Background: Mechanical forces are critical for normal fetal lung development.
Results: Force applied to α6β1 integrin activates TACE and sheds HB-EGF and TGF-α.
Conclusion: Mechanical strain enhances binding of α6β1 integrin to TACE to promote fetal type II cell differentiation.
Significance: Learning how mechanical forces regulate fetal lung development is critical for the discovery of approaches to accelerate lung maturation.
Keywords: ADAM ADAMTS, Cell Differentiation, Cell Signaling, Cell Surface Receptor, Growth Factors, Integrins, Lung, Mechanotransduction
Abstract
Mechanical forces are critical for normal fetal lung development. However, the mechanisms regulating this process are not well-characterized. We hypothesized that strain-induced release of HB-EGF and TGF-α is mediated via integrin-ADAM17/TACE interactions. Employing an in vitro system to simulate mechanical forces in fetal lung development, we showed that mechanical strain of fetal epithelial cells actives TACE, releases HB-EGF and TGF-α, and promotes differentiation. In contrast, in samples incubated with the TACE inhibitor IC-3 or in cells isolated from TACE knock-out mice, mechanical strain did not release ligands or promote cell differentiation, which were both rescued after transfection of ADAM17. Cell adhesion assay and co-immunoprecipitation experiments in wild-type and TACE knock-out cells using several TACE constructs demonstrated not only that integrins α6 and β1 bind to TACE via the disintegrin domain but also that mechanical strain enhances these interactions. Furthermore, force applied to these integrin receptors by magnetic beads activated TACE and shed HB-EGF and TGF-α. The contribution of integrins α6 and β1 to differentiation of fetal epithelial cells by strain was demonstrated by blocking their binding site with specific antibodies and by culturing the cells on membranes coated with anti-integrin α6 and β1 antibodies. In conclusion, mechanical strain releases HB-EGF and TGF-α and promotes fetal type II cell differentiation via α6β1 integrin-ADAM17/TACE signaling pathway. These investigations provide novel mechanistic information on how mechanical forces promote fetal lung development and specifically differentiation of epithelial cells. This information could be also relevant to other tissues exposed to mechanical forces.
Introduction
Mechanical forces are essential for normal fetal lung development (1–7). Throughout gestation, the lung epithelium actively secretes fluid creating a constant distension pressure of around 2.5 mmHg in the potential airspaces (8). In addition, the fetus makes episodic breathing movements generating around 5% changes in the distal lung surface area (9). It is well established that both tonic hydrostatic distension and cyclic mechanical deformation provide physical signals necessary for normal fetal lung development (10–12). However, the precise molecular and cellular mechanisms by which lung cells sense mechanical stimuli to influence lung development are not fully understood. A key component of lung development is the differentiation of type II epithelial cells, the major source of pulmonary surfactant that prevents alveolar collapse during expiration. These cells also participate in fluid homeostasis in the alveolar lumen, host defense, and restoration of normal alveolar epithelium after lung injury (13).
The ErbB family of tyrosine kinase receptors consists of four receptors: epidermal growth factor receptor (EGFR2 or ErbB1), ErbB2, ErbB3, and ErbB4. These receptors are activated by specific ligands. For example, EGF and TGF-α are the ligands for EGFR, HB-EGF binds to EGFR and ErbB4 and neuregulin is the ligand for ErbB3 and ErbB4 (14). Each ligand is synthesized as a transmembrane precursor and is proteolytically cleaved to release the biologically active protein (15). The importance of ErbB receptors in lung development is well documented. EGFR is critical for branching morphogenesis, alveolarization, and differentiation of type II epithelial cells (16–19). ErbB2 and ErbB3 promote fetal lung epithelial cell proliferation (20) and ErbB4 regulates type II cell differentiation (21, 22). Previous studies from our laboratory have shown that mechanical strain, simulating mechanical forces in utero, activates these receptors in fetal type II epithelial cells (23, 24). We further demonstrated that phosphorylation of ErbB receptors and subsequent activation of ERK pathway and differentiation of fetal type II epithelial cells was mediated via shedding of HB-EGF and TGF-α (24, 25). However, the molecular mechanisms by which mechanical strain releases these ligands are unknown.
ADAM (a disintegrin and metalloprotease) proteins are membrane-anchored metalloproteases that process and shed the ectodomains of membrane-anchored growth factors, cytokines and receptors (26). The domain structure of the ADAMs consists of a prodomain, a metalloprotease domain, a disintegrin domain, a cysteine-rich domain, an EGF-like domain, a transmembrane domain and a cytoplasmic tail (27). ADAMs are key components in EGFR signaling (26). Because many transmembrane growth and differentiation factors, including members of the ErbB family of receptors, require ectodomain shedding for proper action in vivo, proteolysis is viewed as a regulatory mechanism in the developing embryos (28). ADAM17/TACE in particular has been shown to be crucial for lung development. Inhibition of TACE by the metalloprotease inhibitor TAPI, or a targeted mutation in Zn (2+)-binding domain of TACE, disrupts two essential epithelial functions in lung development: branching morphogenesis and differentiation (29, 30). Furthermore, neonatal TACE-deficient mice have visible respiratory distress, and their lungs fail to form normal saccular structures. The histopathology of these lungs show fewer peripheral epithelial sacs, deficient septation and thick-walled mesenchyme, resulting in reduced surface for gas exchange (31). Because mice deficient in ADAM17/TACE have pulmonary hypoplasia and display phenotypes similar to mice deficient in EGFR and TGF-α, ADAM17 is considered to be the major supplier of EGFR ligands in vivo (31, 32). In fact, in vitro studies have demonstrated that ADAM17 is the major convertase of epiregulin, TGF-α, amphiregulin, and HB-EGF (33).
Integrins are a family of ubiquitous cell surface receptors that mechanically couple the extracellular matrix to the cytoskeleton (34) and control a variety of cell functions by serving as scaffolds for the assembly of multiprotein signaling complexes within focal adhesion anchoring sites (35, 36). Because integrins preferentially mediate mechanical force transfer across the cell surface, they are ideally positioned to sense mechanical stimuli and, through their interconnections with focal adhesion proteins, transduce them into biochemical signals to modify cell behavior (37, 38). Numerous studies have confirmed that integrins play a central role in mechanotransduction in virtually all cell and tissue types (39–41). Previous experiments from our laboratory have shown that specific integrin subtypes contribute to mechanical strain-induced differentiation of fetal type II epithelial cells (42).
The goal of the present study was to investigate the mechanisms by which mechanical forces release HB-EGF and TGF-α from fetal epithelial cells. Given the key role of TACE in lung development, we hypothesized that ADAM17 is the protease that releases HB-EGF and TGF-α after applying physiologic strain to fetal type II epithelial cells. In addition, as ADAMs are unique among cell-surface proteins to have a disintegrin domain to support integrin-ADAMs interactions (43), we further hypothesized that activation of TACE is mediated via mechanical stimulation of integrin receptors.
EXPERIMENTAL PROCEDURES
TACE Knock-out Mice
The TACE gene was inactivated by deleting the zinc binding domain through homologous recombination as previously described (31). Homozygous TACEΔZn/ΔZn-null mutant (−/−) mice were produced by cross-breeding TACE heterozygous (+/−) mice in a C57BL/6 strain background. TACE genotypes were verified by genomic DNA PCR analysis as documented previously (29).
Cell Isolation and Flexcell Strain Experiments
Animal experiments were performed in compliance with the Lifespan Institutional Animal Care and Use Committee, Providence, RI. Fetal mouse lungs were obtained at embryonic days 17 or 18 from wild-type or TACE knock-out timed-pregnant mice after intra-peritoneal administration of pentobarbital sodium. The presence of a vaginal plug was considered as day 0.5 of pregnancy. Type II cells were isolated as previously described (42). Briefly, after collagenase digestion, cell suspensions were sequentially filtered through 100-, 30-, and 15-μm nylon meshes using screen cups (Sigma-Aldrich). Clumped non-filtered cells from the 30- and 15 μm nylon meshes were collected after several washes with DMEM, plated on Bioflex multiwall Plates (Flexcell International, Hillsborough, NC) precoated with laminin-1 (2 μg/cm2). Monolayers were maintained for an additional 24 h until reached ∼80% confluency and then mounted in a Flexcell FX-4000 Strain Unit. To simulate mechanical forces in fetal lung development, regimens of 5% cyclical strain at intervals of 40 cycles/min or 2.5% continuous distention were used. Cells grown on non-strained membranes were treated in an identical manner and served as controls. In experiments with immobilized antibodies, Bioflex plates were coated with anti-α2 integrin antibody (10 μg/ml) (BD Pharmingen, San Jose, CA, cat. 557017), anti-α6 integrin antibody (10 μg/ml) (AbD Serotec, Raleigh, NC, cat. MCA699EL) or anti-β1-integrin antibody (10 μg/ml) (Millipore, Billerica, MA, cat. MCA2298EL) for 2 h at room temperature, rinsed with PBS, and incubated with 1% BSA in PBS for 1 h at 37 °C. After rinsing the plates twice with PBS and once with DMEM, fresh isolated E17 type II cells were seeded on these antibody-coated substrates in the absence of serum and allowed them to adhere for 4 h before the application of mechanical strain. In some studies, fetal type II cells were incubated with anti-integrin antibodies, or negative control IgG, in suspension at 37 °C for 15 min before being plated on laminin-coated substrates in the continued presence of these blocking antibodies in the following concentrations: integrin α6 (10 μg/ml) (AbD Serotec, Raleigh, NC, cat. MCA699EL) and integrin β1 (10 μg/ml) (Millipore, cat. MCA2298EL).
TACE Activity Assay
TACE activity was determined using the SensoLyte 520 TACE Activity Assay Kit (AnaSpec, San Jose, CA) according to the manufacturer's protocol. This assay uses a 5-FAM (fluorophore)-labeled FRET peptide substrate. Upon cleavage of the FRET peptide by the active enzyme, the fluorescence of 5-FAM is recovered and can be continuously monitored at excitation/emission of 490 nm/520 nm. Fluorescence of the cleavage product was measured in a fluorescence microplate reader (Gemini EM, Molecular Devices, Sunnyvale, CA). 10 μg of cell lysate proteins were used for the assay. Samples were normalized to the protein concentration of the cell lysate. Results are expressed as the percentage of change in fluorescence intensity compared with unstrained, control samples.
Transient Transfection by Electroporation
Type II cells were transiently transfected using Amaxa Nucleofector apparatus (Lonza Inc. Allendale, NJ) as previously described (44). Isolated type II epithelial cells were plated on T75 flasks. The following day, cells were harvested by trypsinization and aliquots of 2 × 106 cells in RPMI with 10% FBS were centrifuged at 100 g for 10 min; supernatants were discarded, and cell pellets were resuspended in 100 μl of Basic Nucleofector Solution (Primary Mammalian Epithelial Cells Protocol, Lonza Inc. Allendale, NJ). Each sample was mixed individually with 2 μg of plasmid DNA. For ligand release, plasmids encoding alkaline phosphatase (AP)-tagged expression vectors for HB-EGF or TGF-α were added (25, 33). Samples were then transferred into the appropriate cuvettes and subjected to electrical pulses using the Amaxa NucleofactorTM II apparatus (Lonza Inc., Allendale, NJ). Samples containing no DNA were treated in an identical manner and served as negative controls (pulse only). After electroporation, samples were transferred into plates precoated with laminin-1 and left undisturbed for 24 h in a culture incubator. Monolayers were then exposed to mechanical strain as described elsewhere.
EGFR Ligand Shedding Assay
Monolayers transfected with AP-tagged EGFR ligands were exposed to different strain protocols. After experiments, cell medium (2 ml/well) from static and strained samples were collected and centrifuged at 16,000 × g for 30 min. Supernatants were saved at −80 °C until further use. Monolayers were lysed by adding 0.5 ml/well of buffer containing 1× PBS, 1% Triton X-100, and protease and phosphatase inhibitors. Lysates were centrifuged and saved at −80 °C. Analysis and quantification of EGFR ligand shedding was performed by running the concentrated supernatants on SDS-polyacrylamide gels and staining the gels for AP activity. Because the AP moiety is N-glycosylated, lectin ConA was used to capture and concentrate the shed proteins. 40 μl of ConA-Sepharose resin (GE Healthcare, Pittsburgh, PA, cat. 17-0440-03) was added (after equilibrated and resuspended 1:1 in PBS plus protease and phosphotase inhibitors) to every 2 ml of cell supernatant and incubated at 4 °C rotating overnight. ConA beads were spun down twice at 800 × g for 1 min to remove the supernatant. Glycoproteins were eluted from the beads by adding 20 μl of elution buffer (50 mm Tris-HCl, pH 8.0, 0.5 m α-d-methyl mannoside), mixed and then incubated at 37 °C for 2 h. Then, 10 μl of 5× SDS-sample loading buffer containing 25 mm of dithiothreitol (DTT) were added. Samples were not boiled as AP is irreversibly inactivated at temperatures higher than 70 °C. Beads were then spun down and supernatants were loaded on 10% SDS-polyacrylamide gel and a constant current of 100 V was applied at 4 °C. When the separation was completed, gels were removed and incubated twice for 30 min in 2.5% Triton X-100, followed by 10 min incubation in alkaline phosphatase buffer (100 mm Tris-HCl, pH 9.5, 100 mm NaCl, and 20 mm MgCl2). AP activity was visualized by incubating the gels at 37 °C in detection solution (by adding 37.5 mg of NBT and 18.5 mg BCIP to the detection buffer (100 mm Tris-HCl, pH 9.5, 100 mm NaCl)). The enzyme reaction was then stopped by removing the detecting buffer. Intensity of the bands was quantified using the Gel-Pro Analyzer 4.0 software (MediaCybernetics, Bethesda, MD). AP activity in the cell lysate was analyzed following the same experimental procedure as described for cell supernatants. Data were expressed as the intensity of each AP supernatant band divided by total AP (supernatant + cell lysate).
Force Application to Integrin Receptors using Magnetic Beads
To apply mechanical force to cells via integrins, we coated ferric oxide (2-μm diameter) beads (Aldrich, cat. 310069) (45–47) with anti-α6 integrin antibody (5 μg/0.6 mg beads) (AbD Serotec, Raleigh, NC, cat. MCA699EL) anti-β1 antibody (5 μg/0.6 mg beads) (AbD Serotec, cat. MCA2298EL), anti-α2 integrin antibody (5 μg/0.6 mg beads) (BD Pharmingen, San Jose, CA, cat. 557017) or AcLDL (5 μg/0.6 mg beads) (Biomedical Technologies Inc, Stoughton, MA, cat. BT-906) in pH 9.4 carbonate buffer as previously described (48). Immediately before an experiment, cells were incubated with coated beads (∼20 beads/cell) for 10 min and then washed multiple times with PBS to remove unbound beads prior to magnetic force application. To maintain proper pH throughout these experiments, studies were carried out in bicarbonate-free medium consisting of Hanks' Balanced Salts (Sigma-Aldrich), 10 mU/liter MEM non-essential amino acids (Sigma-Aldrich), 20 ml/liter MEM essential amino acids (Sigma-Aldrich), 2 mm l-glutamine (Sigma-Aldrich), 10 mm Hepes, pH 7.3, and 1% BSA. A Neodymium Iron Boron (NdFeB) magnet (2.5 cm × 1.25 cm × 0.6 cm, Edmund Optics, Barrington, NJ, cat. NT54-311) was placed on top of the culture Petri lid at 10 mms distance from the cells, and perpendicular to the cell surface to generate forces of ∼0.6 pN/μm2 (47). Forces were applied to cells for a maximum of 30 min to avoid potential bead internalization.
Cell Adhesion Assay
The effect of specific anti-integrin antibodies on cell adhesion were measured in microtiter plates precoated with TACE (20 μg/ml) (R&D Systems, Minneapolis, MN, cat. 2978-AD). Suspended cells were incubated with cycloheximide (20 μg/ml), washed, and incubated with IgG (AbD Serotec, cat. MCA1212EL), anti-α1 (10 μg/ml) (BD Biosciences, San Jose, CA, cat. 555000), anti-α2 (10 μg/ml) (BD Biosciences, San Jose, CA, cat. 5570170), anti-α3 (10 μg/ml) (R&D Systems, Minneapolis, MN, cat. AF2787), anti-α5 (10 μg/ml) (BD Biosciences, San Jose, CA, Cat. 553319), anti-α6 (10 μg/ml) (AbD Serotec, cat. MCA699EL), or anti-β1 antibodies (10 μg/ml) (Millipore, cat. MCA2298EL) for 15 min at 37 °C and then plated on the TACE substrates in the presence of the antibodies and cycloheximide. To investigate the binding domain of fetal type II cells to TACE, plates were coated with proteins containing the pro- and metalloprotease domains (1–477 amino acids) (ENZO Life Sciences cat. BML-SE268-0010) or pro-, metalloprotease and disintegrin domains (1–563 amino acids) (Sino Biological cat. 80350-R08H). After 4 h, nonadherent cells were washed from the substrate with PBS. Cells were fixed with 4% paraformaldehyde in PBS and stained with 0.1% crystal violet (Sigma-Aldrich) in ddH2O for 25 min at room temperature. After several washes with tap water, stained cells were solubilized overnight with 0.5% Triton X-100 (diluted in ddH2O), and the optical density was measured at 590 nm. Cell numbers were derived from a standard curve.
Immunoprecipitation
Wild-type or TACE knock-out fetal type II cells were transfected as described before with ADAM-17-Fc or ADAM-17-MP plasmids (a generous gift from Dr. Humphries, University of Manchester, UK) in combination with either integrin β1 (Addgene, Cambridge, pRK5 β1, plasmid 16042), integrin α6 (Addgene, pRK5 α6, plasmid 16036) or integrin α5 (Addgene, α5 integrin-GFP, plasmid 15238). The cells were left in a culture incubator for 48 h, and they were then exposed to 5% cyclic strain for 30 min; non-strained cells served as controls. After experiments, monolayers were washed with ice-cold PBS and lysed in RIPA buffer (50 mm Tris (pH 7.4), 150 mm NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS) with 1 mm Na3VO4, 1 mm NaF, and protease inhibitor mixture (Thermo Fisher Scientific, Waltham, MA). Protein concentrations were measured and 80 μg of total protein from each sample were incubated with TACE/ADAM17 Ab-1 (Thermo Fisher Scientific, cat. RB-1660-PABX) or anti-integrin β1 (LifeSpan Biosciences, Seattle, WA, cat. LS-C15992/34165), integrin α6 (Novus Biologicals, Littleton, CO, cat. NBP1–85747) or anti-GFP antibodies (Abcam, cat. ab6658) overnight at 4 °C. The following day, protein-G-Sepharose beads (GE Healthcare, Pittsburgh, PA, cat. 17-0618-01) were added and incubated for an additional 2 h at 4 °C with gentle rocking. The beads were collected by centrifugation at 3,000 rpm at 4 °C for 1 min, washed three times with PBS (containing 1 mm Na3VO4, 1 mm NaF, and protein inhibitor mixture), denatured in sample buffer for 10 min at 70 °C, and subjected to 4–12% gradient gel and transferred to PVDF membrane. The immunoprecipitated proteins were detected by Western blot with integrin β1 (LifeSpan Biosciences, cat. LS-C15992/34165), integrin α6 (Novus Biologicals, Littleton, CO, cat. NBP1-85747) or anti-GFP antibodies (Abcam, cat. ab6658) or anti-TACE Ab-1 (Thermo Fisher Scientific, cat. RB-1660-PABX). The membranes were then stripped in buffer (Alpha Diagnostic Intl. Inc. San Antonio, TX) for 15 min at room temperature, blocked, and reprobed with an antibody against TACE/ADAM17 AB-1 or with anti-integrin antibodies as described above.
Real-Time PCR
Total RNA was isolated as previously described (4) and purified further using the Turbo DNA-free kit (Ambion). Total RNA was reverse-transcribed into cDNA using the iScript TM cDNA Synthesis Kit (Bio-Rad) according to the manufacturer's instructions. Pre-designed TaqMan® SP-C (cat. Mm00488144_m1) and SP-B (cat. Mm0045681_m1) primers were purchased from Applied Biosystems. To amplify the cDNA by qRT-PCR, 50 ng of the resulting cDNA were added to a mixture of 10 μl of 2× TaqMan Universal PCR Master Mix (Applied Biosystems, Foster City, CA) and 1 μl of 20× Gene Expression Assay Mix containing forward and reverse primers and TaqMan labeled probe (Applied Biosystems). Samples were normalized to the 18 S rRNA. The reactions were performed in an ABI Prism 7000 Sequence Detection System (Applied Biosystems) with an initial denaturation for 2 min at 50 °C and 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C. All assays were performed in triplicate.
Statistical Analysis
Results are expressed as means ± S.E. from at least three experiments, using different litters for each experiment. Data were analyzed with ANOVA followed by post hoc tests, and Instat 3.0 (GraphPad Software, San Diego, CA) was used for statistical analysis; p < 0.05 was considered statistically significant.
RESULTS
Mechanical Strain Activates TACE
First, we investigated whether mechanical strain stimulates TACE. To simulate mechanical forces in fetal lung development, fetal type II cells were exposed to continuous and cyclic strain. Fig. 1A shows that type II cells isolated during the canalicular stage of lung development (E17) and exposed to 2.5% continuous strain display a 2-fold increase in TACE activation after 1 min (1 ± 0.06 versus 2.17 ± 0.45). Likewise, 5% cyclic strain stimulates TACE by 4-fold after 30 min (1 ± 0.04 versus 3.87 ± 0.66) (Fig. 1B). Specificity of the fluorescence peptide was demonstrated by the absence of TACE activation after 30 min of strain in cells isolated from TACE knock-out mice (Fig. 1C). In cells isolated during the saccular stage of lung development (E18), 2.5% continuous strain stimulates TACE by 3-fold after 5 min (1 ± 0.03 versus 3 ± 0.53) (Fig. 1D) whereas no consistent activation was observed in cells exposed to 5% intermittent strain (Fig. 1E). These data demonstrate that continuous and cyclic strain activates TACE in fetal type II epithelial cells.
FIGURE 1.
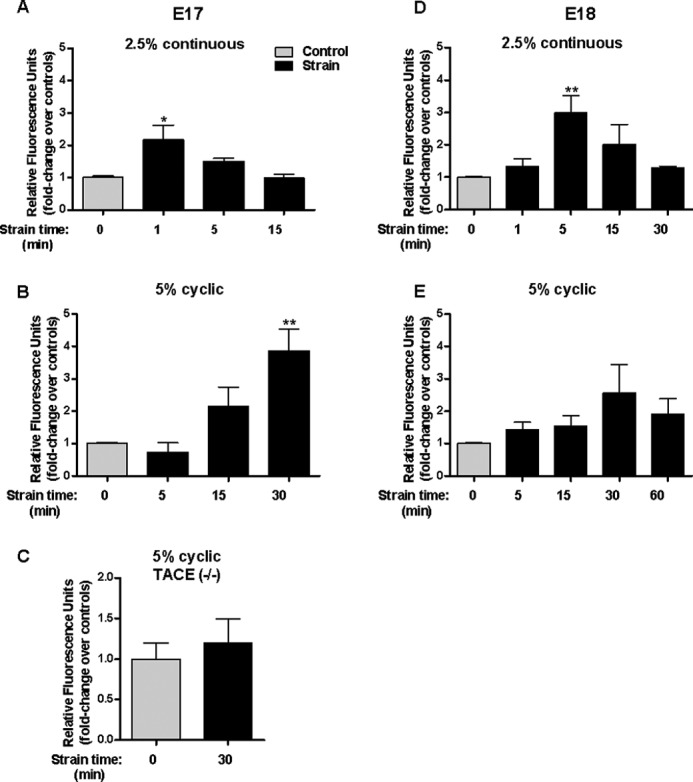
Mechanical strain activates TACE. E17 (A, B, C) and E18 type II cells (D, E) isolated from wild-type (A, B, D, E) and TACE knock-out mice (C) were exposed to 2.5% continuous strain or 5% cyclic strain for the indicated periods of time. Activation of TACE was assessed using an active fluorescence FRET substrate as described in “Experimental Procedures.” Results are expressed as fold-change over unstrained samples. n = 4, *, p < 0.05; ** p < 0.01 versus control.
Strain-induced Release of HB-EGF and TGF-α Is Mediated via TACE
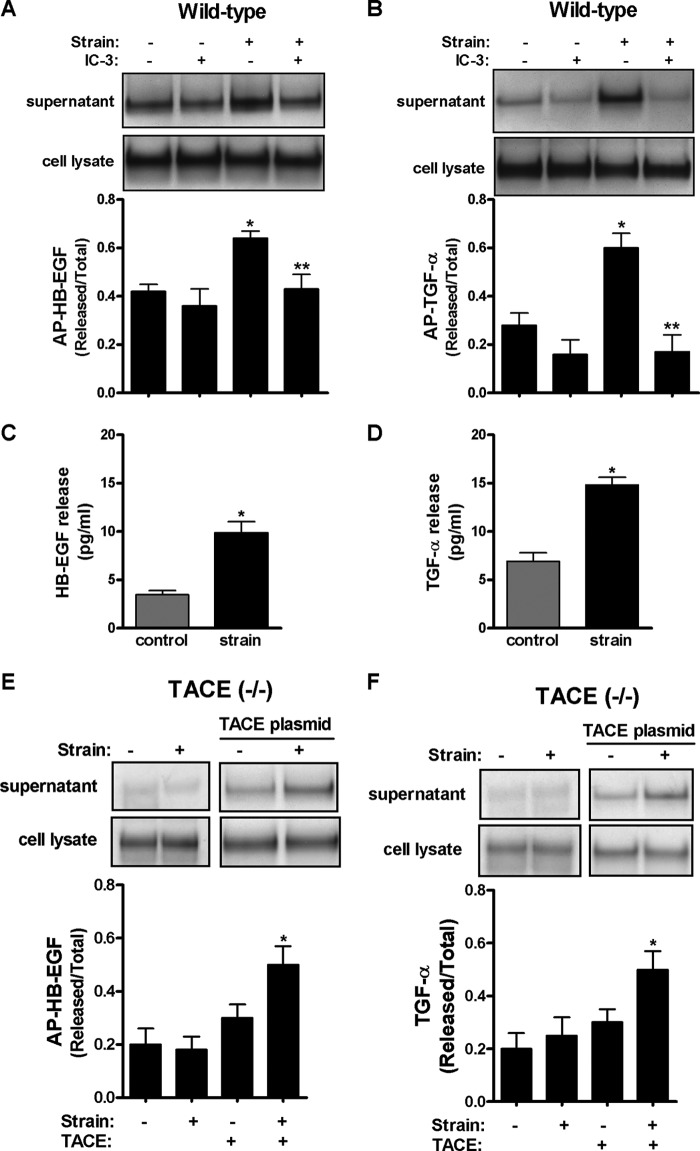
Given the importance of TACE in lung development (31) and as a major convertase of EGFR ligands (33), we evaluated next whether release of HB-EGF and TGF-α is mediated via TACE. E17 type II cells were transfected with plasmids encoding these ligands and then exposed to 5% cyclic strain for 30 min. Fig. 2, A and B shows that mechanical strain increases release of HB-EGF and TGF-α into the supernatant by 50% (0.42 ± 0.03 versus 0.64 ± 0.03) and 200% (0.28 ± 0.05 versus 0.6 ± 0.06), respectively. In contrast, in samples incubated with IC-3, a TACE inhibitor, release of HB-EGF (0.64 ± 0.03 versus 0.43 ± 0.05) or TGF-α (0.6 ± 0.06 versus 0.17 ± 0.07) were significantly decreased when compared with strained samples without inhibitor. Strain-mediated shedding of endogenous HB-EGF and TGF-α was also demonstrated by ELISA (Fig. 2, C and D). The key role of TACE in strain-induced release of ligands was confirmed in fetal type II cells isolated from TACE knock-out mice where mechanical strain did not release HB-EGF or TGF-α into the supernatant. However, shedding of these ligands was rescued after cells were transfected with plasmid encoding TACE (Fig. 2, E and F). These data clearly demonstrate that strain-induced release of HB-EGF and TGF-α is mediated via TACE.
FIGURE 2.
Strain-induced release of HB-EGF and TGF-α is mediated via TACE. A and B, E17 type II cells were transfected by electroporation with plasmids encoding alkaline phosphatase (AP)-tagged HB-EGF (A) or TGF-α (B) ligands in the presence or absence of IC-3 (10 μm), a TACE inhibitor, and then exposed to 5% cyclic strain for 30 min. Samples were processed to assess HB-EGF or TGF-α shedding as described in “Experimental Procedures.” n = 4, *, p < 0.05 versus control; **, p < 0.01 versus strain without inhibitor. C and D, E17 type II cells were exposed to 5% cyclic strain for 30 min. Supernatants were collected, concentrated and processed to detect shedding of mature HB-EGF (R & D, cat. DY259) and TGF-α (R & D, cat. DY239) by ELISA following manufacturer's recommendations. Samples were normalized to the concentration of proteins in the cell lysate. p < 0.05 versus control. Results are from three independent experiments. E and F, E17 type II cells isolated from TACE knock-out mice and transfected or not with a plasmid encoding the full-length of TACE were exposed to similar experimental conditions as described above to investigate shedding of mature HB-EGF (E) or TGF- α (F). n = 4, *, p < 0.05 versus control plus TACE transfection. Upper panels show representative blots.
Strain-induced Fetal Type II Cell Differentiation Is Mediated via TACE
Next, we studied whether TACE is the metalloprotease participating in type II cell differentiation using surfactant protein-C (SP-C) as a marker (Khoor et al., 54). Fetal type II cells were exposed to mechanical strain in the presence of the TACE inhibitor IC-3. Fig. 3, A and B demonstrate that mechanical strain increases SP-C mRNA and protein by 2-fold compared with controls. In contrast, in the presence of IC-3, mechanical strain did not increase SP-C. These results were confirmed in cells isolated from TACE knock-out mice where mechanical strain did not increase SP-C. However, strain-mediated increase of SP-C mRNA and protein were rescued in knock-out cells transfected with TACE (Fig. 3, C and D). Similar results were obtained using SP-B as a marker of differentiation (Fig. 3, E and F). Altogether these results indicate that TACE regulates strain-induced type II cell differentiation.
FIGURE 3.
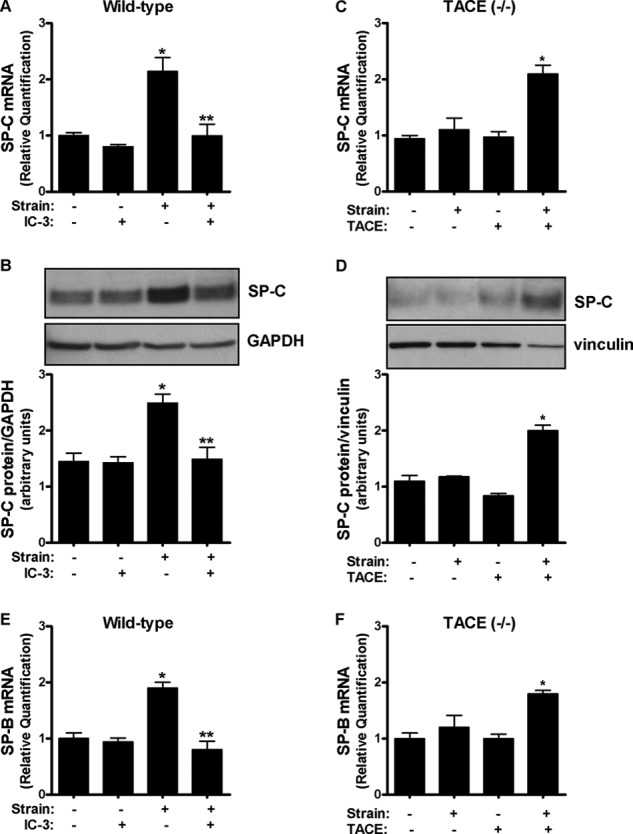
Strain-induced fetal type II cell differentiation is mediated via TACE. A and B, E17 type II cells were exposed to 5% cyclic strain for 24 h in the presence or not of IC-3 (10 μm), a TACE inhibitor. Samples were analyzed by qRT-PCR (A) or Western blot (B) to detect SP-C abundance. n = 4, *, p < 0.05 versus control; **, p < 0.01 versus strain without IC-3. C and D, type II cells isolated from TACE knock-out mice were transfected or not with a plasmid encoding the full-length of TACE and then exposed to similar experimental conditions as described in A and B. n = 4, *, p < 0.05 versus control plus TACE transfection. Upper panels in B and D show representative blots. E and F, E17 cells were exposed to similar experimental conditions described in A and C but SP-B, instead of SP-C, was used as a marker of differentiation.
TACE Is Associated with Integrins α6 and β1 via Disintegrin Domain and Mechanical Strain Increases These Associations
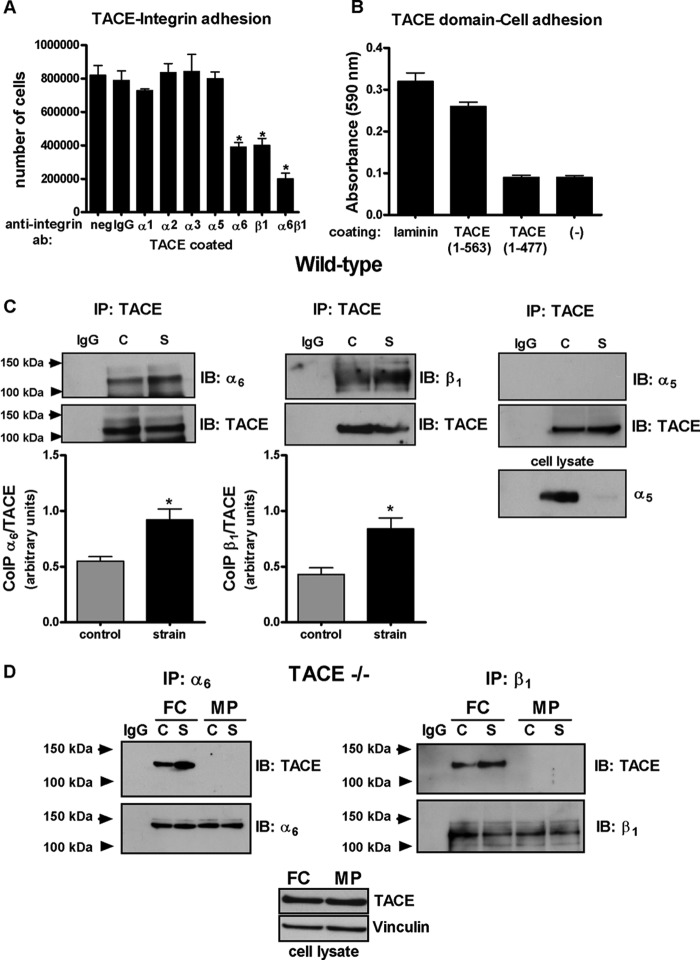
Because TACE contains a disintegrin domain (27), we hypothesized that activation of TACE by strain is mediated via integrins. First, we investigated the ability of TACE to bind to integrins using a quantitative cell adhesion assay. Suspended fetal type II cells were incubated with different anti-integrin antibodies and then plated on wells coated with full- length TACE. Our data show an ∼75% reduction in cell attachment to TACE substrates in cells incubated with anti-α6/β1 antibodies, suggesting that TACE binds to these integrins (Fig. 4A). Next, we evaluated which domain of TACE binds to fetal type II cells. Cells were cultured on substrates coated with recombinant TACE encoding the pro and metalloprotease domains (1–477) or a peptide also including the disintegrin domain (1–563). Our results demonstrate that lack of binding of type II cells to TACE in the absence of disintegrin domain (Fig. 4B). Association between TACE and α6 and β1 integrins under non-strain conditions was also demonstrated by co-immunoprecipitation experiments (Fig. 4C). Furthermore, mechanical strain increased binding of TACE to α6 by 70% (0.55 ± 0.04 versus 0.92 ± 0.1) and to β1 by 100% (0.43 ± 0.06 versus 0.84 ± 0.1) when compared with unstrained samples. Similar results were obtained with samples immunoprecipitated with α6 or β1 integrins and immunoblotted with TACE (data not shown). Specificity of these interactions in fetal type II epithelial cells was also demonstrated by the lack of co-precipitation between TACE and α5 integrin, which has been recently reported to interact in kidney mesangial cells (49) (Fig. 4C). To further investigate the binding site for α6 and β1 integrins on TACE, we performed experiments in fetal type II cells isolated from TACE knock-out mice and transfected with plasmid encoding different domains of ADAM17 (a generous gift from Dr. Martin J. Humphries, University of Manchester, UK) (Bax et al., 59). Fig. 4D shows that mechanical strain in cells transfected with plasmid encoding recombinant ADAM17-Fc (which lacks only the transmembrane and cytoplasmic domains) increases α6 β1 binding to TACE similar to wild-type cells. In contrast, in cells transfected with MP plasmid (lacking the disintegrin domain) there was no binding between α6β1 integrins and TACE. Altogether our data show not only that integrins α6 and β1 bind to TACE via disintegrin domain but also that mechanical strain enhances these interactions.
FIGURE 4.
TACE is associated with integrins α6 and β1 via disintegrin domain and mechanical strain increases these associations. A, cells were preincubated with antibodies against different integrin subtypes as described, and cultured on full-length TACE-coated substrates in the presence of antibody and cycloheximide for 4 h. Results are from 3 different experiments performed in triplicate. *, p < 0.05 versus negative. B, type II cells were plated on wells coated with laminin (positive control), TACE fragment containing the pre-, metalloprotease and disintegrin domains (1–563), TACE fragment with the pre- and metalloprotease domains (1–477), or no substrate (negative control). Adhesion of type II cells to different substrates were quantified by optical density as described in “Experimental Procedures.” C, wild-type E17 type II cells were transfected by electroporation with plasmids encoding ADAM-17-Fc (2 μg) in combination with either integrin α6 (1 μg), integrin β1 (1 μg), or integrin α5 (1 μg) plasmids. 48 h later, cells were exposed or not to 5% cyclic strain for 30 min. Collected proteins were immunoprecipitated with TACE antibody (or negative control IgG) and immunoblotted with anti-integrin α6, β1, or α5 antibodies. Blots were reprobed with anti-TACE antibody. Results are from five independent experiments. *, p < 0.05 versus controls. C, control; S, strain. Upper panels show representative blots. Lower right panel shows a Western blot from total cell lysate demonstrating that α5 integrin is expressed in type II cells and is recognized by this antibody. D, E17 type II cells isolated from TACE knock-out mice were transfected by electroporation with plasmids encoding ADAM-17-Fc (FC) or a truncated form lacking the disintegrin domain (MP) in combination with either integrin α6 or integrin β1 plasmids. 48 h later, cells were exposed or not to 5% cyclic strain for 30 min. Collected proteins were immunoprecipitated with anti-integrin α6 or β1 antibodies (or negative control IgG) and immunoblotted with TACE antibody. Blots were reprobed with anti α6 or β1 antibodies. Blots are representative from two independent experiments. Lower panel shows a Western blot from total cell lysate demonstrating that FC and MP constructs are expressed in TACE knock-out cells.
Force Application to α6 β1 Integrin Receptors Activates TACE and Releases HB-EGF and TGF-α
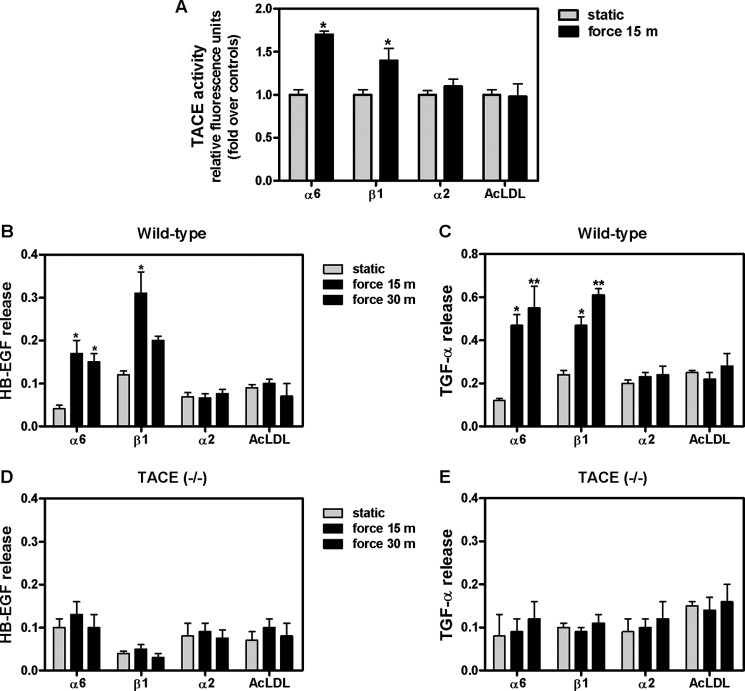
Integrins are well known to function as mechanoreceptors (37); therefore, we evaluated next whether mechanical deformation of these integrin subtypes activates TACE. Monolayers were incubated with magnetic beads coated with antibodies to α6, β1, or α2 integrin or with acetylated low-density lipoprotein (AcLDL, negative control) and then exposed to magnetic field for 15 min. Our data show that mechanical stimulation of cells via α6 and β1 integrins activates TACE by 70 and 40%, respectively, compared with unstrained samples (1 ± 0.06 versus 1.70 ± 0.04 and 1.40 ± 0.14). In contrast, force applied to α2 did not activate TACE (Fig. 5A). Next, we studied whether release of HB-EGF and TGF-α by strain is also mediated via these integrin subtypes. Force applied to integrin α6 for 15 min and 30 min shed HB-EGF by 4-fold when compared with magnetic beads without force (0.04 ± 0.008 versus 0.17 ± 0.03 and 0.15 ± 0.02). Likewise, stimulation of integrin β1 for 15 min released mature HB-EGF into the supernatant by 2.5-fold compared with no force conditions (0.12 ± 0.09 versus 0.31 ± 0.05). In contrast, application of mechanical stress to beads coated with α2 or AcLDL did not release HB-EGF, demonstrating the specificity of the integrin response to force (Fig. 5B). Similarly, force applied to integrin α6 for 15 min and 30 min shed TGF-α by 4-fold when compared with static (0.12 ± 0.09 versus 0.47 ± 0.05 and 0.55 ± 0.1). Mechanical stimulation of β1 for 15 min and 30 min released TGF-α by 2-fold compared with static (0.24 ± 0.02 versus 0.47 ± 0.04 and 0.61 ± 0.03) (Fig. 5C). In contrast, force applied to these integrin receptors in cells isolated from TACE knock-out mice did not release HB-EGF or TGF-α (Fig. 5, D and E). These data strongly suggest that strain-induced release of HB-EGF and TGF-α ligands is mediate via α6 β1-TACE pathway
FIGURE 5.
Application of electromagnetic force to α6 β1 integrin receptors activates TACE and releases HB-EGF and TGF-α. A, E17 type II cells were incubated with magnetic beads coated with integrin α6, β1, α2 antibodies or AcLDL (negative control) and then exposed to magnetic force for 15 min. Activation of TACE was assessed using an active fluorescence FRET substrate. Results are expressed as the percentage of change from unstrained samples. n = 4, *, p < 0.05 versus static. B–E, E17 type II cell monolayers from wild-type (B and C) or TACE knock-out mice (D and E) transfected with plasmids encoding alkaline phosphatase-tagged HB-EGF (B and D) or TGF-α (C and E) were incubated with magnetic beads coated with integrin α6, β1, α2 antibodies and then exposed to electromagnetic force for the indicated periods of time. AcLDL was used as a negative control. Supernatants were collected to measure AP-HB-EGF or TGF-α shedding. Samples were normalized to total AP protein content. Data are from four independent experiments. *, p < 0.05, **, p < 0.01 versus static.
Strain-induced Type II Cell Differentiation Is Mediated via Integrins α6β1
Finally, we investigated whether α6 β1 integrin subunits regulate strain-induced type II cell differentiation. Given that internalization of the beads may occur after being in culture for 1 h (47), we used alternative experimental systems to demonstrate that integrin-TACE pathway also participates in the differentiation of fetal type II epithelial cells. For these experiments, type II cells were incubated in suspension with anti-α6 or anti-β1 antibodies. Cells were then plated on laminin-coated substrates and exposed to cyclic mechanical strain for 24 h. Inhibition of binding of integrin α6 or β1 using specific blocking antibodies decreased SP-C mRNA when compared with strained samples incubated with a nonspecific IgG control (1.37 ± 0.25 and 0.97 ± 0.28 versus 2.43 ± 0.5) (Fig. 6A). To further explore the role of specific integrin subunits in this cellular mechanotransduction response, cells were cultured on membranes coated with different anti-integrin antibodies (instead of with laminin), to constrain the integrin receptor through which the cell initially experiences the mechanical strain. When cells grown on substrates coated with integrin α6 or β1 were exposed to cyclic strain, SP-C mRNA increased by 75% compared with unstrained controls (1 ± 0.06 versus 1.78 ± 0.19 and 1 ± 0.04 versus 1.74 ± 0.19). In contrast, no changes were observed on substrates coated with integrin α2 (Fig. 6B). These data corroborate the functional blocking experiments above and confirm that integrins α6 and β1 selectively mediate mechanotransduction promoting fetal type II cell differentiation.
FIGURE 6.
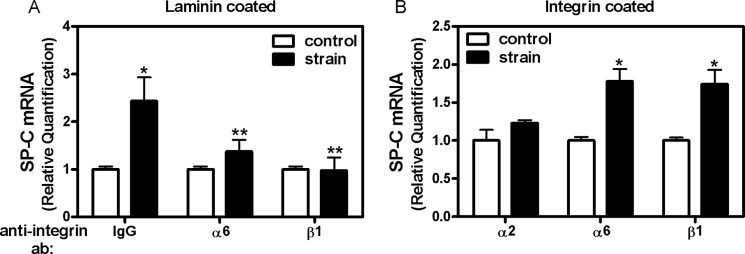
Strain-induced type II cell differentiation is mediated via integrins α6 and β1. A, E17 fetal type II cells were incubated in suspension with antibodies against α6 or β1 or the negative control IgG. Cells were then plated on substrates coated with laminin and exposed to 5% cyclic strain for 24 h. Samples were processed to detect SP-C mRNA expression by qRT-PCR. n = 4; *, p < 0.05 versus control, **, p < 0.05 versus IgG strain. B, E17 type II cells were plated on substrates coated with integrin α2, α6, or β1 antibodies and then exposed to 5% cyclic strain for 24 h. Samples were processed as described above. n = 4; *, p < 0.05 versus their respective controls.
DISCUSSION
Mechanical forces are critical for normal fetal lung development. Previous studies have shown that mechanical strain promotes differentiation of fetal lung epithelial cells via ectodomain shedding of HB-EGF and TGF-α. However, the mechanisms by which mechanical forces release these ligands are unknown. The main findings of this study are: 1) ADAM17/TACE is activated by strain and regulates release of HB-EGF/TGF-α and type II cell differentiation. 2) Integrin subunits α6 and β1 bind to TACE via disintegrin domain and mechanical strain enhances these bindings. 3) Mechanical stimulation of these integrin receptors not only activates TACE but also sheds HB-EGF and TGF-α and promotes differentiation of fetal epithelial cells. All together our data unveil a novel pathway by which mechanical forces promote lung development and specifically differentiation of fetal type II epithelial cells.
It is well established that mechanical forces generated by constant distension pressure and fetal breathing movements are critical for normal fetal lung development (11). One of the potential mechanisms by which mechanical forces promote lung development is by the release of growth factors (50, 51). This hypothesis is supported by previous studies from our laboratory demonstrating that mechanical strain releases soluble factors HB-EGF and TGF-α that are important for differentiation of type II epithelial cells (25). Given that the release of mature, biologically active form requires cleavage at the plasma membrane, we investigated the mechanisms of ectodomain shedding of these ligands by strain. Based on in vitro studies demonstrating that TACE is the major convertase of HB-EGF and TGF-α (33) and the critical role played by TACE in lung development (29–31), we hypothesized that TACE is the protease regulating shedding of these ligands by strain. Using a peptide substrate containing the TACE cleavage site, we found that both continuous and cyclic strain activate TACE. However, activation of TACE depends on type of mechanical stimulation applied, length of strain and gestational age of cells exposed to strain. Previous studies have also documented the activation of TACE in myoblast differentiation by mechanical forces (52) and in the transduction of compression stress in airway epithelial cells (53). Together, these previous studies and our current investigations suggest that TACE plays an important role in mechanotransduction. In addition, our data also show that TACE is important to release mature HB-EGF and TGF-α. These results were demonstrated using a TACE inhibitor and also in cells isolated from TACE knock-out mice. Importantly, TACE was found to be critical for strain-induced differentiation of fetal type II epithelial cells. Using surfactant protein-C as a specific marker of differentiation (54), we observed a significant decrease in the differentiation of type II cells by strain in the absence of TACE, which was rescued after TACE was introduced in knock-out cells. Altogether, our data clearly demonstrate the central role played by TACE in the cleavage of EGFR ligands that are critical for normal lung development and specifically for differentiation of fetal epithelial cells.
ADAMs are transmembrane cell surface proteins with adhesive and proteolytic functions based on their disintegrin and metalloprotease domains (55). This integrin-mediated interaction of the disintegrin-like domains with the cell surface suggests a key role for ADAM-integrin in the regulation of ectodomain shedding (56). Several integrins are known to interact with ADAM disintegrin-like domain (55, 56). EDC-motif D468 within disintegrin-like loop sequence of ADAM17 was shown to be crucial in facilitating integrin-dependent cell adhesion (57). ADAM 17 was also found to interact with α5β1 integrin in human dermal fibroblast cells (58) and to act as a specific ligand for the integrin α5β1 in HeLa cells (59) and mediate cell migration (57). The biological relevance of these connections is reflected for example in the cleavage by ADAM17 of VCAM-1, a key receptor for leukocyte adhesion and migration to inflammatory sites (60).
Our studies demonstrate that integrin α6β1 interacts with ADAM17 in fetal type II cells. These interactions were demonstrated by cell adhesion and co-immunoprecipitation experiments. ADAM-9 was found to mediate cellular adhesion and motility through α6β1integrin (61). Recent studies have also identified that α5β1 regulates release of HB-EGF via ADAM17 in kidney mesangial cells.(49) However, neither cell adhesion or co-immunoprecipitation experiments showed that α5 integrin binds to TACE in fetal lung cells. These results could be explained by tissue-specific role of integrin subtypes in cell signaling. Importantly, we provide the first evidence of integrin-TACE associations by mechanical forces. The application of force to specific surface receptors through bound ligand-coated microbeads provides a unique opportunity to selectively stimulate integrin receptors and assess downstream effects (46, 47). This technique has previously shown for example to up-regulate endothelin-1 gene expression (62) and to activate cyclic AMP signaling pathway (38, 48). To demonstrate whether activation of TACE by strain is mediated via integrin receptors, α6 and β1 subunits were exposed to tensile forces perpendicular to the surface of the cells using ferromagnetic beads (45). Our data demonstrate that mechanical stimulation of α6 and β1 integrins activated TACE. In addition, mechanical deformation of integrin receptors also promoted release of HB-EGF and TGF-α, providing additional support for the link between integrin receptors, TACE activation and release of ligands. However, these experiments cannot conclusively show whether strain-mediated activation of TACE via α6β1 is a direct mechanism or indirect via other intracellular signaling pathways. To address this question, we performed co-immunoprecipitation experiments in TACE-null cells transfected with plasmid encoding different recombinant TACE domains (Fig. 4C). Our data showed that strain-induced binding of α6β1 and TACE was rescued after transfection with recombinant ADAM-17 containing the pro, metalloprotease, and disintegrin domains. In contrast, a truncated form lacking the disintegrin domain was unable to bind to α6 or β1 integrins under static or strain conditions. These experiments demonstrate that integrin α6β1 binds to ADAM17 via disintegrin domain and mechanical strain increases these interactions.
Even though the adhesive domain of ADAMs seems to be crucial for proper generation of a protease-dependent event in vivo (63), investigations evaluating the functional relation between the two distinct functions are limited. Several models of integrin-ADAM interactions have emerged (43, 56). It is possible that integrin ligand properties of the ADAM disintegrins serve to mediate cell-cell interactions much like established integrin counter-receptors, and ADAM-integrin associations facilitate activation of ADAMs and cleavage of proteolytic substrate. Another model is that molecular interactions of the disintegrin domain of ADAMs with integrins serve to sequester the protease and prevent cleavage of the cell surface substrate. Upon conformational change, affinity of the integrin for the ADAMs is altered allowing dissociation of the ADAM from the integrin and subsequent processing of the substrate (56). Prove of this last model has been recently shown in kidney messangial cells, where activation of α5β1 dissociated integrin/ADAM17 binding and increased HB-EGF shedding (49). In contrast, our data support the first mechanistic model where activation of α6β1 by mechanical strain promotes the interaction with ADAM17, resulting in enhanced protease activity with subsequent release of HB-EGF and TGF-α and cell differentiation. Plausible explanations for these apparent contradictory mechanisms regulating shedding of EGFR ligands could be tissue-specificity and/or source of stimulus for integrin activation.
In summary, our data provide evidence of integrin α6β1-TACE interactions as a key regulatory pathway by which mechanical forces promote lung development via release of HB-EGF and TGF-α ligands. We propose a mechanistic model (Fig. 7) by which conformational changes of α6β1 integrin induced by strain will enhance binding of these mechanoreceptors to disintegrin domain of ADAM17 with subsequent increase of metalloprotease activity and cleavage of HB-EGF and TGF-α. Released ligands will bind to EGFR and induce phosphorylation of this receptor, activation of ERK pathway and increased type II cell differentiation, as previously demonstrated (24, 25). Although the scheme shown on Fig. 7 depicts a cis mechanism of transactivation (within the same cell), a trans mechanism of activation (between two cells) is also possible.
FIGURE 7.
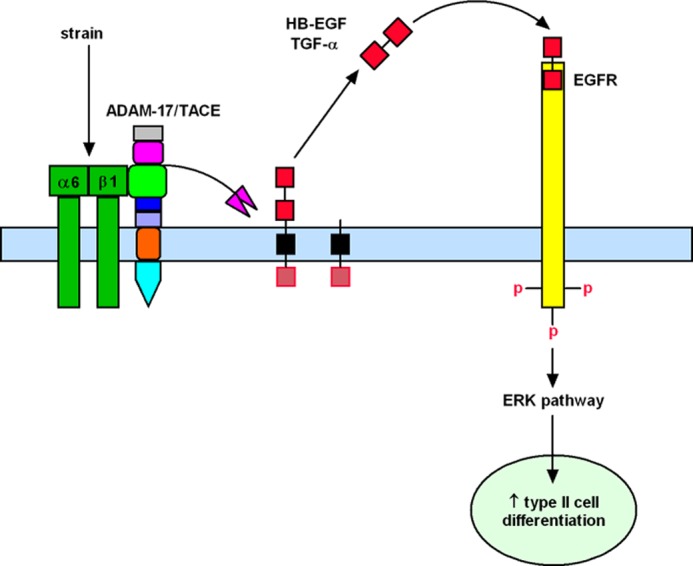
Mechanistic model of strain-induced fetal type II cell differentiation via integrin-TACE pathway. Conformational changes of α6β1 integrin by mechanical strain will enhance binding of these mechanoreceptors to disintegrin domain of ADAM17 (green) with subsequent increase of metalloprotease activity (pink) and cleavage of HB-EGF and TGF-α. Released ligands will bind to EGFR and induce phosphorylation of this receptor, activation of ERK pathway, and increased type II cell differentiation.
Based on the critical role played by mechanical forces during normal fetal lung development, the identification of key regulatory pathways activated by strain in fetal lung may provide a unique opportunity to facilitate development of new approaches to accelerate lung maturation in clinical conditions where lung development is impaired, including pulmonary hypoplasia, bronchopulmonary dysplasia and postnatal lung growth in extreme prematurity. In addition, our results may be also relevant to other areas of cell biology where tissues are exposed to mechanical forces such as blood vessels, heart, bone, kidney, etc.
Acknowledgments
We thank Dr. Martin J. Humphries, University of Manchester, UK for providing the ADAM-17 recombinant constructs and TACE antibody and Dr. Carl P. Blobel, Weill Medical College of Cornell University, New York for providing plasmid encoding alkaline phosphatase-tagged expression vectors for HB-EGF and TGF-α.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 HD052670 (to J. S-E.) and P20 RR018728.
- EGFR
- epidermal growth factor receptor
- ADAM
- a disintegrin and metalloprotease
- AP
- alkaline phosphatase
- SP
- surfactant protein
- TACE
- TNF-α ADAM metalloprotease-converting enzyme.
REFERENCES
- 1. Joe P., Wallen L. D., Chapin C. J., Lee C. H., Allen L., Han V. K., Dobbs L. G., Hawgood S., Kitterman J. A. (1997) Effects of mechanical factors on growth and maturation of the lung in fetal sheep. Am. J. Physiol. 272, L95–L105 [DOI] [PubMed] [Google Scholar]
- 2. Liu M., Post M. (2000) Invited review: mechanochemical signal transduction in the fetal lung. J. Appl. Physiol. 89, 2078–2084 [DOI] [PubMed] [Google Scholar]
- 3. Sanchez-Esteban J., Cicchiello L. A., Wang Y., Tsai S. W., Williams L. K., Torday J. S., Rubin L. P. (2001) Mechanical stretch promotes alveolar epithelial type II cell differentiation. J. Appl. Physiol. 91, 589–595 [DOI] [PubMed] [Google Scholar]
- 4. Sanchez-Esteban J., Tsai S. W., Sang J., Qin J., Torday J. S., Rubin L. P. (1998) Effects of mechanical forces on lung-specific gene expression. Am. J. Med. Sci. 316, 200–204 [PubMed] [Google Scholar]
- 5. Sanchez-Esteban J., Wang Y., Cicchiello L. A., Rubin L. P. (2002) Cyclic mechanical stretch inhibits cell proliferation and induces apoptosis in fetal rat lung fibroblasts. Am. J. Physiol. Lung Cell Mol. Physiol. 282, L448–L456 [DOI] [PubMed] [Google Scholar]
- 6. Torday J. S., Sanchez-Esteban J., Rubin L. P. (1998) Paracrine mediators of mechanotransduction in lung development. Am J. Med. Sci. 316, 205–208 [DOI] [PubMed] [Google Scholar]
- 7. Wirtz H. R., Dobbs L. G. (2000) The effects of mechanical forces on lung functions. Respir Physiol. 119, 1–17 [DOI] [PubMed] [Google Scholar]
- 8. Scarpelli E. M., Condorelli S., Cosmi E. V. (1975) Lamb fetal pulmonary fluid. I. Validation and significance of method for determination of volume and volume change. Pediatr Res. 9, 190–195 [DOI] [PubMed] [Google Scholar]
- 9. Harding R. (1997) in The Lung: Scientific Fountations, 2nd Ed (Crystal R. G., West J. B., Banes P. J., Weiber E. R., eds), pp. 2093–2104, Lippincott-Raven, Philadelphia [Google Scholar]
- 10. Goldstein J. D., Reid L. M. (1980) Pulmonary hypoplasia resulting from phrenic nerve agenesis and diaphragmatic amyoplasia. J. Pediatr. 97, 282–287 [DOI] [PubMed] [Google Scholar]
- 11. Moessinger A. C., Harding R., Adamson T. M., Singh M., Kiu G. T. (1990) Role of lung fluid volume in growth and maturation of the fetal sheep lung. J. Clin. Invest. 86, 1270–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wigglesworth J. S., Desai R. (1979) Effect on lung growth of cervical cord section in the rabbit fetus. Early Hum. Dev. 3, 51–65 [DOI] [PubMed] [Google Scholar]
- 13. Rama K. M., Acarregui M. J., Snyder J. M. (1997) in Lung Growth and Development (McDonald J. A., ed), pp. 119–162, Marcel Dekker, Inc., New York [Google Scholar]
- 14. Singh A. B., Harris R. C. (2005) Autocrine, paracrine and juxtacrine signaling by EGFR ligands. Cell Signal. 17, 1183–1193 [DOI] [PubMed] [Google Scholar]
- 15. Massagué J., Pandiella A. (1993) Membrane-anchored growth factors. Annu. Rev. Biochem. 62, 515–541 [DOI] [PubMed] [Google Scholar]
- 16. Miettinen P. J., Berger J. E., Meneses J., Phung Y., Pedersen R. A., Werb Z., Derynck R. (1995) Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature 376, 337–341 [DOI] [PubMed] [Google Scholar]
- 17. Miettinen P. J., Warburton D., Bu D., Zhao J. S., Berger J. E., Minoo P., Koivisto T., Allen L., Dobbs L., Werb Z., Derynck R. (1997) Impaired lung branching morphogenesis in the absence of functional EGF receptor. Dev. Biol. 186, 224–236 [DOI] [PubMed] [Google Scholar]
- 18. Sibilia M., Kroismayr R., Lichtenberger B. M., Natarajan A., Hecking M., Holcmann M. (2007) The epidermal growth factor receptor: from development to tumorigenesis. Differentiation 75, 770–787 [DOI] [PubMed] [Google Scholar]
- 19. Sibilia M., Wagner E. F. (1995) Strain-dependent epithelial defects in mice lacking the EGF receptor. Science 269, 234–238 [DOI] [PubMed] [Google Scholar]
- 20. Patel N. V., Acarregui M. J., Snyder J. M., Klein J. M., Sliwkowski M. X., Kern J. A. (2000) Neuregulin-1 and human epidermal growth factor receptors 2 and 3 play a role in human lung development in vitro. Am. J. Respir. Cell Mol. Biol. 22, 432–440 [DOI] [PubMed] [Google Scholar]
- 21. Liu W., Purevdorj E., Zscheppang K., von Mayersbach D., Behrens J., Brinkhaus M. J., Nielsen H. C., Schmiedl A., Dammann C. E. (2010) ErbB4 regulates the timely progression of late fetal lung development. Biochim. Biophys. Acta 1803, 832–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zscheppang K., Liu W., Volpe M. V., Nielsen H. C., Dammann C. E. (2007) ErbB4 regulates fetal surfactant phospholipid synthesis in primary fetal rat type II cells. Am. J. Physiol. Lung Cell Mol. Physiol. 293, L429–L435 [DOI] [PubMed] [Google Scholar]
- 23. Sanchez-Esteban J., Wang Y., Gruppuso P. A., Rubin L. P. (2004) Mechanical stretch induces fetal type II cell differentiation via an epidermal growth factor receptor-extracellular-regulated protein kinase signaling pathway. Am. J. Respir. Cell Mol. Biol. 30, 76–83 [DOI] [PubMed] [Google Scholar]
- 24. Huang Z., Wang Y., Nayak P. S., Dammann C. E., Sanchez-Esteban J. (2012) Stretch-induced fetal type II cell differentiation is mediated via ErbB1-ErbB4 interactions. J. Biol. Chem. 287, 18091–18102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang Y., Maciejewski B. S., Soto-Reyes D., Lee H. S., Warburton D., Sanchez-Esteban J. (2009) Mechanical stretch promotes fetal type II epithelial cell differentiation via shedding of HB-EGF and TGF-α. J. Physiol. 587, 1739–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blobel C. P. (2005) ADAMs: key components in EGFR signalling and development. Nat. Rev. Mol. Cell Biol. 6, 32–43 [DOI] [PubMed] [Google Scholar]
- 27. Seals D. F., Courtneidge S. A. (2003) The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 17, 7–30 [DOI] [PubMed] [Google Scholar]
- 28. Kheradmand F., Werb Z. (2002) Shedding light on sheddases: role in growth and development. Bioessays 24, 8–12 [DOI] [PubMed] [Google Scholar]
- 29. Zhao J., Chen H., Peschon J. J., Shi W., Zhang Y., Frank S. J., Warburton D. (2001) Pulmonary hypoplasia in mice lacking tumor necrosis factor-α converting enzyme indicates an indispensable role for cell surface protein shedding during embryonic lung branching morphogenesis. Dev. Biol. 232, 204–218 [DOI] [PubMed] [Google Scholar]
- 30. Zhao J., Chen H., Wang Y. L., Warburton D. (2001) Abrogation of tumor necrosis factor-alpha converting enzyme inhibits embryonic lung morphogenesis in culture. Int. J. Dev. Biol. 45, 623–631 [PubMed] [Google Scholar]
- 31. Peschon J. J., Slack J. L., Reddy P., Stocking K. L., Sunnarborg S. W., Lee D. C., Russell W. E., Castner B. J., Johnson R. S., Fitzner J. N., Boyce R. W., Nelson N., Kozlosky C. J., Wolfson M. F., Rauch C. T., Cerretti D. P., Paxton R. J., March C. J., Black R. A. (1998) An essential role for ectodomain shedding in mammalian development. Science 282, 1281–1284 [DOI] [PubMed] [Google Scholar]
- 32. Lee D. C., Sunnarborg S. W., Hinkle C. L., Myers T. J., Stevenson M. Y., Russell W. E., Castner B. J., Gerhart M. J., Paxton R. J., Black R. A., Chang A., Jackson L. F. (2003) TACE/ADAM17 processing of EGFR ligands indicates a role as a physiological convertase. Ann. N.Y. Acad. Sci. 995, 22–38 [DOI] [PubMed] [Google Scholar]
- 33. Sahin U., Weskamp G., Kelly K., Zhou H. M., Higashiyama S., Peschon J., Hartmann D., Saftig P., Blobel C. P. (2004) Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. J. Cell Biol. 164, 769–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wang N., Butler J. P., Ingber D. E. (1993) Mechanotransduction across the cell surface and through the cytoskeleton. Science 260, 1124–1127 [DOI] [PubMed] [Google Scholar]
- 35. Bershadsky A. D., Balaban N. Q., Geiger B. (2003) Adhesion-dependent cell mechanosensitivity. Annu. Rev. Cell Dev. Biol. 19, 677–695 [DOI] [PubMed] [Google Scholar]
- 36. Clark E. A., Brugge J. S. (1995) Integrins and signal transduction pathways: the road taken. Science 268, 233–239 [DOI] [PubMed] [Google Scholar]
- 37. Ingber D. (1991) Integrins as mechanochemical transducers. Curr. Opin. Cell Biol. 3, 841–848 [DOI] [PubMed] [Google Scholar]
- 38. Meyer C. J., Alenghat F. J., Rim P., Fong J. H., Fabry B., Ingber D. E. (2000) Mechanical control of cyclic AMP signalling and gene transcription through integrins. Nat. Cell Biol. 2, 666–668 [DOI] [PubMed] [Google Scholar]
- 39. Alenghat F. J., Ingber D. E. (2002) Mechanotransduction: all signals point to cytoskeleton, matrix, and integrins. Sci. STKE 2002, pe6. [DOI] [PubMed] [Google Scholar]
- 40. Katsumi A., Orr A. W., Tzima E., Schwartz M. A. (2004) Integrins in mechanotransduction. J. Biol. Chem. 279, 12001–12004 [DOI] [PubMed] [Google Scholar]
- 41. Shyy J. Y., Chien S. (2002) Role of integrins in endothelial mechanosensing of shear stress. Circ. Res. 91, 769–775 [DOI] [PubMed] [Google Scholar]
- 42. Sanchez-Esteban J., Wang Y., Filardo E. J., Rubin L. P., Ingber D. E. (2006) Integrins β1, α6, and α3 contribute to mechanical strain-induced differentiation of fetal lung type II epithelial cells via distinct mechanisms. Am. J. Physiol. Lung Cell Mol. Physiol. 290, L343–L350 [DOI] [PubMed] [Google Scholar]
- 43. White J. M. (2003) ADAMs: modulators of cell-cell and cell-matrix interactions. Curr. Opin. Cell Biol. 15, 598–606 [DOI] [PubMed] [Google Scholar]
- 44. Wang Y., Maciejewski B. S., Lee N., Silbert O., McKnight N. L., Frangos J. A., Sanchez-Esteban J. (2006) Strain-induced fetal type II epithelial cell differentiation is mediated via cAMP-PKA-dependent signaling pathway. Am. J. Physiol. Lung Cell Mol. Physiol. 291, L820–L827 [DOI] [PubMed] [Google Scholar]
- 45. Glogauer M., Ferrier J. (1998) A new method for application of force to cells via ferric oxide beads. Pflugers Arch 435, 320–327 [DOI] [PubMed] [Google Scholar]
- 46. Glogauer M., Ferrier J., McCulloch C. A. (1995) Magnetic fields applied to collagen-coated ferric oxide beads induce stretch-activated Ca2+ flux in fibroblasts. Am. J. Physiol. 269, C1093–C1104 [DOI] [PubMed] [Google Scholar]
- 47. Chan M. W., Chaudary F., Lee W., Copeland J. W., McCulloch C. A. (2010) Force-induced myofibroblast differentiation through collagen receptors is dependent on mammalian diaphanous (mDia). J. Biol. Chem. 285, 9273–9281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matthews B. D., Overby D. R., Alenghat F. J., Karavitis J., Numaguchi Y., Allen P. G., Ingber D. E. (2004) Mechanical properties of individual focal adhesions probed with a magnetic microneedle. Biochem. Biophys. Res. Commun. 313, 758–764 [DOI] [PubMed] [Google Scholar]
- 49. Gooz P., Dang Y., Higashiyama S., Twal W. O., Haycraft C. J., Gooz M. (2012) A disintegrin and metalloenzyme (ADAM) 17 activation is regulated by α5β1 integrin in kidney mesangial cells. PLoS One 7, e33350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Papadakis K., Luks F. I., De Paepe M. E., Piasecki G. J., Wesselhoeft C. W., Jr. (1997) Fetal lung growth after tracheal ligation is not solely a pressure phenomenon. J. Pediatr Surg. 32, 347–351 [DOI] [PubMed] [Google Scholar]
- 51. Luks F. I., Roggin K. K., Wild Y. K., Piasecki G. J., Rubin L. P., Lesieur-Brooks A. M., De Paepe M. E. (2001) Effect of lung fluid composition on type II cellular activity after tracheal occlusion in the fetal lamb. J. Pediatr. Surg. 36, 196–201 [DOI] [PubMed] [Google Scholar]
- 52. Zhan M., Jin B., Chen S. E., Reecy J. M., Li Y. P. (2007) TACE release of TNF-α mediates mechanotransduction-induced activation of p38 MAPK and myogenesis. J. Cell Sci. 120, 692–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shiomi T., Tschumperlin D. J., Park J. A., Sunnarborg S. W., Horiuchi K., Blobel C. P., Drazen J. M. (2011) TNF-α-converting enzyme/a disintegrin and metalloprotease-17 mediates mechanotransduction in murine tracheal epithelial cells. Am. J. Respir. Cell Mol. Biol. 45, 376–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Khoor A., Stahlman M. T., Gray M. E., Whitsett J. A. (1994) Temporal-spatial distribution of SP-B and SP-C proteins and mRNAs in developing respiratory epithelium of human lung. J. Histochem. Cytochem 42, 1187–1199 [DOI] [PubMed] [Google Scholar]
- 55. Lu X., Lu D., Scully M. F., Kakkar V. V. (2007) Structure-activity relationship studies on ADAM protein-integrin interactions. Cardiovasc Hematol Agents Med Chem 5, 29–42 [DOI] [PubMed] [Google Scholar]
- 56. Bridges L. C., Bowditch R. D. (2005) ADAM-Integrin Interactions: potential integrin regulated ectodomain shedding activity. Curr. Pharm. Des. 11, 837–847 [DOI] [PubMed] [Google Scholar]
- 57. Huang J., Bridges L. C., White J. M. (2005) Selective modulation of integrin-mediated cell migration by distinct ADAM family members. Mol. Biol. Cell 16, 4982–4991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dideberg V., Théâtre E., Farnir F., Vermeire S., Rutgeerts P., De Vos M., Belaiche J., Franchimont D., Van Gossum A., Louis E., Bours V. (2006) The TNF/ADAM 17 system: implication of an ADAM 17 haplotype in the clinical response to infliximab in Crohn's disease. Pharmacogenet Genomics 16, 727–734 [DOI] [PubMed] [Google Scholar]
- 59. Bax D. V., Messent A. J., Tart J., van Hoang M., Kott J., Maciewicz R. A., Humphries M. J. (2004) Integrin α5β1 and ADAM-17 interact in vitro and co-localize in migrating HeLa cells. J. Biol. Chem. 279, 22377–22386 [DOI] [PubMed] [Google Scholar]
- 60. Heidemann J., Maaser C., Lügering A., Spahn T. W., Zimmer K. P., Herbst H., Rafiee P., Domschke W., Krieglstein C. F., Binion D. G., Kucharzik T. F. (2006) Expression of vascular cell adhesion molecule-1 (CD 106) in normal and neoplastic human esophageal squamous epithelium. Int. J. Oncol. 28, 77–85 [PubMed] [Google Scholar]
- 61. Nath D., Slocombe P. M., Webster A., Stephens P. E., Docherty A. J., Murphy G. (2000) Meltrin gamma(ADAM-9) mediates cellular adhesion through α(6)β(1 )integrin, leading to a marked induction of fibroblast cell motility. J. Cell Sci. 113, 2319–2328 [DOI] [PubMed] [Google Scholar]
- 62. Chen J., Fabry B., Schiffrin E. L., Wang N. (2001) Twisting integrin receptors increases endothelin-1 gene expression in endothelial cells. Am. J. Physiol. Cell Physiol 280, C1475–C1484 [DOI] [PubMed] [Google Scholar]
- 63. Smith K. M., Gaultier A., Cousin H., Alfandari D., White J. M., DeSimone D. W. (2002) The cysteine-rich domain regulates ADAM protease function in vivo. J. Cell Biol. 159, 893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]