Abstract
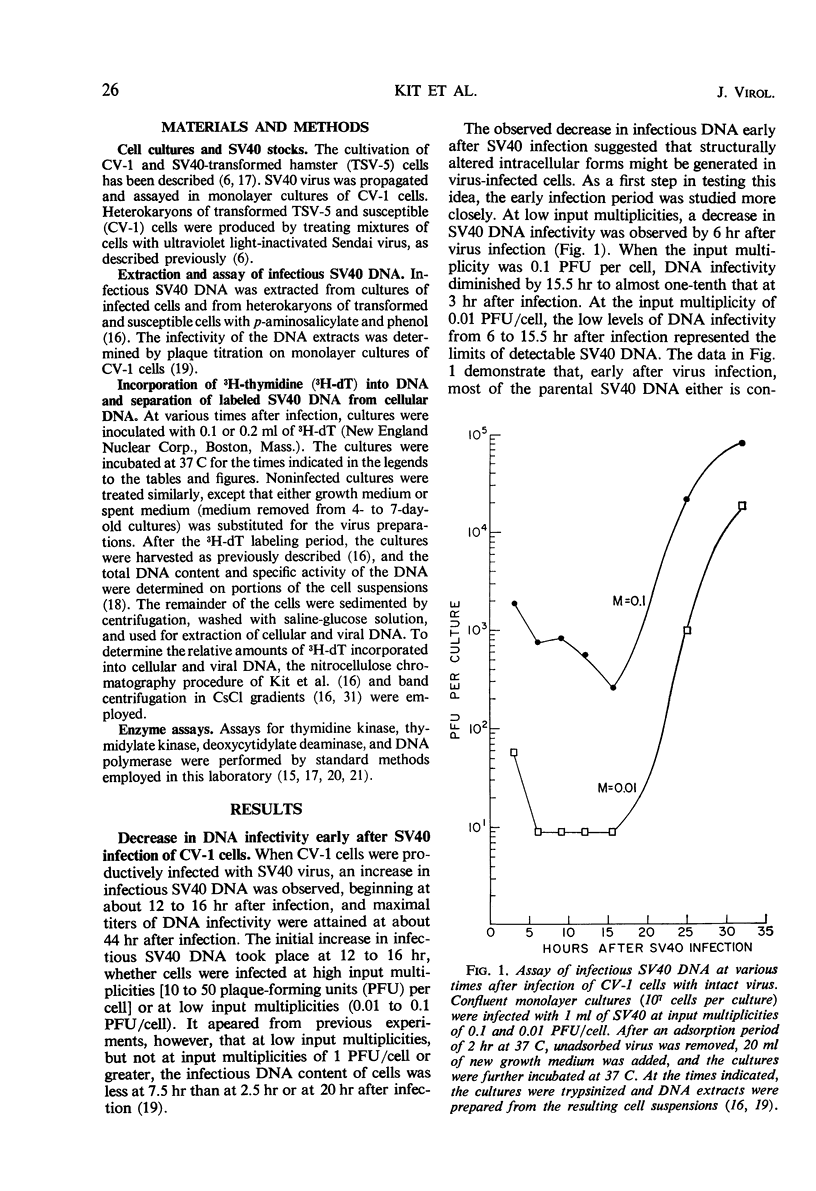
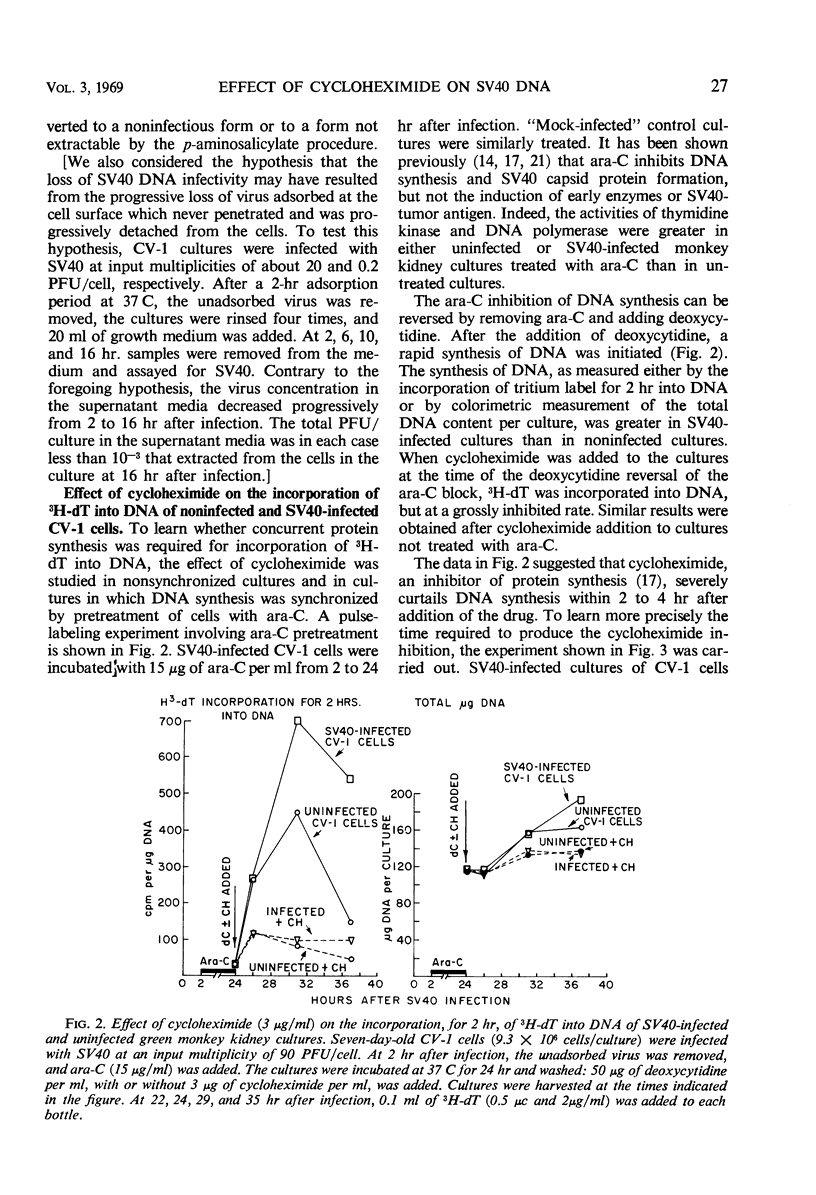
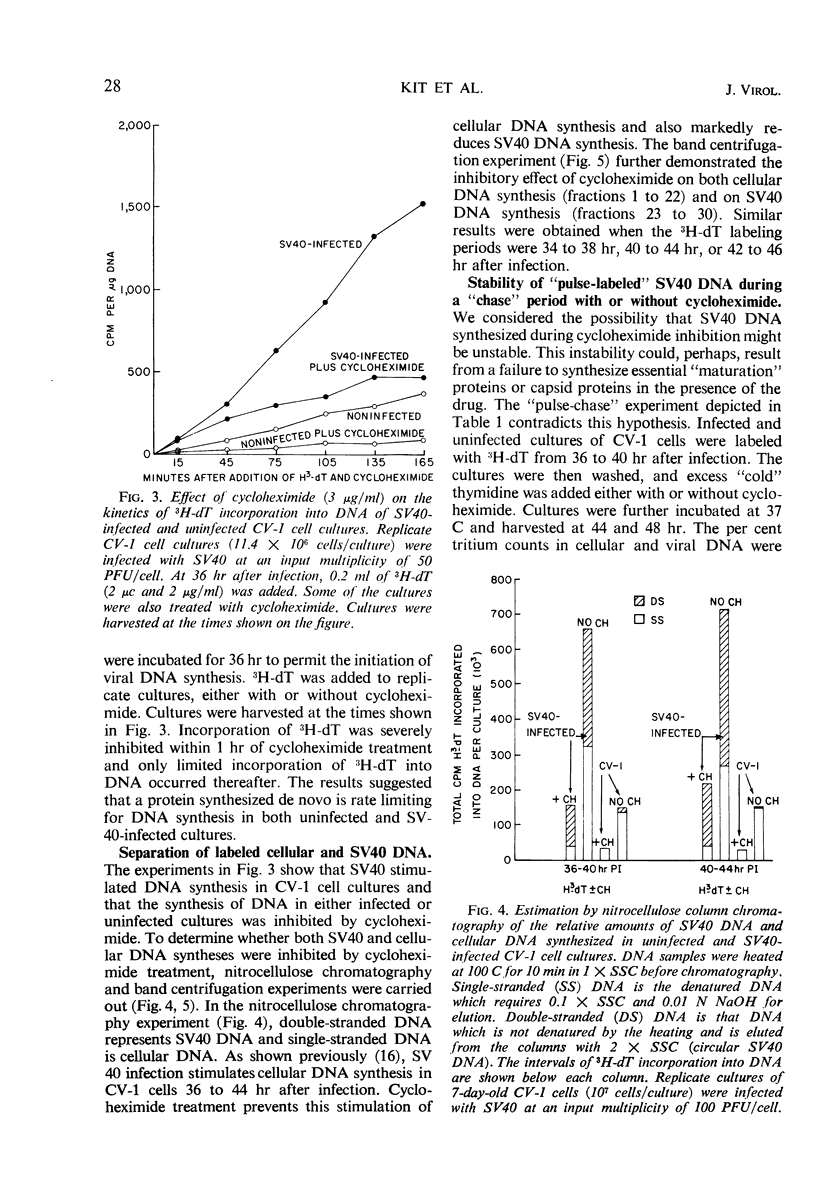
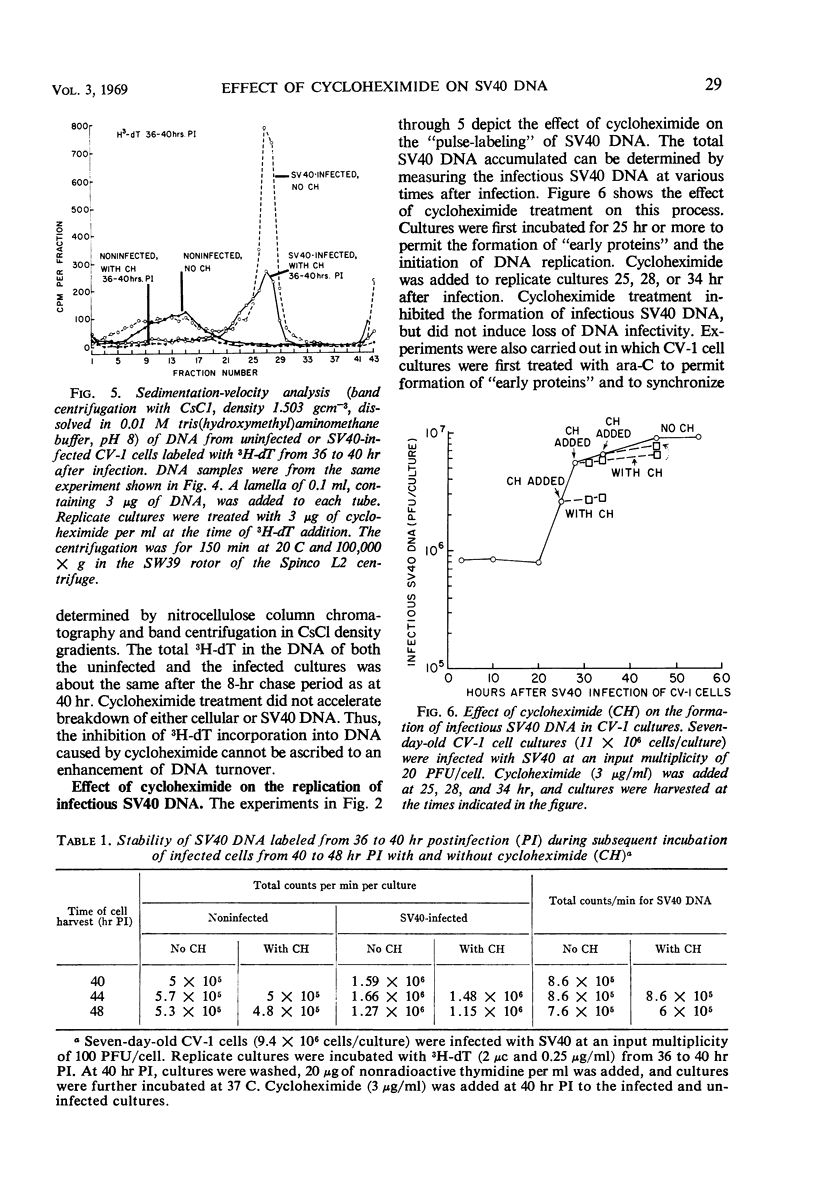
Infectious deoxyribonucleic acid (DNA) was extracted from green monkey kidney (CV-1) cultures at various times after the cultures were infected with simian virus 40 (SV40) at input multiplicities of 0.01 and 0.1 plaque-forming unit (PFU) per cell. A pronounced decrease in infectious DNA was observed from 3 to 16 hr after virus infection, suggesting that structurally altered intracellular forms may have been generated early in infection. Evidence is also presented that SV40 DNA synthesis requires concurrent protein synthesis. DNA replication was studied in the presence and absence of cycloheximide in: (i) SV40-infected and uninfected cultures of CV-1 cells; (ii) cultures synchronized with 1-β-d-arabinofuranosylcytosine (ara-C) for 24 to 30 hr prior to the addition of cycloheximide; and (iii) in heterokaryons of SV40-transformed hamster and susceptible monkey kidney cells. DNA synthesis was determined by pulse-labeling the cultures with 3H-thymidine at various times from 24 to 46 hr after infection. In addition, the total infectious SV40 DNA was measured. Addition of cycloheximide, even after early proteins had been induced, grossly inhibited both SV40 and cellular DNA syntheses. The activities of thymidine kinase, DNA polymerase, deoxycytidylate deaminase, and thymidylate kinase were measured; these enzyme activities remained high for at least 9 hr in the presence of cycloheximide. SV40 DNA prelabeled with 3H-thymidine before the addition of cycloheximide was also relatively stable during the time required for cycloheximide to inhibit further DNA replication.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedson H. S., Cruickshank J. G. Multiple protein functions in the replication of pox virus DNA. J Gen Virol. 1968 Jul;3(1):147–151. doi: 10.1099/0022-1317-3-1-147. [DOI] [PubMed] [Google Scholar]
- Bode V. C., Kaiser A. D. Changes in the structure and activity of lambda DNA in a superinfected immune bacterium. J Mol Biol. 1965 Dec;14(2):399–417. doi: 10.1016/s0022-2836(65)80190-5. [DOI] [PubMed] [Google Scholar]
- Comings D. E., Kakefuda T. Initiation of deoxyribonucleic acid replication at the nuclear membrane in human cells. J Mol Biol. 1968 Apr 14;33(1):225–229. doi: 10.1016/0022-2836(68)90290-8. [DOI] [PubMed] [Google Scholar]
- Dove W. F., Weigle J. J. Intracellular state of the chromosome of bacteriophage lambda. I. The eclipse of infectivity of the bacteriophage DNA. J Mol Biol. 1965 Jul;12(3):620–629. doi: 10.1016/s0022-2836(65)80316-3. [DOI] [PubMed] [Google Scholar]
- Dubbs D. R., Kit S. Isolation of defective lysogens from Simian virus 40-transformed mouse kidney cultures. J Virol. 1968 Nov;2(11):1272–1282. doi: 10.1128/jvi.2.11.1272-1282.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle H., Lark K. G. Chromosome replication in Bacillus subtilis cultures growing at different rates. Proc Natl Acad Sci U S A. 1967 Jan;57(1):95–101. doi: 10.1073/pnas.57.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee L. L., Sinsheimer R. L. The process of infection with bacteriophage phi X174. XVII. Effects of specific metabolic interruptions. J Mol Biol. 1968 Mar 14;32(2):303–320. doi: 10.1016/0022-2836(68)90011-9. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Riggs A. D. On the mechanism of DNA replication in mammalian chromosomes. J Mol Biol. 1968 Mar 14;32(2):327–341. doi: 10.1016/0022-2836(68)90013-2. [DOI] [PubMed] [Google Scholar]
- Jacob F., Ryter A., Cuzin F. On the association between DNA and membrane in bacteria. Proc R Soc Lond B Biol Sci. 1966 Mar 22;164(995):267–278. doi: 10.1098/rspb.1966.0029. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., HSU T. C. Biochemistry of vaccinia-infected mouse fibroblasts (strain L-M). III. Radioautographic and biochemical studies of thymidine-H3 uptake into DNA of L-M cells and rabbit cells in primary culture. Virology. 1963 Jan;19:13–22. doi: 10.1016/0042-6822(63)90019-9. [DOI] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Relationship between protein synthesis and viral deoxyribonucleic acid synthesis. J Virol. 1967 Feb;1(1):110–114. doi: 10.1128/jvi.1.1.110-114.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., De Torres R. A., Dubbs D. R. Arabinofuranosylcytosine-induced stimulation of thymidine kinase and deoxycytidylic deaminase activities of mammalian cultures. Cancer Res. 1966 Sep;26(9):1859–1866. [PubMed] [Google Scholar]
- Kit S., De Torres R. A., Dubbs D. R., Salvi M. L. Induction of cellular deoxyribonuleic acid synthesis by simian virus 40. J Virol. 1967 Aug;1(4):738–746. doi: 10.1128/jvi.1.4.738-746.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Frearson P. M., Melnick J. L. Enzyme induction in SV40-infected green monkey kidney cultures. Virology. 1966 May;29(1):69–83. doi: 10.1016/0042-6822(66)90197-8. [DOI] [PubMed] [Google Scholar]
- Kit S., Kurimura T., Salvi M. L., Dubbs D. R. Activation of infectious SV40 synthesis in transformed cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1239–1246. doi: 10.1073/pnas.60.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Melnick J. L., Anken M., Dubbs D. R., De Torres R. A., Kitahara T. Nonidentiy of some simian virus 40-induced enzymes with tumor antigen. J Virol. 1967 Aug;1(4):684–692. doi: 10.1128/jvi.1.4.684-692.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Piekarski L. J., Dubbs D. R. DNA polymerase induced by Simian virus 40. J Gen Virol. 1967 Apr;1(2):163–173. doi: 10.1099/0022-1317-1-2-163. [DOI] [PubMed] [Google Scholar]
- Knippers R., Sinsheimer R. L. Process of infection with bacteriophage phiX174. XX. Attachment of the parental DNA of bacteriophage phiX174 to a fast-sedimenting cell component. J Mol Biol. 1968 May 28;34(1):17–29. doi: 10.1016/0022-2836(68)90231-3. [DOI] [PubMed] [Google Scholar]
- Lark K. G., Lark C. Regulation of chromosome replication in Escherichia coli: a comparison of the effects of phenethyl alcohol treatment with those of amino acid starvation. J Mol Biol. 1966 Sep;20(1):9–19. doi: 10.1016/0022-2836(66)90113-6. [DOI] [PubMed] [Google Scholar]
- Lark K. G. Regulation of chromosome replication and segregation in bacteria. Bacteriol Rev. 1966 Mar;30(1):3–32. doi: 10.1128/br.30.1.3-32.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUELLER G. C., KAJIWARA K., STUBBLEFIELD E., RUECKERT R. R. Molecular events in the reproduction of animal cells. I. The effect of puromycin on the duplication of DNA. Cancer Res. 1962 Oct;22:1084–1090. [PubMed] [Google Scholar]
- Porter B., Fraser D. K. Involvement of protein and ribonucleic acid in the association of deoxyribonucleic acid with other cell components of Escherichia coli. J Bacteriol. 1968 Jul;96(1):98–104. doi: 10.1128/jb.96.1.98-104.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sved J. A. Telomere attachment of chromosomes. Some genetical and cytological consequences. Genetics. 1966 Apr;53(4):747–756. doi: 10.1093/genetics/53.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor E. W. Control of DNA synthesis in mammalian cells in culture. Exp Cell Res. 1965 Nov;40(2):316–332. doi: 10.1016/0014-4827(65)90265-x. [DOI] [PubMed] [Google Scholar]
- Taylor J. H. Rates of chain growth and units of replication in DNA of mammalian chromosomes. J Mol Biol. 1968 Feb 14;31(3):579–594. doi: 10.1016/0022-2836(68)90429-4. [DOI] [PubMed] [Google Scholar]
- Terasima T., Yasukawa M. Synthesis of G1 protein preceding DNA synthesis in cultured mammalian cells. Exp Cell Res. 1966 Nov-Dec;44(2):669–672. doi: 10.1016/0014-4827(66)90482-4. [DOI] [PubMed] [Google Scholar]
- Weil R., Michel M. R., Ruschmann G. K. Induction of cellular DNA synthesis by polyoma virus. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1468–1475. doi: 10.1073/pnas.53.6.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG E. T., 2nd, SINSHEIMER R. L. NOVEL INTRA-CELLULAR FORMS OF LAMBDA DNA. J Mol Biol. 1964 Dec;10:562–564. doi: 10.1016/s0022-2836(64)80080-2. [DOI] [PubMed] [Google Scholar]


