Abstract
Many studies have demonstrated that hepatic fibrosis is reversible. Regression of liver fibrosis is associated with resorption of fibrous scar and disappearance of collagen producing myofibroblasts. The fate of these myofibroblasts has been recently revealed: Some myofibroblasts undergo senescence and apoptose during reversal of fibrosis, while other myofibroblasts revert to a quiescent-like phenotype. Inactivation of myofibroblasts is a newly described phenomenon1 which now requires mechanistic investigation. Understanding of the mechanism of HSC inactivation upon cessation of fibrogenic stimuli may identify new approaches to revert already existing aHSCs/myofibroblasts into a quiescent-like state. This review summarizes the research on the inactivation of hepatic myofibroblasts.
Keywords: Reversibility of liver fibrosis, Hepatic fibrosis, Inactivation of myofibroblasts, Hepatic stellate cells, HSCs, Collagen, Pathobiology
Introduction
Hepatic fibrosis is an outcome of many chronic liver diseases, including hepatitis B virus (HBV), hepatitis C virus (HCV), alcoholic liver disease and non-alcoholic steatohepatitis (NASH) 2. Hepatic fibrosis is characterized by extensive deposition of extracellular matrix proteins (ECMs), mostly type I collagen, forming a scar. Chronic liver injury damages hepatocytes. Injured or apoptotic hepatocytes secrete factors that facilitate activation and recruitment of inflammatory cells to the injured liver. Activated macrophages secrete IL-6 and TGF-β1, which in turn, activate hepatic myofibroblasts. Myofibroblasts are not present in the normal liver, but in response to injury they transdifferentiate from hepatic stellate cells (HSCs), upregulate collagen and produce the fibrous scar. Myofibroblasts are characterized by expression of a-smooth muscle actin (α-SMA) and type I collagen, and in all clinical and experimental liver fibrosis serve as a major source of the ECM. Thus, activation and proliferation of hepatic myofibroblasts is a key mechanism in the development of liver cirrhosis.
Myofibroblasts are the primary target of anti-fibrotic therapy
Hepatic myofibroblasts are the major source of collagen Type I in fibrotic liver. Therefore, elimination of myofibroblasts or their inactivation is a goal for therapy. Several sources of myofibroblasts have been identified3–6. It is believed that hepatic stellate cells (HSCs) are the major source of fibrogenic myofibroblasts, and contribute > 80% of the collagen producing cells 2, 1,7. Under physiological conditions, HSCs reside in the space of Disse and exhibit a quiescent phenotype (qHSCs). They express neural markers, such as GFAP, synemin, synaptophysin, and nerve growth factor receptor p75, and store vitamin A in lipid droplets3. In response to injury, qHSCs decrease vitamin A storage and peroxisome proliferator-activated receptor gamma (PPARγ) expression, and activate into collagen type I and α-SMA expressing myofibroblasts2. Although the mechanism of HSC activation has been comprehensively studied, insights into the fate of HSCs during regression of liver fibrosis are new.8 In addition to HSCs, portal fibroblasts9,10 and bone marrow (BM)-derived fibrocytes 11 can also contribute to hepatic myofibroblasts.
Reversal of liver fibrosis
Mechanism of regression of liver fibrosis
Sequential liver biopsies from patients with liver fibrosis have demonstrated that removing the underlying etiological agent may reverse hepatic fibrosis in patients with secondary biliary fibrosis 12, Hepatitis C 13, Hepatitis B 14, NASH 15, and autoimmune hepatitis 16. Withdrawal of the etiological source of the chronic injury (e.g. HBV, HCV) 2 results in decrease of pro-inflammatory and fibrogenic (including TGFβ1) cytokines, increased collagenase activity 2,3, decreased ECM production, and the disappearance of activated myofibroblasts.
Studies of liver fibrosis in rodents have confirmed that established liver fibrosis can reverse upon cessation of etiological agent. Reversal of liver fibrosis has been successfully studied in experimental models CCl4 17,18, alcohol and BDL 19 induced liver fibrosis 2. Several events have been identified to be critical for regression of liver fibrosis. These include disappearance of hepatic myofibroblasts/aHSCs, recruitment of collagenese secreting monocytes/macrophages, and prevalence of matrix degrading metalloproteinases over their natural inhibitors TIMPs.
Conditions for reversal of liver fibrosis
BM-derived monocytes and liver resident macrophages play an important role in reversal of liver fibrosis. Selective ablation of CD11b+ cells in mice (CD11b-DTR) during the recovery phase from liver injury significantly attenuates fibrosis resolution, suggesting that macrophages mediate distinct functions at the onset of fibrosis and during recovery. Increased collagenase activity is a primary pathway of fibrosis resolution. At this stage, activated macrophages/Kupffer cells secrete matrix metalloproteinases, e.g. MMP-13 interstitial collagenase, and other enzymes responsible for matrix degradation 6,20. Moreover, increased activity of collagen degrading enzymes during fibrosis resolution correlates with decreased amount of TIMPs, tissue inhibitors of matrix metalloproteinasesis21,22. Activated myofibroblasts/HSCs serve as a significant source of TIMPs, and disappearance of myofibroblasts/HSCs during recovery is associated with reduced production of TIMPs.
An established mechanism for the elimination of activated myofibroblasts is due to senescence23 and apoptosis of activated HSCs 24. Several mechanisms are implicated in the apoptosis of activated HSCs: 1). Activation of death receptor-mediated pathways (Fas or TNFR-1 receptors) and caspases 8 and 3; 2) up-regulation of pro-apoptotic proteins (e.g. p53, Bax, caspase 9); and 3) decrease of pro-survival genes (e.g. Bcl-2) 22. A population of liver associated natural killer (NK) cells and γδT (NKT) cells stimulate apoptosis of activated HSCs. Drugs that induce apoptosis in activated HSCs (glyotoxin, sulfasalazine, IKK inhibitors, and anti-TIMP antibodies) cause liver fibrosis to regress 2,3.
Conditions for irreversible liver fibrosis
Whether end-stage cirrhosis can reverse to a normal liver architecture remains controversial25,26. However, significant improvement in hepatic structure and function provide evidence of regression of liver fibrosis21,27. Perhaps ECM remodeling is limited in cirrhosis by formation of non-reducible cross-linked collagen and an ECM rich with elastin fibers preventing its degradation. This pathophysiological state may lead to a “point of no return” for liver fibrosis26,27. The characteristics of myofibroblast environment play a critical role in myofibroblast survival. For example, stiffness of extracellular matrix and increased contact between myofibroblasts and collagen scar promote survival of myofibroblasts and facilitate their activation28. In support of this notion, transgenic mice expressing an uncleavable form of Collagen Type I are more susceptible to liver fibrosis, and demonstrate a defect in spontaneous resolution of liver fibrosis upon cessation of liver injury29. Similar to these transgenic mice, prolong duration of fibrogenic liver injury often results in formation of persistent and uncleavable scars caused by irreversible crosslinking of collagen fibers. Furthermore, the presence of elastin fibers distinguishes “biochemically mature scars”, which are more likely to persist in recovering liver18. In addition, persistent scars are associated with formation of areas of hypocellularity, suggesting that lack of biodegrading macrophages in these areas may contribute to poor desorption of fibrous scars18.
The role of myofibroblasts in reversal of liver fibrosis
Although activated HSCs undergo senescence 23 and apoptose 17 during the regression of liver fibrosis, the quantitative contribution aHSC apoptosis to disappearance of activated myofibroblasts remained unknown 27. Meanwhile, the cellular population of HSCs is restored in mice recovering from fibrosis, and the source of these quiescent-like HSCs is unknown. Recent studies have suggested that in addition to apoptosis, activated HSCs/myofibroblasts can be eliminating by undergoing inactivation and reverting to a quiescent-like their phenotype. We1, and subsequently others30, have used genetic marking to demonstrate an alternative pathway in which myofibroblasts revert to a quiescent-like phenotype in CCl4-induced liver injury and experimental alcoholic liver disease. These in vivo studies are in concordance with in vitro observations that suggested that cultured HSCs, at least in part, can reverse to a quiescent-like phenotype. The quiescent phenotype of HSCs is associated with expression of lipogenic genes and storage of vitamin A in lipid droplets. Depletion of peroxisome proliferator-activated receptor gamma (PPAR-γ) constitutes a key molecular events for HSC activation, and ectopic expression of this nuclear receptor results in the phenotypic reversal of activated HSC to quiescent cells in culture 31. The treatment of activated HSC with an adipocyte differentiation cocktail or over-expression of SREBP-1c results in up-regulation of adipogenic transcription factors and causes morphologic and biochemical reversal of activated HSC to quiescent-like cells 21,32,33. These in vitro and in vivo studies have provided new insights into the concept of reversibility of liver fibrosis, suggesting that disappearance of activated myofibroblasts is attributed not only to their apoptosis but also to reversal of their phenotype into a quiescent-like.
Methods to study inactivation of HSCs (iHSCs)8
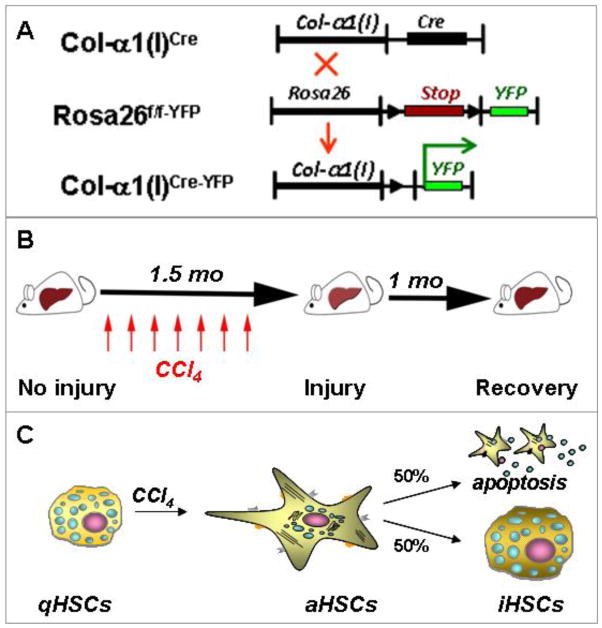
The Cre-lox-system34 provides a unique tool to monitor specific cellular populations and their progeny in mice 35. This system is based on the ability of the small 38-kDa bacteriophage protein Cre-recombinase (Cre) to recognize and excise inverted 13 base pair loxP sequences and the DNA it flanks 34,36. Genetic labeling of a specific cellular population is achieved by crossing mice expressing Cre under control of a cell-specific promoter with reporter Rosa26f/f-YFP mice, ubiquitously expressing the yfp gene in which transcription is blocked by a floxed Stop cassette 36. Genetic Cre-loxP recombination causes excision of the floxed-Stop-floxed sequence from genomic DNA with activation of YFP transcription in the resulting offspring. The cells of interest now irreversibly express YFP so that their phenotypic changes can be monitored in response to injury or stress 37.
Using Cre-LoxP-based genetic labeling of myofibroblasts, we1 and the others30 elucidated the fate of activated (a)HSCs/myofibroblasts during recovery from CCl4-induced liver fibrosis (Figure 1A)8. Genetic labeling of aHSC/myofibroblasts resulted from crossing mice expressing Cre under control of the collagen-α1(I) enhancer/promoter (Colα1(I)Cre mice) with reporter mice (Rosa26f/f-YFP mice). In the offspring (Colα1(I)Cre-YFP mice), all collagen Type I-expressing cells expressed YFP. In uninjured mice, collagen Type I is not expressed in the liver, and this correlates with minimal expression of YFP in livers of Colα1(I)Cre-YFP mice. Following induction of liver injury in these mice, aHSCs and their progeny express Collagen-driven YFP and are permanently labeled by YFP expression1. Phenotypical changes of aHSCs and the mechanism of their inactivation can now be studied during regression of liver fibrosis (Figure 1B)8. Using two models of hepatotoxic-induced liver fibrosis (carbon-tertrachloride (CCl4) and intragastric alcohol feeding), we demonstrated that half of the myofibroblasts escape apoptosis during regression of liver fibrosis, downregulate fibrogenic genes (Col1a1, Col1a2, SMA, TIMP1, TGFβ-RI) and acquire a phenotype similar to, but distinct from, quiescent HSCs1. Similar results were obtained using inducible Cre-based systems, in which genetic labeling of aHSCs was achieved in Collagen-α1(I)-ER-Cre and Vimentin-ER-Cre upon tamoxifen administration.
Figure 1.
Study design to determine the cell fate of aHSCs during regression of CCl4-induced liver fibrosis. Adapted from Kisseleva and Brenner [8]. A. Cre-loxP based genetic labeling marks the fate of Col-α1(I) expressing aHSCs/ myofibroblasts in Col-α1(I)Cre-YFP mice generated by crossing Col-α1(I)Cre and Rosa26f/f-YFP mice. B. Col-α1(I)Cre-YFP mice were subjected to CCl4-induced liver injury (1.5 months), then recuperated upon cessation of injuring agent (for 1 month). Mice were sacrificed and livers were analyzed for the presence of Vitamin A+ YFP+ and Vitamin A+ YFP− HSCs. C. CCl4 induces qHSC activation into aHSCs/myofibroblasts in Col-α1(I)Cre-YFP mice. After CCl4 withdrawal, some aHSCs apoptose while some inactivate (YFP+ iHSCs number <100% of aHSCs)6.
Generation of these novel transgenic mice provided a unique tool to study the fate of hepatic myofibroblasts in fibrotic liver. In addition, several studies have utilized glial fibrillar acidic protein (GFAP)-Cre mice to successfully target HSCs in the liver. Although these GFAP-Cre mice do not discriminate between quiescent, activated and inactivated HSC phenotypes, they were successfully used to label HSCs for quantification purposes or for HSC specific gene deletion. Since HSCs and astrocytes share expression of several neural markers, including GFAP, deletion of this gene in the brain might affect the phenotype in the liver.
Characterization of novel inactivated HSC phenotype
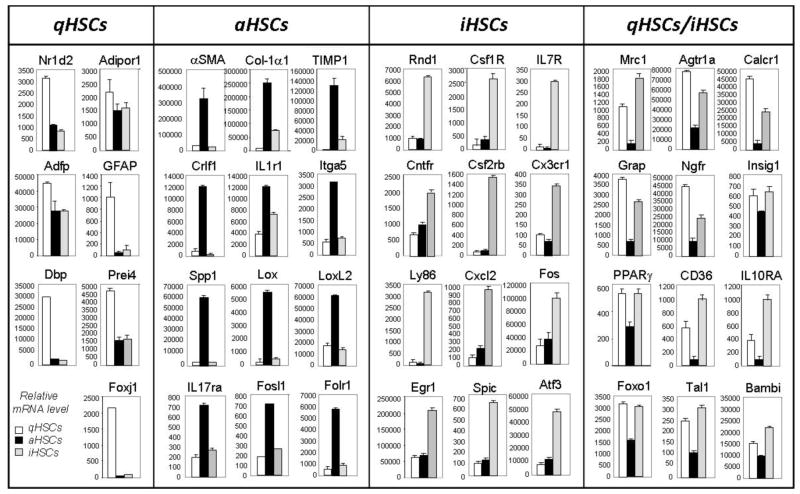
Inactivated HSCs acquire a novel phenotype which has not been previously described and is now designated iHSCs (Figure 1C)8. In particular, iHSCs more rapidly reactivate into myofibroblasts in response to fibrogenic stimuli and more effectively contribute to liver fibrosis. Inactivated HSCs downregulate SMA, Col1a1, Col1a2, TIMP1, TGFβ-RI and obtain features of quiescent-like HSCs due to upregulation of PPAR-γ and Bambi1. Meanwhile, other quiescent-associated genes such as GFAP, Adipor1, Adpf, and Dbp are not re-expressed in iHSCs, suggesting that despite the similarities between qHSCs and iHSCs and their lack of fibrogenic gene expression, iHSCs possess properties distinct from qHSCs.
Inactivation of HSCs is associated with re-expression of lipogenic genes PPAR-γ, Insig1, and CREBP31. Our findings in mice in vivo support previous in vitro studies demonstrating the importance of PPAR-γ for maintaining and re-establishing the quiescent phenotype (qHSCs)31,33. Based on comparison of the global gene expression in qHSCs, aHSCs and iHSCs, we have identified several genes that are differentially expressed in HSCs depending on their stage of activation, and can be used to distinguish iHSCs from qHSCs and aHSCs1. This is of a particular importance for identifying inactivation of human HSCs. Our strategy is based on identification of genes similarly and differentially expressed in qHSCs, aHSCs and iHSCs, and that can be easily detected in a pool of HSC by flow cytometry. Differential expression of cell surface antigens by different HSC types is listed below. Since qHSCs and iHSCs share many features, detection of surface markers may be compared in correlation with induction of phenotype specific transcription factors, or other proteins.
Comparative analysis of phenotype-specific HSC signature genes may provide further insight in inactivation of HSCs in vivo (Figure 2). Specifically, we identified 7 qHSC-specific genes (that are expressed only in qHSCs), 12 aHSC-specific genes (expressed only in aHSCs), 12 iHSC-specific genes (upregulated in iHSCs), and 12 genes which are upregulated in both qHSCs and I HSCs (and therefore are associated with non-fibrogenic properties of HSCs). Furthermore, the following genes Nr1d2, Adipor1, IL17ra, Itga5, Egfr, Crlf1, IL1r1, Rnd1, Csf1R, IL7R, Cntfr, Csf2rb, Cx3cr1, IL10RA, Ly86, CD36, Mrc1, Agtr1a, Calcr1, Grap, and Ngfr are cell surface receptors, so that their expression can be detected using flow cytometry (which can simultaneously detect expression of up to 11–12 surface antigens in a single sample) allowing us to confidently identify and isolate live iHSCs. Furthermore, we identified several phenotype-specific transcription factors expressed in HSCs dependent on the stage of activation/inactivation.
Figure 2.
Differential expression of cell surface antigens by different HSC types. Based on the whole genome microarray, we have identified mRNAs that are specifically upregulated in qHSCs: Nr1d2; Adipor1 - Adiponectin receptor 1, Adfp - Adipose differentiation-related protein, GFAP - Glial fibrillary acidic protein, Dbp - D site of albumin promoter (albumin D-box) binding protein, Prei4; Foxj1 - Forkhead box protein J1. The mRNAs were specifically upregulated in aHSCs: αSMA - Alpha-actin-2 (also known as actin, aortic smooth muscle or alpha smooth muscle actin); Col1 1; TIMP-1 - tissue inhibitor of metalloproteinase 1, Crlf1 – cytokine receptor-like factor 1; IL1r1 - Interleukin 1 receptor, type I (IL1R1, CD121a), Itga5 - Integrin alpha-5; Spp1 - secreted phosphoprotein 1 (Osteopontin (OPN), also known as bone sialoprotein I (BSP-1 or BNSP), early T-lymphocyte activation, ETA-1); Lox - lysyl oxidase; LoxL2 - lysyl oxidase-like 1; IL-17ra - Interleukin 17 receptor A; Fosl1 - fos-like antigen 1; Folr1 - folate receptor 1. The following mRNA s were upregulated in iHSCs: Rnd1 - Rho family GTPase 1; Csf1R - colony stimulating factor 1 receptor; IL7R - interleukin-7 receptor; Cntfr - ciliary neurotrophic factor receptor; Csf2rb - colony stimulating factor 2 receptor, beta, low-affinity (granulocyte-macrophage); Cx3cr1 - CX3C chemokine receptor 1 (fractalkine receptor or G-protein coupled receptor 13, GPR13); Ly86 - lymphocyte antigen 86 (CD180/MD-1), Cxcl2 - chemokine (C-X-C motif) ligand 2 (Cxcl2); Fos - FBJ osteosarcoma oncogene; Egr1 - epidermal growth factor receptor (EGFR; ErbB-1; HER1 in humans); Spic - Spi-C transcription factor (Spi-1/PU.1 related); Atf3 - activating transcription factor 3. Several mRNAs were upregulated both in qHSCs and iHSCs: Mrc1 - mannose receptor 1, Agtr1a - Angiotensin II receptor, type 1 (or AT1 receptor); Calcrl - calcitonin receptor-like; Grap - GRB2-related adapter protein, Ngfr - The Low-Affinity Nerve Growth Factor Receptor (also called the LNGFR or p75 neurotrophin receptor); Insig1 - insulin induced gene 1; PPARγ-peroxisome proliferator activated receptor gamma; CD36; IL-10RA - interleukin 10 receptor, alpha; Foxo1 - forkhead box O1; Tal1 - helix-loop-helix protein; Bambi - BMP and activin membrane-bound inhibitor, homolog (Xenopus laevis).
Conclusions
Inactivation of myofibroblasts during reversal of fibrosis opens new prospects for therapy. Hepatic fibrosis is reversible in patients and in experimental models with decreased fibrous scar and disappearance of the myofibroblast population. However, the fate of the myofibroblasts in patients with liver fibrosis is unknown. Although some myofibroblasts undergo cell death17, an alternative untested hypothesis is that the myofibroblasts revert to their original quiescent phenotype or obtain a new phenotype. Understanding of the origin and biology of fibrogenic myofibroblasts will provide a new target for anti-fibrotic therapy.21
Abbreviations
- HSCs
hepatic stellate cells
- qHSCs
quiescent HSCs
- aHSCs
activated HSCs
- iHSCs
inactivated HSCs
- CCl4
carbon tetrachloride
- α-SMA
α-smooth muscle actin
- Col-α2(I)
Collagen-α2(I)
- Col-α1(I)
Collagen-α1(I)
- Col-α1(1)Cre-YFP mice
Col-α1(I)Cre mice x Rosa26flox-Stop-flox-YFP mice
Footnotes
Human and Animal Rights and Informed Consent
This article does not contain any studies with human subjects performed by any of the authors.
With regard to the authors’ research cited in this paper, all institutional and national guidelines for the care and use of laboratory animals were followed.
Compliance with Ethics Guidelines
Conflict of Interest
Xiao Liu and Jun Xu declare that they have no conflict of interest.
David A. Brenner holds a patent for inducing inactivation of fibrogenic myofibroblasts. Tatiana Kisseleva holds a patent for inducing inactivation of fibrogenic myofibroblasts, and has received research support from National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (R56 DK088837-01A10.
References
Recently published papers of particular interest have been highlighted as:
*Of importance
**Of major importance
- 1**.Kisseleva T, et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:9448–9453. doi: 10.1073/pnas.1201840109. This research article, along with the one by Troeger [30], demonstrated for the first time that aHSCs can inactivate, initiating a new area of research and providing new direction for anti-fibrotic therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kisseleva T, Brenner DA. Hepatic stellate cells and the reversal of fibrosis. Journal of gastroenterology and hepatology. 2006;21 (Suppl 3):S84–87. doi: 10.1111/j.1440-1746.2006.04584.x. [DOI] [PubMed] [Google Scholar]
- 4.Kalluri R, Neilson EG. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomperts BN, Strieter RM. Fibrocytes in lung disease. Journal of leukocyte biology. 2007;82:449–456. doi: 10.1189/jlb.0906587. [DOI] [PubMed] [Google Scholar]
- 6.Fallowfield JA, et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol. 2007;178:5288–5295. doi: 10.4049/jimmunol.178.8.5288. [DOI] [PubMed] [Google Scholar]
- 7.Parola M, Marra F, Pinzani M. Myofibroblast - like cells and liver fibrogenesis: Emerging concepts in a rapidly moving scenario. Molecular aspects of medicine. 2008;29:58–66. doi: 10.1016/j.mam.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 8*.Kisseleva T, Brenner DA. Inactivation of myofibroblasts during regression of liver fibrosis. Cell cycle. 2013;12:381–382. doi: 10.4161/cc.23549. This review article, along with the 2011 review also by Kisseleva and Brenner [21], briefly summarizes the current concept of regression of liver fibrosis as well as the disappearance of aHSCs/myofibroblasts by apoptosis and inactivation, and outlines recent advances in anti-fibrotic therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang L, Tanaka Y, Marumo F, Sato C. Phenotypic change in portal fibroblasts in biliary fibrosis. Liver. 1994;14:76–82. doi: 10.1111/j.1600-0676.1994.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 10.Tuchweber B, Desmouliere A, Bochaton-Piallat ML, Rubbia-Brandt L, Gabbiani G. Proliferation and phenotypic modulation of portal fibroblasts in the early stages of cholestatic fibrosis in the rat. Lab Invest. 1996;74:265–278. [PubMed] [Google Scholar]
- 11.Kisseleva T, Brenner DA. Fibrogenesis of parenchymal organs. Proc Am Thorac Soc. 2008;5:338–342. doi: 10.1513/pats.200711-168DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hammel P, et al. Regression of liver fibrosis after biliary drainage in patients with chronic pancreatitis and stenosis of the common bile duct. The New England journal of medicine. 2001;344:418–423. doi: 10.1056/NEJM200102083440604. [DOI] [PubMed] [Google Scholar]
- 13.Arthur MJ. Reversibility of liver fibrosis and cirrhosis following treatment for hepatitis C. Gastroenterology. 2002;122:1525–1528. doi: 10.1053/gast.2002.33367. [DOI] [PubMed] [Google Scholar]
- 14.Kweon YO, et al. Decreasing fibrogenesis: an immunohistochemical study of paired liver biopsies following lamivudine therapy for chronic hepatitis B. J Hepatol. 2001;35:749–755. doi: 10.1016/s0168-8278(01)00218-5. [DOI] [PubMed] [Google Scholar]
- 15.Dixon JB, Bhathal PS, Hughes NR, O’Brien PE. Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647–1654. doi: 10.1002/hep.20251. [DOI] [PubMed] [Google Scholar]
- 16.Czaja AJ, Carpenter HA. Decreased fibrosis during corticosteroid therapy of autoimmune hepatitis. J Hepatol. 2004;40:646–652. doi: 10.1016/j.jhep.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 17.Iredale JP, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Issa R, et al. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2004;126:1795–1808. doi: 10.1053/j.gastro.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Issa R, et al. Apoptosis of hepatic stellate cells: involvement in resolution of biliary fibrosis and regulation by soluble growth factors. Gut. 2001;48:548–557. doi: 10.1136/gut.48.4.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uchinami H, Seki E, Brenner DA, D’Armiento J. Loss of MMP 13 attenuates murine hepatic injury and fibrosis during cholestasis. Hepatology. 2006;44:420–429. doi: 10.1002/hep.21268. [DOI] [PubMed] [Google Scholar]
- 21*.Kisseleva T, Brenner DA. Anti-fibrogenic strategies and the regression of fibrosis. Best Pract Res Clin Gastroenterol. 2011;25:305–317. doi: 10.1016/j.bpg.2011.02.011. This review article, along with the 2013 review also by Kisseleva and Brenner [8], briefly summarizes the current concept of regression of liver fibrosis as well as the disappearance of aHSCs/myofibroblasts by apoptosis and inactivation, and outlines recent advances in anti-fibrotic therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iredale JP. Hepatic stellate cell behavior during resolution of liver injury. Semin Liver Dis. 2001;21:427–436. doi: 10.1055/s-2001-17557. [DOI] [PubMed] [Google Scholar]
- 23.Krizhanovsky V, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122:286S–289S. doi: 10.1378/chest.122.6_suppl.286s. [DOI] [PubMed] [Google Scholar]
- 25.Friedman SL, Bansal MB. Reversal of hepatic fibrosis -- fact or fantasy? Hepatology. 2006;43:S82–88. doi: 10.1002/hep.20974. [DOI] [PubMed] [Google Scholar]
- 26.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117:539–548. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varga J, Brenner D, Phan SE. Fibrosis Research. Methods and Protocols. Humana Press; Totowa, New Jersey: 2005. [Google Scholar]
- 28.Smit JJ, et al. Homozygous disruption of the murine mdr2 P-glycoprotein gene leads to a complete absence of phospholipid from bile and to liver disease. Cell. 1993;75:451–462. doi: 10.1016/0092-8674(93)90380-9. [DOI] [PubMed] [Google Scholar]
- 29.Issa R, et al. Mutation in collagen-1 that confers resistance to the action of collagenase results in failure of recovery from CCl4-induced liver fibrosis, persistence of activated hepatic stellate cells, and diminished hepatocyte regeneration. FASEB J. 2003;17:47–49. doi: 10.1096/fj.02-0494fje. [DOI] [PubMed] [Google Scholar]
- 30**.Troeger JS, et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012;143:1073–1083. e1022. doi: 10.1053/j.gastro.2012.06.036. This research article, along with the one by Kisseleva [1], demonstrated for the first time that aHSCs can inactivate, initiating a new area of research and providing new direction for anti-fibrotic therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.She H, Xiong S, Hazra S, Tsukamoto H. Adipogenic transcriptional regulation of hepatic stellate cells. J Biol Chem. 2005;280:4959–4967. doi: 10.1074/jbc.M410078200. [DOI] [PubMed] [Google Scholar]
- 32.Tsukamoto H. Fat paradox in liver disease. Keio J Med. 2005;54:190–192. doi: 10.2302/kjm.54.190. [DOI] [PubMed] [Google Scholar]
- 33.Tsukamoto H. Adipogenic phenotype of hepatic stellate cells. Alcohol Clin Exp Res. 2005;29:132S–133S. doi: 10.1097/01.alc.0000189279.92602.f0. [DOI] [PubMed] [Google Scholar]
- 34.Ramirez-Solis R, Liu P, Bradley A. Chromosome engineering in mice. Nature. 1995;378:720–724. doi: 10.1038/378720a0. [DOI] [PubMed] [Google Scholar]
- 35.Perkins AS. Functional genomics in the mouse. Funct Integr Genomics. 2002;2:81–91. doi: 10.1007/s10142-002-0049-3. [DOI] [PubMed] [Google Scholar]
- 36.Sternberg N, Austin S, Hamilton D, Yarmolinsky M. Analysis of bacteriophage P1 immunity by using lambda-P1 recombinants constructed in vitro. Proc Natl Acad Sci U S A. 1978;75:5594–5598. doi: 10.1073/pnas.75.11.5594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sauer B. Inducible gene targeting in mice using the Cre/lox system. Methods. 1998;14:381–392. doi: 10.1006/meth.1998.0593. [DOI] [PubMed] [Google Scholar]