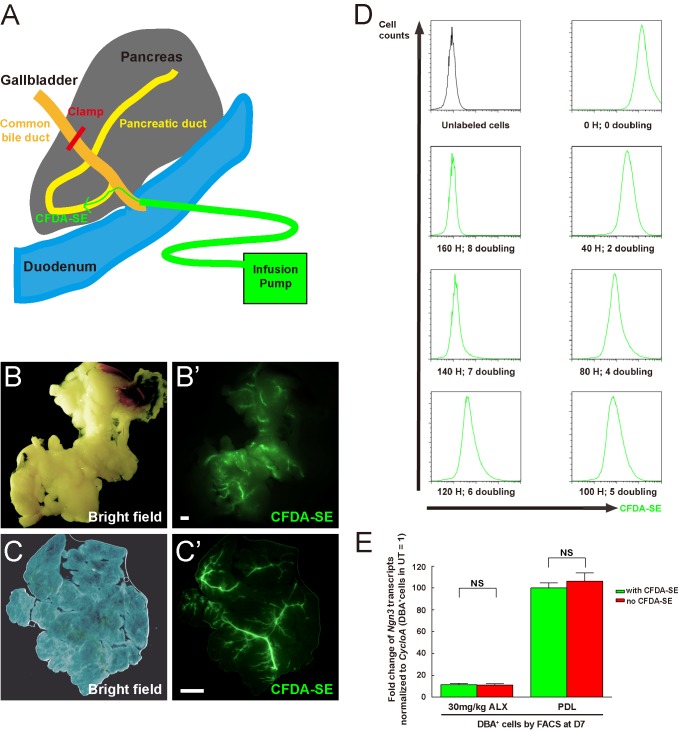
CFDA-SE (Invitrogen) was prepared according to manufacturer's instruction. Pancreatic intraductal CFDA-SE infusion was performed after anesthetizing the animals. Briefly, the duodenum was isolated to expose the common bile duct, after which a microclamp (Roboz, RS-7439) was placed on the common bile duct above the branching of the pancreatic duct. A 31-gauge blunt-ended catheter (World Precision Instruments) was then put into the common bile duct through the sphincter of Oddi in the duodenum, which was then clamped with another microclamp (Roboz, RS-7439) to prevent backflow. The other end of the catheter is connected to a micro-infusion apparatus, which delivers 30 μl of 10 μm CFDA-SE via the catheter at a rate of 1 μl/min. After infusion of CFDA-SE, the hole created by the catheter in the duodenum was closed with 6–0 suture. No animals were lost to surgery or post-surgical complications.
NIH 3T3 cells were grown in 5 mm-glucose DMEM supplemented with 10% FBS, with a cell doubling time of 20 h. 3T3 cells were incubated with different concentration of CFDA-SE for 30 min, after which the cells were washed and the fluorescence levels compared with the sorted CFDA-SE+ cells (green) from the pancreatic digests (30 μl 10 μm CFDA-SE infusion with a speed of 1 μl/min, taking 30 min) by Fluorescence-activated cell sorting (FACS). We found that the 3T3 cells incubated with 8 μm CFDA-SE appeared to have the similar fluorescence level as the in vivo labeled cells. Then the fluorescence level of the 3T3 cells labeled with 8 μm CFDA-SE was examined after serial cell doublings and compared with unlabeled 3T3 cells by FACS.
Pancreatic Digestion and FACS
Pancreatic duct perfusion and subsequent digestion of the pancreas was performed as described previously (45, 46). Pancreatic digests were either incubated with Fluorescein Dolichos Biflorus Agglutinin (DBA, Vector Lab, a duct-binding lectin) for 30 min to allow isolation of green DBA+ duct cells by FACS, or else for Ngn3-Cre; mTmG pancreas sequentially incubated with biotin-DBA (Vector Lab) and streptavidin-cy5 for 30 min to allow isolation of mG+ duct cells and mG− duct cells by FACS. CFDA-SE levels were analyzed by direct fluorescence. Purity of sorted cell factions was evaluated by analysis of expression of cell-type specific markers with RT-qPCR. Beta cell isolation from MIP-GFP mice has been described previously (46).
Laser-capture Microdissection (LCM)
Mouse pancreas was harvested, snap-frozen, sectioned, and mounted on RNase-free membrane-coated microscopy slides (Molecular Machines and Industries, MMI) as described previously (46), followed by 30 min of incubation with DBA to label the duct cells with green fluorescence.
RNA Isolation and RT-qPCR
RNA extraction and RT-qPCR have been described previously (8, 45, 46). Primers were all purchased from Qiagen. They are CycloA (QT00247709), Ngn3 (QT00262850), Synaptophysin (QT01042314), Amylase (QT00179242), Vimentin (QT00159670), Ck19 (QT00156667), Sox9 (QT00163765), Glut2 (QT00103537), FoxO1 (QT00116186), and Cd31 (QT01052044). RT-qPCR values were normalized against CycloA, which proved to be stable across the samples.
Immunohistochemistry
All pancreas samples were fixed in zinc (BD) for 4 h followed by an additional 2 h fixation in 4% formaldehyde, then cryo-protected in 30% sucrose overnight before freezing. mT and mG were detected by direct fluorescence. Primary antibodies for immunostaining are: guinea pig polyclonal insulin and pancreatic polypeptide-specific (Dako); rabbit polyclonal CK19 and somatostatin-specific (Dako) and glucagon-specific (Cell Signaling), and biotin-DBA (Vector Lab). No antigen retrieval was necessary, except for CK19, which needs microwaving. Secondary antibodies for indirect fluorescent staining are Cy2, Cy3, or Cy5 conjugated donkey streptavidin, rabbit, and guinea pig-specific (Jackson ImmunoResearch Labs). Nuclear staining is performed with Hoechst solution (BD). Imaging of cryosections is performed as described previously (8, 45–47).
Data Analysis
The quantification was done on the basis of at least 5 sections that were 100 μm apart. At least 2000 cells were counted for each experimental condition. If the percentage of positive cells was low, counting continued beyond 2000 cells until at least 50 positive cells were counted. All values are depicted as means ± S.E. of the mean. In each condition, 5 mice were analyzed in all groups. All RT-qPCR data are from 5 samples for each condition. All data were statistically analyzed by 2-tailed Student's t-test. Significance was considered when p < 0.05.
RESULTS
Significant Increase in mG+ Duct Cells in Ngn3-Cre; mTmG Mice after Low-dose ALX or PDL
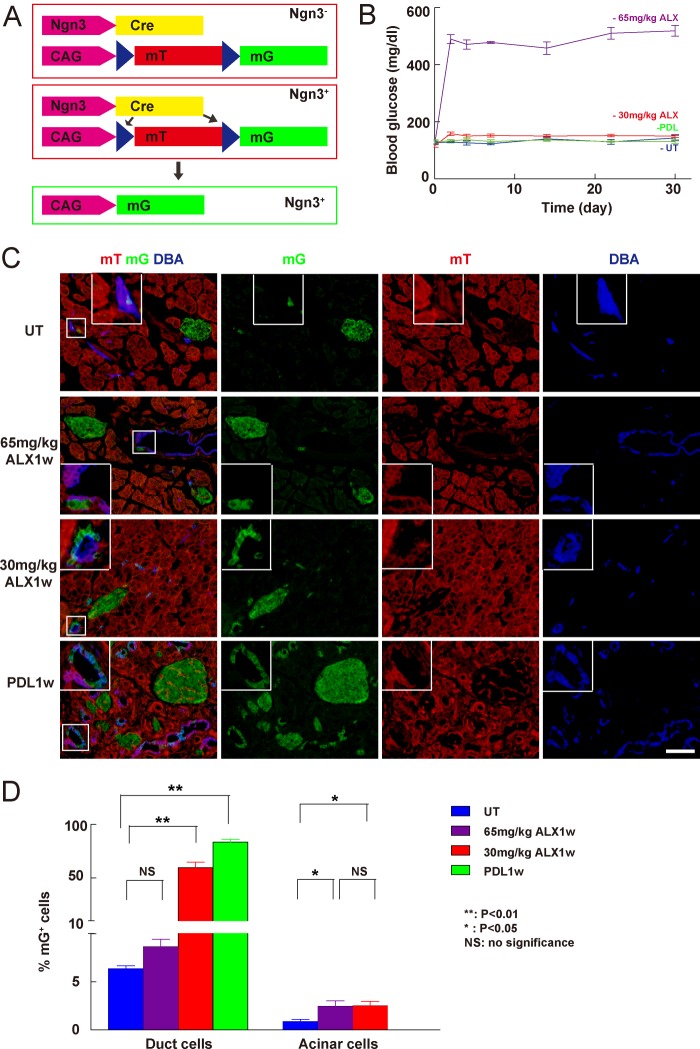
Theoretically, in the pancreas of Ngn3-Cre; mTmG mice, all the non-endocrine cells should express membrane-targeted Tomato red fluorescence (mT) and all the endocrine cells should express membrane-tagged EGFP fluorescence (mG), where the floxed mT cassette was deleted when the Ngn3 promoter was activated during development (Fig. 1A). We did not find any mT+ pancreatic endocrine cells in adult Ngn3-Cre; mTmG mice, suggesting that Ngn3 promoter activity during embryogenesis was strong enough to activate Cre recombinase in all endocrine lineage cells. Of note, we found a very small fraction of mG+ cells in both the duct cell population (6.4 ± 0.5%) and in the acinar cell population (less than 1%). These mG+ duct or acinar cells did not express endocrine markers, suggesting that they may have previously activated Ngn3 during development, but then failed to continue the endocrine differentiation process, possibly due to a low expression level of Ngn3.
FIGURE 1.
Ngn3-Cre; mTmG mice for detecting Ngn3 activation in duct cells. A, schematic of the Ngn3-Cre; mTmG model: All pancreatic cells are mT+ except for endocrine cells that are mG+ due to activation of Ngn3 during development. B, fasting blood glucose levels were measured, showing that sustained hyperglycemia developed in Ngn3-Cre; mTmG mice after 65 mg/kg ALX tail vein injection (purple), while neither 30 mg/kg ALX (red) nor PDL (green) significantly affected glucose levels (untreated control, UT, blue). C, representative confocal fluorescent images of pancreases of untreated (UT) Ngn3-Cre; mTmG mice, or 1 week after treatment with 65 mg/kg, or 30 mg/kg ALX, or PDL. Inset shows representative mG+ duct cells in high magnification. D, quantification of the percentage of mG+ duct cells and acinar cells under various conditions showed a roughly 10-fold increase in the fraction of mG+ duct cells after either 30 mg/kg ALX-treatment or PDL, but not after 65 mg/kg ALX-treatment. *: p < 0.05; **: p < 0.01; NS: no significance. Scale bars are 50 μm.
Since Ngn3 activation has been reported after PDL (35) and after beta-cell-specific toxin treatment (48, 49), we examined the pancreas from these Ngn3-Cre; mTmG mice after treatment with ALX or after PDL. Because Ngn3 activation after beta-cell-toxin treatment has not been reported consistently (48, 49), we suspected that the dosage of the toxin may affect Ngn3 activation. Thus, we tested the effect of two different ALX doses. A high-dose ALX (65 mg/kg) was sufficient to induce sustained hyperglycemia by destroying more than 90% of the beta cells in mice with a C57/6 background. Although neither hyperglycemia nor significantly altered beta cell mass was detected after a low-dose ALX (30 mg/kg) treatment (Fig. 1B and data not shown), we indeed found significant changes in transcripts of certain genes (e.g. up-regulation of Ngn3 and FoxO1 mRNA, down-regulation of Glut2 mRNA) in beta cells, suggesting that the beta cells were injured by low-dose ALX and may then undergo some degree of de-differentiation (50–55) (data not shown). On the other hand, by analysis of stained sections, we found a roughly 10-fold increase in the fraction of mG+ duct cells in the pancreas of Ngn3-Cre; mTmG mice either after 30 mg/kg ALX treatment (59.5 ± 2.3%), or after PDL (83.4 ± 3.6%), but not after high-dose ALX (65 mg/kg) treatment (7.6 ± 0.8%), compared with untreated controls (6.4 ± 0.5%). Similarly, we found a significant increase in the fraction of mG+ acinar cells in the pancreas of Ngn3-Cre; mTmG mice after both 30 mg/kg ALX treatment (2.5 ± 0.2%), or 65 mg/kg ALX treatment (2.4 ± 0.2%), compared with untreated controls (0.7 ± 0.1%) (Fig. 1, C--D). These data suggest that Ngn3 can be activated in duct cells by PDL, or by low-dose ALX, specifically.
Low-dose ALX or PDL Activate Ngn3 in Duct Cells
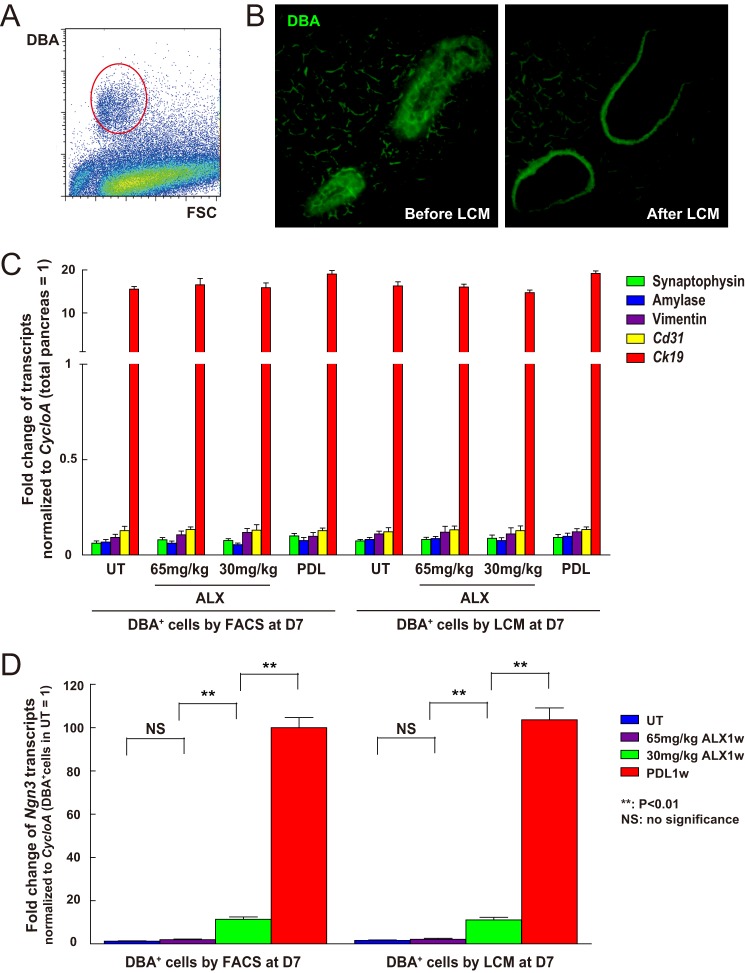
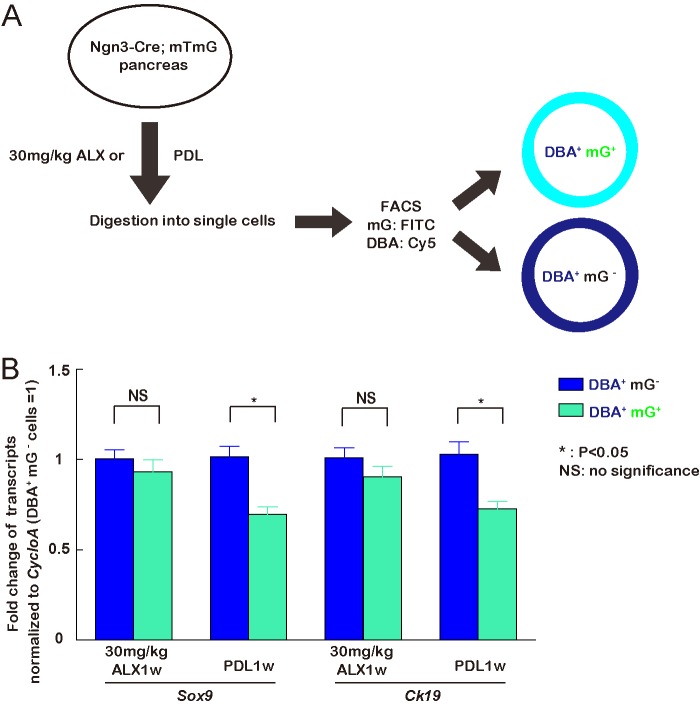
To further confirm Ngn3 gene activation in duct cells by both PDL and low-dose ALX, DBA was used to specifically label duct cells for fluorescence-activated cell sorting (FACS) (Fig. 2A) and for LCM (Fig. 2B). We measured mRNAs for Synaptophysin (marker of endocrine cells), Amylase (marker of acinar cells), Vimentin (marker of mesenchymal cells), Cd31 (marker of endothelial cells), and Ck19 (marker of duct cells) by RT-qPCR in the isolated duct cells to confirm the purity of the duct cell population (Fig. 2C). Then, Ngn3 transcripts were analyzed, confirming the activation of Ngn3 in duct cells by low-dose ALX or PDL (Fig. 2D). We further isolated mG+ DBA+ cells (representing Ngn3-activated duct cells) and mG− DBA+ cells (representing Ngn3-inactivated duct cells) from Ngn3-Cre; mTmG mice 1 week after either low-dose ALX or PDL, and also analyzed transcripts for Ck19 and Sox9, which are predominantly expressed by duct cells in the adult pancreas. We found that both transcripts were significantly down-regulated in Ngn3-activated duct cells after PDL, but the decreases after low-dose ALX did not reach significance (Fig. 3). These data suggest that PDL may be a more potent trigger for duct cell phenotypic changes, consistent with the higher levels of Ngn3 transcript after PDL than after low-dose ALX (Fig. 2D), as well as the higher fraction of Ngn3-lineage tagged duct cells after PDL.
FIGURE 2.
Ngn3 mRNA levels increase in duct cells after low-dose ALX or PDL. A, pancreatic digests were analyzed by flow cytometry following incubation with fluorescein-DBA to label duct cells (circled cell population). B, representative DBA fluorescent images of duct cells before and after LCM: Rim of fluorescence after LCM is nonspecific edge effect. C, RT-qPCR for Synaptophysin, Amylase, Vimentin, Cd31, and Ck19 was performed in FACS- or LCM- prepared cells to assure the purity of the duct cell population (UT, untreated). D, RT-qPCR for Ngn3 showed no change in expression in the ducts from mice treated with 65 mg/kg ALX, but significant up-regulation in the duct cells from mice treated with 30 mg/kg ALX, or especially after PDL. **: p < 0.01; NS: no significance.
FIGURE 3.
Ngn3-activated duct cells after PDL down-regulate Ck19 and Sox9. A, schematic of isolation of Ngn3-activated duct cells and Ngn3-inactivated duct cells 1 week after low-dose ALX or PDL from Ngn3-Cre; mTmG mice. mG+ DBA+ cells represent Ngn3-activated duct cells and mG− DBA+ cells represent Ngn3-inactivated duct cells. B, transcripts of two markers predominantly expressed by duct cells in the adult pancreas, Ck19 and Sox9, were analyzed, showing that both were down-regulated in Ngn3-activated duct cells after PDL, but their change in Ngn3-activated duct cells after low-dose ALX did not reach significance.
Ngn3-activated Duct Cells Do Not Produce Endocrine Hormones
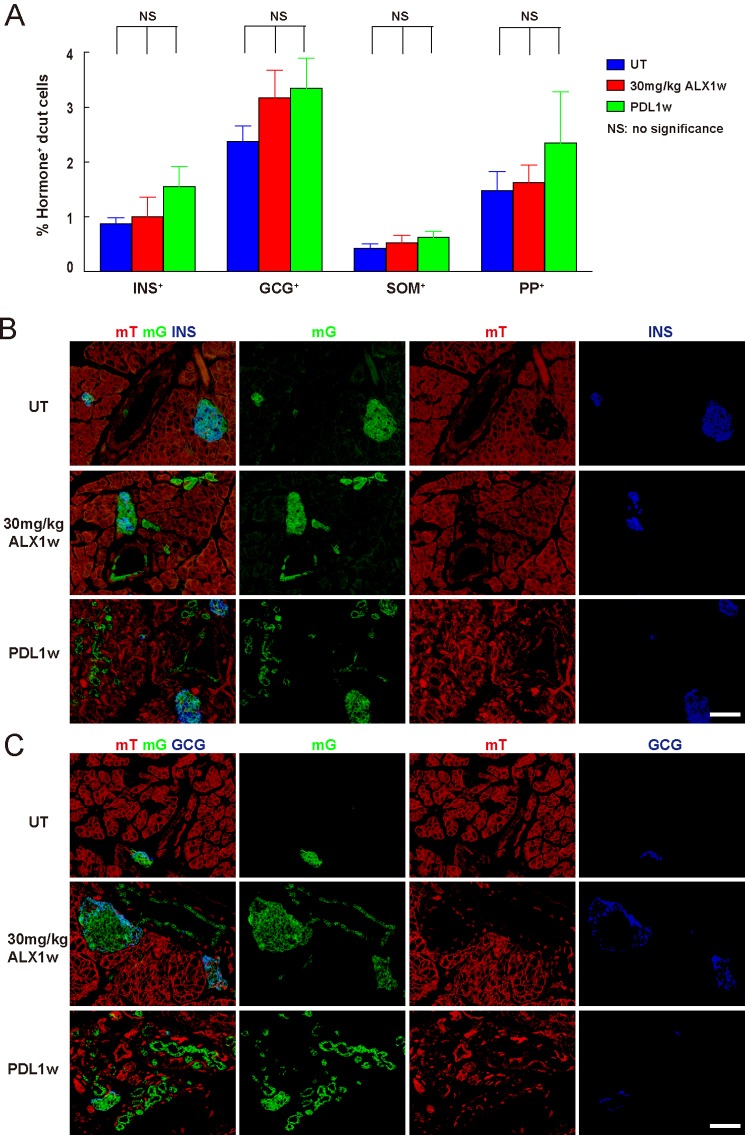
Next we examined whether these Ngn3-activated duct cells may start to produce endocrine hormones, as appears to occur in Ngn3-activated progenitor cells during embryonic pancreatic development (32–34). Since we found this up-regulation of Ngn3 occurs as early as 3 days after either low-dose ALX or after PDL, we expected to see hormone-positive duct cells derived from Ngn3-activated duct cells (hormone+ mG+ duct cells in Ngn3-Cre; mTmG mice) by 1 week after ALX or PDL if the Ngn3-activated duct cells do indeed differentiate into endocrine cells. We did not find an increase in hormone+ mG+ duct cells with either treatment (Fig. 4), suggesting that Ngn3 activation in adult duct cells does not lead to duct-to-endocrine cell transdifferentiation.
FIGURE 4.
Ngn3-activated duct cells do not produce endocrine hormones. A–C, determine whether Ngn3-activated duct cells may start to produce endocrine hormones. A, by quantification of stained confocal-imaged sections, no increase in endocrine-positive cells (insulin: INS; glucagon: GCG; somatostatin: SOM; pancreatic polypeptide: PP) was seen in the DBA+ ducts after either 30 mg/kg ALX or PDL, suggesting that Ngn3 activation in duct cells does not lead to duct-to-endocrine transdifferentiation. B–C, representative confocal fluorescent images of the pancreas of untreated (UT) Ngn3-Cre; mTmG mice, or 1 week after treatment with either 30 mg/kg ALX, or after PDL: insulin (B, INS) and glucagon (C, GCG) staining, respectively, show no evidence of hormone+ mG+ cells in the ducts. NS: no significance. Scale bars are 50 μm.
Long-term Tracking of Pancreatic Duct Cells with CFDA-SE
Since the above experiments could not exclude the possibility that Ngn3-activated duct cells may subsequently migrate out of the ducts prior to analysis, we further examined whether Ngn3-activated duct cells could form endocrine cells outside of the ducts over a relatively long time period. To track Ngn3-activated duct cells, we applied a novel intraductal infusion method to efficiently and specifically label pancreatic duct cells with CFDA-SE, a dye that passively enters the cells and then is retained within the cells (56). At a speed of 1 μl/min, 30 μl of 10 μm CFDA-SE was infused into the pancreatic duct through a fine blunt-ended catheter, leading to efficient labeling of the majority of ducts throughout the entire pancreas (Fig. 5A). One week after infusion, the pancreatic ducts are readily visualized grossly (Fig. 5B) or by whole-mount imaging (Fig. 5C).
FIGURE 5.
An intraductal infusion method to specifically label duct cells. To examine whether Ngn3 activation in duct cells can drive them to an endocrine phenotype over a longer period, an intraductal infusion method was used to label pancreatic duct cells with fluorescent CFDA-SE to track them. A, illustration of how CFDA-SE is infused into the pancreatic duct through a fine blunt-ended catheter. B–C, one week after infusion with CFDA-SE, the pancreatic ducts are readily visualized grossly (B) or by whole-mount (C). D, NIH 3T3 cells (cell doubling time is 20 h) were used to evaluate the effect of cell division on CFDA-SE detection. First, 3T3 cells incubated with 8 μm CFDA-SE were found to have fluorescence level similar to the duct cells in vivo labeled by a 30 μl 10 μm CFDA-SE infusion at a speed of 1 μl/min. The fluorescence levels of the 3T3 cells labeled with 8 μm CFDA-SE were then examined after serial cell doublings and compared with unlabeled 3T3 cells (with quality-control for the change in absolute cell number). The fluorescence is still detectable after 7 doublings, but not after 8 doublings, suggesting that the labeled duct cells with our in vivo intraductal infusion protocol remain detectable for up to 7 cell divisions. E, DBA+ (cy5) duct cells were isolated 1 week after low-dose ALX treatment, or after PDL, with or without CFDA-SE infusion. No significant difference in Ngn3 transcripts in duct cells was detected with CFDA-SE infusion, suggesting that CFDA-SE infusion does not affect Ngn3 activation in duct cells. H: hours. Scale bars are 200 μm.
The fluorescence of individual CFDA-SE-labeled cells should reduce by half after each cell division. Thus, we evaluated the maximal number of cell divisions that would still allow the cellular fluorescence to remain detectable. NIH 3T3 cells (with a 20 h doubling time) were incubated with different concentration of CFDA-SE for 30 min, after which the cells were extensively washed and their fluorescence level compared with sorted green duct cells (30 μl of 10 μm CFDA-SE infused at a speed of 1 μl/min, taking 30 min) from the pancreatic digests by FACS. We found that 3T3 cells incubated with 8 μm CFDA-SE had similar fluorescence intensity as the in vivo freshly-labeled duct cells. The fluorescence intensity of these 3T3 cells was examined after serial cell divisions, with cell counting to confirm cell divisions. Fluorescence of the labeled cells was still detectable after 7 cell divisions, but essentially not after 8 divisions (Fig. 5D). These results suggest that the labeled duct cells in our intraductal infusion system could remain detectable for up to 7 divisions.
To exclude the possibility that CFDA-SE infusion itself may affect Ngn3 activation in duct cells, DBA+ (cy5) duct cells were isolated 1 week after low-dose ALX treatment, or after PDL, with or without CFDA-SE infusion. No significant difference in Ngn3 transcripts in duct cells was detected after either low-dose ALX treatment, or after PDL, with CFDA-SE infusion (Fig. 5E), suggesting that CFDA-SE infusion did not change Ngn3 activation in duct cells.
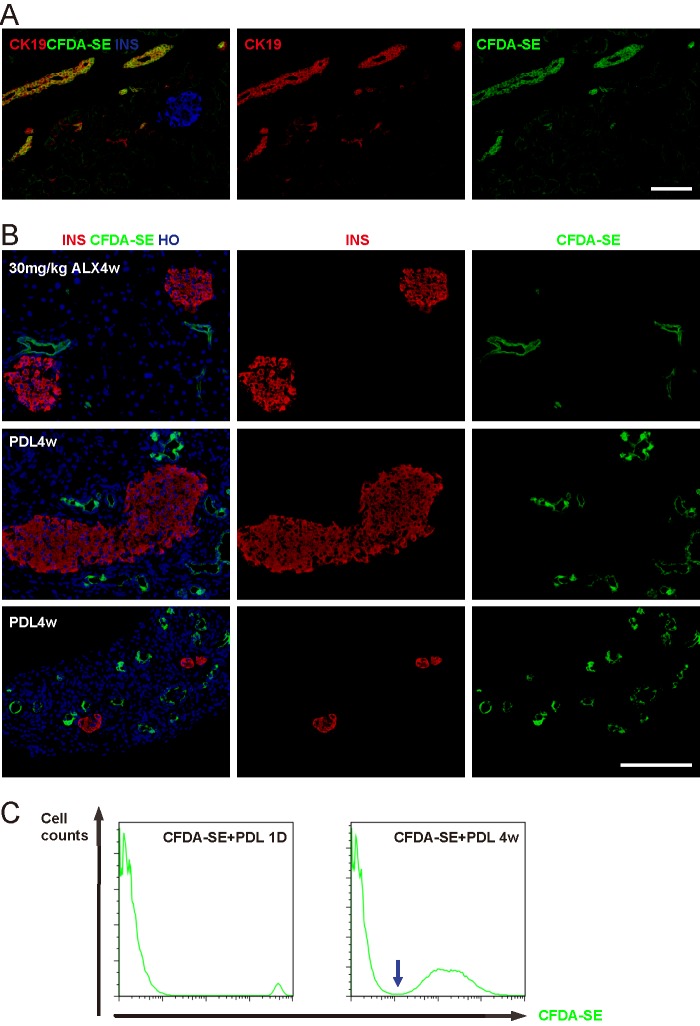
Quantification of CFDA-SE labeling efficiency on sections of the pancreas at 3 days (the earliest time point when Ngn3-Cre; mTmG duct cells were labeled after PDL or low-dose ALX), 1 week and 4 weeks was performed and showed no significant differences, suggesting that CFDA-SE does not selectively label cell fractions with different proliferative potentials. We found that 45.6 ± 4.8% of all duct cells (CK19+) were successfully labeled with CFDA-SE (Fig. 6A). Non-duct cell labeling was not seen. We further determined that the labeling efficiency of the main duct cells was 78.3 ± 6.7%, of intralobular duct cells was 28.4 ± 3.7%, and of centroacinar cells was 2.1 ± 0.2%.
FIGURE 6.
Ngn3-activated duct cells do not become endocrine cells. Representative fluorescent images of mouse pancreas 1 week after CFDA-SE intraductal infusion show the absence of nonspecific labeling. B, one week after CFDA-SE intraductal labeling, the mice received either 30 mg/kg ALX or PDL treatment, and were then sacrificed after another 4 weeks. No dye-labeled beta cells (upper panels) or other endocrine cells (data not shown) were found after 30 mg/kg ALX treatment. Moreover, no dye-labeled beta cells were found in either larger islets (middle panels) or small beta-cell clusters (lower panels) after PDL, while the numerous duct-like structures after PDL were extensively labeled with the dye. C, CFDA-SE signal intensity in labeled duct cells was compared by FACS, 1 day and 4 weeks after combined CFDA-SE infusion and PDL, showing that CFDA-SE intensity significantly decreased after 4 weeks, while still remaining detectable. There was a clear distinction (blue arrow) between CFDA-SE-labeled fluorescent duct cells and unlabeled cells, suggesting that CFDA-SE-labeled duct cells remain detectable after 4 weeks of follow-up. Also, CFDA-SE labeling in individual duct cells became much more variable 4 weeks after labeling and PDL, suggesting a differential proliferation capacity among different duct cells after PDL. Scale bars are 50 μm.
Ngn3-activated Duct Cells Do Not Become Endocrine Cells
One week after CFDA-SE intraductal labeling, the mice received either 30 mg/kg ALX or PDL treatment. The mice were sacrificed after another 4 weeks and assessed for any possible contribution of labeled duct cells to the endocrine cell population. No dye-labeled beta cells (Fig. 6B, upper panel) or other endocrine cells were found after 30 mg/kg ALX. Moreover, we did not find dye-labeled beta cells in either larger islets (Fig. 6B, middle panel) or small beta-cell clusters (Fig. 6B, lower panel) after PDL. The duct-like structures after PDL were extensively labeled with the dye (Fig. 6B, middle and lower panels), suggesting that these structures were mainly derived from the pre-existing duct network, consistent with a recent report (30). Also, these data suggest that the number of proliferation rounds in the duct cells did not exceed our CFDA-SE detection limitation (7 cycles) within 4 weeks. To further confirm this possibility, CFDA-SE signal intensities of labeled duct cells were compared by FACS, 1 day and 4 weeks after combined CFDA-SE infusion and PDL. Our data show that CFDA-SE intensity significantly decreased after 4 weeks, but still remained readily detectable (Fig. 6C). Also, since there was a clear separation between the CFDA-SE-labeled fluorescent population of cells and the unlabeled population (Fig. 6C, arrow), we concluded that duct cells proliferate less than 7 cycles during the 4 week period (Fig. 5D). These data thus demonstrate that the majority, if not all CFDA-SE-labeled duct cells, remain detectable after 4 weeks of follow-up. The CFDA-SE labeling intensity among individual duct cells became much more variable 4 weeks after labeling and PDL, suggesting a differential proliferative capacity among different duct cells after PDL (Fig. 6C).
Acinar Cells Do Not Become Endocrine Cells after PDL
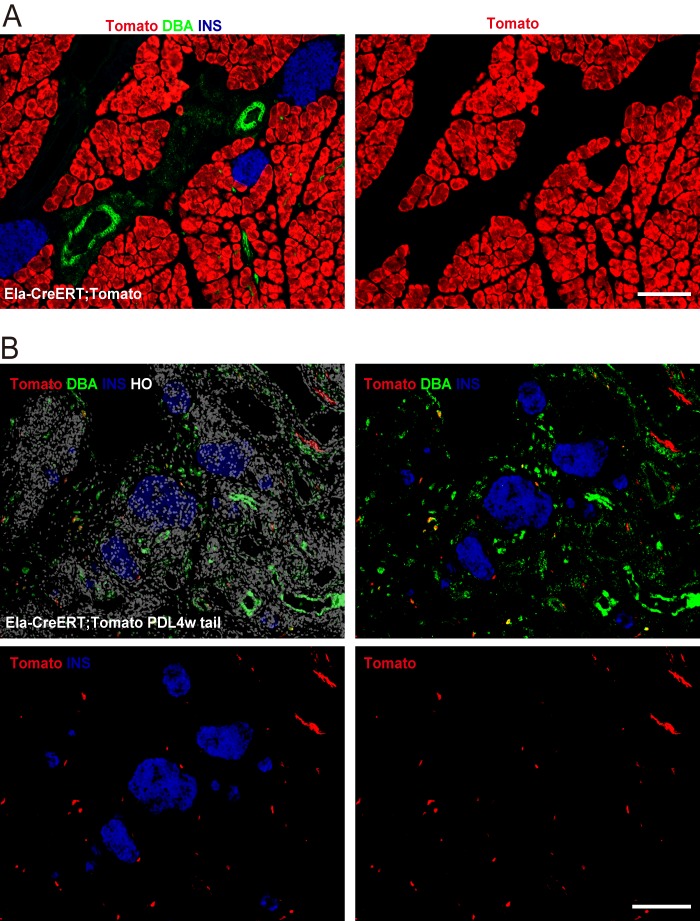
While not the primary focus of our study, we did also look at whether some acinar cells in the ligated tail pancreas after PDL may transdifferentiate into endocrine cells. Here, we used Ela-CreERT; Tomato mice to lineage trace acinar cells. In this model, one low dose (1 mg) of tamoxifen injection successfully labeled 99.6 ± 2.1% acinar cells (Fig. 7A). Tamoxifen-independent labeling of acinar cells should not confound our data interpretation, since we did not see cells other than acinar cells labeled. We did not find evidence of substantial contribution of acinar cells to beta cells 4 weeks after either 30 mg/kg ALX (data not shown) or PDL (Fig. 7B), consistent with previous reports (57, 58). We did not perform longer follow-up (30), since long-term lineage tracing may substantially suffer from misleading labeling of pre-existing beta cells due to leakage of CreER into the nuclei of beta cells (8).
FIGURE 7.
Acinar cells do not become endocrine cells after PDL. Ela-CreERT; Tomato mice were used to lineage trace acinar cells after PDL. A, one low-dose (1 mg) of tamoxifen injection resulted in a 99.6 ± 2.1% labeling efficiency of acinar cells, while no cells other than acinar cells were found to be labeled. B, no Tomato+ INS+ cells were seen 4 weeks after PDL, suggesting no substantial contribution of acinar cells to beta cells. Scale bars are 100 μm.
DISCUSSION
Activation of Ngn3 is a signature for pancreatic endocrine cell neogenesis during embryogenesis (32–34). However, whether Ngn3 activation in the adult pancreas also indicates endocrine cell neogenesis has been debated, and the question relates closely to the central issue of the possible existence of beta cell progenitors in the adult pancreas. Although recent reports revealed that adult beta cells express a low level of Ngn3 (27, 39–41), it remains controversial as to whether Ngn3 activation in adult pancreatic duct cells may be an indicator of duct-to-endocrine neogenesis (8, 25–29, 31, 42).
Here we examined this question in two models of Ngn3 activation in adult pancreatic duct cells (low-dose ALX and PDL). Of note, up-regulation of Ngn3 in duct cells by low-dose ALX may be related to a sub-lethal injury of the beta cells, since the injured beta cells may release cytokines/factors to affect nearby duct cells in a paracrine way. The differential Ngn3 activation in duct cells by different doses of ALX could result from the different phenotype of injured (not dead) beta cells (in 30 mg/kg ALX) versus destroyed (dead) beta cells (in 65 mg/kg ALX). This question is not the primary focus of our study, and here we focused our analysis on Ngn3 transcripts from purified duct cells. There is also significant controversy regarding whether there is a significant change in beta cell mass after PDL (25, 27, 31, 35, 38). We did not perform a beta cell mass analysis here, since beta cell mass quantification after PDL is difficult, due to severe tissue edema and organ remodeling (27, 31, 38).
Previous analyses of duct cell lineages have used a tamoxifen-sensitive Cre-labeling system (25–29, 42). However, RIP-CreERT mice (4) were reported to have either prolonged tamoxifen-induced cre activation (59), or pre-labeling of beta cells in young (60) or aged mice (58), as well as cell-type specific apoptosis (61). Other CreERT strains could potentially suffer from similar flaws related to specificity and sensitivity. Here we used either a straight Cre (no ERT) or a novel non-genetic method to label duct cells (intraductal infusion of CFDA-SE). Once the lipid soluble CFDA-SE enters the cytoplasm of duct cells, intracellular esterases remove the acetate groups of CFDA-SE and convert the molecule to the non-membrane permeable fluorescent ester (CFSE) (56). Due to this covalent coupling reaction, fluorescent CFSE is retained within cells for long periods, and is not transferred to adjacent cells (56). We found that the optimal labeling of duct cells, with no detectable nonspecific non-ductal labeling, is achieved with the infusion of 30 μl of 10 μm CFDA-SE at a speed of 1 μl/min. Of note, a faster infusion or higher dose of CFDA-SE may result in labeling of some periductal cells, perhaps due to CFDA-SE leakage out of the ducts under higher pressure. Thus, since specificity is our highest priority, we were unable to increase dosing to further improve labeling efficiency. Importantly, CFDA-SE labeling seems to be a robust system since we showed that the labeled cells remain detectable after at least 7 doublings.
Using either this dye-labeling or a Ngn3-Cre labeling system, we found that adult Ngn3-activated duct cells do not give rise to beta cells or other endocrine cells, consistent with a previous report (58). Thus, Ngn3 may not be an ideal marker for the identification of beta cell progenitors in adults.
To summarize our findings, although Ngn3 had been previously regarded as a marker of endocrine cell neogenesis from pancreatic ducts in adults (36, 37), our study provides direct evidence othewise. Demonstration of Ngn3 activation alone in duct cells is not sufficient to show adult beta cell neogenesis.
Acknowledgments
We thank Alexis J. Styche, Robert J. Lakomy, Maria Branca, and Lauren Brink for technical assistance in flow cytometry, LCM, confocal microscopy, and mouse genotyping. We also thank Christine Kalinyak for administrative assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 DK064952 and R01 DK083541 (to G. K. G.), the Cochrane-Weber endowed Fund in Diabetes Research (to X. X.), and the Children's Hospital of Pittsburgh Foundation.
- PDL
- pancreatic duct ligation
- Ngn3
- neurogenin3
- CFDA-SE
- carboxyfluorescein diacetate, succinimidyl ester
- ALX
- allxoan
- LCM
- laser-capture microdissection
- FACS
- fluorescence-activated cell sorting
- BAC
- bacterial-artificial-chromosome.
REFERENCES
- 1. Pipeleers D., Keymeulen B., Chatenoud L., Hendrieckx C., Ling Z., Mathieu C., Roep B., Ysebaert D. (2002) A view on beta cell transplantation in diabetes. Ann. N.Y. Acad. Sci. 958, 69–76 [DOI] [PubMed] [Google Scholar]
- 2. Kaestner K. H. (2007) Beta cell transplantation and immunosuppression: can't live with it, can't live without it. J. Clin. Invest. 117, 2380–2382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zaret K. S., Grompe M. (2008) Generation and regeneration of cells of the liver and pancreas. Science 322, 1490–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dor Y., Brown J., Martinez O. I., Melton D. A. (2004) Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41–46 [DOI] [PubMed] [Google Scholar]
- 5. Teta M., Rankin M. M., Long S. Y., Stein G. M., Kushner J. A. (2007) Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev. Cell 12, 817–826 [DOI] [PubMed] [Google Scholar]
- 6. Meier J. J., Butler A. E., Saisho Y., Monchamp T., Galasso R., Bhushan A., Rizza R. A., Butler P. C. (2008) Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes 57, 1584–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Georgia S., Bhushan A. (2004) Beta cell replication is the primary mechanism for maintaining postnatal beta cell mass. J. Clin. Invest. 114, 963–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xiao X., Chen Z., Shiota C., Prasadan K., Guo P., El-Gohary Y., Paredes J., Welsh C., Wiersch J., Gittes G. K. (2013) No evidence for beta cell neogenesis in murine adult pancreas. J. Clin. Invest. 123, 2207–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nir T., Melton D. A., Dor Y. (2007) Recovery from diabetes in mice by beta cell regeneration. J. Clin. Invest. 117, 2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kushner J. A., Weir G. C., Bonner-Weir S. (2010) Ductal origin hypothesis of pancreatic regeneration under attack. Cell Metab. 11, 2–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bonner-Weir S., Weir G. C. (2005) New sources of pancreatic beta-cells. Nat. Biotechnol. 23, 857–861 [DOI] [PubMed] [Google Scholar]
- 12. Hickey R. D., Galivo F., Schug J., Brehm M. A., Haft A., Wang Y., Benedetti E., Gu G., Magnuson M. A., Shultz L. D., Lagasse E., Greiner D. L., Kaestner K. H., Grompe M. (2013) Generation of islet-like cells from mouse gall bladder by direct ex vivo reprogramming. Stem Cell Res. 11, 503–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bramswig N. C., Everett L. J., Schug J., Dorrell C., Liu C., Luo Y., Streeter P. R., Naji A., Grompe M., Kaestner K. H. (2013) Epigenomic plasticity enables human pancreatic alpha to beta cell reprogramming. J. Clin. Invest. 123, 1275–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sachdeva M. M., Stoffers D. A. (2009) Minireview: Meeting the demand for insulin: molecular mechanisms of adaptive postnatal beta-cell mass expansion. Mol. Endocrinol. 23, 747–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cnop M., Hughes S. J., Igoillo-Esteve M., Hoppa M. B., Sayyed F., van de Laar L., Gunter J. H., de Koning E. J., Walls G. V., Gray D. W., Johnson P. R., Hansen B. C., Morris J. F., Pipeleers-Marichal M., Cnop I., Clark A. (2010) The long lifespan and low turnover of human islet beta cells estimated by mathematical modelling of lipofuscin accumulation. Diabetologia 53, 321–330 [DOI] [PubMed] [Google Scholar]
- 16. Kushner J. A. (2013) The role of aging upon beta cell turnover. J. Clin. Invest. 123, 990–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rankin M. M., Kushner J. A. (2009) Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes 58, 1365–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Teta M., Long S. Y., Wartschow L. M., Rankin M. M., Kushner J. A. (2005) Very slow turnover of beta-cells in aged adult mice. Diabetes 54, 2557–2567 [DOI] [PubMed] [Google Scholar]
- 19. Stolovich-Rain M., Hija A., Grimsby J., Glaser B., Dor Y. (2012) Pancreatic beta cells in very old mice retain capacity for compensatory proliferation. J. Biol. Chem. 287, 27407–27414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hang Y., Stein R. (2011) MafA and MafB activity in pancreatic beta cells. Trends Endocrinol. Metab. 22, 364–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kulkarni R. N., Mizrachi E. B., Ocana A. G., Stewart A. F. (2012) Human beta-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes 61, 2205–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wilson M. E., Scheel D., German M. S. (2003) Gene expression cascades in pancreatic development. Mech. Dev. 120, 65–80 [DOI] [PubMed] [Google Scholar]
- 23. Jørgensen M. C., Ahnfelt-Rønne J., Hald J., Madsen O. D., Serup P., Hecksher-Sørensen J. (2007) An illustrated review of early pancreas development in the mouse. Endocr. Rev. 28, 685–705 [DOI] [PubMed] [Google Scholar]
- 24. Gittes G. K. (2009) Developmental biology of the pancreas: a comprehensive review. Dev. Biol. 326, 4–35 [DOI] [PubMed] [Google Scholar]
- 25. Solar M., Cardalda C., Houbracken I., Martín M., Maestro M. A., De Medts N., Xu X., Grau V., Heimberg H., Bouwens L., Ferrer J. (2009) Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev. Cell 17, 849–860 [DOI] [PubMed] [Google Scholar]
- 26. Furuyama K., Kawaguchi Y., Akiyama H., Horiguchi M., Kodama S., Kuhara T., Hosokawa S., Elbahrawy A., Soeda T., Koizumi M., Masui T., Kawaguchi M., Takaori K., Doi R., Nishi E., Kakinoki R., Deng J. M., Behringer R. R., Nakamura T., Uemoto S. (2011) Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat. Genet. 43, 34–41 [DOI] [PubMed] [Google Scholar]
- 27. Kopp J. L., Dubois C. L., Schaffer A. E., Hao E., Shih H. P., Seymour P. A., Ma J., Sander M. (2011) Sox9+ ductal cells are multipotent progenitors throughout development but do not produce new endocrine cells in the normal or injured adult pancreas. Development 138, 653–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kopinke D., Brailsford M., Shea J. E., Leavitt R., Scaife C. L., Murtaugh L. C. (2011) Lineage tracing reveals the dynamic contribution of Hes1+ cells to the developing and adult pancreas. Development 138, 431–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Inada A., Nienaber C., Katsuta H., Fujitani Y., Levine J., Morita R., Sharma A., Bonner-Weir S. (2008) Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc. Natl. Acad. Sci. U.S.A. 105, 19915–19919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pan F. C., Bankaitis E. D., Boyer D., Xu X., Van de Casteele M., Magnuson M. A., Heimberg H., Wright C. V. (2013) Spatiotemporal patterns of multipotentiality in Ptf1a-expressing cells during pancreas organogenesis and injury-induced facultative restoration. Development 140, 751–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rankin M. M., Wilbur C. J., Rak K., Shields E. J., Granger A., Kushner J. A. (2013) beta-Cells Are Not Generated in Pancreatic Duct Ligation-Induced Injury in Adult Mice. Diabetes 62, 1634–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schwitzgebel V. M., Scheel D. W., Conners J. R., Kalamaras J., Lee J. E., Anderson D. J., Sussel L., Johnson J. D., German M. S. (2000) Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development 127, 3533–3542 [DOI] [PubMed] [Google Scholar]
- 33. Jensen J., Heller R. S., Funder-Nielsen T., Pedersen E. E., Lindsell C., Weinmaster G., Madsen O. D., Serup P. (2000) Independent development of pancreatic alpha- and beta-cells from neurogenin3-expressing precursors: a role for the notch pathway in repression of premature differentiation. Diabetes 49, 163–176 [DOI] [PubMed] [Google Scholar]
- 34. Gu G., Dubauskaite J., Melton D. A. (2002) Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development 129, 2447–2457 [DOI] [PubMed] [Google Scholar]
- 35. Xu X., D'Hoker J., Stangé G., Bonné S., De Leu N., Xiao X., Van de Casteele M., Mellitzer G., Ling Z., Pipeleers D., Bouwens L., Scharfmann R., Gradwohl G., Heimberg H. (2008) Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 132, 197–207 [DOI] [PubMed] [Google Scholar]
- 36. Ackermann Misfeldt A., Costa R. H., Gannon M. (2008) Beta-cell proliferation, but not neogenesis, following 60% partial pancreatectomy is impaired in the absence of FoxM1. Diabetes 57, 3069–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Figeac F., Ilias A., Bailbe D., Portha B., Movassat J. (2012) Local in vivo GSK3beta knockdown promotes pancreatic beta cell and acinar cell regeneration in 90% pancreatectomized rat. Mol. Ther. 20, 1944–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chintinne M., Stangé G., Denys B., Ling Z., In 't Veld P., Pipeleers D. (2012) Beta cell count instead of beta cell mass to assess and localize growth in beta cell population following pancreatic duct ligation in mice. PLoS One 7, e43959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang S., Jensen J. N., Seymour P. A., Hsu W., Dor Y., Sander M., Magnuson M. A., Serup P., Gu G. (2009) Sustained Neurog3 expression in hormone-expressing islet cells is required for endocrine maturation and function. Proc. Natl. Acad. Sci. U.S.A. 106, 9715–9720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dror V., Nguyen V., Walia P., Kalynyak T. B., Hill J. A., Johnson J. D. (2007) Notch signalling suppresses apoptosis in adult human and mouse pancreatic islet cells. Diabetologia 50, 2504–2515 [DOI] [PubMed] [Google Scholar]
- 41. Jenny M., Uhl C., Roche C., Duluc I., Guillermin V., Guillemot F., Jensen J., Kedinger M., Gradwohl G. (2002) Neurogenin3 is differentially required for endocrine cell fate specification in the intestinal and gastric epithelium. EMBO J. 21, 6338–6347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kopp J. L., Dubois C. L., Hao E., Thorel F., Herrera P. L., Sander M. (2011) Progenitor cell domains in the developing and adult pancreas. Cell Cycle 10, 1921–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ji B., Song J., Tsou L., Bi Y., Gaiser S., Mortensen R., Logsdon C. (2008) Robust acinar cell transgene expression of CreErT via BAC recombineering. Genesis 46, 390–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schonhoff S. E., Giel-Moloney M., Leiter A. B. (2004) Neurogenin 3-expressing progenitor cells in the gastrointestinal tract differentiate into both endocrine and non-endocrine cell types. Dev. Biol. 270, 443–454 [DOI] [PubMed] [Google Scholar]
- 45. Xiao X., Wiersch J., El-Gohary Y., Guo P., Prasadan K., Paredes J., Welsh C., Shiota C., Gittes G. K. (2013) TGFβ Receptor Signaling Is Essential for Inflammation-Induced but Not beta-Cell Workload-Induced beta-Cell Proliferation. Diabetes 62, 1217–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xiao X., Guo P., Chen Z., El-Gohary Y., Wiersch J., Gaffar I., Prasadan K., Shiota C., Gittes G. K. (2013) Hypoglycemia reduces vascular endothelial growth factor a production by pancreatic Beta cells as a regulator of Beta cell mass. J. Biol. Chem. 288, 8636–8646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Scharfmann R., Xiao X., Heimberg H., Mallet J., Ravassard P. (2008) Beta cells within single human islets originate from multiple progenitors. PLoS One 3, e3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liang X. D., Guo Y. Y., Sun M., Ding Y., Wang N., Yuan L., De W. (2011) Streptozotocin-induced expression of Ngn3 and Pax4 in neonatal rat pancreatic alpha-cells. World J. Gastroenterol. 17, 2812–2820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kodama S., Toyonaga T., Kondo T., Matsumoto K., Tsuruzoe K., Kawashima J., Goto H., Kume K., Kume S., Sakakida M., Araki E. (2005) Enhanced expression of PDX-1 and Ngn3 by exendin-4 during beta cell regeneration in STZ-treated mice. Biochem. Biophys. Res. Commun. 327, 1170–1178 [DOI] [PubMed] [Google Scholar]
- 50. Talchai C., Xuan S., Lin H. V., Sussel L., Accili D. (2012) Pancreatic beta cell dedifferentiation as a mechanism of diabetic beta cell failure. Cell 150, 1223–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kitamura T., Nakae J., Kitamura Y., Kido Y., Biggs W. H., 3rd, Wright C. V., White M. F., Arden K. C., Accili D. (2002) The forkhead transcription factor Foxo1 links insulin signaling to Pdx1 regulation of pancreatic beta cell growth. J. Clin. Invest. 110, 1839–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nakae J., Kitamura T., Silver D. L., Accili D. (2001) The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J. Clin. Invest. 108, 1359–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Okamoto H., Hribal M. L., Lin H. V., Bennett W. R., Ward A., Accili D. (2006) Role of the forkhead protein FoxO1 in beta cell compensation to insulin resistance. J. Clin. Invest. 116, 775–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Weinberg N., Ouziel-Yahalom L., Knoller S., Efrat S., Dor Y. (2007) Lineage tracing evidence for in vitro dedifferentiation but rare proliferation of mouse pancreatic beta-cells. Diabetes 56, 1299–1304 [DOI] [PubMed] [Google Scholar]
- 55. Haeusler R. A., Kaestner K. H., Accili D. (2010) FoxOs function synergistically to promote glucose production. J. Biol. Chem. 285, 35245–35248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bronner-Fraser M. (1985) Alterations in neural crest migration by a monoclonal antibody that affects cell adhesion. J. Cell Biol. 101, 610–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Desai B. M., Oliver-Krasinski J., De Leon D. D., Farzad C., Hong N., Leach S. D., Stoffers D. A. (2007) Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J. Clin. Invest. 117, 971–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Blaine S. A., Ray K. C., Anunobi R., Gannon M. A., Washington M. K., Means A. L. (2010) Adult pancreatic acinar cells give rise to ducts but not endocrine cells in response to growth factor signaling. Development 137, 2289–2296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reinert R. B., Kantz J., Misfeldt A. A., Poffenberger G., Gannon M., Brissova M., Powers A. C. (2012) Tamoxifen-Induced Cre-loxP Recombination Is Prolonged in Pancreatic Islets of Adult Mice. PLoS One 7, e33529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu Y., Suckale J., Masjkur J., Magro M. G., Steffen A., Anastassiadis K., Solimena M. (2010) Tamoxifen-independent recombination in the RIP-CreER mouse. PLoS One 5, e13533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhu Y., Huang Y. F., Kek C., Bulavin D. V. (2013) Apoptosis differently affects lineage tracing of Lgr5 and Bmi1 intestinal stem cell populations. Cell Stem Cell 12, 298–303 [DOI] [PubMed] [Google Scholar]