Background: Notch signaling controls cell fate decisions in all metazoan organisms.
Results: The Notch1 receptor exhibits a binding affinity for the ligand Delta-like 4 at least an order of magnitude stronger than for its close homologue, Delta-like 1.
Conclusion: Intrinsic binding preferences exist for specific Notch receptor-ligand pairs.
Significance: Binding selectivity among different potential receptor-ligand complexes may influence receptor-mediated responses of developmental signaling pathways.
Keywords: Cell Signaling, Cell Surface Receptor, Cell-Cell Interaction, Notch Pathway, Receptor Structure-Function, Recombinant Protein Expression, Biolayer Interferometry, Receptor-Ligand Interaction
Abstract
Notch signaling makes critical contributions to cell fate determination in all metazoan organisms, yet remarkably little is known about the binding affinity of the four mammalian Notch receptors for their three Delta-like and two Jagged family ligands. Here, we utilized signaling assays and biochemical studies of purified recombinant ligand and receptor molecules to investigate the differences in signaling behavior and intrinsic affinity between Notch1-Dll1 and Notch1-Dll4 complexes. Systematic deletion mutagenesis of the human Notch1 ectodomain revealed that epidermal growth factor (EGF) repeats 6–15 are sufficient to maintain signaling in a reporter assay at levels comparable with the full-length receptor, and identified important contributions from EGF repeats 8–10 in conveying an activating signal in response to either Dll1 or Dll4. Truncation studies of the Dll1 and Dll4 ectodomains showed that the MNNL-EGF3 region was both necessary and sufficient for full activation. Plate-based and cell binding assays revealed a specific, calcium-dependent interaction between cell-surface and recombinant Notch receptors and ligand molecules. Finally, direct measurement of the binding affinity of Notch1 EGF repeats 6–15 for Dll1 and Dll4 revealed that Dll4 binds with at least an order of magnitude higher affinity than Dll1. Together, these studies give new insights into the features of ligand recognition by Notch1, and highlight how intrinsic differences in the biochemical behavior of receptor-ligand complexes can influence receptor-mediated responses of developmental signaling pathways.
Introduction
Notch signaling makes critical contributions to cell fate determination in all metazoan organisms. Because Notch receptors and their ligands are transmembrane proteins, Notch signals are transduced at sites of cell-cell contact, where signals can promote or suppress proliferation, differentiation, or apoptosis, with the outcome dictated by cellular context (1, 2). Mutations of Notch receptors or their ligands have been found in developmental anomalies, neurodegenerative diseases, and cancer, highlighting the importance of precisely regulating the intensity and duration of signaling in a variety of human tissues and organ systems (3–5).
Notch receptors are large, single-pass transmembrane proteins that are processed by furin-like proteases at site S1 during maturation to yield non-covalently linked heterodimers at the cell surface (6). These receptors are resistant to further proteolysis until ligands of the Delta, Serrate, and Lag-2 (DSL)3 family bind. Upon ligand engagement, Notch receptors become susceptible to cleavage by ADAM family metalloproteases at a juxtamembrane site (S2). After S2 cleavage, the truncated Notch receptor is further processed near the inner membrane leaflet (at site S3) by γ-secretase, which releases the intracellular portion of the receptor from the membrane (7–9). The intracellular portion of Notch then translocates to the nucleus, where it assembles into a ternary complex with the transcription factor RBPJ and a co-activator from the Mastermind family to induce transcription of Notch target genes (10, 11).
Mammals have four Notch receptors, three Delta-like (Dll) ligands, and two Jagged ligands, both homologous to Drosophila Serrate (12). The extracellular region of mammalian Notch receptors includes 29–36 N-terminal EGF-like repeats, many of which contain consensus calcium-binding motifs (Fig. 1, A and B). Previous studies have identified EGF repeats 11 and 12 as essential for binding to ligand molecules (13), but additional EGF repeats may also contribute (14). The five Delta-like and Jagged ligands (collectively referred to as “DSL” ligands) also exhibit a modular architecture, including an N-terminal MNNL domain, a cysteine-rich DSL module, and 6–16 EGF-like repeats (Fig. 1, C and D). Jagged ligands also contain a cysteine-rich region immediately preceding the transmembrane domain, whereas Delta ligands do not. The MNNL and DSL domains of Drosophila Delta, along with EGF2, appear to be required for aggregation of Delta- and Notch-expressing cells (15), whereas deletion mutagenesis of mouse Jagged1 indicates that the DSL domain is required for binding to mouse Notch2 in both cell-based and solid-phase binding assays, with EGF repeats 1 and 2 reported to influence affinity (16).
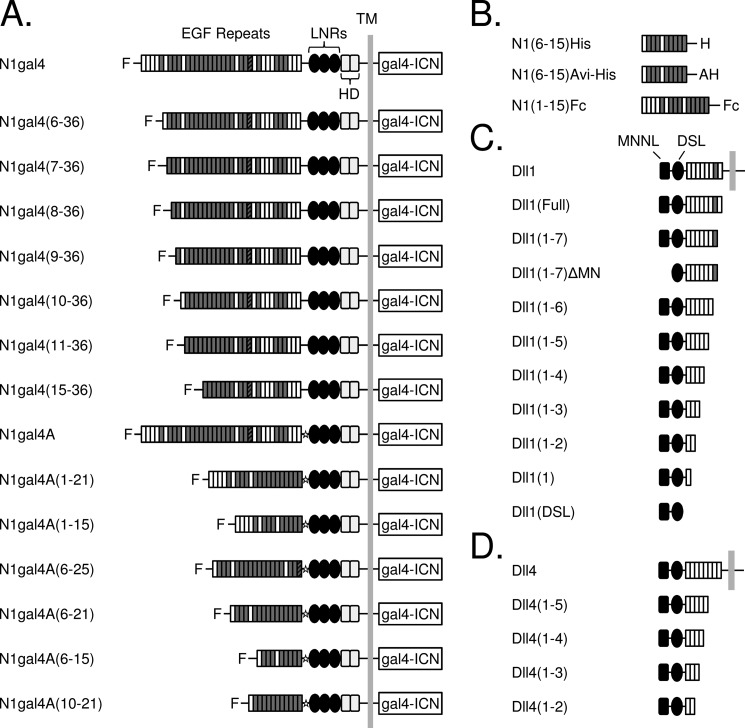
FIGURE 1.
Domain organization of ligand and receptor constructs. A, Notch molecules used in signaling assays. The N1gal4 construct was created by fusing a gal4 promoter to the intracellular region of Notch1 (gal4-ICN), and EGF repeats were systematically truncated from the N terminus. Repeats containing a consensus calcium-binding motif are shaded in gray, and EGF25, which contains an extra cysteine residue, is indicated with a striped pattern. “F” indicates the presence of an N-terminal FLAG epitope. C-terminal truncations were constructed using a Notch1-gal4A variant, which contains a restriction site (indicated with a star) between the EGF repeat region and the NRR. B, Notch molecules used in binding assays. The Notch extracellular truncation spanning EGF repeats 6–15, inclusive, was designed for use in protein-protein binding experiments, along with a N1(1–15)Fc fusion protein (R&D Biosystems). C and D, ligand molecules used in binding and signaling assays. C, human Delta-like 1 was systematically truncated from the C terminus, along with a variant lacking the N-terminal MNNL domain. D, C-terminal truncations of human Delta-like 4. LNRs, LIN12-Notch repeats; HD, heterodimerization domain.
Notch signaling can also be modulated by glycosylation of the EGF repeats of the receptor. Addition of O-linked fucose groups at consensus acceptor sites, catalyzed by an O-fucosyl transferase enzyme (O-Fuc-T1) (17, 18), serves to prime subsequent addition of N-acetylglucosamine by fringe glycosyltransferases (19, 20). It has been reported that N-acetylglucosamine addition modulates ligand specificity, enhancing binding of Delta, and inhibiting binding of Serrate to Drosophila Notch (21). Similarly, binding of murine Dll1 to Notch1 has also been reported to be enhanced by fringe in cell-surface binding assays, and is correlated with increased Notch1 signaling in cells (22). Additionally, O-glucosylation by Rumi glycosyltransferases is required for receptor activation in both Drosophila and mammals (23–25). Although it is clear that glycosylation of Notch receptors has profound effects on signaling and also appears to affect ligand binding, the basis for these effects remains poorly understood.
Little is known about the binding affinity of the four mammalian Notch receptors for their three Delta-like and two Jagged family ligands. A weak interaction has been observed between EGF repeats 11–13 of Notch1 and the DSL-EGF3 portion of Jagged1, both purified after refolding from bacterial expression, suggesting that these fragments may represent the minimum length interacting regions for this receptor-ligand pair (26). A similar weak interaction between EGF repeats 11–14 from Notch1 and a Dll1 construct spanning from MNNL-EGF3 was analyzed by surface plasmon resonance, which yielded an estimated KD of 130 μm (27). These affinities are lower than ELISA-based measurements performed with larger ectodomain fragments analyzed after mammalian expression as Fc-fusion proteins, which lie in the low nanomolar range (16, 28). These seemingly disparate findings can be reconciled by the existence of additional interactions involving regions of the ligand and receptor outside of the minimal-interacting region, differences in valency due to the use of fusion proteins, or differences resulting from post-translational modifications of the receptor (or ligand) occurring in mammalian cells.
Even less is known about whether intrinsic binding preferences exist among different potential ligand-receptor pairs, but it does appear that preferences for certain Notch-ligand partnerships exist in vivo. For example, the Notch1-Dll4 interaction is a key regulator of angiogenesis (29–31) and is responsible for induction of T lymphopoiesis in the thymus (32, 33); recent work has also implicated the Jagged2-Notch3 interaction in mediating the α/β versus γ/δ fate decision later in T cell maturation (34). In contrast, the Dll1-Notch2 interaction is important for marginal zone B-cell development (35, 36) and mast cell adhesion at inflammatory sites (37). Dll1 and Dll4 appear to play distinct, non-overlapping roles in vascular signaling (33, 38), and Notch1-dependent Dll4-mediated tip cell sprouting in angiogenesis is counteracted by Jagged1 (39). However, it remains unclear whether these preferences are based solely on spatial and temporal differences in expression patterns (reviewed in Refs. 40 and 41), or if there are underlying intrinsic differences in affinity among various ligand-receptor complexes.
Here, we utilized signaling assays and biochemical studies of purified recombinant ligand and receptor molecules to investigate the differences in signaling behavior and intrinsic affinity between Notch1-Dll1 and Notch1-Dll4 complexes. Systematic deletion mutagenesis of the human Notch1 ectodomain revealed that EGF repeats 6–15 are sufficient to maintain signaling in a reporter assay at levels comparable with the full-length receptor, and identified important contributions from EGF repeats 8–10 in conveying an activating signal in response to either Dll1 or Dll4. Truncation studies of the Dll1 and Dll4 ectodomains showed that the MNNL-EGF3 region was both necessary and sufficient for full activation. Plate-based and cell binding assays revealed a specific, calcium-dependent interaction between cell surface and recombinant Notch receptors and ligand molecules. Finally, direct measurement of the binding affinity of the EGF6–15 region of Notch1 for Dll1 and Dll4 revealed that the Dll4 ectodomain binds with at least an order of magnitude higher affinity than that of Dll1. Together, these studies give new insights into the features of ligand recognition by Notch1, and suggest that intrinsic differences in the biochemical behavior of receptor-ligand complexes influence receptor-mediated responses of developmental signaling pathways.
EXPERIMENTAL PROCEDURES
Materials and cDNA Constructs
For protein expression and purification, ectodomain fragments of human Delta-like 1 (DLL1; GenBankTM ID 118582508), human Delta-like 4 (DLL4; GenBank ID 9506545), and human Notch1 (GenBank ID 148833507) were subcloned into a modified pLEXm mammalian expression vector (42) containing a 15-amino acid residue BirA recognition sequence followed by a His6 tag at the C terminus. A Notch1 fragment encompassing EGF repeats 6–15 was also subcloned into pcDNA3.1 (Invitrogen) with a C-terminal His6 tag. For signaling experiments, human Notch1 ectodomain variants were constructed in the Notch1-gal4 chimeric backbone described previously (N1gal4) (43) or a variant that contains an additional AvrII site between residues 1429 and 1430, encoding Pro-Arg (N1gal4A). All constructs were confirmed via DNA sequencing. A Notch1-Fc fusion protein containing EGF repeats 1–15 (Notch1 1–15-Fc) was also purchased (R&D Systems) for use as a positive control in some binding assays.
Protein Expression and Purification
For large scale protein production, HEK-293S suspension cells were maintained in Freestyle 293 medium (Invitrogen) supplemented with 5% fetal bovine serum and 1% penicillin/streptomycin (Invitrogen) at 37 °C. Cells were grown to a cell density of 106 cells/ml, and then transiently transfected with ligand or Notch DNA (1 mg/liter of culture) and polyethyleneimine (Polysciences) at a 3:1 PEI/DNA ratio. For biotinylation of Avi-tagged proteins, cells were co-transfected with BirA DNA (1 mg/liter of culture). For production of Notch1 proteins, cells were also co-transfected with DNA encoding the O-fucosyl transferase, O-Fuc-T1 (44), which enhanced proper Notch1 folding and secretion.
Transfected cells were cultured in hybridoma serum-free media (Invitrogen) and incubated for 72–96 h. The culture supernatant was separated from the cells by centrifugation (6,000 × g at 4 °C for 30 min) and filtration. Following addition of phenylmethylsulfonyl fluoride (0.2 mm) to inhibit proteolysis, the supernatant was concentrated by ultrafiltration using a Prep/Scale-TFF cartridge (Millipore), and dialyzed into buffer containing 50 mm Tris, 500 mm NaCl, and 5 mm CaCl2, pH 8.0 (Buffer A). The concentrated supernatant was then bound to pre-washed Ni-NTA beads (Qiagen), using ∼3 ml of resin/liter of culture media, and incubated with gentle rocking for at least 2 h. The beads were transferred into a gravity column, washed with 1 column volume of buffer A supplemented with 20 mm imidazole, and bound protein was then eluted with increasing concentrations of imidazole. Fractions containing partially purified protein, as assessed via SDS-PAGE, were concentrated using an Ultracel-10K fitration device (Amicon). After concentration, proteins were further purified by size exclusion chromatography on a Superdex 200 HR 10/30 column in 50 mm Tris, 300 mm NaCl, and 5 mm CaCl2, pH 8.0. The quality and purity of the resulting proteins were assessed via non-reducing SDS-PAGE, and fractions containing a single predominant band were flash frozen in liquid nitrogen and stored at −80 °C. Working stocks of protein were stable at 4 °C for at least 1 month. The efficiency of biotinylation was estimated by immunoprecipitation with SoftLink soft release Avidin resin (Promega).
Luciferase Reporter Assays
The ability of purified ligand proteins to activate Notch1 signaling was assessed in luciferase reporter gene assays using a U2OS Flp-in Notch1-Gal4 stable cell line (43). On day 1, cells in 6-well dishes were transfected with a mixture containing Lipofectamine 2000 (Invitrogen), 2.5 μg of a Gal4-firefly luciferase reporter, and 50 ng of an internal control pRL-TK Renilla luciferase plasmid (Invitrogen). Each well of a non-tissue culture-treated 96-well plate was precoated with 400 ng of RetroNectin (Takara) and, if using avidin capture, 400 ng of streptavidin (Roche Applied Science), and incubated overnight at 4 °C. After washing with buffer, unbiotinylated ligands were allowed to attach to the plate by incubation at room temperature for 30 min. When biotinylated ligands were used, the RetroNectin surface was blocked with 2% BSA for 30 min at room temperature, and then ligands (14 nm) were captured by incubation at room temperature for 30 min. Stable N1gal4 cells were then split onto the ligand-coated plate in the presence of tetracycline (1 μg/ml). On day 3, following 24 h of culture, firefly and Renilla luciferase activities were measured in whole cell extracts using the Dual Luciferase kit (Promega) and a specially configured luminometer (Turner Systems). Each measurement was normalized to a human IgG control surface, for which the signal was arbitrarily set to a value of 1.
To assess the activity of truncated Notch1 receptors, the luciferase reporter assay was performed using U2OS cells transiently transfected with plasmid DNA encoding the different N1gal4 chimeric proteins, in addition to a Gal4 reporter plasmid and the Renilla luciferase internal control plasmid. On day 2, the cells were split into 96-well plates containing OP9 or OP9-Dll1 feeder cells (a kind gift from Dr. Juan-Carlos Zuniga-Pflucker), or MS5 or MS5-Dll4 cells (a kind gift from Nadia Carlesso). Luciferase activity was assessed as described above, after 24 h of co-culture, and responses were normalized to non-ligand bearing OP9 or MS5 cells. To show that reporter activity was dependent on γ-secretase cleavage, compound E was used as a γ-secretase inhibitor (GSI) at 1 μm in numerous experiments. The compound was applied when transfected cells were added to the plated ligand-bearing cells. All data points within experiments were obtained in triplicate, and each experiment was repeated at least three times.
Measurement of Protein Expression
To assess the levels of truncated Notch receptor proteins at the cell surface, the transfected U2OS cells were trypsinized, washed twice with TBS + 0.1% azide, and incubated with an antibody specific for the Notch1 negative regulatory region (WC-75 (45)) at 1:100 dilution for 1 h on ice. After two additional washes, the cells were incubated with 1:100 goat anti-human IgG FITC (Molecular Probes) for 1 h on ice. The cells were then washed twice, resuspended in TBS + azide, and analyzed on a Guava easyCyte 5HT Flow Cytometer (Millipore). The distribution of observed fluorescence was then used to compare levels of cell surface receptor protein for each truncation analyzed.
Flow Cytometry Binding Assay and Fluorescence Microscopy
N1gal4 U2OS cells were induced with tetracycline (1 μg/ml) overnight, then trypsinized, washed twice with TBS, and blocked in TBS + 3% BSA on ice for 30 min. Yellow fluorescent avidin beads (Spherotech; 0.4–0.6 μm) were prepared for capture of biotinylated ligands by washing with calcium-free TBS to remove phosphate buffer, and then incubated with 2 μm biotinylated ligand for 1 h in TBS containing 5 mm CaCl2 (TBS-Ca). Cells were spun down, resuspended in TBS-Ca, and incubated with the corresponding ligand-bead complexes for 1 h. An additional aliquot of cells was also incubated with WC-613, an antibody specific to the Notch ligand-binding region (45), followed by goat anti-human IgG FITC (Molecular Probes) to confirm Notch1 surface expression. Cells were washed 2–3 times, resuspended in TBS-Ca, and analyzed using a Guava easyCyte 5HT Flow Cytometer (Millipore). All incubations were done on ice unless stated otherwise.
To visualize ligand-cell binding, biotinylated ligands (200 nm) were clustered on beads and incubated with cells, as described above. The cell/bead mixtures were then fixed with 1% paraformaldehyde for 20 min on ice, plated onto glass microscope slides with a drop of VECTASHIELD mounting medium containing DAPI (Vector Laboratories), sealed under a 22-mm glass coverslip, and then imaged and photographed on a Nikon Eclipse Ti fluorescence microscope linked to a Photometrics CoolSNAP EZ camera.
ELISA Binding Assay
96-Well plates were coated with Notch molecules (0.5–1.0 μg/ml) by incubating at 4 °C overnight, then washed and blocked with 3% BSA for 1 h. Biotinylated ligands (10–20 nm) were preclustered 4:1 with NeutrAvidin-HRP (Thermo Scientific) by incubating at 4 °C for at least 1 h. The ligand/HRP mixture was adjusted to 3% BSA and added to the Notch-coated plates, which were then rotated at room temperature for 1 h. After another 15 min block and 3–4 washes with TBS, QuantaBlu fluorogenic peroxidase substrate (Thermo Scientific) was added to the wells, and the emission spectra were recorded using a SpectraMax microplate reader (Molecular Devices). The integrity of the plated Notch was assessed using WC-613 and anti-human IgG HRP (Promega) as a secondary antibody. Unless stated otherwise, all experiments were performed in the presence of 5 mm calcium.
Biolayer Interferometry
Protein binding affinities were quantified by biolayer interferometry using the BLItz instrument (ForteBio). Streptavidin biosensors were loaded with biotinylated Notch molecules, equilibrated in buffer for 1 min to establish a stable baseline, then dipped into samples of varying ligand concentrations until equilibrium was reached. All protein-binding experiments were done in HBS-P buffer (GE Healthcare), containing 0.005% P20 surfactant and supplemented with 5 mm CaCl2 unless stated otherwise. Negative control experiments were performed by binding the loaded Notch receptor biosensor with WC-613 blocking antibody before ligand binding, or performing the binding step in EDTA. Equilibrium binding curves were fitted with one-site binding models using GraphPad Prism.
Mass Spectometry
Notch protein (5 μg) was reduced with DTT (10 mm final concentration), alkylated with iodoacetamide (22.5 mm), and digested with 500 ng of chymotrypsin overnight at 37 °C. Digested peptides (∼2 pmol) were injected onto a self-packed pre-column (4 cm POROS10R2), resolved on an analytical column with integrated ESI emitter tip (46) (30 μm inner diameter packed with 12 cm C18) and eluted into the mass spectrometer (LTQ OrbitrapVelos, ThermoFisher Scientific) using an HPLC gradient (NanoAcquity UPLC, Milford, MA; 0–35% B in 120 min; A = 0.2 m acetic acid in water; B = 0.2 m acetic acid in acetonitrile). Peptides were subjected to MS2 by CAD (electron multiplier detection, relative collision energy 35%, q = 0.25) as well as HCD (image current detection, resolution at m/z 400 = 7500, relative collision energy 35%). Raw data files were converted to Mascot Generic Format using in-house m/z scripts (47) and these files were searched using Mascot version 2.2.1 against a forward-reversed human NCBI refseq database. Individual searches were performed for each type of possible carbohydrate modification, and modified peptide hits were manually validated.
Surface Plasmon Resonance
Surface plasmon resonance experiments were performed on a BIAcore 3000 in HBS-P running buffer (10 mm HEPES, pH 7.4, 150 mm NaCl, and 0.005% P20 surfactant), supplemented with 5 mm CaCl2 unless stated otherwise. Biotinylated N1(6–15) was immobilized on a streptavidin-coated chip surface to ∼500 resonance units at a flow rate of 50 μl/min. Biotin (30 μl/min for 1 min) was used to block free streptavidin sites in both the Notch and reference flow cells. Responses were obtained by injecting various ligand concentrations (10 nm to 20 μm) over the flow cells at a rate of 30 μl/min for 3 min, followed by dissociation in buffer until the response reached baseline (∼3 min). Reference cell responses were manually subtracted, and equilibrium binding curves were fitted as described above.
RESULTS
Identification of a Minimum Length Notch1 Receptor That Retains Ligand Responsiveness
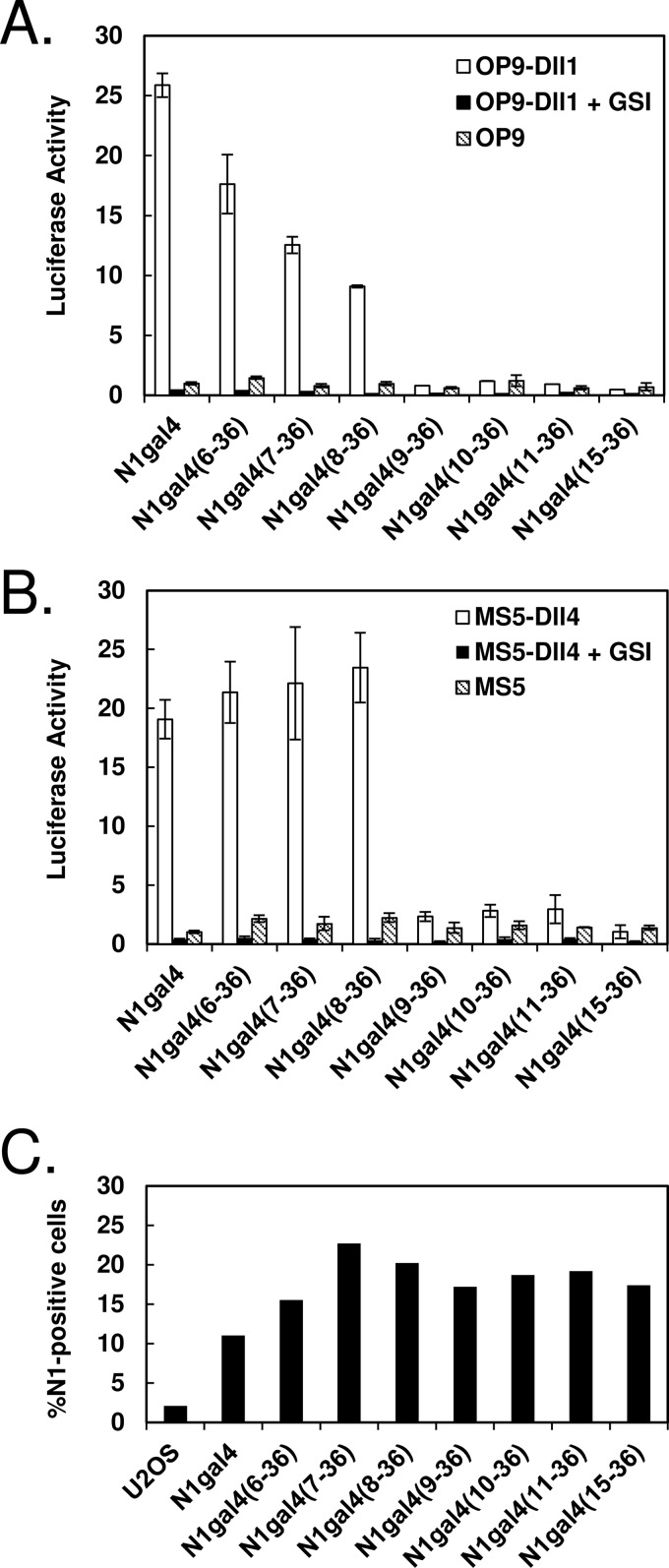
To define a region of extracellular Notch required for full signaling capacity, truncation mutants of the Notch ligand-binding region were made in the context of Notch1-gal4 chimeras. In these proteins, the intracellular ankyrin repeat domain of Notch1 is replaced with the DNA-binding domain of gal4, permitting receptor activity to be read out on a gal4 luciferase reporter gene (Fig. 1A). Systematic truncation of successive N-terminal EGF repeats began at EGF6, as EGF repeats 1–5 have been reported to be dispensable for Notch-ligand binding in Drosophila (14). Cells transiently transfected with the various N1gal4 chimeras were stimulated by co-culture with OP9 cells stably expressing Dll1 (OP9-Dll1 cells), or MS5 cells stably expressing Dll4 (MS5-Dll4 cells), and compared with stimulation by non-ligand bearing OP9 or MS5 cells, respectively. The pattern of ligand responsiveness of the different truncated receptors was similar regardless of whether signaling was stimulated by OP9-Dll1 cells (Fig. 2A) or MS5-Dll4 cells (Fig. 2B).
FIGURE 2.
Signaling of N-terminal Notch truncations. A and B, ligand-induced activation of N-terminal N1gal4 truncations. U2OS cells were transiently transfected with receptor variants, co-cultured with ligand-expressing or control cells, and the resulting activation was measured via luciferase activity. A, Notch N-terminal truncations activated with OP9-Dll1 cells. Gradual loss of activity can be seen with removal of EGF repeats 6 and 7, and complete loss of activity with removal of EGF8. B, Notch N-terminal truncations activated with MS5-Dll4 cells. Activity equivalent to full-length N1gal4 is observed upon deletion of EGF repeats 6 and 7, but deletion of EGF8 abolishes signaling. All results are shown normalized to co-culture with non-ligand-bearing OP9 or MS5 cells, and treatment with the γ-secretase inhibitor compound E (GSI, 1 μm) inhibits activation in all receptor constructs tested. C, surface expression of truncated receptors. Surface expression was detected via flow cytometry, using an antibody to the Notch1 NRR (WC-75) and a FITC-conjugated secondary antibody. Percentage of Notch1-positive cells is indicated on the y axis.
Near wild-type signaling activity is still observed after removal of the first five EGF modules with both OP9-Dll1 (Fig. 2A) and MS5-Dll4 cells (Fig. 2B). A gradual decrease in reporter activity is then seen upon sequential removal of EGF repeats 6 and 7 when OP9-Dll1 cells are used as a source of ligand, such that the N1(8–36) receptor retains ∼40% of full-length Notch1 activity (Fig. 2A). In contrast, when MS5-Dll4 cells are used as a source of ligand, the responsiveness of Notch1 receptors lacking as many as the first 7 EGF repeats is indistinguishable from full-length Notch. However, when the first 8 EGF repeats are removed, the resulting N1(9–36) receptor exhibits little residual signaling activity with either ligand. Further deletion of EGF repeats 9 and 10 also yields truncated receptors with no responsiveness to either ligand. Deletion of EGF repeats 1–15, which encompasses the EGF repeat 11–12 region previously determined to be critical for ligand binding (13, 14), also produces a receptor with no ligand-induced signaling activity, and treatment with the GSI inhibitor compound E abolishes activity in all receptor truncations.
To exclude the possibility that differences in signaling could be attributable to differences in protein expression, surface protein levels were assessed by flow cytometry. Consistent levels of surface expression were detected by flow cytometry (Fig. 2C), indicating that the assay faithfully reported on the intrinsic signaling activity of the various truncated receptors tested.
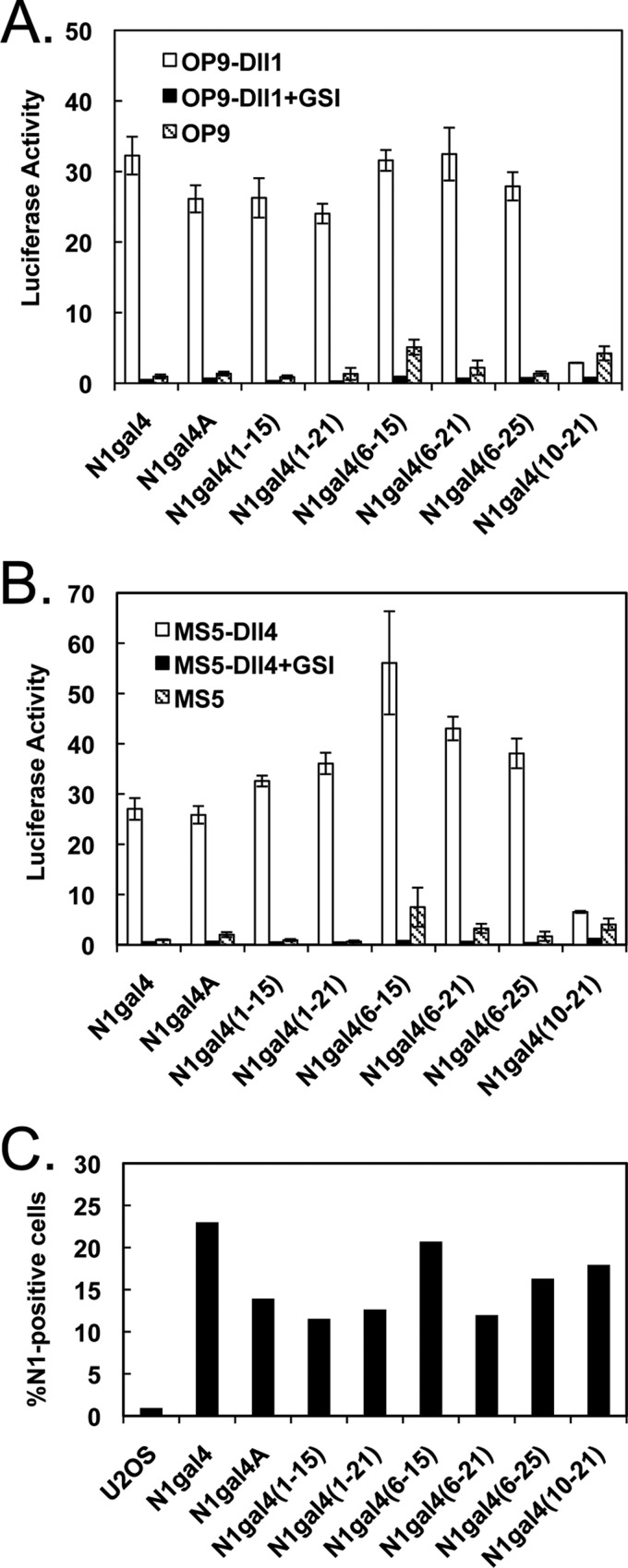
To construct C-terminal truncations, the N1gal4A variant was produced by inserting an AvrII restriction site between EGF36 and the LNR repeat region (Fig. 1A). Internal deletion mutants were then cloned into N1gal4A, comprising EGF repeats 1–21 and 1–15; the C-terminal repeats were chosen based on the location of the consensus calcium-binding sequences and known ligand-binding repeats. Both C-terminal truncations showed activity indistinguishable from the N1gal4A parent molecule, when stimulated with either OP9-Dll1 or MS5-Dll4, arguing that EGF repeats 16–36 are not necessary for full Notch activation (Fig. 3, A and B). Additionally, when the N-terminal deletion of EGF repeats 1–5 was combined with these C-terminal truncations, the resulting molecules also displayed activation similar to full-length N1gal4A, whereas a Notch1 variant lacking EGF repeats 1–9 and 22–36 showed no ligand-dependent activation, consistent with the effect of removing EGF repeats 1–9 alone. Surface expression was also evaluated for the set of C-terminal truncations, all of which showed a relatively consistent degree of expression (Fig. 3C). The Notch1 EGF6–15 region, therefore, appears to be sufficient to generate full-length Notch signaling activity.
FIGURE 3.
Signaling of C-terminal Notch truncations. A and B, ligand-induced activation of C-terminal N1gal4 truncations. Luciferase assays were performed as described in the legend to Fig. 2. A, Notch C-terminal truncations activated with OP9-Dll1 cells. Activity comparable with full-length N1gal4A is observed in C-terminal truncations up to EGF15, and truncating from both ends resulted in the identification of the EGF repeat 6–15 fragment, the smallest Notch1 fragment identified with activity comparable with the full-length ectodomain. B, Notch C-terminal truncations activated with MS5-Dll4 cells. The observed activation pattern is similar to OP9-Dll1 results. All data are shown normalized to co-culture with non-ligand-bearing OP9 or MS5 cells, and treatment with 1 μm GSI inhibits activation in all receptor constructs tested. C, surface expression of truncated receptors. Surface expression was detected via flow cytometry, using an antibody to the Notch1 NRR (WC-75) and a FITC-conjugated secondary antibody. Percentage of Notch1-positive cells is indicated on the y axis.
Identification of the Minimum Length Region of Dll1 and Dll4 Needed to Stimulate Notch1 Signaling
Previous studies using cell aggregation, as well as cell- and plate-based binding assays, have reported that the N-terminal MNNL and DSL domains of both fly and mammalian Notch ligands are required for receptor binding (15, 16), but less is known about the ligand regions needed to induce a signaling response in Notch-expressing cells. Therefore, to define the region of mammalian Delta-like ligands required to initiate optimal Notch signaling, EGF repeats were systematically truncated from the C terminus up to the DSL domain of Dll1 and Dll4 (Fig. 1, C and D). Additionally, an N-terminal deletion construct of Dll1 was created by removing the MNNL domain (Dll1(1–7)ΔMN). Each ligand protein was expressed in HEK-293S cells (as described in Ref. 42), captured on Ni-NTA resin, and purified via size exclusion chromatography. The presence of a single band on non-reducing SDS-PAGE confirmed that these steps were sufficient to yield protein of uniformly high purity and quality for subsequent assays (Fig. 4, A and B).
FIGURE 4.
Signaling of ligand truncations. A, non-reducing SDS-PAGE gel of purified Dll1 proteins. B, non-reducing SDS-PAGE gel of purified Dll4 proteins. Multiple bands are present as a result of N-linked glycosylation, which was not observed in Dll1. C, signaling induced by Dll1 truncations. Purified Dll1 proteins were plated at an equivalent molar concentration and used to activate N1gal4 U2OS stable cells. Resulting luciferase activity is shown on the y axis, normalized to activation with plated human IgG. C-terminal truncation up to EGF3 had little effect on signaling, whereas removal of the N-terminal MNNL domain (ΔMN) abolished activity. D, signaling induced by Dll4 truncations, performed as in C. C-terminal truncations up to EGF3 also maintained full signaling capacity, whereas removal of EGF3 abolished signaling. E and F, concentration dependence of ligand-induced signaling. Dll1(1–5) and Dll4(1–5) were plated at varying concentrations and incubated with U2OS cells transfected with either full-length N1gal4 or N1gal4(6–15). The resulting luciferase activity is shown as a function of concentration, fitted with a one-site exponential binding model. Calculated EC50 values for N1gal4 were 227 ± 175 nm (Dll1) and 47 ± 22 nm (Dll4); for N1gal4(6–15), 281 ± 82 nm (Dll1) and 47 ± 33 nm (Dll4).
To assess the ability of purified Dll1 molecules to induce Notch signaling, we used a luciferase reporter assay in which N1gal4 U2OS stable cells were incubated in the presence of various ligand molecules immobilized on a 96-well plate. These studies revealed that sequential elimination of EGF repeats 4–8 of Dll1 did not reduce signaling below full-length levels (Fig. 4C). Additional deletion of EGF3, however, diminished signaling by more than 50% when compared with full-length Dll1, and further deletion beyond EGF3 reduced signaling to background levels. In addition, removal of the MNNL domain (Dll1(1–7)ΔMN) also eliminated detectable signaling activity above background levels. Similar reporter assays carried out with a panel of Dll4 truncations (Fig. 4D) mirrored the pattern observed with Dll1 as the plated ligand, showing that the region required to induce a Notch1 signal is shared between the two major mammalian Delta-like ligands.
To compare directly how Notch1 responds to Dll1 and Dll4, we measured the N1gal4 signal stimulated in response to the Dll1(1–5) and Dll4(1–5) ligands as a function of concentration. These proteins were chosen instead of the full ligand ectodomains because they were as active as the full ectodomains in signaling assays. They were also more stable during storage, less aggregation-prone than the full ectodomains, and generally better behaved molecules biochemically. Both ligands exhibited a clear concentration dependence, yielding EC50 values of 227 ± 175 nm for Dll1 and 47 ± 22 nm for Dll4, when fit with a one-phase exponential binding model (Fig. 4E). These results show that purified Dll1 is less intrinsically active than Dll4 in inducing a Notch1 signal. Similar fits were obtained using N1gal4(6–15) instead of full-length N1gal4 (Fig. 4F), confirming that ligand-induced signaling of the N1(6–15) fragment faithfully reflects the dose-response behavior of full-length Notch1.
Direct Visualization of Ligand Binding to Cells Expressing Notch1 Receptors
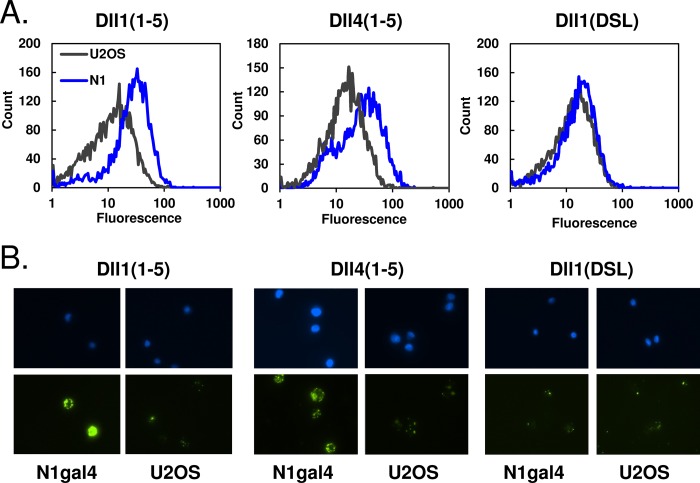
Binding of Notch EGF repeats 11–13 and 11–14 to Delta-like 1-expressing cells has been previously observed by flow cytometry (27). We adopted a similar approach to test binding of our recombinant ligand molecules to full-length Notch1 on cells. Purified Dll1 and Dll4 fragments were coupled to avidin-coated fluorescent beads, and then subsequently bound to Notch1-expressing cells. Analysis by flow cytometry showed that incubation of Dll1(1–5) or Dll4(1–5) with Notch1-expressing cells produced a significant shift in fluorescence intensity (Fig. 5A), indicating that these ligands bind preferentially to cells expressing the Notch receptor. The observed shift was not observed with a shorter, non-signaling ligand (the DSL domain of Dll1), as predicted from the reporter assay data. These results confirm that purified Dll1(1–5) and Dll4(1–5) bind to full-length Notch1 on the cell surface.
FIGURE 5.
Ligand binding to Notch cells. A, detection of purified ligands binding to Notch-bearing cells. Biotinylated ligands were coupled to yellow fluorescent avidin beads and incubated with Notch1-gal4 U2OS or U2OS control cells. When analyzed via flow cytometry, the N1gal4 cells incubated with Dll1(1–5) and Dll4(1–5) showed a shift in fluorescence relative to U2OS control cells, which was not observed with the shorter Dll1(DSL) ligand. B, visualization of ligand-cell binding with fluorescence microscopy. Top and bottom rows show DAPI and FITC staining, respectively. Beads coated with Dll1(1–5) or Dll4(1–5) both bound preferentially to Notch-bearing cells over control cells, whereas Dll1(DSL)-coated beads did not.
Binding of these ligand-coated beads to Notch1-expressing cells was also visualized by fluorescence microscopy, which showed beads clustered on the cell surface in discrete puncta (Fig. 5B). As expected, Dll1(1–5)- and Dll4(1–5)-coated beads could be seen binding preferentially to Notch-expressing cells, whereas Dll1(DSL)-coated beads showed background levels of binding to both Notch-expressing and U2OS control cells.
Expression and Binding of Purified Notch Protein
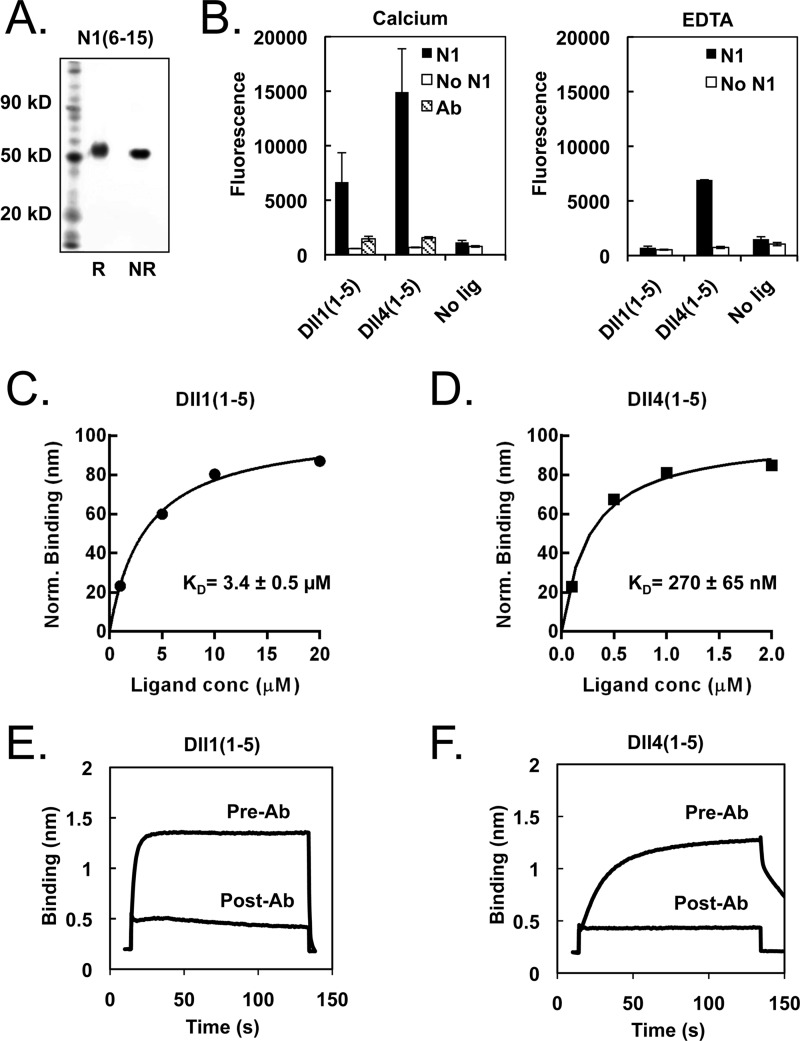
After we determined that EGF repeats 6–15 of Notch1 were necessary and sufficient for signaling in response to ligand-expressing cells, the cDNA for EGF repeats 6–15 of Notch1 (N1(6–15)) was cloned into a mammalian expression vector behind the Notch1 signal sequence and in-frame with a C-terminal His6 or Avi-His6 tag (Fig. 1B) for production of purified protein. To achieve proper folding and secretion of N1(6–15) proteins in HEK-293S suspension culture, it was necessary to co-express O-Fuc-T1, an O-fucosyl-transferase and Notch-specific chaperone. After purification on Ni-NTA affinity resin and size exclusion chromatography, the recombinant N1(6–15) ran as a single band on non-reducing SDS-PAGE (Fig. 6A).
FIGURE 6.
Measurement of Notch-ligand interactions using purified proteins. A, SDS-PAGE gel of purified N1(6–15) protein under reducing and non-reducing conditions. B, ELISA binding assay. Functional ELISAs were performed on plated N1(6–15), bound with biotinylated Dll1(1–5) or Dll4(1–5) preclustered with Neutravidin-HRP. The resulting interaction is calcium-dependent and inhibited by blocking antibody. C and D, quantification of Notch-ligand binding affinity via biolayer interferometry (BLI). Biotinylated N1(6–15) was immobilized on a streptavidin biosensor and bound to varying concentrations of Dll1(1–5) or Dll4(1–5). The resulting concentration-response plots were fitted with a one-site exponential binding model and normalized for comparison, yielding KD values of 270 ± 65 nm for N1-Dll4 and 3.4 ± 0.5 μm for N1-Dll1 interactions. E and F, specificity of observed Notch-ligand binding. Treatment with the ligand-blocking antibody WC-613 (1 μg/ml) inhibited binding of Dll1 (5 μm) or Dll4 (2 μm) to N1(6–15).
O-Linked glycosylation of Drosophila Notch can exert differential effects on the responsiveness of the receptor to Delta and Serrate ligands (19, 21, 48) and Fringe-mediated addition of N-acetylglucosamine to O-fucosylated Notch, in particular, appears to enhance binding of Delta and inhibit binding of Serrate to fly Notch (14). To determine whether these modifications are present in our binding studies, the glycosylation state of N1(6–15) was analyzed by mass spectrometry. Evidence of fucosylation was observed at several EGF repeats within the 6–15 region, and a mass change consistent with O-linked addition of an N-acetylglucosamine residue to EGF14 was also observed (Table 1, supplemental Fig. S1). However, there was no evidence for extension of O-fucose residues with N-acetylglucosamine. These data indicate that the N1(6–15) molecule used in these experiments lacks Fringe modifications.
TABLE 1.
Sugar modifications identified on N1(6–15) by tandem mass spectrometry of chymotryptic peptides
| Peptide sequence | Notch residues | Modification | Location |
|---|---|---|---|
| YCECPHGRTGLL | 358–369 | Fucose | EGF9 |
| TGPRCEIDVNECVSNPCQNDATCLDQIGEF | 445–474 | Fucose | EGF12 |
| GSCKDGVATFTCL | 578–590 | Fucose | EGF15 |
| CTEDVDECQLMPNACQNGGTCHNTHGGY | 292–319 | Fucose | EGF8 |
| HGATCHDRVASF | 346–357 | Fucose | EGF9 |
| TGTHCEVDIDECDPDPCHY | 559–577 | HexNAc | EGF14 |
| TCVCTEGYTGTHCEVDIDECDPDPCHY | 551–577 | HexNAc | EGF14 |
| GSFECQCLQGY | 434–444 | Hexose | EGF11 |
| DVDECASTPCKDGAKCL | 528–544 | Xyl-Xyl-hexose | EGF14 |
The activity of N1(6–15) was then tested in a solid-phase binding assay. Notch molecules were immobilized on a plate, and incubated with biotinylated ligand molecules bound to NeutrAvidin-HRP. Both Dll1(1–5) and Dll4(1–5) exhibited detectable binding to the N1(6–15) protein (Fig. 6B); of note, binding was observed only when the biotinylated ligand molecules were pre-clustered with HRP, and not when the two reagents were applied sequentially. The observed signal was inhibited by addition of WC-613, a Notch-specific ligand-blocking antibody (45), and was substantially diminished by EDTA, indicating that the interaction of N1(6–15) with the Dll1 and Dll4 proteins is specific and partially calcium dependent. Similar results were obtained when a commercial N1(1–15)-Fc protein was plated on the solid surface (not shown).
Quantitative Analysis of Notch-Ligand Interactions
We next measured the affinity of N1(6–15) for both Dll1 and Dll4 by biolayer interferometry, to directly compare the two values. Each ligand exhibited a saturation-binding curve that could be fit readily by a single-site binding model, which yielded KD values of 3.4 ± 0.5 μm for Dll1(1–5) and 270 ± 65 nm for Dll4(1–5) (Fig. 6, C and D). Association of Dll1 or Dll4 with Notch1 was blocked in the presence of ligand-blocking anti-Notch1 antibody (Fig. 6, E and F). Neither ligand showed binding in the presence of EDTA, nor to the Notch1 NRR (data not shown), confirming that the observed binding of both ligands to Notch1 is specific.
To cross-validate these results, we also performed surface plasmon resonance measurements of the Notch1-Dll4 and Notch1-Dll1 interactions. Fitting the concentration-response plots yielded estimated KD values of 12.4 ± 6.0 μm for N1:Dll1 and 450 ± 31 nm for N1:Dll4, consistent with the biolayer interferometry measurements (data not shown). Together, these binding data reveal that Notch1 has an affinity for Dll4 that is more than 10-fold greater than its affinity for Dll1.
DISCUSSION
The existence of four mammalian Notch receptors and five canonical ligands results in the potential formation of 20 different receptor-ligand combinations. Receptor glycosylation at O-linked sites, which occurs within the critical region for ligand binding and differentially influences the responsiveness of receptors to Delta and Serrate/Jagged family ligands, further expands the range of mature receptor-ligand possibilities. Remarkably, however, little is known about intrinsic differences in binding affinities among the different receptor-ligand pairs, perhaps due to the challenges in producing sufficient amounts of purified proteins for detailed biochemical study.
Here, we used a systematic deletion analysis to identify the region of Notch1 that is sufficient to maintain a response to Dll1 and Dll4 comparable with that of the full-length receptor, and thereby zero in on an ectodomain fragment that fully encompasses the critical region. Notch EGF repeats 11–12 have long been thought to be necessary and sufficient for Notch-ligand binding (13, 49, 50), and our results confirm that deletion of these repeats abolishes detectable Notch signaling. In addition, we have shown that Notch EGF repeats 8–10 are required for Dll1- and Dll4-induced Notch signaling, and that EGF repeats 6 and 7 may play a minor role as well, at least in the response to Dll1.
Other recent studies are consistent with our conclusion that repeats beyond EGF repeats 11–12 play an important role in Notch-ligand interactions. The jigsaw mutation, a newly described Drosophila Notch allele, harbors a mutation in EGF repeat 8 (V361M) that selectively impairs Serrate binding and signaling activity, whereas it exhibits negligible effects on Delta binding and signaling (51). Similarly, in cell-based binding assays, a Drosophila Notch construct comprising EGF repeats 10–13 exhibits very little binding to Delta- or Serrate-expressing S2 cells, relative to full-length Notch, and isolated deletion of either EGF repeats 1–9 or 25–36 also appears to impair binding (14). The latter portion overlaps with the Abruptex region (EGF repeats 24–29), a stretch of Notch EGF repeats in which point mutations can cause gain-of-function phenotypes with complex complementation patterns (52). To explain these phenotypes, it has been suggested that this region may normally play an inhibitory role in Notch signaling via Notch-Notch interactions (53) or cis-inhibitory Notch-ligand interactions (54). Our reporter assay data indicates that the Abruptex region of Notch1 is dispensable for trans-activation, as removal of Notch EGF repeats 26–36 or 22–36 still produces signaling equivalent to full-length Notch. Any potential effect on cis-inhibition remains to be explored, but if the Abruptex region is involved in Notch-Notch interactions, those interactions do not appear to impact the maximum observed signal strength in our assays with the human Notch1 receptor.
A similar mutagenesis strategy was used to determine which regions of Dll1 and Dll4 are required to induce a full-strength signaling response in Notch1-expressing cells. These mapping studies pointed to the importance of the MNNL domain, as well as the DSL domain and EGF repeats 1–3, in inducing a strong signaling response from receptors bearing the Notch1 ectodomain. These observations are consistent with previously published studies, which have established that the MNNL and DSL of Drosophila Delta and mouse Jagged1 are required for binding, whereas EGF repeats 1–2 also play important modulatory roles (15, 16). These results are also in line with the known pattern of mutations in the JAG1 ligand observed in Alagille syndrome, in which mutations are most frequently observed in the MNNL domain, DSL domain, and the first two EGF repeats (see Ref. 55 for a review).
Despite the wealth of available studies on Notch signaling in cells and model organisms, quantitative binding data for Notch-ligand interactions remains relatively sparse and inconsistent. ELISA-based assays have been used to estimate a KD of ∼0.7 nm for a mouse Jagged1 ectodomain interacting with Notch2 EGF repeats 1–15 (16), and a KD of 1.87 nm for fly Delta binding to Notch EGF repeats 11–20 (53). These measurements seem consistent with the relatively high adhesion rate observed between Notch and Delta-expressing cells (56). However, surface plasmon resonance-based measurements of the interaction between bacterially expressed (i.e. lacking sugar modifications) Notch1(11–14) and a Dll1 fragment, spanning from the MNNL domain to EGF3, yielded an estimated KD of 130 μm, several orders of magnitude higher than the ELISA values.
Quantitative studies of Notch-ligand interactions have also encountered challenges due to the various O-linked sugars that decorate the Notch ectodomain. The N1(6–15) protein used in these experiments is co-expressed with O-fuc-T1, which modifies serine and threonine residues at consensus sites with O-linked fucose. However, the HEK-293S cells used to produce N1(6–15) are effectively Fringe-null,4 and did not elaborate O-fucose residues with secondary N-acetylglucosamine moieties (Table 1, supplemental Fig. S1). Thus, the binding affinities reported here correspond to the interaction of Dll1 and Dll4 with O-fucosylated N1(6–15) that lacks Fringe modification. Because Fringe modification has been reported to enhance the binding of Delta to Notch in cell-based assays, relative to Serrate ligands (21, 22), it should now be possible to quantify these effects directly by comparing the binding of Dll1 and Dll4 to N1(6–15) before and after modification with recombinant Fringe. Intriguingly, Radtke and co-workers (57) observed that Dll1-Fc binding to Notch1-expressing cells was enhanced by co-expression with Lunatic Fringe, whereas binding of Dll4-Fc to Notch1 cells was relatively unaffected, suggesting that Fringe modification may be another way to fine-tune the Notch-ligand subtype specificity. Comparison of Dll1 and Dll4 binding to Fringe-modified Notch in our purified protein assay could yield important insights into this mechanism.
Several other sugar modifications were also observed on the N1(6–15) protein, including a primary N-acetylglucosamine (GlcNAc) on EGF14. The presence of an O-GlcNAc modification has been reported on EGF20 of Drosophila Notch, and within EGF repeats 1–10 and 22–31 (58), and O-GlcNAcylation has been shown to mediate epithelial cell-matrix interactions at the cell surface. However, mutation of the Eogt O-GlcNAc transferase has not been found to affect any Notch-dependent processes, so it remains to be seen what effect, if any, this modification has on Notch signaling (59). Additionally, a Xyl-Xyl-Gal modification was observed on EGF14, which is expected based on the Rumi consensus sequence, and a single hexose modification was observed on EGF11, which is not (60). Finally, O-fucosylation is seen on EGF repeats 8, 9, and 12, which is consistent with the O-Fuc-T1 consensus sequence and previously published literature (61–63). Additional O-fucose modifications were observed between C5 and C6 on EGF9, and between C3 and C4 on EGF15, possibly due to forced co-expression of O-Fuc-T1.
Our measured values of KD likely reflect monovalent binding of Notch1 to both Dll1 and Dll4, and fall between the high affinities reported in the ELISA-based measurements with Fc-fused ligand proteins, and the low affinities reported using smaller ligand and receptor fragments. In the solid phase studies, the influence of avidity effects likely overestimates the receptor-ligand monovalent KD (also evident in our own ELISA; Fig. 6B). In contrast, the presence of additional Notch1 EGF repeats, particularly EGF repeats 8–10 identified here as essential for signaling, likely accounts in part for the increased affinity (along with a potential contribution from O-fucosylation) in the interaction of Dll1 with Notch1 repeats 6–15, as compared with Notch1 repeats 11–14.
The observed interaction between Notch1 and Dll4 plays a critical role in many physiological processes, including vascular development and T lymphopoiesis. Notch1 and Dll4 null mice share several similarities, such as defects in arterial branching, stenosis of the large arteries, and failure to remodel the primitive vascular plexus, suggesting that Dll4 is a key ligand mediating Notch1 signaling in the vasculature (64, 65). Dll4 is also the physiologic ligand in the induction of T-cell commitment (66), and tests of ligand binding activity showed that Dll4-Fc fusion proteins exhibit higher avidity than Dll1-Fc fusions for binding to both thymocytes and Notch1 transiently transfected cells (57).
Although previous reports have described a propensity for Notch1-Dll4 binding in cell culture and in vivo, our study presents the first quantitative comparison of purified Dll1 and Dll4 binding to Notch in vitro. The observation that the affinity of Dll4 for Notch1 is more than 10-fold tighter than that of Dll1 provides a clear biochemical foundation for these observations, and suggests that the relative importance of Dll4 as a Notch1 ligand derives not only from patterns of ligand gene expression, but also from inherent differences in Notch1 affinity. These results are consistent with signaling studies of Dll4/Jagged1 chimeras, which found that differences in signal magnitude were determined by the ligand extracellular regions, whereas the intracellular regions were necessary but interchangeable (67).
So what is the source of the Notch1-affinity difference between Dll1 and Dll4? Alignment of the MNNL-EGF3 regions reveals high sequence identity throughout the EGF repeats (66.7–82.5%), whereas identity between the MNNL domains is relatively low (53.1%), with a large proportion of sequence gaps in the alignment (11.9%), and identity between the DSL domains is lowest of all (45.7%). Additionally, several mutations in the Jagged1 DSL domain have been shown to abolish ligand-induced Notch signaling, supporting a key role for this region in ligand-receptor binding (26). Thus, the MNNL and/or DSL domains are the most likely source of this affinity difference, but conclusive resolution of this question must await either detailed structural studies or follow-up investigation using Dll1/Dll4 chimeric proteins.
Due to the physiological importance of Notch1-Dll4 signaling, selective targeting of this complex over other Notch1-ligand complexes has potential therapeutic significance. Dll4 is preferentially expressed in tumor vasculature, relative to normal blood vessels (68), and Dll4-targeting antibodies have already shown promise in inhibiting tumor growth (29, 30). Inhibitory Dll4 antibodies have also been shown to attenuate atherosclerosis and metabolic disorders in mice (5). Blocking a specific ligand-receptor complex would result in improved specificity, whereas mitigating on-pathway side effects, warranting a more complete exploration of ligand-receptor subtype interactions in the near future.
Acknowledgments
We thank Pam Stanley and Robert Haltiwanger for providing O-Fuc-T1 constructs.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 CA092433 (to S. C. B.) and P01 CA119070.

This article contains supplemental Fig. S1.
P. Stanley, personal communication.
- DSL
- Delta, Serrate, and Lag-2
- GSI
- γ-secretase
- Ni-NTA
- nickel-nitrilotriacetic acid.
REFERENCES
- 1. Artavanis-Tsakonas S., Rand M. D., Lake R. J. (1999) Notch signaling. Cell fate control and signal integration in development. Science 284, 770–776 [DOI] [PubMed] [Google Scholar]
- 2. Bray S. J. (2006) Notch signalling. A simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 7, 678–689 [DOI] [PubMed] [Google Scholar]
- 3. Aster J. C., Pear W. S., Blacklow S. C. (2008) Notch signaling in leukemia. Annu. Rev. Pathol. 3, 587–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Penton A. L., Leonard L. D., Spinner N. B. (2012) Notch signaling in human development and disease. Semin. Cell Dev. Biol. 23, 450–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fukuda D., Aikawa E., Swirski F. K., Novobrantseva T. I., Kotelianski V., Gorgun C. Z., Chudnovskiy A., Yamazaki H., Croce K., Weissleder R., Aster J. C., Hotamisligil G. S., Yagita H., Aikawa M. (2012) Notch ligand Delta-like 4 blockade attenuates atherosclerosis and metabolic disorders. Proc. Natl. Acad. Sci. U.S.A. 109, E1868–E1877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blaumueller C. M., Qi H., Zagouras P., Artavanis-Tsakonas S. (1997) Intracellular cleavage of Notch leads to a heterodimeric receptor on the plasma membrane. Cell 90, 281–291 [DOI] [PubMed] [Google Scholar]
- 7. Mumm J. S., Schroeter E. H., Saxena M. T., Griesemer A., Tian X., Pan D. J., Ray W. J., Kopan R. (2000) A ligand-induced extracellular cleavage regulates γ-secretase-like proteolytic activation of Notch1. Mol. Cell 5, 197–206 [DOI] [PubMed] [Google Scholar]
- 8. De Strooper B., Annaert W., Cupers P., Saftig P., Craessaerts K., Mumm J. S., Schroeter E. H., Schrijvers V., Wolfe M. S., Ray W. J., Goate A., Kopan R. (1999) A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature 398, 518–522 [DOI] [PubMed] [Google Scholar]
- 9. Struhl G., Greenwald I. (1999) Presenilin is required for activity and nuclear access of Notch in Drosophila. Nature 398, 522–525 [DOI] [PubMed] [Google Scholar]
- 10. Schroeter E. H., Kisslinger J. A., Kopan R. (1998) Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 393, 382–386 [DOI] [PubMed] [Google Scholar]
- 11. Jarriault S., Brou C., Logeat F., Schroeter E. H., Kopan R., Israel A. (1995) Signalling downstream of activated mammalian Notch. Nature 377, 355–358 [DOI] [PubMed] [Google Scholar]
- 12. Gordon W. R., Arnett K. L., Blacklow S. C. (2008) The molecular logic of Notch signaling. A structural and biochemical perspective. J. Cell Sci. 121, 3109–3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rebay I., Fleming R. J., Fehon R. G., Cherbas L., Cherbas P., Artavanis-Tsakonas S. (1991) Specific EGF repeats of Notch mediate interactions with Delta and Serrate. Implications for Notch as a multifunctional receptor. Cell 67, 687–699 [DOI] [PubMed] [Google Scholar]
- 14. Xu A., Lei L., Irvine K. D. (2005) Regions of Drosophila Notch that contribute to ligand binding and the modulatory influence of Fringe. J. Biol. Chem. 280, 30158–30165 [DOI] [PubMed] [Google Scholar]
- 15. Parks A. L., Stout J. R., Shepard S. B., Klueg K. M., Dos Santos A. A., Parody T. R., Vaskova M., Muskavitch M. A. (2006) Structure-function analysis of Delta trafficking, receptor binding and signaling in Drosophila. Genetics 174, 1947–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shimizu K., Chiba S., Kumano K., Hosoya N., Takahashi T., Kanda Y., Hamada Y., Yazaki Y., Hirai H. (1999) Mouse jagged1 physically interacts with Notch2 and other Notch receptors. Assessment by quantitative methods. J. Biol. Chem. 274, 32961–32969 [DOI] [PubMed] [Google Scholar]
- 17. Wang Y., Spellman M. W. (1998) Purification and characterization of a GDP-fucose:polypeptide fucosyltransferase from Chinese hamster ovary cells. J. Biol. Chem. 273, 8112–8118 [DOI] [PubMed] [Google Scholar]
- 18. Okajima T., Irvine K. D. (2002) Regulation of Notch signaling by O-linked fucose. Cell 111, 893–904 [DOI] [PubMed] [Google Scholar]
- 19. Brückner K., Perez L., Clausen H., Cohen S. (2000) Glycosyltransferase activity of Fringe modulates Notch-Delta interactions. Nature 406, 411–415 [DOI] [PubMed] [Google Scholar]
- 20. Moloney D. J., Panin V. M., Johnston S. H., Chen J., Shao L., Wilson R., Wang Y., Stanley P., Irvine K. D., Haltiwanger R. S., Vogt T. F. (2000) Fringe is a glycosyltransferase that modifies Notch. Nature 406, 369–375 [DOI] [PubMed] [Google Scholar]
- 21. Panin V. M., Papayannopoulos V., Wilson R., Irvine K. D. (1997) Fringe modulates Notch-ligand interactions. Nature 387, 908–912 [DOI] [PubMed] [Google Scholar]
- 22. Yang L. T., Nichols J. T., Yao C., Manilay J. O., Robey E. A., Weinmaster G. (2005) Fringe glycosyltransferases differentially modify Notch1 proteolysis induced by Delta1 and Jagged1. Mol. Biol. Cell 16, 927–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Acar M., Jafar-Nejad H., Takeuchi H., Rajan A., Ibrani D., Rana N. A., Pan H., Haltiwanger R. S., Bellen H. J. (2008) Rumi is a CAP10 domain glycosyltransferase that modifies Notch and is required for Notch signaling. Cell 132, 247–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leonardi J., Fernandez-Valdivia R., Li Y. D., Simcox A. A., Jafar-Nejad H. (2011) Multiple O-glucosylation sites on Notch function as a buffer against temperature-dependent loss of signaling. Development 138, 3569–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fernandez-Valdivia R., Takeuchi H., Samarghandi A., Lopez M., Leonardi J., Haltiwanger R. S., Jafar-Nejad H. (2011) Regulation of mammalian Notch signaling and embryonic development by the protein O-glucosyltransferase Rumi. Development 138, 1925–1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cordle J., Johnson S., Tay J. Z., Roversi P., Wilkin M. B., de Madrid B. H., Shimizu H., Jensen S., Whiteman P., Jin B., Redfield C., Baron M., Lea S. M., Handford P. A. (2008) A conserved face of the Jagged/Serrate DSL domain is involved in Notch trans-activation and cis-inhibition. Nat. Struct. Mol. Biol. 15, 849–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cordle J., Redfieldz C., Stacey M., van der Merwe P. A., Willis A. C., Champion B. R., Hambleton S., Handford P. A. (2008) Localization of the Delta-like-1-binding site in human Notch-1 and its modulation by calcium affinity. J. Biol. Chem. 283, 11785–11793 [DOI] [PubMed] [Google Scholar]
- 28. Pei Z., Baker N. (2008) Competition between Delta and the Abruptex domain of Notch. BMC Dev. Biol. 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ridgway J., Zhang G., Wu Y., Stawicki S., Liang W. C., Chanthery Y., Kowalski J., Watts R. J., Callahan C., Kasman I., Singh M., Chien M., Tan C., Hongo J. A., de Sauvage F., Plowman G., Yan M. (2006) Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature 444, 1083–1087 [DOI] [PubMed] [Google Scholar]
- 30. Noguera-Troise I., Daly C., Papadopoulos N. J., Coetzee S., Boland P., Gale N. W., Lin H. C., Yancopoulos G. D., Thurston G. (2006) Blockade of Dll4 inhibits tumour growth by promoting non-productive angiogenesis. Nature 444, 1032–1037 [DOI] [PubMed] [Google Scholar]
- 31. Hellström M., Phng L. K., Hofmann J. J., Wallgard E., Coultas L., Lindblom P., Alva J., Nilsson A. K., Karlsson L., Gaiano N., Yoon K., Rossant J., Iruela-Arispe M. L., Kalén M., Gerhardt H., Betsholtz C. (2007) Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445, 776–780 [DOI] [PubMed] [Google Scholar]
- 32. Mohtashami M., Shah D. K., Nakase H., Kianizad K., Petrie H. T., Zúñiga-Pflücker J. C. (2010) Direct comparison of Dll1- and Dll4-mediated Notch activation levels shows differential lymphomyeloid lineage commitment outcomes. J. Immunol. 185, 867–876 [DOI] [PubMed] [Google Scholar]
- 33. Hozumi K., Mailhos C., Negishi N., Hirano K., Yahata T., Ando K., Zuklys S., Holländer G. A., Shima D. T., Habu S. (2008) Delta-like 4 is indispensable in thymic environment specific for T cell development. J. Exp. Med. 205, 2507–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Van de Walle I., Waegemans E., De Medts J., De Smet G., De Smedt M., Snauwaert S., Vandekerckhove B., Kerre T., Leclercq G., Plum J., Gridley T., Wang T., Koch U., Radtke F., Taghon T. (2013) Specific Notch receptor-ligand interactions control human TCR-αβ/γδ development by inducing differential Notch signal strength. J. Exp. Med. 210, 683–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saito T., Chiba S., Ichikawa M., Kunisato A., Asai T., Shimizu K., Yamaguchi T., Yamamoto G., Seo S., Kumano K., Nakagami-Yamaguchi E., Hamada Y., Aizawa S., Hirai H. (2003) Notch2 is preferentially expressed in mature B cells and indispensable for marginal zone B lineage development. Immunity 18, 675–685 [DOI] [PubMed] [Google Scholar]
- 36. Hozumi K., Negishi N., Suzuki D., Abe N., Sotomaru Y., Tamaoki N., Mailhos C., Ish-Horowicz D., Habu S., Owen M. J. (2004) Delta-like 1 is necessary for the generation of marginal zone B cells but not T cells in vivo. Nat. Immunol. 5, 638–644 [DOI] [PubMed] [Google Scholar]
- 37. Murata A., Okuyama K., Sakano S., Kajiki M., Hirata T., Yagita H., Zúñiga-Pflücker J. C., Miyake K., Akashi-Takamura S., Moriwaki S., Niida S., Yoshino M., Hayashi S. (2010) A Notch ligand, Delta-like 1 functions as an adhesion molecule for mast cells. J. Immunol. 185, 3905–3912 [DOI] [PubMed] [Google Scholar]
- 38. Sörensen I., Adams R. H., Gossler A. (2009) DLL1-mediated Notch activation regulates endothelial identity in mouse fetal arteries. Blood 113, 5680–5688 [DOI] [PubMed] [Google Scholar]
- 39. Benedito R., Roca C., Sörensen I., Adams S., Gossler A., Fruttiger M., Adams R. H. (2009) The Notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell 137, 1124–1135 [DOI] [PubMed] [Google Scholar]
- 40. Shah D. K., Zúñiga-Pflücker J. C. (2012) Notch receptor-ligand interactions during T cell development, a ligand endocytosis-driven mechanism. Curr. Top. Microbiol. Immunol. 360, 19–46 [DOI] [PubMed] [Google Scholar]
- 41. Hofmann J. J., Iruela-Arispe M. L. (2007) Notch signaling in blood vessels. Who is talking to whom about what? Circ. Res. 100, 1556–1568 [DOI] [PubMed] [Google Scholar]
- 42. Aricescu A. R., Lu W., Jones E. Y. (2006) A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr. D Biol. Crystallogr. 62, 1243–1250 [DOI] [PubMed] [Google Scholar]
- 43. Gordon W. R., Vardar-Ulu D., L'Heureux S., Ashworth T., Malecki M. J., Sanchez-Irizarry C., McArthur D. G., Histen G., Mitchell J. L., Aster J. C., Blacklow S. C. (2009) Effects of S1 cleavage on the structure, surface export, and signaling activity of human Notch1 and Notch2. PLoS One 4, e6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Luo Y., Haltiwanger R. S. (2005) O-Fucosylation of Notch occurs in the endoplasmic reticulum. J. Biol. Chem. 280, 11289–11294 [DOI] [PubMed] [Google Scholar]
- 45. Aste-Amézaga M., Zhang N., Lineberger J. E., Arnold B. A., Toner T. J., Gu M., Huang L., Vitelli S., Vo K. T., Haytko P., Zhao J. Z., Baleydier F., L'Heureux S., Wang H., Gordon W. R., Thoryk E., Andrawes M. B., Tiyanont K., Stegmaier K., Roti G., Ross K. N., Franlin L. L., Wang F., Chastain M., Bett A. J., Audoly L. P., Aster J. C., Blacklow S. C., Huber H. E. (2010) Characterization of Notch1 antibodies that inhibit signaling of both normal and mutated Notch1 receptors. PLoS One 5, e9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ficarro S. B., Zhang Y., Lu Y., Moghimi A. R., Askenazi M., Hyatt E., Smith E. D., Boyer L., Schlaeger T. M., Luckey C. J., Marto J. A. (2009) Improved electrospray ionization efficiency compensates for diminished chromatographic resolution and enables proteomics analysis of tyrosine signaling in embryonic stem cells. Anal. Chem. 81, 3440–3447 [DOI] [PubMed] [Google Scholar]
- 47. Askenazi M., Parikh J. R., Marto J. A. (2009) mzAPI. A new strategy for efficiently sharing mass spectrometry data. Nat. Methods 6, 240–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fleming R. J., Gu Y., Hukriede N. A. (1997) Serrate-mediated activation of Notch is specifically blocked by the product of the gene fringe in the dorsal compartment of the Drosophila wing imaginal disc. Development 124, 2973–2981 [DOI] [PubMed] [Google Scholar]
- 49. Lieber T., Wesley C. S., Alcamo E., Hassel B., Krane J. F., Campos-Ortega J. A., Young M. W. (1992) Single amino acid substitutions in EGF-like elements of Notch and Delta modify Drosophila development and affect cell adhesion in vitro. Neuron 9, 847–859 [DOI] [PubMed] [Google Scholar]
- 50. de Celis J. F., Barrio R., del Arco A., García-Bellido A. (1993) Genetic and molecular characterization of a Notch mutation in its Delta- and Serrate-binding domain in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 90, 4037–4041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yamamoto S., Charng W. L., Rana N. A., Kakuda S., Jaiswal M., Bayat V., Xiong B., Zhang K., Sandoval H., David G., Wang H., Haltiwanger R. S., Bellen H. J. (2012) A mutation in EGF repeat-8 of Notch discriminates between Serrate/Jagged and Delta family ligands. Science 338, 1229–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. de Celis J. F., Garcia-Bellido A. (1994) Modifications of the Notch function by Abruptex mutations in Drosophila melanogaster. Genetics 136, 183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pei Z., Baker N. E. (2008) Competition between Delta and the Abruptex domain of Notch. BMC Dev. Biol. 8, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. de Celis J. F., Bray S. J. (2000) The Abruptex domain of Notch regulates negative interactions between Notch, its ligands and Fringe. Development 127, 1291–1302 [DOI] [PubMed] [Google Scholar]
- 55. Chillakuri C. R., Sheppard D., Lea S. M., Handford P. A. (2012) Notch receptor-ligand binding and activation. Insights from molecular studies. Semin. Cell Dev. Biol. 23, 421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ahimou F., Mok L. P., Bardot B., Wesley C. (2004) The adhesion force of Notch with Delta and the rate of Notch signaling. J. Cell Biol. 167, 1217–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Besseyrias V., Fiorini E., Strobl L. J., Zimber-Strobl U., Dumortier A., Koch U., Arcangeli M. L., Ezine S., Macdonald H. R., Radtke F. (2007) Hierarchy of Notch-Delta interactions promoting T cell lineage commitment and maturation. J. Exp. Med. 204, 331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Matsuura A., Ito M., Sakaidani Y., Kondo T., Murakami K., Furukawa K., Nadano D., Matsuda T., Okajima T. (2008) O-Linked N-acetylglucosamine is present on the extracellular domain of Notch receptors. J. Biol. Chem. 283, 35486–35495 [DOI] [PubMed] [Google Scholar]
- 59. Sakaidani Y., Nomura T., Matsuura A., Ito M., Suzuki E., Murakami K., Nadano D., Matsuda T., Furukawa K., Okajima T. (2011) O-Linked N-acetylglucosamine on extracellular protein domains mediates epithelial cell-matrix interactions. Nat. Commun. 2, 583. [DOI] [PubMed] [Google Scholar]
- 60. Rana N. A., Nita-Lazar A., Takeuchi H., Kakuda S., Luther K. B., Haltiwanger R. S. (2011) O-Glucose trisaccharide is present at high but variable stoichiometry at multiple sites on mouse Notch1. J. Biol. Chem. 286, 31623–31637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lei L., Xu A., Panin V. M., Irvine K. D. (2003) An O-fucose site in the ligand binding domain inhibits Notch activation. Development 130, 6411–6421 [DOI] [PubMed] [Google Scholar]
- 62. Xu A., Haines N., Dlugosz M., Rana N. A., Takeuchi H., Haltiwanger R. S., Irvine K. D. (2007) In vitro reconstitution of the modulation of Drosophila Notch-ligand binding by Fringe. J. Biol. Chem. 282, 35153–35162 [DOI] [PubMed] [Google Scholar]
- 63. Shao L., Moloney D. J., Haltiwanger R. (2003) Fringe modifies O-fucose on mouse Notch1 at epidermal growth factor-like repeats within the ligand-binding site and the Abruptex region. J. Biol. Chem. 278, 7775–7782 [DOI] [PubMed] [Google Scholar]
- 64. Krebs L. T., Shutter J. R., Tanigaki K., Honjo T., Stark K. L., Gridley T. (2004) Haploinsufficient lethality and formation of arteriovenous malformations in Notch pathway mutants. Genes Dev. 18, 2469–2473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gale N. W., Dominguez M. G., Noguera I., Pan L., Hughes V., Valenzuela D. M., Murphy A. J., Adams N. C., Lin H. C., Holash J., Thurston G., Yancopoulos G. D. (2004) Haploinsufficiency of delta-like 4 ligand results in embryonic lethality due to major defects in arterial and vascular development. Proc. Natl. Acad. Sci. U.S.A. 101, 15949–15954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Koch U., Fiorini E., Benedito R., Besseyrias V., Schuster-Gossler K., Pierres M., Manley N. R., Duarte A., Macdonald H. R., Radtke F. (2008) Delta-like 4 is the essential, nonredundant ligand for Notch1 during thymic T cell lineage commitment. J. Exp. Med. 205, 2515–2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Abe N., Hozumi K., Hirano K., Yagita H., Habu S. (2010) Notch ligands transduce different magnitudes of signaling critical for determination of T-cell fate. Eur. J. Immunol. 40, 2608–2617 [DOI] [PubMed] [Google Scholar]
- 68. Patel N. S., Dobbie M. S., Rochester M., Steers G., Poulsom R., Le Monnier K., Cranston D. W., Li J. L., Harris A. L. (2006) Up-regulation of endothelial Delta-like 4 expression correlates with vessel maturation in bladder cancer. Clin. Cancer Res. 12, 4836–4844 [DOI] [PubMed] [Google Scholar]